Porphyrin-Based MOF Thin Film on Transparent Conducting Oxide: Investigation of Growth, Porosity and Photoelectrochemical Properties
Abstract
1. Introduction
2. Results and Discussion
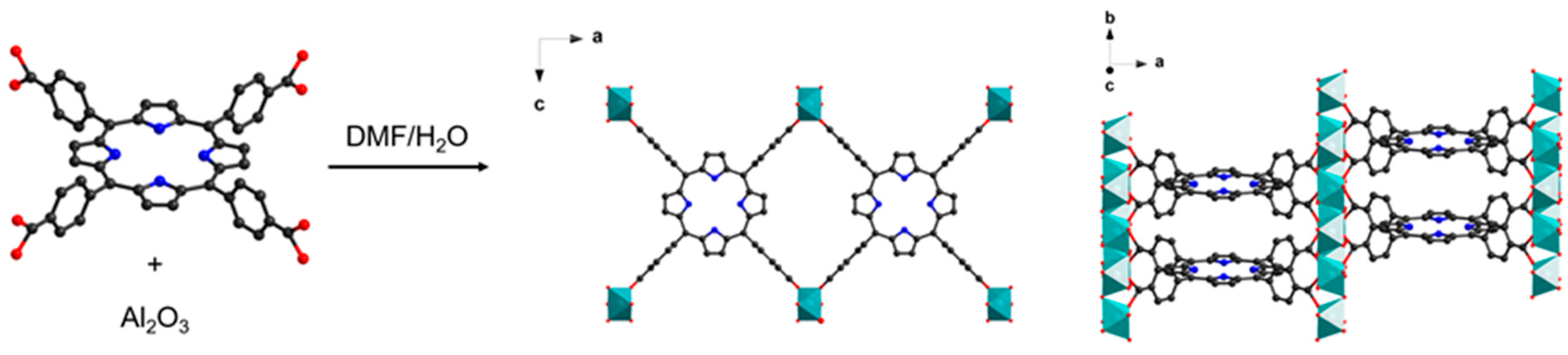
2.1. Solvent Effect on the Conversion of Alumina to Al-PMOF
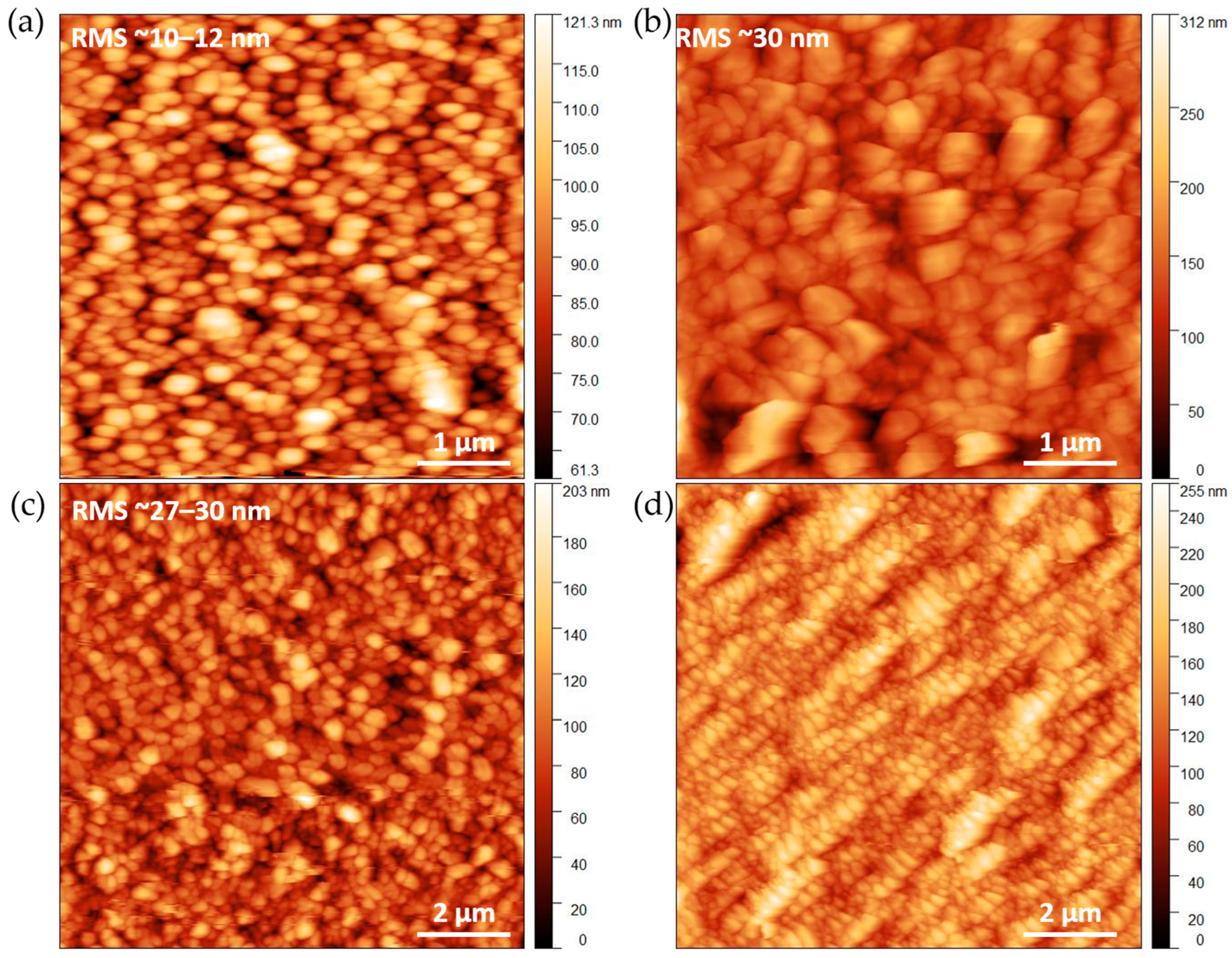
2.2. Impact of the Alumina Thickness on Al-PMOF Morphology
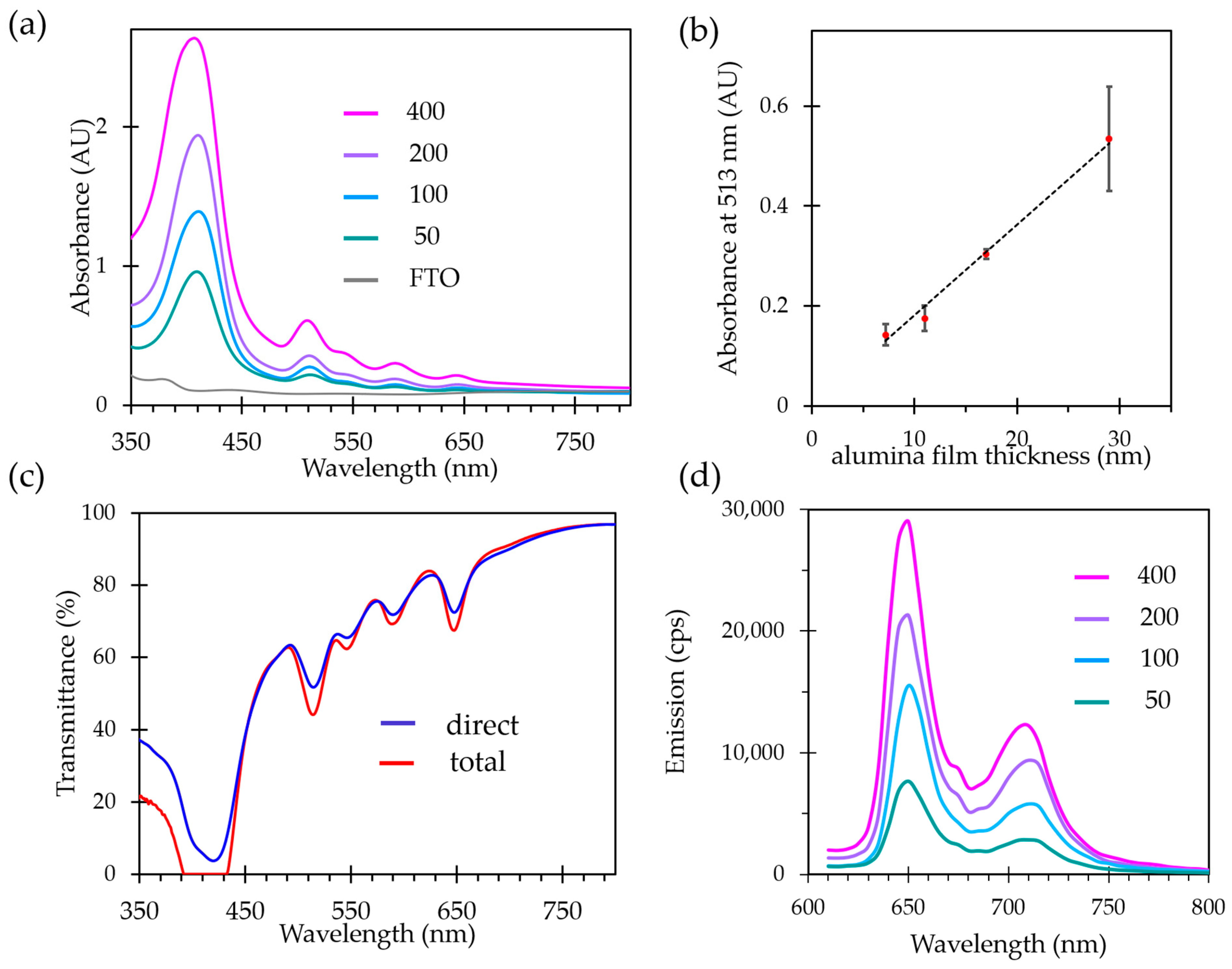
2.3. Optical Properties
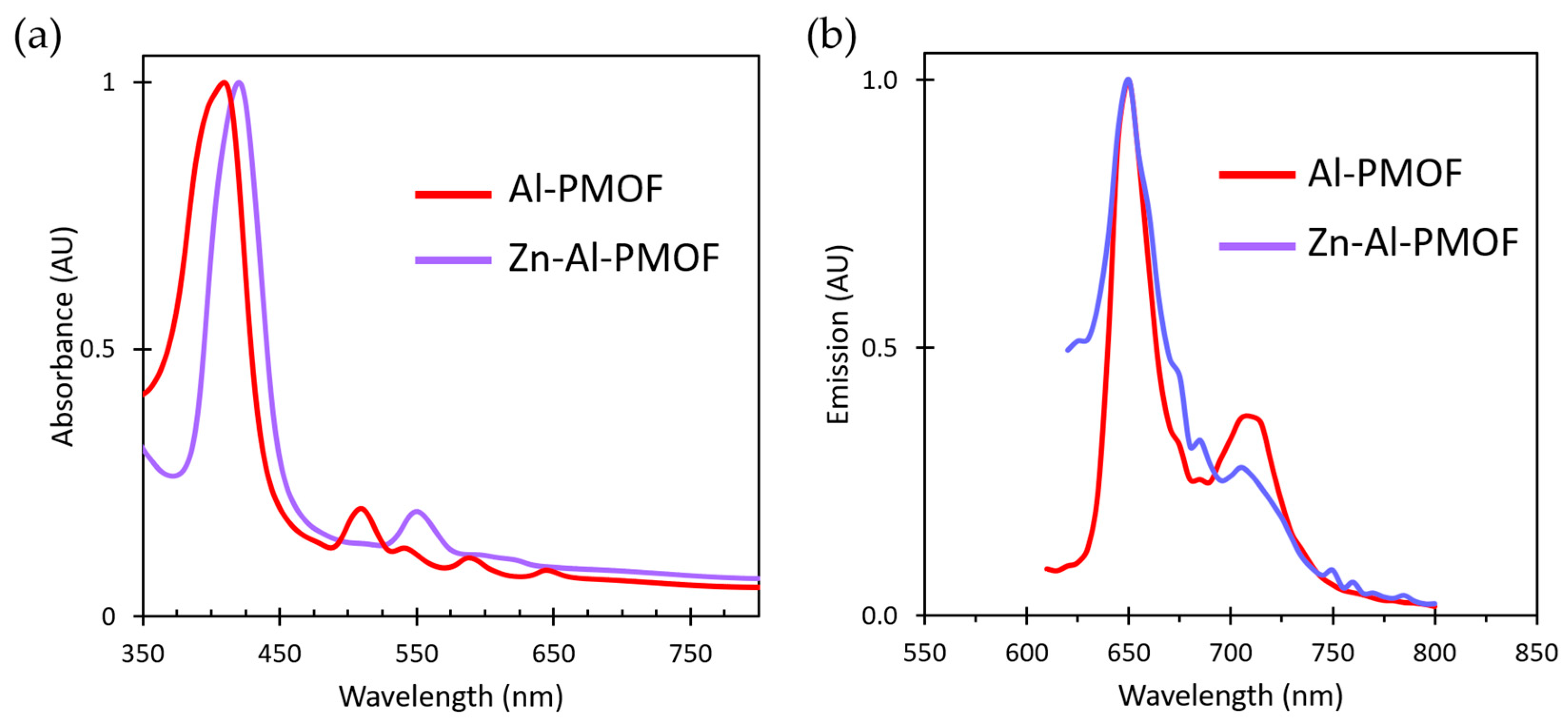
2.4. Accessible Porosity and Post Modification
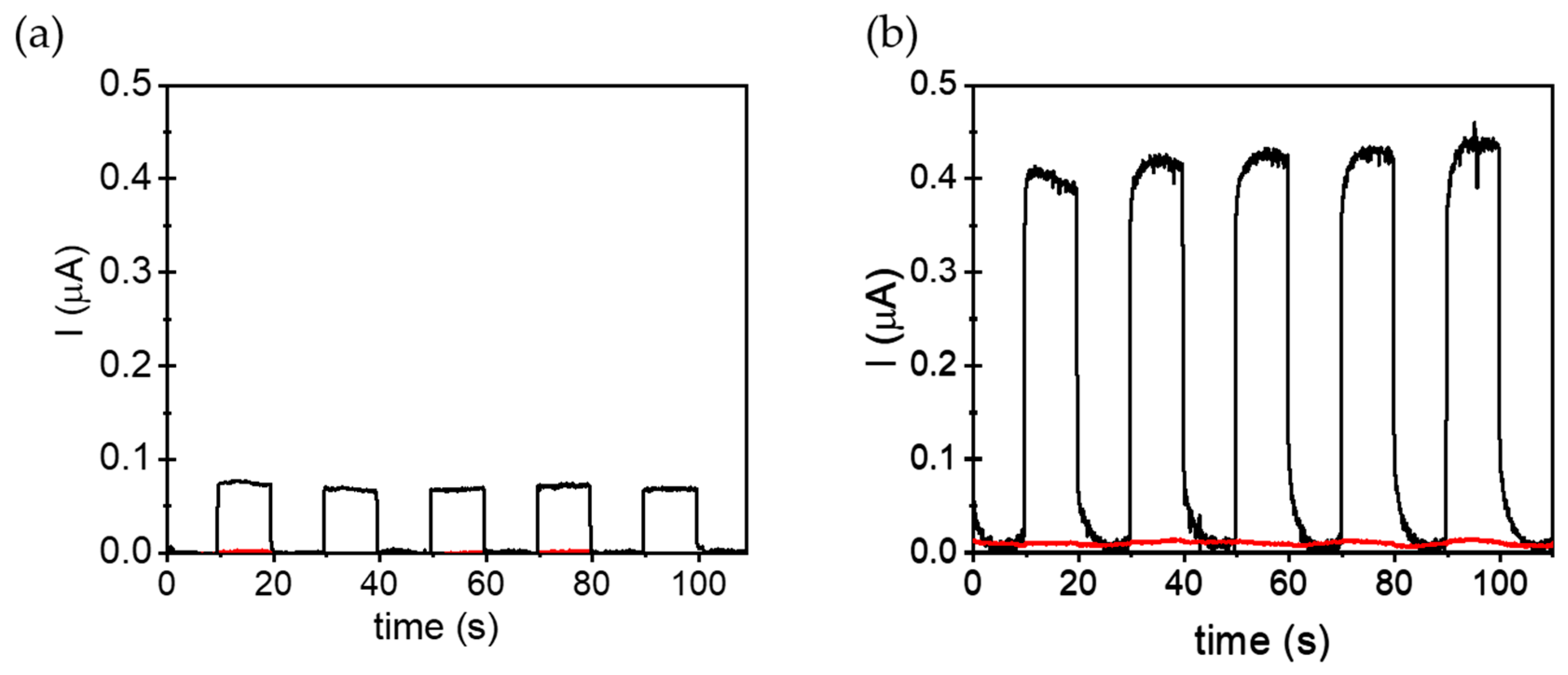
2.5. Photoelectrochemical Characterizations
3. Materials and Methods
3.1. Characterizations
3.2. Thin Film Growth Procedures
3.2.1. Alumina Growth by ALD
3.2.2. Synthesis of Al-PMOF Thin Films
3.2.3. Vapor Phase Infiltration (VPI) of Al-PMOF Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Reboul, J.; Furukawa, S.; Horike, N.; Tsotsalas, M.; Hirai, K.; Uehara, H.; Kondo, M.; Louvain, N.; Sakata, O.; Kitagawa, S. Mesoscopic Architectures of Porous Coordination Polymers Fabricated by Pseudomorphic Replication. Nat. Mater. 2012, 11, 717–723. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Turner, S.; Tu, M.; Wannapaiboon, S.; Schneemann, A.; Meyer, R.; Ludwig, A.; Van Tendeloo, G.; Fischer, R.A. Self-Directed Localization of ZIF-8 Thin Film Formation by Conversion of ZnO Nanolayers. Adv. Funct. Mater. 