Influence of Ligand Environment Stoichiometry on NIR-Luminescence Efficiency of Sm3+, Pr3+ and Nd3+ Ions Coordination Compounds
Abstract
1. Introduction
2. Results
2.1. Crystal Structure
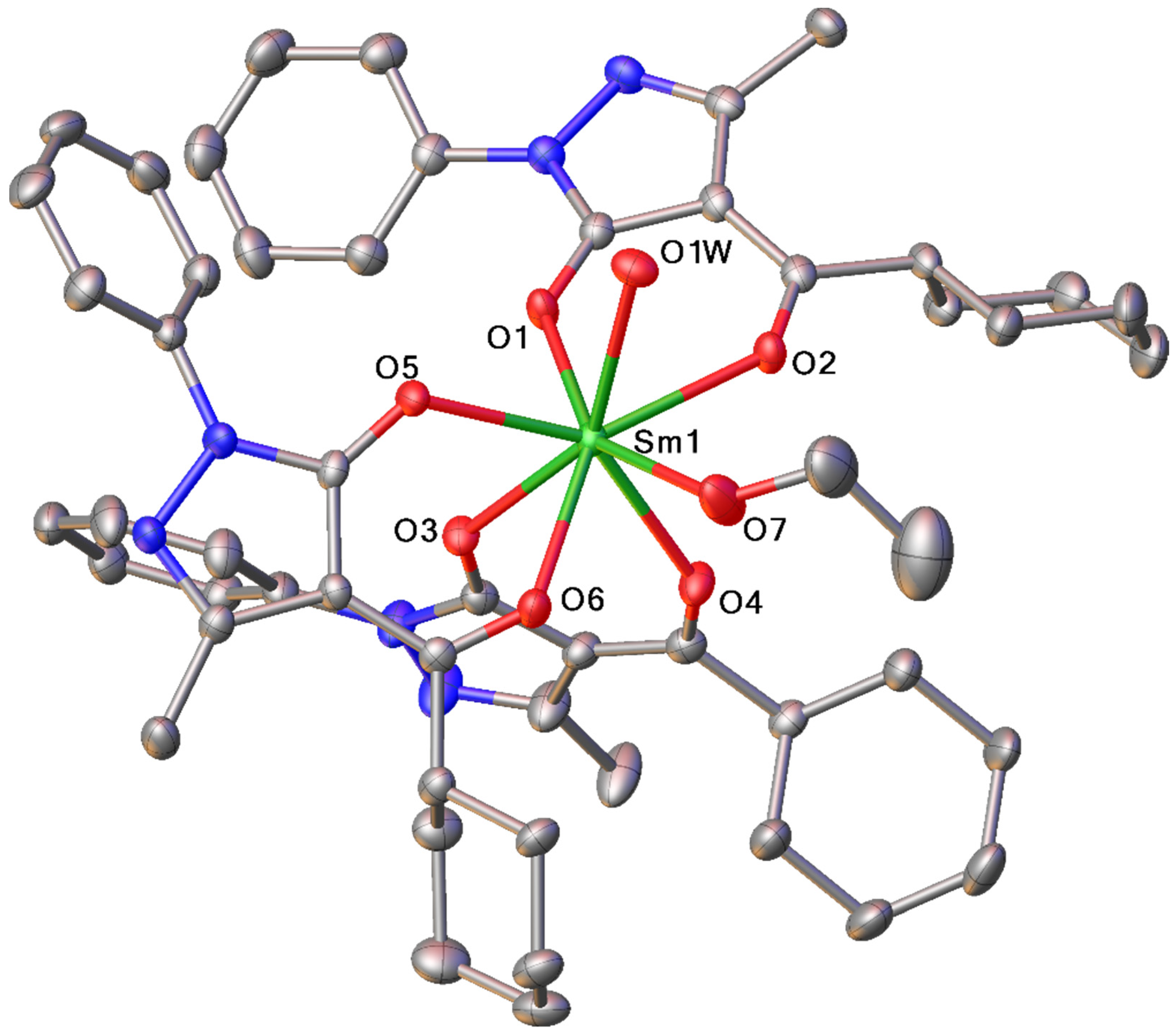
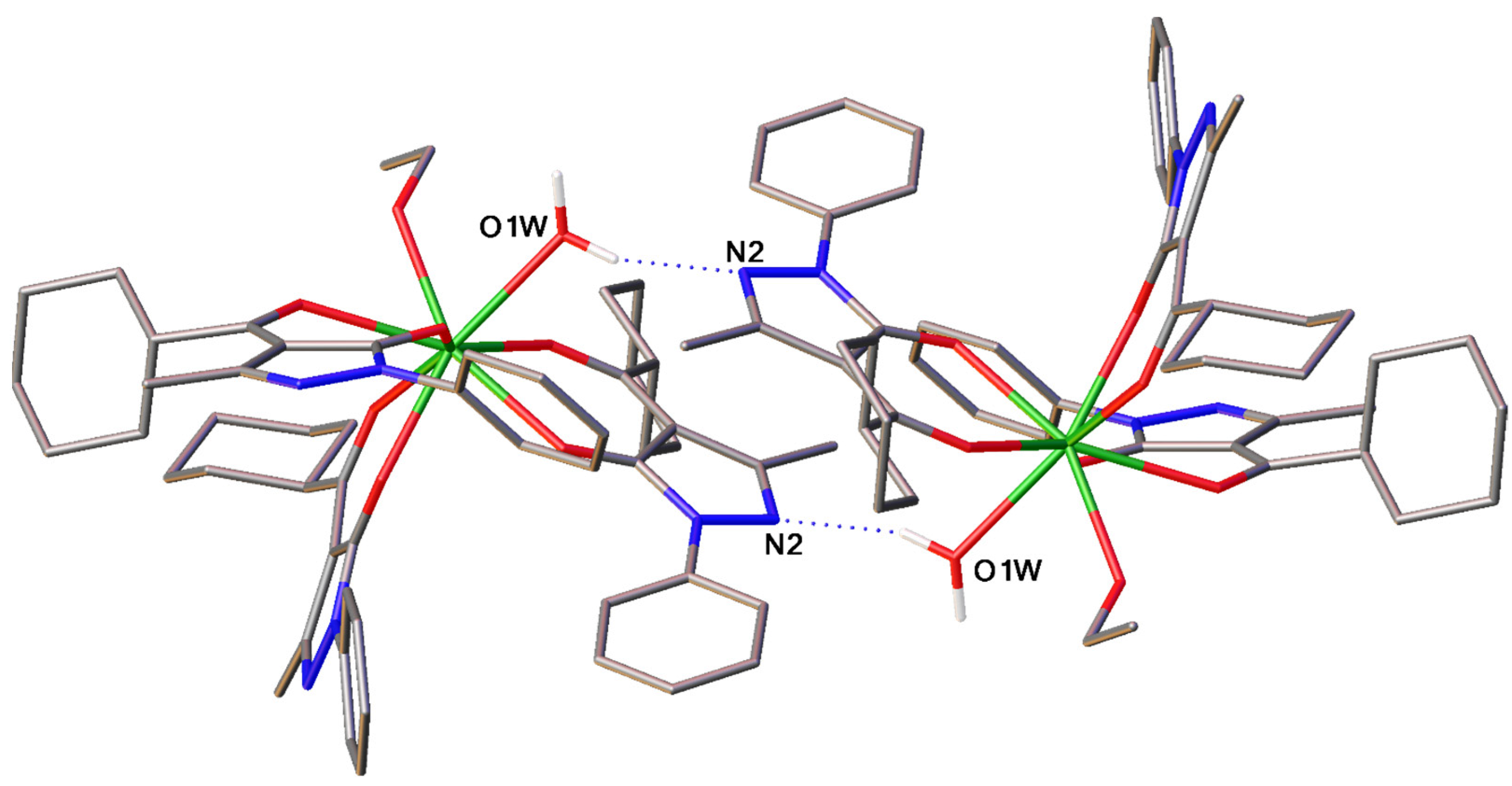
2.1.1. Tris-Complexes
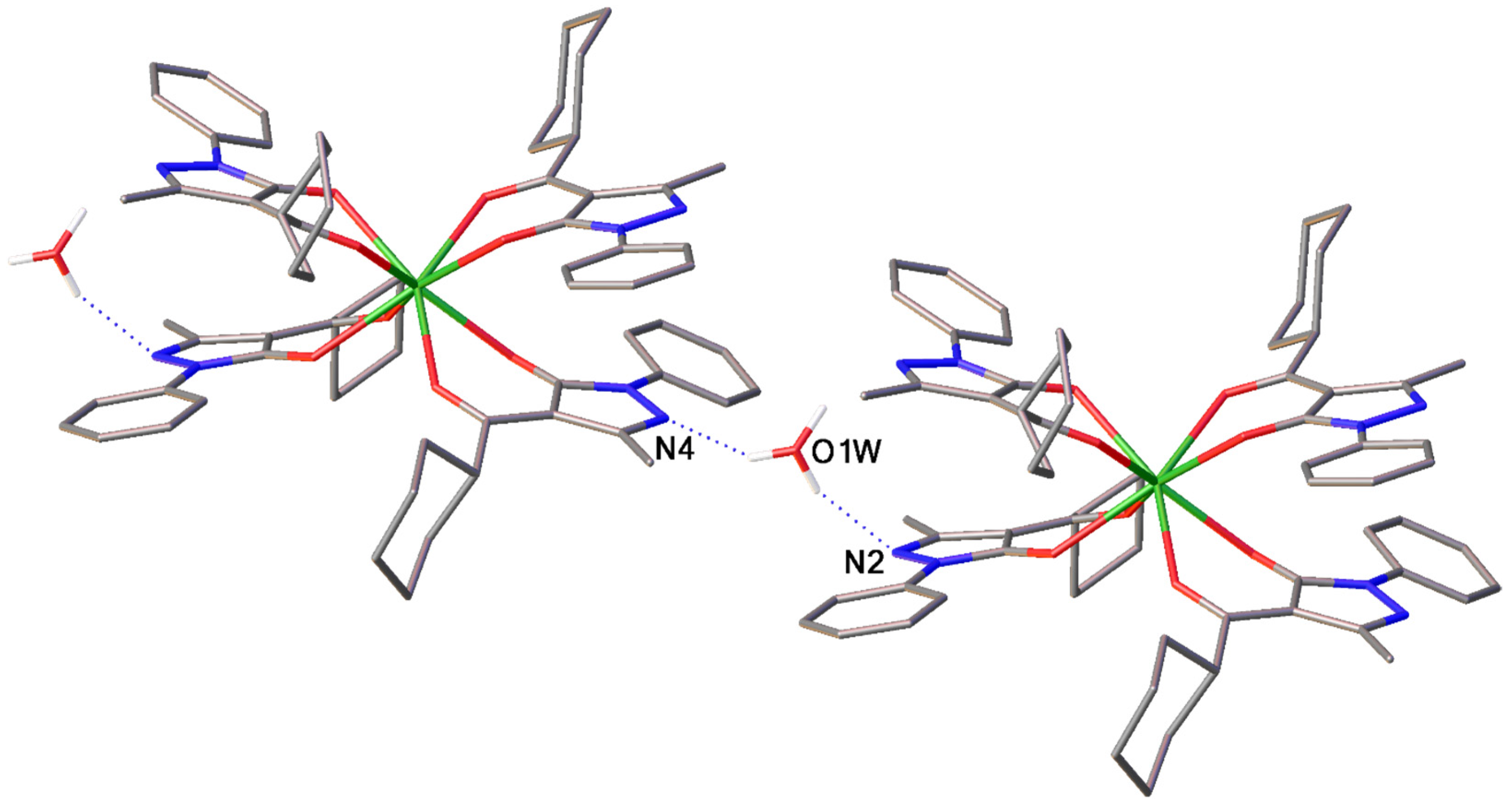
2.1.2. Tetrakis-Complexes
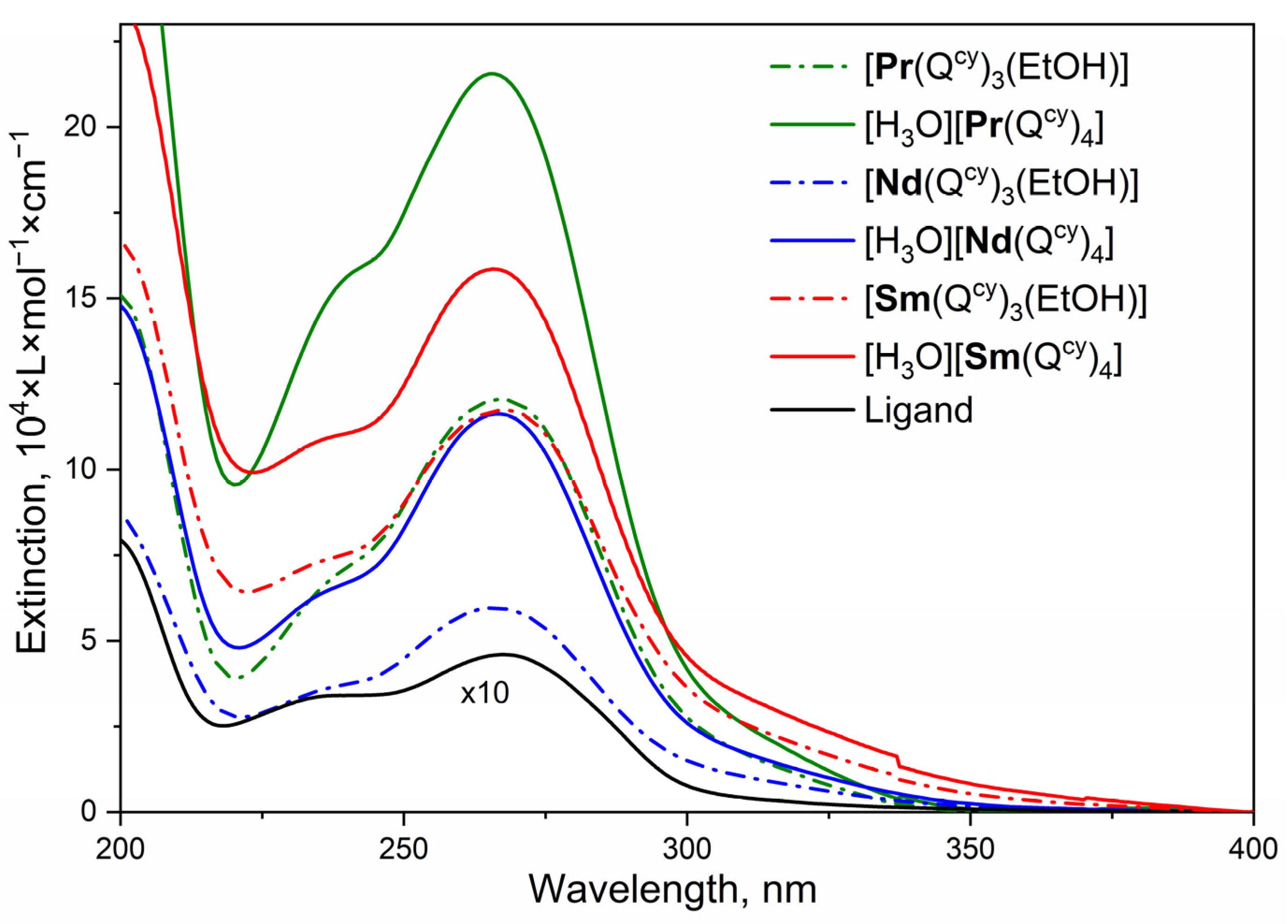
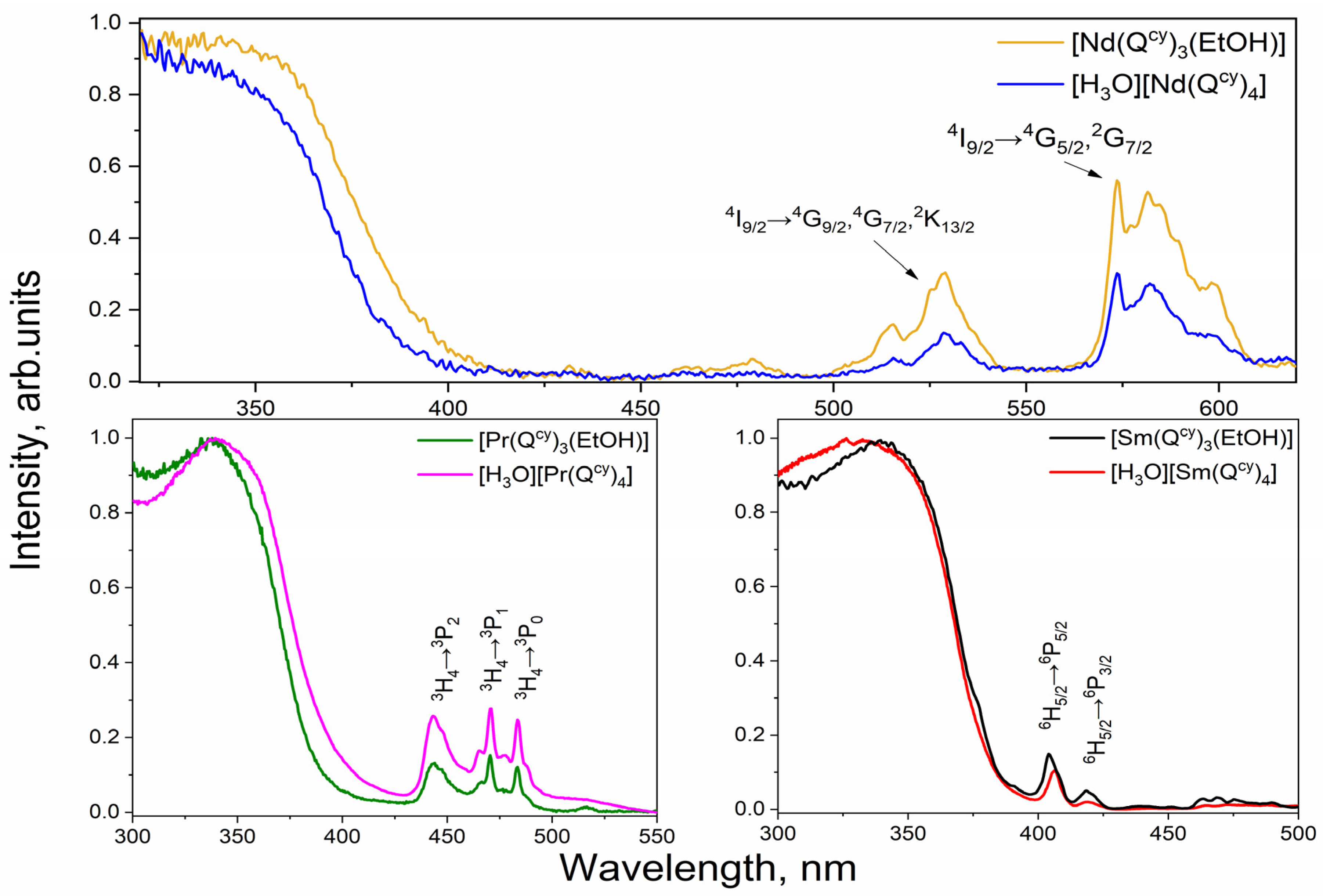
2.2. Optical Absorption
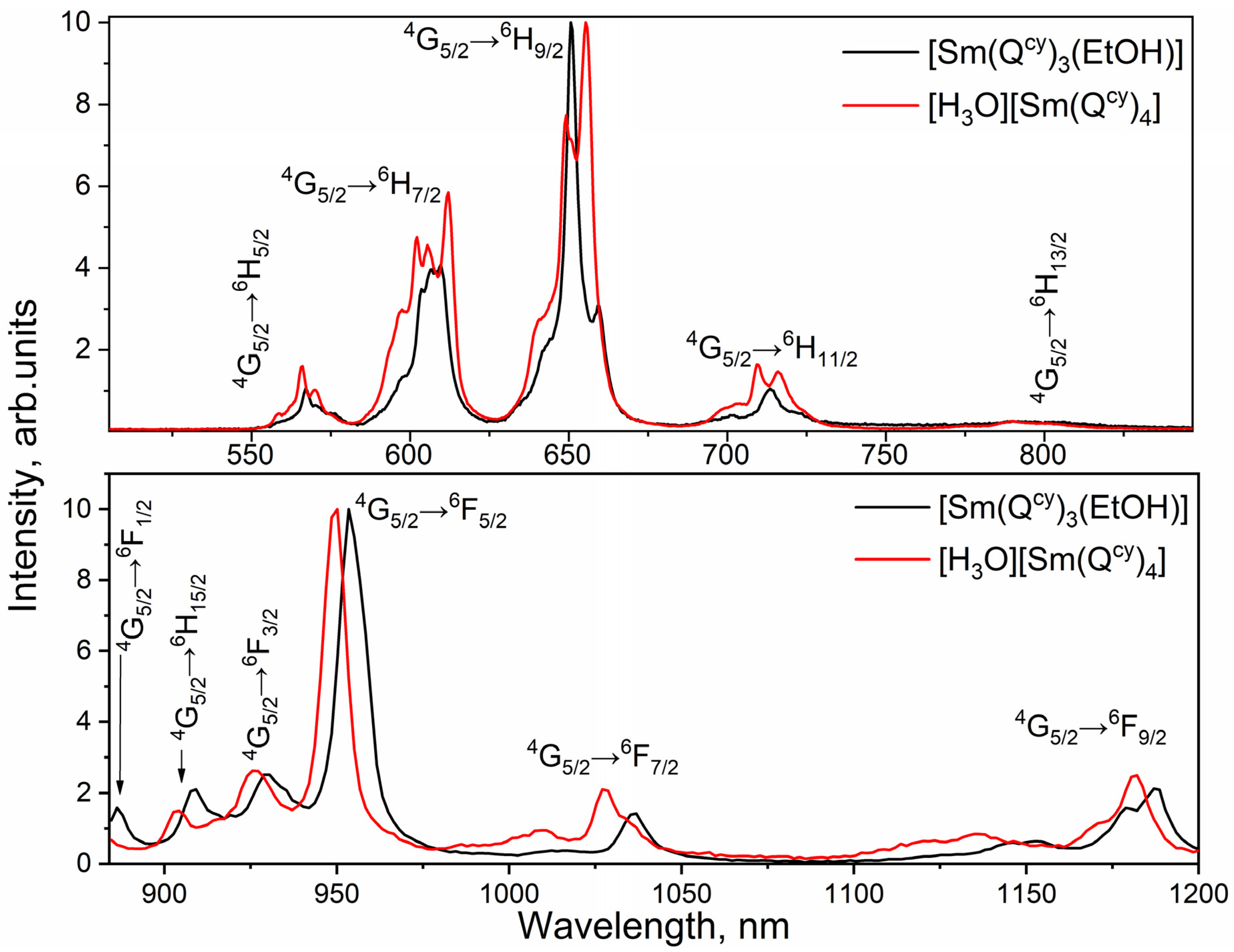
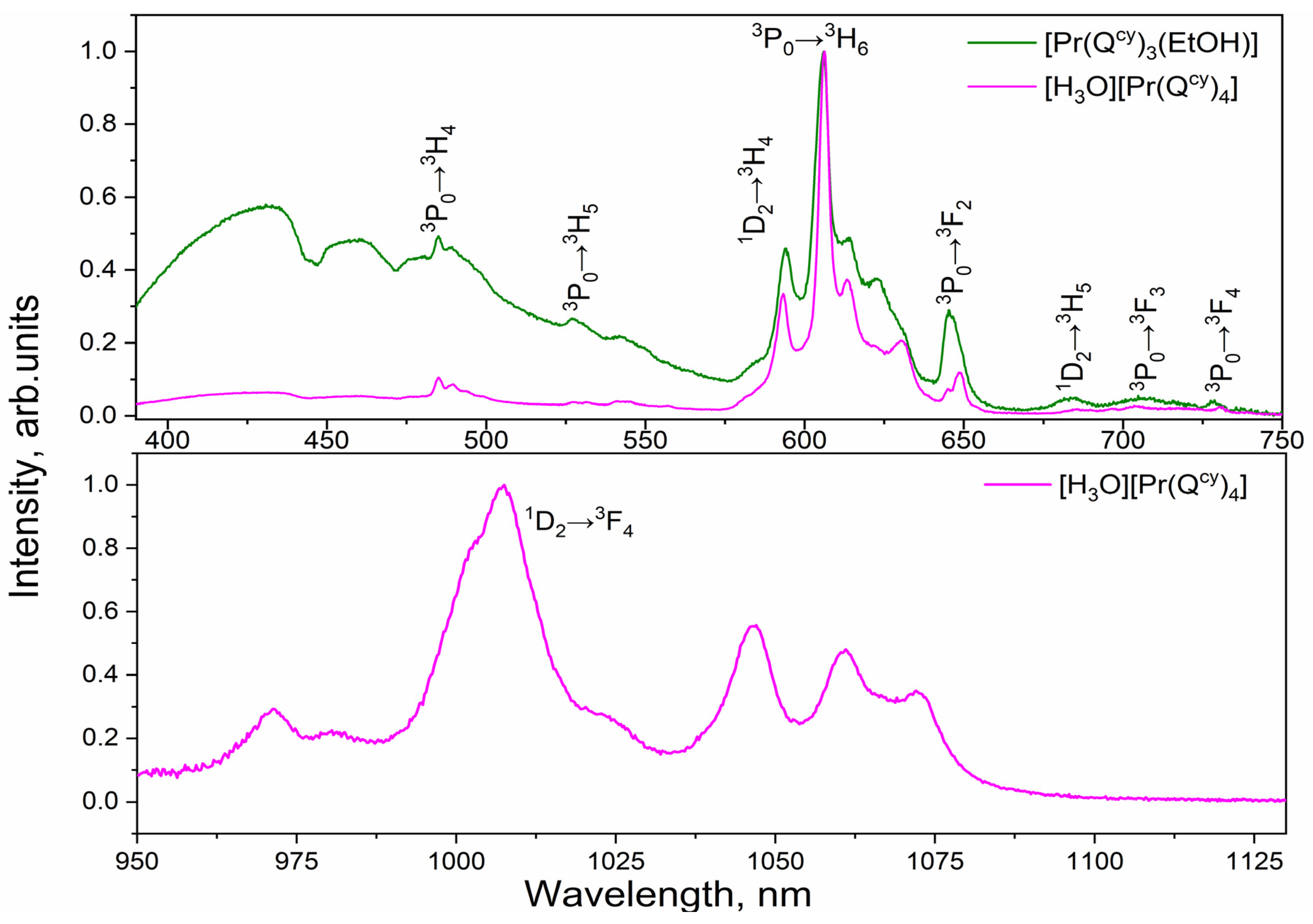
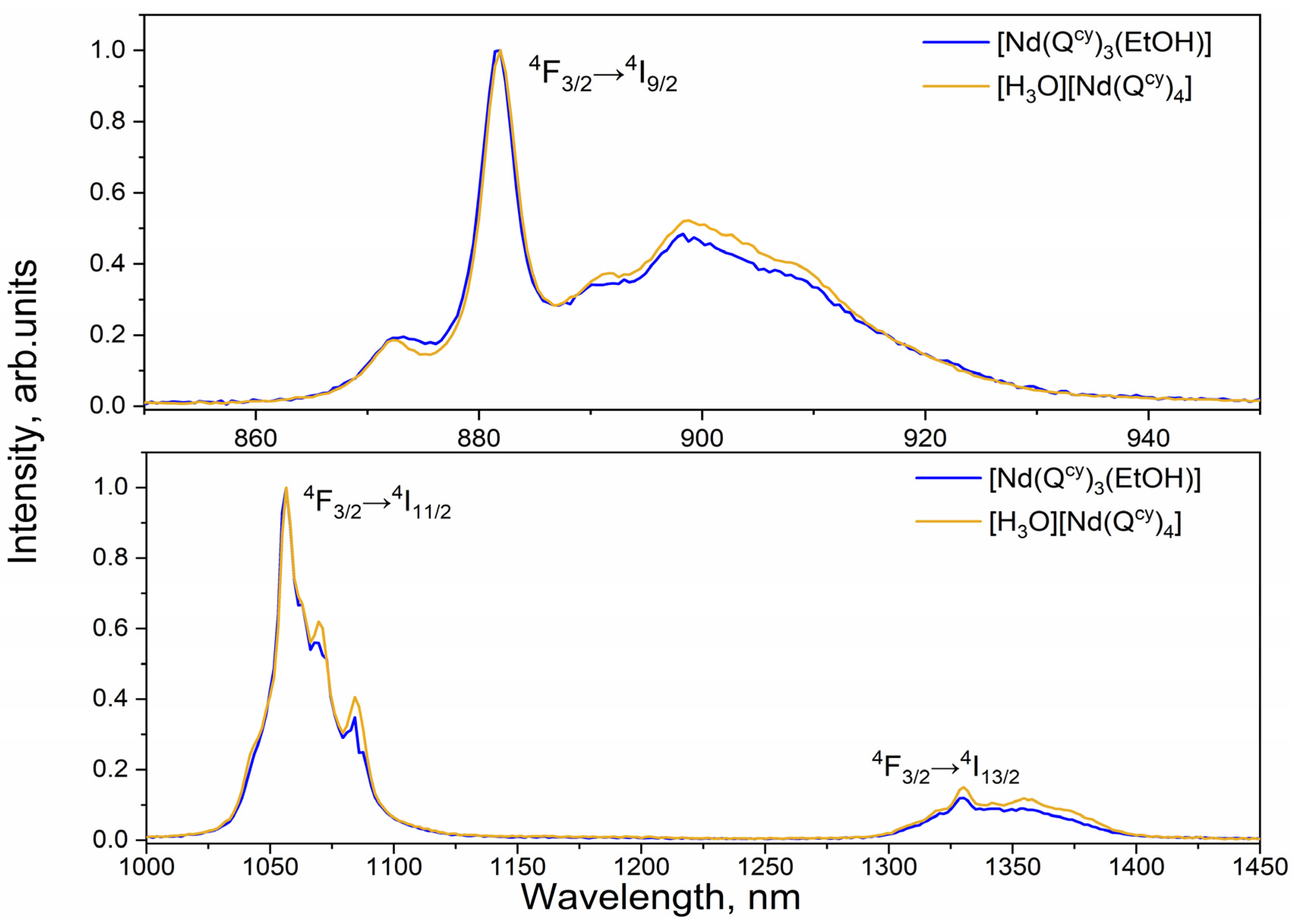
2.3. Photoluminescence
2.4. Photoluminescence Excitation
2.5. Judd–Ofelt Analysis
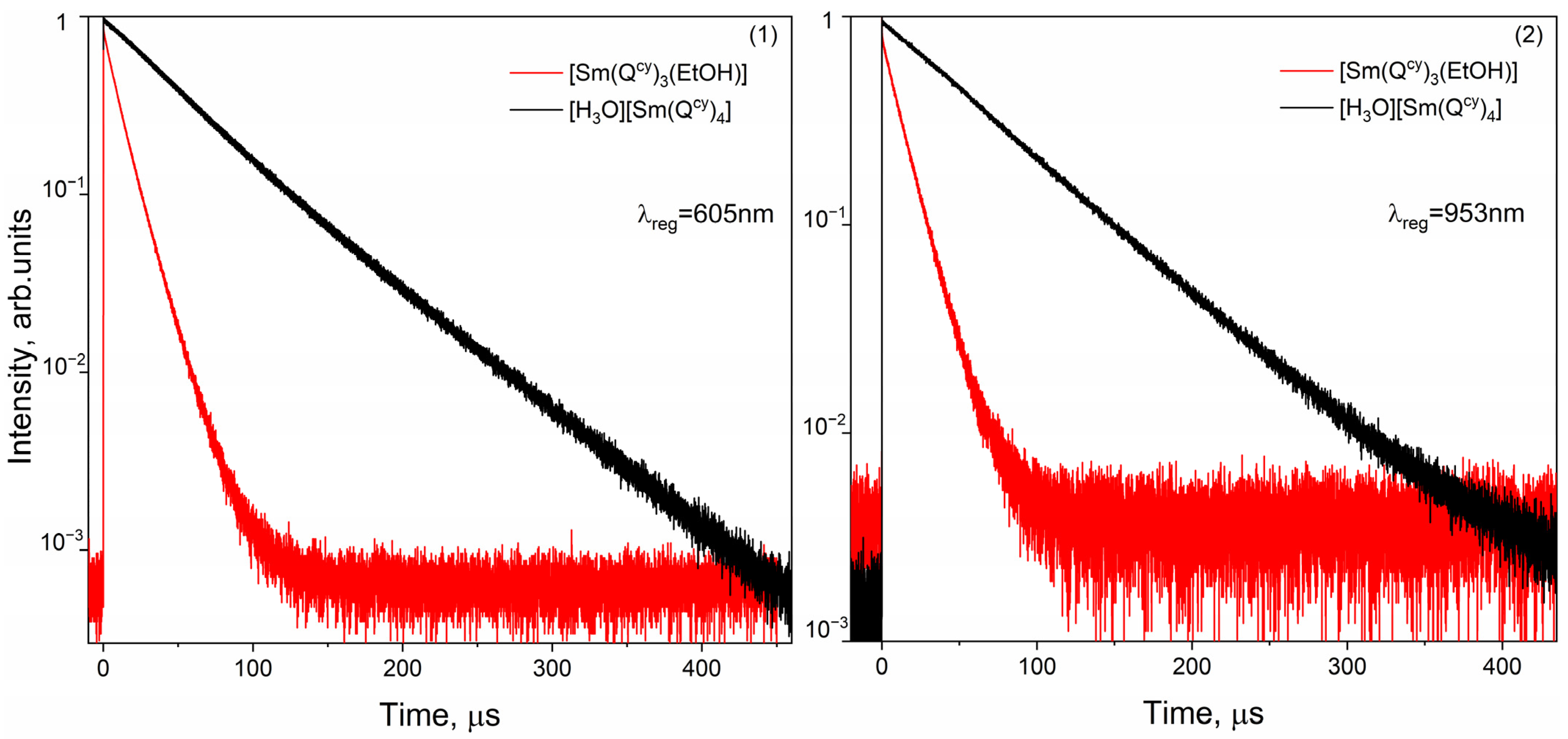
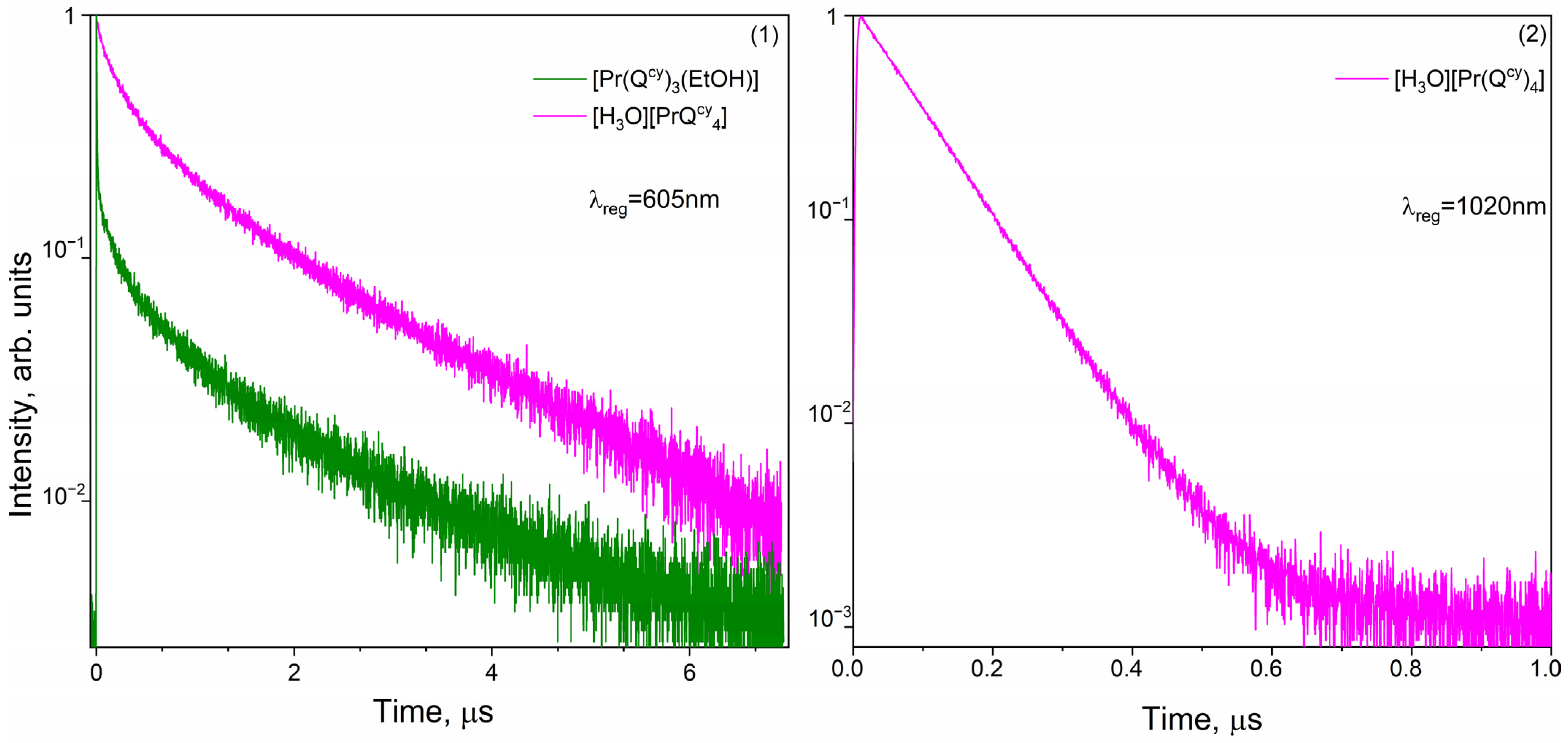
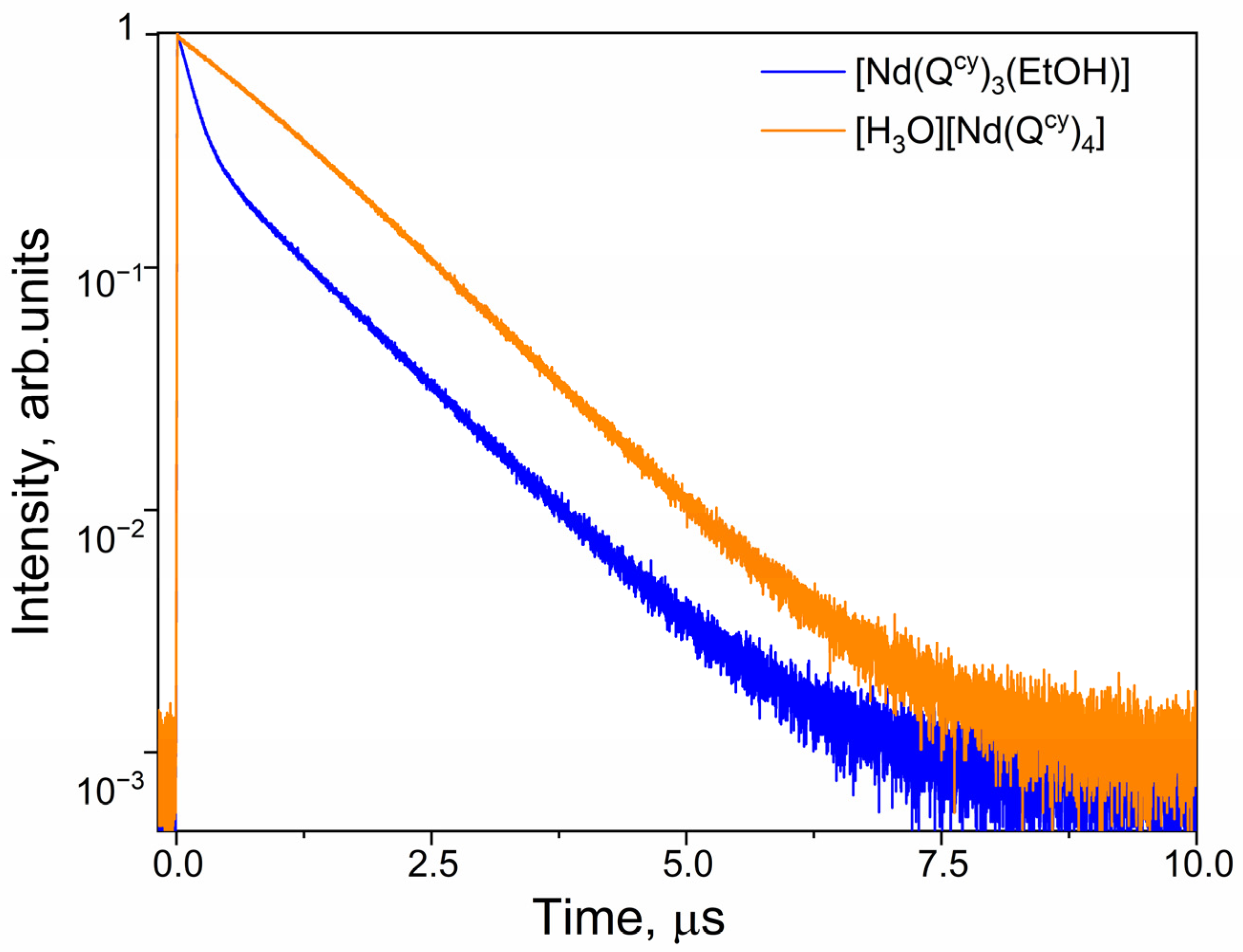
2.6. Luminescent Decays and Quantum Yields
3. Materials and Methods
3.1. Experimental Setups
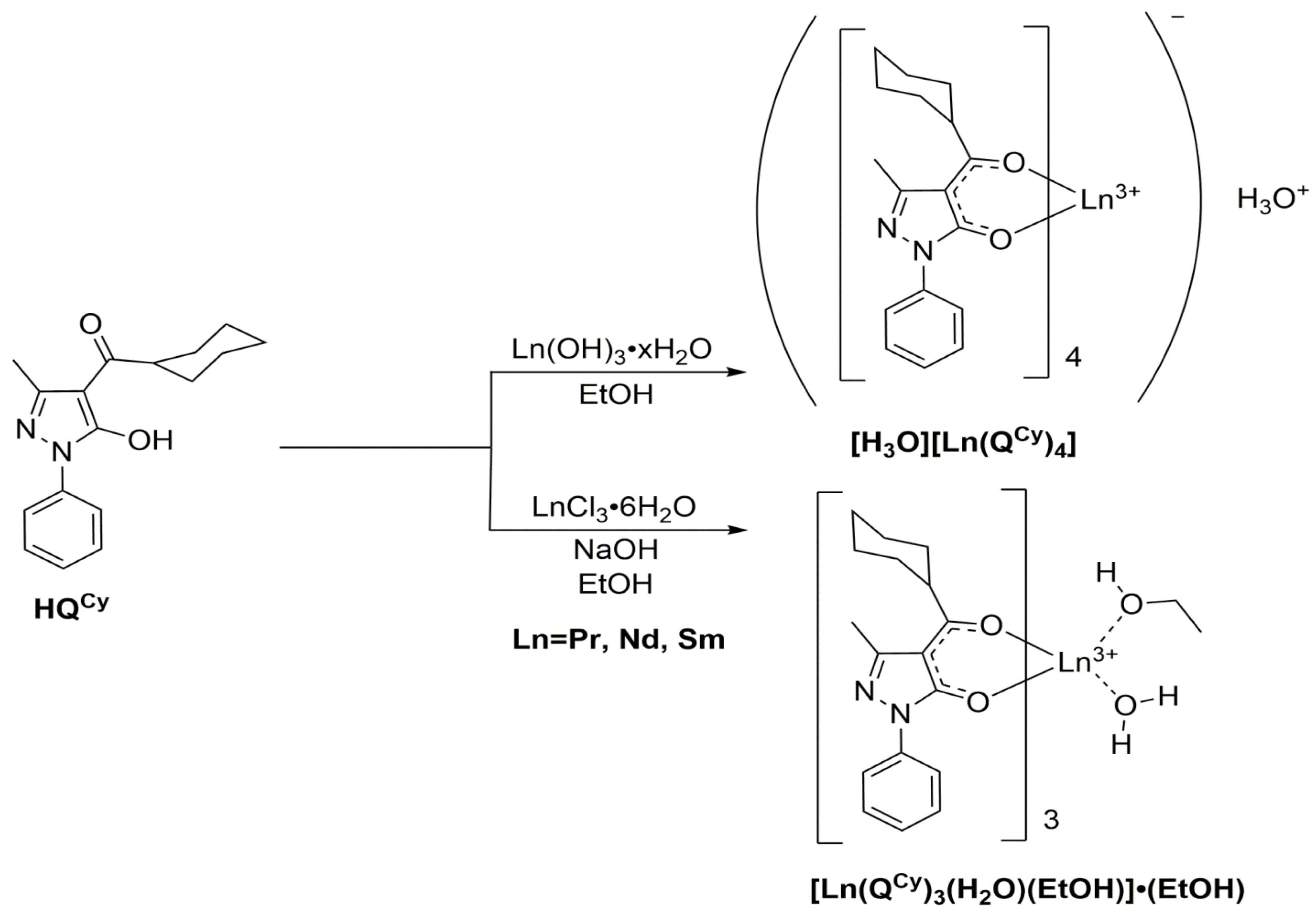
3.2. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Metlin, M.T.; Goryachii, D.O.; Aminev, D.F.; Datskevich, N.P.; Korshunov, V.M.; Metlina, D.A.; Pavlov, A.A.; Mikhalchenko, L.V.; Kiskin, M.A.; Garaeva, V.V.; et al. Bright Yb3+ Complexes for Efficient Pure Near-Infrared OLEDs. Dye. Pigment. 2021, 195, 109701. [Google Scholar] [CrossRef]
- Sizov, V.S.; Komissar, D.A.; Metlina, D.A.; Aminev, D.F.; Ambrozevich, S.A.; Nefedov, S.E.; Varaksina, E.A.; Metlin, M.T.; Mislavskií, V.V.; Taydakov, I.V. Effect of Ancillary Ligands on Visible and NIR Luminescence of Sm3+ β-Diketonate Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117503. [Google Scholar] [CrossRef] [PubMed]
- Belousov, Y.A.; Korshunov, V.M.; Metlin, M.T.; Metlina, D.A.; Kiskin, M.A.; Aminev, D.F.; Datskevich, N.P.; Drozdov, A.A.; Pettinari, C.; Marchetti, F.; et al. Towards Bright Dysprosium Emitters: Single and Combined Effects of Environmental Symmetry, Deuteration, and Gadolinium Dilution. Dye. Pigment. 2022, 199, 110078. [Google Scholar] [CrossRef]
- Metlin, M.T.; Ambrozevich, S.A.; Metlina, D.A.; Vitukhnovsky, A.G.; Taydakov, I.V. Luminescence of Pyrazolic 1,3-Diketone Pr3+ Complex with 1,10-Phenanthroline. J. Lumin. 2017, 188, 365–370. [Google Scholar] [CrossRef]
