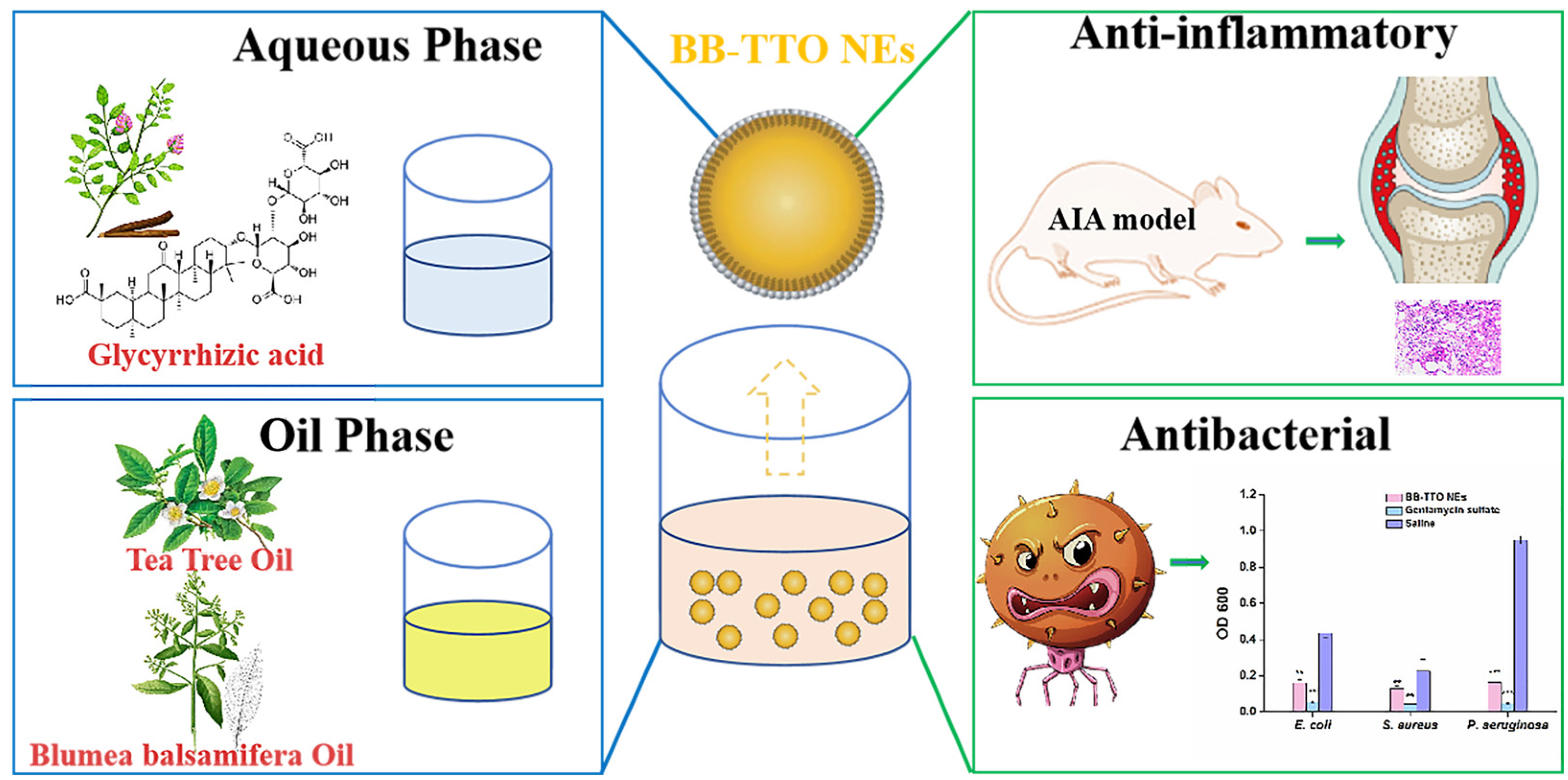
Fabrication and Biological Activities of All-in-One Composite Nanoemulsion Based on Blumea balsamifera Oil-Tea Tree Oil
Abstract
1. Introduction
2. Results and Discussions
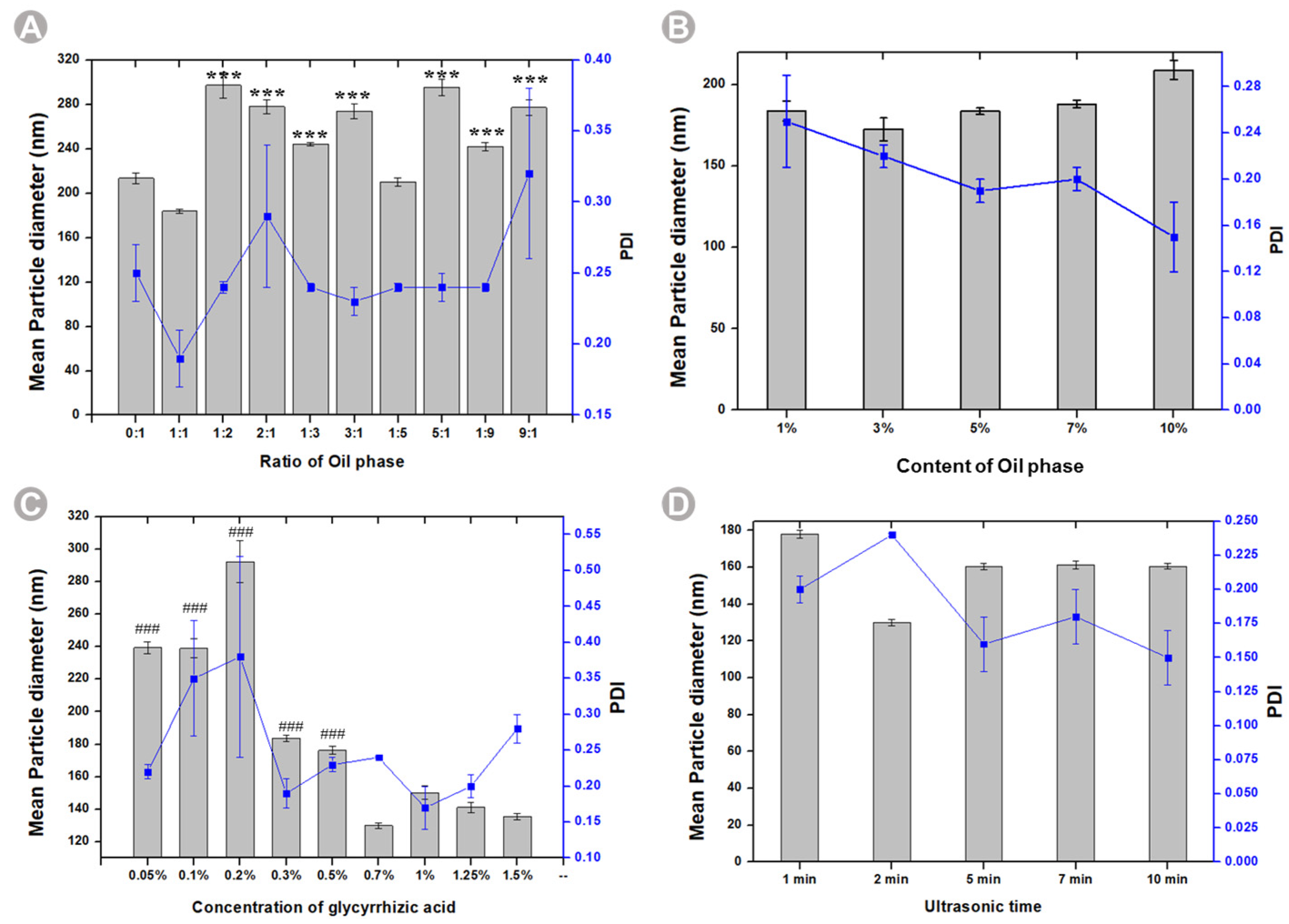
2.1. Single Factor Experiments to Optimize the Prescription of BB-TTO NEs
2.2. Characterization of BB-TTO NEs
2.2.1. Morphological Analysis
2.2.2. Mean Particle Size, PDI, and Zeta Potential
2.2.3. Type Identification
2.2.4. pH, Viscosity, and Turbidity
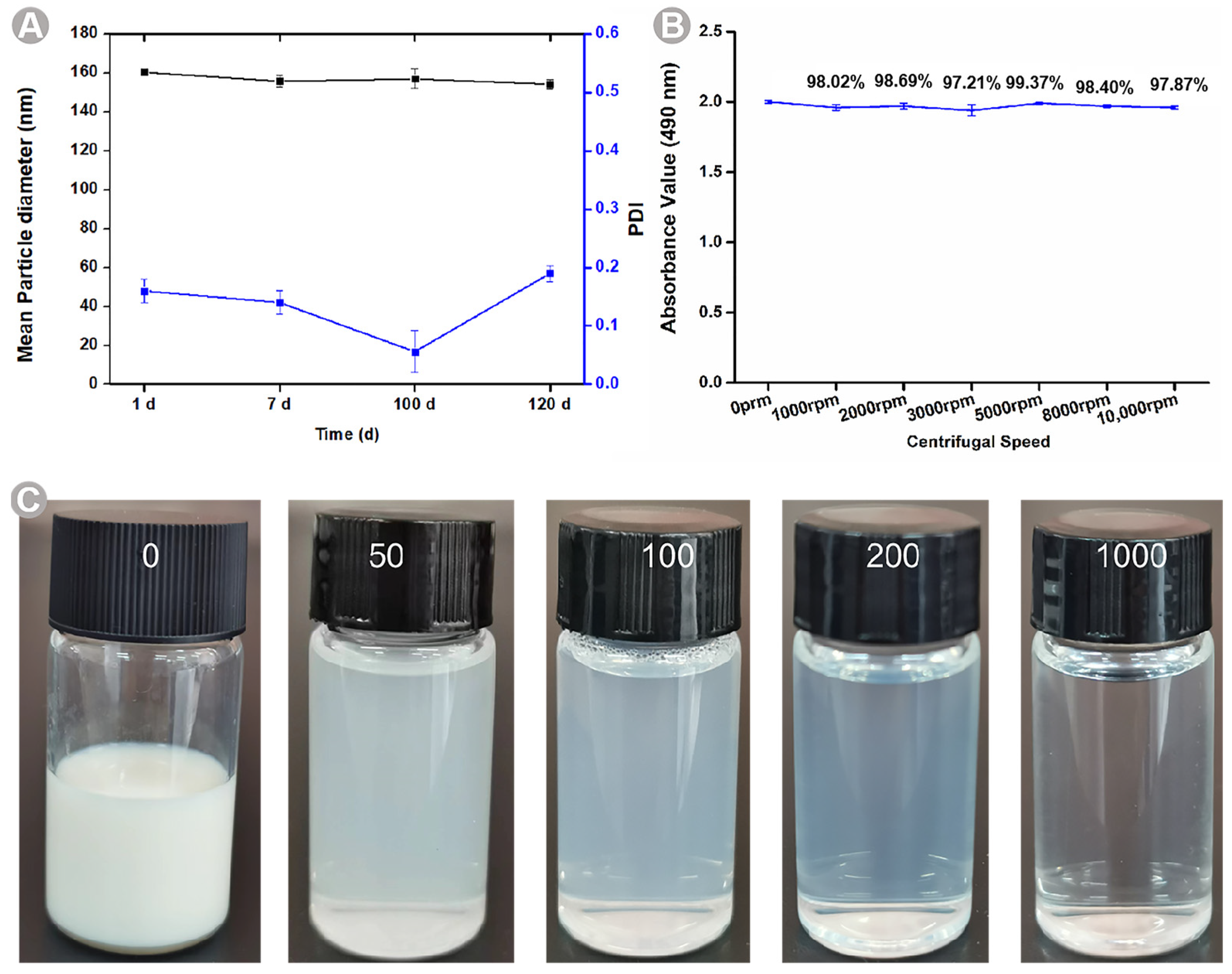
2.3. Stability Studies
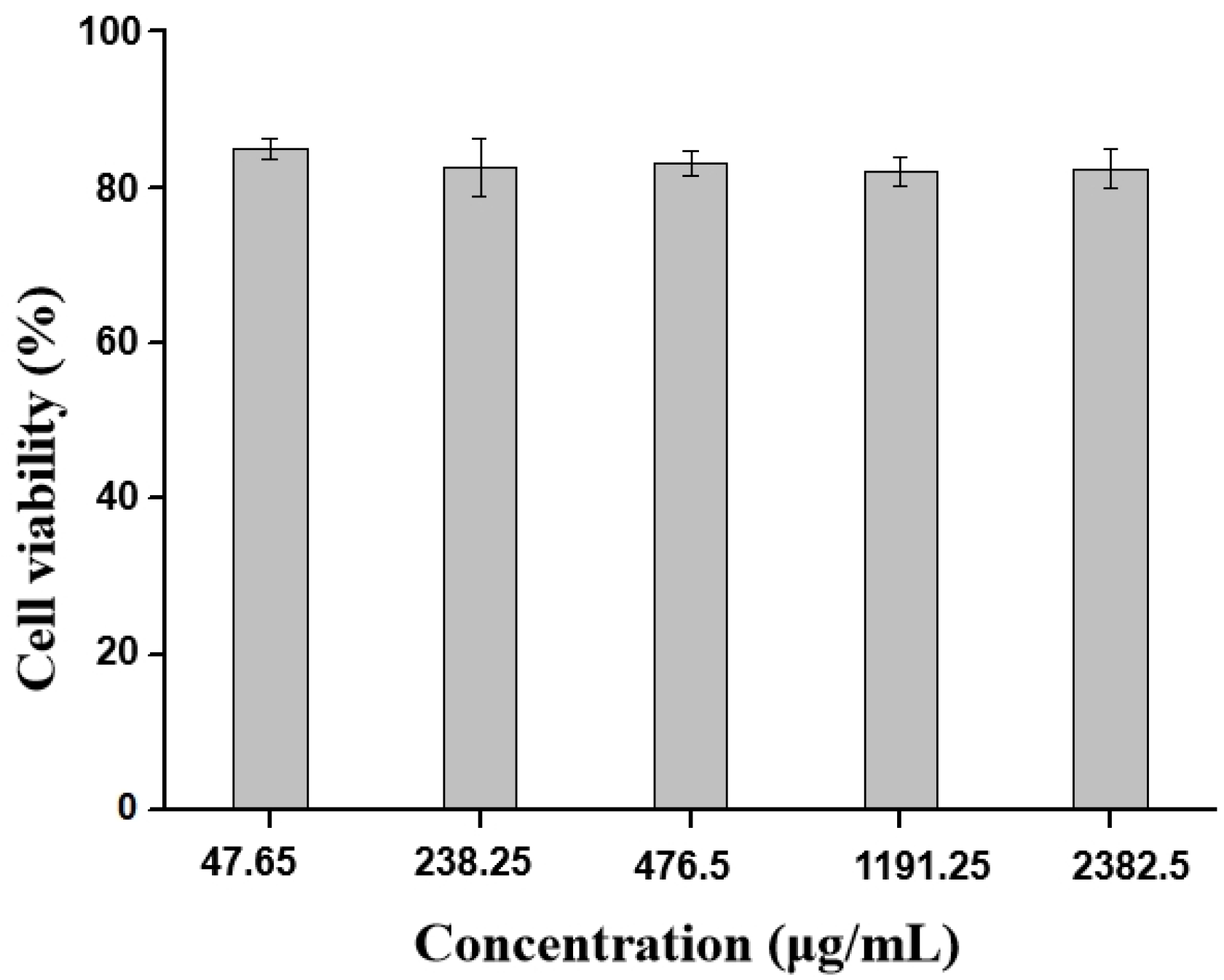
2.4. Cytotoxicity Studies of BB-TTO NEs
2.5. Antibacterial Evaluation
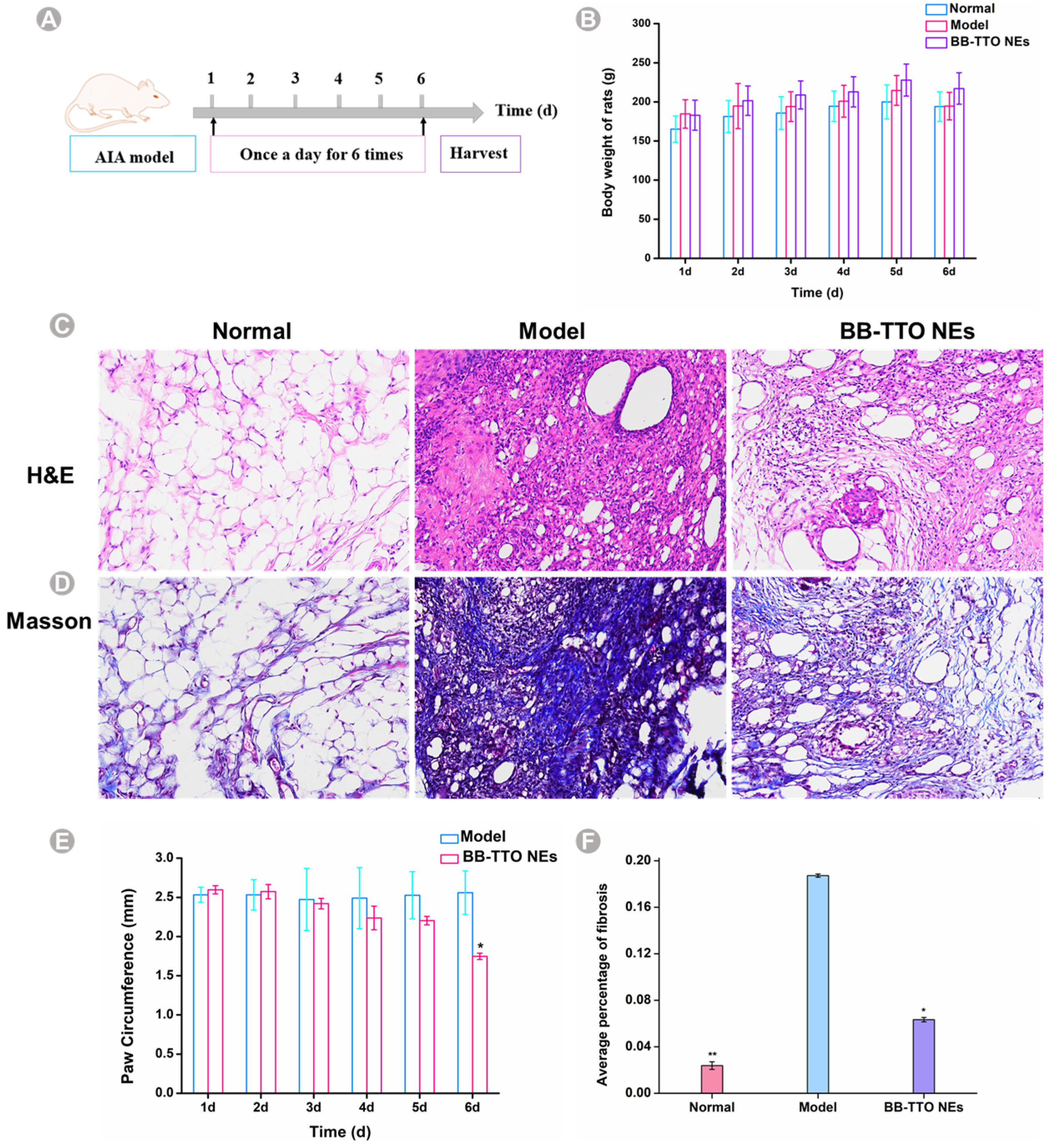
2.6. Anti-Inflammatory Activity
3. Materials and Method
3.1. Materials
3.2. Preparation of BB-TTO NEs
3.3. Single-Factor Experiments to Optimize the Prescription of BB-TTO NEs
3.4. Characterization of Physicochemical Properties of BB-TTO NEs
3.5. Stability Studies
3.6. Cytotoxicity Studies of BB-TTO NEs
3.7. Antibacterial Evaluation
3.8. Anti-Inflammatory Activity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Amorim, C.A. Nanoemulsion applications in photodynamic therapy. J. Control. Release 2022, 351, 164–173. [Google Scholar] [CrossRef]
- Harimurti, N.; Nasikin, M.; Mulia, K. Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts. Molecules 2021, 26, 196. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, A.U.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability-The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Liu, H.; Hu, L. Nanoemulsion-based delivery approaches for nutraceuticals: Fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021, 12, 1933–1953. [Google Scholar] [CrossRef]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef]
- Sah, M.K.; Gautam, B.; Pokhrel, K.P.; Ghani, L.; Bhattarai, A. Quantification of the Quercetin Nanoemulsion Technique Using Various Parameters. Molecules 2023, 28, 2540. [Google Scholar] [CrossRef]