2014, 24, 4804–4811. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Grenci, G.; Gorp, H.V.; Vanderlinden, W.; Feyter, S.D.; Falcaro, P.; Vos, D.D.; Vereecken, P.; Ameloot, R. Chemical Vapour Deposition of Zeolitic Imidazolate Framework Thin Films. Nat. Mater. 2016, 15, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Bechelany, M.; Drobek, M.; Vallicari, C.; Abou Chaaya, A.; Julbe, A.; Miele, P. Highly Crystalline MOF-Based Materials Grown on Electrospun Nanofibers. Nanoscale 2015, 7, 5794–5802. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lee, D.T.; Shi, K.; Wang, S.; Barton, H.F.; Zhu, J.; Yan, J.; Ke, Q.; Parsons, G.N. Fabrication of a Freestanding Metal Organic Framework Predominant Hollow Fiber Mat and Its Potential Applications in Gas Separation and Catalysis. J. Mater. Chem. A 2020, 8, 3803–3813. [Google Scholar] [CrossRef]
- Lee, D.T.; Jamir, J.D.; Peterson, G.W.; Parsons, G.N. Protective Fabrics: Metal-Organic Framework Textiles for Rapid Photocatalytic Sulfur Mustard Simulant Detoxification. Matter 2020, 2, 404–415. [Google Scholar] [CrossRef]
- Lee, D.T.; Dai, Z.; Peterson, G.W.; Hall, M.G.; Pomerantz, N.L.; Hoffman, N.; Parsons, G.N. Highly Breathable Chemically Protective MOF-Fiber Catalysts. Adv. Funct. Mater. 2022, 32, 2108004. [Google Scholar] [CrossRef]
