Photocatalytic Self-Fenton System of g-C3N4-Based for Degradation of Emerging Contaminants: A Review of Advances and Prospects
Abstract
1. Introduction
2. Fundamental Mechanisms of Degradation of Pollutants by Photocatalysis Self-Fenton Technology
2.1. Mechanisms of the H2O2 Production by Photocatalyst
2.2. Mechanisms of the Fe2+/H2O2-Mediated Fenton Reaction to Remove Emerging Pollutants
2.3. The Influence Factors of H2O2 Generation
2.4. The Influence Factors of Fenton Reaction
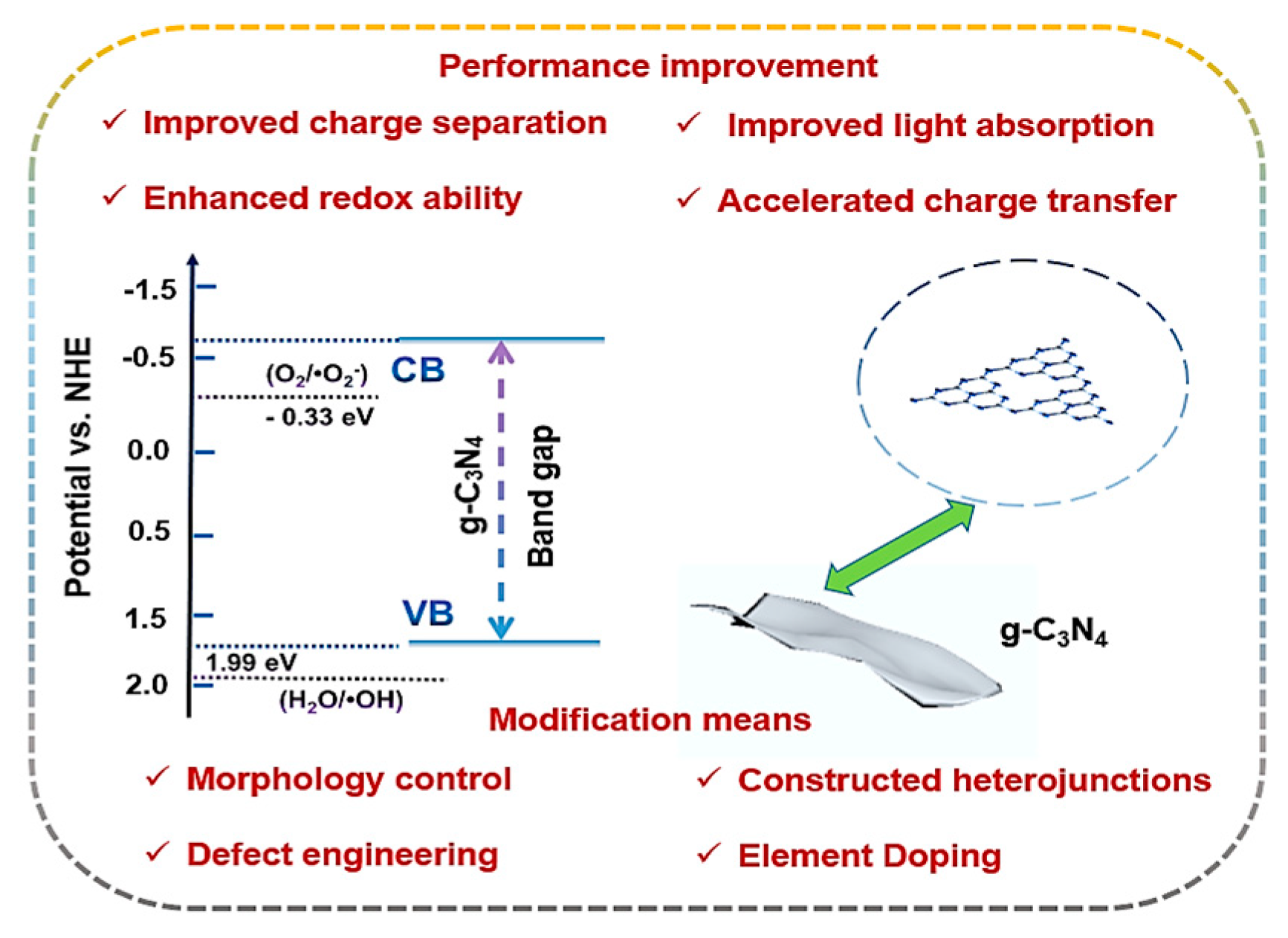
3. Graphite Carbon Nitride (g-C3N4) Material
3.1. Synthetic Methods and Morphology of g-C3N4
3.2. Band Structure of g-C3N4
4. Various Modification Strategies for Photocatalytic Self-Fenton Based on g-C3N4
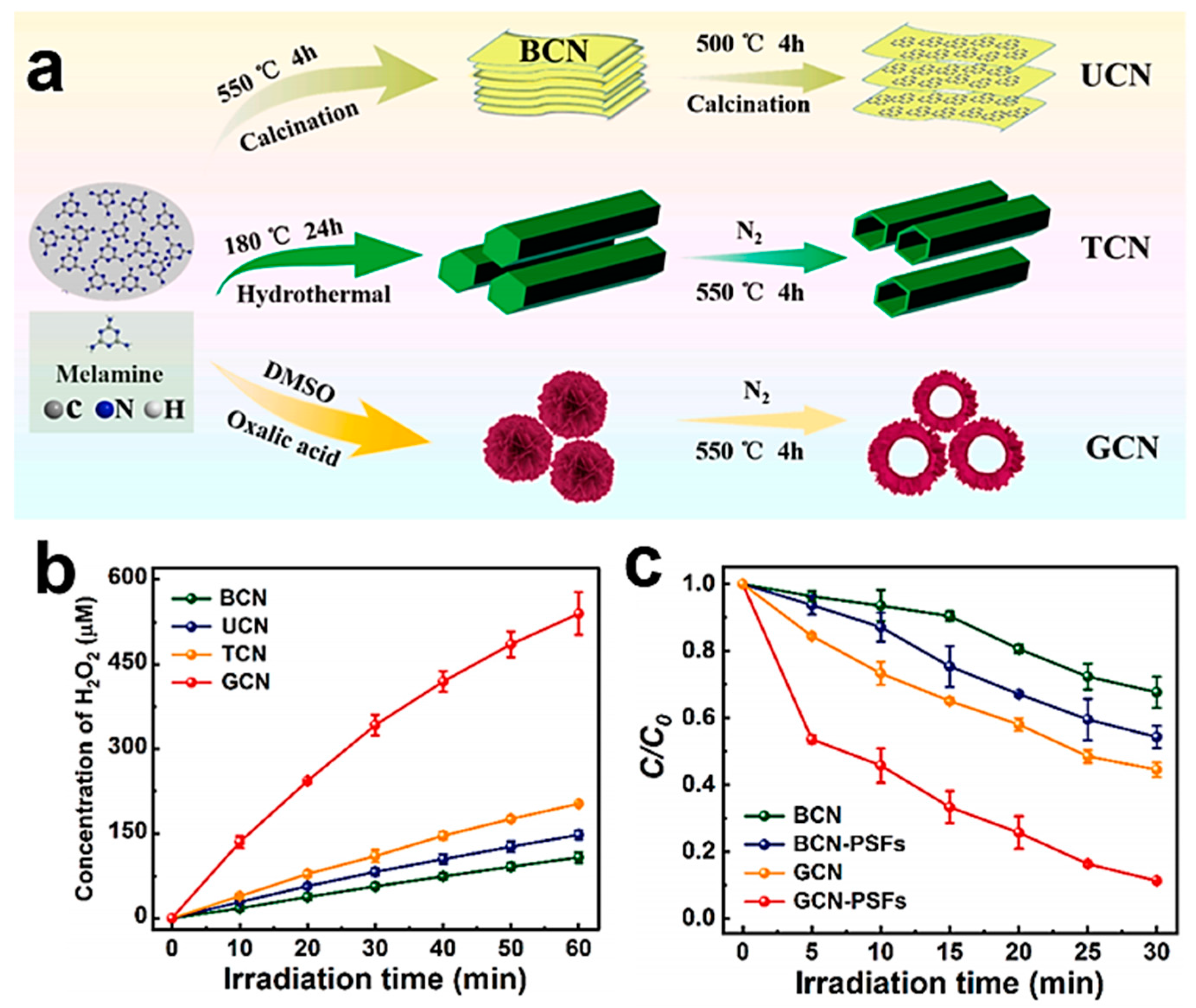
4.1. Morphology Control
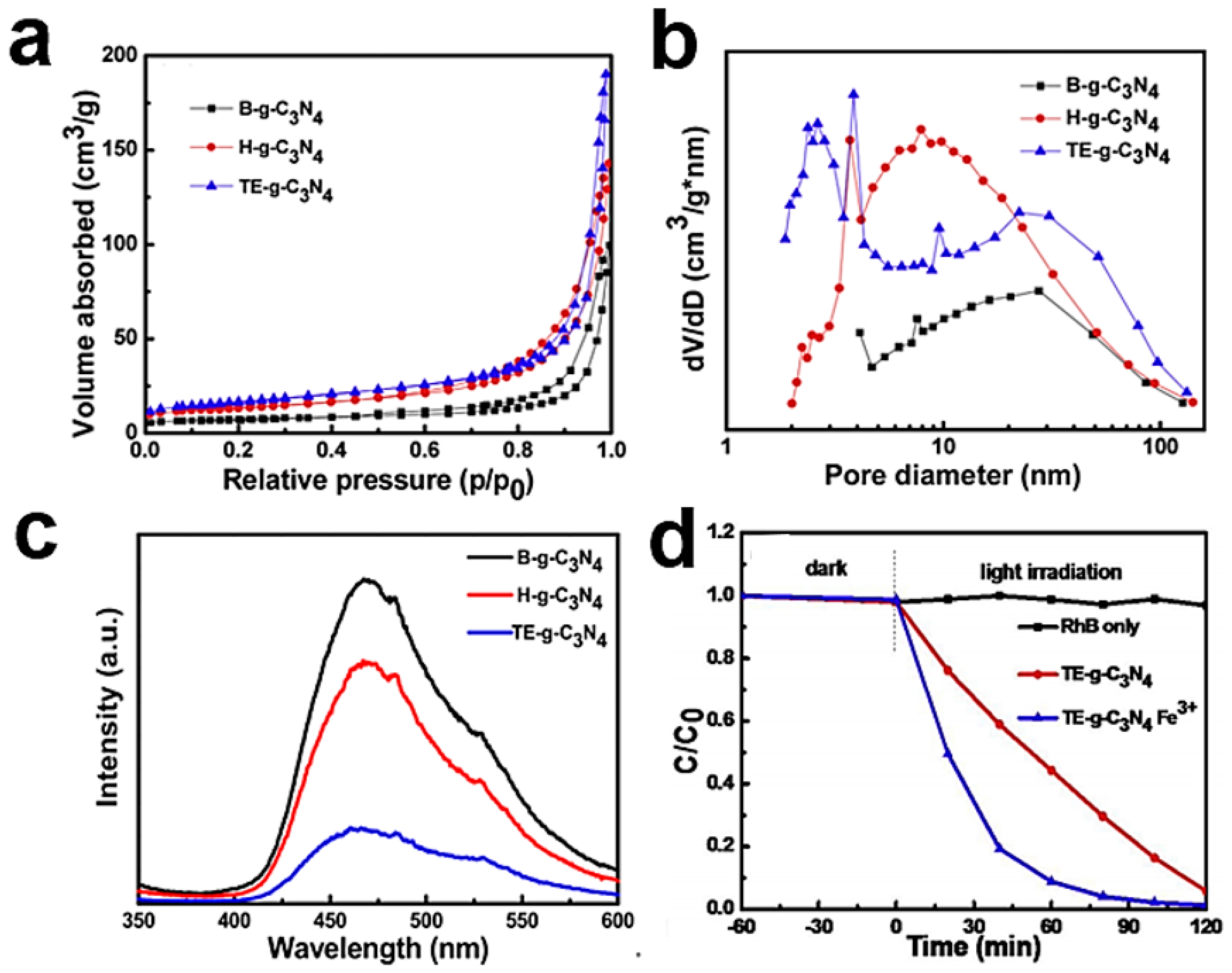
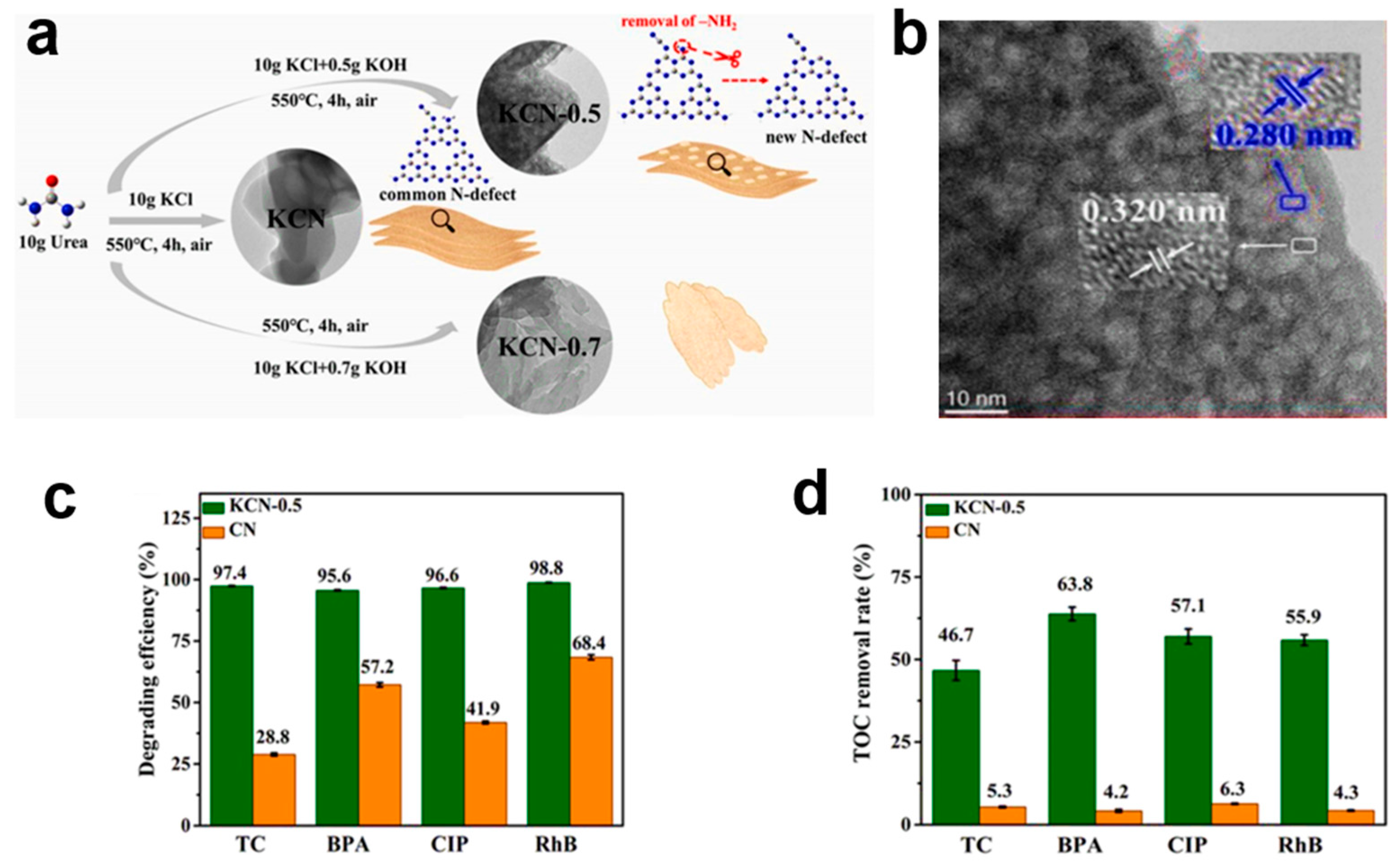
4.2. Defect Engineering
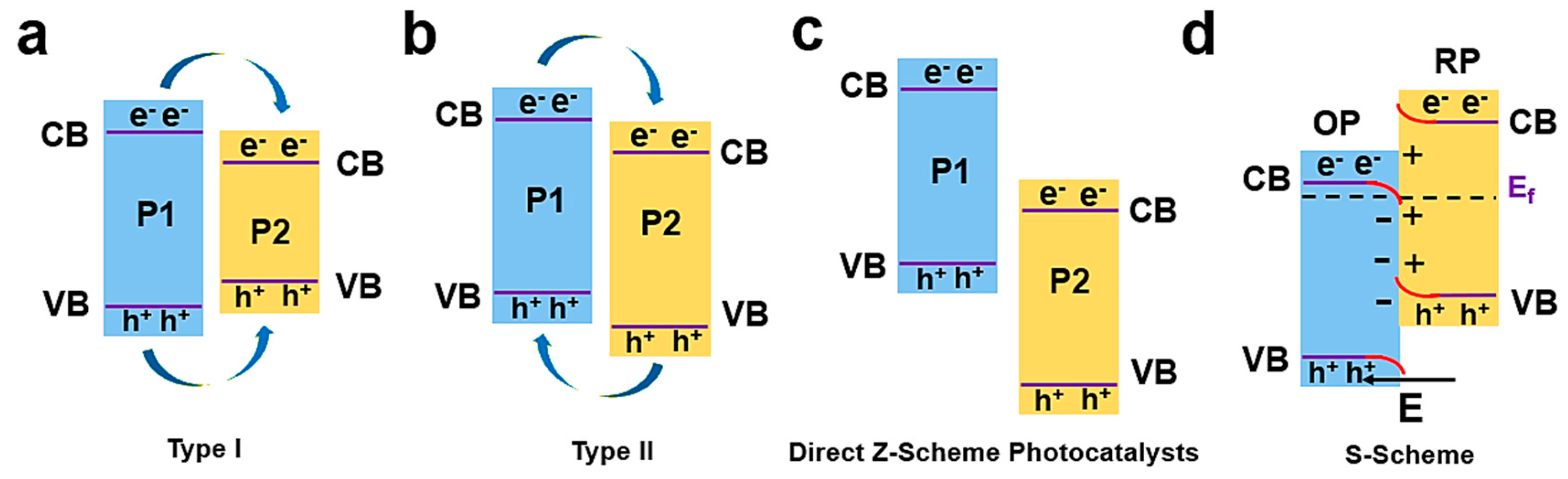
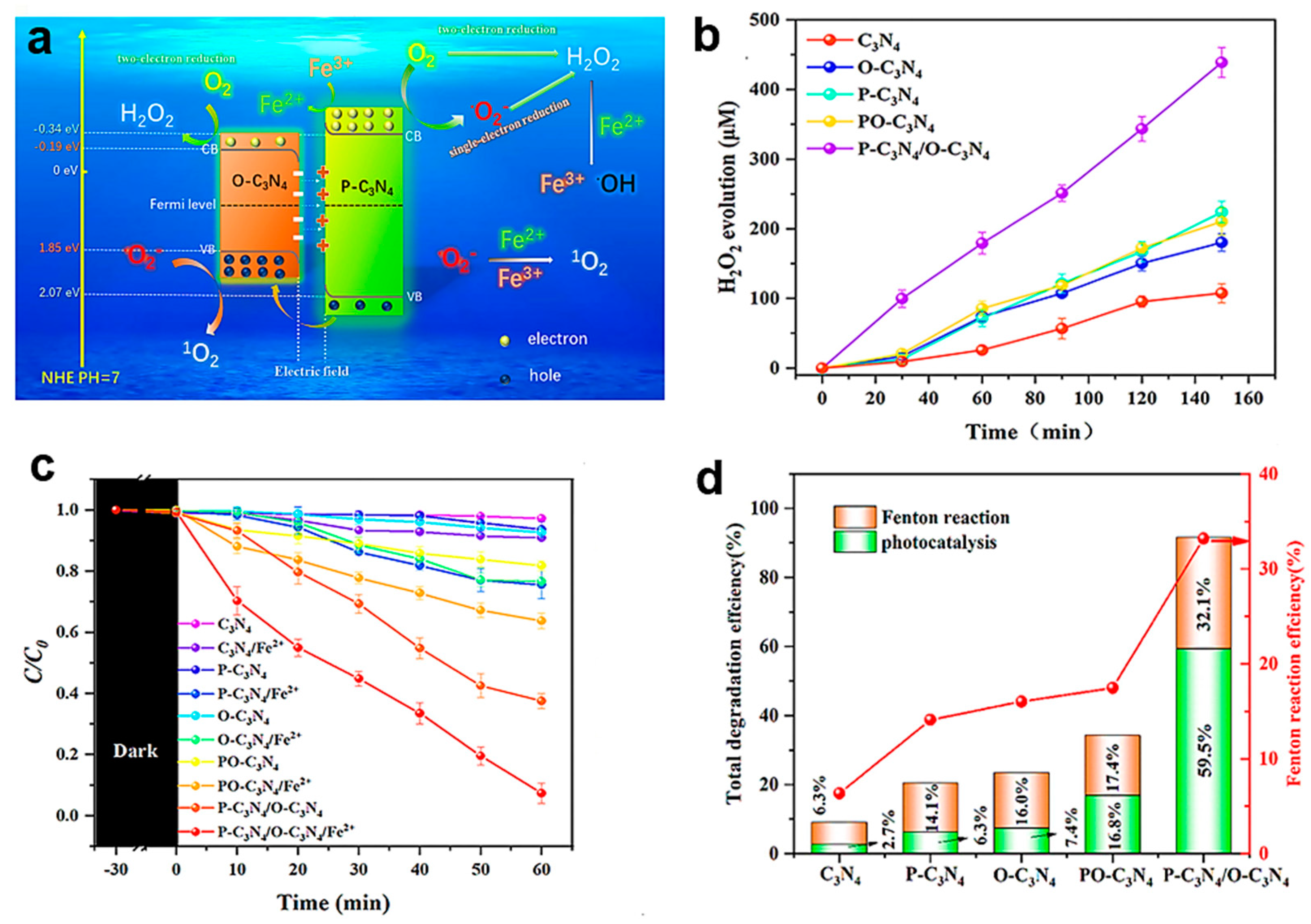
4.3. Constructed Heterojunctions
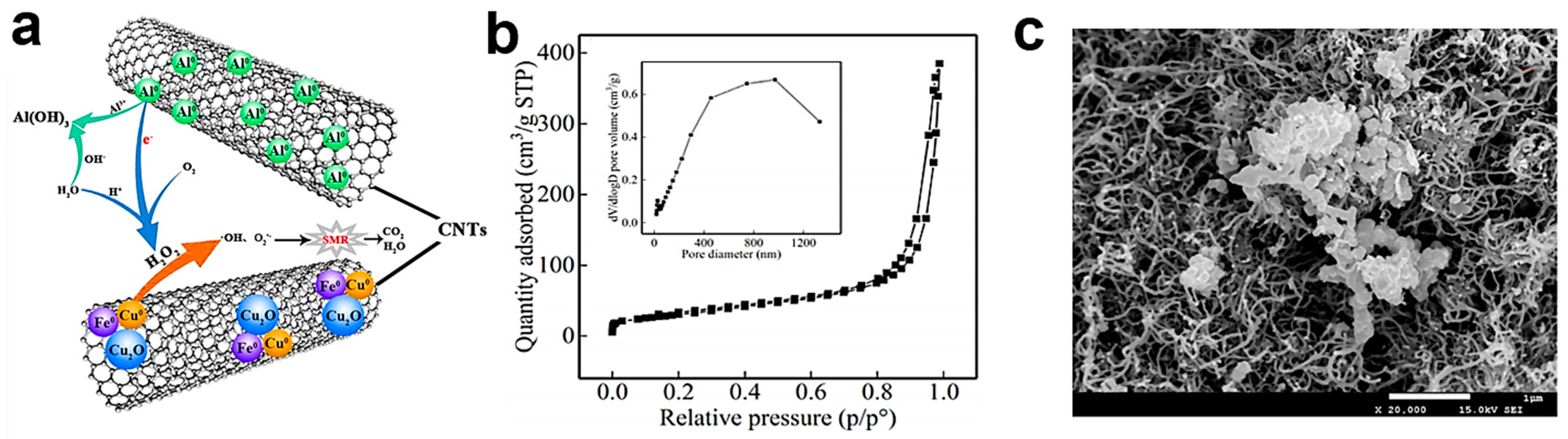
4.4. Element Doping
4.5. Others
4.5.1. Heterojunction of g-C3N4 with Other Materials
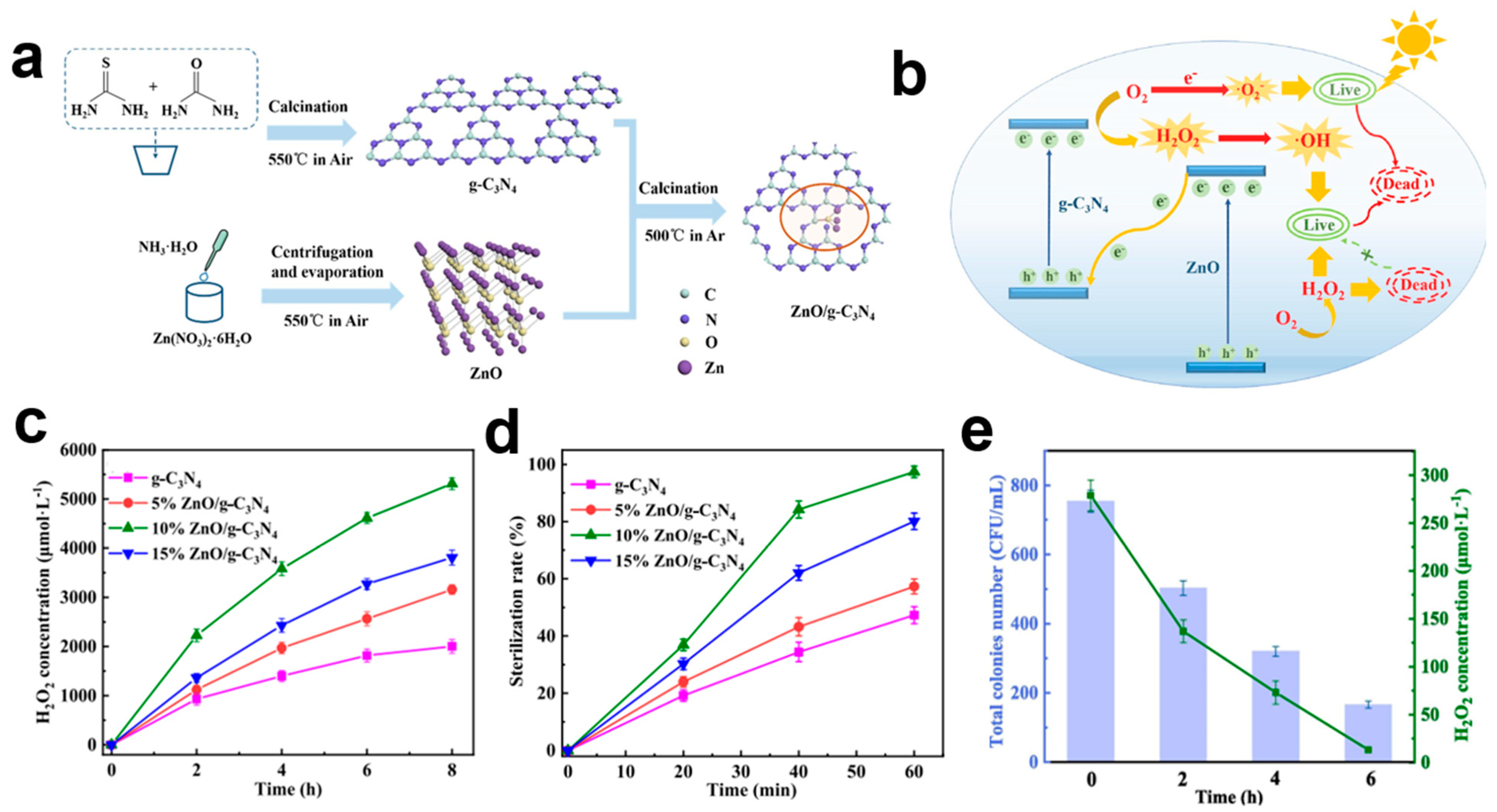
4.5.2. g-C3N4-Based Magnetic Photocatalysts
5. Conclusions and Prospects
- (1)
- In the photocatalytic self-Fenton process, the catalyst generates H2O2 by external interaction with Fe2+ in the photocatalytic phase, generating active species such as •OH by in situ activation, and promotes Fe2+/Fe3+ cycling for the efficient degradation of pollutants. However, most self-Fenton systems have problems such as metal leaching or agglomeration and difficult separation and recovery of Fe3+ after the reaction, leading to low