Abstract
Perilla frutescens is an annual herb of the Labiatae family and is widely grown in several countries in Asia. Perilla frutescens is a plant that is used medicinally in its entirety, as seen in its subdivision into perilla seeds, perilla stalks, and perilla leaves, which vary more markedly in their chemical composition. Several studies have shown that Perilla frutescens has a variety of pharmacological effects, including anti-inflammatory, antibacterial, detoxifying, antioxidant, and hepatoprotective. In the absence of a review of Perilla frutescens for the treatment of cancer. This review provides an overview of the chemical composition and molecular mechanisms of Perilla frutescens for cancer treatment. It was found that the main active components of Perilla frutescens producing cancer therapeutic effects were perilla aldehyde (PAH), rosmarinic acid (Ros A), lignan, and isoestrogen (IK). In addition to these, extracts of the leaves and fruits of Perilla frutescens are also included. Among these, perilla seed oil (PSO) has a preventive effect against colorectal cancer due to the presence of omega-3 polyunsaturated fatty acids. This review also provides new ideas and thoughts for scientific innovation and clinical applications related to Perilla frutescens.
1. Introduction
Cancer is one of the world’s leading diseases in terms of mortality [1], the second leading cause of death, and a major public health problem worldwide [2,3]. As of 2019, prostate cancer, colorectal cancer, liver cancer, and lung cancer are the four types with the highest incidence and mortality rates in men, while breast cancer and cervical cancer are the most common among female cancer patients, according to related research studies [4]. The incidence and mortality of cancer in different regions are significantly different [1,3], which is closely related to the factors leading to cancer occurrence. There have been many literature reports on the risk factors for cancer, including diet, lifestyle, family genetic history, ionizing radiation, and other nine important categories [2,5]. In addition to the more common cancer treatment methods such as surgery, chemotherapy, and radiotherapy [6], the current effective cancer treatment methods also include gene therapy [7,8] and immunotherapy [9].
Based on the existence of drug resistance and toxicity, scientists are actively looking for herbs or plant metabolites that can have anticancer potential [10,11]. Numerous studies have shown that chemicals derived from plants can have preventive and therapeutic effects on cancer [12]. Flavonoids [13,14], natural phenolic compounds from herbs and dietary plants [15], polyphenols and their compositions [16,17], and dietary unsaturated fatty acids [18] all have preventive and anti-tumor effects. Bioactive compounds derived from plants, such as tanshinone, astragaloside, berberine, ginsenoside, and matrine, can inhibit tumor growth, metastasis, and invasion by regulating the expression of abnormal miRNA and further affect tumor progression, the microenvironment, and drug resistance related to various cancers [19,20,21].
Perilla frutescens is an annual herb of the Labiaceae family [22]. As a common traditional herb of the same origin as medicine and food, it is widely cultivated in China, Korea, Japan, Vietnam, and other countries [23]. As a medicinal herb, Perilla frutescens can be subdivided into perilla seed, perilla leaf, and perilla stem. The active components of these three parts were found to have some differences after study [24,25]. Active ingredients include alkaloids [26], phenylpropane [27], terpenoids [28], polyphenols [29], flavonoids [30], anthocyanins, coumarins, carotenoids, neolignans [31,32], fatty acids, tocopherols, phytosterols, glucosides, peptides, and other related ingredients [33]. Because of the diversity of its active ingredients, Perilla frutescens has a wide range of pharmacological effects. According to relevant studies on Perilla frutescens, its pharmacological effects mainly include insecticidal effects [34], anti-allergic effects [35,36], anti-depressant effects [37], liver protection effects [38], hair growth promotion effects [39], blood lipid lowering effects, neuroprotective effects [40], anti-inflammatory effects [26], antioxidant effects [41], anticancer effects [42], and anti-tumor and antibacterial effects [43]. This review mainly summarizes the anti-cancer effect of Perilla frutescens systematically and provides ideas for the treatment of various tumors. The specific process is shown in Figure 1.
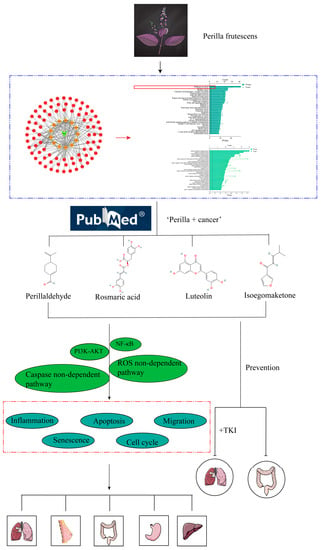
Figure 1.
Flowchart. The antitumor effect of Perilla frutescens was verified by web pharmacology analysis of Perilla frutescens. Literature search, review, and synthesis of the literature to summarize the mechanism of action and related signaling pathways of Perilla frutescens as well as active ingredients against tumors. In the red box are cancer-related pathways in the KEGG pathway.
2. Network Diagram of Anti-Tumor Effect of Perilla frutescens
In order to confirm the anti-tumor effect of Perilla frutescens, a network pharmacological analysis of Perilla frutescens was carried out based on its multi-component, multi-target, and multi-level properties. According to OD ≥ 0.3 and DL ≥ 0.18, 13 active components (see Supplementary File S1 for details) and 144 related proteins were screened from the TCMSP database (https://old.tcmsp-e.com/tcmsp.php (accessed on 11 May 2023)). The obtained protein name was entered in the Multiple Proteins section of the STRING database (https://cn.string-db.org (accessed on 12 May 2023)), and the Homo sapiens species was selected. Download the obtained gene name and match it with the protein name. Topological network maps of the 13 active components and 144 corresponding genes were constructed using Cytoscape software 3.9.1 (see Supplementary File S2 for details). In the figure, the plant name is the green module, the orange template is the active ingredient, and the red module is the corresponding gene. KEGG and GO analysis was performed on 144 target genes through the DAVID database (https://david.ncifcrf.gov (accessed on 12 May 2023)). The KEGG results showed that nine of the top 30 signaling pathways were associated with cancer. GO analysis showed that it was closely related to apoptosis, migration, proliferation, and other related processes (see Supplementary File S3 for details). It mainly includes a series of cancer-related signaling pathways, such as lung endometrial cancer, breast cancer, non-small cell lung cancer, pancreatic cancer, gallbladder cancer, and bladder cancer, as shown in Figure 2. These signaling pathways demonstrate that the relevant active components of Perilla frutescens can produce anti-tumor effects through these cancer-related pathways.
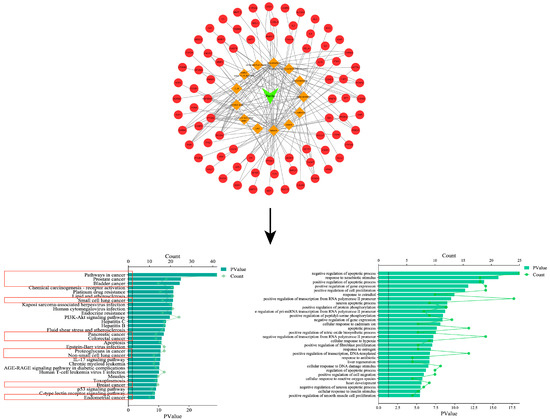
Figure 2.
Perilla-active composition target plot and GO and KEGG analyses. In the red box are the signaling pathways associated with various types of cancer in the KEGG enrichment analysis.
3. Active Ingredients
As an important annual herb in the Labiaceae family, Perilla frutescens has rich chemical constituents and biological functions and is widely used in food and medicine fields [44]. Related studies have found that the active components of Perilla frutescens can be divided into 14 main active components (see Figure 3). The detailed composition is shown in Table 1.