- Dey, T.K.; Bose, P.; Paul, S.; Karmakar, B.C.; Saha, R.N.; Gope, A.; Koley, H.; Ghosh, A.; Dutta, S.; Dhar, P.; et al. Protective efficacy of fish oil nanoemulsion against non-typhoidal Salmonella mediated mucosal inflammation and loss of barrier function. Food Funct. 2022, 13, 10083–10095. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, M.; Zhang, W.; Zhang, C.; Ai, Y.; Liang, X.; Shi, Y.; Chen, Y.; Zhang, L.; He, T. Chemical composition of Blumea balsamifera and Magnolia sieboldii essential oils and prevention of UV-B radiation-induced skin photoaging. Nat. Prod. Res. 2021, 35, 5977–5980. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, Y.; Long, L.; Cai, Y.; Liao, J.; Peng, J.; Wang, L. Antibacterial effect of Blumea balsamifera (L.) DC. essential oil against Staphylococcus aureus. Arch. Microbiol. 2021, 203, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Masyudi, M.; Hanafiah, M.; Usman, S.; Marlina, M. Effectiveness of gel formulation of capa leaf (Blumea balsamifera L.) on wound healing in white rats. Vet. World 2022, 15, 2059–2066. [Google Scholar] [CrossRef]
- Ullah, A.; Saito, Y.; Ullah, S.; Haider, M.K.; Nawaz, H.; Duy-Nam, P.; Kharaghani, D.; Kim, I.S. Bioactive Sambong oil-loaded electrospun cellulose acetate nanofibers: Preparation, characterization, and in-vitro biocompatibility. Int. J. Biol. Macromol. 2021, 166, 1009–1021. [Google Scholar] [CrossRef]
- Voelker, J.; Shepherd, M.; Mauleon, R. A high-quality draft genome for Melaleuca alternifolia (tea tree): A new platform for evolutionary genomics of myrtaceous terpene-rich species. GigaByte 2021, 2021, gigabyte28. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, L.; Li, H.; Xiong, Z.; Fu, Z.; Zhang, Z.; Xie, W.; Cui, H.; Zhang, S.; Tang, Y.; et al. Construction of tea tree oil/salicylic acid/palygorskite hybrids for advanced antibacterial and anti-inflammatory performance. J. Mater. Chem. B 2023, 11, 4260–4273. [Google Scholar] [CrossRef]
- Zhu, L.; Machmudah, S.; Wahyudiono Kanda, H.; Goto, M. Reduced-Pressure Process for Fabricating Tea Tree Oil-Polyvinylpyrrolidone Electrospun Fibers. Polymers 2022, 14, 743. [Google Scholar] [CrossRef]
