Isolation and Identification of β-Glucosidases-Producing Non-Saccharomyces Yeast Strains and Its Influence on the Aroma of Fermented Mango Juice
Abstract
1. Introduction
2. Results and Discussion
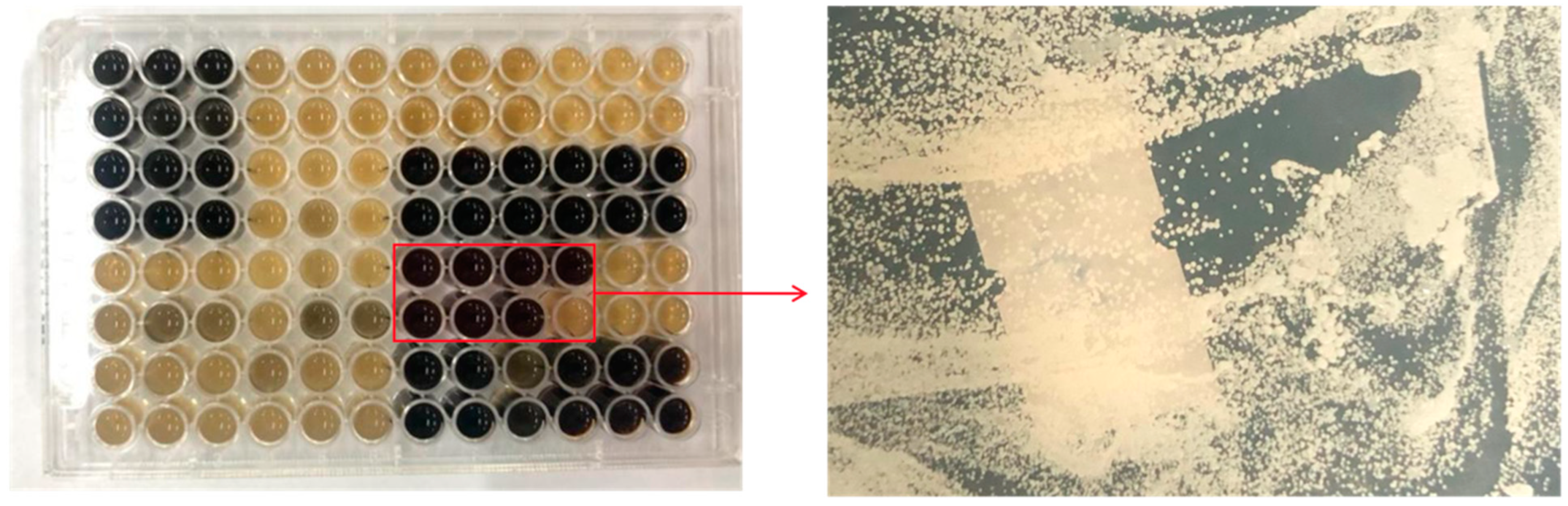
2.1. Screening of Non-Saccharomyces Yeasts
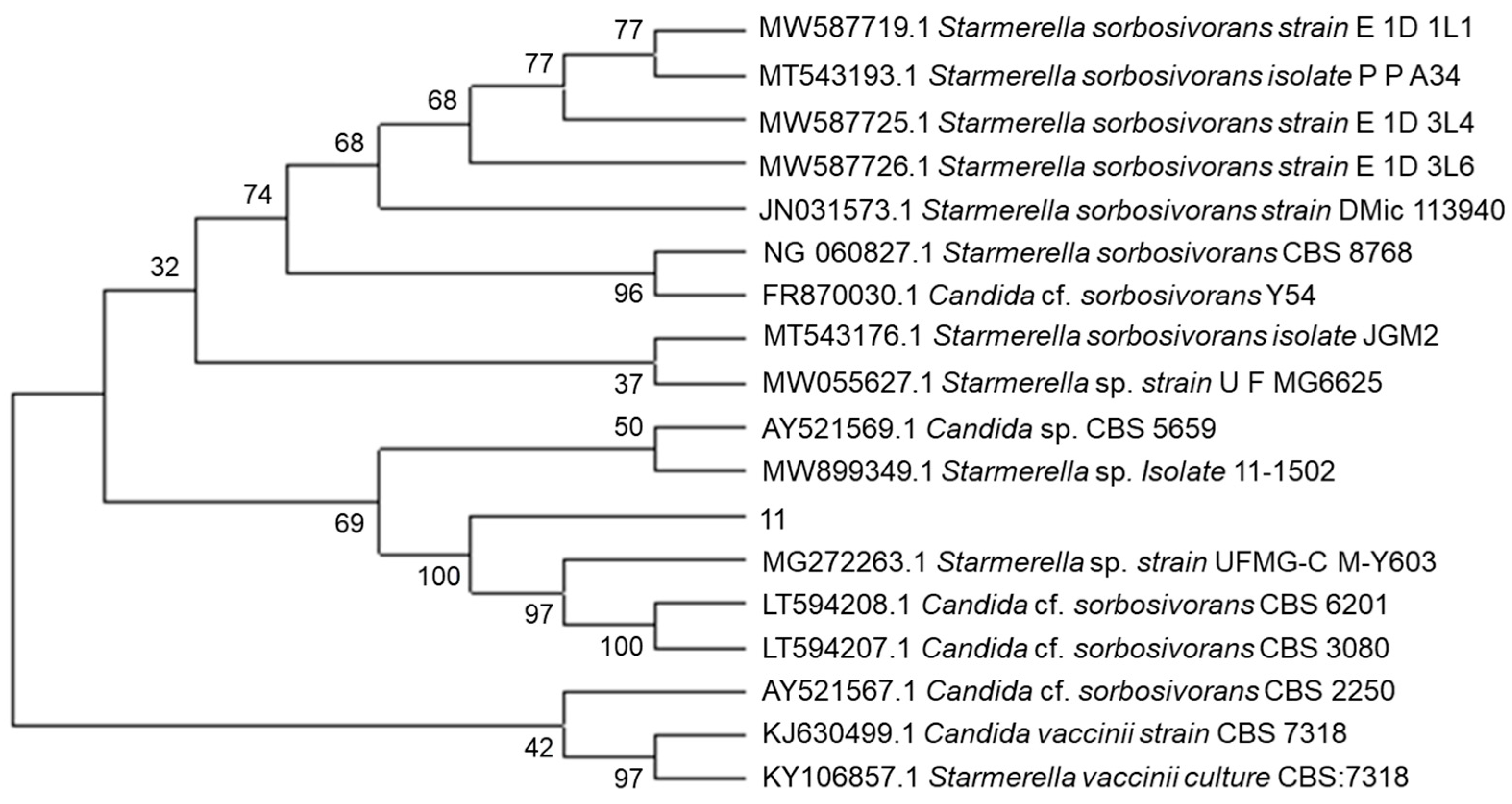
2.2. Molecular Biology Identification
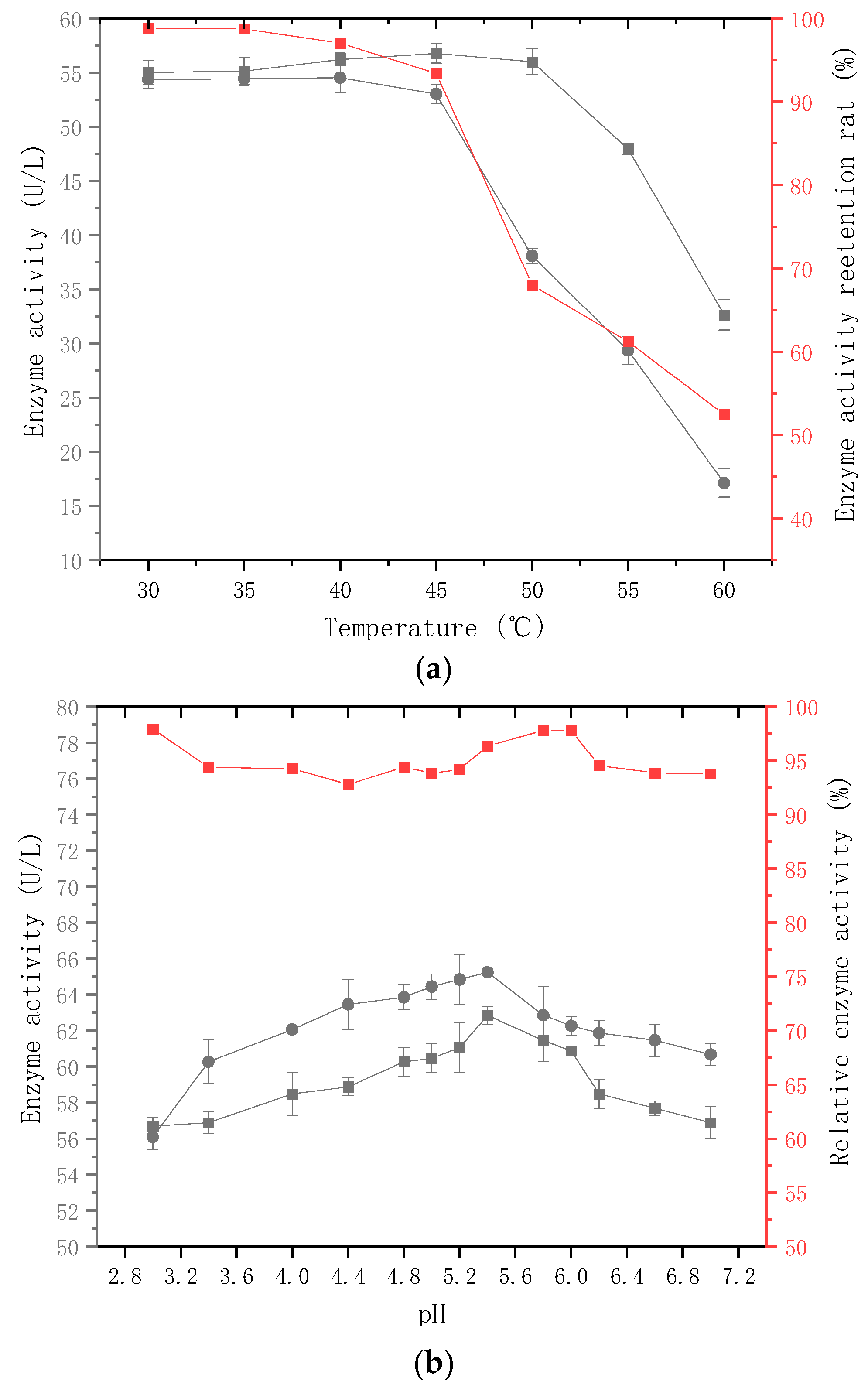
2.3. Enzymatic Properties of β-Glucosidase Produced by the Strain
