Long-Chain Molecules with Agro-Bioactivities and Their Applications
Abstract
1. Introduction
2. Long-Chain Molecules Exist Widely in Nature
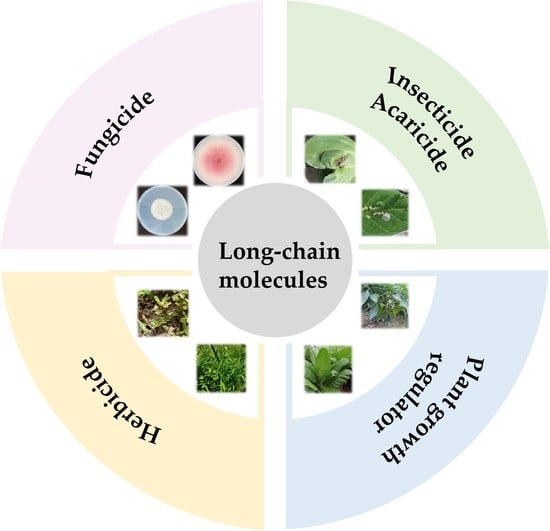
3. Long-Chain Molecules with Agro-Bioactivities
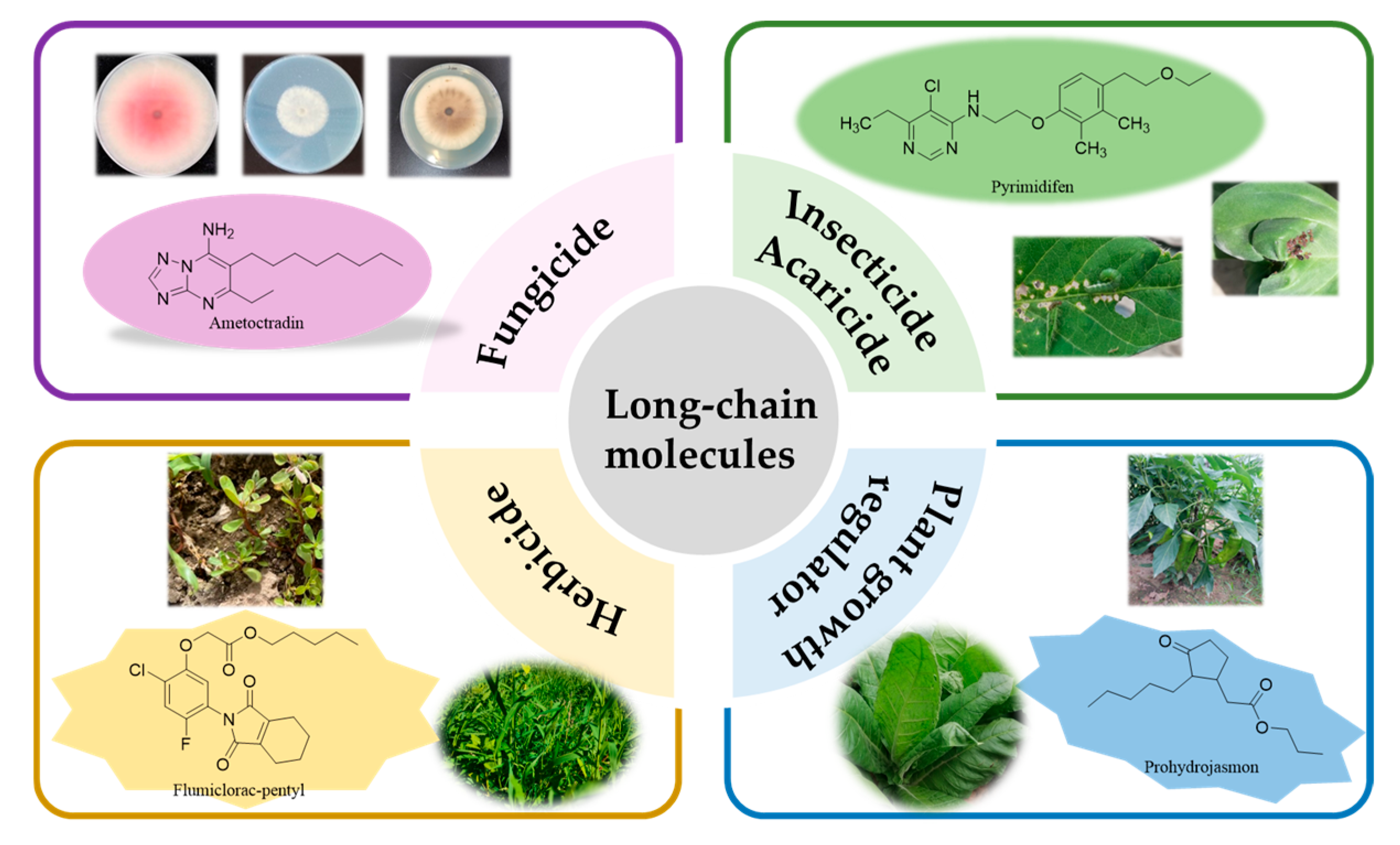
3.1. Phytopathogenic Fungicides Containing a Long Chain
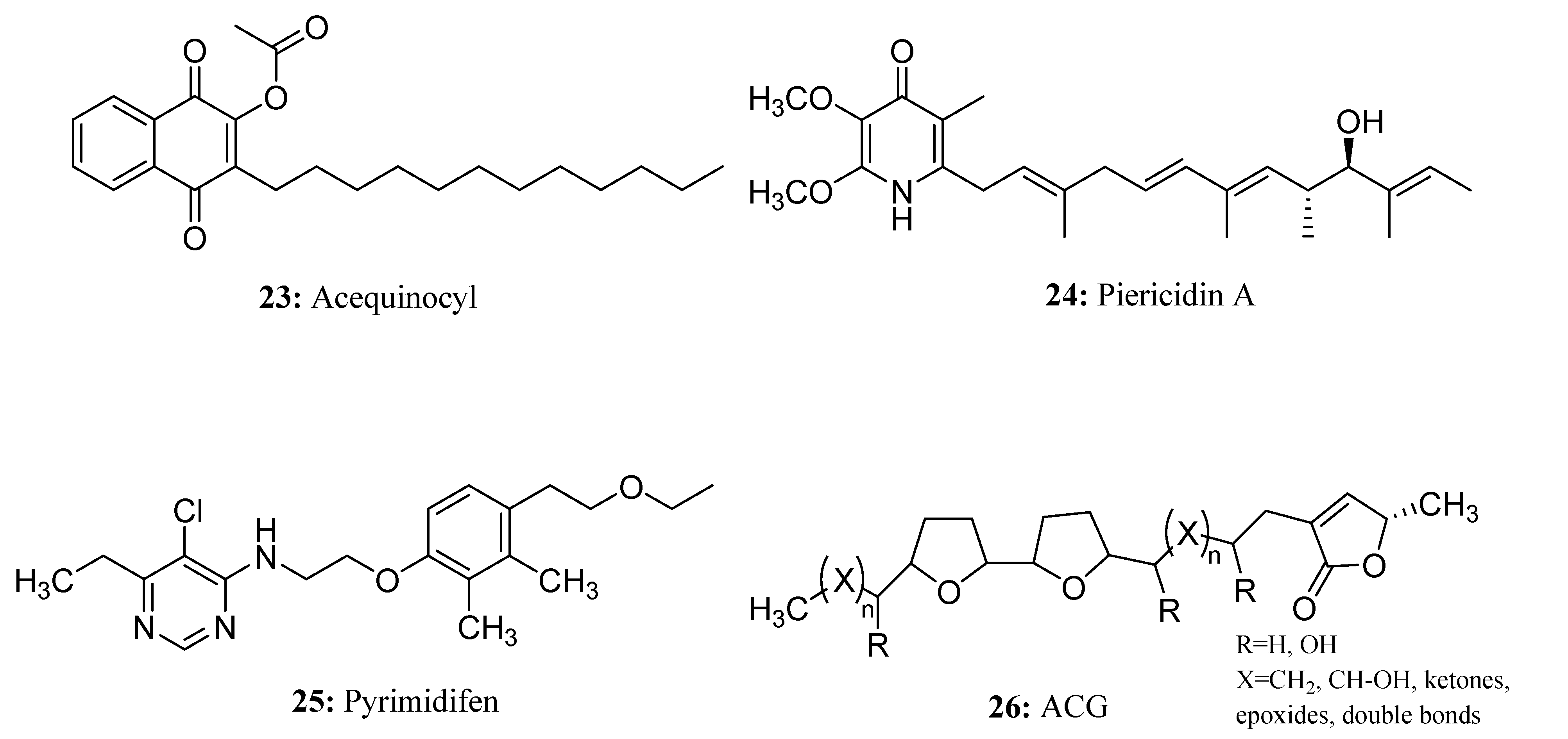
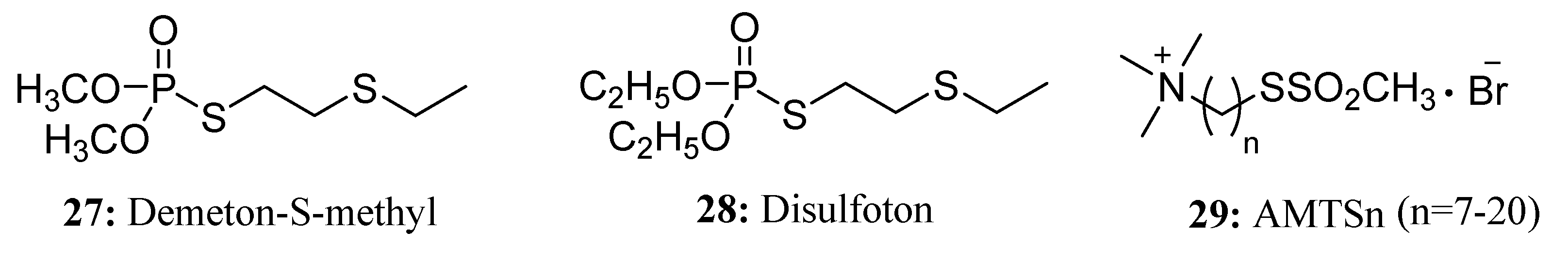
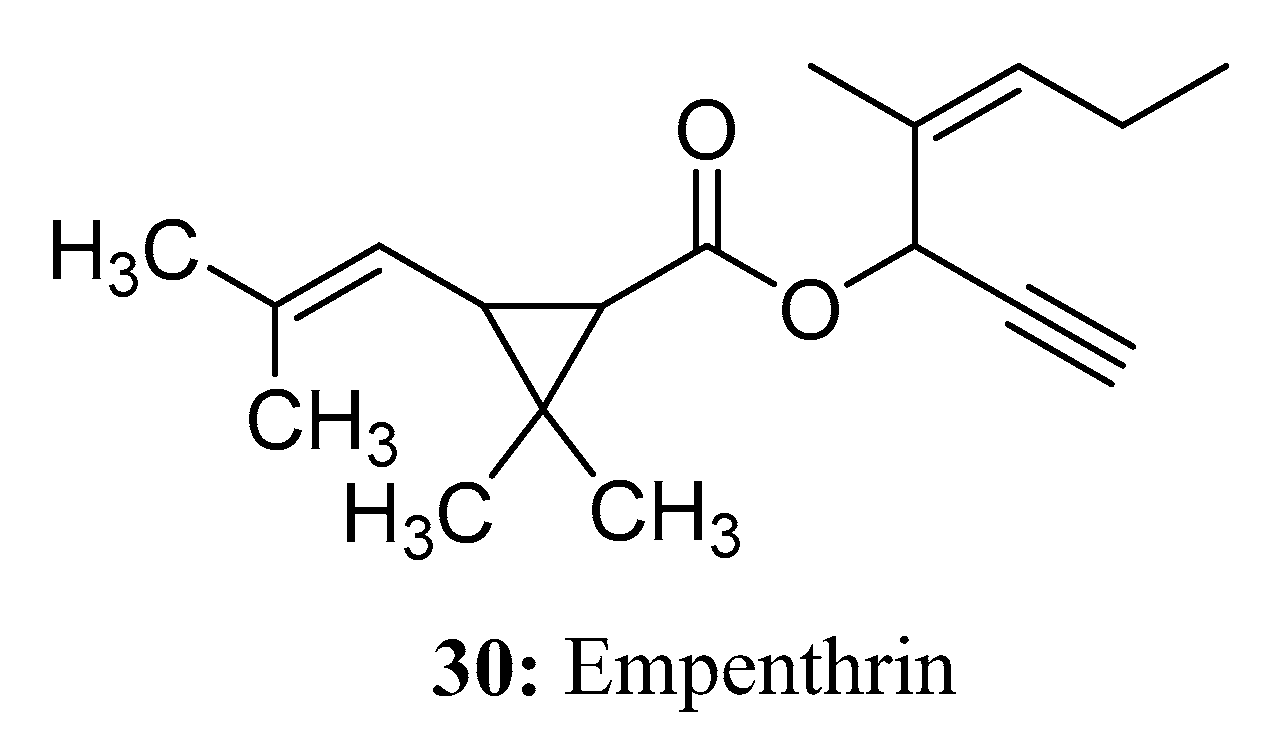
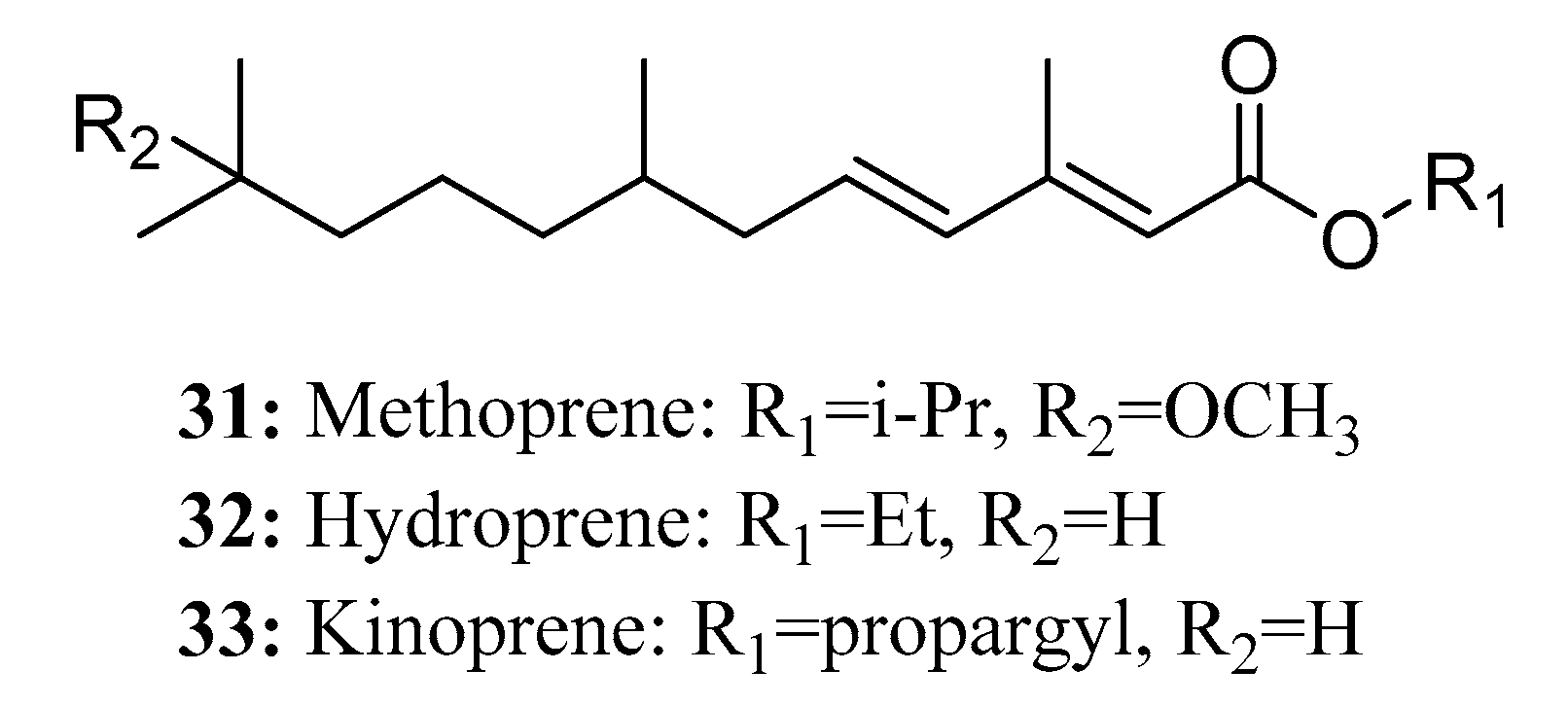
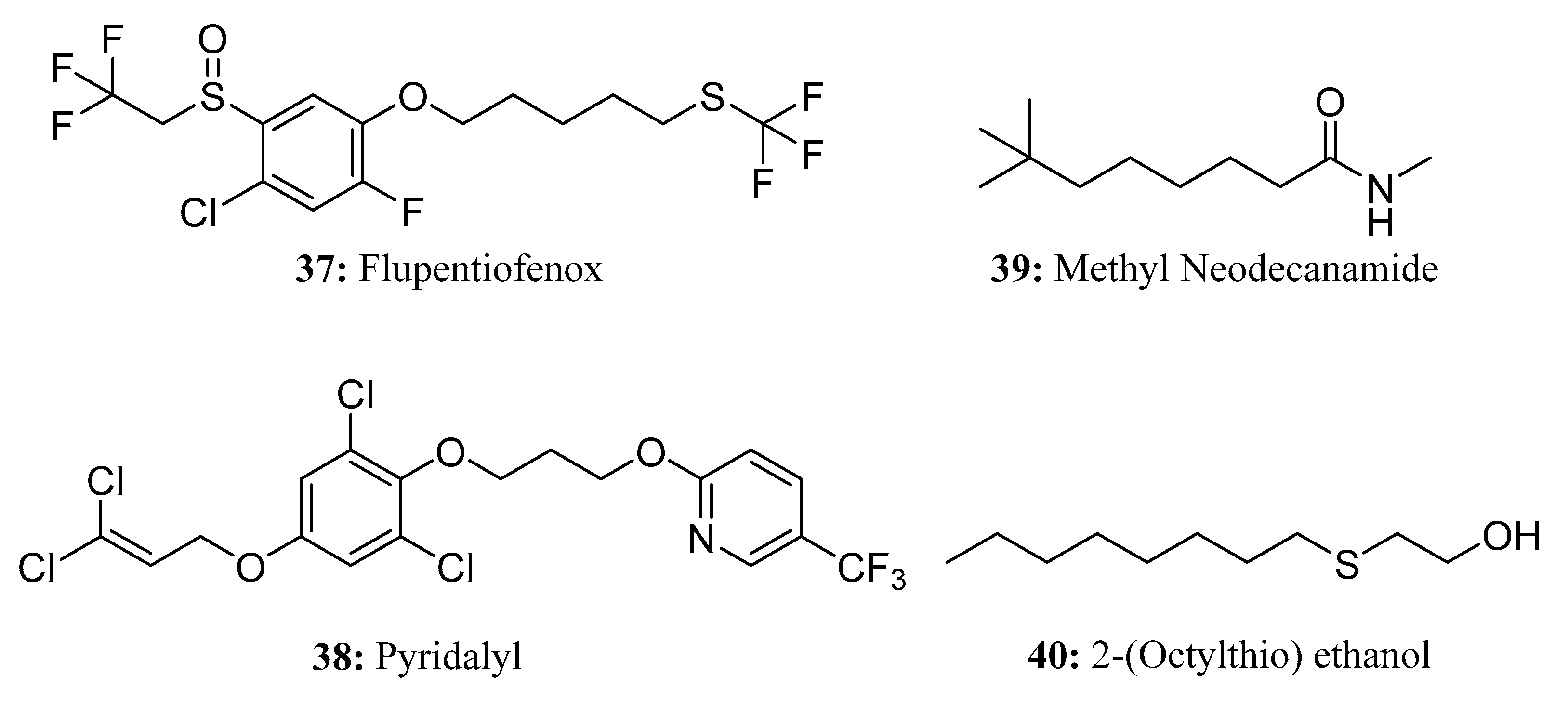
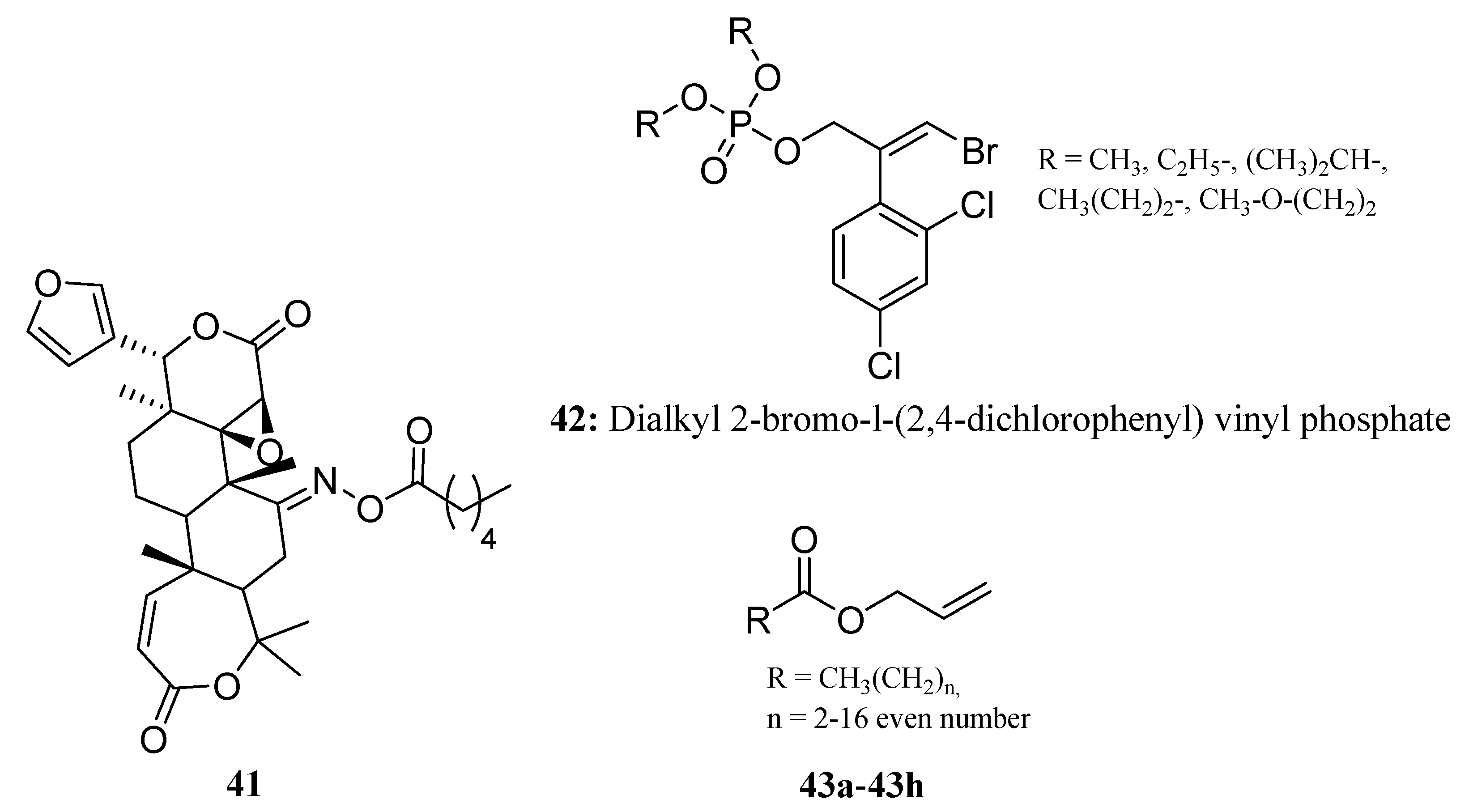
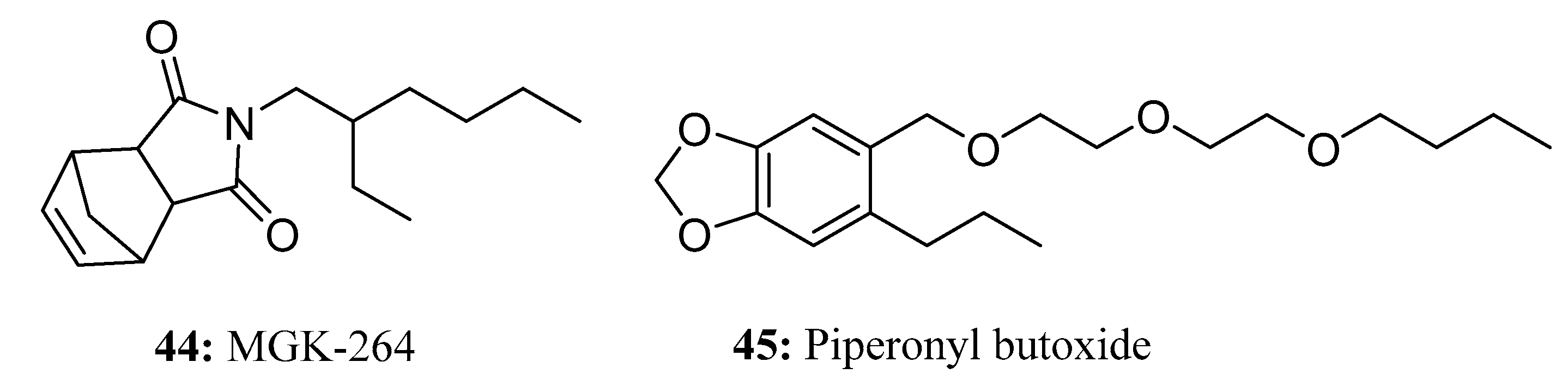
3.2. Insecticides/Acaricides Containing a Long Chain
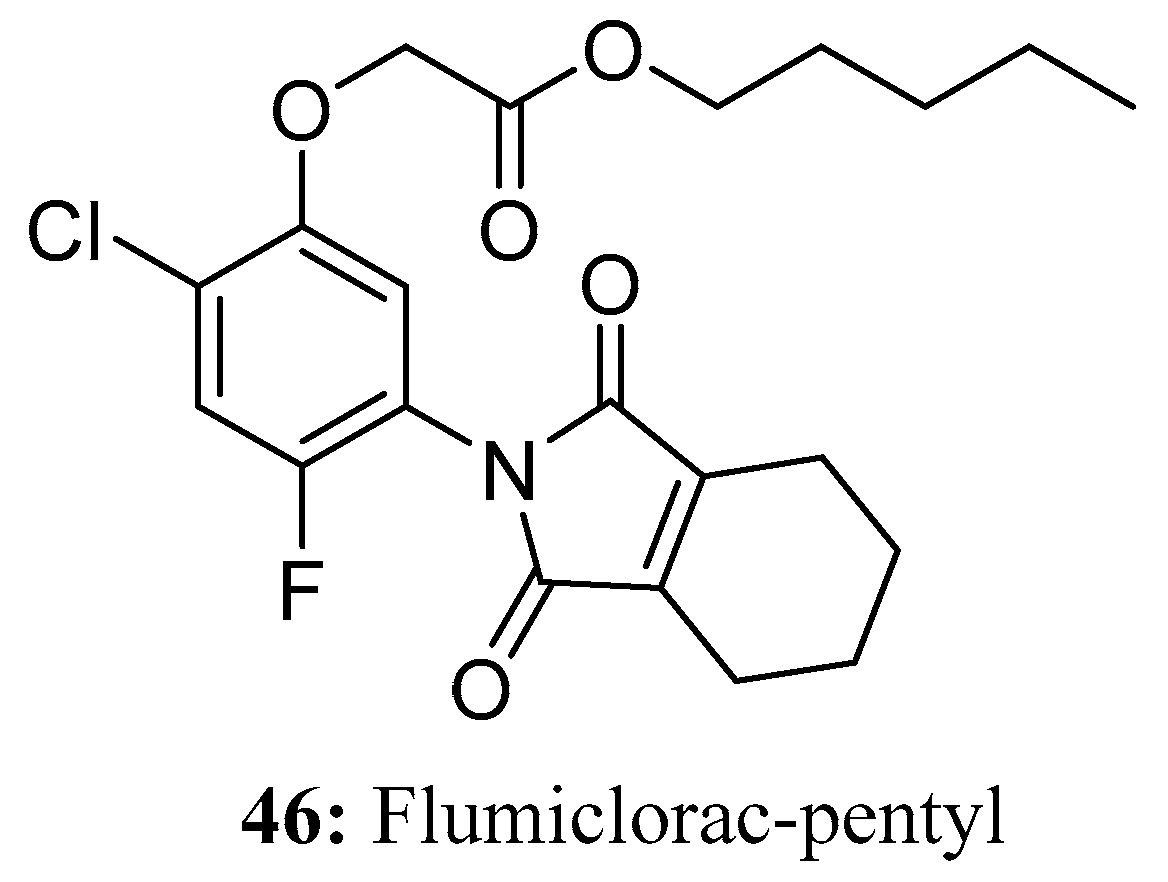
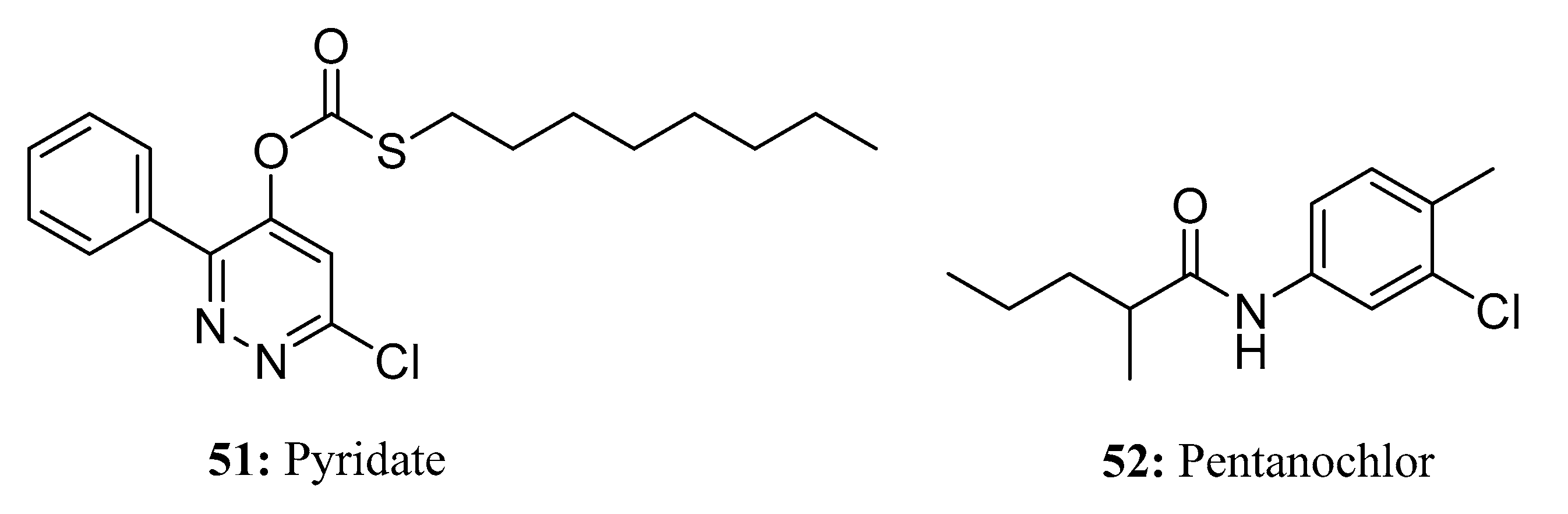
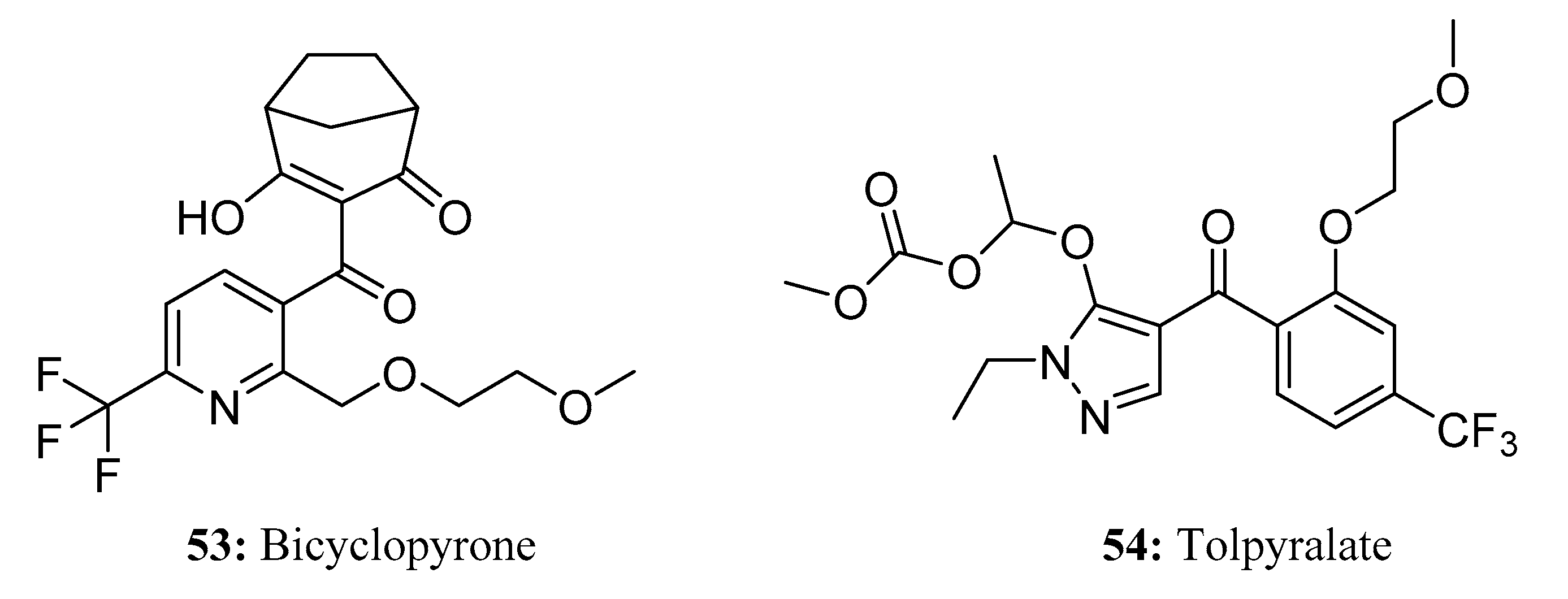
3.3. Herbicides Containing a Long Chain
3.4. Plant Growth Regulators Containing a Long Chain
3.5. Long-Chain Molecules with Rodenticidal Activity
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Long, Z.-Q.; Yang, L.-L.; Zhang, J.-R.; Liu, S.-T.; Xie, J.; Wang, P.-Y.; Zhu, J.-J.; Shao, W.-B.; Liu, L.-W.; Yang, S. Fabrication of Versatile Pyrazole Hydrazide Derivatives Bearing a 1,3,4-Oxadiazole Core as Multipurpose Agricultural Chemicals against Plant Fungal, Oomycete, and Bacterial Diseases. J. Agric. Food Chem. 2021, 69, 8380–8393. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Zhu, J.-K.; Yin, X.-D.; Yan, Y.-F.; Wang, Y.-L.; Shang, X.-F.; Liu, Y.-Q.; Zhao, Z.-M.; Peng, J.-W.; Liu, H. Design, Synthesis, and Antifungal Evaluation of Novel Quinoline Derivatives Inspired from Natural Quinine Alkaloids. J. Agric. Food Chem. 2019, 67, 11340–11353. [Google Scholar] [CrossRef] [PubMed]
- Pahutski, T.F.; Ahmad, O.K.; Marshall, E.A.; Joraski, K.; Barry, J.D.; Keathley, C.; Cordova, D.; Benner, E.; Nesnow, D.; Christianson, L.; et al. Discovery of novel (N-aryl-4-methylpiperidinyl)pyrazoles: A new class of potent lepidopteran insecticides. Pest Manag. Sci. 2023, 79, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, M.; Saikia, S.; Bordoloi, P.K.; Kolita, B.; Dutta, P.P.; Bhuyan, P.D.; Dutta, S.C.; Rao, P.G. Isolation, characterization and antifungal activity of very long chain alkane derivatives from Cinnamomum obtusifolium, Elaeocarpus lanceifolius and Baccaurea sapida. J. Mol. Struct. 2017, 1142, 200–210. [Google Scholar] [CrossRef]
- Scott, S.; Cahoon, E.B.; Busta, L. Variation on a theme: The structures and biosynthesis of specialized fatty acid natural products in plants. Plant J. 2022, 111, 954–965. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Stoyanova-Ivanova, B.; Khadzhieva, P.; Popov, S. Composition, structure and biogenesis of the ketones in rose flower wax. Phytochemistry 1969, 8, 1549. [Google Scholar] [CrossRef]
- Kokpol, U.; Chavasiri, W.; Chittawong, V.; Bruce, M.; Cunningham, G.; Miles, D. Long chain aliphatic alcohols and saturated carboxylic acids from heartwood of Rhizophora apiculata. Phytochemistry 1993, 33, 1129–1131. [Google Scholar] [CrossRef]
- Misra, T.N.; Singh, R.S.; Pandey, H.S.; Prasad, C.; Singh, B.P. Antifungal essential oil and a long chain alcohol from Achyranthes aspera. Phytochemistry 1992, 31, 1811–1812. [Google Scholar] [CrossRef]
- Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal Activities of Four Fatty Acids against Plant Pathogenic Fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Vidal, D.M.; von Rymon-Lipinski, A.-L.; Ravella, S.; Groenhagen, U.; Herrmann, J.; Zaburannyi, N.; Zarbin, P.H.G.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; et al. Long-Chain Alkyl Cyanides: Unprecedented Volatile Compounds Released by Pseudomonas and Micromonospora Bacteria. Angew. Chem. Int. Ed. Engl. 2017, 56, 4342–4346. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, S.; Gan, M.; Chen, M.; Li, L.; Wang, S.; Zi, J.; Fan, X.; Liu, Y.; Si, Y.; et al. Yaoshanenolides A and B: New Spirolactones from the Bark of Machilus yaoshansis. Org. Lett. 2012, 14, 1004–1007. [Google Scholar] [CrossRef]
- Komae, H.; Hayashi, N.; Kosela, S. Palmitone from the fruits of Lindera citriodora. Phytochemistry 1971, 10, 3311. [Google Scholar] [CrossRef]
- Ho, C.-L.; Hsu, K.-P.; Wang, E.I.-C.; Su, Y.-C. Composition and Antimicrobial Activity of the Leaf Essential Oil of Machilus obovatifolia from Taiwan. J. Essent. Oil Res. 2009, 21, 471–475. [Google Scholar] [CrossRef]
- Nowicka, B.; Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1587–1605. [Google Scholar] [CrossRef] [PubMed]
- Abby, S.S.; Kazemzadeh, K.; Vragniau, C.; Pelosi, L.; Pierrel, F. Advances in bacterial pathways for the biosynthesis of ubiquinone. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148259. [Google Scholar] [CrossRef]
- Siebert, M.; Severin, K.; Heide, L. Formation of 4-hydroxybenzoate in Escherichia coli: Characterization of the ubiC gene and its encoded enzyme chorismate pyruvate-lyase. Microbiology 1994, 140, 897–904. [Google Scholar] [CrossRef]
- White, M.D.; Payne, K.A.P.; Fisher, K.; Marshall, S.A.; Parker, D.; Rattray, N.J.W.; Trivedi, D.K.; Goodacre, R.; Rigby, S.E.J.; Scrutton, N.S.; et al. UbiX is a flavin prenyltransferase required for bacterial ubiquinone biosynthesis. Nature 2015, 522, 502–506. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, B.; Zhang, X.; Fan, X.; Niu, L.; Li, X.; Wang, J.; Teng, M. Structural and biochemical studies reveal UbiG/Coq3 as a class of novel membrane-binding proteins. Biochem. J. 2015, 470, 105–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Wang, C.; Liu, Y.; Fan, X.; Zhang, L.; Niu, L.; Teng, M.; Li, X. Structural insights into the methyl donor recognition model of a novel membrane-binding protein UbiG. Sci. Rep. 2016, 6, 23147. [Google Scholar] [CrossRef]
- Kwon, O.; Kotsakis, A.; Meganathan, R. Ubiquinone (coenzyme Q) biosynthesis in Escherichia coli: Identification of the ubiF gene. FEMS Microbiol. Lett. 2000, 186, 157–161. [Google Scholar] [CrossRef][Green Version]