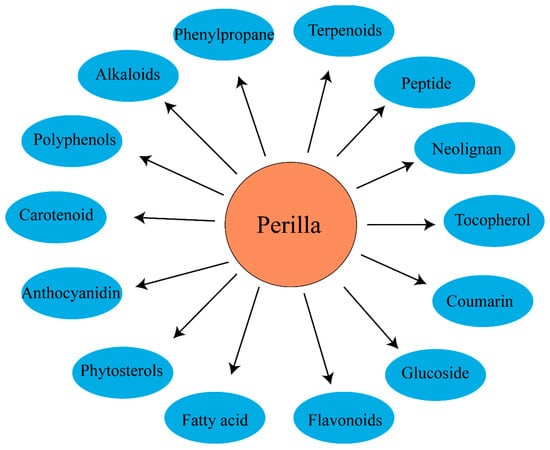
Figure 3.
The chemical classes of Perilla frutescens compounds.
3.1. Alkaloids
Alkaloids exist in a variety of traditional Chinese herbs; for example, hyoscypane alkaloids [45] are mainly found in Solanaceae, dibenzyl isoquinoline alkaloids [46] are mainly found in the seeds and fruits of lotus, and Evodia alkaloids are mainly found in Evodia officinalis of the rutaceae [47]. In recent years, the presence of the alkaloid compound neoechinulin A in Perilla frutescens has been found, which can produce anti-inflammatory effects on RAW267.4 cells stimulated by lipopolysaccharide.
3.2. Phenylpropane
Phenylpropane compounds are secondary metabolites of plants derived from phenylalanine, an aromatic amino acid in most plants, or tyrosine in some monocotyledon plants. Some studies have found that Perilla frutescens collected from Taiwan contained elemin, a phenylpropanoid component, while this component was not detected in Japanese Perilla frutescens [48]. Some studies have found that phenylpropanoid compounds can be separated from the ethanol extract of Perilla leaves, including allyl tetramethoxybenzene, elietin, and myristin, among which elietin and myristin have been found to inhibit the production of pro-inflammatory cytokines in pneumonia in a concentration-dependent manner within a certain concentration range [49].
3.3. Terpenoids
Terpenoids are the most common compounds in Perilla frutescens, of which the monoterpenoid PAH is the main component of Perilla leaf essential oil, which can improve the in vivo function of intestinal inflammation through JNK-mediated cytokine ajay [50]. At the same time, cytoplasmic DNA-induced innate immune responses can be inhibited by inhibiting cGAS activity [51]. The triterpenoid camelliol C [52] was identified from Perilla seed species, and a series of pentacyclic triterpenes were discovered, including ursolic acid [53], oleanolic acid, corosolic acid, 3surface acid, marlinic acid, and 3-surface equine linolenic acid [54], all of which have anti-tumor effects [55].
3.4. Polyphenol Compounds
Common polyphenols in Perilla frutescens are Ros A and caffeic acid, which have been proven to have various pharmacological activities, such as anti-inflammatory [49], anti-anxiety, anti-depressive [37], hepatoprotective [56], and anticancer [42].
3.5. Flavonoids
Perilla leaves are composed of many types of active ingredients, but mainly flavonoids. Studies have confirmed that it acts as an anti-inflammatory agent in vivo and in vitro in specific dermatitis models [57]. The most common compound of Perilla frutescens flavonoids is luteolin, which has been confirmed to have anti-inflammatory, anti-itch [58], anti-allergic [59], anti-cytotoxic [60], and antibacterial [43] activities.
3.6. Anthocyanins, Coumarins, Carotenoids, and Neolignans
Anthocyanin pigments are the main cause of red Perilla leaves [61]. Two new lignans identified in Perilla frutescens, magnosalin and andamanicin, can act as inhibitors of tumor necrosis factor and nitric oxide synthesis in RAW264.7 cells induced by lipopolysaccharide [32]. Aesculin, as a coumarin, was first discovered in Perilla frutescens and has been found to have a certain relationship with anti-inflammatory effects [30].
3.7. Fatty Acids, Tocopherols, and Phytosterols
PSO is rich in active ingredients and contains a large amount of unsaturated fatty acids, which are drug and food homologies [62]. The contents of five fatty acids in Perilla seeds were identified, including palmitic acid (PA) (10.9–13.1%), stearic acid (SA) (70.3–99.11%), oleic acid (OA) (1.21–9.10%), oleic acid (1.21–9.10%), linoleic acid (LA) (2.23–4.54%), and linolenic acid (LNA) (3.75–4.100%) [63]. Omega-3 polyunsaturated fatty acids are a general term that includes alpha-linolenic acid, which is abundant in perilla oil (PO) [64]. This omega-3 polyunsaturated fatty acid has been proven to be associated with anti-inflammatory [65] and lipid metabolism disorders [66]. The intake of such dietary fatty acids can improve the intestinal flora [67] as well as intestinal inflammation and metabolic disorders in diabetic patients [68]. Tocopherols and phytosterols are rich sources of health-promoting compounds in Perilla frutescens [69,70]. Unsaponifiable substances, including tocopherols and phytosterols, have antioxidant and health-promoting properties [71].
3.8. Glucoside and Peptide
Twelve secondary metabolites isolated from perillafrutoside A, perillafrutoside B, and ten other known compounds were found in perillafrutoside A, among which perillafrutoside A can inhibit the growth of Enterococcus faecalis [72]. Monoterpene glucosides, perillosides A and C, obtained from perilli leaves, have also been found to be aldose reductases. One of the richest sources of peptides from Perilla seeds, peptides obtained from Perilla seeds can improve muscle synthesis and motor performance in mice [73]. Two novel antioxidant peptides were purified and identified from Perilla seeds to inhibit lipid peroxidation in the liver [74].

Table 1.
Representative compounds of the main components of each class.
Table 1.
Representative compounds of the main components of each class.
| Active Ingredients | Species | References |
|---|---|---|
| Alkaloids | Neoechinulin A | [26] |
| Benzene propane | Eleuthero | [49] |
| Myricetin | [49] | |
| Eugenol | [75] | |
| Terpenoids | Perillone | [76] |
| Perillaldehyde | [77] | |
| Polyphenols | Rosmarinic acid | [78] |
| Flavonoids | Luteolin | [78] |
| Apigenin | [29] | |
| Isoestradiol | [27] | |
| Baicalin | [79] | |
| Anthocyanins | Malonylstilbene | [79] |
| Perillin | [80] | |
| Carotenoids | Lolliolactone | [81] |
| Isoxolactone | [81] | |
| Neolignan | Mullein | [82] |
| Gooseberry | [82] | |
| Coumarins | Heptazine | [30] |
| 6,7-Dihydroxycoumarin | [83] | |
| Fatty acids | Lauric acid | [84] |
| Palm oleic acid | [85] | |
| Tocopherol | Delta-tocopherol | [86] |
| Gamma tocopherol | [86] | |
| Beta tocopherol | [86] | |
| Alpha tocopherol | [86] | |
| Glucoside | Perilla lactone A | [81] |
| Perillolactone B | [81] | |
| Loganin | [87] | |
| Phytosterols | Vegetable oil sterols | [86] |
| Soysterol | [86] | |
| beta-Sitosterol | [86] |
4. Anti-Cancer Compound Structural Formula
Related studies have found that Perilla frutescens mainly generates anti-tumor activity against liver cancer, lung cancer, and breast cancer through a series of related mechanisms such as PAH [88], IK [89], luteolin [90], Ros A [91], ethanol extract of Perilla leaves [92], Perilla extract [93], PO [94], etc. The structural formula of related compounds is shown in Figure 4.
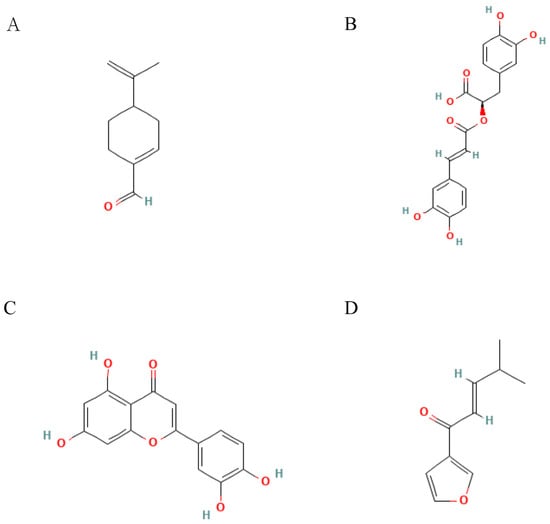
Figure 4.
