Diverse Coordination Chemistry of the Whole Series Rare-Earth L-Lactates: Synthetic Features, Crystal Structure, and Application in Chemical Solution Deposition of Ln2O3 Thin Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of RE Lactates
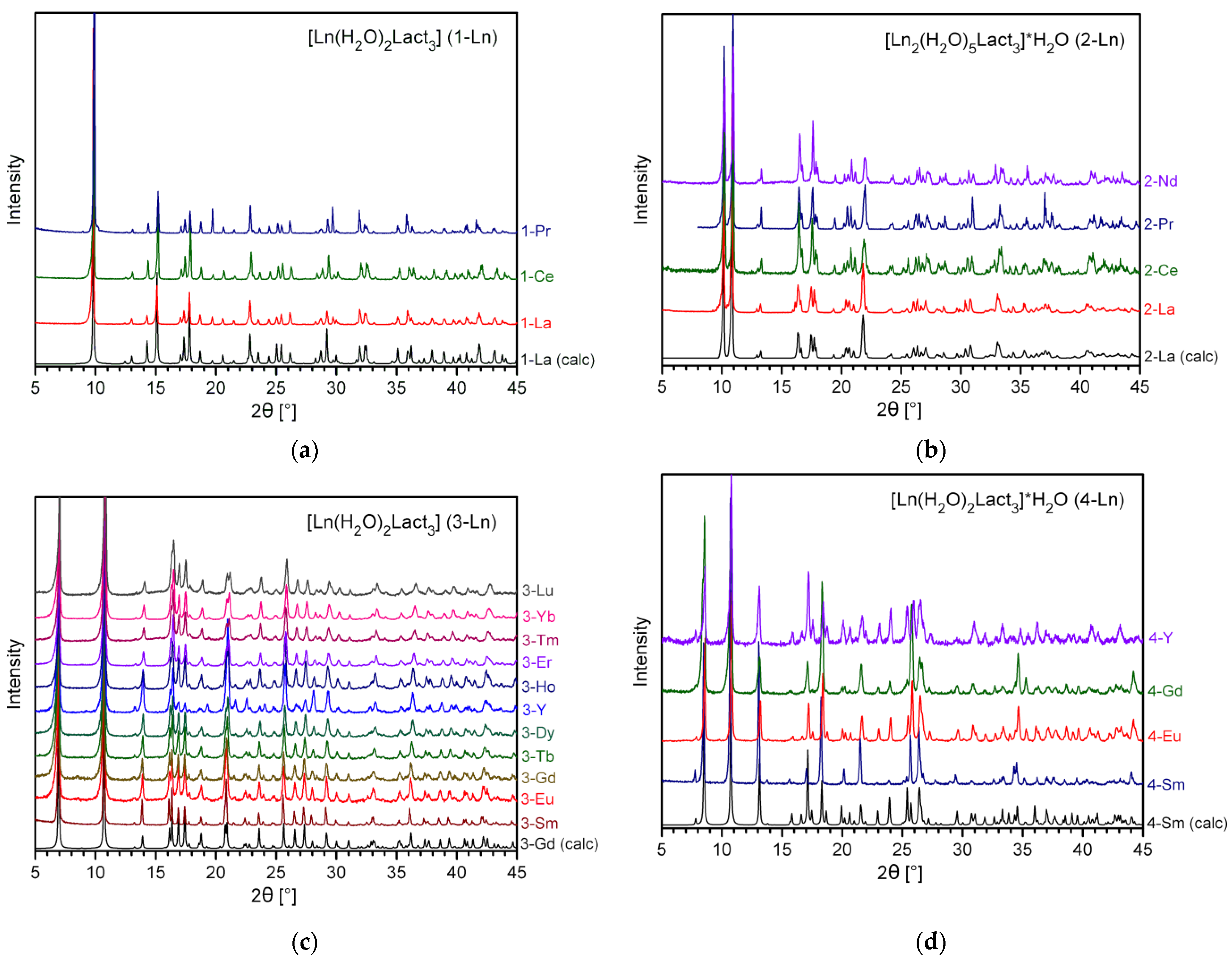
2.2. Crystal Structures of RE Lactates
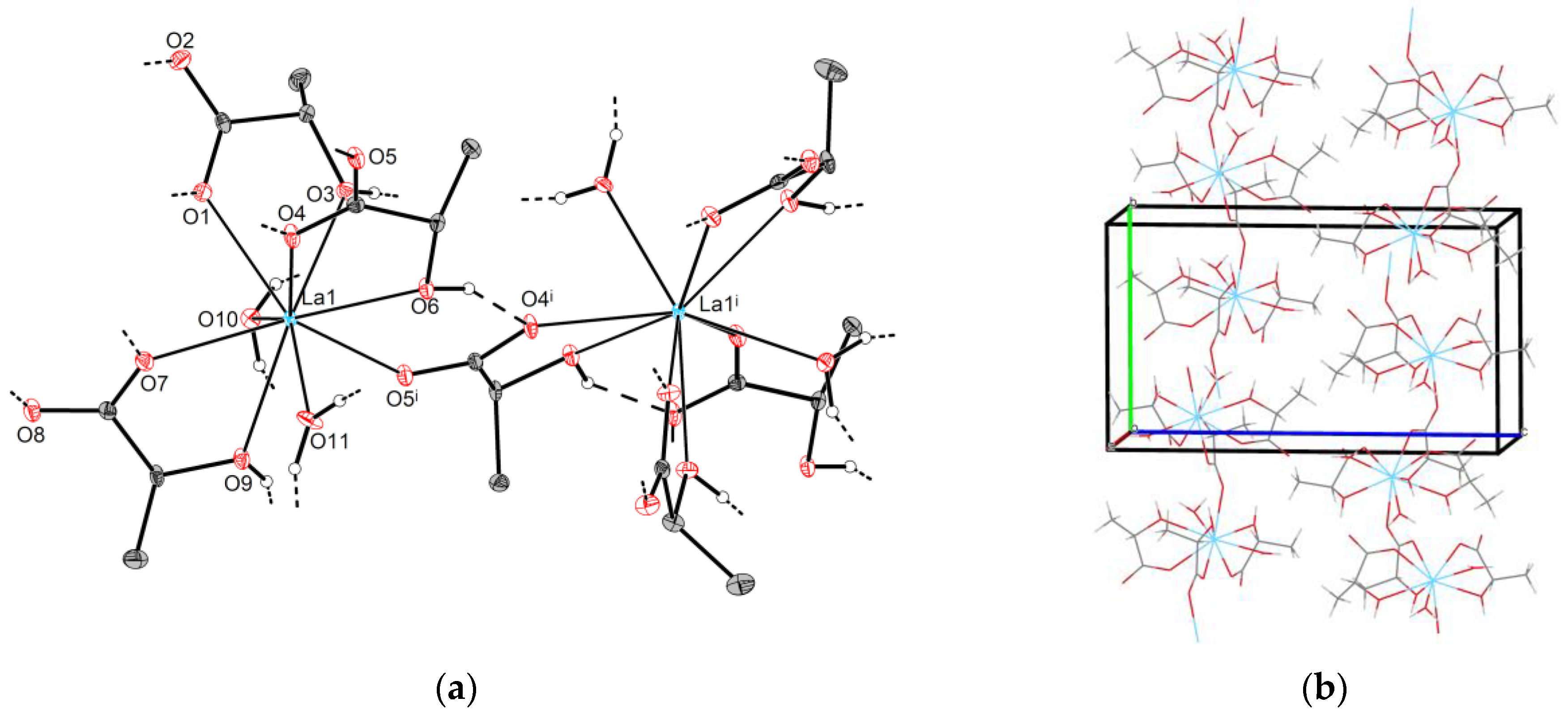
2.2.1. Crystal Structures of Type 1-Ln (Ln = La, Pr)
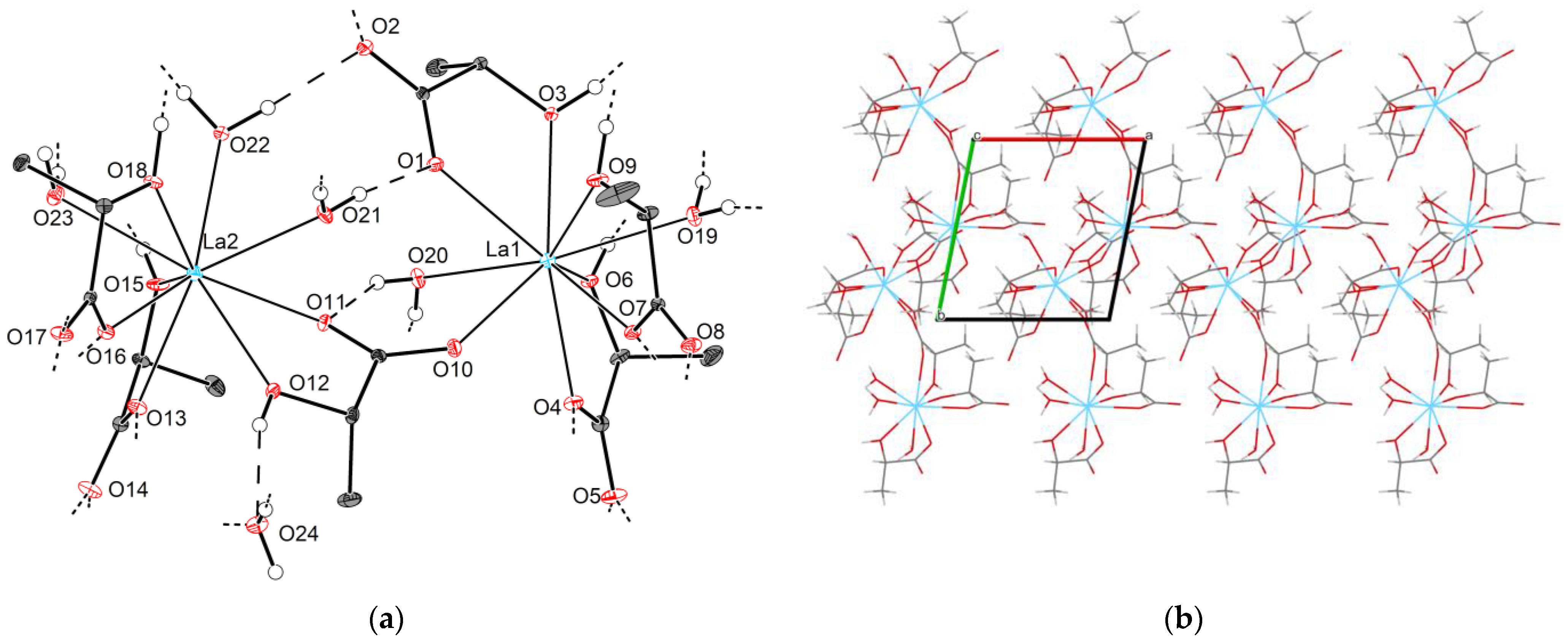
2.2.2. Crystal Structures of Type 2-Ln (Ln = La, Ce, Pr)
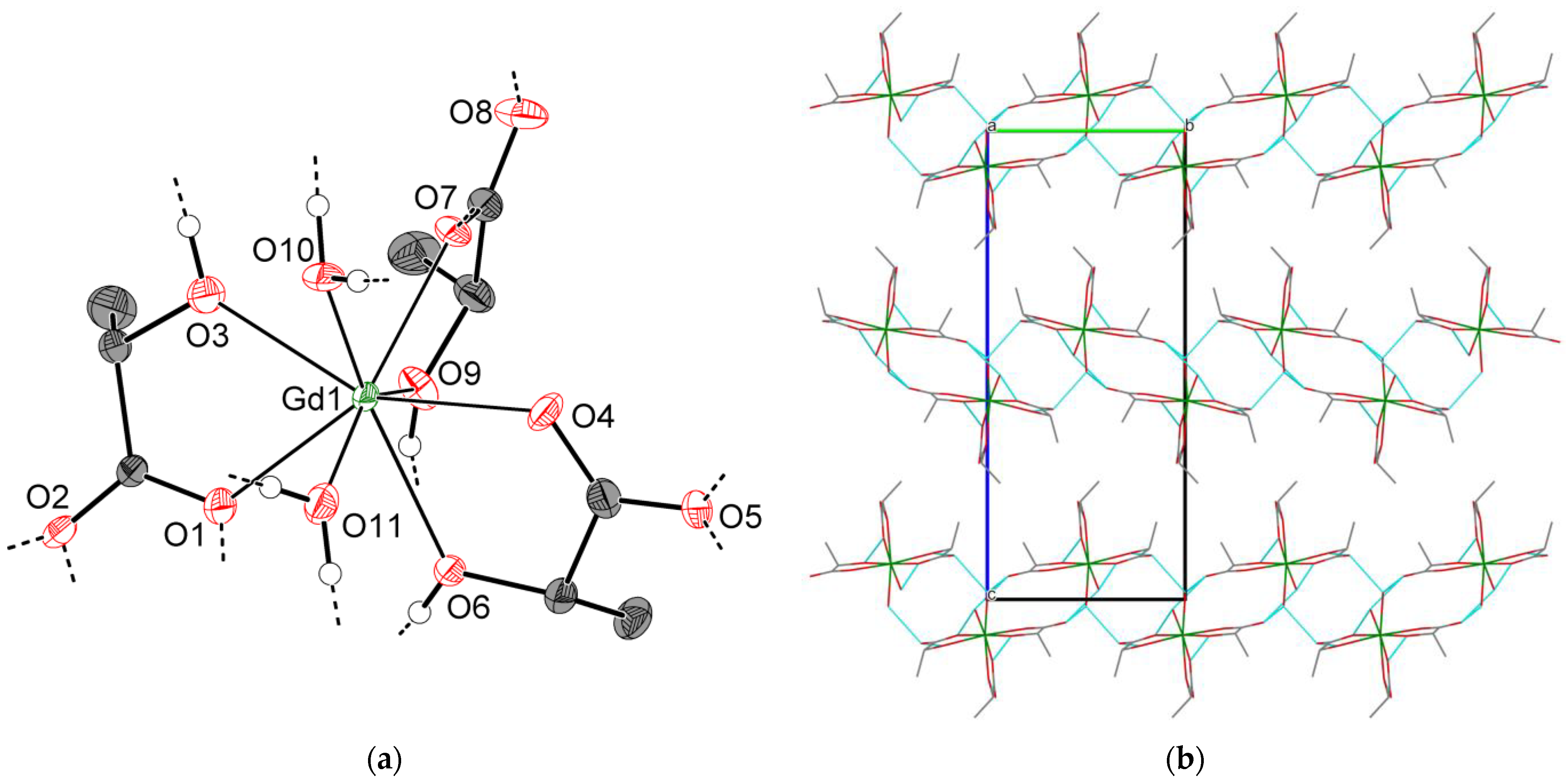
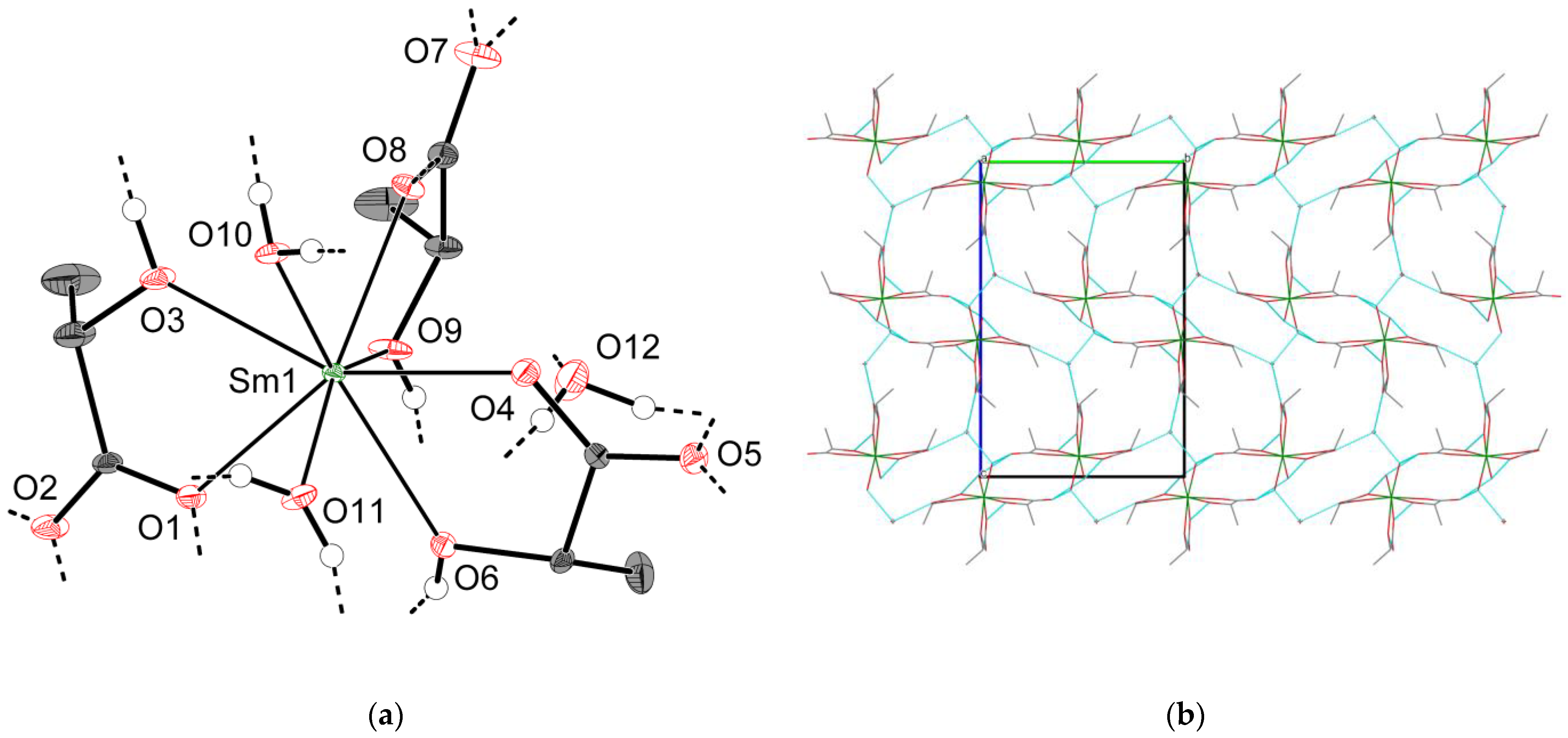
2.2.3. Crystal Structures of Types 3-Ln and 4-Ln (Ln = Sm–Lu, Y)
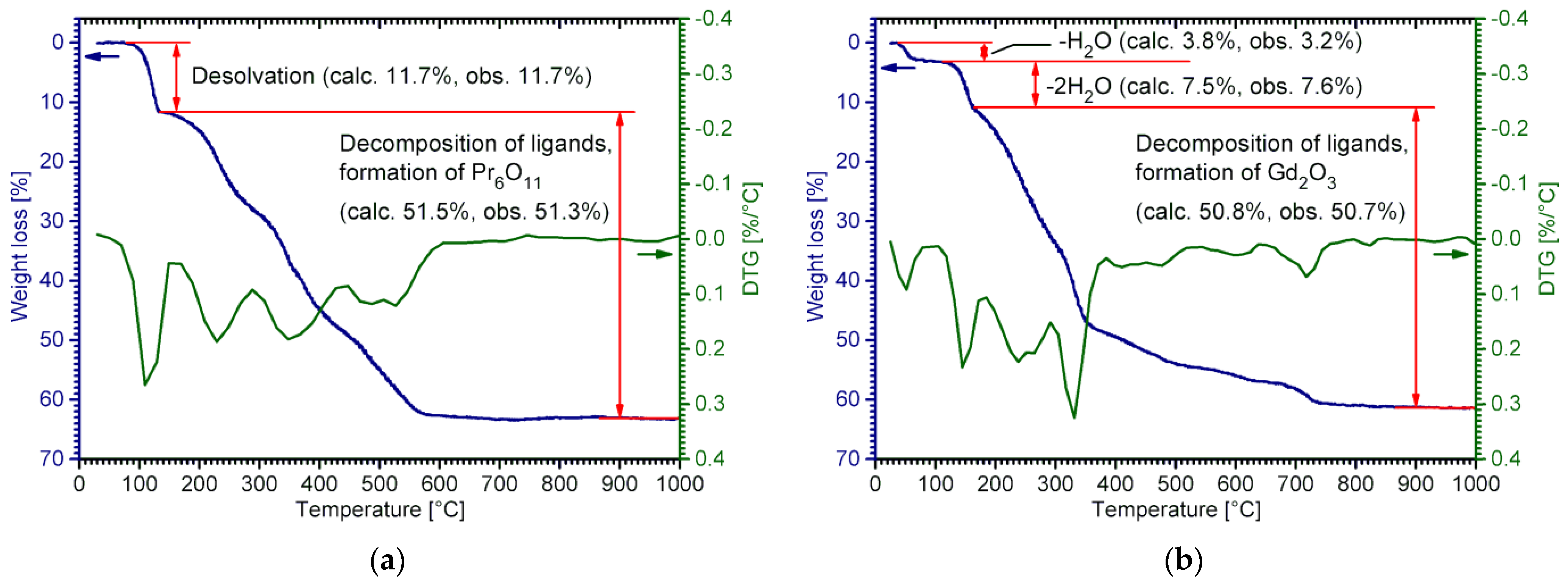
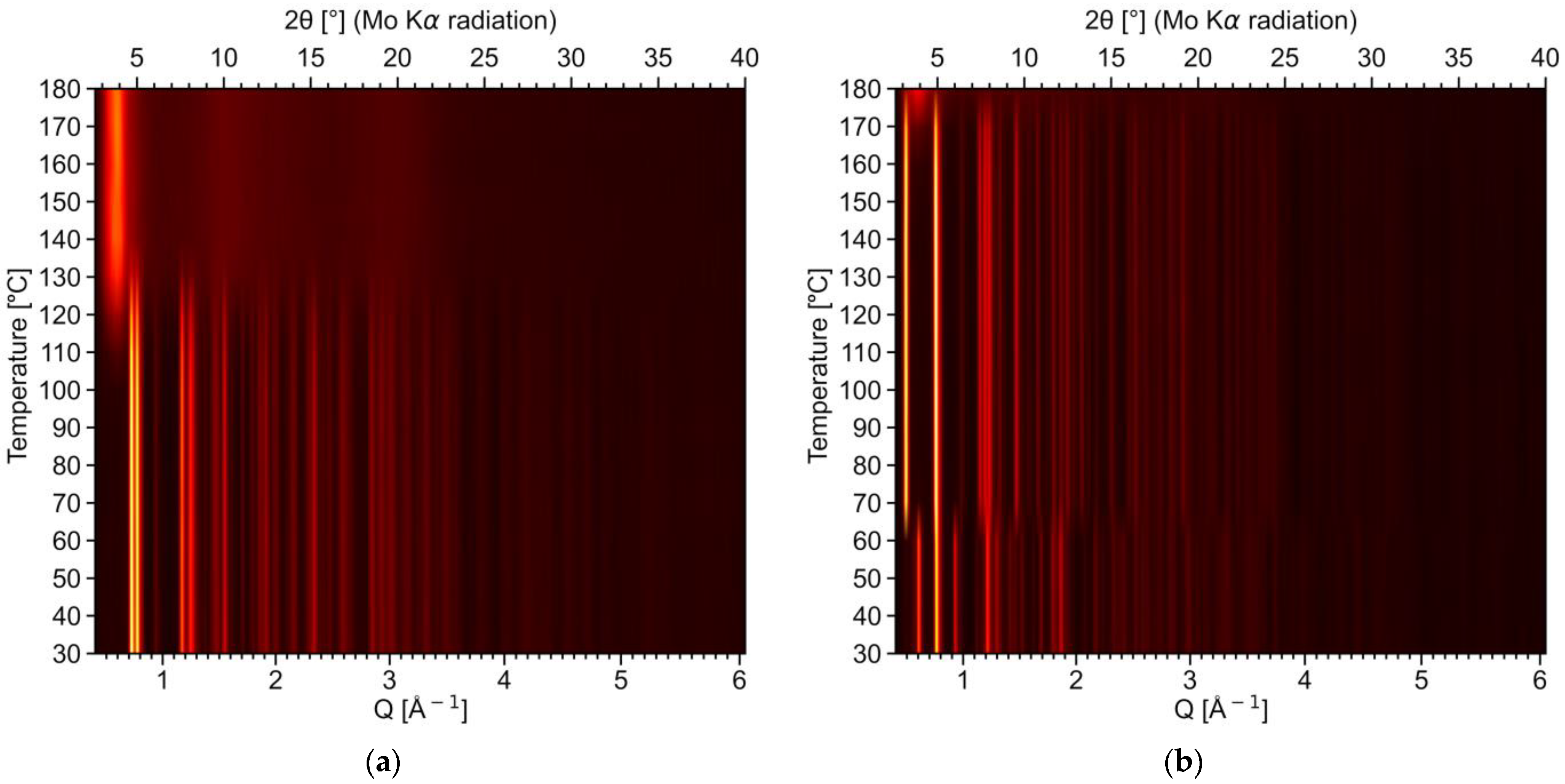