- Zuzarte, M.; Vitorino, C.; Salgueiro, L.; Girão, H. Plant Nanovesicles for Essential Oil Delivery. Pharmaceutics 2022, 14, 2581. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Sneha, K.; Ashwani, K. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food. Sci. Emerg. 2022, 76, 102914. [Google Scholar]
- Hatice, Y.; Yesim, O.; Esmeray, K. Antimicrobial influence of nanoemulsified lemon essential oil and pure lemon essential oil on food-borne pathogens and fish spoilage bacteria. Int. J. Food Microbiol. 2019, 306, 108266. [Google Scholar]
- Tang, M.; Liu, F.; Wang, Q.; Wang, D.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W. Physicochemical characteristics of ginger essential oil nanoemulsion encapsulated by zein/NaCas and antimicrobial control on chilled chicken. Food Chem. 2022, 374, 131624. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Sobral, P.J.D.A.; Aquino, A.; Neves, M.A.D.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones-A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid. Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, A.; Sharma, R.; Aurora, R.; Jain, S.K. Biosurfactants as a Novel Additive in Pharmaceutical Formulations: Current Trends and Future Implications. Curr. Drug Metab. 2020, 21, 885–901. [Google Scholar] [CrossRef]
- Da Silva, G.H.; Fernandes, M.A.; Trevizan, L.N.F.; de Lima, F.T.; Eloy, J.O.; Chorilli, M. A Critical Review of Properties and Analytical Methods for the Determination of Docetaxel in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2018, 48, 517–527. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhang, X.; Deng, M.; Xie, L.; Zhang, L.; Li, X. Tea saponins as natural stabilizers for the production of hesperidin nanosuspensions. Int. J. Pharm. 2020, 583, 119406. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Muriel Mundo, J.; McClements, D.J. Factors impacting lipid digestion and nutraceutical bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): Emulsifier type. Food Res. Int. 2020, 137, 109739. [Google Scholar] [CrossRef]