material utilization and environmental pollution, so it is important to design and synthesize environmentally stable catalysts. Secondary contamination is avoided by constructing metal atom dispersion to hinder the leaching of iron atoms as well as the rational use of iron materials such as adding spinel ferrite materials to the system to form heterojunctions with the catalyst. The preparation of catalysts that are easy to synthesize, stable, easy to separate, and prevent metal ion leaching plays an important role in improving the performance of photocatalytic self-Fenton systems.
- (2)
- As a photocatalytic system, the photocatalytic self-Fenton system is based on three basic processes of photocatalysis, whose photogenerated carrier separation and migration efficiency and photo-response range affect its activity. Currently, most g-C3N4-based photocatalysts are limited to the visible light region, and the near-infrared light, which accounts for 50% of the solar spectrum, is not effectively utilized, resulting in low overall light utilization of the catalyst. Although coupling with semiconductors and heteroatom doping can adjust the band gap to improve light absorption, the improvement in the light absorption range is limited based on the inherent nature of semiconductors. Therefore, it is a good strategy to achieve a broad-spectrum photo-response range by combining with near-infrared response materials containing metal elements such as Ag and Cu to generate a local plasmon resonance effect or up-conversion process.
- (3)
- In addition to the catalyst properties themselves, external factors such as the pH of the reaction system and reaction solvent have significant effects on the catalyst activity. The conventional Fenton and Fenton-like reaction systems mainly generate active species such as hydroxyl groups via H2O2 and Fe2+ under acidic conditions, which limit the scope of catalyst applications, thus, it is important to design and synthesize catalysts with a wide pH response. Strategies such as the formation of iron complexes and the use of Fenton-like systems with a wide pH range can be used to prepare photocatalytic self-Fenton systems for the efficient degradation of pollutants.
- (4)
- Sacrificial agents play an important role in the photocatalytic Fenton system, acting as electron acceptors or donors, trapping photogenerated electrons or holes and promoting photogenerated carrier separation efficiency. However, the widespread use of sacrificial agents lacks economic benefits, so the reaction performance can be improved by adding some functional small molecules that are not sacrificial agents. For example, when ethylenediaminetetraacetic acid (EDTA) is added to the reaction system, the electron-rich carboxyl groups in EDTA form hydrogen bonds with O2, which increases the electron density and bond length of O2 molecules, thus facilitating O2 activation and significantly increasing the H2O2 yield of the reaction system. In addition, functional small molecules such as oxalic acid and furfuryl alcohol were applied in the photocatalytic H2O2 production system, which significantly improved the H2O2 yield. Therefore, it is a direction worth exploring to effectively utilize the synergy between functional molecules and catalysts to improve the H2O2 yield and thus promote the subsequent Fenton reaction for the efficient degradation of pollutants.