- Chehade, M.H.; Loiseau, L.; Lombard, M.; Pecqueur, L.; Ismail, A.; Smadja, M.; Golinelli-Pimpaneau, B.; Mellot-Draznieks, C.; Hamelin, O.; Aussel, L.; et al. ubiI, a New Gene in Escherichia coli Coenzyme Q Biosynthesis, Is Involved in Aerobic C5-hydroxylation. J. Biol. Chem. 2013, 288, 20085–20092. [Google Scholar] [CrossRef]
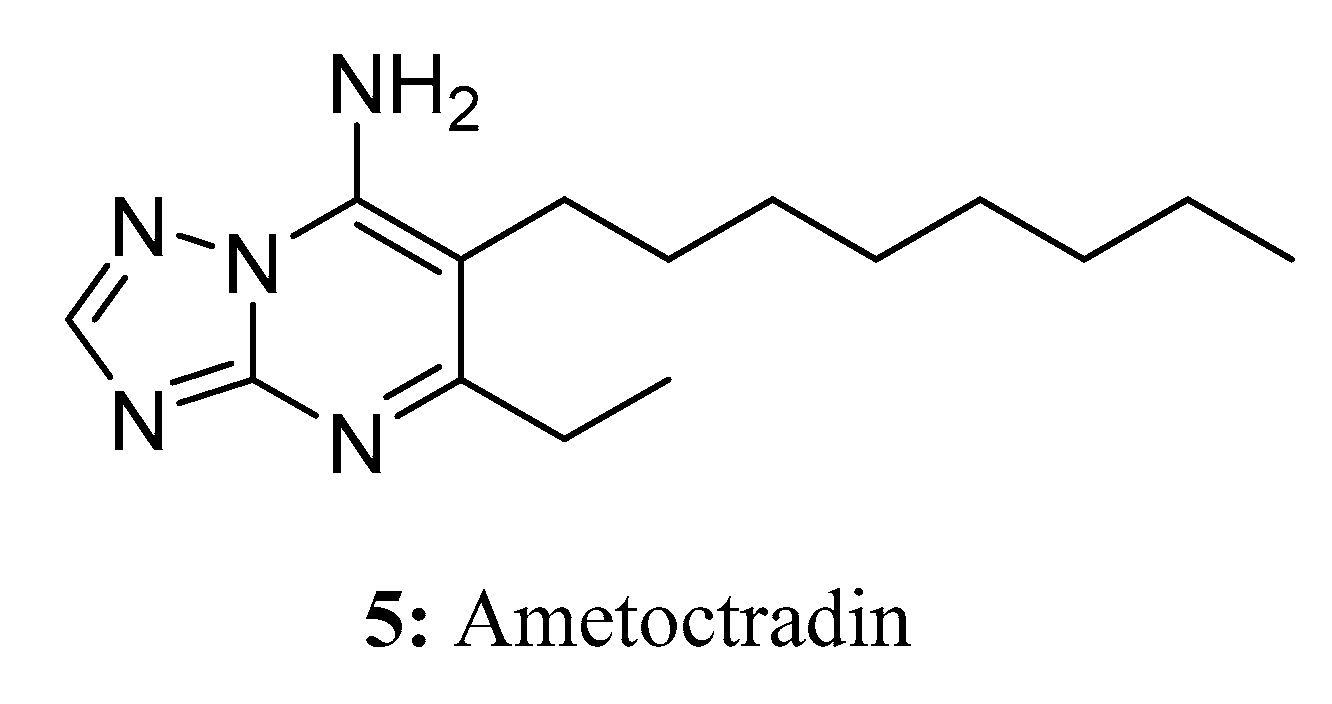
- Fehr, M.; Wolf, A.; Stammler, G. Binding of the respiratory chain inhibitor ametoctradin to the mitochondrial bc1 complex. Pest Manag. Sci. 2016, 72, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.-S.; Fu, X.-C.; Sun, X.-F.; Li, Q.; Li, Z.-N. Synthesis of ametoctradin and its bioactivity. Nongyao 2012, 51, 645-646–674. [Google Scholar]
- Gao, X.; Hu, S.; Liu, Z.; Zhu, H.; Yang, J.; Han, Q.; Fu, Y.; Miao, J.; Gu, B.; Liu, X. Analysis of resistance risk and resistance-related point mutations in Cyt b of QioI fungicide ametoctradin in Phytophthora litchii. Pest Manag. Sci. 2022, 78, 2921–2930. [Google Scholar] [CrossRef]
- Dreinert, A.; Wolf, A.; Mentzel, T.; Meunier, B.; Fehr, M. The cytochrome bc complex inhibitor Ametoctradin has an unusual binding mode. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 567–576. [Google Scholar] [CrossRef]
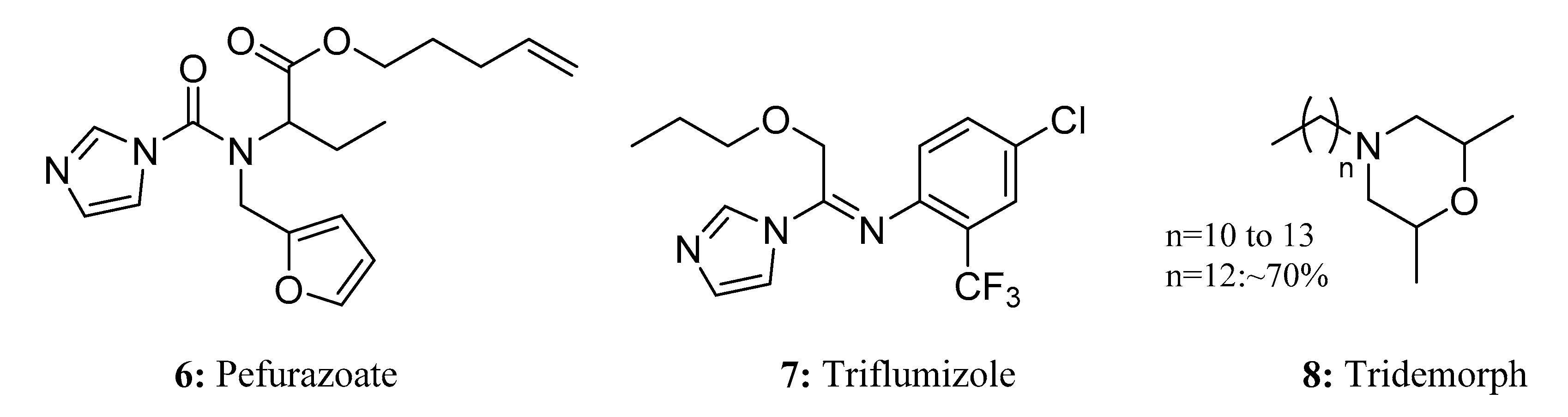
- Takenaka, M.; Kimura, S.; Tanaka, T.; Wada, T. Chiral Synthesis of Pefurazoate Enantiomers and Their Antifungal Activity to Gibberella fujikuroi. J. Pestic. Sci. 1992, 17, 205–211. [Google Scholar] [CrossRef]
- Takenaka, M.; Nishimura, T.; Hayashi, K. Enantioselective Antifungal Activity of Pefurazoate against Pathogens of Rice Seed Diseases. Nippon. Noyaku Gakkaishi 2001, 26, 347–353. [Google Scholar] [CrossRef][Green Version]
- Xi, J.; Shao, J.; Wang, Y.; Wang, X.; Yang, H.; Zhang, X.; Xiong, D. Acute toxicity of triflumizole to freshwater green algae Chlorella vulgaris. Pestic. Biochem. Physiol. 2019, 158, 135–142. [Google Scholar] [CrossRef]
- Nakata, A.; Hashimoto, S.; Ikura, K.; Katsuura, K. Development of a New Fungicide, Triflumizole. Nippon. Noyaku Gakkaishi 1991, 16, 301–313. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Song, J.-G.; Lee, I.-K.; Yeo, W.-H.; Yun, B.-S. Bacillus sp. BS061 Suppresses Powdery Mildew and Gray Mold. Mycobiology 2013, 41, 108–111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markoglou, A.N.; Ziogas, B.N. Genetic control of resistance to tridemorph inustilago maydis. Phytoparasitica 2000, 28, 349–360. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Liu, Y. Residual behavior and risk assessment of tridemorph in banana conditions. Food Chem. 2018, 244, 71–74. [Google Scholar] [CrossRef]
- Kerkenaar, A.; Uchiyama, M.; Versluis, G. Specific effects of tridemorph on sterol biosynthesis in Ustilago maydis. Pestic. Biochem. Physiol. 1981, 16, 97–104. [Google Scholar] [CrossRef]
- Guin, D.; Mittal, S.; Bozymski, B.; Shukla, D.; Gruebele, M. Dodine as a Kosmo-Chaotropic Agent. J. Phys. Chem. Lett. 2019, 10, 2600–2605. [Google Scholar] [CrossRef]
- Lian, C.; Xu, W.; Luo, Y.; Zhu, X.; Fan, Y.; Redshaw, C.; Tao, Z.; Xiao, X. Detection of the pesticide dodine using a cucurbit [10] uril-based fluorescent probe. Microchem. J. 2021, 167, 106309. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, P.C. Does Fungicide “Dodine” Unfold Protein like Kosmo-Chaotropic Agent? J. Phys. Chem. B 2019, 123, 8240–8246. [Google Scholar] [CrossRef]
- Feng, Y.; Han, J.; Liang, L.; Ma, X.; Zhang, A.; Bian, Y. Residues, storage stability, and long-term dietary risk assessment of Xinjunan in cucumber, tomato, and citrus. Int. J. Environ. Anal. Chem. 2022. ahead of print. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Li, Y.; Lai, Q. The Development and Application of Novel Technology of Xinjunan in the Soil-borne Disease Control. Shijie Nongyao 2020, 42, 46–48. [Google Scholar] [CrossRef]
- Han, C.; Hu, B.; Chen, S.; Wang, N.; Hou, J.; Jin, N.; Shen, Y. Determination of Xinjunan pesticide residue in foodstuffs of plant origin by a modified QuEChERS method and ultra performance liquid chromatography-tandem mass spectrometry. LWT 2021, 151, 112101. [Google Scholar] [CrossRef]
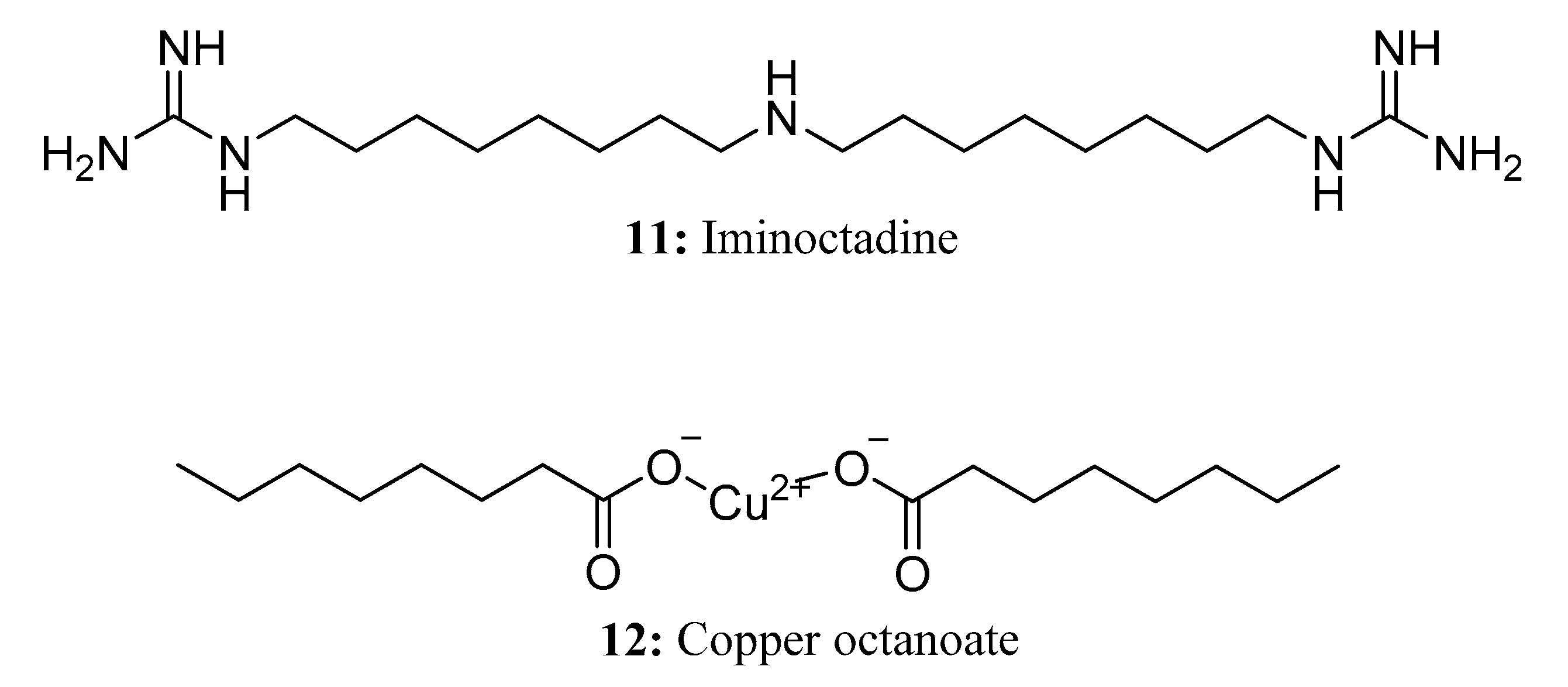
- Smarzewska, S.; Metelka, R.; Guziejewski, D.; Skowron, M.; Skrzypek, S.; Brycht, M.; Ciesielski, W. Voltammetric behaviour and quantitative determination of pesticide iminoctadine. Anal. Methods 2014, 6, 1884–1889. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance guazatine (variant assessed guazatine acetates). EFSA J. 2010, 8, 1708. [Google Scholar] [CrossRef]
- Brown, G.E. Efficacy of Guazatine and Iminoctadine for Control of Postharvest Decays of Oranges. Plant Dis. 1988, 72, 906. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, L.; Ma, D.; Gao, Y.; Mu, W.; Liu, F. A bioactivity and biochemical analysis of iminoctadine tris (albesilate) as a fungicide against Corynespora cassiicola. Pestic. Biochem. Physiol. 2019, 158, 121–127. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crops Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Bika, R.; Jennings, C.; Palmer, C.; Simmons, T. Comparative Performance of Chemical and Biological-based Products in Management of Algal Leaf Spot on Magnolia. Horttechnology 2020, 30, 733–740. [Google Scholar] [CrossRef]
- Clifford, D.R.; Gendle, P.; Holgate, M.E.; Hunter, T. Comparison of paint and gel formulations for the treatment of Nectria cankers on apple trees. Ann. Appl. Biol. 1987, 110, 471–487. [Google Scholar] [CrossRef]
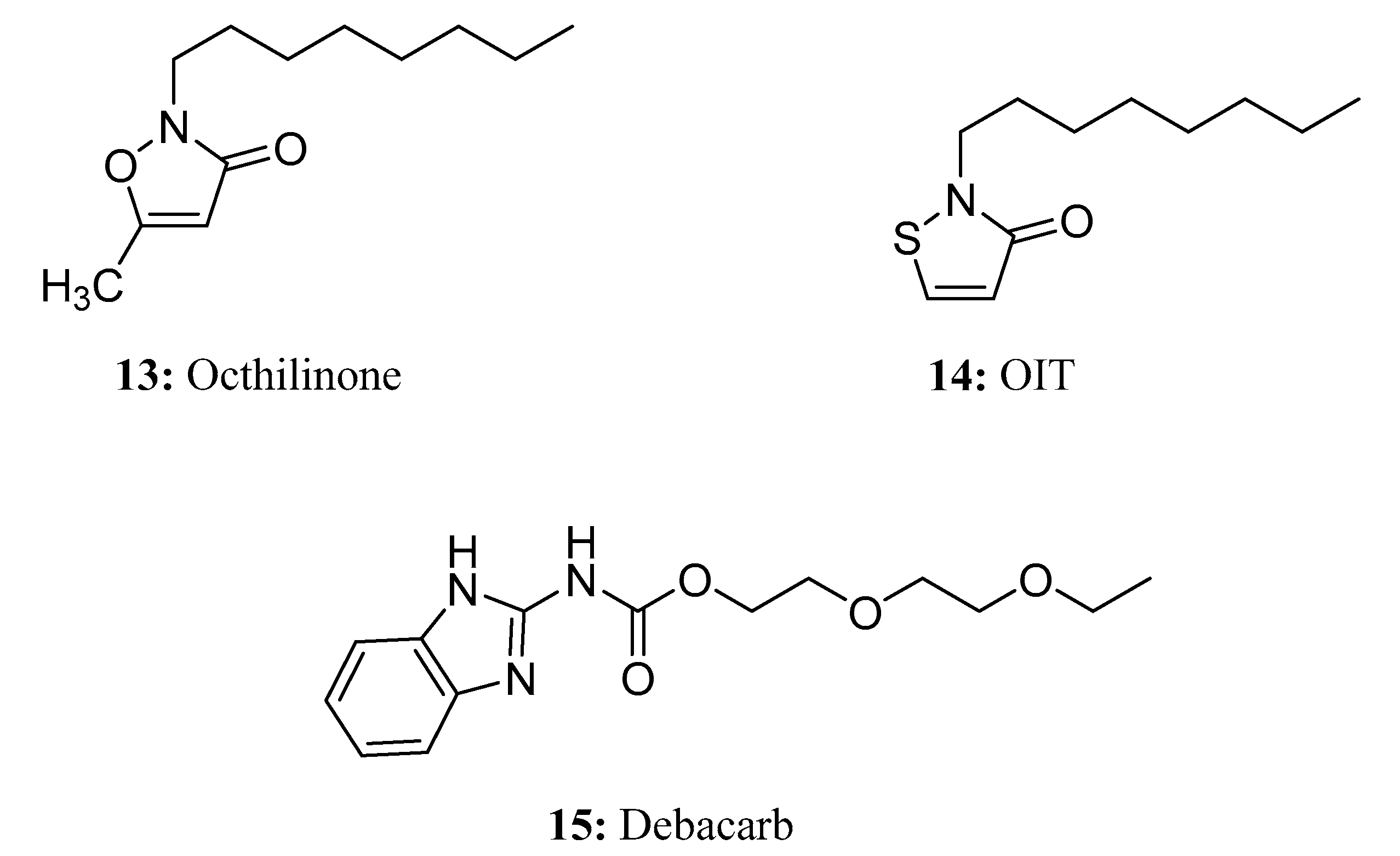
- Kresmann, S.; Arokia, A.H.R.; Koch, C.; Sures, B. Ecotoxicological potential of the biocides terbutryn, octhilinone and methylisothiazolinone: Underestimated risk from biocidal pathways? Sci. Total. Environ. 2018, 625, 900–908. [Google Scholar] [CrossRef]
- Nagorka, R.; Gleue, C.; Scheller, C.; Moriske, H.-J.; Straff, W. Isothiazolone emissions from building products. Indoor Air 2015, 25, 68–78. [Google Scholar] [CrossRef]