- Shu, G.; Khalid, N.; Chen, Z.; Neves, M.A.; Barrow, C.J.; Nakajima, M. Formulation and characterization of astaxanthin-enriched nanoemulsions stabilized using ginseng saponins as natural emulsifiers. Food Chem. 2018, 255, 67–74. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, C.; Gao, X.; Tan, C.; Bai, H.; Ning, K. Multi-omics profiling reveals comprehensive microbe-plant-metabolite regulation patterns for medicinal plant Glycyrrhiza uralensis Fisch. Plant Biotechnol. J. 2022, 20, 1874–1887. [Google Scholar] [CrossRef]
- Tan, D.; Tseng, H.H.L.; Zhong, Z.; Wang, S.; Vong, C.T.; Wang, Y. Glycyrrhizic Acid and Its Derivatives: Promising Candidates for the Management of Type 2 Diabetes Mellitus and Its Complications. Int. J. Mol. Sci. 2022, 23, 10988. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Thuy, L.T.; Lee, Y.; Piao, C.; Choi, J.S.; Lee, M. Dual-Functional Dendrimer Micelles with Glycyrrhizic Acid for Anti-Inflammatory Therapy of Acute Lung Injury. ACS Appl. Mater. Interfaces 2021, 13, 47313–47326. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Ma, H.L.; Varanda, L.C.; Perussi, J.R.; Carrilho, E. Hypericin-loaded oil-in-water nanoemulsion synthesized by ultrasonication process enhances photodynamic therapy efficiency. J. Photochem. Photobiol. B 2021, 223, 112303. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, J.; Qin, Y.; Xu, X.; Jin, Z. Characterization and Mechanisms of Novel Emulsions and Nanoemulsion Gels Stabilized by Edible Cyclodextrin-Based Metal-Organic Frameworks and Glycyrrhizic Acid. J. Agric. Food Chem. 2019, 67, 391–398. [Google Scholar] [CrossRef]
- Sinsuebpol, C.; Changsan, N. Effects of Ultrasonic Operating Parameters and Emulsifier System on Sacha Inchi Oil Nanoemulsion Characteristics. J. Oleo Sci. 2020, 69, 437–448. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhang, X.; Gui, L.; Wu, J.; Feng, Q.; Peng, S.; Zhao, M. Dimethyl 2,2′-[2,2′-(ethane-1,1-diyl)bis(1H-indole-3,2-diyl)]-diacetate: A small molecule capable of nano-scale assembly, inhibiting venous thrombosis and inducing no bleeding side effect. Int. J. Nanomedicine 2018, 13, 7835–7844. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Salleh, H.M.; Moniruzzaman, M. Preparation, Characterization and Biological Activities of an Oil-in-Water Nanoemulsion from Fish By-Products and Lemon Oil by Ultrasonication Method. Molecules 2022, 27, 6725. [Google Scholar] [CrossRef]