Author Contributions
Funding
Conflicts of Interest
References
- Alexander, J.A.N.; Worrall, L.J.; Hu, J.; Vuckovic, M.; Satishkumar, N.; Poon, R.; Sobhanifar, S.; Rosell, F.I.; Jenkins, J.; Chiang, D.; et al. Structural basis of broad-spectrum beta-lactam resistance in Staphylococcus aureus. Nature 2023, 613, 375–382. [Google Scholar] [CrossRef]
- Mojica, M.F.; Rossi, M.A.; Vila, A.J.; Bonomo, R.A. The urgent need for metallo-beta-lactamase inhibitors: An unattended global threat. Lancet Infect. Dis. 2022, 22, e28–e34. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, A.; Alcantar, M.A.; Donghia, N.M.; Cardenas, P.; Avila-Pacheco, J.; Collins, J.J. An engineered live biotherapeutic for the prevention of antibiotic-induced dysbiosis. Nat. Biomed. Eng. 2022, 6, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Chen, H.; Srinivasa Raghavan, D.S.; Ting, Y.-P. Removal behaviors of antibiotics in a hybrid microfiltration-forward osmotic membrane bioreactor for real municipal wastewater treatment. Chem. Eng. J. 2021, 417, 129146. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two Different Roles of Metallic Ag on Ag/AgX/BiOX (X = Cl, Br) Visible Light Photocatalysts: Surface Plasmon Resonance and Z-Scheme Bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hernandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Dionysiou, D.D.; Olsson, O.; Fatta-Kassinos, D. A path to clean water. Science 2018, 361, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K. Photocatalytic degradation of agricultural N-heterocyclic organic pollutants using immobilized nanoparticles of titania. J. Hazard. Mater. 2007, 145, 65–71. [Google Scholar] [CrossRef]
- Wei, T.; Fan, Z.; Zhao, G. Enhanced adsorption and degradation of nonylphenol on electron-deficient centers of photocatalytic surfaces. Chem. Eng. J. 2020, 388, 124168. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; He, H.; Cai, A.; Xi, S.; Du, J.; Zhang, F.; Fan, X.; Peng, W.; Li, Y. Wastewater flocculation substrate derived three-dimensional ordered macroporous Co single-atom catalyst for singlet oxygen-dominated peroxymonosulfate activation. Appl. Catal. B Environ. 2023, 335, 122886. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite Oxide Catalysts for Advanced Oxidation Reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Jery, A.E.; Najam, T.; Nazir, M.A.; Wei, L.; Hussain, E.; Hussain, S.; Rebah, F.B.; Javed, M.S. Surface engineering of MOF-derived FeCo/NC core-shell nanostructures to enhance alkaline water-splitting. Int. J. Hydrog. Energy 2022, 47, 5036–5043. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; Rehman, A.u. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; Rehman, A.u. Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts 2023, 13, 813. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Bashir, M.S.; Javed, M.S.; Bashir, M.A.; Imran, M.; Azhar, U.; Shah, S.S.A.; Rehman, A.u. Kinetics, isothermal and mechanistic insight into the adsorption of eosin yellow and malachite green from water via tri-metallic layered double hydroxide nanosheets. Korean J. Chem. Eng. 2022, 39, 216–226. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zheng, Y.; Shang, C.; Yin, R. Concentration-dependent chloride effect on radical distribution and micropollutant degradation in the sulfate radical-based AOPs. J. Hazard. Mater. 2022, 430, 128450. [Google Scholar] [CrossRef]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part I.—The ferrous ion reaction. Trans. Faraday Soc. 1951, 47, 462–500. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Guo, F.; Shi, Y.; Li, L.; Xu, Z.; Yan, X.; Shi, W. Fe-doped g-C3N4 derived from biowaste material with Fe-N bonds for enhanced synergistic effect between photocatalysis and Fenton degradation activity in a broad pH range. J. Alloys Compd. 2022, 900, 163410. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Hao, C.; Guo, F.; Tang, Y. Heterogeneous photo-Fenton process over magnetically recoverable MnFe2O4/MXene hierarchical heterostructure for boosted degradation of tetracycline. Mater. Today Commun. 2022, 33, 104449. [Google Scholar] [CrossRef]
- Lu, C.; Wang, J.; Cao, D.; Guo, F.; Hao, X.; Li, D.; Shi, W. Synthesis of magnetically recyclable g-C3N4/NiFe2O4 S-scheme heterojunction photocatalyst with promoted visible-light-response photo-Fenton degradation of tetracycline. Mater. Res. Bull. 2023, 158, 112064. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Wang, J.; Sun, H.; Shi, Y.; Guo, F.; Lu, C. Magnetically retrievable CdS/reduced graphene oxide/ZnFe2O4 ternary nanocomposite for self-generated H2O2 towards photo-Fenton removal of tetracycline under visible light irradiation. Sep. Purif. Technol. 2022, 292, 120987. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Wang, Z.; Lou, Y.; Pan, C.; Zhu, Y. Unprecedentedly efficient mineralization performance of photocatalysis-self-Fenton system towards organic pollutants over oxygen-doped porous g-C3N4 nanosheets. Appl. Catal. B Environ. 2022, 312, 121438. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Guo, Z.; Yang, T.; Cao, Y.; Xiang, Y.; Chen, H.; Xi, M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chem. Eng. J. 2020, 379, 122324. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Wang, J.; Li, Y.; Zhang, G. Facet-Dependent Activation of Oxalic Acid over Magnetic Recyclable Fe2O4 for Efficient Pollutant Removal under Visible Light Irradiation: Enhanced Catalytic Activity, DFT Calculations, and Mechanism Insight. Environ. Sci. Technol. 2022, 56, 18008–18017. [Google Scholar] [CrossRef]
- Yan, Q.; Lian, C.; Huang, K.; Liang, L.; Yu, H.; Yin, P.; Zhang, J.; Xing, M. Constructing an Acidic Microenvironment by MoS2 in Heterogeneous Fenton Reaction for Pollutant Control. Angew. Chem. Int. Ed. Engl. 2021, 60, 17155–17163. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, A.; Shan, C.; Gao, G.; Pan, B. Enhanced Fe(III)-mediated Fenton oxidation of atrazine in the presence of functionalized multi-walled carbon nanotubes. Water Res. 2018, 137, 37–46. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, C.; Tang, S.; Sun, M.; Zhang, Y.; Rao, Y.; Wang, Z.; Ke, J. Fe(3+)-sulfite complexation enhanced persulfate Fenton-like process for antibiotic degradation based on response surface optimization. Sci. Total Environ. 2020, 727, 138773. [Google Scholar] [CrossRef]
- Dong, C.; Yang, Y.; Hu, X.; Cho, Y.; Jang, G.; Ao, Y.; Wang, L.; Shen, J.; Park, J.H.; Zhang, K. Self-cycled photo-Fenton-like system based on an artificial leaf with a solar-to-H2O2 conversion efficiency of 1.46. Nat. Commun. 2022, 13, 4982. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Y.; Wang, H.; Ma, H.; Wu, D.; Ren, X.; Wei, Q.; Xu, J.J. Self-Supply of H2O2 and O2 by Hydrolyzing CaO2 to Enhance the Electrochemiluminescence of Luminol Based on a Closed Bipolar Electrode. Anal. Chem. 2020, 92, 12693–12699. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Sun, X.; He, K.; Chen, Z.; Yuan, H.; Guo, F.; Shi, W. Construction of visible-light-response photocatalysis-self-Fenton system for the efficient degradation of amoxicillin based on industrial waste red mud/CdS S-scheme heterojunction. Sep. Purif. Technol. 2023, 324, 124600. [Google Scholar] [CrossRef]
- Lotfi, S.; Fischer, K.; Schulze, A.; Schafer, A.I. Photocatalytic degradation of steroid hormone micropollutants by TiO2-coated polyethersulfone membranes in a continuous flow-through process. Nat. Nanotechnol. 2022, 17, 417–423. [Google Scholar] [CrossRef]
- Zhang, M.; Nie, S.; Cheng, T.; Feng, Y.; Zhang, C.; Zheng, L.; Wu, L.; Hao, W.; Ding, Y. Enhancing the macroscopic polarization of CdS for piezo-photocatalytic water splitting. Nano Energy 2021, 90, 106635. [Google Scholar] [CrossRef]
- Chu, C.; Miao, W.; Li, Q.; Wang, D.; Liu, Y.; Mao, S. Highly efficient photocatalytic H2O2 production with cyano and SnO2 co-modified g-C3N4. Chem. Eng. J. 2022, 428, 132531. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Yan, C.; Guo, F. Facile synthesis of 2D/2D Co3(PO4)2/g-C3N4 heterojunction for highly photocatalytic overall water splitting under visible light. Chem. Eng. J. 2020, 382, 122960. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Huang, X.; Li, M.; Tang, Y.; Guo, F. Low cost red mud modified graphitic carbon nitride for the removal of organic pollutants in wastewater by the synergistic effect of adsorption and photocatalysis. Sep. Purif. Technol. 2020, 237, 116477. [Google Scholar] [CrossRef]
- Ren, W.; Chang, Q.; Li, N.; Yang, J.; Hu, S. Carbon dots-modulated covalent triazine frameworks with exceptionally rapid hydrogen peroxide production in water. Chem. Eng. J. 2023, 451, 139035. [Google Scholar] [CrossRef]
- Feng, C.; Tang, L.; Deng, Y.; Wang, J.; Luo, J.; Liu, Y.; Ouyang, X.; Yang, H.; Yu, J.; Wang, J. Synthesis of Leaf-Vein-Like g-C3N4 with Tunable Band Structures and Charge Transfer Properties for Selective Photocatalytic H2O2 Evolution. Adv. Funct. Mater. 2020, 30, 2001922. [Google Scholar] [CrossRef]