- Lustig, W.P.; Li, J. Luminescent Metal–Organic Frameworks and Coordination Polymers as Alternative Phosphors for Energy Efficient Lighting Devices. Coord. Chem. Rev. 2018, 373, 116–147. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) Hybrid Inorganic–Organic Materials with Intriguing Stimuli Responsive and Optoelectronic Properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Q.; An, Z.; Wei, Y.; Liu, X. Electroluminescence from Europium(III) Complexes. Coord. Chem. Rev. 2015, 293–294, 228–249. [Google Scholar] [CrossRef]
- Dalal, A.; Nehra, K.; Hooda, A.; Singh, D.; Kumar, P.; Kumar, S.; Malik, R.S.; Rathi, B. Luminous Lanthanide Diketonates: Review on Synthesis and Optoelectronic Characterizations. Inorg. Chim. Acta 2023, 550, 121406. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide NIR Luminescence for Telecommunications, Bioanalyses and Solar Energy Conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Mason, S.F. Mechanisms for F-f Transition Probabilities in Lanthanide Coordination Compounds. Inorg. Chim. Acta 1984, 94, 88. [Google Scholar] [CrossRef]
- Weissman, S.I. Intramolecular Energy Transfer The Fluorescence of Complexes of Europium. J. Chem. Phys. 1942, 10, 214–217. [Google Scholar] [CrossRef]
- Kettle, S.F.A. Physical Inorganic Chemistry; Springer Berlin Heidelberg: Berlin, Heidelberg, 1996; ISBN 978-0-7167-4514-3. [Google Scholar]
- Girotto, E.; Pereira, A.; Arantes, C.; Cremona, M.; Bortoluzzi, A.J.; Salla, C.A.M.; Bechtold, I.H.; Gallardo, H. Efficient Terbium Complex Based on a Novel Pyrazolone Derivative Ligand Used in Solution-Processed OLEDs. J. Lumin. 2019, 208, 57–62. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, F.; Hao, F.; Bian, Z.; Ding, B.; Zhu, Y.; Chen, F.; Huang, C. A Highly Efficient OLED Based on Terbium Complexes. Org. Electron. 2009, 10, 939–947. [Google Scholar] [CrossRef]
- Thorne, J.R.G.; Rey, J.M.; Denning, R.G.; Watkins, S.E.; Etchells, M.; Green, M.; Christou, V. Excited State Dynamics of Organo-Lanthanide Electroluminescent Phosphors: The Properties of Tb(Tb-Pmp) 3 and Gd(Tb-Pmp) 3. J. Phys. Chem. A 2002, 106, 4014–4021. [Google Scholar] [CrossRef]