- Gul, U.; Khan, M.I.; Madni, A.; Sohail, M.F.; Rehman, M.; Rasul, A.; Peltonen, L. Olive oil and clove oil-based nanoemulsion for topical delivery of terbinafine hydrochloride: In vitro and ex vivo evaluation. Drug Deliv. 2022, 29, 600–612. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Seibert, J.B.; Rodrigues, I.V.; Carneiro, S.P.; Amparo, T.R.; Lanza, J.S.; Frézard, F.J.G.; de Souza, G.H.B.; Santos, O.D.H.D. Seasonality study of essential oil from leaves of Cymbopogon densiflorus and nanoemulsion development with antioxidant activity. Flavour Fragr. J. 2019, 34, 5–14. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sust. Energ. Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. The Relation of pH and Skin Cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Win, T.; Jaffri, J.M.; Edueng, K.; Taher, M. Palm Olein Emulsion: A Novel Vehicle for Topical Drug Delivery of Betamethasone 17-Valerate. AAPS PharmSciTech 2018, 19, 371–383. [Google Scholar] [CrossRef]
- Saki, E.; Murthy, V.; Khandanlou, R.; Wang, H.; Wapling, J.; Weir, R. Optimisation of Calophyllum inophyllum seed oil nanoemulsion as a potential wound healing agent. BMC Complement Med. Ther. 2022, 22, 285. [Google Scholar] [CrossRef]
- Hou, P.; Pu, F.; Zou, H.; Diao, M.; Zhao, C.; Xi, C.; Zhang, T. Whey protein stabilized nanoemulsion: A potential delivery system for ginsenoside Rg3 whey protein stabilized nanoemulsion: Potential Rg3 delivery system. Food Biosci. 2019, 31, 100427. [Google Scholar] [CrossRef]
- He, S.; Jacobsen, J.; Nielsen, C.U.; Genina, N.; Østergaard, J.; Mu, H. Exploration of in vitro drug release testing methods for saquinavir microenvironmental pH modifying buccal films. Eur. J. Pharm. Sci. 2021, 163, 105867. [Google Scholar] [CrossRef]