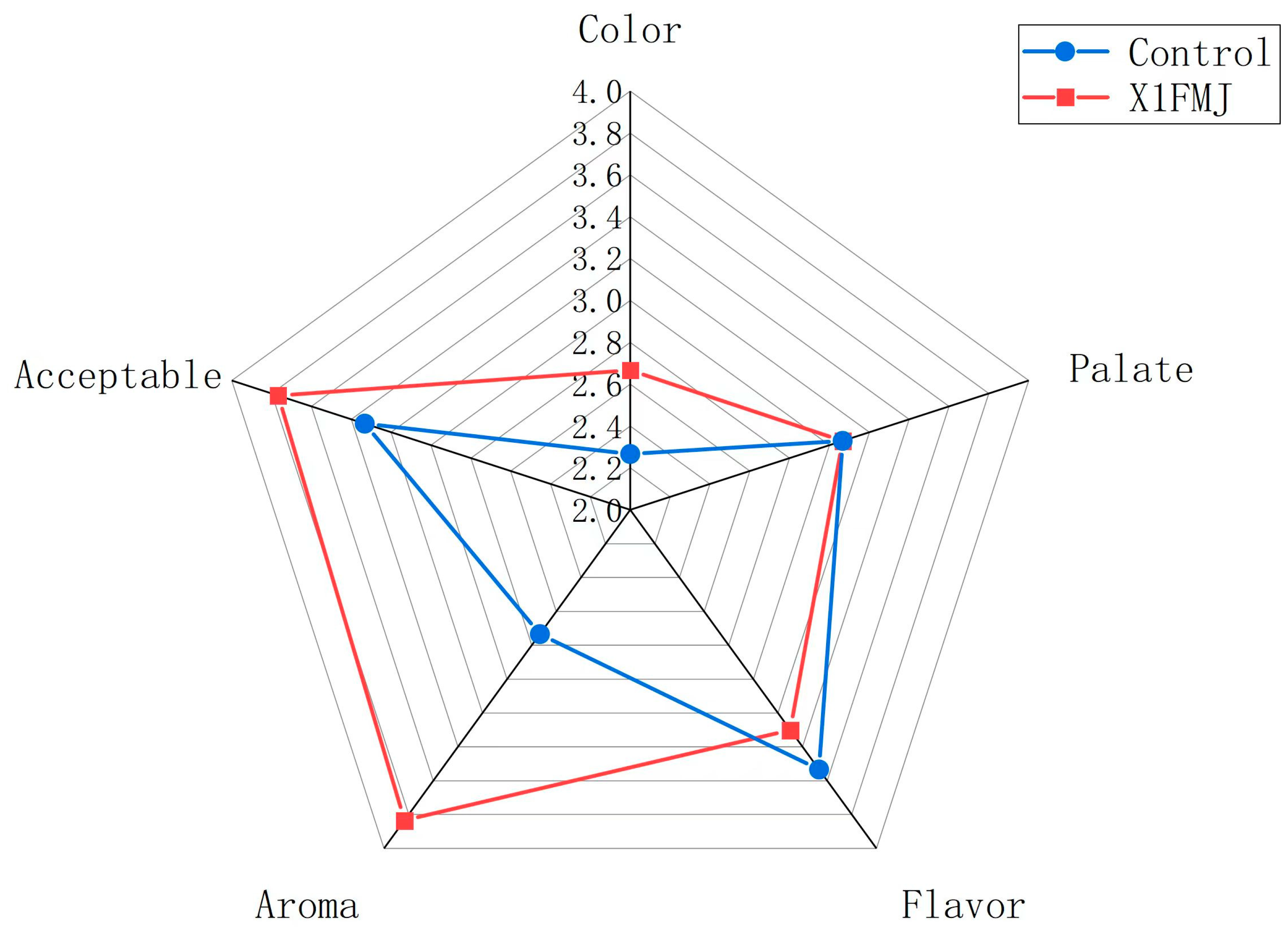
2.4. Sensory Analysis
2.5. Analysis of Aroma Composition by GC-MS
2.5.1. Results of OPLS–DA

2.5.2. Analysis of Differential Metabolites
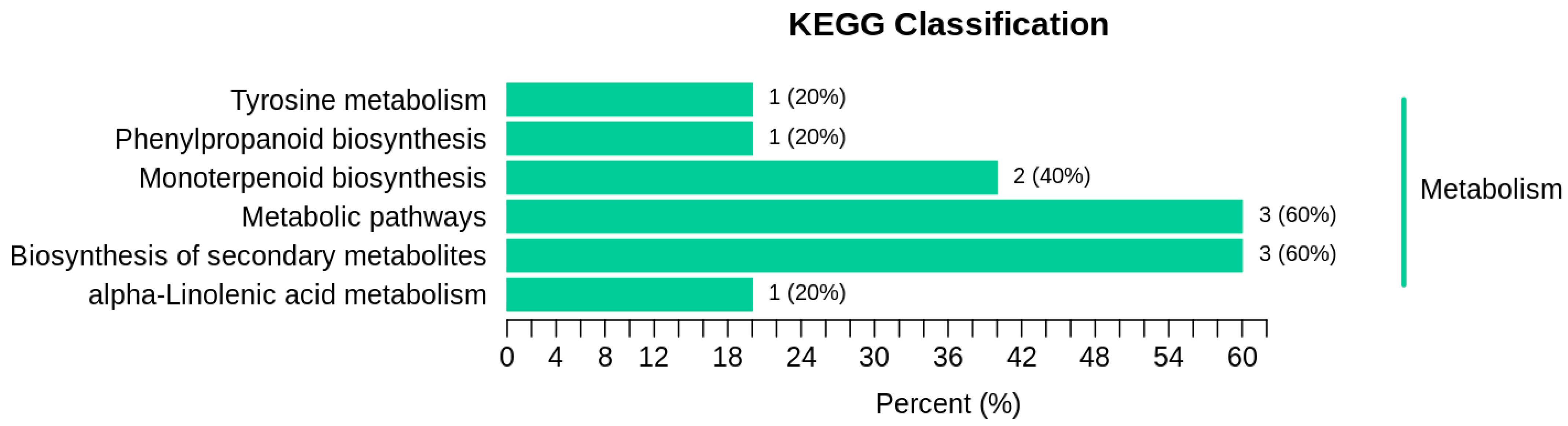
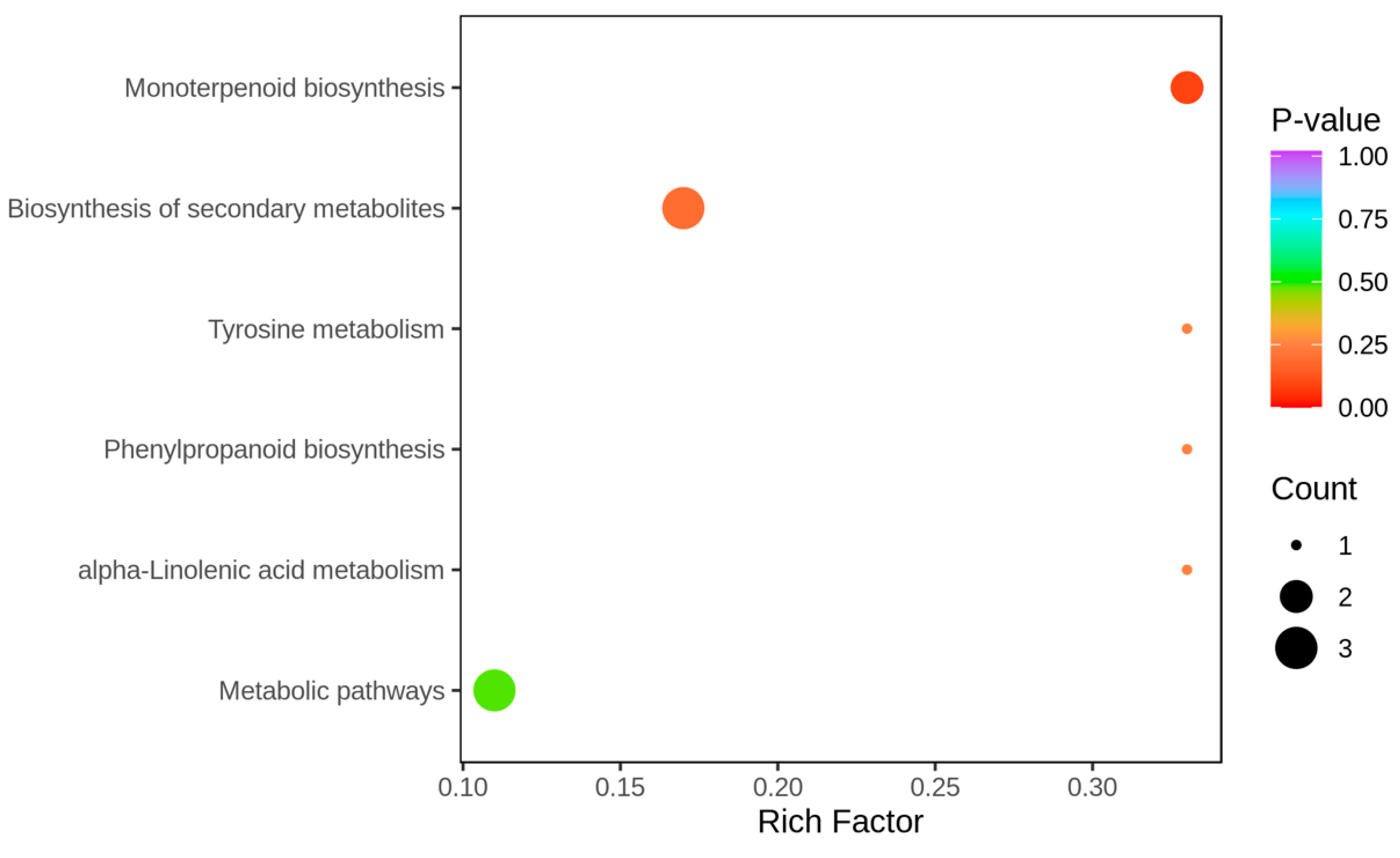
2.5.3. Research on Differential Metabolite Metabolic Pathways
3. Materials and Methods
3.1. Isolation and Prescreening of Non-Saccharomyces Yeasts
3.2. Molecular Biological Identification of Non-Saccharomyces Yeasts
3.3. Beta-Glucosidase Property Analysis
3.3.1. Determination of Optimal Temperature and Thermal Stability of Enzymatic Reactions
3.3.2. Optimum Temperature and Thermal Stability of Enzyme Catalysis
3.3.3. Optimum pH and pH Stability of Enzyme Catalysis
3.4. MJ Preparation and Fermentation
3.5. Sensory Assessment
3.6. Volatile Aromatic Compound (VOC) Analysis
3.7. Differential Metabolite Screening
3.8. KEGG Annotation and Enrichment Analysis
3.9. Data Processing
- (1)
- UV (unit variance scaling), also known as Z-score standardization or auto-scaling, is a method for standardizing data based on the mean and standard deviation of the original data. The processed data adhere to a standard normal distribution, meaning that the mean is 0 and the standard deviation is 1. The calculation method involves dividing by the variable’s standard deviation after data centralization, using the following formula: x’ = (x – µ)/σ, where µ represents the mean and σ signifies the standard deviation.