Structural formulae of representative compounds: (A) Perillaldehyde, (B) Rosmaric acid, (C) Luteolin, and (D) Isoegomaketone.
5. Anti-Cancer Effect
Inducing cell apoptosis, blocking the cell cycle, reducing cell inflammation and oxidative stress, inhibiting cell metastasis, growth, and proliferation, and inducing cell senescence are the main pathways and phenotypes of the anti-tumor effects of Perilla frutescens, as shown in Table 2.
5.1. Cell Transfer
Metastases are a hallmark of cancer and cause the largest number of cancer-related deaths [95]. Epithelial-mesenchymal transformation (EMT) is a process by which epithelial cells acquire mesenchymal characteristics. In cancer, EMT is associated with tumor occurrence, invasion, metastasis, and treatment resistance [96]. Relevant studies have shown that isoproterenol (ISO) increases the migration and invasion of MDA-MB-231 human breast cancer cells and Hep3B human hepatocyte cancer cells [97]. The ethanol extract of Perilla leaf can reverse cancer cell metastasis induced by adrenergic agonists through the SRCT231F-mediated EMT pathway [92]. PAH is one of the active components of Perilla frutescens, which can affect prostate cancer-induced bone metastasis by inhibiting the NF-κB pathway [98].
5.2. Apoptosis
Apoptosis is an orderly and coordinated cellular process that occurs under physiological and pathological conditions. The mechanism of apoptosis is complex and involves many pathways. Defects can occur at any point in these pathways, leading to malignant transformation of affected cells, tumor metastasis, and anti-cancer drug resistance [99]. Ethanol extract of Perilla leaf can inhibit the growth of HCT116 and H1299 cells in a dose-dependent manner, inhibit cell colony formation, increase the G1 cell population, change nuclear morphology, and induce cell apoptosis [100]. The YAP/WW domain contains transcription factors (TAZ) that are critical for cell proliferation, survival, and self-renewal. It has also been shown to have an important carcinogenic effect on various tumors [101]. Perilla leaf extract (PLE) can induce phosphorylation of YAP/TAZ, resulting in its inactivation, and thus produce anti-tumor effects. The results suggest that PLE inhibits cell growth and increases apoptosis in breast cancer (BC) cells by inactivating YAP activity in a LATS1/2-dependent manner [102]. In the treatment of melanoma cells, IK can produce ROS, up-regulate the expression of Bax and Bcl-2, inhibit the growth of melanoma cells, and induce apoptosis.
5.3. Cell Cycle
Abnormal cell cycle progression is one of the basic mechanisms of tumorigenesis [103]. The Perilla frutescens derivative 8-hydroxy-5,7-dimethoxyflavanone (PDMF) can induce the phosphorylation of p15 and increase the expression of p21, caspase-3, and caspase-9. PDMF can trigger G2/M cell cycle arrest and apoptosis driven by p53 [104].
5.4. Cell Senescence
In most species, aging may induce a number of degenerative diseases characterized by a debilitating loss of tissue or cell function [105]. However, for the aging of cancer cells, its basic feature is stable proliferation arrest induced by various stressors [106]. PDMF can induce senescence in A549 human adenocarcinoma cells through the p21-p549 pathway but has no effect on normal bronchial epithelial cells [107].
5.5. Oxidative Stress Response and Cellular Inflammation
Tumor necrosis factor TNF-α is a major inflammatory cytokine that is particularly important in the development of tumors [108]. Endothelial microparticles are important factors in inflammation-related diseases. Studies have found that phenolic compounds contained in ethyl acetate and ethanol extracts extracted from Perilla fruit can reduce endothelial microparticles induced by TNF-α, thereby protecting endothelial cells from vascular inflammation [109]. Perilla extract can improve colitis induced by sodium dextran sulfate (DSS) in mice by inhibiting the expression of inflammation-related proteins such as COX-1. NF-κB and STAT3 are major transcriptional regulators of inflammatory signaling. Perilla extract inhibits DDS-induced NF-κB and STAT3, thereby reducing pro-inflammatory signaling [93]. Oxidative stress is a state caused by disruption of the balance between ROS production and antioxidant defense [110]. The Ros A component in PSO can reduce the production of ROS in the A549 cell line and the mRNA levels of related IL-6, IL-8, COX-2, etc., resulting in decreased expression of TNF-α induced NF-κB, JNK, MnSOD, and FOXO1 signaling pathways [91]. The ethyl acetate and ethanol extracts of Perilla frutescens can inhibit the production of ROS and have a protective effect on lipid peroxidation, indicating their potential to protect against oxidative stress in liver diseases [111].
5.6. Cell Growth
Cell growth is one of the key markers in cancer. Amp-activated protein kinase (AMPK) is associated with autophagy in unused tissues. PAH can activate AMPK by increasing phosphorylation at THr172, resulting in the increase of AMPK-related proteins such as caspase-3 and p53, resulting in increased autophagy levels and inhibiting the growth of gastric cancer [112].
5.7. Cell Proliferation
The abnormal proliferation of cancer cells is an important sign of a tumor and also an important reason for the expansion of cancer cells’ colonies. The PI3K/AKT signaling pathway is one of the frequently activated signaling pathways in the process of cancer, which is closely related to the occurrence and development of tumors. Studies have shown that IK isolated from Perilla extract can inhibit liver cancer (HCC) tumor proliferation by inhibiting pAKT levels without affecting total AKT levels and blocking the PI3K/AKT pathway [113].

Table 2.
Antitumor effects of Perilla frutescens and its derivatives.
Table 2.
Antitumor effects of Perilla frutescens and its derivatives.
