Photochromic Azobenzene Inverse Opal Film toward Dynamic Anti-Fake Pattern
Abstract
1. Introduction
2. Results and Discussion
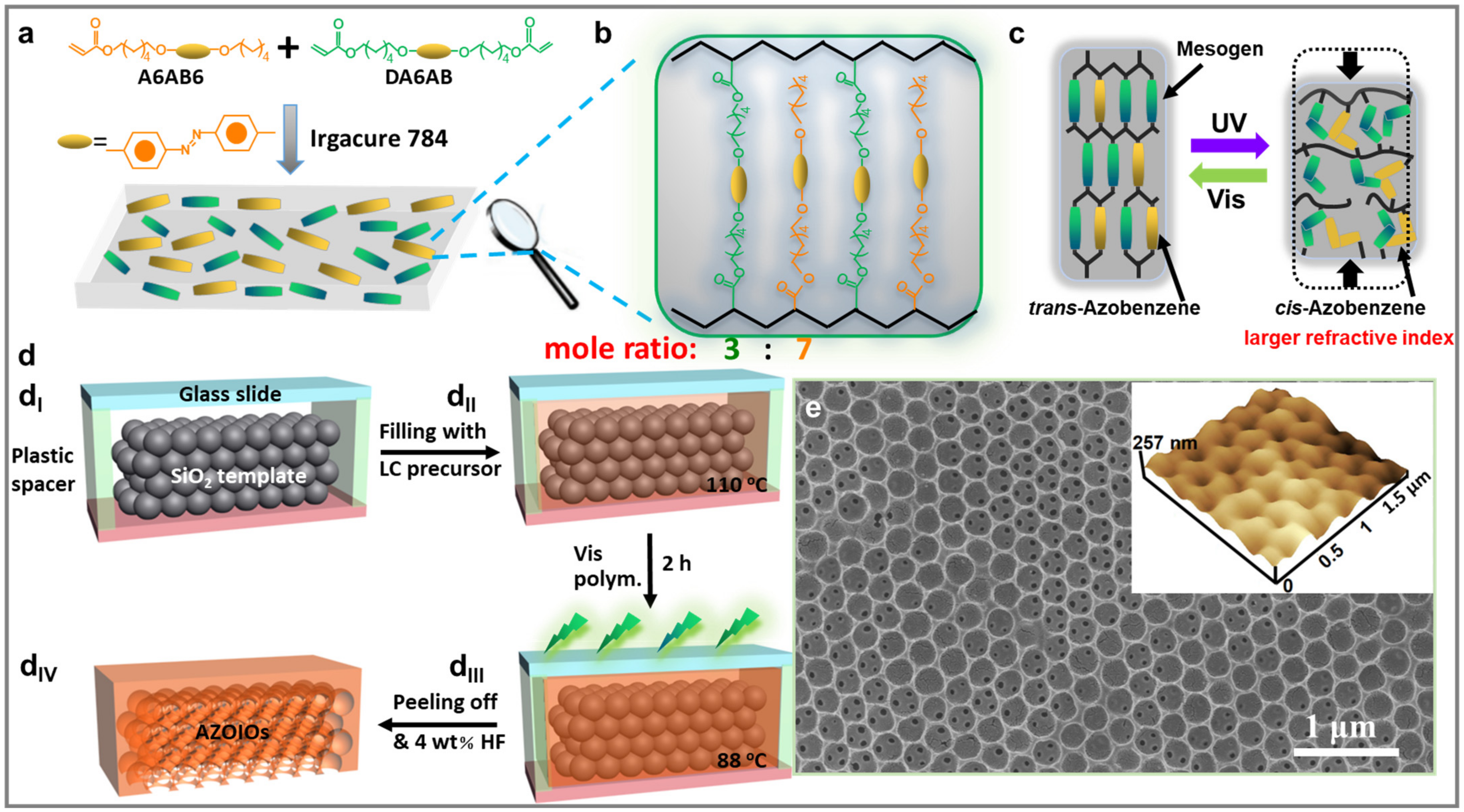
2.1. Fabrication Process of AZOIOs
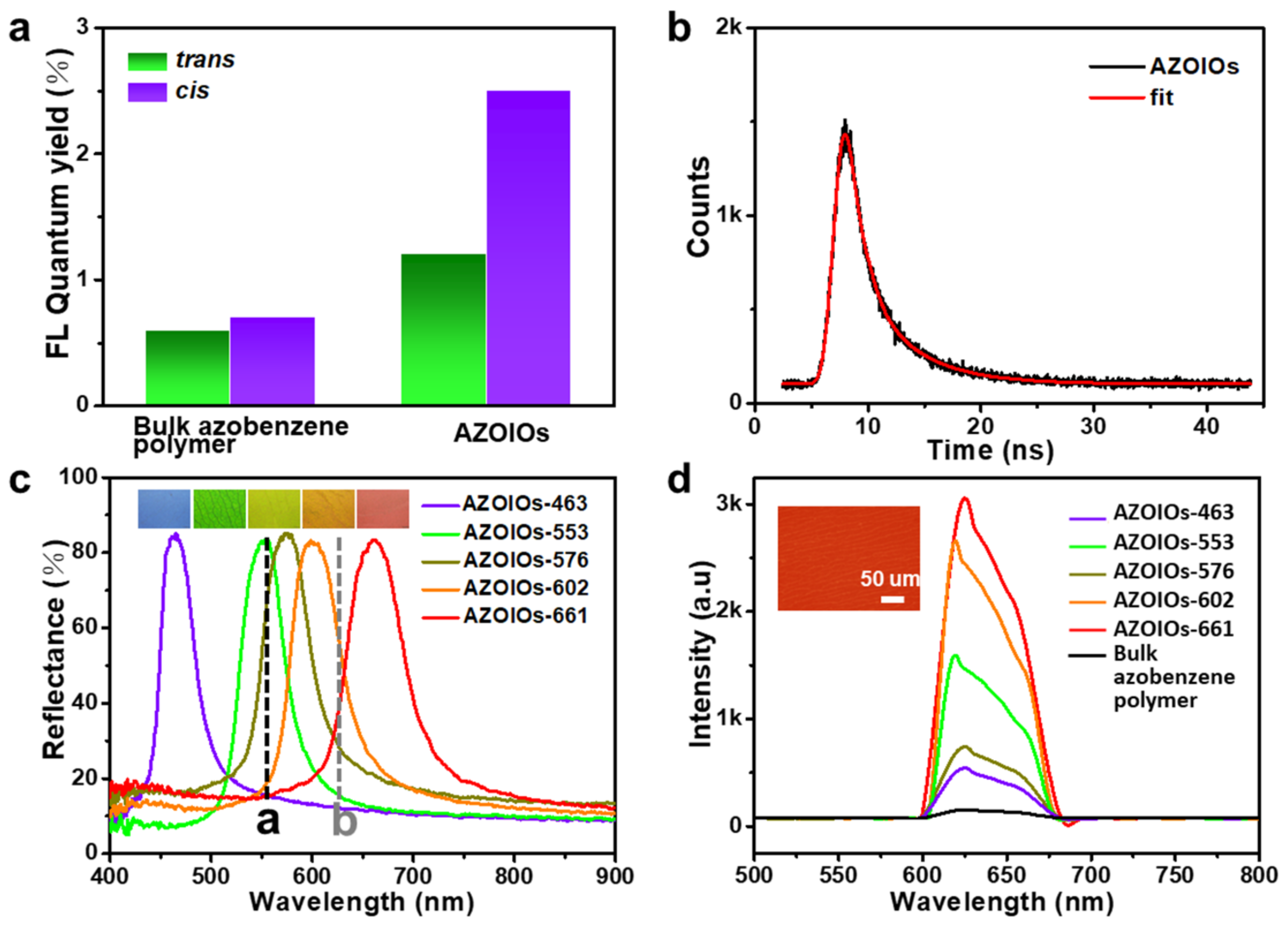
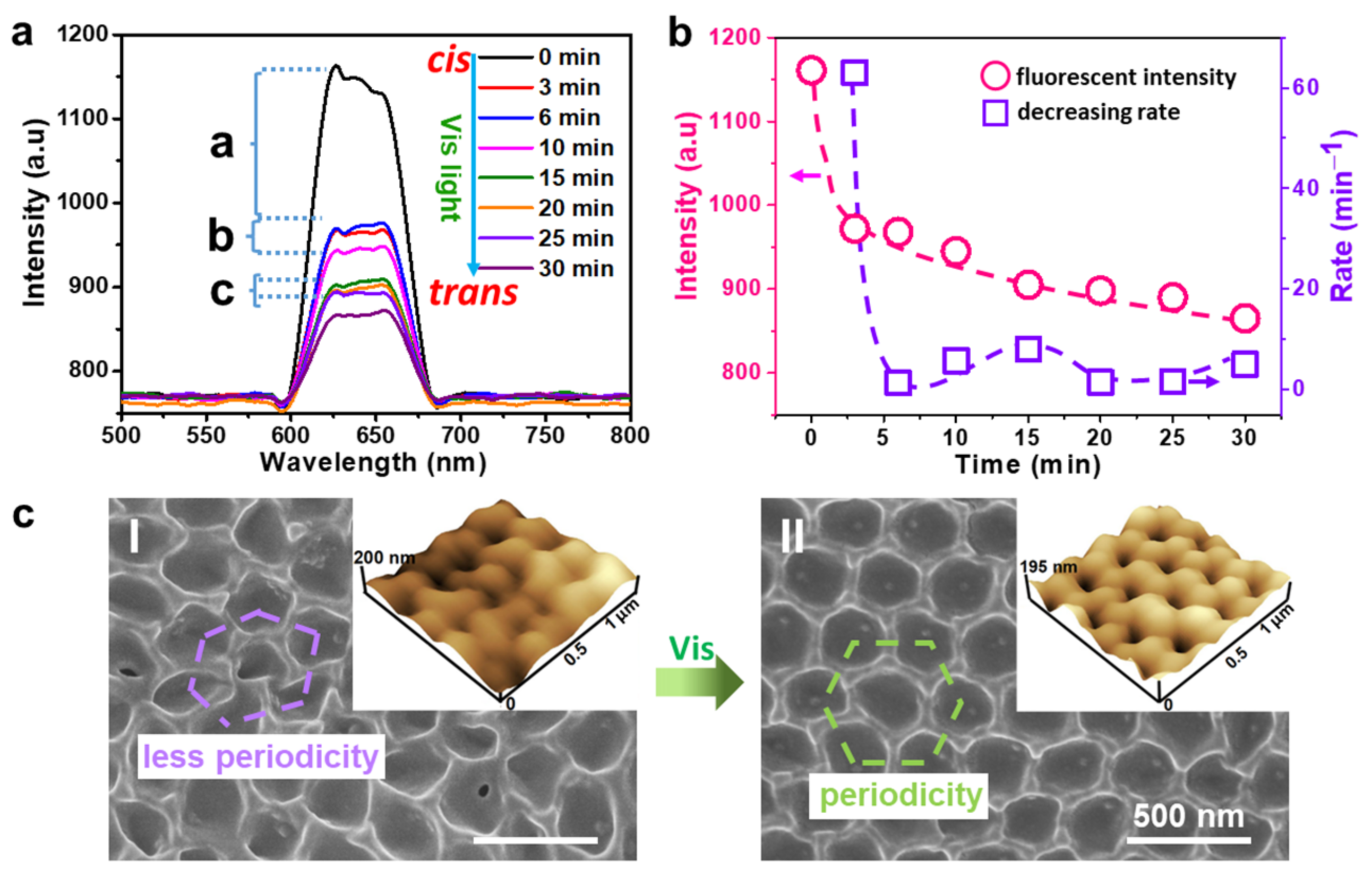
2.2. The Modulation Effect of AZOIOs’ Bandgap and Photo-Isomerization on Fluorescent Properties
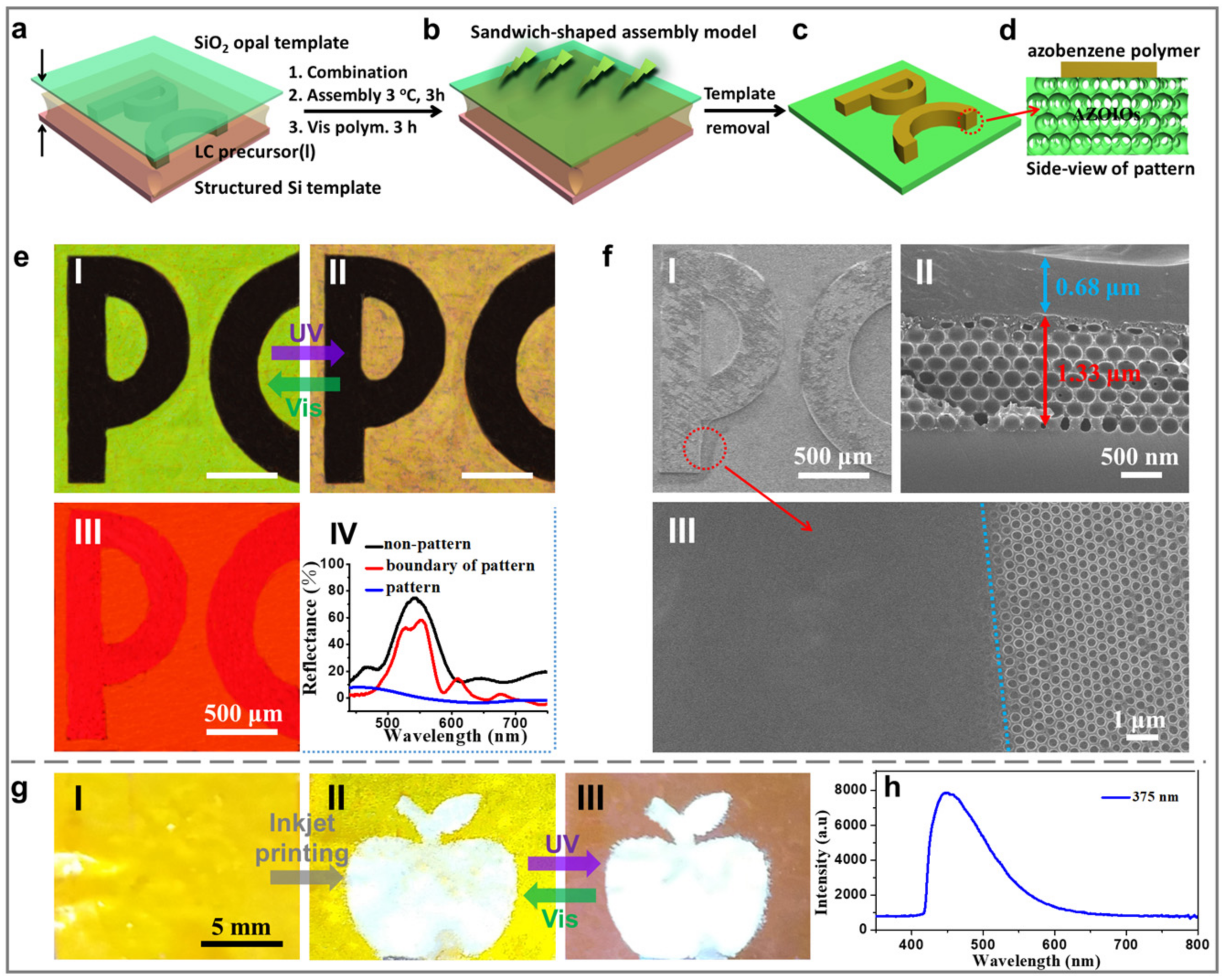
2.3. Applications in Anti-Fake Patterns
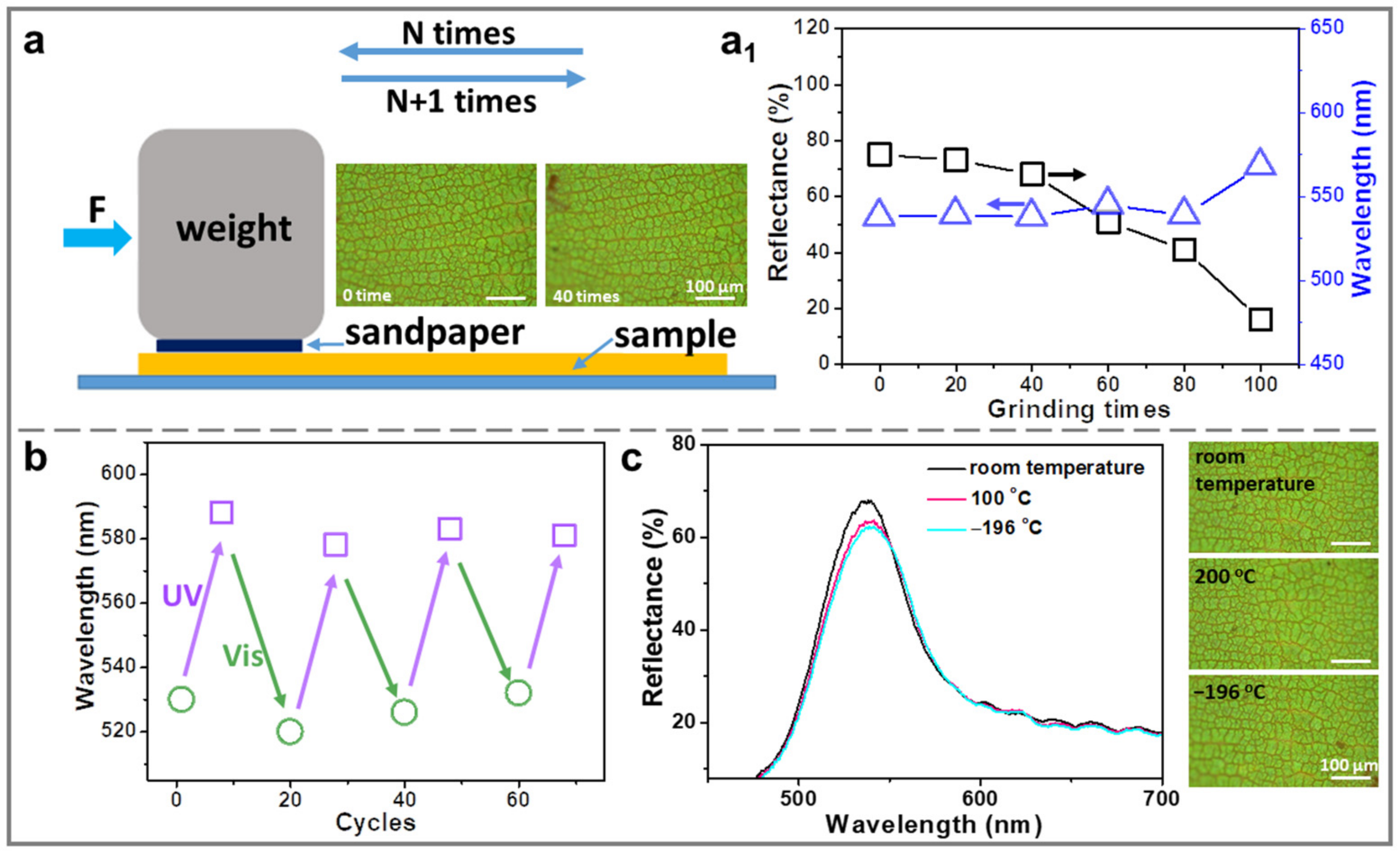
2.4. Stability Test of AZOIOs
2.5. Conclusions
3. Materials and Methods
3.1. Self-Assembly of SiO2 Opal Template
3.2. Fabrication of Azobenzene Monomer/Crosslinker
3.3. Fabrication of Azobenzene Inverse Opals
3.4. Groove-Structured Si Templates
3.5. Manufacturing of Anti-Fake Pattern by Structured Si Template
3.6. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zeng, Q.; Liu, S.; Lin, H.; Zhao, K.; Bai, X.; Zhao, K.; Hu, P.; Wang, B.; Donnio, B. Pyrene-fused poly-aromatic regioisomers: Synthesis, columnar mesomorphism, and optical properties. Molecules 2023, 28, 1721. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Ullah, S.; Khan, M.F.A.; Mustaqeem, M.; Paracha, R.N.; Rehman, M.F.U.; Kanwal, F.; ul Hassan, S.S.; Bungau, S. Fluorescent and phosphorescent nitrogen-containing heterocycles and crown ethers: Biological and pharmaceutical applications. Molecules 2022, 27, 6631. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Sun, J.; Ma, C.; Wan, S.; Li, Y.; Gostl, R.; Herrmann, A.; Liu, K.; Zhang, H. Engineered near-infrared fluorescent protein assemblies for robust bioimaging and therapeutic applications. Adv. Mater. 2020, 32, e2000964. [Google Scholar] [CrossRef] [PubMed]
- Zalmi, G.A.; Jadhav, R.W.; Mirgane, H.A.; Bhosale, S.V. Recent advances in aggregation-induced emission active materials for sensing of biologically important molecules and drug delivery system. Molecules 2022, 27, 150. [Google Scholar] [CrossRef]
- Burilov, V.; Fatykhova, A.; Mironova, D.; Sultanova, E.; Nugmanov, R.; Artemenko, A.; Volodina, A.; Daminova, A.; Evtugyn, V.; Solovieva, S.; et al. Oxyethylated fluoresceine-(thia)calix[4]arene conjugates: Synthesis and visible-light photoredox catalysis in water-organic media. Molecules 2023, 28, 261. [Google Scholar] [CrossRef]
- Choi, H.; Kim, M.; Jang, J.; Hong, S. Visible-light-induced cysteine-specific bioconjugation: Biocompatible thiol-ene click chemistry. Angew. Chem. Int. Ed. 2020, 59, 22514–22522. [Google Scholar] [CrossRef]
- Shimomura, M.; Kunitake, T. Fluorescence and photoisomerization of azobenzene-gontaining bilayer membranes. J. Am. Chem. Soc. 1987, 109, 5175–5183. [Google Scholar] [CrossRef]
- Bo, Q.; Zhao, Y. Fluorescence from an azobenzene-containing diblock copolymer micelle in solution. Langmuir 2007, 23, 5746–5751. [Google Scholar] [CrossRef]