- Mayorquin, J.S.; Carrillo, J.D.; Twizeyimana, M.; Peacock, B.B.; Sugino, K.Y.; Na, F.; Wang, D.H.; Kabashima, J.N.; Eskalen, A. Chemical Management of Invasive Shot Hole Borer and Fusarium Dieback in California Sycamore (Platanus racemosa) in Southern California. Plant Dis. 2018, 102, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Singh, R.; Sati, O.P.; Walia, S.; Garg, N. Synthesis and antimicrobial activity of esters of 3-ethoxy-4-hydroxybenzaldehyde oxime. Toxicol. Environ. Chem. 2016, 99, 1–9. [Google Scholar] [CrossRef]
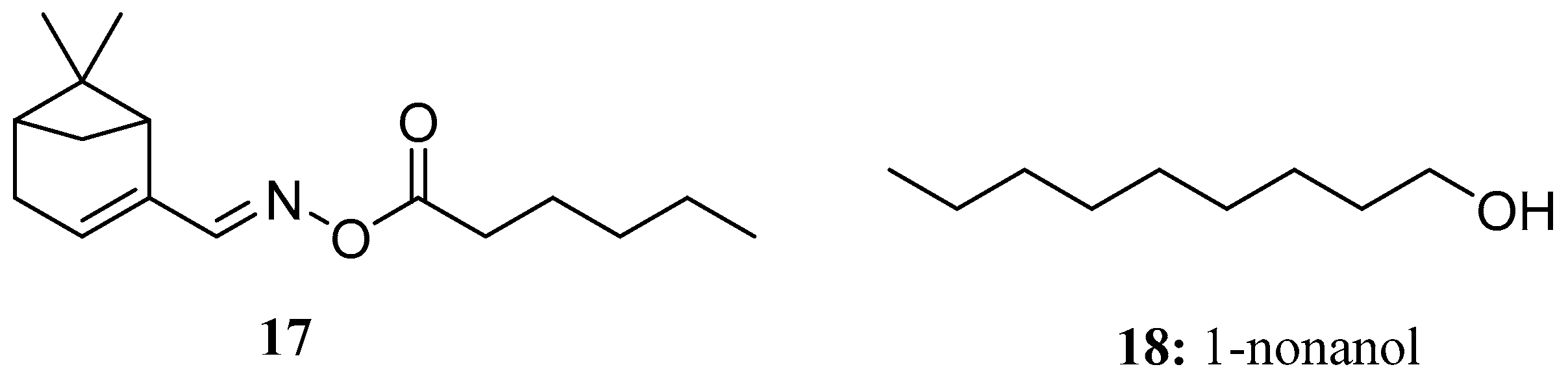
- Lin, G.-S.; Bai, X.; Duan, W.; Cen, B.; Huang, M.; Lu, S. High Value-Added Application of Sustainable Natural Forest Product α-Pinene: Synthesis of Myrtenal Oxime Esters as Potential KARI Inhibitors. ACS Sustain. Chem. Eng. 2019, 7, 7862–7868. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Qin, Y.-L.; Li, S.-F.; Lv, Y.-Y.; Zhai, H.-C.; Hu, Y.-S.; Cai, J.-P. Antifungal mechanism of 1-nonanol against Aspergillus flavus growth revealed by metabolomic analyses. Appl. Microbiol. Biotechnol. 2021, 105, 7871–7888. [Google Scholar] [CrossRef]
- Liu, W.-S.; Wang, C.-H.; Sun, J.-F.; Hou, G.-G.; Wang, Y.-P.; Qu, R.-J. Synthesis, Characterization and Antibacterial Properties of Dihydroxy Quaternary Ammonium Salts with Long Chain Alkyl Bromides. Chem. Biol. Drug Des. 2015, 85, 91–97. [Google Scholar] [CrossRef]
- Muhizi, T.; Grelier, S.; Coma, V. Synthesis of N-Alkyl-β-d-glucosylamines and Their Antimicrobial Activity against Fusarium proliferatum, Salmonellatyphimurium, and Listeria innocua. J. Agric. Food Chem. 2009, 57, 11092–11099. [Google Scholar] [CrossRef]
- Mandal, S.; Kanrar, B.; Das, S.; Bhattacharyya, A. Analytical Method Validation for the Determination of Meptyldinocap As 2,4-Dinitrooctylphenol Metabolite in Mango and Soil Using LC−MS/MS and Dissipation Study of the Fungicide in Indian Mango Field Ecosystem. J. Agric. Food Chem. 2010, 58, 8911–8917. [Google Scholar] [CrossRef]
- Černohlávková, J.; Jarkovský, J.; Hofman, J. Effects of fungicides mancozeb and dinocap on carbon and nitrogen mineralization in soils. Ecotoxicol. Environ. Saf. 2009, 72, 80–85. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Lofgren, C.S.; Williams, D.F. Fluoroaliphatic Sulfones: A New Class of Delayed-action Insecticides for Control of Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 1985, 78, 1190–1197. [Google Scholar] [CrossRef]
- Nascimento, R.A.; Nunoo, D.B.; Bizkarguenaga, E.; Schultes, L.; Zabaleta, I.; Benskin, J.P.; Spanó, S.; Leonel, J. Sulfluramid use in Brazilian agriculture: A source of per- and polyfluoroalkyl substances (PFASs) to the environment. Environ. Pollut. 2018, 242, 1436–1443. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koura, Y.; Kariya, H.; Ohsaki, N.; Watanabe, T. AKD-2023: A novel miticide. Biological activity and mode of action. Pestic. Sci. 1999, 55, 659–660. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, P.; Van Leeuwen, T.; Khajehali, J.; Vanholme, B.; Tirry, L. Mutations in the mitochondrial cytochromeb of Tetranychus urticae Koch (Acari: Tetranychidae) confer cross-resistance between bifenazate and acequinocyl. Pest Manag. Sci. 2009, 65, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Dekeyser, M. Acaricide mode of action. Pest Manag. Sci. 2005, 61, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fenical, W. The unique chemistry and biology of the piericidins. J. Antibiot. 2016, 69, 582–593. [Google Scholar] [CrossRef]
- Bridges, H.R.; Fedor, J.G.; Blaza, J.N.; Di Luca, A.; Jussupow, A.; Jarman, O.D.; Wright, J.J.; Agip, A.-N.A.; Gamiz-Hernandez, A.P.; Roessler, M.M.; et al. Structure of inhibitor-bound mammalian complex I. Nat. Commun. 2020, 11, 5261. [Google Scholar] [CrossRef]
- Tamura, S.; Takahashi, N.; Miyamoto, S.; Mori, R.; Suzuki, S.; Nagatsu, J. Isolation and Physiological Activities of Piericidin A, A Natural Insecticide Produced by Streptomyces. Agric. Biol. Chem. 1963, 27, 576–582. [Google Scholar] [CrossRef]
- Eisinger, M.; Almog, Y. Pyrimidifen intoxication. Ann. Emerg. Med. 2003, 42, 289–291. [Google Scholar] [CrossRef]
- Bai, Y.-l. Acaricides with different modes of action. Xiandai Nongyao 2005, 4, 27–30. [Google Scholar]
- Alali, F.Q.; Liu, X.-X.; McLaughlin, J.L. Annonaceous Acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- Zafra-Polo, M.C.; González, M.C.; Estornell, E.; Sahpaz, S.; Cortes, D. Acetogenins from annonaceae, inhibitors of mitochondrial complex I. Phytochemistry 1996, 42, 253–271. [Google Scholar] [CrossRef]
- Lümmen, P. Complex I inhibitors as insecticides and acaricides. Biochim. Biophys. Acta Bioenerg. 1998, 1364, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bazire, A.; Gillon, E.; Lockridge, O.; Vallet, V.; Nachon, F. The kinetic study of the inhibition of human cholinesterases by demeton-S-methyl shows that cholinesterase-based titration methods are not suitable for this organophosphate. Toxicol. Vitr. 2011, 25, 754–759. [Google Scholar] [CrossRef]
- Dubois, K.P.; Plazak, G.J. The acute toxicity and anticholinesterase action of O,O-dimethyl S-ethyl-2-sulfinylethyl phosphorothioate (Meta-Systox R) and related compounds. Toxicol. Appl. Pharmacol. 1962, 4, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Szeto, S.Y.; Vernon, R.S.; Brown, M.J. Degradation of disulfoton in soil and its translocation into asparagus. J. Agric. Food Chem. 1983, 31, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Futagami, K.; Otsubo, K.; Nakao, Y.; Aoyama, T.; Iimori, E.; Urakami, S.; Ide, M.; Oishi, R. Acute Organophosphate Poisoning After Disulfoton Ingestion. J. Toxicol. Clin. Toxicol. 1995, 33, 151–155. [Google Scholar] [CrossRef]
- Usui, K.; Hayashizaki, Y.; Minagawa, T.; Hashiyada, M.; Nakano, A.; Funayama, M. Rapid determination of disulfoton and its oxidative metabolites in human whole blood and urine using QuEChERS extraction and liquid chromatography–tandem mass spectrometry. Leg. Med. 2012, 14, 309–316. [Google Scholar] [CrossRef]