2.3. Thermal Decomposition of RE Lactates
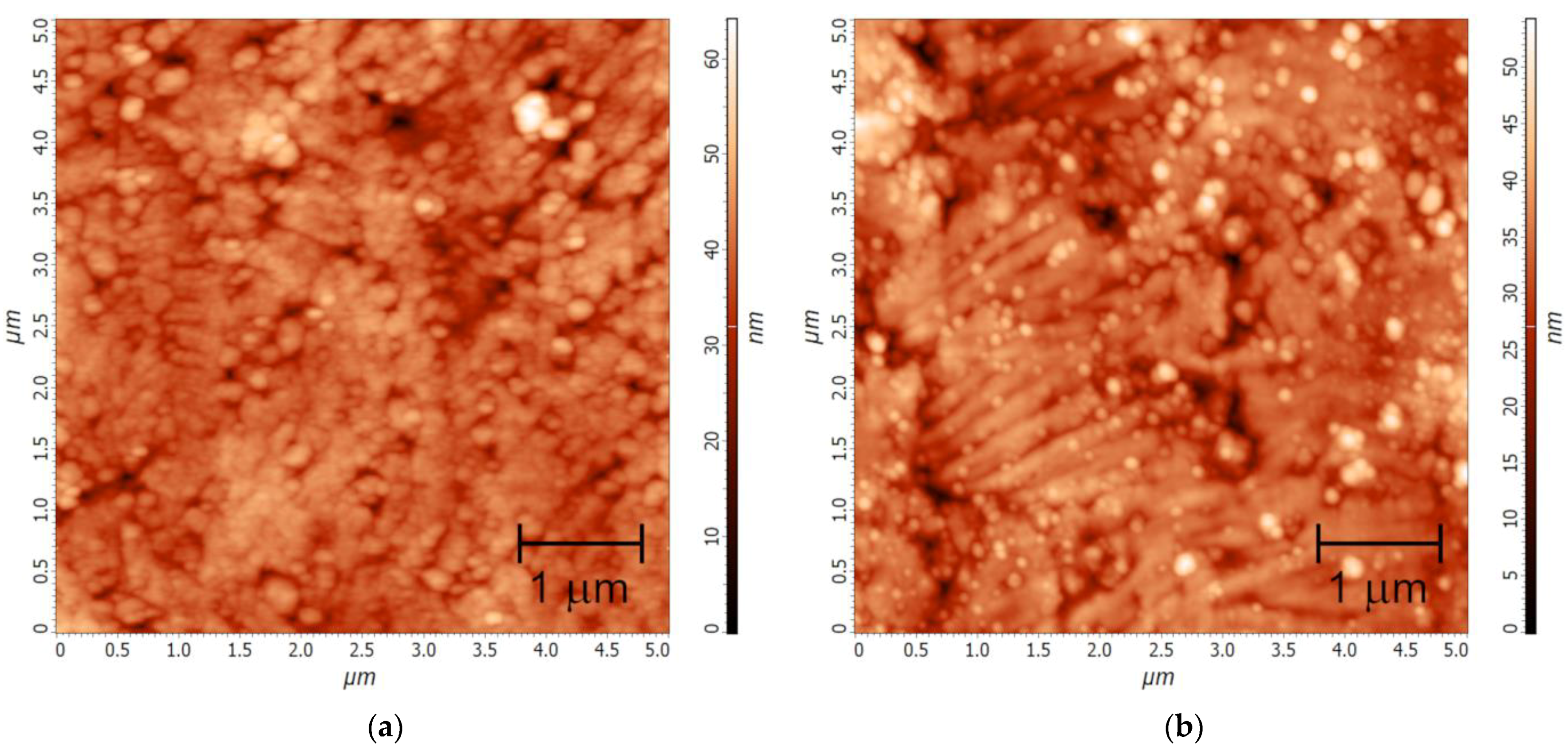
2.4. Ln2O3 Thin Films
3. Materials and Methods
3.1. Synthesis and Crystal Growth of RE Lactates
- [La(H2O)2Lact3]∞ (1-La). Calc. for C9H19LaO11 (%): La, 31.42; C, 24.45; H, 4.33. Found (%): La, 31.3; C, 24.8; H, 4.5. FTIR (ATR, ν, cm−1): 3338sh, 3264, 2992w, 2935vw, 2876vw (νOH, νCH); 1705w; 1698w; 1668w (δH2O); 1590sh, 1557s (νasCOO); 1486sh, 1477sh, 1465 (δasCH3); 1439vw; 1425w, 1391 (νsCOO, δCOH); 1375w; 1365, 1359sh, 1351sh (δsCH3); 1319; 1284; 1242s; 1130s; 1117s; 1093; 1055; 1037; 937; 864; 815; 773, 765 (δCOO); 660.
- [Ce(H2O)2Lact3]∞ (1-Ce). Calc. for C9H19CeO11 (%): Ce, 31.60; C, 24.38; H, 4.32. Found (%): Ce, 31.5; C, 24.3; H, 4.3. FTIR (ATR, ν, cm−1): 