- Cacucciolo, V.; Shintake, J.; Kuwajima, Y.; Maeda, S.; Floreano, D.; Shea, H. Stretchable pumps for soft machines. Nature 2019, 572, 516–519. [Google Scholar] [CrossRef]
- Huang, W.; Huang, X.; Majidi, C.; Jawed, M.K. Dynamic simulation of articulated soft robots. Nat. Commun. 2020, 11, 2233. [Google Scholar] [CrossRef]
- Wilson, M.; Sola, J.; Carlone, A.; Goldup, S.; Lebrasseur, N.; Leigh, D. An autonomous chemically fuelled small-molecule motor. Nature 2016, 534, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, L.; Zhou, Y.; Zhang, H. Fully room temperature reprogrammable, recyclable, and photomobile soft actuators from physically cross-linked main-chain azobenzene liquid crystalline polymers. Molecules 2023, 28, 4174. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hua, M.; Wu, S.; Du, Y.; Pei, X.; Zhu, X.; Zhou, F.; He, X. Bioinspired high-power-density strong contractile hydrogel by programmable elastic recoil. Sci. Adv. 2020, 6, eabd2520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, H.; Mao, J.; Chirarattananon, P.; Helbling, E.; Hyun, N.; Clarke, D.; Wood, R. Controlled flight of a microrobot powered by soft artificial muscles. Nature 2019, 575, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Goodnight, D.; Gao, Z.; Cavusoglu, A.; Sabharwal, N.; DeLay, M.; Driks, A.; Sahin, O. Scaling up nanoscale water-driven energy conversion into evaporation-driven engines and generators. Nat. Commun. 