- (2)
- Zero-centered calculation method. The means of variables are subtracted from the original data, using the following formula: x’ = x − µ, where µ is the mean.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pan, X.; Wu, J.; Zhang, W.; Liu, J.; Yang, X.; Liao, X.; Lao, F. Effects of sugar matrices on the release of key aroma compounds in fresh and high hydrostatic pressure processed Tainong mango juices. Food Chem. 2021, 338, 128117. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A. Odour-active compounds in mango (Mangifera indica L. cv. Corazón). Int. J. Food Sci. Technol. 2012, 47, 1944–1950. [Google Scholar] [CrossRef]
- Quijano, C.E.; Salamanca, G.; Pino, J.A. Aroma volatile constituents of Colombian varieties of mango (Mangifera indica L.). Flavour Fragr. J. 2007, 22, 401–406. [Google Scholar] [CrossRef]
- Munafo, J.P., Jr.; Didzbalis, J.; Schnell, R.J.; Schieberle, P.; Steinhaus, M. Characterization of the major aroma-active compounds in mango (Mangifera indica L.) cultivars Haden, White Alfonso, Praya Sowoy, Royal Special, and Malindi by application of a comparative aroma extract dilution analysis. J. Agric. Food Chem. 2014, 62, 4544–4551. [Google Scholar] [CrossRef]
- Naknean, P.; Juntorn, N.; Yimyuan, T. Influence of clarifying agents on the quality of pasteurised palmyra palm sap (Borassus flabellifer Linn.). Int. J. Food Sci. Technol. 2014, 49, 1175–1183. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef]
- Winterhalter, P.; Skouroumounis, G.K. Glycoconjugated aroma compounds: Occurrence, role and biotechnological transformation. Adv. Biochem. Eng. Biotechnol. 1997, 55, 73–105. [Google Scholar]
- Haslbeck, K.; Jerebic, S.; Zarnkow, M. Characterization of the Unfertilized and Fertilized Hop Varieties Progress and Hallertauer Tradition—Analysis of Free and Glycosidic-Bound Flavor Compounds and beta-Glucosidase Activity. Brew. Sci. 2017, 70, 148–158. [Google Scholar]
- Zhang, T.; Fang, K.; Ni, H.; Li, T.; Li, L.J.; Li, Q.B.; Chen, F. Aroma enhancement of instant green tea infusion using beta-glucosidase and beta-xylosidase. Food Chem. 2020, 315, 126287. [Google Scholar] [CrossRef]
- Vernocchi, P.; Patrignani, F.; Ndagijimana, M.; Lopez, C.C.; Suzzi, G.; Gardini, F.; Lanciotti, R. Trebbiano wine produced by using Saccharomyces cerevisiae strains endowed with β-glucosidase activity. Ann. Microbiol. 2014, 65, 1565–1571. [Google Scholar] [CrossRef]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Lu, Y.; Chua, J.Y.; Huang, D.; Lee, P.R.; Liu, S.Q. Chemical consequences of three commercial strains of Oenococcus oeni co-inoculated with Torulaspora delbrueckii in durian wine fermentation. Food Chem. 2017, 215, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, M.; Xie, N.; Huang, M.; Feng, Y. Community structure of yeast in fermented soy sauce and screening of functional yeast with potential to enhance the soy sauce flavor. Int. J. Food Microbiol. 2022, 370, 109652. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food Agr. 2015, 94, 2951–2957. [Google Scholar] [CrossRef]