- Li, Z.-F.; Zhou, L.; Yu, J.-B.; Zhang, H.-J.; Deng, R.-P.; Peng, Z.-P.; Guo, Z.-Y. Synthesis, Structure, Photoluminescence, and Electroluminescence Properties of a New Dysprosium Complex. J. Phys. Chem. C 2007, 111, 2295–2300. [Google Scholar] [CrossRef]
- Li, X.-L.; Li, J.; Zhu, C.; Han, B.; Liu, Y.; Yin, Z.; Li, F.; Liu, C.-M. An Intense Luminescent Dy(iii) Single-Ion Magnet with the Acylpyrazolonate Ligand Showing Two Slow Magnetic Relaxation Processes. New J. Chem. 2018, 42, 16992–16998. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Metlin, M.T.; Metlina, D.A.; Kiskin, M.A.; Yakushev, I.A.; Polikovskiy, T.A.; Taydakov, I.V.; Drozdov, A.A.; Marchetti, F.; Pettinari, C. Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence. Polymers 2023, 15, 867. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Belousov, Y.A.; Lyssenko, K.A.; Varaksina, E.; Drozdov, A.A.; Marchetti, F.; Pettinari, R.; Pettinari, C. Synthesis, Phosphorescence and Luminescence Properties of Novel Europium and Gadolinium Tris-Acylpyrazolonate Complexes. Inorg. Chim. Acta 2020, 502, 119279. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Tsorieva, A.V.; Gontcharenko, V.E.; Zanizdra, S.R.; Metlin, M.T.; Polikovskiy, T.A.; Taydakov, I.V. Photophysical Properties of Eu3+ β-Diketonates with Extended π-Conjugation in the Aromatic Moiety. Inorganics 2022, 11, 15. [Google Scholar] [CrossRef]
- Varaksina, E.A.; Kiskin, M.A.; Lyssenko, K.A.; Puntus, L.N.; Korshunov, V.M.; Silva, G.S.; Freire, R.O.; Taydakov, I.V. Tuning the Luminescence Efficiency by Perfluorination of Side Chains in Eu3+ Complexes with β-Diketones of the Thiophene Series. Phys. Chem. Chem. Phys. 2021, 23, 25748–25760. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Liu, L.; Liu, G.; Jia, D.; Xu, G. A Series of Pyrazolone Lanthanide (III) Complexes: Synthesis, Crystal Structures and Fluorescence. Inorg. Chim. Acta 2007, 360, 1995–2001. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Drozdov, A.; Troyanov, S.; Voloshin, A.I.; Shavaleev, N.M. Synthesis, Structure and Luminescence Properties of New Rare Earth Metal Complexes with 1-Phenyl-3-Methyl-4-Acylpyrazol-5-Ones. J. Chem. Soc. Dalton Trans. 2002, 1409. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Metlin, M.T.; Ambrozevich, S.A.; Golovanov, I.S.; Gontcharenko, V.E.; Selyukov, A.S.; Taydakov, I.V. Impact of Ligand-Centered Excited States on Luminescence Sensitization in Pr 3 + Complexes with β -Diketones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119863. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Q.; He, Y.; Fu, G.; Li, W.; Miao, T.; Lü, X. Single-Molecule White-Light of Tris-Pyrazolonate-Dy3+ Complexes. Inorg. Chem. Commun. 2019, 109, 107573. [Google Scholar] [CrossRef]
- Xin, H.; Shi, M.; Zhang, X.M.; Li, F.Y.; Bian, Z.Q.; Ibrahim, K.; Liu, F.Q.; Huang, C.H. Carrier-Transport, Photoluminescence, and Electroluminescence Properties Comparison of a Series of Terbium Complexes with Different Structures. Chem. Mater. 2003, 15, 3728–3733. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, M.; Liu, Z.; Li, F.; Yi, T.; Huang, C. Luminescence Modulation of a Terbium Complex with Anions and Its Application as a Reagent. Eur. J. Inorg. Chem. 2006, 2006, 2277–2284. [Google Scholar] [CrossRef]
- Polikovskiy, T.; Korshunov, V.; Gontcharenko, V.; Kiskin, M.; Belousov, Y.; Pettinari, C.; Taydakov, I. Dynamics of the Ligand Excited States Relaxation in Novel β-Diketonates of Non-Luminescent Trivalent Metal Ions. IJMS 2023, 24, 8131. [Google Scholar] [CrossRef]
- Shen, L.; Shi, M.; Li, F.; Zhang, D.; Li, X.; Shi, E.; Yi, T.; Du, Y.; Huang, C. Polyaryl Ether Dendrimer with a 4-Phenylacetyl-5-Pyrazolone-Based Terbium(III) Complex as Core: Synthesis and Photopysical Properties. Inorg. Chem. 2006, 45, 6188–6197. [Google Scholar] [CrossRef]
- Safronova, A.V.; Bochkarev, L.N.; Baranov, E.V. Synthesis of Lanthanide Pyrazolonate Complexes by the Reactions of 1-Phenyl-3-Methyl-4-(2,2-Dimethylpropan-1-Oyl)Pyrazol-5-One with Metallic Lanthanides. Crystal Structures of [Ln(Bu t -PMP)3]2 (Ln = Gd, Tb, and Tm). Russ J Coord Chem 2013, 39, 537–543. [Google Scholar] [CrossRef]