3335sh, 3256, 2993w, 2936vw, 2877vw (νOH, νCH); 1705w; 1695sh; 1664w (δH2O); 1588sh, 1557s (νasCOO); 1485sh, 1478sh, 1464 (δasCH3); 1439w; 1425w, 1388 (νsCOO, δCOH); 1364, 1359, 1351 (δsCH3); 1318; 1283; 1243s; 1126s; 1117s; 1093; 1055; 1036; 937; 864; 815; 774, 764 (δCOO); 660.
- [Pr(H2O)2Lact3]∞ (1-Pr). Calc. for C9H19O11Pr (%): Pr, 31.73. Found (%): Pr, 31.6. FTIR (ATR, ν, cm−1): 3338sh, 3255, 2995w, 2939vw, 2879vw (νOH, νCH); 1704vw; 1694vw; 1662w (δH2O); 1591sh, 1558 (νasCOO); 1480, 1466 (δasCH3); 1440w; 1426w, 1391 (νsCOO, δCOH); 1375w; 1365, 1358, 1352sh (δsCH3); 1330; 1319; 1284; 1245s; 1130s; 1118s; 1094; 1056; 1037; 938; 865; 817; 774, 764 (δCOO); 660.
- [La2(H2O)5Lact6]∙H2O (2-La). Calc. for C18H42La2O24 (%): La, 30.19; C, 23.49; H, 4.60. Found (%): La, 30.4; C, 23.6; H, 4.5. FTIR (ATR, ν, cm−1): 3562sh, 3303sh, 3170, 2985vw, 2941vw (νOH, νCH); 1665w, 1653sh, 1645sh (δH2O); 1568s (νasCOO); 1475 (δasCH3); 1424sh, 1395 (νsCOO, δCOH); 1378sh; 1363 (δsCH3); 1320, 1277sh, 1268; 1232w; 1127; 1116s; 1089; 1041s; 932; 865; 845w; 774 (δCOO); 663.
- [Ce2(H2O)5Lact6]∙H2O (2-Ce). Calc. for C18H42Ce2O24 (%): Ce, 30.37; C, 23.43; H, 4.59. Found (%): Ce, 31.2; C, 23.0; H, 4.6. FTIR (ATR, ν, cm−1): 3319sh, 3203, 2986w, 2941vw (νOH, νCH); 1662vw, 1645sh (δH2O); 1564s (νasCOO); 1471vw, 1465sh, 1456sh (δasCH3); 1423sh, 1395 (νsCOO, δCOH); 1387sh; 1377sh; 1362 (δsCH3); 1319; 1280sh; 1266w; 1230sh; 1124sh; 1116s; 1089; 1041s; 932; 865; 845w; 819w; 774 (δCOO); 707sh; 660.