- Zanela da Silva Marques, T.Z.; Santos-Oliveira, R.; de Siqueira, L.B.d.O.; da Silva Cardoso, V.; de Freitas, Z.M.F.; Barros, R.d.C.d.S.A.; Villa, A.L.V.; Monteiro, M.S.d.S.d.B.; dos Santos, E.P.; Ricci, E., Jr. Development and characterization of a nanoemulsion containing propranolol for topical delivery. Int. J. Nanomed. 2018, 13, 2827–2837. [Google Scholar] [CrossRef]
- Walia, N.; Zhang, S.; Wismer, W.; Chen, L. A low energy approach to develop nanoemulsion by combining pea protein and Tween 80 and its application for vitamin D delivery, Food Hydrocoll. HLTH 2022, 2, 100078. [Google Scholar] [CrossRef]
- Abakar, H.O.M.; Bakhiet, S.E.; Abadi, R.S.M. Antimicrobial activity and minimum inhibitory concentration of Aloe vera sap and leaves using different extracts. J. Pharmacogn. Phytochem. 2017, 6, 298–303. [Google Scholar]
- Merghni, A.; Lassoued, M.A.; Voahangy Rasoanirina, B.N.; Moumni, S.; Mastouri, M. Characterization of Turpentine nanoemulsion and assessment of its antibiofilm potential against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2022, 166, 105530. [Google Scholar] [CrossRef]
- Nie, Y.; Pan, Y.; Jiang, Y.; Xu, D.; Yuan, R.; Zhu, Y.; Zhang, Z. Stability and bioactivity evaluation of black pepper essential oil nanoemulsion. Heliyon 2023, 9, e14730. [Google Scholar] [CrossRef]
- Saeedi, M.; Moghbeli, M.R.; Vahidi, O. Chitosan/glycyrrhizic acid hydrogel: Preparation, characterization, and its potential for controlled release of gallic acid. Int. J. Biol. Macromol. 2023, 231, 123197. [Google Scholar] [CrossRef]
- Li, W.R.; Li, H.L.; Shi, Q.S.; Sun, T.L.; Xie, X.B.; Song, B.; Huang, X.M. The dynamics and mechanism of the antimicrobial activity of tea tree oil against bacteria and fungi. Appl. Microbiol. Biotechnol. 2016, 100, 8865–8875. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Peng, Y.; Wang, Z.; Qiang, X.; Zhao, Q. Synthesis, Antiviral, and Antibacterial Activity of the Glycyrrhizic Acid and Glycyrrhetinic Acid Derivatives. Russ. J. Bioorg. Chem. 2022, 48, 906–918. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-inflammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, J.; Li, W.; Yang, L.; Dong, H.; Zhang, X. Programmable Polymeric Microneedles for Combined Chemotherapy and Antioxidative Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 55559–55568. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar]