| Type of Drug | Type of Cancer | Model | IC50 or Dose | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Perilla frutescens leaf extract | Colon cancer | HCT116 human colon cancer cells | Dose: 87.5–350 μg/mL | Inhibits the growth, colony formation, and adhesion of human colon and lung cancer cells and the migration of human lung cancer cells. | [100] |
| Lung cancer | H1299 human non-small cell lung cancer cells | Dose: 87.5–350 μg/mL | |||
| Perilla frutescens leaf extract | Triple negative breast cancer | HEK293A, MDA-MB-231, MCF10A and BT549 cells | HEK293A IC50: 584.3 μg/mL MDA-MB-231 IC50: 268.9 μg/mL MCF10A IC50: 650.8 μg/mL BT549 IC50: 307.1 μg/mL | Increased YAP phosphorylation and reduced YAP-TEAD-mediated transcriptional activity. | [102] |
| IK | Prostate cancer | RC-58T/h/SA#4 cells | Dose: 10–200 ng/mL | Enhancement of tumor necrosis factor-related apoptosis-inducing ligands (TRAIL)-mediated apoptosis through upregulation of DR5 by an ROS-independent pathway. | [89] |
| Perillaldehyde | Prostate cancer | RAW264.7 and PC-3 cells | Dose: 0.5–5 μM | Activation of the NF-κB pathway of nuclear factor-κB ligands and receptor activators to inhibit cancer cell-induced osteoclast formation. | [98] |
| IK | Liver cancer | Huh-7 and Hep3B cells and nude mouse models of hepatocellular carcinoma | Dose: 10 nmol/L | Significantly inhibited cell viability and xenograft tumor formation in HCC cells and inhibited AKT phosphorylation, but not AKT and p38 expression. | [113] |
| Perillaldehyde | Stomach cancer | MFC murine-derived cells and GC9811-P human gastric cancer cells | Dose: 0.1–5 mM | PAH activates AMPK by increasing Thr172 phosphorylation and activity; PAH increases the expression of beclin-1, LC3-II, caspase-3, and p53. | [112] |
| PSO and Ros A | Lung cancer | A549 lung adenocarcinoma cells | Dose PSO: 0–400 μg/mL Dsoe Ros A: 0–40 μg/mL | PSO and Ros A scavenge TNF-α induced ROS levels, resulting in reduced expression of MnSOD, FOXO1, NF-κB, and JNK signaling pathways. | [91] |
| PO | Breast cancer, colon cancer | Female SD rats | Dose: 10%PO | Alpha-linolenic acid-rich PO diet inhibits the development of breast, colon, and kidney tumors. | [114] |
| PDMF | Lung cancer | Human lung adenocarcinoma A549 cells | Dose: 30–75 μg/mL | Triggering p53-driven G2/M cell cycle arrest and apoptosis. | [104] |
| PDMF | Lung cancer | A549 human lung adenocarcinoma cells | Activation of the p21-p549 pathway in A53 cells; p53 is particularly important for cellular senescence | [107] | |
| Ethanolic extract of Perilla frutescens (EPF) | Liver cancer | Human hepatocellular carcinoma HuH7 cells | IC50: 3.43 mg/mL | Protective effect of ethanol extract on the production of reactive oxygen species and lipid peroxidation in FeCl3–induction of HuH7 cells in a dose-dependent manner | [111] |
| Perilla frutescens leaf extract | Skin tumors | - | Dose: 0.05% | Significant reduction in tumor incidence and diversity. | [115] |
| IK | Melanoma | B16 melanoma cells | Dose: 10–100 μM | IK-induced apoptosis involves the production of ROS and the upregulation of Bax and Bcl-2 expression, leading to the release of cytochrome c and AIF. IK inhibits melanoma cell growth and induces apoptosis through the activation of ROS-mediated cysteinase-dependent and non-dependent pathways. | [116] |
| Perilla extract | Liver cancer | Human hepatocellular carcinoma HepG2 cells | Dose: 105 μg/mL | Expression of a large number of apoptosis-related genes is regulated in a time-dependent manner. | [42] |
| Perilla extract | Skin cancer | Two-stage skin carcinogenesis model in mice | Dose: 2.0 mg/mice | Part of the anti-cancer effect of perilla extract is due to RA through two separate mechanisms: inhibition of the inflammatory response and scavenging of reactive oxygen radicals. | [117] |
| PO | Liver cancer | Diethylnitrosamine (DEN)-induced hepatocellular carcinoma in male F344 rats | Dose: 5% | PO enriched with n-6 and n-3 PUFA altered the membrane fatty acid composition of the liver and inhibited the development of hepatocellular carcinoma in rats. | [118] |
| Luteolin | Colon cancer | HT-29 human colon cancer cells | Dose: 0–60 μmol/L | By activating caspase-3, -7, and -9, the cleavage of poly (ADP-ribose) polymerase was enhanced, the expressions of p21 (CIP1/WAF1), survivin, Mcl-1, Bcl-x(L), and Mdm-2 were decreased, and the activities of cyclin-dependent kinase (CDK)4 and CDK2 were inhibited. | [90] |
| PO | Breast cancer | PhIP-induced mammary carcinogenesis model in rats | Dose: 0.1% | CFA-P may retard the development of PhIP-induced breast tumors, inhibit the formation of PhIP-DNA adducts, and reduce breast carcinogenesis in the context of post-initiation inhibition of cell proliferation. | [119] |
| IK | Colon cancer | DLD1 colon cancer cells | Dose: 10–100 μM | IK treatment led to the cleavage of caspases-3, -8, and -9 in a dose- and time-dependent manner. IK treatment also led to cleavage of Bid and translocation of Bax. IK induced apoptosis via cystathione-dependent and caspase-non-dependent pathways in DLD1 cells. | [120] |
| IK | Melanoma | SK-MEL-2 human melanoma Cells | Dose: 100 μM | IK-induced ROS production regulated cell growth inhibition and induced apoptosis through cysteinase-dependent and non-independent pathways by modulating PI2K/AKT signaling in SK-MEL-3 cells. Reduced protein levels of Bax and cytochrome c as well as PARP cleavage, while protein levels of Bcl-2 were increased. | [121] |
| Ros A | Liver cancer | Hep G1 human liver cancer cells | IC50: 50 μM | Ros A dose-dependently attenuated aflatoxin- and hectoroxin-induced ROS production and inhibition of DNA and protein synthesis. Similarly, prevention of apoptosis by reduction of DNA fragmentation and inhibition of cysteinase-3 activation. | [122] |
| EPF | Liver cancer | MDA-MB-231 human breast cancer cells | Dose: 2.5–10 μg/mL | EPF inhibits the ability of adrenergic agonists to promote cancer cell metastasis by inhibiting Src-mediated EMT. | [92] |
| Breast cancer | Hep3B human hepatocellular carcinoma cells | Dose: 25–100 μg/mL |
6. Summary of Anticancer Mechanism
Perilla frutescens and its active components or derivatives mainly produce anti-tumor effects on cell growth, proliferation, inflammation, cycle, apoptosis, and metastasis through ROS, NF-κB, PI3K/AKT, JNK, and other pathways, as shown in Figure 5.
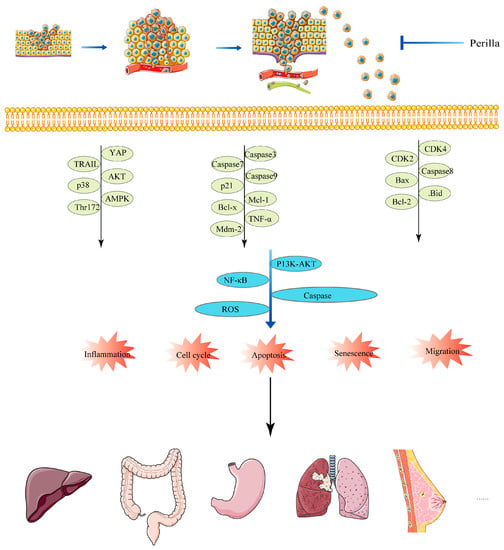
Figure 5.
Anticancer mechanism of Perilla frutescens.
7. Preventative Effects
Relevant studies have shown that unsaturated fatty acids can be used as adjuvant therapeutic agents in cancer treatment [123]. Omega-3 polyunsaturated fatty acids (PUFAs) are considered immune nutrients and are commonly used in the nutritional treatment of cancer patients due to their rich biological effects [124]. The intake of such dietary oils is particularly important for human health. PO is a complex of unsaturated fatty acids from Perilla. PO is rich in the omega-3 polyunsaturated fatty acid alpha-linolenic acid, which can effectively reduce the risk of colon cancer [125,126] Table 3.

Table 3.
The preventive effect of perilla and its derivatives on tumors.
Table 3.
The preventive effect of perilla and its derivatives on tumors.
| Composition | Cancer | Models | IC50 or Dose | Conclusion | Mechanisms | References |
|---|---|---|---|---|---|---|
| PDMF | Lung cancer | A549 human adenocarcinoma of the lung | Dose: 10–125 μM | PDMF and anti-cancer tyrosine kinase inhibitors (TKI) synergistically inhibit the proliferation of A549 cells. | Synergy | [127] |
| PO | Colon cancer | Female F3 rats | Dose: 9%, 32%, 40%. | The relatively small amount of PO, accounting for 25% of total dietary fat, may have a significant beneficial effect in reducing the risk of colon cancer. | Preventive role | [125] |
| PO | Colon Cancer | Male F344 rats | Dose: 3%, 6%, 12% | PO significantly reduced ras expression and AgNORs count (a biomarker of cell proliferation) in colonic mucosa. A significant increase in n-3 polyunsaturated fatty acids in the membrane phospholipid fraction and a decrease in PGE2 levels were observed in the colonic mucosa of rats fed with PO. | Preventive role | [94] |
| PO | Colon cancer | Male F344 rats | Dose: 3%, 12% | β-Carotene plus PO also inhibited the number of silver-stained nucleolar organizer regions and the expression of ras mRNA (a biomarker of cell proliferation) in the colonic mucosa. | Synergy, preventive role | [128] |
| PO | Colon cancer | Male F20 rats | Dose: 10%, 20% | Dietary PO significantly inhibits the development of small bowel and colon tumors in APC (min) mice. | Preventive role | [129] |
8. Summary and Outlook
Cancer is a kind of malignant disease that is difficult to treat, not only because of its diversification in proliferation and metastasis but also because cancer cells have strong adaptability [130]. Chinese herbs are an effective source of adjuvant cancer treatment and have been found to treat or prevent cancer in a variety of ways. Relevant studies have shown that various plant extracts and plant active ingredients can activate various pathways in cancer cells, including apoptosis [131]. Phenolic compounds extracted from herbs can inhibit or weaken the occurrence, progression, and metastasis of cancer [132]. Artemisinin and its derivatives have the therapeutic potential to induce iron death in cancer cells [133]. Many studies have shown that plants such as garlic, olives, and pomegranates are effective in preventing colon cancer [134].