- Qader, B.; Baron, M.; Hussain, I.; Sevilla, J.; Johnson, R.P.; Gonzalez-Rodriguez, J. Electrochemical determination of disulfoton using a molecularly imprinted poly-phenol polymer. Electrochimica Acta 2018, 295, 333–339. [Google Scholar] [CrossRef]
- Lammerink, J.; Banfield, R.A. Effect of disulfoton on growth of aphid-free oilseed rape. N. Z. J. Exp. Agric. 1979, 7, 221–223. [Google Scholar] [CrossRef]
- Li, H.; Pan, L.; Yu, C.; Zhang, X.; Cui, X.; Luo, T.; Cao, Z.; Wang, J.; Li, Q. Development and Validation for Simultaneous Determination of Disulfoton and Its Five Metabolites in Seven Agro-Products Using Liquid Chromatography-Tandem Mass Spectrometry Combined with QuEChERS Extraction Method. Chromatographia 2022, 85, 529–537. [Google Scholar] [CrossRef]
- Tabrizi, A.B.; Abdollahi, A. Determination of Organothiophosphate Insecticides in Environmental Water Samples by a Very Simple and Sensitive Spectrofluorimetric Method. Bull. Environ. Contam. Toxicol. 2015, 95, 536–541. [Google Scholar] [CrossRef]
- Parham, H.; Saeed, S. Resonance Rayleigh scattering method for determination of ethion using silver nanoparticles as probe. Talanta 2015, 131, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Khaled, E.; Kamel, M.S.; Hassan, H.; Abdel-Gawad, H.; Aboul-Enein, H.Y. Performance of a portable biosensor for the analysis of ethion residues. Talanta 2014, 119, 467–472. [Google Scholar] [CrossRef]
- Pang, Y.-P.; Singh, S.K.; Gao, Y.; Lassiter, T.L.; Mishra, R.K.; Zhu, K.Y.; Brimijoin, S. Selective and Irreversible Inhibitors of Aphid Acetylcholinesterases: Steps Toward Human-Safe Insecticides. PLoS ONE 2009, 4, e4349. [Google Scholar] [CrossRef] [PubMed]
- Kryger, G.; Harel, M.; Giles, K.; Toker, L.; Velan, B.; Lazar, A.; Kronman, C.; Barak, V.; Ariel, N.; Shafferman, A.; et al. Structures of recombinant native and E202Q mutant human acetylcholinesterase complexed with the snake-venom toxin fasciculin-II. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.-P. Novel Acetylcholinesterase Target Site for Malaria Mosquito Control. PLoS ONE 2006, 1, e58. [Google Scholar] [CrossRef]
- Pezzementi, L.; Rowland, M.; Wolfe, M.; Tsigelny, I. Inactivation of an invertebrate acetylcholinesterase by sulfhydryl reagents: The roles of two cysteines in the catalytic gorge of the enzyme. Invertebr. Neurosci. 2006, 6, 47–55. [Google Scholar] [CrossRef]
- Isobe, N.; Suzuki, T.; Nishikawa, J.-I.; Kaneko, H.; Nakatsuka, I.; Yoshitake, A. Metabolism of Empenthrin Isomers in Rats. J. Pestic. Sci. 1992, 17, 27–37. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsuda, S.; Okuno, Y. Practical application of empenthrin as a mothproofer of textile. Sen’i Gakkaishi 1984, 40, T254–T262. [Google Scholar] [CrossRef]
- Kanamaru, H.; Kawahara, K.; Nishioka, K. Stereoselective synthesis of empenthrin, a novel insecticide against fabric pests, in a regioselectively 14C-labeled form. Radioisotopes 1991, 40, 136–141. [Google Scholar] [CrossRef][Green Version]
- Degitz, S.J.; Durhan, E.J.; Tietge, J.E.; Kosian, P.A.; Holcombe, G.W.; Ankley, G.T. Developmental toxicity of methoprene and several degradation products in Xenopus laevis. Aquat. Toxicol. 2003, 64, 97–105. [Google Scholar] [CrossRef]
- Naruse, S.; Washidu, Y.; Miura, K.; Shinoda, T.; Minakuchi, C. Methoprene-tolerant is essential for embryonic development of the red flour beetle Tribolium castaneum. J. Insect Physiol. 2020, 121, 104017. [Google Scholar] [CrossRef] [PubMed]
- Wijayaratne, L.; Fields, P.G. Effect of methoprene on the heat tolerance and cold tolerance of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2010, 46, 166–173. [Google Scholar] [CrossRef]
- Bai, H.; Gelman, D.B.; Palli, S.R. Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles gambiae. Pest Manag. Sci. 2010, 66, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Daglish, G.J.; Holloway, J.C.; Nayak, M.K. Implications of methoprene resistance for managing Rhyzopertha dominica (F.) in stored grain. J. Stored Prod. Res. 2013, 54, 8–12. [Google Scholar] [CrossRef]
- Aubuchon, M.D.; Mullen, G.R.; Eubanks, M.D. Efficacy of broadcast and perimeter applications of S-methoprene bait on the red imported fire ant in grazed pastures. J. Econ. Entomol. 2006, 99, 621–625. [Google Scholar] [CrossRef]
- Moser, B.A.; Koehler, P.G.; Patterson, R.S. Effect of Methoprene and Diflubenzuron on Larval Development of the Cat Flea (Siphonaptera: Pulicidae). J. Econ. Entomol. 1992, 85, 112–116. [Google Scholar] [CrossRef]
- Wilson, T.G. The molecular site of action of juvenile hormone and juvenile hormone insecticides during metamorphosis: How these compounds kill insects. J. Insect Physiol. 2004, 50, 111–121. [Google Scholar] [CrossRef]
- Mohandass, S.; Arthur, F.; Zhu, K.; Throne, J. Hydroprene: Mode of action, current status in stored-product pest management, insect resistance, and future prospects. Crops Prot. 2006, 25, 902–909. [Google Scholar] [CrossRef]
- Mori, K.; Takigawa, T.; Manabe, Y.; Tominaga, M.; Matsui, M.; Kiguchi, K.; Akai, H.; Ohtaki, T. Synthesis of compounds with juvenile hormone activity. XXI. Effect of the molecular chain length on biological activity of juvenile hormone analogs. Agric. Biol. Chem. 1975, 39, 259–265. [Google Scholar] [CrossRef][Green Version]
- Kisida, H.; Hatakoshi, M.; Itaya, N.; Nakayama, I. New Insect Juvenile Hormone Mimics: Thiolcarbamates. Agric. Biol. Chem. 1984, 48, 2889–2891. [Google Scholar] [CrossRef]
- Jones, K.A. Environmentally-Safe Bicarbonate-Containing Pesticide Compositions. WO9400982A1, 20 January 1994. [Google Scholar]
- Serre, I.; Cabanne, F.; Gauvrit, C. seed oil derivatives as adjuvants: Influence of methyl to octadecyl oleates on the penetration of herbicides through various plant cuticles. Meded.-Fac. Landbouwkd. Toegepaste Biol. Wet. 1993, 58, 795–802. [Google Scholar]
- Davidson, W.S.; Saxena, R.K.; Gupta, R. The fungistatic action of oleic acid. Curr. Sci. 1999, 76, 1137–1140. [Google Scholar]