- Lan, H.; Salmi, L.D.; Rönkkö, T.; Parshintsev, J.; Jussila, M.; Hartonen, K.; Kemell, M.; Riekkola, M.-L. Integrated Atomic Layer Deposition and Chemical Vapor Reaction for the Preparation of Metal Organic Framework Coatings for Solid-Phase Microextraction Arrow. Anal. Chim. Acta 2018, 1024, 93–100. [Google Scholar] [CrossRef]
- Stassin, T.; Stassen, I.; Marreiros, J.; Cruz, A.J.; Verbeke, R.; Tu, M.; Reinsch, H.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J.; et al. Solvent-Free Powder Synthesis and MOF-CVD Thin Films of the Large-Pore Metal–Organic Framework MAF-6. Chem. Mater. 2020, 32, 1784–1793. [Google Scholar] [CrossRef]
- Tu, M.; Kravchenko, D.E.; Xia, B.; Rubio-Giménez, V.; Wauteraerts, N.; Verbeke, R.; Vankelecom, I.F.J.; Stassin, T.; Egger, W.; Dickmann, M.; et al. Template-Mediated Control over Polymorphism in the Vapor-Assisted Formation of Zeolitic Imidazolate Framework Powders and Films. Angew. Chem. Int. Ed. 2021, 60, 7553–7558. [Google Scholar] [CrossRef]
- Yang, F.; Hu, W.; Yang, C.; Patrick, M.; Cooksy, A.L.; Zhang, J.; Aguiar, J.A.; Fang, C.; Zhou, Y.; Meng, Y.S.; et al. Tuning Internal Strain in Metal–Organic Frameworks via Vapor Phase Infiltration for CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 4572–4580. [Google Scholar] [CrossRef]