Prior to writing this review, relevant information was consulted. Related reviews were searched in PubMed with the keywords “Perilla frutescens,” “Perilla frutescens and caner,” “Perilla frutescens and carcinoma,” “Perilla frutescens frutescens,” and “Perilla frutescens and tumor.” Few reviews were found in the last 5 years, and none were related to cancer treatment. Among the relevant reviews that have been reviewed, there are three related to the pharmacological and phytochemical effects of Perilla frutescens, which mainly summarize the phytomedicinal, ethnobotanical, phytochemical, and pharmacological effects of Perilla frutescens [24,135,136]. Three reviews were related to the active components of Perilla frutescens, namely, perillone and IK [137], Ros A [138], and PAH [88]. The review of Ros A gives an overview of its anti-cancer potential, but Ros A is derived from a variety of herbs, including rosemary and Perilla frutescens.
In this review, the active components of Perilla frutescens were summarized according to the relevant literature and the reading summary of the literature. The anticancer effects of related targets were demonstrated by biogenic analysis. The molecular mechanism and preventive effect of the components of Perilla frutescens with anticancer activity, including PAH, Ros A, luteolin, PO, etc. This review also gave a general description of Perilla frutescens’s treatment of cancer-related phenotypes, such as cell proliferation, cell metastasis, cell cycle, etc. It was found that Perilla frutescens mainly targeted the cellular inflammation and oxidative stress responses of cancer cells as the main targets to produce anticancer activity. In the process of writing the review, it was found that there were no relevant studies on the attenuated and synergistic effects of Perilla frutescens as an adjuvant therapy for cancer. Perilla frutescens contains many kinds of effective components, but there are few anticancer studies on PAH, PSO, perillone, and so on, which are unique to Perilla frutescens, and most studies focus on Ros A and IK.
Through the literature review, it was found that Perilla frutescens has a unique active ingredient, PO, which can prevent cancer. Through the systematic summary of PO, it was found that it is rich in omega-3 polyunsaturated fatty acids, which can have a positive effect on human health and prevent the occurrence of some diseases. Starting from this basis, according to these properties of PO, health care products and drugs can be developed to improve the quality of life, prevent major diseases, and make significant contributions to human health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28155883/s1. Supplementary File S1: The 13 active components of Perilla frutescens and their corresponding OB and DL values. Supplementary File S2: The protein and gene names corresponding to 13 active components of Perilla frutescens. Supplementary File S3: is titled KEGG and GO enrichment analysis results.
Author Contributions
Conceptualization, L.Y. and Y.N.; Writing—Original Draft Preparation, S.H.; Writing—Review and Editing, G.C. and N.N.; Translation, Y.D. and D.L.; Construction, Y.Y.; Tabulation, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by Ningxia Natural Science Foundation (project no. 2022AAC02039; 2023AAC03175; 2023AAC03222), “Young Scholars of Western China” (Class A) West Light Foundation of the Chinese Academy of Sciences (project no. XAB2019AW13) and Ningxia Key Research and Development Program (project no. 2023BEG02015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this paper, and further inquiries can be directed to the corresponding author (E-mail: 20080017@nxmu.edu.cn).
Acknowledgments
The authors acknowledge any support given which is not covered by the author contribution or funding sections.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yusefi, A.R.; Bagheri Lankarani, K.; Bastani, P.; Radinmanesh, M.; Kavosi, Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac. J. Cancer Prev. 2018, 19, 591–603. [Google Scholar] [CrossRef]
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.; Burmester, J.K. Gene therapy for cancer treatment: Past, present and future. Clin. Med. Res. 2006, 4, 218–227. [Google Scholar] [CrossRef]
- Zaimy, M.A.; Saffarzadeh, N.; Mohammadi, A.; Pourghadamyari, H.; Izadi, P.; Sarli, A.; Moghaddam, L.K.; Paschepari, S.R.; Azizi, H.; Torkamandi, S.; et al. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017, 24, 233–243. [Google Scholar] [CrossRef]
- Leibovici, J.; Itzhaki, O.; Huszar, M.; Sinai, J. Targeting the tumor microenvironment by immunotherapy: Part 2. Immunotherapy 2011, 3, 1385–1408. [Google Scholar] [CrossRef]
- Fatma, H.; Siddique, H.R. Research and Patents Status of Selected Phytochemicals Against Cancer: How Close and How Far? Recent Pat. Anticancer Drug Discov. 2023, 18, 428–447. [Google Scholar] [CrossRef]
- Shin, S.A.; Moon, S.Y.; Kim, W.Y.; Paek, S.M.; Park, H.H.; Lee, C.S. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Farooq, M.; Haseeb, M.; Choi, S. Role of Plant-Derived Active Constituents in Cancer Treatment and Their Mechanisms of Action. Cells 2022, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar] [PubMed]
- Mohi-Ud-Din, R.; Mir, R.H.; Sabreen, S.; Jan, R.; Pottoo, F.H.; Singh, I.P. Recent Insights into Therapeutic Potential of Plant-Derived Flavonoids against Cancer. Anticancer Agents Med. Chem. 2022, 22, 3343–3369. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Abel, S.; Riedel, S.; Gelderblom, W.C. Dietary PUFA and cancer. Proc. Nutr. Soc. 2014, 73, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of plant-derived bioactive compounds and related microRNAs in cancer therapy. Phytother. Res. 2021, 35, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Arora, S.; Averett, C.; Singh, S.; Singh, A.P. Modulation of microRNAs by phytochemicals in cancer: Underlying mechanisms and translational significance. Biomed. Res. Int. 2015, 2015, 848710. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Bhatt, K.C. Diversity distribution and collection of genetic resources of cultivated and weedy type in Perilla frutescens (L.) Britton var. frutescens and their uses in Indian Himalaya. Genet. Resour. Crop Evol. 2008, 55, 883–892. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol. 2017, 108, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef]
- Wang, P.; Jin, B.; Lian, C.; Guo, K.; Ma, C. Comparative Analysis of Polycyclic Aromatic Hydrocarbons and Halogenated Polycyclic Aromatic Hydrocarbons in Different Parts of Perilla frutescens (L.) Britt. Molecules 2022, 27, 3133. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, H.; Jiang, K.; Wang, Q.Q.; Zheng, Y.H.; Tang, W.; Tan, C.H. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells. Fitoterapia 2018, 130, 61–65. [Google Scholar] [CrossRef]
- Park, Y.D.; Jin, C.H.; Choi, D.S.; Byun, M.W.; Jeong, I.Y. Biological evaluation of isoegomaketone isolated from Perilla frutescens and its synthetic derivatives as anti-inflammatory agents. Arch. Pharm. Res. 2011, 34, 1277–1282. [Google Scholar] [CrossRef]