- Duan, W.; Zhao, B.; Liu, Y. Cyhalothrin Emulsified Concentrate with Methyl Oleate as Solvent and Its Preparation Method. CN105532652, 4 May 2016. [Google Scholar]
- Li, X.; Jiang, J.; Wang, Q.; Qi, P.; Liu, X.; Li, R.; Zhou, K.; Li, H.; Chen, Z.; Jin, L. Tolfenpyrad Electrostatic Spray Liquid and Its Application in Controlling Tea Leafhopper. CN111357749, 3 July 2020. [Google Scholar]
- Wang, Y.; Hu, X.; Wang, L. Methyl Oleate Emulsifier for Pesticide and Preparation Method Thereof. CN110558315, 13 December 2019. [Google Scholar]
- Dai, G.; Chen, Y. Application of Ethyl oleate and Ethyl Oleate Fungicide. CN104957135, 7 October 2015. [Google Scholar]
- Dai, G.; Chen, Y. Application of Ethyl Oleate and Ethyl Oleate Acaricide. CN104996423, 28 October 2015. [Google Scholar]
- Gray, K.C.; Heider, P.; McGough, P.; Ondari, M.; Devaraj, J.; Yang, Q.; Frycek, G.; Graham, B.; Neuman, J.; Lorsbach, B.A.; et al. Development of a Scalable Process for the Insecticidal Candidate Tyclopyrazoflor. Part 3. A Scalable Synthesis of Methyl 3-((3,3,3-Trifluoropropyl)thio)propanoate via Thiol–Ene Chemistry. Org. Process. Res. Dev. 2019, 23, 2142–2147. [Google Scholar] [CrossRef]
- Buysse, A.M.; Niyaz, N.M.; Zhang, Y.; Walsh, M.J.; Kubota, A.; Hunter, R.; Trullinger, T.K.; Lowe, C.T.; Knueppel, D.; Demeter, D.A.; et al. Preparation of Pyridinylpyrazolamine Derivatives as Pesticides and Their Pesticidal Compositions. WO2013162715A2, 31 October 2013. [Google Scholar]
- Yang, Q.; Li, X.; Lorsbach, B.A.; Muhuhi, J.M.; Roth, G.A.; Gray, K.; Podhorez, D.E. Development of a Scalable Process for the Insecticidal Candidate Tyclopyrazoflor. Part 2. Fit-for-Purpose Optimization of the Route to Tyclopyrazoflor Featuring [3 + 2] Cyclization of 3-Hydrazinopyridine·2HCl and Methyl Acrylate. Org. Process. Res. Dev. 2019, 23, 2133–2141. [Google Scholar] [CrossRef]
- Chen, M.; Li, Z.; Shao, X.; Maienfisch, P. Bioisosteric-Replacement-Driven Lead Optimization of Tyclopyrazoflor. J. Agric. Food Chem. 2022, 70, 11123–11137. [Google Scholar] [CrossRef]
- Wang, J.; Yu, S.; Wang, L.; Liu, T.; Yang, X.; Hu, X.; Wang, Y. Capsaicin decreases fecundity in the Asian malaria vector Anopheles stephensi by inhibiting the target of rapamycin signaling pathway. Parasites Vectors 2022, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Edelson, J.V.; Duthie, J.; Roberts, W. Toxicity of biorational insecticides: Activity against the green peach aphid, Myzus persicae (Sulzer). Pest Manag. Sci. 2002, 58, 255–260. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P.; Wei, L.; Kang, R.; Chen, L.; Zhang, M.; Tan, E.K.; Liu, W. Capsaicin Functions as Drosophila Ovipositional Repellent and Causes Intestinal Dysplasia. Sci. Rep. 2020, 10, 9963. [Google Scholar] [CrossRef]
- Domon, K.; Toriyabe, K.; Ogawa, Y.; Bessho, J.; Kawamoto, K.; Watanabe, A.; Komatsu, M.; Matsuda, T.; Ito, S. Preparation of Alkylphenylsulphide Derivatives as Pest Control Agents. WO2013157229A1, 24 October 2013. [Google Scholar]
- Powell, G.F.; Ward, D.A.; Prescott, M.C.; Spiller, D.G.; White, M.R.; Turner, P.C.; Earley, F.G.; Phillips, J.; Rees, H.H. The molecular action of the novel insecticide, Pyridalyl. Insect Biochem. Mol. Biol. 2011, 41, 459–469. [Google Scholar] [CrossRef]
- Sakamoto, N.; Hirose, T.; Saito, S.; Umeda, K. Discovery and development of pyridalyl. J. Pestic. Sci. 2012, 37, 265–266, 283–287. [Google Scholar] [CrossRef]
- Sakamoto, N.; Matsuo, S.; Suzuki, M.; Hirose, T.; Tsushima, K.; Umeda, K. Preparation of Dihalopropene Insecticides and Acaricides. WO9611909A1, 25 April 1996. [Google Scholar]
- Isayama, S.; Saito, S.; Kuroda, K.; Umeda, K.; Kasamatsu, K. Pyridalyl, a novel insecticide: Potency and insecticidal selectivity. Arch. Insect Biochem. Physiol. 2005, 58, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Saito, S.; Hirose, T.; Suzuki, M.; Matsuo, S.; Izumi, K.; Nagatomi, T.; Ikegami, H.; Umeda, K.; Tsushima, K.; et al. The discovery of pyridalyl: A novel insecticidal agent for controlling lepidopterous pests. Pest Manag. Sci. 2003, 60, 25–34. [Google Scholar] [CrossRef]
- Steltenkamp, R.J.; Hamilton, R.L.; Cooper, R.A.; Schal, C. Alkyl and Aryl Neoalkanamides: Highly Effective Insect Repellents. J. Med. Entomol. 1992, 29, 141–149. [Google Scholar] [CrossRef]
- Rasmussen, H.T.; Friedman, S.K.; Mustilli, A.J.; McDonough, R.; McPherson, B.P. Analysis of Methyl Neodecanamide in Lake Water by Reversed-Phase High Performance Liquid Chromatography and Gas Chromatography-Mass Spectrometry. J. Liq. Chromatogr. 1994, 17, 589–601. [Google Scholar] [CrossRef]
- Campbell, M.M. A test for repellency to non-biting flies and a comparison of repellents using Musca domestica L. Pestic. Sci. 1983, 14, 199–212. [Google Scholar] [CrossRef]
- Yu, X.; Shi, D.; Zhi, X.; Li, Q.; Yao, X.; Xu, H. Synthesis and quantitative structure–activity relationship (QSAR) study of C7-oxime ester derivatives of obacunone as insecticidal agents. RSC Adv. 2015, 5, 31700–31707. [Google Scholar] [CrossRef]
- Fulde, S.; Kroczynaski, J.; Malinowski, H. Structure-activity relationship for some substituted dialkyl vinyl phosphates. Pestic. Sci. 1980, 11, 20–22. [Google Scholar] [CrossRef]
- Escribà, M.; Barbut, M.; Eras, J.; Canela, R.; Avilla, J.; Balcells, M. Synthesis of Allyl Esters of Fatty Acids and Their Ovicidal Effect on Cydia pomonella (L.). J. Agric. Food Chem. 2009, 57, 4849–4853. [Google Scholar] [CrossRef]
- Sahay, N.; Agarwal, R. MGK-264- pyrethroid synergism against Lymnaea acuminata. Chemosphere 1997, 35, 1011–1021. [Google Scholar] [CrossRef]
- Singh, K.; Singh, A.; Singh, D.K. The use of piperonyl butoxide and MGK-264 to improve the efficacy of some plant-derived molluscicides. Pestic. Sci. 1998, 54, 145–149. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y. Synergistic effects of MGK-264. Zhonghua Weisheng Shachong Yaoxie 2007, 13, 468–469. [Google Scholar]