- Belloch, C.; Orlic, S.; Barrio, E.; Querol, A. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int. J. Food Microbiol. 2008, 122, 188–195. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- de Ovalle, S.; Brena, B.; Gonzalez-Pombo, P. Influence of beta glucosidases from native yeast on the aroma of Muscat and Tannat wines. Food Chem. 2021, 346, 128899. [Google Scholar] [CrossRef]
- Yan, T.R.; Lin, C.L. Purification and characterization of a glucose-tolerant beta-glucosidase from Aspergillus niger CCRC 31494. Biosci. Biotechnol. Biochem. 1997, 61, 965–970. [Google Scholar] [CrossRef]
- San, A.T.; Joyce, D.C.; Hofman, P.J.; Macnish, A.J.; Webb, R.I.; Matovic, N.J.; Williams, C.M.; De Voss, J.J.; Wong, S.H.; Smyth, H.E. Stable isotope dilution assay (SIDA) and HS-SPME-GCMS quantification of key aroma volatiles for fruit and sap of Australian mango cultivars. Food Chem. 2017, 221, 613–619. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; White, M.M. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Balmaseda, A.; Bordons, A.; Reguant, C.; Bautista-Gallego, J. Non-Saccharomyces in Wine: Effect Upon Oenococcus oeni and Malolactic Fermentation. Front. Microbiol. 2018, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, H.; Wang, W.; Jiao, W.; Chen, W.; Zhong, Q.P.; Yun, Y.H.; Chen, W. Characterization of Volatile Profiles and Marker Substances by HS-SPME/GC-MS during the Concentration of Coconut Jam. Foods 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
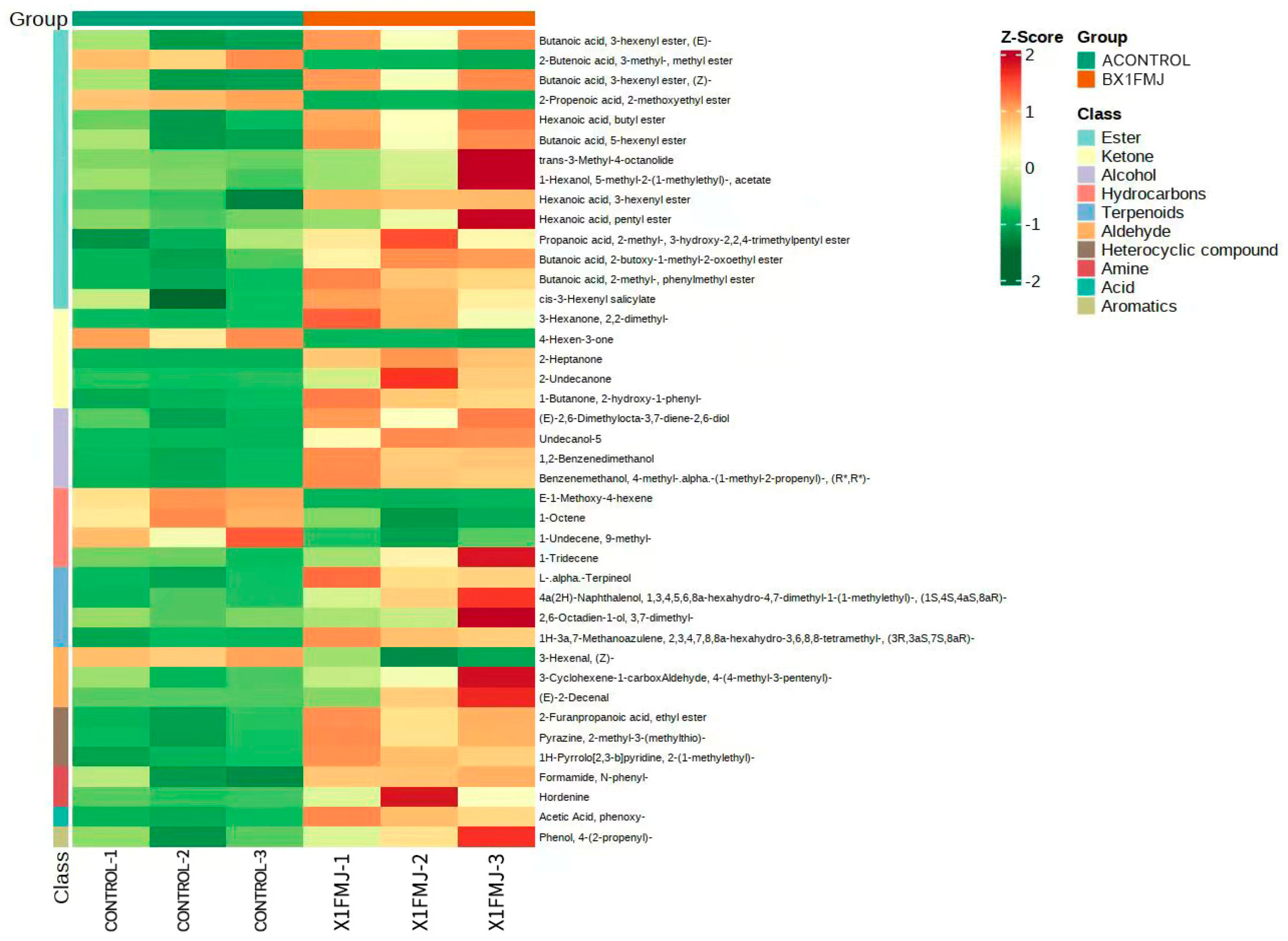

| Index | Compounds | Class I |
|---|---|---|
| XMW1069 | Butanoic acid, 3-hexenyl ester, (E)- | Ester |
| XMW0881 | 2-Butenoic acid, 3-methyl-, methyl ester | Ester |
| KMW0366 | Butanoic acid, 3-hexenyl ester, (Z)- | Ester |
| XMW0180 | 2-Propenoic acid, 2-methoxyethyl ester | Ester |
| KMW0381 | Hexanoic acid, butyl ester | Ester |
| XMW0304 | Butanoic acid, 5-hexenyl ester | Ester |
| KMW0490 | trans-3-Methyl-4-octanolide | Ester |
| D457 | 1-Hexanol, 5-methyl-2-(1-methylethyl)-, acetate | Ester |
| XMW1097 | Hexanoic acid, 3-hexenyl ester | Ester |
| XMW1244 | Hexanoic acid, pentyl ester | Ester |
| XMW0617 | Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | Ester |
| XMW0962 | Butanoic acid, 2-methyl-, phenylmethyl ester | Ester |
| w44 | Butanoic acid, 2-butoxy-1-methyl-2-oxoethyl ester | Ester |
| XMW0479 | cis-3-Hexenyl salicylate | Ester |
| D422 | Undecanol-5 | Alcohol |
| XMW0443 | (E)-2,6-Dimethylocta-3,7-diene-2,6-diol | Alcohol |
| XMW0107 | 1,2-Benzenedimethanol | Alcohol |
| XMW0728 | Benzenemethanol, 4-methyl-. alpha.-(1-methyl-2-propenyl)-, (R*, R*)- | Alcohol |
| XMW0648 | 3-Hexanone, 2,2-dimethyl- | Ketone |
| XMW0201 | 4-Hexen-3-one | Ketone |
| KMW0113 | 2-Heptanone | Ketone |
| KMW0474 | 2-Undecanone | Ketone |
| XMW0856 | 1-Butanone, 2-hydroxy-1-phenyl- | Ketone |
| D81 | 2-Furanpropanoic acid, ethyl ester | Heterocyclic compound |
| KMW0068 | 3-Hexenal, (Z)- | Heterocyclic compound |
| D323 | Pyrazine, 2-methyl-3-(methylthio)- | Heterocyclic compound |