- Nam, B.; So, Y.; Kim, H.Y.; Kim, J.B.; Jin, C.H.; Han, A.R. A New Monoterpene from the Leaves of a Radiation Mutant Cultivar of Perilla frutescens var. crispa with Inhibitory Activity on LPS-Induced NO Production. Molecules 2017, 22, 1471. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, J.H.; Lee, M.H.; Lee, B.W.; Kwon, H.S.; Park, C.H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Yamamoto, Y.; Yoshinaka, N.; Namba, M.; Matsuo, H.; Okuyama, T.; Yoshigai, E.; Okumura, T.; Nishizawa, M.; Ikeya, Y. A new flavanone and other flavonoids from green perilla leaf extract inhibit nitric oxide production in interleukin 1β-treated hepatocytes. Biosci. Biotechnol. Biochem. 2015, 79, 138–146. [Google Scholar] [CrossRef] [PubMed]
- He, Y.K.; Yao, Y.Y.; Chang, Y.N. Characterization of Anthocyanins in Perilla frutescens var. acuta Extract by Advanced UPLC-ESI-IT-TOF-MSn Method and Their Anticancer Bioactivity. Molecules 2015, 20, 9155–9169. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Son, H.J.; Lee, S.H.; Sohn, D.H. Two neolignans from Perilla frutescens and their inhibition of nitric oxide synthase and tumor necrosis factor-alpha expression in murine macrophage cell line RAW 264.7. Bioorg. Med. Chem. Lett. 2002, 12, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ohira, K.; Miyatake, K.; Nakano, Y.; Nakayama, M. Inhibitory effects of perillosides A and C, and related monoterpene glucosides on aldose reductase and their structure-activity relationships. Chem. Pharm. Bull. 1995, 43, 920–926. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Yang, K.; Wu, Y.; Zhang, W.J.; Wang, Y.; Geng, Z.F.; Chen, H.P.; Jiang, H.Y.; Du, S.S.; Deng, Z.W.; et al. Chemical composition and insecticidal activities of the essential oil of Perilla frutescens (L.) Britt. aerial parts against two stored product insects. Eur. Food Res. Technol. 2014, 239, 481–490. [Google Scholar] [CrossRef]
- Oh, H.A.; Park, C.S.; Ahn, H.J.; Park, Y.S.; Kim, H.M. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011, 236, 99–106. [Google Scholar] [CrossRef]
- Kamei, R.; Fujimura, T.; Matsuda, M.; Kakihara, K.; Hirakawa, N.; Baba, K.; Ono, K.; Arakawa, K.; Kawamoto, S. A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions. Biochem. Biophys. Res. Commun. 2017, 483, 674–679. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Inazu, M.; Egashira, T.; Matsumiya, T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002, 449, 261–267. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Sanbongi, C.; Kato, Y.; Osawa, T.; Yoshikawa, T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic. Biol. Med. 2002, 33, 798–806. [Google Scholar] [CrossRef]
- Li, J.J.; Li, Z.; Gu, L.J.; Choi, K.J.; Kim, D.S.; Kim, H.K.; Sung, C.K. The promotion of hair regrowth by topical application of a Perilla frutescens extract through increased cell viability and antagonism of testosterone and dihydrotestosterone. J. Nat. Med. 2018, 72, 96–105. [Google Scholar] [CrossRef]
- Zhao, G.; Yao-Yue, C.; Qin, G.W.; Guo, L.H. Luteolin from Purple Perilla mitigates ROS insult particularly in primary neurons. Neurobiol. Aging 2012, 33, 176–186. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Kuo, C.L.; Wang, J.P.; Cheng, J.S.; Huang, Z.W.; Chen, C.F. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J. Ethnopharmacol. 2007, 112, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ogawa, T. Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem. 2002, 66, 921–924. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lin, Q.Q.; Tu, Z.C.; Zhang, L. The influence of in vitro gastrointestinal digestion on the Perilla frutescens leaf extract: Changes in the active compounds and bioactivities. J. Food Biochem. 2020, 44, e13530. [Google Scholar] [CrossRef]
- Huang, J.P.; Wang, Y.J.; Tian, T.; Wang, L.; Yan, Y.; Huang, S.X. Tropane alkaloid biosynthesis: A centennial review. Nat. Prod. Rep. 2021, 38, 1634–1658. [Google Scholar] [CrossRef]
- Bharathi Priya, L.; Huang, C.Y.; Hu, R.M.; Balasubramanian, B.; Baskaran, R. An updated review on pharmacological properties of neferine—A bisbenzylisoquinoline alkaloid from Nelumbo nucifera. J. Food Biochem. 2021, 45, e13986. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, D.; Chu, C.; Li, X.; Wang, X.; Jia, Y.; Hua, H.; Xu, F. Antiproliferative Effects of Alkaloid Evodiamine and Its Derivatives. Int. J. Mol. Sci. 2018, 19, 3403. [Google Scholar] [CrossRef]
- Ito, M.; Kiuchi, F.; Yang, L.L.; Honda, G. Perilla citriodora from Taiwan and its phytochemical characteristics. Biol. Pharm. Bull. 2000, 23, 359–362. [Google Scholar] [CrossRef]
- Lim, H.J.; Woo, K.W.; Lee, K.R.; Lee, S.K.; Kim, H.P. Inhibition of Proinflammatory Cytokine Generation in Lung Inflammation by the Leaves of Perilla frutescens and Its Constituents. Biomol. Ther. 2014, 22, 62–67. [Google Scholar] [CrossRef]
- Uemura, T.; Yashiro, T.; Oda, R.; Shioya, N.; Nakajima, T.; Hachisu, M.; Kobayashi, S.; Nishiyama, C.; Arimura, G.I. Intestinal Anti-Inflammatory Activity of Perillaldehyde. J. Agric. Food Chem. 2018, 66, 3443–3448. [Google Scholar] [CrossRef]
- Chu, L.; Li, C.; Li, Y.; Yu, Q.; Yu, H.; Li, C.; Meng, W.; Zhu, J.; Wang, Q.; Wang, C.; et al. Perillaldehyde Inhibition of cGAS Reduces dsDNA-Induced Interferon Response. Front. Immunol. 2021, 12, 655637. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, D.; Ji, Z.; Fan, B.; She, Y.; Zhang, X.; Duan, L.; Shen, Q. Characterization of a Group of 2,3-Oxidosqualene Cyclase Genes Involved in the Biosynthesis of Diverse Triterpenoids of Perilla frutescens. J. Agric. Food Chem. 2023, 71, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef]
- Cho, J.; Tremmel, L.; Rho, O.; Camelio, A.M.; Siegel, D.; Slaga, T.J.; DiGiovanni, J. Evaluation of pentacyclic triterpenes found in Perilla frutescens for inhibition of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Oncotarget 2015, 6, 39292–39306. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hong, C.O.; Lee, G.P.; Kim, C.T.; Lee, K.W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef]
- Komatsu, K.I.; Takanari, J.; Maeda, T.; Kitadate, K.; Sato, T.; Mihara, Y.; Uehara, K.; Wakame, K. Perilla leaf extract prevents atopic dermatitis induced by an extract of Dermatophagoides farinae in NC/Nga mice. Asian Pac. J. Allergy Immunol. 2016, 34, 272–277. [Google Scholar] [CrossRef][Green Version]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef]
- Gaihre, Y.R.; Iwamoto, A.; Oogai, S.; Hamajima, H.; Tsuge, K.; Nagata, Y.; Yanagita, T. Perilla pomace obtained from four different varieties have different levels and types of polyphenols and anti-allergic activity. Cytotechnology 2022, 74, 341–349. [Google Scholar] [CrossRef]
- Choi, E.M. Luteolin protects osteoblastic MC3T3-E1 cells from antimycin A-induced cytotoxicity through the improved mitochondrial function and activation of PI3K/Akt/CREB. Toxicol. In Vitro 2011, 25, 1671–1679. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kono, M.; Ito, A.; Ito, M. Anthocyanins in perilla plants and dried leaves. Phytochemistry 2018, 147, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Yu, D.; Jin, L.; Gong, X.; Zhang, B. Perilla oil regulates intestinal microbiota and alleviates insulin resistance through the PI3K/AKT signaling pathway in type-2 diabetic KKAy mice. Food Chem. Toxicol. 2020, 135, 110965. [Google Scholar] [CrossRef]
- Park, H.; Sa, K.J.; Hyun, D.Y.; Lee, S.; Lee, J.K. Identifying SSR Markers Related to Seed Fatty Acid Content in Perilla Crop (Perilla frutescens L.). Plants 2021, 10, 1404. [Google Scholar] [CrossRef]