- Li, M.; Zhang, L.; Liu, Z.; Zhang, L.; Xing, R.; Yin, S.; Li, X.; Zhang, N.; Wang, P. Sanse Powder Essential Oil Nanoemulsion Negatively Regulates TRPA1 by AMPK/mTOR Signaling in Synovitis: Knee Osteoarthritis Rat Model and Fibroblast-Like Synoviocyte Isolates. Mediat. Inflamm. 2021, 2021, 4736670. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Aarabi, M.H.; Aghighi, F.; Kazemi, M.; Vakili, Z.; Memarzadeh, M.R.; Talaei, S.A. Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: Behavioral, biochemical, and histopathological evidence. BMC Complement Med. Ther. 2021, 21, 57. [Google Scholar] [CrossRef]
- Richard, S.A. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediat. Inflamm. 2021, 2021, 6699560. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sultana, A.; Khan, M.F.; Boonhok, R.; Afroz, S. Tea tree oil, a vibrant source of neuroprotection via neuroinflammation inhibition: A critical insight into repurposing Melaleuca alternifolia by unfolding its characteristics. J. Zhejiang Univ. Sci. B 2023, 24, 554–573. [Google Scholar] [CrossRef]
- Liao, J.; Xie, X.; Wang, W.; Gao, Y.; Cai, Y.; Peng, J.; Li, T.; Yi, Q.; He, C.; Wang, L. Anti-inflammatory Activity of Essential Oil from Leaves of Blumea balsamifera (L.) DC through Inhibiting TLR4/NF-kB Signaling Pathways and NLRP3 Inflammasome Activation in LPS-induced RAW264.7 Macrophage Cells. J. Essent. Oil Bear. Pl. 2021, 24, 160–176. [Google Scholar] [CrossRef]
- Lal, D.K.; Kumar, B.; Saeedan, A.S.; Ansari, M.N. An Overview of Nanoemulgels for Bioavailability Enhancement in Inflammatory Conditions via Topical Delivery. Pharmaceutics 2023, 15, 1187. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Kesavan Pillai, S.; Naushad Emmambux, M. Development of hemp seed oil nanoemulsions loaded with ascorbyl palmitate: Effect of operational parameters, emulsifiers, and wall materials. Food Chem. 2023, 400, 134052. [Google Scholar] [CrossRef]