- Safronova, A.V.; Bochkarev, L.N.; Malysheva, I.P.; Baranov, E.V. Facile Synthesis of Rare-Earth Pyrazolonates by the Reaction of Rare-Earth Metals with 1-Phenyl-3-Methyl-4-Isobutyryl-5-Pyrazolone. Crystal Structures of [Ln(PMIP)3]2 (Ln=Y, Gd, Tb, Er, Tm). Inorg. Chim. Acta 2012, 392, 454–458. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Drozdov, A.; Semenov, S.; Troyanov, S.I.; Zolin, V. A New Rare-Earth Metal Acylpyrazolonate Containing the Zundel Ion Stabilized by Strong Hydrogen Bonding. Inorg. Chem. Commun. 2006, 9, 634–637. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Natanti, P.; Drozdov, A.; Semenov, S.; Troyanov, S.I.; Zolin, V. Syntheses, Spectroscopic Characterization and X-Ray Structural Studies of Lanthanide Complexes with Adamantyl Substituted 4-Acylpyrazol-5-One. Inorg. Chim. Acta 2006, 359, 4063–4070. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Cingolani, A.; Drozdov, A.; Timokhin, I.; Troyanov, S.I.; Tsaryuk, V.; Zolin, V. Syntheses, Structural and Spectroscopic Investigation (IR, NMR and Luminescence) of New Terbium and Europium Acylpyrazolonates. Inorg. Chim. Acta 2004, 357, 4181–4190. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A. Lanthanide Acylpyrazolonates: Synthesis, Properties and Structural Features. Russ. Chem. Rev. 2012, 81, 1159–1169. [Google Scholar] [CrossRef]
- Bai, H.-Y.; Ma, J.-F.; Yang, J.; Liu, Y.-Y.; Ma, J.-C. Effect of Anions on the Self-Assembly of Cd(II)-Containing Coordination Polymers Based on a Novel Flexible Tetrakis (Imidazole) Ligand. Cryst. Growth Des. 2010, 10, 995–1016. [Google Scholar] [CrossRef]
- Mara, D.; Artizzu, F.; Laforce, B.; Vincze, L.; Van Hecke, K.; Van Deun, R.; Kaczmarek, A.M. Novel Tetrakis Lanthanide β-Diketonate Complexes: Structural Study, Luminescence Properties and Temperature Sensing. J. Lumin. 2019, 213, 343–355. [Google Scholar] [CrossRef]
- Arppe, R.; Hyppänen, I.; Perälä, N.; Peltomaa, R.; Kaiser, M.; Würth, C.; Christ, S.; Resch-Genger, U.; Schäferling, M.; Soukka, T. Quenching of the Upconversion Luminescence of NaYF 4: Yb3+, Er3+ and NaYF4: Yb3+,Tm3+ Nanophosphors by Water: The Role of the Sensitizer Yb3+ in Non-Radiative Relaxation. Nanoscale 2015, 7, 11746–11757. [Google Scholar] [CrossRef]
- Wang, Y.; Darapaneni, P.; Kizilkaya, O.; Dorman, J.A. Role of Ce in Manipulating the Photoluminescence of Tb Doped Y2Zr2O7. Inorg. Chem. 2020, 59, 2358–2366. [Google Scholar] [CrossRef]