- Genesio, G.; Maynadié, J.; Carboni, M.; Meyer, D. Recent Status on MOF Thin Films on Transparent Conductive Oxides Substrates (ITO or FTO). New J. Chem. 2018, 42, 2351–2363. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal–Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [PubMed]
- Lions, M.; Tommasino, J.-B.; Chattot, R.; Abeykoon, B.; Guillou, N.; Devic, T.; Demessence, A.; Cardenas, L.; Maillard, F.; Fateeva, A. Insights into the Mechanism of Electrocatalysis of the Oxygen Reduction Reaction by a Porphyrinic Metal Organic Framework. Chem. Commun. 2017, 53, 6496–6499. [Google Scholar] [CrossRef]
- Shang, S.; Xiong, W.; Yang, C.; Johannessen, B.; Liu, R.; Hsu, H.; Gu, Q.; Leung, M.; Shang, J. Atomically Dispersed Iron Metal Site in a Porphyrin-Based Metal-Organic Framework for Photocatalytic Nitrogen Fixation. ACS Nano 2021, 15, 9670–9678. [Google Scholar] [CrossRef]
- Abeykoon, B.; Devic, T.; Grenèche, J.-M.; Fateeva, A.; Sorokin, A.B. Confinement of Fe–Al-PMOF Catalytic Sites Favours the Formation of Pyrazoline from Ethyl Diazoacetate with an Unusual Sharp Increase of Selectivity upon Recycling. Chem. Commun. 2018, 54, 10308–10311. [Google Scholar] [CrossRef]
- Zhao, Y.; Kornienko, N.; Liu, Z.; Zhu, C.; Asahina, S.; Kuo, T.-R.; Bao, W.; Xie, C.; Hexemer, A.; Terasaki, O.; et al. Mesoscopic Constructs of Ordered and Oriented Metal–Organic Frameworks on Plasmonic Silver Nanocrystals. J. Am. Chem. Soc. 2015, 137, 2199–2202. [Google Scholar] [CrossRef]