- [Pr2(H2O)5Lact6]∙H2O (2-Pr). Calc. for C18H42O24Pr2 (%): Pr, 30.49. Found (%): Pr, 30.6. FTIR (ATR, ν, cm−1): 3569vw, 3328sh, 3199, 2985vw, 2941vw (νOH, νCH); 1660, 1650sh (δH2O); 1567s, 1556sh (νasCOO); 1475 (δasCH3); 1426sh, 1398 (νsCOO, δCOH); 1389sh; 1384sh; 1376sh; 1363 (δsCH3); 1320; 1306sh; 1279w; 1266; 1126; 1116s; 1090; 1042s; 933w; 866; 848w; 816wv; 774 (δCOO); 711sh; 663.
- [Nd2(H2O)5Lact6]∙H2O (2-Nd). Calc. for C18H42Nd2O24 (%): Nd, 30.99. Found (%): Nd, 32.0. FTIR (ATR, ν, cm−1): 3319sh, 3195, 2980sh, 2975sh, 2967sh, 2942vw, 2936sh, 2897w, 2880w (νOH, νCH); 1659w, 1651sh (δH2O); 1570s, 1559sh (νasCOO); 1471, 1450vw (δasCH3); 1423sh, 1399 (νsCOO, δCOH); 1385sh; 1363 (δsCH3); 1320; 1280; 1266; 1223; 1126; 1116s; 1092; 1042s; 933; 866; 849; 812sh; 775 (δCOO); 710sh; 672; 663.
- [Sm(H2O)2Lact3] (3-Sm). Calc. for C9H19O11Sm (%): Sm, 33.15; C, 23.83; H, 4.22. Found (%): Sm, 33.2; C, 23.6; H, 4.3. FTIR (ATR, ν, cm−1): 3399, 3159sh, 3054, 2997, 2989, 2980sh, 2957w, 2943sh, 2883vw (νOH, νCH); 1668sh (δH2O); 1577s (νasCOO); 1481, 1470, 1456sh (δasCH3); 1427sh, 1407sh, 1404sh, 1391s (νsCOO, δCOH); 1365, 1358 (δsCH3); 1320sh; 1314s; 1281; 1270sh; 1263; 1111s; 1094; 1045s; 932; 863; 782, 776sh (δCOO); 759; 709; 665sh.
- [Eu(H2O)2Lact3] (3-Eu). Calc. for C9H19EuO11 (%): Eu, 33.38. Found (%): Eu, 33.7. FTIR (ATR, ν, cm−1): 3403, 3158sh, 3058, 2997, 2989, 2980sh, 2958w, 2938w, 2885sh (νOH, νCH); 1663sh (δH2O); 1583s (νasCOO); 1482, 1469, 1457sh (δasCH3); 1427sh, 1408sh, 1393s (νsCOO, δCOH); 1365, 1359 (δsCH3); 1321sh; 1314s; 1282; 1272; 1265; 1112s; 1095; 1046s; 932; 863; 783, 776sh (δCOO); 758; 712; 651.
- [Gd(H2O)2Lact3] (3-Gd). Calc. for C9H19GdO11 (%): Gd, 34.15. Found (%): Gd, 34.1. FTIR (ATR, ν, cm−1): 3403, 3161sh, 3074, 3013vw, 2998w, 2978vw, 2960w, 2946w, 2845vw (νOH, νCH); 1668sh (δH2O); 1585s (νasCOO); 1480, 1465 (δasCH3); 1409sh, 1394s (νsCOO, δCOH); 1372, 1358 (δsCH3); 1314s; 1278sh; 1263; 1115s; 1092sh; 1045s; 933; 864; 787, 768, 753sh (δCOO); 710; 651.
- [Tb(H2O)2Lact3] (3-Tb). Calc. for C9H19O11Tb (%): Tb, 34.39. Found (%): Tb, 34.1. FTIR (ATR, ν, cm−1): 3403, 3151sh, 3061, 2997, 2989, 2981sh, 2955w, 2938w, 2883sh (νOH, νCH); 1661sh (δH2O); 1580s (νasCOO); 1483, 1468, 1456sh (δasCH3); 1428sh, 1409sh, 1393s (νsCOO, δCOH); 1365, 1359 (δsCH3); 1321sh; 1315s; 1281sh; 1271sh; 1264; 1112s; 1096; 1046s; 933; 864; 783, 776sh (δCOO); 759sh; 715; 666sh; 651.
- [Dy(H2O)2Lact3] (3-Dy). Calc. for C9H19DyO11 (%): Dy, 34.89. Found (%): Dy, 35.3. FTIR (ATR, ν, cm−1): 3405, 3161sh, 3061, 2997w, 2990w, 2959sh, 2941sh, 2881vw (νOH, νCH); 1581s (νasCOO); 1485, 1468 (δasCH3); 1413sh, 1394s (νsCOO, δCOH); 1366w, 1359 (δsCH3); 1315s; 1282sh; 1273sh; 1265; 1123sh; 1112s; 1096; 1047s; 934; 864; 784, 775sh, 760sh (δCOO); 716; 651.
- [Ho(H2O)2Lact3] (3-Ho). Calc. for C9H19HoO11 (%): Ho, 35.23; C, 23.09; H, 4.09. Found (%): Ho, 35.9; C, 22.8; H, 4.2. FTIR (ATR, ν, cm−1): 3405, 3158sh, 3064, 2981, 2967, 2942sh, 2880vw (νOH, νCH); 1666sh (δH2O); 1583s (νasCOO); 1485, 1469 (δasCH3); 1415sh, 1395s (νsCOO, δCOH); 1366, 1359 (δsCH3); 1323sh; 1315; 1283sh; 1273sh; 1266; 1124sh; 1113s; 1096; 1047s; 934; 865; 785, 775sh (δCOO); 719; 651.
- [Er(H2O)2Lact3] (3-Er). Calc. for C9H19ErO11 (%): Er, 35.55. Found (%): Er, 35.6. FTIR (ATR, ν, cm−1): 3406, 3157sh, 3059, 3019vw, 2997sh, 2990, 2981sh, 2961w, 2940vw, 2878sh (νOH, νCH); 1666sh (δH2O); 1584s (νasCOO); 1487, 1469 (δasCH3); 1434sh, 1416sh, 1397s (νsCOO, δCOH); 1378sh; 1366, 1359 (δsCH3); 1324sh; 1316; 1283; 1273sh; 1267; 1125w; 1113s; 1096; 1047s; 935; 865; 786, 776sh (δCOO); 722; 673vw; 654.
- [Tm(H2O)2Lact3] (3-Tm). Calc. for C9H19O11Tm (%): Tm, 35.78. Found (%): Tm, 37.2. FTIR (ATR, ν, cm−1): 3408, 3158sh, 3062, 2996sh, 2989, 2983sh, 2966w, 2950vw 2931vw, 2879vw (νOH, νCH); 1663sh (δH2O); 1583s (νasCOO); 1487, 1469 (δasCH3); 1434sh, 1413sh, 1396s (νsCOO, δCOH); 1380; 1366, 1359 (δsCH3); 1322sh; 1316s; 1282sh; 1272sh; 1266; 1113s; 1097; 1048s; 935; 865; 785, 777sh (δCOO); 723.
- [Yb(H2O)2Lact3] (3-Yb). Calc. for C9H19O11Yb (%): Yb, 36.33. Found (%): Yb, 35.6. FTIR (ATR, ν, cm−1): 3419, 3167sh, 3062, 3001, 2983, 2941, 2892w (νOH, νCH); 1663sh (δH2O); 1583s (νasCOO); 1487, 1471 (δasCH3); 1434sh, 1414sh, 1398s (νsCOO, δCOH); 1379; 1364 (δsCH3); 1318s; 1284; 1267; 1126; 1114s; 1102sh; 1098sh; 1048s; 935; 866; 786, 777sh (δCOO); 722; 651.
- [Lu(H2O)2Lact3] (3-Lu). Calc. for C9H19LuO11 (%): Lu, 36.59. Found (%): Lu, 37.8. FTIR (ATR, ν, cm−1): 3408, 3146sh, 3066, 2996sh, 2990, 2983sh, 2941sh, 2879vw (νOH, νCH); 1667sh (δH2O); 1584s (νasCOO); 1485, 1470 (δasCH3); 1434sh, 1414sh, 1399s (νsCOO, δCOH); 1380; 1366, 1360 (δsCH3); 1323sh; 1316s; 1282sh, 1273sh, 1268; 1113s; 1098; 1049s; 936; 866; 786, 776sh (δCOO); 727.