- Hamazaki, K. Role of Omega-3 Polyunsaturated Fatty Acids in Mental Health—Studies from Japan. J. Oleo Sci. 2019, 68, 511–515. [Google Scholar] [CrossRef]
- Thomas, S.S.; Cha, Y.S.; Kim, K.A. Protective Effect of Diet-Supplemented and Endogenously Produced Omega-3 Fatty Acids against HFD-Induced Colon Inflammation in Mice. Foods 2022, 11, 2124. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Liu, X.; Xie, Y.; Xia, H.; Wang, S.; Sun, G. Effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acids on erythrocyte fatty acid composition in type 2 diabetic patients. Lipids Health Dis. 2022, 21, 20. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Hu, M.; Wang, J.; Xia, H.; Yang, X.; Yang, L.; Sun, G. Perilla Oil Supplementation Improves Hypertriglyceridemia and Gut Dysbiosis in Diabetic KKAy Mice. Mol. Nutr. Food Res. 2018, 62, e1800299. [Google Scholar] [CrossRef]
- Kangwan, N.; Pratchayasakul, W.; Kongkaew, A.; Pintha, K.; Chattipakorn, N.; Chattipakorn, S.C. Perilla Seed Oil Alleviates Gut Dysbiosis, Intestinal Inflammation and Metabolic Disturbance in Obese-Insulin-Resistant Rats. Nutrients 2021, 13, 3141. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Park, J.G.; Kim, H.Y.; Ha, S.H.; Lee, B.; Park, S.U.; Seo, W.D.; Kim, J.K. Metabolite Profiling and Chemometric Study for the Discrimination Analyses of Geographic Origin of Perilla (Perilla frutescens) and Sesame (Sesamum indicum) Seeds. Foods 2020, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Torri, L.; Bondioli, P.; Folegatti, L.; Rovellini, P.; Piochi, M.; Morini, G. Development of Perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chem. 2019, 286, 584–591. [Google Scholar] [CrossRef]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Protective Effects of Unsaponifiable Matter from Perilla Seed Meal on UVB-induced Damages and the Underlying Mechanisms in Human Skin Fibroblasts. Antioxidants 2019, 8, 644. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tam, L.T.; Pham Thi Mai, H.; Tran Thi Hong, H.; Ninh, T.N.; Cuong, D.V.; Nguyen Xuan, C.; Tran, H.Q. Antimicrobial secondary metabolites from the aerial parts of Perilla frutescens. Nat. Prod. Res. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Wei, Y.; Ma, Y.; Wang, Y.; Huang, L.; Wang, Y. Hydrolyzed peptides from purple perilla (Perilla frutescens L. Britt.) seeds improve muscle synthesis and exercise performance in mice. J. Food Biochem. 2020, 44, e13461. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, L.; Cai, T.; Chen, Q.; Ma, Q.; Yang, J.; Meng, C.; Hong, J. Purification and identification of two novel antioxidant peptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates. PLoS ONE 2018, 13, e0200021. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Tavaszi-Sarosi, S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019, 275, 730–738. [Google Scholar] [CrossRef]
- Ito, M.; Honda, G.; Sydara, K. Perilla frutescens var. frutescens in northern Laos. J. Nat. Med. 2008, 62, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bumblauskiené, L.; Jakstas, V.; Janulis, V.; Mazdzieriené, R.; Ragazinskiené, O. Preliminary analysis on essential oil composition of Perilla L. cultivated in Lithuania. Acta Pol. Pharm. 2009, 66, 409–413. [Google Scholar] [PubMed]
- Zhou, X.J.; Yan, L.L.; Yin, P.P.; Shi, L.L.; Zhang, J.H.; Liu, Y.J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2008, 14, 133–140. [Google Scholar] [CrossRef]
- Yamazaki, M.; Nakajima, J.; Yamanashi, M.; Sugiyama, M.; Makita, Y.; Springob, K.; Awazuhara, M.; Saito, K. Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 2003, 62, 987–995. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.-H.; Zhou, S.; Gao, H.; Li, G.-L.; Guo, W.-J.; Fang, X.-Y.; Wang, W. Perillanolides A and B, new monoterpene glycosides from the leaves of Perilla frutescens. Rev. Bras. Farmacogn. 2017, 27, 564–568. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Kon’kova, N.G.; Zakharenko, A.M.; Golokhvast, K.S. Polyphenols of Perilla frutescens of the family Lamiaceae identified by tandem mass spectrometry. Vavilovskii Zhurnal Genet. Sel. 2022, 26, 637–644. [Google Scholar] [CrossRef]
- Masahiro, T.; Risa, M.; Harutaka, Y.; Kazuhiro, C. Novel Antioxidants Isolated from Perilla frutescens Britton var. crispa (Thunb.). Biosci. Biotechnol. Biochem. 1996, 60, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Zhang, J.; Mou, Z.L.; Hao, S.L.; Zhang, Z.Q. Microwave-assisted one-step extraction-derivatization for rapid analysis of fatty acids profile in herbal medicine by gas chromatography-mass spectrometry. Analyst 2012, 137, 5135–5143. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.Y.; Na, J.K.; Seong, E.S.; Yu, C.Y. Metabolite profiling based on lipophilic compounds for quality assessment of perilla (Perilla frutescens) cultivars. J. Agric. Food Chem. 2012, 60, 2257–2263. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, B.; Kim, S.; Kim, M.-S.; Kim, H.; Hwang, S.-R.; Kim, K.; Lee, J.H. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal. 2017, 25, 776–788. [Google Scholar] [CrossRef]
- Erhunmwunsee, F.; Pan, C.; Yang, K.; Li, Y.; Liu, M.; Tian, J. Recent development in biological activities and safety concerns of perillaldehyde from perilla plants: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6328–6340. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.D.; Jeong, I.Y.; Lee, M.K.; Seo, K.I. Sensitization of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant primary prostate cancer cells by isoegomaketone from Perilla frutescens. J. Nat. Prod. 2014, 77, 2438–2443. [Google Scholar] [CrossRef]
- Lim, D.Y.; Jeong, Y.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, H.J.; Chi, G.Y.; Choi, Y.H.; Park, S.H. An Ethanol Extract of Perilla frutescens Leaves Suppresses Adrenergic Agonist-Induced Metastatic Ability of Cancer Cells by Inhibiting Src-Mediated EMT. Molecules 2023, 28, 3414. [Google Scholar] [CrossRef]
- Park, D.D.; Yum, H.W.; Zhong, X.; Kim, S.H.; Kim, S.H.; Kim, D.H.; Kim, S.J.; Na, H.K.; Sato, A.; Miura, T.; et al. Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-κB, STAT3, and Nrf2 as Putative Targets. Front. Pharmacol. 2017, 8, 482. [Google Scholar] [CrossRef]
- Onogi, N.; Okuno, M.; Komaki, C.; Moriwaki, H.; Kawamori, T.; Tanaka, T.; Mori, H.; Muto, Y. Suppressing effect of perilla oil on azoxymethane-induced foci of colonic aberrant crypts in rats. Carcinogenesis 1996, 17, 1291–1296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, S.; LingHu, X.; Wang, Y.; Wang, B.; Zhong, S.; Xie, S.; Xu, X.; Yu, A.; Nagai, A.; et al. Perillaldehyde inhibits bone metastasis and receptor activator of nuclear factor-κB ligand (RANKL) signaling-induced osteoclastogenesis in prostate cancer cell lines. Bioengineered 2022, 13, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Kwak, Y.; Ju, J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr. Res. Pract. 2015, 9, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Guan, K.L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.L.; Shin, Y.S.; Choi, S.H.; Oh, S.; Kim, K.; Jeong, H.S.; Mo, J.S. Extracts of Perilla frutescens var. Acuta (Odash.) Kudo Leaves Have Antitumor Effects on Breast Cancer Cells by Suppressing YAP Activity. Evid. Based Complement. Alternat. Med. 2021, 2021, 5619761. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hafeez, A.A.; Fujimura, T.; Kamei, R.; Hirakawa, N.; Baba, K.; Ono, K.; Kawamoto, S. A methoxyflavanone derivative from the Asian medicinal herb (Perilla frutescens) induces p53-mediated G(2)/M cell cycle arrest and apoptosis in A549 human lung adenocarcinoma. Cytotechnology 2018, 70, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Jochems, F.; Thijssen, B.; De Conti, G.; Jansen, R.; Pogacar, Z.; Groot, K.; Wang, L.; Schepers, A.; Wang, C.; Jin, H.; et al. The Cancer SENESCopedia: A delineation of cancer cell senescence. Cell Rep. 2021, 36, 109441. [Google Scholar] [CrossRef]