2015, 6, 7346–7353. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Shen, X.; Wu, P.; Schafer, C.; Guo, J.; Wang, C. Ultrafast assembly of nanoparticles to form smart polymeric photonic crystal films: A new platform for quick detection of solution compositions. Nanoscale 2019, 11, 1253–1261. [Google Scholar] [CrossRef]
- Juneja, S.; Zhang, B.; Nujhat, N.; Wang, A.X. Quantitative sensing of domoic acid from shellfish using biological photonic crystal enhanced SERS substrates. Molecules 2022, 27, 8364. [Google Scholar] [CrossRef]
- Guo, M.; Yu, X.; Zhao, J.; Wang, J.; Qing, R.; Liu, J.; Wu, X.; Zhu, L.; Chen, S. Versatile titanium dioxide inverse opal composite photonic hydrogel films towards multi-solvents chip sensors. Sens. Actuators B Chem. 2021, 347, 130639. [Google Scholar] [CrossRef]
- Li, H.; Fang, W.; Zhao, Z.; Li, A.; Li, Z.; Li, M.; Li, Q.; Feng, X.; Song, Y. Droplet precise self-splitting on patterned adhesive surfaces for simultaneous multidetection. Angew. Chem. Int. Ed. 2020, 59, 10535–10539. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Ikeda, T.; Jiang, L. Liquid-phase super photoactuator through the synergetic effects of a Janus structure and solvent/thermal/photo responses. Adv. Funct. Mater. 2021, 31, 2105728. [Google Scholar] [CrossRef]
- Liu, J.; Wan, L.; Zhang, M.; Jiang, K.; Song, K.; Wang, J.; Ikeda, T.; Jiang, L. Electrowetting-induced morphological evolution of metal-organic inverse opals toward a water-lithography approach. Adv. Funct. Mater. 2017, 27, 1605221. [Google Scholar] [CrossRef]