- [Y(H2O)2Lact3] (3-Y). Calc. for C9H19O11Y (%): Y, 22.67; C, 27.57; H, 4.88. Found (%): Y, 23.4; C, 27.3; H, 4.9. FTIR (ATR, ν, cm−1): 3407, 3159sh, 3062, 2996sh, 2990, 2980sh, 2941sh, 2881vw (νsCOO, δCOH); 1583 (νasCOO); 1484, 1469 (δasCH3); 1416sh, 1396s (νsCOO, δCOH); 1377sh, 1365, 1359 (δsCH3); 1323sh; 1315s; 1283sh; 1275sh; 1266; 1124sh; 1113s; 1096; 1047s; 934; 865; 785, 775sh (δCOO); 719; 652.
- [Sm(H2O)2Lact3]∙H2O (4-Sm). Calc. for C9H21O12Sm (%): Sm, 31.88. Found (%): Sm, 31.8. FTIR (ATR, ν, cm–1): 3492vw, 3399w, 3157sh, 3068, 2997w, 2989w, 2979sh, 2959vw, 2941sh. 2883vw (νOH, νCH); 1634sh (δH2O); 1582s (νasCOO); 1480, 1467 (δasCH3); 1407sh, 1391s (νsCOO, δCOH); 1367, 1358 (δsCH3); 1322sh; 1314s; 1280sh; 1271sh; 1264; 1114s; 1094; 1045s; 932; 863; 782, 774sh (δCOO); 707; 650.
- [Eu(H2O)2Lact3]∙H2O (4-Eu). Calc. for C9H21EuO12 (%): Eu, 32.11. Found (%): Eu, 32.2. FTIR (ATR, ν, cm–1): 3492vw, 3402vw, 3157sh, 3084, 3014w, 2999w, 2978w, 2960vw, 2949vw (νOH, νCH); 1636 (δH2O); 1588s (νasCOO); 1479, 1465 (δasCH3); 1409s, 1396sh (νsCOO, δCOH); 1372, 1358 (δsCH3); 1315s; 1278; 1264; 1116s; 1092sh; 1045s; 931; 864; 788, 768 (δCOO); 705.
- [Y(H2O)2Lact3]∙H2O (4-Y). Calc. for C9H21O12Y (%): Y, 21.68. Found (%): Y, 21.5. FTIR (ATR, ν, cm–1): 3492vw, 3405sh, 3170sh, 3086, 3014w. 2999w, 2979w, 2963w, 2946w (νOH, νCH); 1639 (δH2O); 1591s (νasCOO); 1482sh, 1466 (δasCH3); 1412, 1401 (νsCOO, δCOH); 1373, 1359 (δsCH3); 1316s; 1279; 1265; 1115s; 1092w; 1051sh; 1045s; 934; 866; 790, 769 (δCOO); 708w; 677w.
3.2. Preparation of Precursor Solutions and Deposition of Ln2O3 Thin Films
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mishra, S.; Daniele, S. Metal–Organic Derivatives with Fluorinated Ligands as Precursors for Inorganic Nanomaterials. Chem. Rev. 2015, 115, 8379–8448. [Google Scholar] [CrossRef]
- ten Elshof, J.E. Chemical Solution Deposition of Oxide Thin Films. In Epitaxial Growth of Complex Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–100. [Google Scholar]
- Kessler, V.G. Aqueous Route to TiO2-Based Nanomaterials Using pH-Neutral Carboxylate Precursors. J. Sol-Gel Sci. Technol. 2013, 68, 464–470. [Google Scholar] [CrossRef]
- Schneller, T.; Waser, R.; Kosec, M.; Payne, D. Chemical Solution Deposition of Functional Oxide Thin Films; Schneller, T., Waser, R., Kosec, M., Payne, D., Eds.; Springer: Vienna, Austria, 2013; Volume 9783211993, ISBN 978-3-211-99310-1. [Google Scholar]
- Lee, H.Y.; Kim, S.I.; Lee, Y.C.; Hong, Y.P.; Park, Y.H.; Ko, K.H. New Chemical Route for YBCO Thin Films. IEEE Trans. Appl. Supercond. 2003, 13, 2743–2746. [Google Scholar] [CrossRef]
- Hasenkox, U.; Mitze, C.; Waser, R.; Arons, R.R.; Pommer, J.; Güntherodt, G. Chemical Solution Deposition of Epitaxial La1−x(Ca, Sr)XMnO3 Thin Films. J. Electroceram. 1999, 3, 255–260. [Google Scholar] [CrossRef]
- Kendin, M.; Tsymbarenko, D. Synthesis and Thermal Decomposition of Rare Earth Isovalerates and Their Solutions with Amines as an Effective Pathway to Obtain Oxide Nanomaterials. J. Anal. Appl. Pyrolysis 2019, 140, 367–375. [Google Scholar] [CrossRef]
- Schwartz, R.W. Chemical Solution Deposition of Perovskite Thin Films. Chem. Mater. 1997, 9, 2325–2340. [Google Scholar] [CrossRef]
- Tsymbarenko, D.M.; Martynova, I.A.; Malkerova, I.P.; Alikhanyan, A.S.; Kuzmina, N.P. Mixed Ligand Acetate, Propionate, and Pivalate Complexes of Rare Earth Metals with Monoethanolamine: A New Approach to the Synthesis, Composition, Structure, and Use for the Preparation of Oxide Materials. Russ. J. Coord. Chem. 2016, 42, 662–678. [Google Scholar] [CrossRef]
- Aspinall, H.C. Requirements of Precursors for MOCVD and ALD of Rare Earth Oxides. In Rare Earth Oxide Thin Films. Topics in Applied Physics; Fanciulli, M., Scarel, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 106, pp. 53–72. ISBN 3540357963. [Google Scholar]
- Jones, A.C.; Aspinall, H.C.; Chalker, P.R. Chapter 8. Chemical Vapour Deposition of Metal Oxides for Microelectronics Applications. In Chemical Vapour Deposition: Precursors, Processes and Applications; Jones, A.C., Hitchman, M.L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 357–412. [Google Scholar]
- Jones, A.C.; Aspinall, H.C.; Chalker, P.R. Molecular Design of Improved Precursors for the MOCVD of Oxides Used in Microelectronics. Surf. Coat. Technol. 2007, 201, 9046–9054. [Google Scholar] [CrossRef]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of Rare Earth Elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Ouchi, A.; Suzuki, Y.; Ohki, Y.; Koizumi, Y. Structure of Rare Earth Carboxylates in Dimeric and Polymeric Forms. Coord. Chem. Rev. 1988, 92, 29–43. [Google Scholar] [CrossRef]
- Gomez Torres, S.; Meyer, G. Anhydrous Neodymium(III) Acetate. Z. Anorg. Allg. Chem. 2008, 634, 231–233. [Google Scholar] [CrossRef]
- Malaestean, I.L.; Kutluca-Alıcı, M.; Ellern, A.; van Leusen, J.; Schilder, H.; Speldrich, M.; Baca, S.G.; Kögerler, P. Linear, Zigzag, and Helical Cerium(III) Coordination Polymers. Cryst. Growth Des. 2012, 12, 1593–1602. [Google Scholar] [CrossRef]
- Tsymbarenko, D.; Martynova, I.; Grebenyuk, D.; Shegolev, V.; Kuzmina, N. One-Dimensional Coordination Polymers of Whole Row Rare Earth Tris-Pivalates. J. Solid State Chem. 2018, 258, 876–884. [Google Scholar] [CrossRef]
- Kendin, M.; Tsymbarenko, D. 2D-Coordination Polymers Based on Rare-Earth Propionates of Layered Topology Demonstrate Polytypism and Controllable Single-Crystal-to-Single-Crystal Phase Transitions. Cryst. Growth Des. 2020, 20, 3316–3324. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Ryzhkov, N.; Tsymbarenko, D. Novel Mononuclear Mixed Ligand Complexes of Heavy Lanthanide Trifluoroacetates with Diethylenetriamine. J. Fluor. Chem. 2017, 202, 82–90. [Google Scholar] [CrossRef]
- Vermeir, P.; Cardinael, I.; Bäcker, M.; Schaubroeck, J.; Schacht, E.; Hoste, S.; Van Driessche, I. Fluorine-Free Water-Based Sol–Gel Deposition of Highly Epitaxial YBa2Cu3O7−δ Films. Supercond. Sci. Technol. 2009, 22, 075009. [Google Scholar] [CrossRef]
- Vermeir, P.; Cardinael, I.; Schaubroeck, J.; Verbeken, K.; Bäcker, M.; Lommens, P.; Knaepen, W.; D’haen, J.; De Buysser, K.; Van Driessche, I. Elucidation of the Mechanism in Fluorine-Free Prepared YBa2Cu3O7−δ Coatings. Inorg. Chem. 2010, 49, 4471–4477. [Google Scholar] [CrossRef]