- Freund, R.; Lanza, A.E.; Canossa, S.; Gemmi, M.; Goscianska, J.; Cauda, V.; Oschatz, M.; Wuttke, S. Understanding the Chemistry of Metal Oxide to Metal–Organic Framework Reactions for Morphology Control. Chem. Mater. 2023, 35, 1891–1900. [Google Scholar] [CrossRef]
- Seybold, P.G.; Gouterman, M. Porphyrins: XIII: Fluorescence Spectra and Quantum Yields. J. Mol. Spectrosc. 1969, 31, 1–13. [Google Scholar] [CrossRef]
- Khairutdinov, R.F.; Serpone, N. Photoluminescence and Transient Spectroscopy of Free Base Porphyrin Aggregates. J. Phys. Chem. B 1999, 103, 761–769. [Google Scholar] [CrossRef]
- Guo, B.; Cai, X.; Xu, S.; Fateminia, S.M.A.; Liu, J.; Liang, J.; Feng, G.; Wu, W.; Liu, B. Decoration of Porphyrin with Tetraphenylethene: Converting a Fluorophore with Aggregation-Caused Quenching to Aggregation-Induced Emission Enhancement. J. Mater. Chem. B 2016, 4, 4690–4695. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimada, T.; Tachibana, H.; Inoue, H.; Takagi, S. Regulation of the Collisional Self-Quenching of Fluorescence in Clay/Porphyrin Complex by Strong Host–Guest Interaction. J. Phys. Chem. A 2012, 116, 12065–12072. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Ruseckas, A.; Shaw, P.E.; Barcena, H.S.; Burn, P.L.; Samuel, I.D.W. Thickness Dependence of the Fluorescence Lifetime in Films of Bisfluorene-Cored Dendrimers. J. Phys. Chem. C 2008, 112, 20463–20468. [Google Scholar] [CrossRef]