- Liu, L.-L.; Chen, F.; Wu, J.-H.; Ke, M.-K.; Cui, C.; Chen, J.-J.; Yu, H.-Q. Edge electronic vacancy on ultrathin carbon nitride nanosheets anchoring O2 to boost H2O2 photoproduction. Appl. Catal. B Environ. 2022, 302, 120845. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Wang, D.; Zhang, F.; Zhao, Y.; Yan, M.; Zheng, C.; Wang, Q.; Long, M.; Chen, C. Modification of g-C3N4 with hydroxyethyl cellulose as solid proton donor via hydrogen bond to enhance H2O2 production. Appl. Catal. B Environ. 2022, 318, 121749. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Teter, D.M.; Hemley, R.J. Low-Compressibility Carbon Nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Ma, J.; Wang, K.; Wang, C.; Chen, X.; Zhu, W.; Zhu, G.; Yao, W.; Zhu, Y. Photocatalysis-self-Fenton system with high-fluent degradation and high mineralization ability. Appl. Catal. B Environ. 2020, 276, 119150. [Google Scholar] [CrossRef]
- Wu, Y.; Che, H.; Liu, B.; Ao, Y. Promising Materials for Photocatalysis-Self-Fenton System: Properties, Modifications, and Applications. Small Struct. 2023, 4, 2200371. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.; Park, S.O.; Hwang, J.M.; Hong, Y.; Sharma, P.; Jeon, W.C.; Cho, Y.; Yang, C.; Kwak, S.K.; et al. High performance H2O2 production achieved by sulfur-doped carbon on CdS photocatalyst via inhibiting reverse H2O2 decomposition. Appl. Catal. B Environ. 2021, 284, 119690. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Zhang, Y.; Yang, S.; Han, K.; Dong, F.; Ma, T.; Huang, H. Three-in-One Oxygen Vacancies: Whole Visible-Spectrum Absorption, Efficient Charge Separation, and Surface Site Activation for Robust CO2 Photoreduction. Angew. Chem. Int. Ed. Engl. 2019, 58, 3880–3884. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Cao, J.; Wang, H.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. Efficient production of H2O2 via two-channel pathway over ZIF-8/C3N4 composite photocatalyst without any sacrificial agent. Appl. Catal. B Environ. 2020, 278, 119289. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving efficient H2O2 production by a visible-light absorbing, highly stable photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Wan, Y.; Zhang, Y.; Qi, Z.; Wu, X.; Xu, H. Acetylene and Diacetylene Functionalized Covalent Triazine Frameworks as Metal-Free Photocatalysts for Hydrogen Peroxide Production: A New Two-Electron Water Oxidation Pathway. Adv. Mater. 2020, 32, 1904433. [Google Scholar] [CrossRef]
- Kofuji, Y.; Ohkita, S.; Shiraishi, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Graphitic Carbon Nitride Doped with Biphenyl Diimide: Efficient Photocatalyst for Hydrogen Peroxide Production from Water and Molecular Oxygen by Sunlight. ACS Catal. 2016, 6, 7021–7029. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Shao, B.; Liu, Z.; Liang, Q.; Wu, T.; He, Q.; Huang, J.; Peng, Z.; et al. Bifunctional template-mediated synthesis of porous ordered g-C3N4 decorated with potassium and cyano groups for effective photocatalytic H2O2 evolution from dual-electron O2 reduction. Chem. Eng. J. 2022, 427, 132032. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Slater, T.J.A.; Sharma, M.; Bowker, M.; Catlow, C.R.A. Enhanced H2O2 Production via Photocatalytic O2 Reduction over Structurally-Modified Poly(heptazine imide). Chem. Mater. 2022, 34, 5511–5521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C.; et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Chen, S.; Quan, X. Enhanced Photocatalytic H2O2 Production over Carbon Nitride by Doping and Defect Engineering. ACS Catal. 2020, 10, 14380–14389. [Google Scholar] [CrossRef]
- Luo, J.; Fan, C.; Tang, L.; Liu, Y.; Gong, Z.; Wu, T.; Zhen, X.; Feng, C.; Feng, H.; Wang, L.; et al. Reveal Brønsted–Evans–Polanyi relation and attack mechanisms of reactive oxygen species for photocatalytic H2O2 production. Appl. Catal. B Environ. 2022, 301, 120757. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, M.; Zhang, Z.; Yao, W.; Tan, H.; Zhu, Y. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ. Sci. 2018, 11, 2581–2589. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Zhang, L. Constructed 3D hierarchical micro-flowers CoWO4@Bi2WO6 Z-scheme heterojunction catalyzer: Two-channel photocatalytic H2O2 production and antibiotics degradation. Chem. Eng. J. 2021, 420, 127639. [Google Scholar] [CrossRef]
- Isaka, Y.; Kawase, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Two-Phase System Utilizing Hydrophobic Metal-Organic Frameworks (MOFs) for Photocatalytic Synthesis of Hydrogen Peroxide. Angew. Chem. Int. Ed. Engl. 2019, 58, 5402–5406. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jiang, Y.; Tang, K.; Zhang, Y.; Andersen, H.R. Efficient recovery of dissolved Fe(II) from near neutral pH Fenton via microbial electrolysis. J. Hazard. Mater. 2022, 436, 129196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, G.; Ji, Q.; Lan, H.; Qu, J.; Liu, H. Carbon nanodot-modified FeOCl for photo-assisted Fenton reaction featuring synergistic in-situ H2O2 production and activation. Appl. Catal. B Environ. 2020, 266, 118665. [Google Scholar] [CrossRef]
- Xu, J.; Long, Y.; Shen, D.; Feng, H.; Chen, T. Optimization of Fenton treatment process for degradation of refractory organics in pre-coagulated leachate membrane concentrates. J. Hazard. Mater. 2017, 323, 674–680. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, Y. Elimination of nitric oxide using new Fenton process based on synergistic catalysis: Optimization and mechanism. Chem. Eng. J. 2019, 372, 92–98. [Google Scholar] [CrossRef]
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; et al. Degradation of sulfamethoxazole by super-hydrophilic MoS2 sponge co-catalytic Fenton: Enhancing Fe(2+)/Fe(3+) cycle and mass transfer. J. Hazard. Mater. 2023, 458, 131878. [Google Scholar] [CrossRef]
- Dou, X.; Li, Q.; Shi, H. Ag nanoparticle-decorated 2D/2D S-scheme g-C3N4/Bi2WO6 heterostructures for an efficient photocatalytic degradation of tetracycline. CrystEngComm 2021, 23, 4638–4647. [Google Scholar] [CrossRef]
- Liu, W.; Iwasa, N.; Fujita, S.; Koizumi, H.; Yamaguchi, M.; Shimada, T. Porous graphitic carbon nitride nanoplates obtained by a combined exfoliation strategy for enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2020, 499, 143901. [Google Scholar] [CrossRef]
- Chen, L.; Maigbay, M.A.; Li, M.; Qiu, X. Synthesis and modification strategies of g-C3N4 nanosheets for photocatalytic applications. Adv. Powder Mater. 2023, 100150, in press. [Google Scholar] [CrossRef]
- Xu, C.Q.; Li, K.; Zhang, W.D. Enhancing visible light photocatalytic activity of nitrogen-deficient g-C3N4 via thermal polymerization of acetic acid-treated melamine. J. Colloid. Interface Sci. 2017, 495, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, F.; Pan, J.; Huang, W.; Wang, K.; Shi, W. One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process. Chem. Eng. J. 2021, 406, 126844. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Sun, H.; Li, M.; Shi, W. High-efficiency photocatalytic water splitting by a N-doped porous g-C3N4 nanosheet polymer photocatalyst derived from urea and N,N-dimethylformamide. Inorg. Chem. Front. 2020, 7, 1770–1779. [Google Scholar] [CrossRef]
- Shi, W.; Shu, K.; Sun, H.; Ren, H.; Li, M.; Chen, F.; Guo, F. Dual enhancement of capturing photogenerated electrons by loading CoP nanoparticles on N-deficient graphitic carbon nitride for efficient photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2020, 246, 116930. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A 2008, 94, 387–392. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, B.; Cheng, X.; Zhang, F.; Wan, Q.; Liu, L.; Tan, X.; Tan, D.; Zheng, L.; Zhang, J. Ultra-small UiO-66-NH2 nanoparticles immobilized on g-C3N4 nanosheets for enhanced catalytic activity. Green. Energy Environ. 2022, 7, 512–518. [Google Scholar] [CrossRef]
- Fang, W.; Liu, J.; Yu, L.; Jiang, Z.; Shangguan, W. Novel (Na, O) co-doped g-C3N4 with simultaneously enhanced absorption and narrowed bandgap for highly efficient hydrogen evolution. Appl. Catal. B Environ. 2017, 209, 631–636. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, Y.; Lei, J.; Wang, L.; Liu, Y.; Zhang, J. Self-modification of g-C3N4 with its quantum dots for enhanced photocatalytic activity. Catal. Sci. Technol. 2018, 8, 2617–2623. [Google Scholar] [CrossRef]
- Luo, L.; Li, K.; Zhang, A.; Shi, H.; Zhang, G.; Ma, J.; Zhang, W.; Tang, J.; Song, C.; Guo, X. Controllable assembly of single/double-thin-shell g-C3N4 vesicles via a shape-selective solid-state templating method for efficient photocatalysis. J. Mater. Chem. A 2019, 7, 17815–17822. [Google Scholar] [CrossRef]
- Zhang, H.; Bao, C.; Hu, X.; Wen, Y.; Li, K.; Zhang, H. Synthesis of tunnel structured g-C3N4 through a facile vapor deposition method using SBA-15 and KIT-6 as templates and their photocatalytic degradation of tetracycline hydrochloride and phenol. J. Environ. Chem. Eng. 2022, 10, 107871. [Google Scholar] [CrossRef]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel-Crafts reaction of benzene. Angew. Chem. Int. Ed. Engl. 2006, 45, 4467–4471. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Grenu, B.; Torres, J.; Garcia-Gonzalez, J.; Munoz-Pina, S.; de Los Reyes, R.; Costero, A.M.; Amoros, P.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. ChemSusChem 2021, 14, 208–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Li, Y.; Wu, B.; Luo, X.; Ouyang, S.; Luo, S.; Al Kheraif, A.A.; Lin, J. A g-C3N4@Au@SrAl2O4:Eu2+,Dy3+composite as an efficient plasmonic photocatalyst for round-the-clock environmental purification and hydrogen evolution. J. Mater. Chem. A 2019, 7, 19173–19186. [Google Scholar] [CrossRef]