- Maeda, A.; Fujimura, T.; Hirakawa, N.; Baba, K.; Kawamoto, S. A Methoxyflavanone from Perilla frutescens Induces Cellular Senescence in A549 Human Lung Adenocarcinoma Cells but Not in Normal Human Bronchial Epithelial Cells. Biol. Pharm. Bull. 2022, 45, 1581–1584. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Paradee, N.; Utama-Ang, N.; Uthaipibull, C.; Porter, J.B.; Garbowski, M.W.; Srichairatanakool, S. Extracts of Thai Perilla frutescens nutlets attenuate tumour necrosis factor-α-activated generation of microparticles, ICAM-1 and IL-6 in human endothelial cells. Biosci. Rep. 2020, 40, BSR20192110. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Paradee, N.; Howes, M.R.; Utama-Ang, N.; Chaikitwattna, A.; Hider, R.C.; Srichairatanakool, S. A chemically characterized ethanolic extract of Thai Perilla frutescens (L.) Britton fruits (nutlets) reduces oxidative stress and lipid peroxidation in human hepatoma (HuH7) cells. Phytother. Res. 2019, 33, 2064–2074. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Feng, Q.; Huang, X.; Wang, X.; Peng, Y.; Zhao, Z.; Liu, Z. Perilaldehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. J. Cell. Biochem. 2019, 120, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Han, J.; Zheng, W.; Ma, W. Extract of Perilla frutescens inhibits tumor proliferation of HCC via PI3K/AKT signal pathway. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Masuda, A.; Ito, N.; Kamano, K.; Okuyama, H. Effects of dietary perilla oil, soybean oil and safflower oil on 7,12-dimethylbenz[a]anthracene (DMBA) and 1,2-dimethyl-hydrazine (DMH)-induced mammary gland and colon carcinogenesis in female SD rats. Carcinogenesis 1990, 11, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol. Pharm. Bull. 2003, 26, 560–563. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lee, J.H.; Moon, K.D.; Jeong, I.Y.; Ahn, D.U.; Lee, M.K.; Seo, K.I. Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway. Food Chem. Toxicol. 2014, 65, 97–104. [Google Scholar] [CrossRef]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef]
- Okuno, M.; Tanaka, T.; Komaki, C.; Nagase, S.; Shiratori, Y.; Muto, Y.; Kajiwara, K.; Maki, T.; Moriwaki, H. Suppressive effect of low amounts of safflower and perilla oils on diethylnitrosamine-induced hepatocarcinogenesis in male F344 rats. Nutr. Cancer 1998, 30, 186–193. [Google Scholar] [CrossRef]
- Futakuchi, M.; Cheng, J.L.; Hirose, M.; Kimoto, N.; Cho, Y.M.; Iwata, T.; Kasai, M.; Tokudome, S.; Shirai, T. Inhibition of conjugated fatty acids derived from safflower or perilla oil of induction and development of mammary tumors in rats induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Cancer Lett. 2002, 178, 131–139. [Google Scholar] [CrossRef]
- Cho, B.O.; Jin, C.H.; Park, Y.D.; Ryu, H.W.; Byun, M.W.; Seo, K.I.; Jeong, I.Y. Isoegomaketone induces apoptosis through caspase-dependent and caspase-independent pathways in human DLD1 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1306–1311. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lee, J.H.; Moon, K.D.; Jeong, I.Y.; Yee, S.T.; Lee, M.K.; Seo, K.I. Isoegomaketone induces apoptosis in SK-MEL-2 human melanoma cells through mitochondrial apoptotic pathway via activating the PI3K/Akt pathway. Int. J. Oncol. 2014, 45, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, C.; Galvano, F.; Pierdomenico, L.; Speroni, E.; Guerra, M.C. Effects of rosmarinic acid against aflatoxin B1 and ochratoxin-A-induced cell damage in a human hepatoma cell line (Hep G2). J. Appl. Toxicol. 2004, 24, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Asefy, Z.; Tanomand, A.; Hoseinnejhad, S.; Ceferov, Z.; Oshaghi, E.A.; Rashidi, M. Unsaturated fatty acids as a co-therapeutic agents in cancer treatment. Mol. Biol. Rep. 2021, 48, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef]
- Narisawa, T.; Fukaura, Y.; Yazawa, K.; Ishikawa, C.; Isoda, Y.; Nishizawa, Y. Colon cancer prevention with a small amount of dietary perilla oil high in alpha-linolenic acid in an animal model. Cancer 1994, 73, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Narisawa, T.; Takahashi, M.; Kotanagi, H.; Kusaka, H.; Yamazaki, Y.; Koyama, H.; Fukaura, Y.; Nishizawa, Y.; Kotsugai, M.; Isoda, Y.; et al. Inhibitory effect of dietary perilla oil rich in the n-3 polyunsaturated fatty acid alpha-linolenic acid on colon carcinogenesis in rats. Jpn. J. Cancer Res. 1991, 82, 1089–1096. [Google Scholar] [CrossRef]
- Abd El-Hafeez, A.A.; Fujimura, T.; Kamei, R.; Hirakawa, N.; Baba, K.; Ono, K.; Kawamoto, S. Synergistic tumor suppression by a Perilla frutescens-derived methoxyflavanone and anti-cancer tyrosine kinase inhibitors in A549 human lung adenocarcinoma. Cytotechnology 2018, 70, 913–919. [Google Scholar] [CrossRef]
- Komaki, C.; Okuno, M.; Onogi, N.; Moriwaki, H.; Kawamori, T.; Tanaka, T.; Mori, H.; Muto, Y. Synergistic suppression of azoxymethane-induced foci of colonic aberrant crypts by the combination of beta-carotene and perilla oil in rats. Carcinogenesis 1996, 17, 1897–1901. [Google Scholar] [CrossRef][Green Version]
- Rao, C.V.; Patlolla, J.M.; Cooma, I.; Kawamori, T.; Steele, V.E. Prevention of familial adenomatous polyp development in APC min mice and azoxymethane-induced colon carcinogenesis in F344 Rats by ω-3 fatty acid rich perilla oil. Nutr. Cancer 2013, 65 (Suppl. S1), 54–60. [Google Scholar] [CrossRef]
- Sodir, N.M.; Evan, G.I. Finding cancer’s weakest link. Oncotarget 2011, 2, 1307–1313. [Google Scholar] [CrossRef]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Wahle, K.W.; Brown, I.; Rotondo, D.; Heys, S.D. Plant phenolics in the prevention and treatment of cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, N.; Yang, T.; Yan, J.; Wang, W.; Li, X. The Potential Mechanisms by which Artemisinin and Its Derivatives Induce Ferroptosis in the Treatment of Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 1458143. [Google Scholar] [CrossRef] [PubMed]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal Plants in the Prevention and Treatment of Colon Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef]
- Liu, S.; Jin, X.; Shang, Y.; Wang, L.; Du, K.; Chen, S.; Li, J.; He, J.; Fang, S.; Chang, Y. A comprehensive review of the botany, ethnopharmacology, phytochemistry, pharmacology, toxicity and quality control of Perillae Fructus. J. Ethnopharmacol. 2023, 304, 116022. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Feng, C.; Zhang, J.; Qin, Y.; Meng, L. Advances in the Pharmacological Activities and Effects of Perilla Ketone and Isoegomaketone. Evid. Based Complement. Alternat. Med. 2022, 2022, 8809792. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).