- Queraltó, A.; Banchewski, J.; Pacheco, A.; Gupta, K.; Saltarelli, L.; Garcia, D.; Alcalde, N.; Mocuta, C.; Ricart, S.; Pino, F.; et al. Combinatorial Screening of Cuprate Superconductors by Drop-On-Demand Inkjet Printing. ACS Appl. Mater. Interfaces 2021, 13, 9101–9112. [Google Scholar] [CrossRef]
- Rasi, S.; Ricart, S.; Obradors, X.; Puig, T.; Roura-Grabulosa, P.; Farjas, J. Effect of Triethanolamine on the Pyrolysis of Metal-Propionate-Based Solutions. J. Anal. Appl. Pyrolysis 2019, 143, 104685. [Google Scholar] [CrossRef]
- Zhao, Y.; Torres, P.; Tang, X.; Norby, P.; Grivel, J.-C. Growth of Highly Epitaxial YBa2Cu3O7−δ Films from a Simple Propionate-Based Solution. Inorg. Chem. 2015, 54, 10232–10238. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Zhang, Y.; Liu, R.; Zhen, X. Synthesis, Sintering and Characterization of PLZST Perovskite Prepared by a Lactate Precursor Route. J. Eur. Ceram. Soc. 2006, 26, 323–329. [Google Scholar] [CrossRef]
- Gromilov, S.A.; Piryazev, D.A.; Tatarchuk, V.V. Crystal Structure of Zinc Lactate Dihydrate—A By-Product of the Micellar Synthesis of ZnO2 Nanoparticles from Zinc Acetate and Hydroperite. J. Struct. Chem. 2021, 62, 571–576. [Google Scholar] [CrossRef]
- Ke, Z.; Fan, X.; You-ying, D.; Chen, F.; Zhang, L.; Yang, K.; Li, B.; Kong, Y. Crystal Structure and Solution Thermodynamics of the Lactate Complex Zn[(C3H5O3)2(H2O)2]·H2O(S). Chem. Thermodyn. Therm. Anal. 2022, 5, 100024. [Google Scholar] [CrossRef]
- Singh, K.D.; Jain, S.C.; Sakore, T.D.; Biswas, A.B. The Crystal and Molecular Structure of Zinc Lactate Trihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 990–993. [Google Scholar] [CrossRef]
- Carballo, R.; Covelo, B.; Vázquez-López, E.M.; Castiñeiras, A.; Niclós, J. The Interaction of α-Hydroxycarboxylic Acids with Metal Ions: Lactic Acid (H2L1) and 2-Methyllactic Acid (H2L2) with Cobalt(II) Crystal and Molecular Structures of [Co(HL1)2(H2O)2]·H2O and [Co(HL2)2(H2O)2]. Z. Anorg. Allg. Chem. 2002, 628, 468–472. [Google Scholar] [CrossRef]
- Lis, T. Structure of Manganese(II) L-Lactate Dihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 937–939. [Google Scholar] [CrossRef]
- Larsen, E.M.; Homeier, E.H. Zirconium(IV) and Hafnium(IV) Complexes of α-Hydroxy Carboxylates, Lactates, Mandelates, and Isopropylmandelates. Stereospecificity in Eight-Coordinate Complexes. Inorg. Chem. 1972, 11, 2687–2692. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, H.; Wang, X.; Qu, Z.; Xiong, R.; Xue, X.; Xue, Z.; You, X. 2D Chiral Uranyl(VI) Coordination Polymers with Second-Harmonic Generation Response and Ferroelectric Properties. Eur. J. Inorg. Chem. 2003, 2003, 3712–3715. [Google Scholar] [CrossRef]
- Demartin, F.; Biagioli, M.; Strinna-Erre, L.; Panzanelli, A.; Micera, G. Molecular Structure of a Mono-Peroxo Vanadium(V) Complex Formed by d,l-Lactic Acid. Inorganica Chim. Acta 2000, 299, 123–127. [Google Scholar] [CrossRef]
- Kakihana, M.; Tomita, K.; Petrykin, V.; Tada, M.; Sasaki, S.; Nakamura, Y. Chelating of Titanium by Lactic Acid in the Water-Soluble Diammonium Tris(2-Hydroxypropionato)Titanate(IV). Inorg. Chem. 2004, 43, 4546–4548. [Google Scholar] [CrossRef]
- Hati, S.; Batchelor, R.J.; Einstein, F.W.B.; Tracey, A.S. Vanadium(V) Complexes of α-Hydroxycarboxylic Acids in Aqueous Solution. Inorg. Chem. 2001, 40, 6258–6265. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S.M.; Lah, M.S.; Pecoraro, V.L. Manganese Complexes of α-Hydroxy Acids. Inorg. Chem. 1991, 30, 8–15. [Google Scholar] [CrossRef]
- Petrykin, V.; Kakihana, M.; Yoshioka, K.; Sasaki, S.; Ueda, Y.; Tomita, K.; Nakamura, Y.; Shiro, M.; Kudo, A. Synthesis and Structure of New Water-Soluble and Stable Tantalum Compound: Ammonium Tetralactatodiperoxo-μ-Oxo-Ditantalate(V). Inorg. Chem. 2006, 45, 9251–9256. [Google Scholar] [CrossRef] [PubMed]
- Carballo, R.; Covelo, B.; Fernández-Hermida, N.; Lago, A.B.; Vázquez-López, E.M. Synthesis of Mixed-Ligand Complexes of Copper(II) with Lactate and 2,2′-Dipyridylamine: Study of the Effect of Weak Interactions on Their Crystal Packing. Z. Anorg. Allg. Chem. 2007, 633, 1791–1795. [Google Scholar] [CrossRef]
- Balboa, S.; Borrás, J.; Brandi, P.; Carballo, R.; Castiñeiras, A.; Lago, A.B.; Niclós-Gutiérrez, J.; Real, J.A. Three-Dimensional Mixed-Ligand Coordination Polymers with Ferromagnetically Coupled Cyclic Tetranuclear Copper(II) Units Bonded by Weak Interactions. Cryst. Growth Des. 2011, 11, 4344–4352. [Google Scholar] [CrossRef]
- Fursova, E.; Romanenko, G.; Ovcharenko, V. Unpredictable Polynuclear Ni(II) Lactate Formation. Polyhedron 2013, 62, 274–277. [Google Scholar] [CrossRef]
- Carballo, R.; Covelo, B.; Fernández-Hermida, N.; García-Martínez, E.; Lago, A.B.; Vázquez-López, E.M. Solid State Coordination Chemistry of Copper(II) Lactate Complexes with the Twisted Ligands 4,4′-Dipyridyldisulfide and Bis(4-Pyridylthio)Methane. J. Mol. Struct. 2008, 892, 427–432. [Google Scholar] [CrossRef]
- Qu, Z.-R.; Ye, Q.; Zhao, H.; Fu, D.-W.; Ye, H.-Y.; Xiong, R.-G.; Akutagawa, T.; Nakamura, T. Homochiral Laminar Europium Metal–Organic Framework with Unprecedented Giant Dielectric Anisotropy. Chem.-A Eur. J. 2008, 14, 3452–3456. [Google Scholar] [CrossRef]
- Ye, Q.; Fu, D.-W.; Tian, H.; Xiong, R.-G.; Chan, P.W.H.; Huang, S.D. Multiferroic Homochiral Metal−Organic Framework. Inorg. Chem. 2008, 47, 772–774. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.; Baranchikov, A.E.; Churakov, A.V.; Kopitsa, G.P.; Silvestrova, A.A.; Golikova, M.V.; Ivanova, O.S.; Gorshkova, Y.E.; Ivanov, V.K. The First Amorphous and Crystalline Yttrium Lactate: Synthesis and Structural Features. RSC Adv. 2021, 11, 30195–30205. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chen, L.-Q.; Lin, L.-R.; Long, L.-S.; Zheng, L.-S. Doped Luminescent Lanthanide Coordination Polymers Exhibiting Both White-Light Emission and Thermal Sensitivity. Inorg. Chem. 2021, 60, 6986–6990. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zheng, T.-F.; Chen, J.-L.; Wen, H.-R.; Liu, S.-J.; Hu, T.-L. A pH-Stable TbIII -Based Metal–Organic Framework as a Turn-On and Blue-Shift Fluorescence Sensor toward Benzaldehyde and Salicylaldehyde in Aqueous Solution. Inorg. Chem. 2022, 61, 16177–16184. [Google Scholar] [CrossRef] [PubMed]