- Li, K.; Sun, C.; Chen, Z.; Qu, H.; Xie, H.; Zhong, Q. Fe-carbon dots enhance the photocatalytic nitrogen fixation activity of TiO2@CN heterojunction. Chem. Eng. J. 2022, 429, 132440. [Google Scholar] [CrossRef]
- Xiong, J.; Li, X.; Huang, J.; Gao, X.; Chen, Z.; Liu, J.; Li, H.; Kang, B.; Yao, W.; Zhu, Y. CN/rGO@BPQDs high-low junctions with stretching spatial charge separation ability for photocatalytic degradation and H2O2 production. Appl. Catal. B Environ. 2020, 266, 118602. [Google Scholar] [CrossRef]
- Shi, W.; Shu, K.; Huang, X.; Ren, H.; Li, M.; Chen, F.; Guo, F. Enhancement of visible-light photocatalytic degradation performance over nitrogen-deficient g-C3N4/KNbO3 heterojunction photocatalyst. J. Chem. Technol. Biotechnol. 2020, 95, 1476–1486. [Google Scholar] [CrossRef]
- Guo, F.; Sun, H.; Huang, X.; Shi, W.; Yan, C. Fabrication of TiO2/high-crystalline g-C3N4 composite with enhanced visible-light photocatalytic performance for tetracycline degradation. J. Chem. Technol. Biotechnol. 2020, 95, 2684–2693. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, C.; Ping, B.; Li, G.; Chen, J.; Sun, Z.; Zhang, Y.; Ruan, Q.; Tao, L. A hydroxyl-induced carbon nitride homojunction with functional surface for efficient photocatalytic production of H2O2. Appl. Catal. B Environ. 2023, 324, 122216. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, Y.; Xu, Y.; Zhang, Q.; Shi, X.; Li, D.; Tian, D.; Jiang, D. Improved charge transfer in polymeric carbon nitride synergistically induced by the aromatic rings modification and Schottky junctions for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2023, 463, 142395. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Sun, H.; Chen, L. Prominent co-catalytic effect of CoP nanoparticles anchored on high-crystalline g-C3N4 nanosheets for enhanced visible-light photocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2020, 395, 125118. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Sun, H.; Li, M.; Shi, W.; Lin, X. A one-pot sealed ammonia self-etching strategy to synthesis of N-defective g-C3N4 for enhanced visible-light photocatalytic hydrogen. Int. J. Hydrog. Energy 2020, 45, 30521–30532. [Google Scholar] [CrossRef]
- Wang, Y.; Song, H.; Chen, J.; Chai, S.; Shi, L.; Chen, C.; Wang, Y.; He, C. A novel solar photo-Fenton system with self-synthesizing H2O2: Enhanced photo-induced catalytic performances and mechanism insights. Appl. Surf. Sci. 2020, 512, 145650. [Google Scholar] [CrossRef]
- Shi, W.; Yang, S.; Sun, H.; Wang, J.; Lin, X.; Guo, F.; Shi, J. Carbon dots anchored high-crystalline g-C3N4 as a metal-free composite photocatalyst for boosted photocatalytic degradation of tetracycline under visible light. J. Mater. Sci. 2020, 56, 2226–2240. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, F.; Pan, J.; Sun, H.; Gao, L.; Deng, J.; Zhu, X.; Shi, W. Fabrication of visible-light-response face-contact ZnSnO3@g-C3N4 core–shell heterojunction for highly efficient photocatalytic degradation of tetracycline contaminant and mechanism insight. J. Mater. Sci. 2020, 56, 4366–4379. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Kwon, S.; Zhang, F.; Stephen, B.; Kim, K.K.; Jung, R.; Kwon, S.; Chung, K.-B.; Yang, W. Photocatalytic improvement of Mn-adsorbed g-C3N4. Appl. Catal. B Environ. 2017, 206, 271–281. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Z.; Qiu, B.; Xing, M.; Zhang, J. Emerging Cocatalysts on g-C3N4 for Photocatalytic Hydrogen Evolution. Small 2021, 17, 2101070. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Huang, X.; Chen, Z.; Cao, L.; Cheng, X.; Chen, L.; Shi, W. Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Sun, H.; Shi, Y.; Luo, H.; Jing, S.; Fan, Y.; Guo, F.; Lu, C. Fabrication of ternary CoO/g-C3N4/Co3O4 nanocomposite with p-n-p type heterojunction for boosted visible-light photocatalytic performance. J. Chem. Technol. Biotechnol. 2021, 96, 1854–1863. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of Functional Group Modifications on the Photocatalytic Performance of g-C3N4. Small 2023, 19, 2300109. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, W.; Chen, H.; Ji, H. A review on g-C3N4 decorated with silver for photocatalytic energy conversion. Chin. Chem. Lett. 2023, 108567, in press. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Huang, X.; Cao, L.; Cheng, X.; Shi, W.; Chen, L. Cu3P nanoparticles decorated hollow tubular carbon nitride as a superior photocatalyst for photodegradation of tetracycline under visible light. Sep. Purif. Technol. 2021, 275, 119223. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Huang, X.; Cao, L.; Cheng, X.; Shi, W.; Chen, L. Ternary Ni2P/Bi2MoO6/g-C3N4 composite with Z-scheme electron transfer path for enhanced removal broad-spectrum antibiotics by the synergistic effect of adsorption and photocatalysis. Chin. J. Chem. Eng. 2022, 44, 157–168. [Google Scholar] [CrossRef]
- Xu, J.; Dai, G.; Chen, B.; He, D.; Situ, Y.; Huang, H. Construction of Ti(3+)-TiO2-C3N4por compound coupling photocatalysis and Fenton-like process: Self-driven Fenton-like process without extra H2O2 addition. Chemosphere 2020, 241, 125022. [Google Scholar] [CrossRef]
- Su, S.; Xing, Z.; Zhang, S.; Du, M.; Wang, Y.; Li, Z.; Chen, P.; Zhu, Q.; Zhou, W. Ultrathin mesoporous g-C3N4/NH2-MIL-101(Fe) octahedron heterojunctions as efficient photo-Fenton-like system for enhanced photo-thermal effect and promoted visible-light-driven photocatalytic performance. Appl. Surf. Sci. 2021, 537, 147890. [Google Scholar] [CrossRef]
- Li, Y.; Luo, N.; Tian, Z.; Li, H.; Yang, M.; Shang, W.; Yifeng, S.; Qu, M.; Zhou, A. H2O2-free photo-Fenton degradation of organic pollutants on thermally exfoliated g-C3N4. Colloids Surf. Physicochem. Eng. Asp. 2020, 586, 124190. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Liu, Y.; Liu, Y. Fenton-like degradation of sulfamerazine at nearly neutral pH using Fe-Cu-CNTs and Al0-CNTs for in-situ generation of H2O2/OH/O2−. Chem. Eng. J. 2020, 396, 125329. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Sampaio, M.J.; Teixo, J.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Photo-Fenton degradation assisted by in situ generation of hydrogen peroxide using a carbon nitride photocatalyst. J. Water Process Eng. 2020, 37, 101467. [Google Scholar] [CrossRef]
- Geng, X.; Wang, L.; Zhang, L.; Wang, H.; Peng, Y.; Bian, Z. H2O2 production and in situ sterilization over a ZnO/g-C3N4 heterojunction photocatalyst. Chem. Eng. J. 2021, 420, 129722. [Google Scholar] [CrossRef]
- Li, C.; Tan, X.; Ma, J. Concerted high innergenerated-H2O2 photocatalysis and Photo-Fenton degradation of organic pollutants over SCNO@CdS. J. Photochem. Photobiol. A Chem. 2021, 420, 113477. [Google Scholar] [CrossRef]
- Li, J.; Mei, Y.; Ma, S.; Yang, Q.; Jiang, B.; Xin, B.; Yao, T.; Wu, J. Internal-electric-field induced high efficient type-I heterojunction in photocatalysis-self-Fenton reaction: Enhanced H2O2 yield, utilization efficiency and degradation performance. J. Colloid Interface Sci. 2022, 608, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, H.; Li, G.; Li, J.; Wong, P.K.; An, T. Visible Light-Induced Marine Bacterial Inactivation in Seawater by an In Situ Photo-Fenton System without Additional Oxidants: Implications for Ballast Water Sterilization. ACS EST Water 2021, 1, 1483–1494. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Che, H.; Gao, X.; Ao, Y.; Wang, P. Boosting 2e− oxygen reduction reaction in garland carbon nitride with carbon defects for high-efficient photocatalysis-self-Fenton degradation of 2,4-dichlorophenol. Appl. Catal. B Environ. 2022, 307, 121185. [Google Scholar] [CrossRef]
- Shi, W.; Sun, W.; Liu, Y.; Zhang, K.; Sun, H.; Lin, X.; Hong, Y.; Guo, F. A self-sufficient photo-Fenton system with coupling in-situ production H2O2 of ultrathin porous g-C3N4 nanosheets and amorphous FeOOH quantum dots. J. Hazard Mater. 2022, 436, 129141. [Google Scholar] [CrossRef]
- Jian, L.; Zhao, H.; Dong, Y.; Xu, J.; Mao, Q.; Ji, R.; Yan, Z.; Pan, C.; Wang, G.; Zhu, Y. Graphite carbon ring modified carbon nitride with a strong built-in electric field for high photocatalysis-self-Fenton performance. Catal. Sci. Technol. 2022, 12, 7379–7388. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.; Lin, H.; Wang, C.; Xu, J. Enhanced Fenton-like degradation of Rhodamine B and Congo red by benzene and K+ co-doped carbon nitride with in situ-generated H2O2. J. Taiwan Inst. Chem. Eng. 2022, 131, 104179. [Google Scholar] [CrossRef]
- Chen, L.; He, X.-X.; Gong, Z.-H.; Li, J.-L.; Liao, Y.; Li, X.-T.; Ma, J. Significantly improved photocatalysis-self-Fenton degradation performance over g-C3N4 via promoting Fe(III)/Fe(II) cycle. Rare Met. 2022, 41, 2429–2438. [Google Scholar] [CrossRef]