- Kim, H.; Lee, W.; Chang, T.; Huffaker, D. Room-temperature InGaAs nanowire array band-edge lasers on patterned silicon-on-insulator platforms. Phys. Status Solidi RRL 2018, 13, 1800489. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, F.; Jia, B.; Li, G.; Huang, X. Inverse design of photonic topological insulators with extra-wide bandgaps. Phys. Status Solidi RRL 2019, 13, 1900175. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, B.; Xie, J.; Liu, E.; Li, H.; Ge, R.; Gao, F.; Liu, J. Variation of topological edge states of 2D honeycomb lattice photonic crystals. Phys. Status Solidi RRL 2020, 14, 2000202. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Yu, Y. Dual-responsive inverse opal films based on a crosslinked liquid crystal polymer containing azobenzene. J. Mater. Chem. C 2014, 2, 10262–10267. [Google Scholar] [CrossRef]
- Hong, J.; Park, J.; Chun, C.; Kim, D. Photoinduced tuning of optical stop bands in azopolymer based inverse opal photonic crystals. Adv. Funct. Mater. 2007, 17, 2462–2469. [Google Scholar] [CrossRef]
- Ube, T.; Minagawa, K.; Ikeda, T. Interpenetrating polymer networks of liquid-crystalline azobenzene polymers and poly(dimethylsiloxane) as photomobile materials. Soft. Matter. 2017, 13, 5820–5823. [Google Scholar] [CrossRef]