- Kohli, A.; Alpar, H. Potential use of nanoparticles for transcutaneous vaccine delivery: Effect of particle size and charge. Int. J. Pharm. 2004, 275, 13–17. [Google Scholar] [CrossRef]
- Said Suliman, A.; Tom, R.; Palmer, K.; Tolaymat, I.; Younes, H.M.; Arafat, B.; Elhissi, A.M.A.; Najlah, M. Development, characterization and stability evaluation of ciprofloxacin-loaded parenteral nutrition nanoemulsions. Pharm. Dev. Technol. 2020, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; He, P.; Zhao, J.; He, C.; Jiang, M.; Zhang, Z.; Zhang, Z.; Sun, X. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J. Control. Release 2021, 336, 537–548. [Google Scholar] [CrossRef]

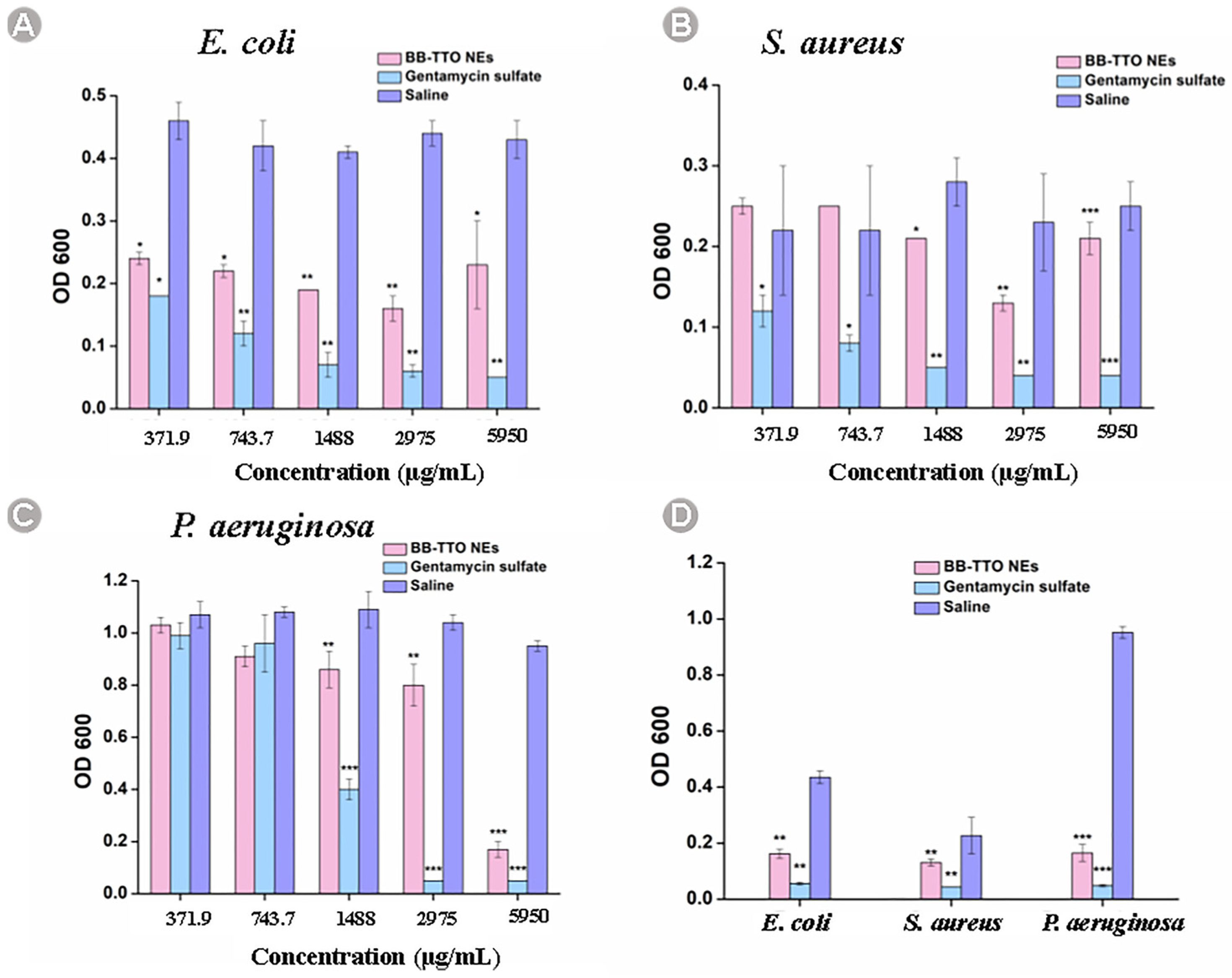
| Strain | MIC (μg/mL) |
|---|---|
| E. coli | 2975 |
| S. aureus | 2975 |
| P.aeruginosa | 5950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Chen, T.; Feng, T.; Zhang, J.; Meng, Z.; Zhang, N.; Luo, G.; Wang, Z.; Pang, Y.; Zhou, Y. Fabrication and Biological Activities of All-in-One Composite Nanoemulsion Based on Blumea balsamifera Oil-Tea Tree Oil. Molecules 2023, 28, 5889. https://doi.org/10.3390/molecules28155889
Zhu Y, Chen T, Feng T, Zhang J, Meng Z, Zhang N, Luo G, Wang Z, Pang Y, Zhou Y. Fabrication and Biological Activities of All-in-One Composite Nanoemulsion Based on Blumea balsamifera Oil-Tea Tree Oil. Molecules. 2023; 28(15):5889. https://doi.org/10.3390/molecules28155889
Chicago/Turabian StyleZhu, Yue, Teng Chen, Tingting Feng, Jiaojiao Zhang, Zejing Meng, Ning Zhang, Gang Luo, Zuhua Wang, Yuxin Pang, and Ying Zhou. 2023. "Fabrication and Biological Activities of All-in-One Composite Nanoemulsion Based on Blumea balsamifera Oil-Tea Tree Oil" Molecules 28, no. 15: 5889. https://doi.org/10.3390/molecules28155889
APA StyleZhu, Y., Chen, T., Feng, T., Zhang, J., Meng, Z., Zhang, N., Luo, G., Wang, Z., Pang, Y., & Zhou, Y. (2023). Fabrication and Biological Activities of All-in-One Composite Nanoemulsion Based on Blumea balsamifera Oil-Tea Tree Oil. Molecules, 28(15), 5889. https://doi.org/10.3390/molecules28155889