- Chen, B.; Xu, J.; Dai, G.; Sun, X.; Situ, Y.; Huang, H. Accelerated Fe(III)/Fe(II) cycle couples with in-situ generated H2O2 boosting visible light-induced Fenton-like oxidation. Sep. Purif. Technol. 2022, 299, 121688. [Google Scholar] [CrossRef]
- Jiang, G.; You, X.; An, B.; Zhu, B.; Liu, F.; Duan, X.; Wang, Y.; Zhao, R. Constructing direct Z-scheme heterojunctions of defective MoS2-v on carbon nitride nanotubes for high-performance hydrogen peroxide production and iron-free photo-Fenton-like reactions over a wide pH range. Appl. Surf. Sci. 2023, 618, 156656. [Google Scholar] [CrossRef]
- Mao, Y.; Lin, L.; Chen, Y.; Yang, M.; Zhang, L.; Dai, X.; He, Q.; Jiang, Y.; Chen, H.; Liao, J.; et al. Preparation of site-specific Z-scheme g-C3N4/PAN/PANI@LaFeO3 cable nanofiber membranes by coaxial electrospinning: Enhancing filtration and photocatalysis performance. Chemosphere 2023, 328, 138553. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Zhao, H.; Jian, L.; Pan, C.; Dong, Y.; Zhu, Y. Coral-like B-doped g-C3N4 with enhanced molecular dipole to boost photocatalysis-self-Fenton removal of persistent organic pollutants. J. Hazard. Mater. 2023, 449, 131017. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zheng, M.; Zhou, X.; Zhang, D.; Shi, Y.; Li, C.; Yang, M. Carbon vacancies in porous g-C3N4 nanosheets induced robust H2O2 production for highly efficient photocatalysis-self-Fenton system for metronidazole degradation. Chem. Eng. J. 2023, 464, 142584. [Google Scholar] [CrossRef]
- Lu, T.; Zhao, H.; Jian, L.; Ji, R.; Pan, C.; Wang, G.; Dong, Y.; Zhu, Y. Photocatalysis-self-Fenton system over edge covalently modified g-C3N4 with high mineralization of persistent organic pollutants. Environ. Res. 2023, 222, 115361. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, C.; Zhang, Q.; Ao, Y. Constructing photocatalysis-self-Fenton system over a defective twin C3N4: In-situ producing H2O2 and mineralizing organic pollutants. Appl. Catal. B Environ. 2023, 331, 122716. [Google Scholar] [CrossRef]
- Xu, J.; Cao, S.; Brenner, T.; Yang, X.; Yu, J.; Antonietti, M.; Shalom, M. Supramolecular Chemistry in Molten Sulfur: Preorganization Effects Leading to Marked Enhancement of Carbon Nitride Photoelectrochemistry. Adv. Funct. Mater. 2015, 25, 6265–6271. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Zhang, G.; Cui, P.; Wang, X.; Jiang, X.; Zhao, J.; Luo, Y.; Jiang, J. Combining photocatalytic hydrogen generation and capsule storage in graphene based sandwich structures. Nat. Commun. 2017, 8, 16049. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, Y.; Li, L.; Sun, H.; Amin, M.D.S.; Guo, F.; Wen, H.; Shi, W. Fabrication of 2D/2D Z-scheme highly crystalline carbon nitride/δ-Bi2O3 heterojunction photocatalyst with enhanced photocatalytic degradation of tetracycline. J. Alloys Compd. 2022, 895, 162667. [Google Scholar] [CrossRef]
- Li, Y.; Jin, R.; Xing, Y.; Li, J.; Song, S.; Liu, X.; Li, M.; Jin, R. Macroscopic Foam-Like Holey Ultrathin g-C3N4Nanosheets for Drastic Improvement of Visible-Light Photocatalytic Activity. Adv. Energy Mater. 2016, 6, 1601273. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Z.; Shi, Y.; Cao, L.; Cheng, X.; Shi, W.; Chen, L.; Lin, X. A ragged porous hollow tubular carbon nitride towards boosting visible-light photocatalytic hydrogen production in water and seawater. Renew. Energy 2022, 188, 1–10. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Amin, S.; Guo, F.; Shi, W.; Li, Y. Engineering of 2D/3D architectures type II heterojunction with high-crystalline g-C3N4 nanosheets on yolk-shell ZnFe2O4 for enhanced photocatalytic tetracycline degradation. Mater. Res. Bull. 2022, 150, 111789. [Google Scholar] [CrossRef]
- Qin, L.; Maciejewska, B.M.; Subroto, T.; Morton, J.A.; Porfyrakis, K.; Tzanakis, I.; Eskin, D.G.; Grobert, N.; Fezzaa, K.; Mi, J. Ultrafast synchrotron X-ray imaging and multiphysics modelling of liquid phase fatigue exfoliation of graphite under ultrasound. Carbon 2022, 186, 227–237. [Google Scholar] [CrossRef]
- Yang, J.L.; Ma, G.P.; Yang, R.; Yang, S.Q.; Fu, L.Z.; Cheng, A.C.; Wang, M.S.; Zhang, S.H.; Shen, K.F.; Jia, R.Y.; et al. Simple and rapid detection of Salmonella serovar Enteritidis under field conditions by loop-mediated isothermal amplification. J. Appl. Microbiol. 2010, 109, 1715–1723. [Google Scholar] [CrossRef]
- Bousek, J.; Scroccaro, D.; Sima, J.; Weissenbacher, N.; Fuchs, W. Influence of the gas composition on the efficiency of ammonia stripping of biogas digestate. Bioresour. Technol. 2016, 203, 259–266. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, J.; Fu, X.; Wang, X. Metal-free photocatalytic degradation of 4-chlorophenol in water by mesoporous carbon nitride semiconductors. Catal. Sci. Technol. 2012, 2, 1396–1402. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; She, X.; Xu, L.; Xia, J.; Xu, Y.; Song, Y.; Huang, L.; Li, H. Graphene-analogue carbon nitride: Novel exfoliation synthesis and its application in photocatalysis and photoelectrochemical selective detection of trace amount of Cu2+. Nanoscale 2014, 6, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C.; Kang, S.; Dong, M.; Li, X. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B Environ. 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Dang, X.; Yang, R.; Wang, Z.; Wu, S.; Zhao, H. Efficient visible-light activation of molecular oxygen to produce hydrogen peroxide using P doped g-C3N4 hollow spheres. J. Mater. Chem. A 2020, 8, 22720–22727. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Liu, J.D.; Chen, Y.; Liu, X.K.; Liu, W.; Ma, T.; Li, Y.; Zheng, X.S.; Yao, T.; et al. Unconventional CN vacancies suppress iron-leaching in Prussian blue analogue pre-catalyst for boosted oxygen evolution catalysis. Nat. Commun. 2019, 10, 2799. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Ho, W.; Han, S.; Wang, P.; Lee, S.; Zhang, Z. Modulation of Sulfur Vacancies at ZnIn2S4-δ/g-C3N4 Heterojunction Interface for Successive C-H Secession in Photocatalytic Gaseous Formaldehyde Complete Oxidation. Appl. Catal. B Environ. 2023, 338, 123048. [Google Scholar] [CrossRef]
- Gu, Z.; Cui, Z.; Wang, Z.; Qin, K.S.; Asakura, Y.; Hasegawa, T.; Tsukuda, S.; Hongo, K.; Maezono, R.; Yin, S. Carbon vacancies and hydroxyls in graphitic carbon nitride: Promoted photocatalytic NO removal activity and mechanism. Appl. Catal. B Environ. 2020, 279, 119376. [Google Scholar] [CrossRef]
- Chi, H.; Liu, J.; Zhang, X.; Xue, X.; Zhang, D.; Lin, X.; Huang, P.; Sun, L.; Xiong, J.; Cai, P.; et al. Synergetic defects boost charge separation in CN for enhanced photocatalytic water splitting. J. Mater. Chem. C 2020, 8, 9366–9372. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, Y.; Ruan, Q.; Wang, S.; Yu, J.; Guo, X.; Zhang, W.; Xie, H.; Zhang, Z.; Huang, Y. Plasma-induced hierarchical amorphous carbon nitride nanostructure with two N2 C-site vacancies for photocatalytic H2O2 production. Appl. Catal. B Environ. 2022, 311, 121372. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Wang, C.; Gan, L.; Chen, X.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.; Zhou, X.; et al. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, W.; He, F.; Liu, K.; Cao, H.; Yan, H. One-step synthesis of nitrogen-defective graphitic carbon nitride for improving photocatalytic hydrogen evolution. J. Hazard. Mater. 2021, 410, 124594. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, S.; Wang, G.; Wang, J.; Gao, T.; Su, Y.; Wong, C.-P. Electrostatic self-assembly combined with microwave hydrothermal strategy: Construction of 1D/1D carbon nanofibers/crystalline g-C3N4 heterojunction for boosting photocatalytic hydrogen production. Nano Energy 2022, 99, 107432. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Y.; Shi, W.; Guo, F. High-crystalline/amorphous g-C3N4 S-scheme homojunction for boosted photocatalytic H2 production in water/simulated seawater: Interfacial charge transfer and mechanism insight. Appl. Surf. Sci. 2022, 593, 153281. [Google Scholar] [CrossRef]
- Sun, X.; Shi, Y.; Lu, J.; Shi, W.; Guo, F. Template-free self-assembly of three-dimensional porous graphitic carbon nitride nanovesicles with size-dependent photocatalytic activity for hydrogen evolution. Appl. Surf. Sci. 2022, 606, 154841. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Wang, F. Oxygen-controlled photo-reforming of biopolyols to CO over Z-scheme CdS@g-C3N4. Chem. 2022, 8, 465–479. [Google Scholar] [CrossRef]
- Su, Y.; Yu, X.; Fu, X.; Zhu, Q.; Liu, L.; Zhu, Y.; Zhang, Y. Embedding Ag nanoparticles to construct BiOI/Ag/PANI with enhanced photoelectrocatalytic activity: A demonstration of the switch from type-II to Z-scheme. Electrochim. Acta 2020, 344, 136144. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.; Shi, Y.; Sun, H.; Li, L.; Guo, F.; Wen, H. Carbon dots as solid-state electron mediator and electron acceptor in S-scheme heterojunction for boosted photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 595, 153482. [Google Scholar] [CrossRef]