- Dickins, R.S.; Love, C.S.; Puschmann, H. Bidentate Lactate Binding in Aqueous Solution in a Cationic, Heptadentate Lanthanide Complex: An Effective Chiral Derivatising Agent. Chem. Commun. 2001, 1, 2308–2309. [Google Scholar] [CrossRef]
- Martynova, I.A.; Tsymbarenko, D.M.; Kuz’mina, N.P. Yttrium Tris-Propionate Monohydrate: Synthesis, Crystal Structure, and Thermal Stability. Russ. J. Coord. Chem. Khimiya 2014, 40, 565–570. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, Z. Rare Earth Complexes with Carboxylic Acids. In Rare Earth Coordination Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 91–136. ISBN 9780470824856. [Google Scholar]
- Scales, N.; Zhang, Y.; Bhadbhade, M.; Karatchevtseva, I.; Kong, L.; Lumpkin, G.R.; Li, F. Neodymium Coordination Polymers with Propionate, Succinate and Mixed Succinate–Oxalate Ligands: Synthesis, Structures and Spectroscopic Characterization. Polyhedron 2015, 102, 130–136. [Google Scholar] [CrossRef]
- Grivel, J.-C.; Zhao, Y.; Tang, X.; Pallewatta, P.G.P.A.; Watenphul, A.; Zimmermann, M.V. Thermal Decomposition of Yttrium(III) Valerate in Argon. J. Anal. Appl. Pyrolysis 2014, 106, 125–131. [Google Scholar] [CrossRef]
- Bußkamp, H.; Deacon, G.B.; Hilder, M.; Junk, P.C.; Kynast, U.H.; Lee, W.W.; Turner, D.R. Structural Variations in Rare Earth Benzoate Complexes: Part I. Lanthanum. CrystEngComm 2007, 9, 394–411. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Zobel, M.; Polentarutti, M.; Ungur, L.; Kendin, M.; Zakharov, K.; Degtyarenko, P.; Vasiliev, A.; Tsymbarenko, D. A Family of Lanthanide Hydroxo Carboxylates with 1D Polymeric Topology and Ln4 Butterfly Core Exhibits Switchable Supramolecular Arrangement. Inorg. Chem. 2021, 60, 8049–8061. [Google Scholar] [CrossRef]
- Li, Y.; Yan, P.; Hou, G.; Li, H.; Chen, P.; Li, G. Luminescence of Unique 1D Tube-like Lactate Lanthanide Coordination Polymers. J. Organomet. Chem. 2013, 723, 176–180. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem.-A Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef]
- Gomez Torres, S.; Pantenburg, I.; Meyer, G. Direct Oxidation of Europium Metal with Acetic Acid: Anhydrous Europium(III) Acetate, Eu(OAc)3, Its Sesqui-Hydrate, Eu(OAc)3(H2O)1.5, and the “Hydrogendiacetate”, [Eu(H(OAc)2)3](H2O). Z. Anorg. Allg. Chem. 2006, 632, 1989–1994. [Google Scholar] [CrossRef]
- Kepert, C.J.; Wei-Min, L.; Junk, P.C.; Skelton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. X (‘Maximally’) Hydrated Rare Earth Acetates. Aust. J. Chem. 1999, 52, 437. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, Y.; Zhen, S.; Jiang, G.; Chen, Y.; Wu, W.; Zhang, Z.; Hong, Z.; Jin, Z. Influence of Seed Layer Materials on the Texture of IBAD-MgO Layer. IEEE Trans. Appl. Supercond. 2018, 28, 1–5. [Google Scholar] [CrossRef]
- Lee, S.; Petrykin, V.; Molodyk, A.; Samoilenkov, S.; Kaul, A.; Vavilov, A.; Vysotsky, V.; Fetisov, S. Development and Production of Second Generation High Tc Superconducting Tapes at SuperOx and First Tests of Model Cables. Supercond. Sci. Technol. 2014, 27, 044022. [Google Scholar] [CrossRef]
- Martynova, I.A.; Tsymbarenko, D.M.; Kamenev, A.A.; Mudretsova, S.N.; Streletsky, A.N.; Vasiliev, A.L.; Kuzmina, N.P.; Kaul, A.R. Chemical Deposition of Smooth Nanocrystalline Y2O3 Films from Solutions of Metal-Organic Precursors. Russ. Chem. Bull. 2013, 62, 1454–1458. [Google Scholar] [CrossRef]
- Tsymbarenko, D.; Grebenyuk, D.; Burlakova, M.; Zobel, M. Quick and Robust PDF Data Acquisition Using a Laboratory Single-Crystal X-Ray Diffractometer for Study of Polynuclear Lanthanide Complexes in Solid Form and in Solution. J. Appl. Crystallogr. 2022, 55, 890–900. [Google Scholar] [CrossRef]
- Martynova, I.; Tsymbarenko, D.; Kamenev, A.; Kuzmina, N.; Kaul, A. Synthesis and Characterization of Amorphous Yttrium Oxide Layers by Metal Organic Chemical Solution Deposition. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 447–451. [Google Scholar] [CrossRef]
- Tsymbarenko, D.M.; Martynova, I.A.; Ryzhkov, N.V.; Kuz’mina, N.P. Aluminum Hydroxocarboxylates in Solution Deposition of Planarization Alumina Films. Russ. J. Gen. Chem. 2017, 87, 1209–1216. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, Version 5.10, Structure Determination Software Suite; Bruker AXS: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPRO Softwave System, Version 1.171.40.67a; Rigaku Oxford Diffraction, Rigaku Corporation: Wroclaw, Poland, 2019.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- SCALE3 ABSPACK; Version 1.0.4, GUI 1.03; An Oxford Diffraction Program; Oxford Diffraction Ltd.: Abingdon, UK, 2005.
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Z. Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashigullin, R.; Kendin, M.; Martynova, I.; Tsymbarenko, D. Diverse Coordination Chemistry of the Whole Series Rare-Earth L-Lactates: Synthetic Features, Crystal Structure, and Application in Chemical Solution Deposition of Ln2O3 Thin Films. Molecules 2023, 28, 5896. https://doi.org/10.3390/molecules28155896
Gashigullin R, Kendin M, Martynova I, Tsymbarenko D. Diverse Coordination Chemistry of the Whole Series Rare-Earth L-Lactates: Synthetic Features, Crystal Structure, and Application in Chemical Solution Deposition of Ln2O3 Thin Films. Molecules. 2023; 28(15):5896. https://doi.org/10.3390/molecules28155896
Chicago/Turabian StyleGashigullin, Ruslan, Mikhail Kendin, Irina Martynova, and Dmitry Tsymbarenko. 2023. "Diverse Coordination Chemistry of the Whole Series Rare-Earth L-Lactates: Synthetic Features, Crystal Structure, and Application in Chemical Solution Deposition of Ln2O3 Thin Films" Molecules 28, no. 15: 5896. https://doi.org/10.3390/molecules28155896
APA StyleGashigullin, R., Kendin, M., Martynova, I., & Tsymbarenko, D. (2023). Diverse Coordination Chemistry of the Whole Series Rare-Earth L-Lactates: Synthetic Features, Crystal Structure, and Application in Chemical Solution Deposition of Ln2O3 Thin Films. Molecules, 28(15), 5896. https://doi.org/10.3390/molecules28155896