| XMW0701 | 1H-Pyrrolo [2,3-b] pyridine, 2-(1-methylethyl)- | Heterocyclic compound |
| XMW0279 | (E)-1-Methoxy-4-hexene | Hydrocarbons |
| XMW0775 | 1-Undecene, 9-methyl- | Hydrocarbons |
| XMW1114 | 1-Octene | Hydrocarbons |
| D266 | 1-Tridecene | Hydrocarbons |
| NMW0071 | l-alpha-Terpineol | Terpenoids |
| XMW0854 | 4a(2H)-Naphthalenol,1,3,4,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S,4S,4aS,8aR)- | Terpenoids |
| NMW0104 | 2,6-Octadien-1-ol, 3,7-dimethyl- | Terpenoids |
| XMW1412 | 1H-3a,7-Methanoazulene, 2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-, (3R,3aS,7S,8aR)- | Terpenoids |
| XMW0711 | Formamide, N-phenyl- | Amine |
| NMW0218 | Hordenine | Amine |
| GMW0087 | (E)-2-Decenal | Aldehyde |
| D425 | 3-Cyclohexene-1-carboxAldehyde,4-(4-methyl-3-pentenyl)- | Aldehyde |
| KMW0433 | Phenol, 4-(2-propenyl)- | Aromatics |
| NMW0171 | Acetic Acid, phenoxy- | Acid |
| Index | Content (µg/L × 103) | VIP | p-Value | Type | Fold Change | Cpd ID | KEGG Map | |
|---|---|---|---|---|---|---|---|---|
| Control | X1FMJ | |||||||
| XMW1069 | 5.98 × 105 | 1.72 × 106 | 1.28 | 1.49 × 10−2 | up | 2.87 | - | - |
| XMW0881 | 8.52 × 105 | 1.45 × 105 | 1.45 | 2.17 × 10−3 | down | 1.71 × 10−1 | - | - |
| KMW0366 | 6.24 × 105 | 1.79 × 106 | 1.28 | 1.54 × 10−2 | up | 2.87 | - | - |
| XMW0180 | 3.22 × 105 | 3.53 × 104 | 1.44 | 4.40 × 10−4 | down | 1.10 × 10−1 | - | - |
| KMW0381 | 2.62 × 104 | 5.64 × 104 | 1.36 | 1.59 × 10−2 | up | 2.15 | - | - |
| XMW0304 | 3.26 × 105 | 9.58 × 105 | 1.28 | 1.53 × 10−2 | up | 2.93 | - | - |
| KMW0490 | 1.56 × 104 | 4.76 × 104 | 1.12 | 2.83 × 10−1 | up | 3.06 | - | - |
| D457 | 1.59 × 104 | 4.87 × 104 | 1.06 | 3.03 × 10−1 | up | 3.07 | D88387 | - |
| XMW1097 | 1.97 × 104 | 4.21 × 104 | 1.37 | 1.13 × 10−2 | up | 2.14 | - | - |
| XMW1244 | 1.45 × 104 | 4.28 × 104 | 1.14 | 2.56 × 10−1 | up | 2.96 | - | - |
| XMW0617 | 6.74 × 104 | 1.45 × 105 | 1.24 | 2.83 × 10−2 | up | 2.14 | - | - |
| XMW0962 | 5.90 × 104 | 2.18 × 105 | 1.42 | 3.14 × 10−3 | up | 3.69 | - | - |
| w44 | 2.50 × 104 | 6.71 × 104 | 1.41 | 6.91 × 10−3 | up | 2.68 | - | - |
| XMW0479 | 2.13 × 104 | 5.43 × 104 | 1.11 | 3.60 × 10−2 | up | 2.55 | - | - |
| XMW0648 | 3.36 × 104 | 1.89 × 105 | 1.38 | 4.19 × 10−2 | up | 5.64 | - | - |
| XMW0201 | 5.12 × 105 | 4.67 × 104 | 1.44 | 1.18 × 10−2 | down | 9.11 × 10−2 | - | - |
| KMW0113 | 9.00 | 4.86 × 105 | 1.45 | 2.89 × 10−3 | up | 5.40 × 104 | C08380 | - |
| KMW0474 | 2.97 × 103 | 2.12 × 105 | 1.41 | 9.32 × 10−2 | up | 7.15 × 10 | C01875 | - |
| XMW0856 | 9.26 × 104 | 3.85 × 105 | 1.41 | 5.58 × 10−3 | up | 4.16 | - | - |
| D81 | 4.76 × 104 | 9.77 × 104 | 1.41 | 1.30 × 10−3 | up | 2.05 | - | - |
| KMW0068 | 4.27 × 104 | 1.75 × 104 | 1.36 | 1.52 × 10−2 | down | 4.09 × 10−1 | C16310 | ko00592, ko01110 |
| D323 | 2.23 × 104 | 4.60 × 104 | 1.41 | 1.74 × 10−3 | up | 2.06 | - | - |
| XMW0701 | 1.76 × 104 | 4.26 × 104 | 1.41 | 4.59 × 10−4 | up | 2.42 | - | - |
| XMW0279 | 7.06 × 104 | 8.42 × 103 | 1.44 | 7.09 × 10−3 | down | 1.19 × 10−1 | - | - |