- Pham, V.V.; Truong, T.K.; Hai, L.V.; La, H.P.P.; Nguyen, H.T.; Lam, V.Q.; Tong, H.D.; Nguyen, T.Q.; Sabbah, A.; Chen, K.-H.; et al. S-Scheme α-Fe2O3/g-C3N4 Nanocomposites as Heterojunction Photocatalysts for Antibiotic Degradation. ACS Appl. Nano Mater. 2022, 5, 4506–4514. [Google Scholar] [CrossRef]
- Wei, H.; Meng, F.; Yu, W.; Li, J.; Zhang, H. Highly efficient photocatalytic degradation of levofloxacin by novel S-scheme heterojunction Co3O4/Bi2MoO6@g-C3N4 hollow microspheres: Performance, degradation pathway and mechanism. Sep. Purif. Technol. 2023, 318, 123940. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, B.; Qin, X.; Ho, W.; Yu, J. Zinc porphyrin/g-C3N4 S-scheme photocatalyst for efficient H2O2 production. Chem. Eng. J. 2023, 467, 143528. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zhai, H.; Zhang, Q.; Huang, B.; Wang, Z.; Liu, Y.; Dai, Y.; Qin, X.; Zhang, X. Synthesis of synergetic phosphorus and cyano groups (CN) modified g-C3N4 for enhanced photocatalytic H2 production and CO2 reduction under visible light irradiation. Appl. Catal. B Environ. 2018, 232, 521–530. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Chen, Z.; Cai, Y.; Guo, F.; Du, X. Environmentally friendly supermolecule self-assembly preparation of S-doped hollow porous tubular g-C3N4 for boosted photocatalytic H2 production. Ceram. Int. 2023, 49, 11989–11998. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Guo, F.; Li, Y.; Shi, W. Synergistic coupling of piezoelectric and plasmonic effects regulates the Schottky barrier in Ag nanoparticles/ultrathin g-C3N4 nanosheets heterostructure to enhance the photocatalytic activity. Appl. Surf. Sci. 2023, 616, 156466. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Zhong, W.; Chen, Z.; Wei, Y.; Guo, F.; Chen, L.; Du, X. Boosted built-in electric field and active sites based on Ni-doped heptazine/triazine crystalline carbon nitride for achieving high-efficient photocatalytic H2 evolution. J. Mol. Struct. 2023, 1280, 135076. [Google Scholar] [CrossRef]
- Xing, Z.; Shi, K.; Parsons, Z.S.; Feng, X. Interplay of Active Sites and Microenvironment in High-Rate Electrosynthesis of H2O2 on Doped Carbon. ACS Catal. 2023, 13, 2780–2789. [Google Scholar] [CrossRef]
- Hu, S.; Qu, X.; Li, P.; Wang, F.; Li, Q.; Song, L.; Zhao, Y.; Kang, X. Photocatalytic oxygen reduction to hydrogen peroxide over copper doped graphitic carbon nitride hollow microsphere: The effect of Cu(I)-N active sites. Chem. Eng. J. 2018, 334, 410–418. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Q.; Zhou, Y.; Ye, Q.; Jiang, D.; Tian, D.; Li, D. Boosting charge transfer in Au-decorated B/K co-doped CN nanosheets towards enhanced photocatalytic CO2 reduction. Mater. Chem. Front. 2023, 7, 2049–2058. [Google Scholar] [CrossRef]
- Muelas-Ramos, V.; Sampaio, M.J.; Silva, C.G.; Bedia, J.; Rodriguez, J.J.; Faria, J.L.; Belver, C. Degradation of diclofenac in water under LED irradiation using combined g-C3N4/NH2-MIL-125 photocatalysts. J. Hazard. Mater. 2021, 416, 126199. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, L.; Shi, Y.; Shi, W.; Yang, X.; Li, H. Achieving superior anticorrosion and antibiofouling performance of polyaniline/graphitic carbon nitride composite coating. Prog. Org. Coat. 2023, 179, 107512. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Shi, Y.; Guo, F.; Yang, X.; Shi, W. A hydrophobic high-crystalline g-C3N4/epoxy resin composite coating with excellent durability and stability for long-term corrosion resistance. Mater. Today Commun. 2023, 35, 105692. [Google Scholar] [CrossRef]
- Zhang, X.; Maddock, J.; Nenoff, T.M.; Denecke, M.A.; Yang, S.; Schroder, M. Adsorption of iodine in metal-organic framework materials. Chem. Soc. Rev. 2022, 51, 3243–3262. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Harish, S.; Archana, J.; Navaneethan, M. Fabrication of novel hybrid Z-Scheme WO3@g-C3N4@MWCNT nanostructure for photocatalytic degradation of tetracycline and the evaluation of antimicrobial activity. Chemosphere 2022, 287, 132050. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Yin, H.; Li, S.-L.; Gan, L.-Y.; Wang, P. Pt-embedded in monolayer g-C3N4 as a promising single-atom electrocatalyst for ammonia synthesis. J. Mater. Chem. A 2019, 7, 11908–11914. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B Environ. 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, A. Recent advancements of g-C3N4-based magnetic photocatalysts towards the degradation of organic pollutants: A review. Nanotechnology 2021, 33, 072004. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Ren, Q.; Liu, X.; Jin, Z.; Ding, Y.; Zhu, H.; Li, J.; Chen, R. Fenton activity on RhB degradation of magnetic g-C3N4/diatomite/Fe3O4 composites. Appl. Surf. Sci. 2021, 543, 148844. [Google Scholar] [CrossRef]
- Wang, S.; Long, J.; Jiang, T.; Shao, L.; Li, D.; Xie, X.; Xu, F. Magnetic Fe3O4/CeO2/g-C3N4 composites with a visible-light response as a high efficiency Fenton photocatalyst to synergistically degrade tetracycline. Sep. Purif. Technol. 2021, 278, 119609. [Google Scholar] [CrossRef]


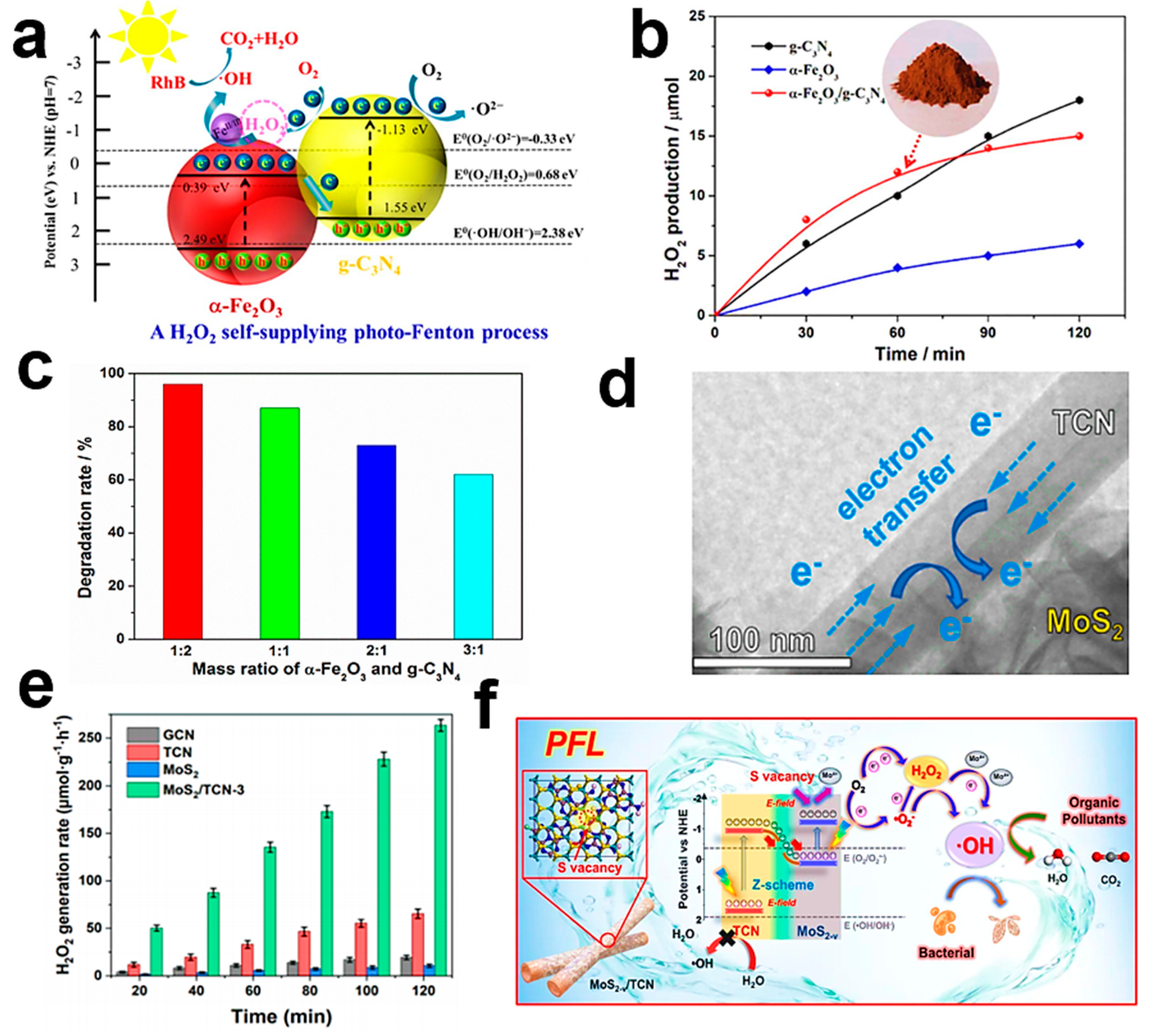
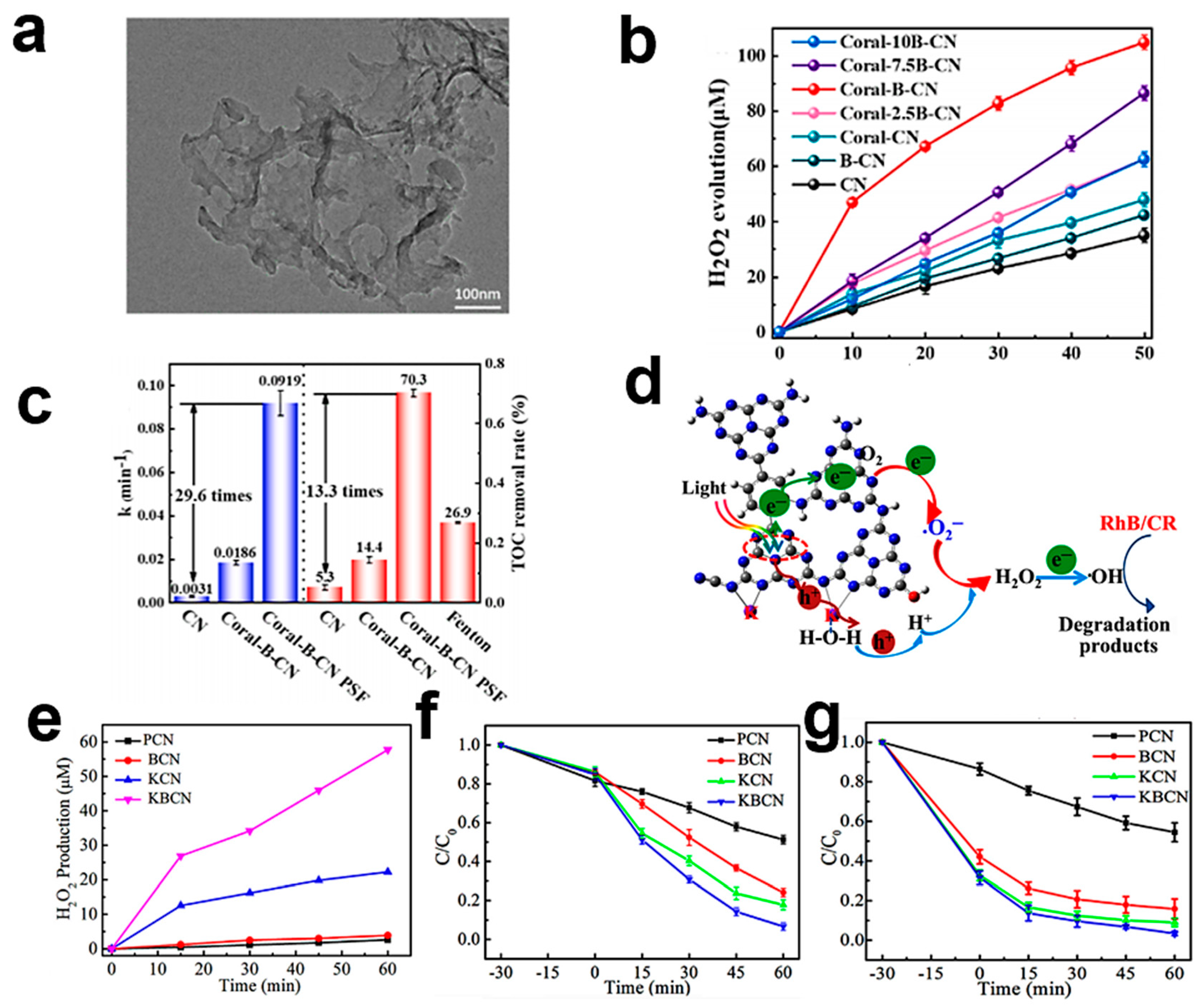

| Year | Photocatalysts | Light Source | Iron Ion Source | Sacrificial Agent | Pollutants | H2O2 Yield | Pollutant Removal (%) | TOC Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 2020 | P-g-C3N4 | Vis | FeSO4·7H2O | - | 2,4-DCP | 23.5 μM h−1 | 91 | 42.7 | [46] |
| Ti3+-TiO2-C3N4por | Vis | - | - | MO | 6.32 mM h−1 | 97.9 | 89 | [105] | |
| M101-Ux | Vis | MIL101 | - | 2,6-DCP/2,4,5-TCP | 23 μM h−1 | 98.7/97.3 | 16/14 | [106] | |
| TE-g-C3N4 | Vis | FeCl3 | - | RhB | N/A | ~100 | - | [107] | |
| α-Fe2O3/g-C3N4 | Vis | α-Fe2O3 | IPA | RhB/TC | 7.5 μM h−1 | 96/95 | 92/86 | [93] | |
| Al0-CNTs/CNTs-Fe-Cu | Vis | FeSO4 7H2O | - | SMR | N/A | 85 | 60 | [108] | |
| Metal-free GCN | Vis | Fe(II) | Isopropyl alcohol | GA | N/A | ~93 | 88.8 | [109] | |
| 2021 | ZnO/g-C3N4 | Vis | FeSO4 | IPA | Bactericidal | 5312.45 μM L−1 | 97.4 | - | [110] |
| SCNO@CdS | Vis | FeCl2·4H2O | IPA | RhB | 79,971.0 μM g−1 h−1 | ~83 | - | [111] | |
| P-C3N4/O-C3N4 | Vis | FeSO4 7H2O | IPA | MTZ | 179 μM h−1 | 91.6 | - | [112] | |
| g-C3N4/PDI | Vis | FeSO4 7H2O | - | Bactericidal | 112.95 μM h−1 | ~100 | - | [113] | |
| 2022 | GCN | Vis | FeSO4 7H2O | IPA | 2,4-DCP | 21.59 mM h−1 g−1 | 88.8 | - | [114] |
| UPCN | Vis | FeCl3·6H2O | IPA | OTC | 23.91 μM L−1 | 86.23 | 48..6 | [115] | |
| Cg-C3N4 | Vis | FeSO4 7H2O | IPA | 4-CP | 217.26 μM g−1 h−1 | ~98 | 59.64 | [116] | |
| KBCN | Vis | - | - | RhB/CR | 57.7 μM h−1 | 93.3/96.6 | - | [117] | |
| OPCN | Vis | FeCl3·6H2O | IPA | 2,4-DCP | 25.06 μM h−1 | 93 | 42.22 | [24] | |
| CUCN | Vis | FeSO4 7H2O | - | RhB | 14.81 μM h−1 | up to 100 | 63.77 | [118] | |
| TFMS-CP | Vis | FeCl3 | IPA | PH/HQ/MO | 1.02 μM h−1 | 92.6/84.6/88.2 | - | [119] | |
| 2023 | MoS2-v/TCN | Vis | - | IPA | RhB | 1879 μM g−1 h−1 | 98.5 | - | [120] |
| PC@PL | Vis | LaFeO3 | - | MB | 7.86 μM L−1 | 97 | - | [121] | |
| Coral-B-CN | Vis | FeSO4 7H2O | - | 4-CP | 314.55 μM g−1 h−1 | 99.6 | 70.3 | [122] | |
| Cv-PCNNS | Vis | FeSO4 7H2O | IPA | MTZ | 984.8 μM L−1 h−1 | 90.7 | 62 | [123] | |
| CPBA-CN | Vis | FeCl2·4H2O | - | 4-CP | 156.4 μM h−1 | ~97 | 74.6 | [124] | |
| KCN | Vis | - | IPA | BPA | 1.76 mM h−1 | 95.6 | 63.8 | [125] |
| Abbreviated Pre-Name | Abbreviated Name |
|---|---|
| Hydrogen peroxide | H2O2 |
| Reactive oxygen species | ROS |
| Superoxide anion radical | |
| Singlet oxygen | 1O2 |
| Graphite carbon nitride | g-C3N4 |
| Oxygen reduction reaction | ORR |
| Advanced oxidation processes | AOPs |
| Hydroxyl radicals | ∙OH |
| Valence band | VB |
| Conduction band | CB |
| 2,4-dichlorophenol | 2,4-DCP |
| Methyl Orange | MO |
| 2,4,5-Trichlorophenol | 2,4,5-TCP |
| Rhodamine B | RhB |
| Tetracycline | TC |
| 2,6-dichlorophen | 2,6-DCP |
| Sulfamerazine | SMR |
| Gallic acid | GA |
| Metronidazole | MTZ |
| 4-Chlorophenol | 4-CP |
| Phenol | PH |
| Hydroquinone | HQ |
| Oxytetracycline | OTC |
| Methylene Blue | MB |
| Metronidazole | MTZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yan, Y.; Lu, C.; Lin, X.; Fu, Z.; Shi, W.; Guo, F. Photocatalytic Self-Fenton System of g-C3N4-Based for Degradation of Emerging Contaminants: A Review of Advances and Prospects. Molecules 2023, 28, 5916. https://doi.org/10.3390/molecules28155916
Chen Z, Yan Y, Lu C, Lin X, Fu Z, Shi W, Guo F. Photocatalytic Self-Fenton System of g-C3N4-Based for Degradation of Emerging Contaminants: A Review of Advances and Prospects. Molecules. 2023; 28(15):5916. https://doi.org/10.3390/molecules28155916
Chicago/Turabian StyleChen, Zhouze, Yujie Yan, Changyu Lu, Xue Lin, Zhijing Fu, Weilong Shi, and Feng Guo. 2023. "Photocatalytic Self-Fenton System of g-C3N4-Based for Degradation of Emerging Contaminants: A Review of Advances and Prospects" Molecules 28, no. 15: 5916. https://doi.org/10.3390/molecules28155916
APA StyleChen, Z., Yan, Y., Lu, C., Lin, X., Fu, Z., Shi, W., & Guo, F. (2023). Photocatalytic Self-Fenton System of g-C3N4-Based for Degradation of Emerging Contaminants: A Review of Advances and Prospects. Molecules, 28(15), 5916. https://doi.org/10.3390/molecules28155916