- Bae, J.-W.; Kwon, W.-S. Piperonyl butoxide, a synergist of pesticides can elicit male-mediated reproductive toxicity. Reprod. Toxicol. 2021, 100, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Marchand, P.A.; Dimier-Vallet, C.; Vidal, R. Biorational substitution of piperonyl butoxide in organic production: Effectiveness of vegetable oils as synergists for pyrethrums. Environ. Sci. Pollut. Res. 2018, 25, 29936–29942. [Google Scholar] [CrossRef]
- Lake, B.G.; Price, R.J.; Scott, M.P.; Chatham, L.R.; Vardy, A.; Osimitz, T.G. Piperonyl butoxide: Mode of action analysis for mouse liver tumour formation and human relevance. Toxicology 2020, 439, 152465. [Google Scholar] [CrossRef]
- Matsunaga, H.; Tomigahara, Y.; Kaneko, H.; Nakatsuka, I.; Yamane, S. Metabolism of pentyl 2-chloro-4-fluoro-5-(3,4,5,6-tetrahydrophthalimido)phenoxyacetate (flumiclorac pentyl, S-23031) in rats. 3. Identification of a reduced form metabolite of flumiclorac pentyl (S-23031) in rats. Nippon. Noyaku Gakkaishi 1997, 22, 133–135. [Google Scholar] [CrossRef][Green Version]
- Soltani, N.; Brown, L.R.; Sikkema, P.H. Weed Control in White Bean with Pethoxamid Tank-Mixes Applied Preemergence. Int. J. Agron. 2018, 2018, 2402696. [Google Scholar] [CrossRef]
- Schlosser, H.G.; Hunt, B.; Teicher, H.B. Herbicidal Combination of Pethoxamid and Picloram. WO2014202092A1, 24 December 2014. [Google Scholar]
- Okamoto, H.; Kato, S.; Kobutani, T.; Ogasawara, M.; Konnai, M.; Takematsu, T. Herbicidally Active N-(1-Arylethenyl)-2-chloroacetamides Bearing an Alkyloxyalkyl Moiety. Agric. Biol. Chem. 1991, 55, 2737–2743. [Google Scholar] [CrossRef]
- Dhareesank, A.; Kobayashi, K.; Usui, K. Phytotoxic activity of pethoxamid in soil under different moisture conditions. Weed Biol. Manag. 2005, 5, 197–202. [Google Scholar] [CrossRef]
- Kumar, J.; Patel, A.; Tiwari, S.; Tiwari, S.; Srivastava, P.K.; Prasad, S.M. Pretilachlor toxicity is decided by discrete photo-acclimatizing conditions: Physiological and biochemical evidence from Anabaena sp. and Nostoc muscorum. Ecotoxicol. Environ. Saf. 2018, 156, 344–353. [Google Scholar] [CrossRef]
- Wu, C.; Lou, X.; Xu, X.; Huang, A.; Zhang, M.; Ma, L. Thermodynamics and Kinetics of Pretilachlor Adsorption on Organobentonites for Controlled Release. ACS Omega 2020, 5, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lou, X.; Huang, A.; Zhang, M.; Ma, L. Thermodynamics and kinetics of pretilachlor adsorption: Implication to controlled release from organobentonites. Appl. Clay Sci. 2020, 190, 105566. [Google Scholar] [CrossRef]
- Swatch, G.K.; Singh, D.P.; Khattar, J.S.; Mohapatra, P.K. Interaction of pretilachlor with PS-II activity of the cyanobacterium Desmonostoc muscorum PUPCCC 405.10. J. Basic Microbiol. 2020, 60, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kaur, P.; Bhullar, M.S. Persistence behaviour of pretilachlor in puddled paddy fields under subtropical humid climate. Environ. Monit. Assess. 2015, 187, 524. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Yu, R.; Zhao, X.; Wang, Q.; Cai, L. Pretilachlor has the potential to induce endocrine disruption, oxidative stress, apoptosis and immunotoxicity during zebrafish embryo development. Environ. Toxicol. Pharmacol. 2016, 42, 125–134. [Google Scholar] [CrossRef]
- Diyanat, M.; Saeidian, H.; Baziar, S.; Mirjafary, Z. Preparation and characterization of polycaprolactone nanocapsules containing pretilachlor as a herbicide nanocarrier. Environ. Sci. Pollut. Res. 2019, 26, 21579–21588. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Wang, H.; Wu, S.; Li, J.; Jin, C.; Xu, H. Polyurea microencapsulate suspension: An efficient carrier for enhanced herbicidal activity of pretilachlor and reducing its side effects. J. Hazard. Mater. 2021, 402, 123744. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Deng, S.; Wang, H.; Ou, X.; Huang, L.; Li, J.; Jin, C. Pretilachlor Releasable Polyurea Microcapsules Suspension Optimization and Its Paddy Field Weeding Investigation. Front. Chem. 2020, 8, 826. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Ngoc, S.T.T.; Duong, D.; Le Ngoc, P. Effect of Pretilachlor on Weedy Rice and Other Weeds in Wet-Seeded Rice Cultivation in South Vietnam. Plant Prod. Sci. 2015, 17, 315–320. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Li, Y.; Zhu, S.; Li, Y.; Yi, J.; Ouyang, Z.; Liu, B.; Mehmood, K.; Hussain, R.; et al. Exposure to the herbicide butachlor activates hepatic stress signals and disturbs lipid metabolism in mice. Chemosphere 2021, 283, 131226. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhou, Z.; Wu, X.; Bhatt, P.; Chen, S. Current insights into the microbial degradation for butachlor: Strains, metabolic pathways, and molecular mechanisms. Appl. Microbiol. Biotechnol. 2021, 105, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Matthes, B.; Böger, P. Chloroacetamides Affect the Plasma Membrane. Z. Naturforsch. C J. Biosci. 2002, 57, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Trenkamp, S.; Martin, W.; Tietjen, K. Specific and differential inhibition of very-long-chain fatty acid elongases from Arabidopsis thaliana by different herbicides. Proc. Natl. Acad. Sci. USA 2004, 101, 11903–11908. [Google Scholar] [CrossRef] [PubMed]
- Beste, C.E. Herbicide Handbook of the Weed Science Society of America, 5th ed.; Weed Science Society of America: Champaign, IL, USA, 1983. [Google Scholar]
- Reddy, S.R.; Babu, T.R.; Sreenivasulu, B. Electrochemical Investigation of carbonyl group containing pesticide monalide in water samples by using differential pulse adsorptive stripping voltammetry. Int. J. Res. Pharm. Life Sci. 2013, 1, 43–47. [Google Scholar]
- Li, L.; Ye, F. Photosynthesis inhibitor herbicides. Pestic. Sci. Adm. 2010, 31, 25–28. [Google Scholar]
- Morgan, W.C.; Stevenson, B.C.; Morgans, A.S. Selective post-emergence chemical control of Solanum nigrum L. and other broad leaved weeds in direct seeded processing tomatoes. J. Hortic. Sci. 1991, 66, 737–746. [Google Scholar] [CrossRef]