| XMW0775 | 2.35 × 104 | 6.56 × 103 | 1.33 | 3.53 × 10−2 | down | 2.79 × 10−1 | - | - |
| XMW1114 | 8.26 × 104 | 3.79 × 104 | 1.39 | 3.27 × 10−3 | down | 4.59 × 10−1 | D91846 | - |
| D266 | 6.57 × 103 | 2.82 × 104 | 1.25 | 1.83 × 10−1 | up | 4.29 | D92374 | - |
| NMW0071 | 1.39 × 105 | 3.29 × 105 | 1.40 | 8.80 × 10−3 | up | 2.37 | C11393 | ko00902, ko01100, ko01110 |
| XMW0854 | 7.73 × 103 | 1.82 × 104 | 1.36 | 8.41 × 10−2 | up | 2.35 | - | - |
| NMW0104 | 2.73 × 104 | 7.42 × 104 | 1.07 | 2.96 × 10−1 | up | 2.71 | C01500 | ko00902, ko01100, ko01110 |
| XMW0711 | 1.87 × 104 | 4.66 × 104 | 1.30 | 3.10 × 10−2 | up | 2.49 | D70279 | - |
| NMW0218 | 1.09 × 105 | 2.30 × 105 | 1.27 | 1.37 × 10−1 | up | 2.12 | C06199 | ko00350, ko01100 |
| GMW0087 | 1.50 × 104 | 1.28 × 105 | 1.28 | 1.75 × 10−1 | up | 8.53 | - | - |
| D425 | 1.65 × 104 | 3.57 × 104 | 1.20 | 1.78 × 10−1 | up | 2.17 | - | - |
| KMW0433 | 3.29 × 104 | 7.75 × 104 | 1.25 | 7.30 × 10−2 | up | 2.36 | C16930 | ko00940 |
| NMW0171 | 3.33 × 104 | 1.22 × 105 | 1.42 | 2.91 × 10−3 | up | 3.67 | C02181 | - |
| XMW0775 | 2.35 × 104 | 6.56 × 103 | 1.33 | 3.53 × 10−2 | down | 2.79 × 104 | - | - |
| XMW0443 | 5.44 × 104 | 1.19 × 105 | 1.38 | 1.88 × 10−2 | up | 2.19 | - | - |
| XMW0107 | 4.93 × 104 | 2.26 × 105 | 1.43 | 2.13 × 10−3 | up | 4.58 | - | - |
| XMW0728 | 2.53 × 105 | 1.21 × 106 | 1.42 | 2.92 × 10−3 | up | 4.80 | - | - |
| D422 | 1.14 × 104 | 9.71 × 104 | 1.45 | 2.44 × 10−2 | up | 8.52 | - | - |
| XMW1412 | 6.72 × 104 | 1.72 × 105 | 1.42 | 8.88 × 10−4 | up | 2.56 | - | - |
| KEGG_Pathway | ko_ID | Sig_Compound | Compound | Sig_Compound_All | Compound_All |
|---|---|---|---|---|---|
| alpha-Linolenic acid metabolism | ko00592 | 1 | 3 | 5 | 55 |
| Biosynthesis of secondary metabolites | ko01110 | 3 | 18 | 5 | 55 |
| Monoterpenoid biosynthesis | ko00902 | 2 | 6 | 5 | 55 |
| Metabolic pathways | ko01100 | 3 | 27 | 5 | 55 |
| Phenylpropanoid biosynthesis | ko00940 | 1 | 3 | 5 | 55 |
| Tyrosine metabolism | ko00350 | 1 | 3 | 5 | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Zhong, Q. Isolation and Identification of β-Glucosidases-Producing Non-Saccharomyces Yeast Strains and Its Influence on the Aroma of Fermented Mango Juice. Molecules 2023, 28, 5890. https://doi.org/10.3390/molecules28155890
Miao Y, Zhong Q. Isolation and Identification of β-Glucosidases-Producing Non-Saccharomyces Yeast Strains and Its Influence on the Aroma of Fermented Mango Juice. Molecules. 2023; 28(15):5890. https://doi.org/10.3390/molecules28155890
Chicago/Turabian StyleMiao, Yuemei, and Qiuping Zhong. 2023. "Isolation and Identification of β-Glucosidases-Producing Non-Saccharomyces Yeast Strains and Its Influence on the Aroma of Fermented Mango Juice" Molecules 28, no. 15: 5890. https://doi.org/10.3390/molecules28155890
APA StyleMiao, Y., & Zhong, Q. (2023). Isolation and Identification of β-Glucosidases-Producing Non-Saccharomyces Yeast Strains and Its Influence on the Aroma of Fermented Mango Juice. Molecules, 28(15), 5890. https://doi.org/10.3390/molecules28155890