- Wang, Y.; Darapaneni, P.; Ofoegbuna, T.; Gupta, S.K.; Kizilkaya, O.; Mao, Y.; Dorman, J.A. Effect of Oxide Ion Distribution on a Uranium Structure in Highly U-Doped RE 2 Hf 2 O 7 (RE = La and Gd) Nanoparticles. Inorg. Chem. 2020, 59, 14070–14077. [Google Scholar] [CrossRef]
- R Bajgiran, K.; Darapaneni, P.; Melvin, A.T.; Dorman, J.A. Effects of Weak Electric Field on the Photoluminescence Behavior of Bi3+ -Doped YVO 4: Eu3+ Core–Shell Nanoparticles. J. Phys. Chem. C 2019, 123, 13027–13035. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic Energy Levels in the Trivalent Lanthanide Aquo Ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthanide Luminescence; Hänninen, P., Härmä, H., Eds.; Springer Series on Fluorescence; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; Volume 7, pp. 1–45. ISBN 978-3-642-21022-8. [Google Scholar]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Metlina, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Selyukov, A.S.; Datskevich, N.P.; Aminev, D.F.; Goryachii, D.O.; Lyssenko, K.A.; Pavlov, A.A.; Dmitrienko, A.O.; et al. Bright NIR-Luminescent Nd 3 + Complexes with Pyrazole-Substituted 1,3-Diketones Demonstrated an Unusual Spectral Lines Branching Ratios. Dye. Pigment. 2020, 181, 108558. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Spectral Intensities of the Trivalent Lanthanides and Actinides in Solution. II. Pm3+, Sm3+, Eu3+, Gd3+, Tb3+, Dy3+, and Ho3+. J. Chem. Phys. 1968, 49, 4412–4423. [Google Scholar] [CrossRef]
- Kozma, I.Z.; Krok, P.; Riedle, E. Direct Measurement of the Group-Velocity Mismatch and Derivation of the Refractive-Index Dispersion for a Variety of Solvents in the Ultraviolet. J. Opt. Soc. Am. B 2005, 22, 1479. [Google Scholar] [CrossRef]
- Jørgensen, C.K.; Reisfeld, R. Judd-Ofelt Parameters and Chemical Bonding. J. Less Common Met. 1983, 93, 107–112. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Lyle, S.J.; Rahman, M.M. Complexometric Titration of Yttrium and the Lanthanons—IA Comparison of Direct Methods. Talanta 1963, 10, 1177–1182. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pizzabiocca, A.; Drozdov, A.A.; Troyanov, S.I.; Zhuravlev, C.O.; Semenov, S.N.; Belousov, Y.A.; Timokhin, I.G. Syntheses, Structures, and Spectroscopy of Mono- and Polynuclear Lanthanide Complexes Containing 4-Acyl-Pyrazolones and Diphosphineoxide. Inorg. Chim. Acta 2010, 363, 4038–4047. [Google Scholar] [CrossRef]