- Ghosh, M.; Nath, S.; Hajra, A.; Sinha, S. Fluorescence Self-Quenching of Tetraphenylporphyrin in Liquid Medium. J. Lumin. 2013, 141, 87–92. [Google Scholar] [CrossRef]
- Leng, C.Z.; Losego, M.D. Vapor Phase Infiltration (VPI) for Transforming Polymers into Organic–Inorganic Hybrid Materials: A Critical Review of Current Progress and Future Challenges. Mater. Horiz. 2017, 4, 747–771. [Google Scholar] [CrossRef]
- De, S.; Quan, G.C.; Gikonyo, B.; Martineau-Corcos, C.; Bousige, C.; Veyre, L.; Devic, T.; Marichy, C.; Fateeva, A. Vapor-Phase Infiltration inside a Microporous Porphyrinic Metal–Organic Framework for Postsynthesis Modification. Inorg. Chem. 2020, 59, 10129–10137. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Comprehensive Review of Photophysical Parameters (ε, Φf, Τs) of Tetraphenylporphyrin (H2TPP) and Zinc Tetraphenylporphyrin (ZnTPP)—Critical Benchmark Molecules in Photochemistry and Photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2021, 46, 100401. [Google Scholar] [CrossRef]
- Magdaong, N.C.M.; Taniguchi, M.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Photophysical Properties and Electronic Structure of Zinc(II) Porphyrins Bearing 0–4 Meso-Phenyl Substituents: Zinc Porphine to Zinc Tetraphenylporphyrin (ZnTPP). J. Phys. Chem. A 2020, 124, 7776–7794. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gikonyo, B.; Liu, F.; Hawila, S.; Demessence, A.; Baldovi, H.G.; Navalón, S.; Marichy, C.; Fateeva, A. Porphyrin-Based MOF Thin Film on Transparent Conducting Oxide: Investigation of Growth, Porosity and Photoelectrochemical Properties. Molecules 2023, 28, 5876. https://doi.org/10.3390/molecules28155876
Gikonyo B, Liu F, Hawila S, Demessence A, Baldovi HG, Navalón S, Marichy C, Fateeva A. Porphyrin-Based MOF Thin Film on Transparent Conducting Oxide: Investigation of Growth, Porosity and Photoelectrochemical Properties. Molecules. 2023; 28(15):5876. https://doi.org/10.3390/molecules28155876
Chicago/Turabian StyleGikonyo, Ben, Fangbing Liu, Saly Hawila, Aude Demessence, Herme G. Baldovi, Sergio Navalón, Catherine Marichy, and Alexandra Fateeva. 2023. "Porphyrin-Based MOF Thin Film on Transparent Conducting Oxide: Investigation of Growth, Porosity and Photoelectrochemical Properties" Molecules 28, no. 15: 5876. https://doi.org/10.3390/molecules28155876
APA StyleGikonyo, B., Liu, F., Hawila, S., Demessence, A., Baldovi, H. G., Navalón, S., Marichy, C., & Fateeva, A. (2023). Porphyrin-Based MOF Thin Film on Transparent Conducting Oxide: Investigation of Growth, Porosity and Photoelectrochemical Properties. Molecules, 28(15), 5876. https://doi.org/10.3390/molecules28155876