- Ube, T.; Takado, K.; Ikeda, T. Photomobile materials with interpenetrating polymer networks composed of liquid-crystalline and amorphous polymers. J. Mater. Chem. C 2015, 3, 8006–8009. [Google Scholar] [CrossRef]
- Yu, Y.; Nakano, M.; Ikeda, T. Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Wang, J.; Zhou, G.; Ikeda, T.; Jiang, L. Inkless rewritable photonic crystals paper enabled by a light-driven azobenzene mesogen switch. ACS Appl. Mater. Interfaces 2021, 13, 12383–12392. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Freymann, G.; Choi, S.; Kitaev, V.; Ozin, G. Amplified photochemistry with slow photons. Adv. Mater. 2006, 18, 1915–1919. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Pan, Z.; Cui, L.; Xu, L.; Wang, R.; Song, Y.; Jiang, L. Amplifying fluorescence sensing based on inverse opal photonic crystal toward trace TNT detection. J. Mater. Chem. 2011, 21, 1730–1735. [Google Scholar] [CrossRef]
- Liu, J.; Ren, J.; Xie, Z.; Guan, B.; Wang, J.; Ikeda, T.; Jiang, L. Multi-functional organosilane-polymerized carbon dot inverse opals. Nanoscale 2018, 10, 4642–4649. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, C.; Cong, H.; Yang, Q.; Wu, Y.; Su, B.; Zhao, Y.; Wang, J.; Jiang, L. Hybrid top-down/bottom-up strategy using superwettability for the fabrication of patterned colloidal assembly. ACS Appl. Mater. Interfaces 2016, 8, 4985–4993. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Su, B.; Jiang, L. Interfacial material system exhibiting superwettability. Adv. Mater. 2014, 26, 6872–6897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Meng, F.; Feng, J.; Wang, J.; Wu, Y.; Jiang, L. Manipulation of colloidal particles in three dimensions via microfluid engineering. Adv. Mater. 2018, 30, 1707291. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Abrams, N.; Lewis, B.; Halaoui, L.; Mallouk, T.; Benkstein, K.; Lagemaat, J.; Frank, A. Standing wave enhancement of red absorbance and photocurrent in dye-sensitized titanium dioxide photoelectrodes coupled to photonic crystals. J. Am. Chem. Soc. 2003, 125, 6306–6310. [Google Scholar] [CrossRef]
- Mihi, A.; Míguez, H. Surface resonant modes in colloidal photonic crystals. Phys. Rev. B 2005, 71, 125131. [Google Scholar] [CrossRef]
- Li, G.; Cheng, R.; Cheng, H.; Yu, X.; Ling, L.; Wang, C.; Chen, S. Microfluidic synthesis of robust carbon dots-functionalized photonic crystals. Chem. Eng. J. 2021, 405, 126539. [Google Scholar] [CrossRef]
- Liu, J.; Shang, Y.; Zhang, D.; Xie, Z.; Hu, R.; Wang, J. Single-material solvent-sensitive fluorescent actuator from carbon dots inverse opals based on gradient dewetting. Chin. J. Poly. Sci. 2017, 35, 1043–1050. [Google Scholar] [CrossRef]