- Wu, C.-S.; Huang, J.-L.; Sun, Y.-S.; Yang, D.-Y. Mode of Action of 4-Hydroxyphenylpyruvate Dioxygenase Inhibition by Triketone-type Inhibitors. J. Med. Chem. 2002, 45, 2222–2228. [Google Scholar] [CrossRef]
- Raspail, C.; Graindorge, M.; Moreau, Y.; Crouzy, S.; Lefèbvre, B.; Robin, A.Y.; Dumas, R.; Matringe, M. 4-Hydroxyphenylpyruvate Dioxygenase Catalysis: Identification of catalytic residues and production of a hydroxylated intermediate shared with a structurally unrelated enzyme. J. Biol. Chem. 2011, 286, 26061–26070. [Google Scholar] [CrossRef]
- Lee, D.L.; Knudsen, C.G.; Michaely, W.J.; Chin, H.-L.; Nguyen, N.H.; Carter, C.G.; Cromartie, T.H.; Lake, B.H.; Shribbs, J.M.; Fraser, T. The structure-activity relationships of the triketone class of p-hydroxyphenylpyruvate dioxygenase inhibiting herbicides. Pestic. Sci. 1998, 54, 377–384. [Google Scholar] [CrossRef]
- Kovaleva, E.G.; Lipscomb, J.D. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat. Chem. Biol. 2008, 4, 186–193. [Google Scholar] [CrossRef]
- Brownlee, J.M.; Johnson-Winters, K.; Harrison, D.H.T.; Moran, G.R. Structure of the Ferrous Form of (4-Hydroxyphenyl)pyruvate Dioxygenase from Streptomyces avermitilis in Complex with the Therapeutic Herbicide, NTBC. Biochemistry 2004, 43, 6370–6377. [Google Scholar] [CrossRef]
- Beaudegnies, R.; Edmunds, A.J.; Fraser, T.E.; Hall, R.G.; Hawkes, T.R.; Mitchell, G.; Schaetzer, J.; Wendeborn, S.; Wibley, J. Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors—A review of the triketone chemistry story from a Syngenta perspective. Bioorg. Med. Chem. 2009, 17, 4134–4152. [Google Scholar] [CrossRef]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; van Almsick, A. 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors in Combination with Safeners: Solutions for Modern and Sustainable Agriculture. Angew. Chem. Int. Ed. 2013, 52, 9388–9398. [Google Scholar] [CrossRef]
- Shimoharada, H.; Tsukamoto, M.; Ikeguchi, M.; Kikugawa, H.; Sano, M.; Kitahara, Y.; Kominami, H.; Okita, T. Preparation of Benzoylpyrazole Derivatives as Herbicides. WO2007069771, 21 June 2007. [Google Scholar]
- Langdon, N.M.; Soltani, N.; Raedar, A.J.; Robinson, D.E.; Hooker, D.C.; Sikkema, P.H. Influence of Adjuvants on the Efficacy of Tolpyralate plus Atrazine for the Control of Annual Grass and Broadleaf Weeds in Corn with and without Roundup WeatherMAX. Am. J. Plant Sci. 2020, 11, 465–495. [Google Scholar] [CrossRef]
- Langdon, N.M.; Soltani, N.; Raedar, A.J.; Hooker, D.C.; Robinson, D.E.; Sikkema, P.H. Tolpyralate + Atrazine Applied Preemergence Provides Residual GR Canada Fleabane [Conyza canadensis (L.) Cronq.] Control Similar to Current Industry Standards. Agric. Sci. 2020, 11, 417–424. [Google Scholar] [CrossRef]
- Metzger, B.A.; Soltani, N.; Raeder, A.J.; Hooker, D.C.; Robinson, D.E.; Sikkema, P.H. Effect of hybrid varieties, application timing, and herbicide rate on field corn tolerance to tolpyralate plus atrazine. Weed Sci. 2019, 67, 475–484. [Google Scholar] [CrossRef]
- Dayan, F.; Owens, D.K.; Duke, S. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef]
- Malev, V.V.; Kaulin, Y.; Bezrukov, S.M.; Gurnev, P.; Takemoto, J.Y.; Shchagina, L.V. Kinetics of opening and closure of syringomycin E channels formed in lipid bilayers. Membr. Cell Biol. 2001, 14, 813–829. [Google Scholar]
- Chaimovitsh, D.; Abu-Abied, M.; Belausov, E.; Rubin, B.; Dudai, N.; Sadot, E. Microtubules are an intracellular target of the plant terpene citral. Plant J. 2010, 61, 399–408. [Google Scholar] [CrossRef]
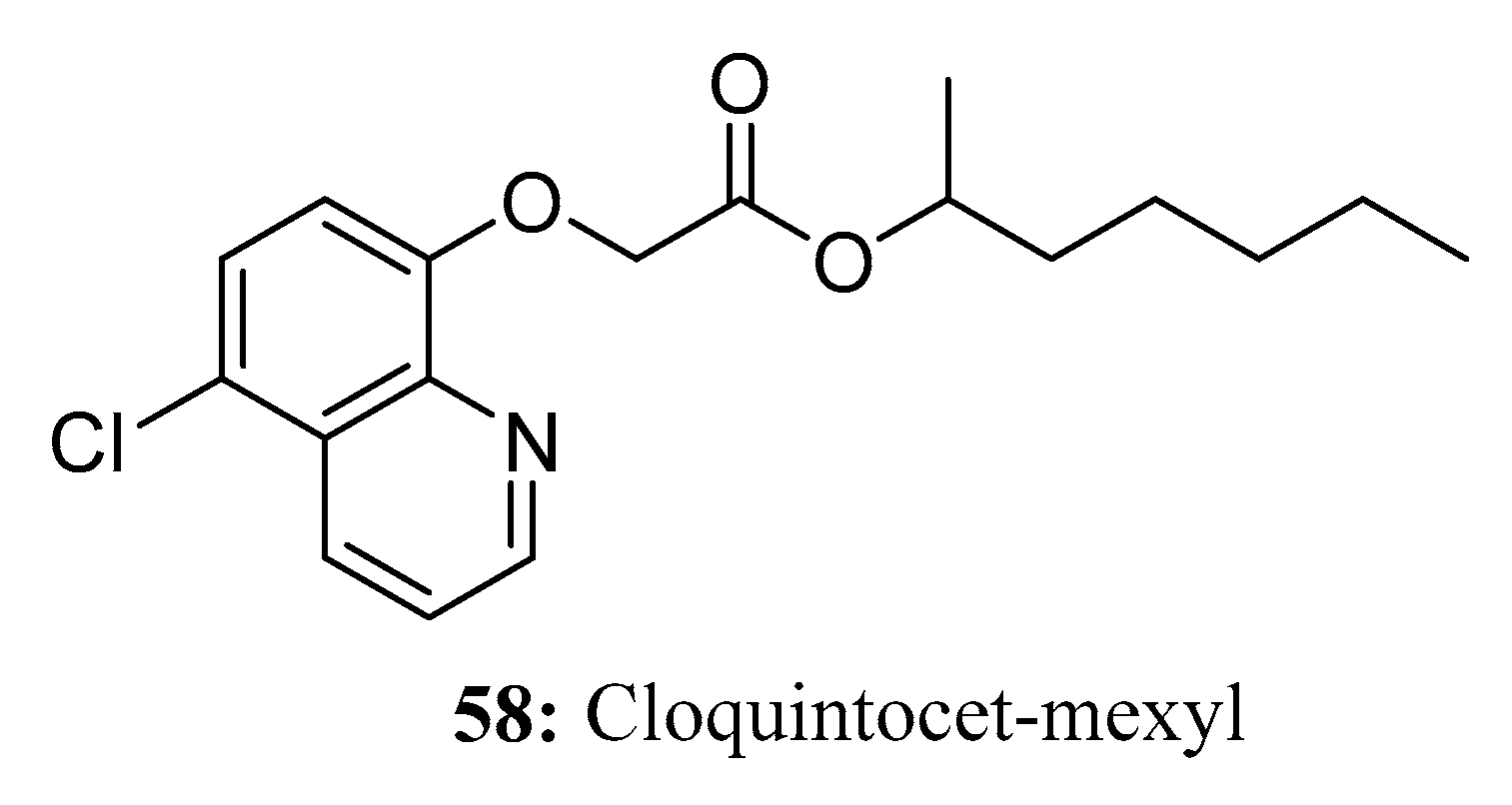
- Li, M.; Di, X.; Jiang, Z. Enantioselective separation, analysis and stereoselective dissipation of the chiral pesticide cloquintocet-mexyl using a modified QuEChERS method by high-performance liquid chromatography tandem mass spectrometry. Chemosphere 2022, 291, 133084. [Google Scholar] [CrossRef]
- Dzardanov, D.V.; Elinevskaya, L.S.; Roldughin, V.I. The influence of the nature and composition of mixed surfactants on the stability of herbicide emulsions based on fenoxaprop-P-ethyl and cloquintocet-mexyl. Colloid J. 2014, 76, 675–682. [Google Scholar] [CrossRef]
- Taylor, V.L.; Cummins, I.; Brazier-Hicks, M.; Edwards, R. Protective responses induced by herbicide safeners in wheat. Environ. Exp. Bot. 2013, 88, 93–99. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, W.; Xu, X.; Zhou, Z.; Liu, D. Direct chiral resolution of cloquintocet-mexyl and its application to in vitro degradation combined with clodinafop-propargyl. Biomed. Chromatogr. 2011, 26, 1058–1061. [Google Scholar] [CrossRef]
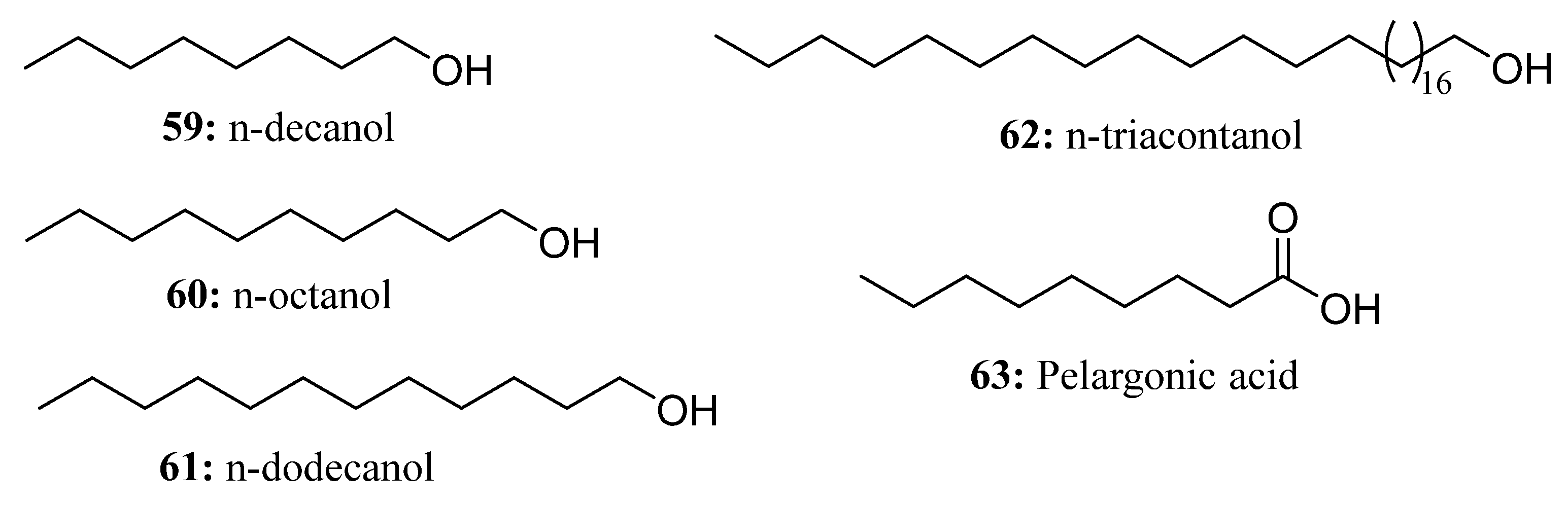
- Zhao, X.; Wang, H. International Tobacco Bud Inhibitors Overview. Tob. Sci. Technol. 1991, 38–39. [Google Scholar]
- Cai, K.; Li, Z.; Jin, A. Preliminary report of n-decanol inhibition test on tobacco buds. Tob. Sci. Technol. 1986, 16. [Google Scholar] [CrossRef]
- Karam, E.A.; Keramat, B. Foliar spray of triacontanol improves growth by alleviating oxidative damage in coriander under salinity. Indian J. Plant Physiol. 2017, 22, 120–124. [Google Scholar] [CrossRef]
- Ahmad, J.; Ali, A.A.; Al-Huqail, A.A.; Qureshi, M.I. Triacontanol attenuates drought-induced oxidative stress in Brassica juncea L. by regulating lignification genes, calcium metabolism and the antioxidant system. Plant Physiol. Biochem. 2021, 166, 985–998. [Google Scholar] [CrossRef]
- Naeem, M.; Khan, M.M.A.; Moinuddin. Triacontanol: A potent plant growth regulator in agriculture. J. Plant Interact. 2012, 7, 129–142. [Google Scholar] [CrossRef]
- Ali, H.M.M.; Perveen, S. Effect of foliar applied triacontanol on wheat (Triticum aestivum L.) under arsenic stress: A study of changes in growth, yield and photosynthetic characteristics. Physiol. Mol. Biol. Plants 2020, 26, 1215–1224. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef]