| [Pr(Qcy)3(H2O)(EtOH)]∙(EtOH) | [H3O][Pr(Qcy)4] | ||||
|---|---|---|---|---|---|
| 3P0 ⟶ 2S+1LJ | Wavelength, nm | Arad, s−1 | bcalc, % | Arad, s−1 | bcalc, % |
| 1D2 | 3051 | 26.4 | 0.03 | 8.5 | 0.02 |
| 3F4 | 697 | 1165.5 | 1.6 | 1996.7 | 5.4 |
| 3F2 | 635 | 56,964.5 | 81.1 | 18,349.5 | 49.7 |
| 3H6 | 608 | 6415.1 | 9.1 | 6886.3 | 18.6 |
| 3H4 | 482 | 5656.8 | 8.1 | 9690.4 | 26.2 |
| trad = 14.2 µs; | trad = 27.1 µs; | ||||
| 3P1 ⟶ 2S+1LJ | Wavelength, nm | Arad, s−1 | bcalc, % | Arad, s−1 | bcalc, % |
| 1D2 | 2223 | 359.7 | 0.2 | 115.9 | 0.1 |
| 3F4 | 688 | 2973.2 | 1.5 | 5093.23 | 4.7 |
| 3F3 | 669 | 97,011.6 | 49.2 | 34,600.8 | 31.8 |
| 3F2 | 611 | 58,151.7 | 29.5 | 18,731.9 | 17.3 |
| 3H6 | 586 | 12,305.9 | 6.2 | 13,209.9 | 12.2 |
| 3H5 | 520 | 20,134.5 | 10.2 | 26,390.4 | 24.3 |
| 3H4 | 468 | 6083.7 | 3.08 | 10,421.8 | 9.6 |
| trad = 5.1 µs; | trad = 9.2 µs; | ||||
| 1D2 ⟶ 2S+1LJ | Wavelength, nm | Arad, s−1 | bcalc, % | Arad, s−1 | bcalc, % |
| 3F4 | 995 | 30,580.5 | 74.6 | 10,155.6 | 51.9 |
| 3F3 | 956 | 1880.1 | 4.6 | 708.9 | 3.6 |
| 3F2 | 843 | 1685.3 | 4.1 | 1279.6 | 6.5 |
| 3H6 | 796 | 757.8 | 1.8 | 1131.9 | 5.8 |
| 3H5 | 678 | 47.9 | 0.1 | 65.7 | 0.3 |
| 3H4 | 592 | 6048.3 | 14.8 | 6206.9 | 31.8 |
| trad = 24.3 µs; | trad = 51.2 µs; | ||||
| [Nd(Qcy)3(H2O)(EtOH)]∙(EtOH) | [H3O][Nd(Qcy)4] | ||||
|---|---|---|---|---|---|
| 4F3/2 ⟶ 2S+1LJ | Wavelength, nm | Arad, s−1 | bcalc, % | Arad, s−1 | bcalc, % |
| 4I15/2 | 880 | 1890.2 | 48.2 | 2951.6 | 52.6 |
| 4I13/2 | 1060 | 1717.7 | 43.8 | 2056.5 | 40.8 |
| 4I11/2 | 1330 | 295.9 | 7.5 | 314.9 | 6.2 |
| 4I9/2 | 1830 | 15.2 | 0.4 | 16.2 | 0.3 |
| trad = 255.2 µs; | trad = 198.5 µs; | ||||
| [Sm(Qcy)3(H2O)(EtOH)]∙(EtOH) | [H3O][Sm(Qcy)4] * | ||||
|---|---|---|---|---|---|
| 4G5/2 ⟶ 2S+1LJ | Wavelength, nm | Arad, s−1 | bcalc, % | Arad, s−1 | bcalc, % |
| 6H5/2 | 565 | 42.4 | 2.5 | 8.6 | 2.7 |
| 6H7/2 | 610 | 201.4 | 12.1 | 46.2 | 14.9 |
| 6H9/2 | 650 | 1006.7 | 60.4 | 178.7 | 57.6 |
| 6H11/2 | 715 | 57.1 | 3.4 | 14.3 | 4.6 |