- Wu, P.; Wei, C.; Yang, W.; Lin, L.; Pei, W.; Wang, J.; Jiang, L. Rewritable PEDOT film based on water-writing and electroerasing. ACS Appl. Mater. Interfaces 2021, 13, 41220–41230. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, T.; Zhang, T.; Li, H.; Li, A.; Li, Z.; Lai, X.; Hou, X.; Wang, Y.; Shi, L.; et al. Facile full-color printing with a single transparent ink. Sci. Adv. 2021, 7, eabh1992. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, Z.; Shang, Y.; Ren, J.; Hu, R.; Guan, B.; Wang, J.; Ikeda, T.; Jiang, L. Lyophilic but nonwettable organosilane-polymerized carbon dots inverse opals with closed-cell structure. ACS Appl. Mater. Interfaces 2018, 10, 6701–6710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Han, Z.; Wu, P.; Shang, Y.; Chen, J.; Jia, P. Photochromic Azobenzene Inverse Opal Film toward Dynamic Anti-Fake Pattern. Molecules 2023, 28, 5881. https://doi.org/10.3390/molecules28155881
Liu J, Han Z, Wu P, Shang Y, Chen J, Jia P. Photochromic Azobenzene Inverse Opal Film toward Dynamic Anti-Fake Pattern. Molecules. 2023; 28(15):5881. https://doi.org/10.3390/molecules28155881
Chicago/Turabian StyleLiu, Junchao, Zhitong Han, Pingping Wu, Yuanyuan Shang, Jiansheng Chen, and Pan Jia. 2023. "Photochromic Azobenzene Inverse Opal Film toward Dynamic Anti-Fake Pattern" Molecules 28, no. 15: 5881. https://doi.org/10.3390/molecules28155881
APA StyleLiu, J., Han, Z., Wu, P., Shang, Y., Chen, J., & Jia, P. (2023). Photochromic Azobenzene Inverse Opal Film toward Dynamic Anti-Fake Pattern. Molecules, 28(15), 5881. https://doi.org/10.3390/molecules28155881