- Islam, S.; Zaid, A.; Mohammad, F. Role of Triacontanol in Counteracting the Ill Effects of Salinity in Plants: A Review. J. Plant Growth Regul. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Borowski, E.; Blamowski, Z.K. The effects of triacontanol ‘TRIA’ and Asahi SL on the development and metabolic activity of sweet basil (Ocimum basilicum L.) plants treated with chilling. Folia Hortic. 2009, 21, 39–48. [Google Scholar] [CrossRef]
- Perveen, S.; Shahbaz, M.; Ashraf, M. Influence of foliar-applied triacontanol on growth, gas exchange characteristics, and chlorophyll fluorescence at different growth stages in wheat under saline conditions. Photosynthetica 2013, 51, 541–551. [Google Scholar] [CrossRef]
- Perveen, S.; Iqbal, M.; Parveen, A.; Akram, M.S.; Shahbaz, M.; Akber, S.; Mehboob, A. Exogenous triacontanol-mediated increase in phenolics, proline, activity of nitrate reductase, and shoot k+ confers salt tolerance in maize (Zea mays L.). Braz. J. Bot. 2016, 40, 1–11. [Google Scholar] [CrossRef]
- Coleman, R.; Penner, D. Organic Acid Enhancement of Pelargonic Acid. Weed Technol. 2008, 22, 38–41. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Herbicides based on pelargonic acid: Herbicides of the bioeconomy. Biofuels, Bioprod. Biorefining 2019, 13, 1476–1482. [Google Scholar] [CrossRef]
- Williams, M.W. New Chemical Approaches for Control of Biennial Bearing of Apples. ACS Symp. Ser. 1994, 557, 16–25. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres-Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez-Moreiras, A.M.; Verdeguer, M. Phytotoxic Effects of Three Natural Compounds: Pelargonic Acid, Carvacrol, and Cinnamic Aldehyde, against Problematic Weeds in Mediterranean Crops. Agronomy 2020, 10, 791. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural Compounds as Next-Generation Herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
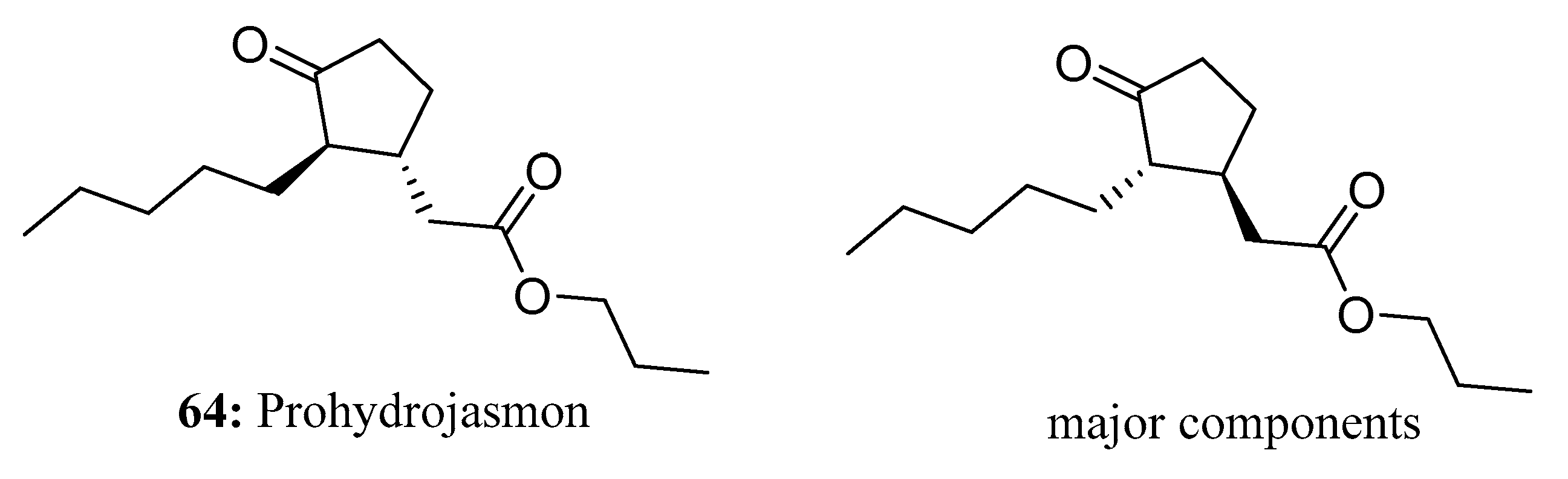
- Azis, H.R.; Takahashi, S.; Koshiyama, M.; Fujisawa, H.; Isoda, H. Effect of Prohydrojasmon on the Growth of Eggplant and Komatsuna. Plants 2020, 9, 1368. [Google Scholar] [CrossRef]
- Azis, H.R.; Etteieb, S.; Takahashi, S.; Koshiyama, M.; Fujisawa, H.; Isoda, H. Effect of prohydrojasmon on total phenolic content, anthocyanin accumulation and antioxidant activity in komatsuna and lettuce. Biosci. Biotechnol. Biochem. 2020, 84, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Sajjadi, S.E.; Parang, K. A Review (Research and Patents) on Jasmonic Acid and Its Derivatives. Arch. Pharm. 2014, 347, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates: A review. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Creelman, R.; Mullet, J. Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc. Natl. Acad. Sci. USA 1995, 92, 4114–4119. [Google Scholar] [CrossRef]
- Takahashi, S.; Namioka, Y.; Azis, H.R.; Sano, T.; Aono, M.; Koshiyama, M.; Fujisawa, H.; Isoda, H. Prohydrojasmon Promotes the Accumulation of Phenolic Compounds in Red Leaf Lettuce. Plants 2021, 10, 1920. [Google Scholar] [CrossRef]
- Sato, K.; Ikoma, Y. Improvement in Handpicking Efficiency of Satsuma Mandarin Fruit with Combination Treatments of Gibberellin, Prohydrojasmon and Ethephon. Hortic. J. 2017, 86, 283–290. [Google Scholar] [CrossRef]
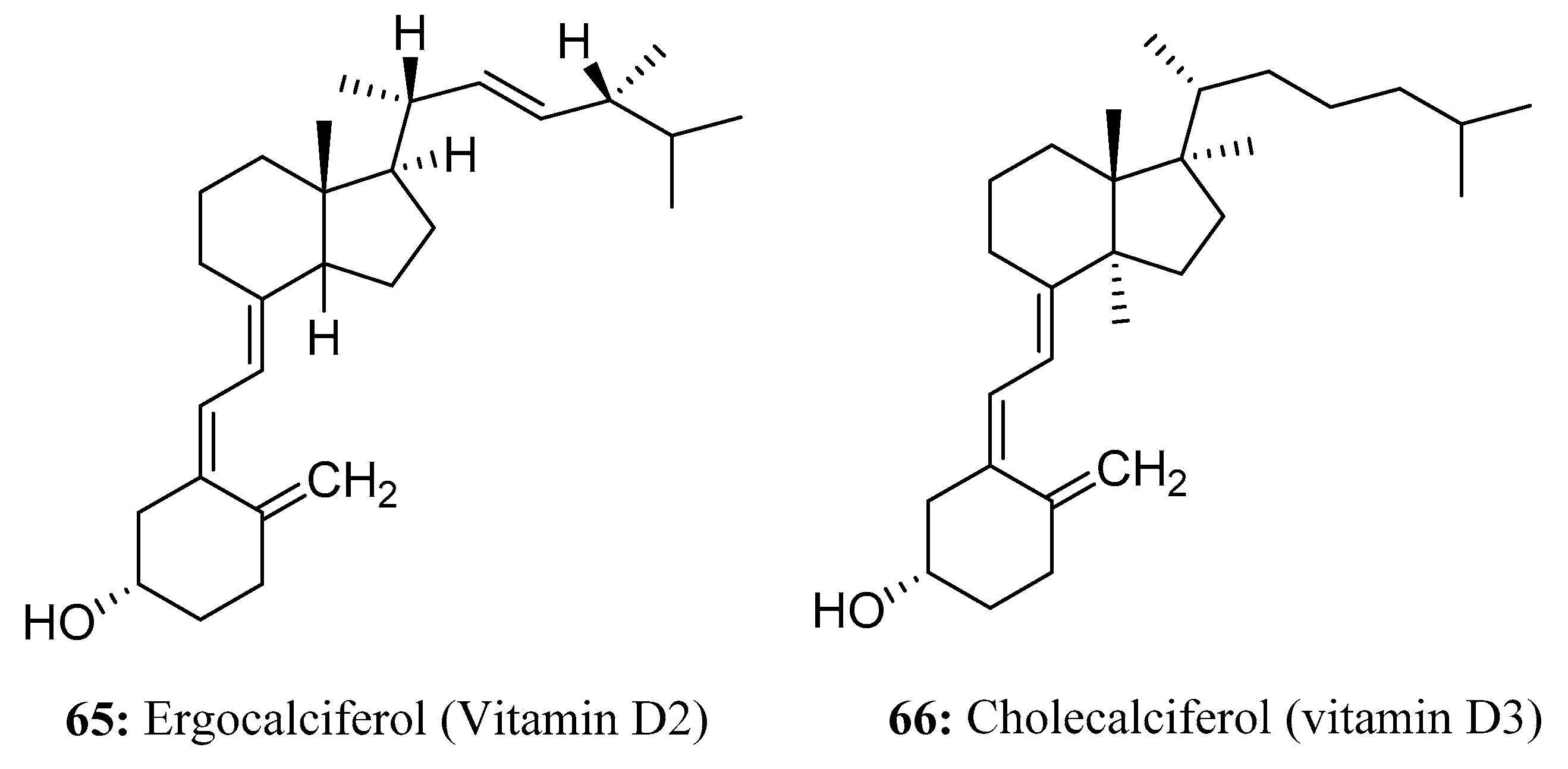
- Román-Hidalgo, C.; Villar-Navarro, M.; Falcón-García, G.; Carbonero-Aguilar, M.; Bautista-Palomas, J.; Bello-López, M.; Martín-Valero, M.; Fernández-Torres, R. Selective, rapid and simultaneous determination of ergosterol and ergocalciferol in mushrooms by UPLC-Q-TOF-MS. J. Pharm. Biomed. Anal. 2020, 194, 113748. [Google Scholar] [CrossRef]
- Greaves, J.H.; Redfern, R.; King, R.E. Some properties of calciferol as a rodenticide. Epidemiol. Infect. 1974, 73, 341–351. [Google Scholar] [CrossRef][Green Version]
- Harris, W.D.; Zukel, J.W. Chemical Structure-Activity Relationship, Chemical Structure of Series of Organic Sulfites and Its Toxicity to Two-Spotted Spider Mite. J. Agric. Food Chem. 1954, 2, 140–142. [Google Scholar] [CrossRef]
- Maxwell, K.E.; Piper, V.D. Molecular Structure of Nonionic Surfactants in Relation to Laboratory Insecticidal Activity. J. Econ. Entomol. 1968, 61, 1633–1636. [Google Scholar] [CrossRef]
- Santos, F.O.; Lima, H.G.; Rosa, S.D.S.S.; das Mercês, N.B.; Serra, T.M.; Uzeda, R.S.; Reis, I.M.A.; Botura, M.B.; Branco, A.; Batatinha, M.J.M. In vitro acaricide and anticholinesterase activities of digitaria insularis (Poaceae) against Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2018, 255, 102–106. [Google Scholar] [CrossRef]
- Peterson, G.S.; Kandil, M.A.; Abdallah, M.D.; Farag, A.-A.A. Isolation and characterisation of biologically-active compounds from some plant extracts. Pestic. Sci. 1989, 25, 337–342. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Molina, S.C.; Muñoz, E.; Lamilla, C.; Alarcon, J.; Palacios, S.M.; Carpinella, M.C.; Avila, J.G. The insecticidal, molting disruption and insect growth inhibitory activity of extracts from Condalia microphylla Cav. (Rhamnaceae). Ind. Crops Prod. 2013, 42, 78–86. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Z.; Lu, H.; Liu, S.; Ding, W.; Li, J.; Xiong, Y.; Li, C. New diphenyl ethers from a fungus Epicoccum sorghinum L28 and their antifungal activity against phytopathogens. Bioorg. Chem. 2021, 115, 105232. [Google Scholar] [CrossRef] [PubMed]




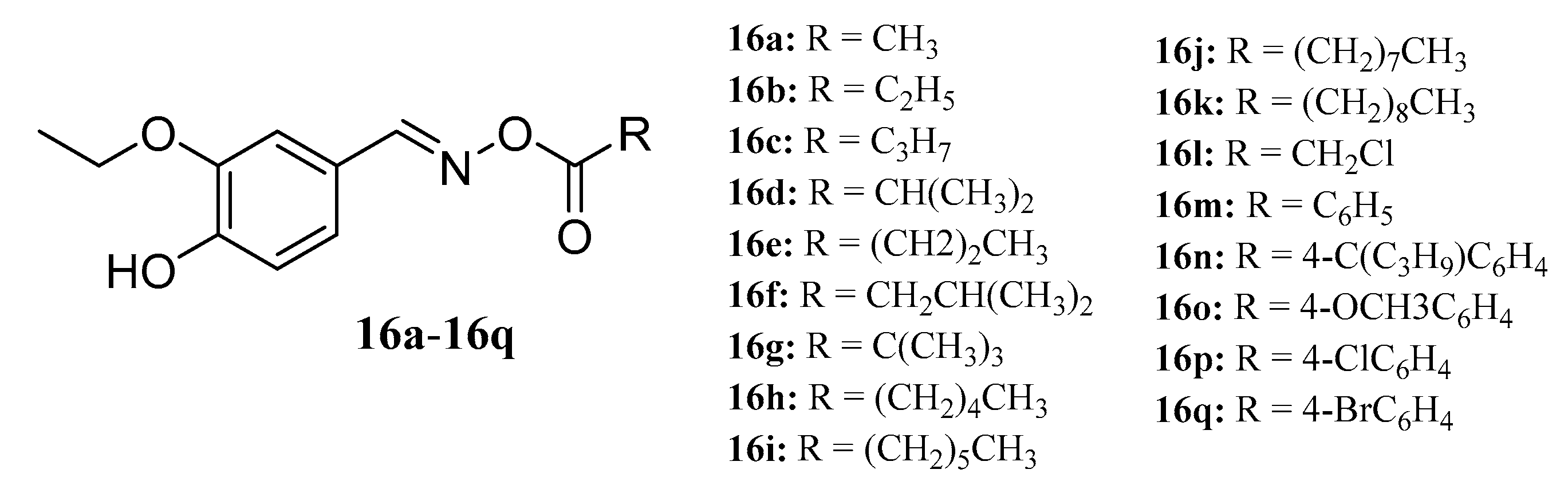






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Qin, Z. Long-Chain Molecules with Agro-Bioactivities and Their Applications. Molecules 2023, 28, 5880. https://doi.org/10.3390/molecules28155880
Yin F, Qin Z. Long-Chain Molecules with Agro-Bioactivities and Their Applications. Molecules. 2023; 28(15):5880. https://doi.org/10.3390/molecules28155880
Chicago/Turabian StyleYin, Fahong, and Zhaohai Qin. 2023. "Long-Chain Molecules with Agro-Bioactivities and Their Applications" Molecules 28, no. 15: 5880. https://doi.org/10.3390/molecules28155880
APA StyleYin, F., & Qin, Z. (2023). Long-Chain Molecules with Agro-Bioactivities and Their Applications. Molecules, 28(15), 5880. https://doi.org/10.3390/molecules28155880