| 6H13/2 | 800 | 5.8 | 0.3 | 0.8 | 0.3 |
| 6F3/2 | 936 | 36.3 | 2.2 | 6.1 | 1.9 |
| 6F5/2 | 949 | 202.8 | 12.1 | 34.9 | 11.2 |
| 6F7/2 | 1036 | 5.5 | 0.3 | 1.5 | 0.5 |
| 6F9/2 | 1180 | 108.2 | 6.4 | 18.7 | 6.0 |
| trad = 0.6 ms; | trad = 3.2 ms; | ||||
| Complex | , nm | , ns | , ns | Φ, % |
|---|---|---|---|---|
| [Nd(Qcy)3(H2O)(EtOH)]∙(EtOH) | 1056 | 146 | 1046 | 1.3 |
| 1056 | 1183 | – | 1.3 | |
| [Pr(Qcy)3(H2O)(EtOH)]∙(EtOH) | 605 | 5 | 184 | 0.4 |
| 1020 | 5 | 58 | – | |
| 605 | 81 | 392 | 0.4 | |
| 1020 | 85 | – | – | |
| [Sm(Qcy)3(H2O)(EtOH)]∙(EtOH) | 650 | 13 × 103 | – | 1.3 |
| 953 | 14 × 103 | – | 0.5 | |
| 650 | 55 × 103 | – | 2.0 | |
| 953 | 66 × 103 | – | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polikovskiy, T.; Korshunov, V.; Metlin, M.; Gontcharenko, V.; Metlina, D.; Datskevich, N.; Kiskin, M.; Belousov, Y.; Tsorieva, A.; Taydakov, I. Influence of Ligand Environment Stoichiometry on NIR-Luminescence Efficiency of Sm3+, Pr3+ and Nd3+ Ions Coordination Compounds. Molecules 2023, 28, 5892. https://doi.org/10.3390/molecules28155892
Polikovskiy T, Korshunov V, Metlin M, Gontcharenko V, Metlina D, Datskevich N, Kiskin M, Belousov Y, Tsorieva A, Taydakov I. Influence of Ligand Environment Stoichiometry on NIR-Luminescence Efficiency of Sm3+, Pr3+ and Nd3+ Ions Coordination Compounds. Molecules. 2023; 28(15):5892. https://doi.org/10.3390/molecules28155892
Chicago/Turabian StylePolikovskiy, Trofim, Vladislav Korshunov, Mikhail Metlin, Viktoria Gontcharenko, Darya Metlina, Nikolay Datskevich, Mikhail Kiskin, Yury Belousov, Alisia Tsorieva, and Ilya Taydakov. 2023. "Influence of Ligand Environment Stoichiometry on NIR-Luminescence Efficiency of Sm3+, Pr3+ and Nd3+ Ions Coordination Compounds" Molecules 28, no. 15: 5892. https://doi.org/10.3390/molecules28155892
APA StylePolikovskiy, T., Korshunov, V., Metlin, M., Gontcharenko, V., Metlina, D., Datskevich, N., Kiskin, M., Belousov, Y., Tsorieva, A., & Taydakov, I. (2023). Influence of Ligand Environment Stoichiometry on NIR-Luminescence Efficiency of Sm3+, Pr3+ and Nd3+ Ions Coordination Compounds. Molecules, 28(15), 5892. https://doi.org/10.3390/molecules28155892







