Synthesis of Porous Activated Carbon Doped with Tetramethylammonium Hydroxide: Evaluation of Excellent Gasoline Vapor Adsorption Performance and Activation Mechanism
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Activation Temperature, Time, and Activator Dosage
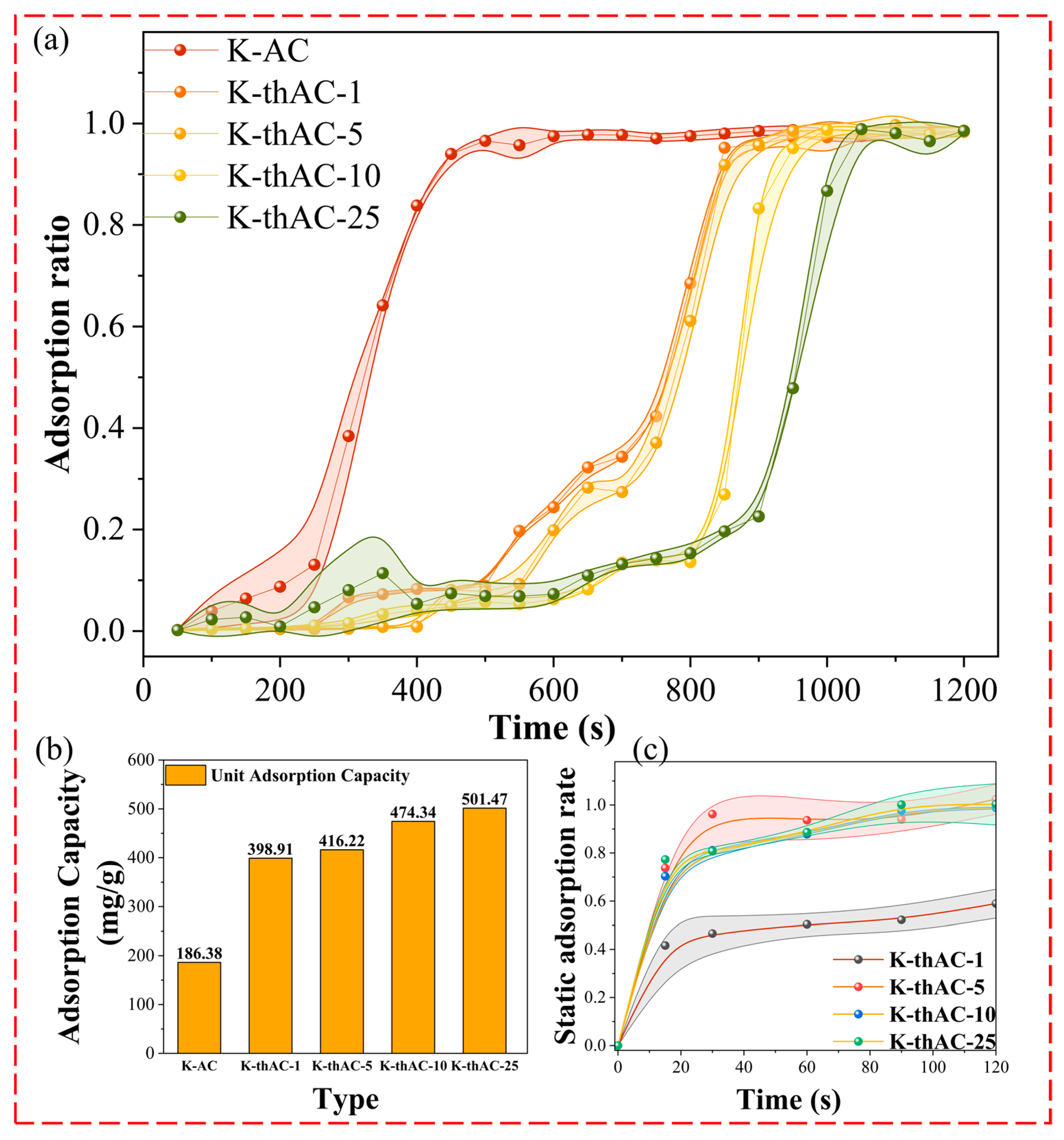
2.2. Dynamic Adsorption Test and Static Adsorption Test
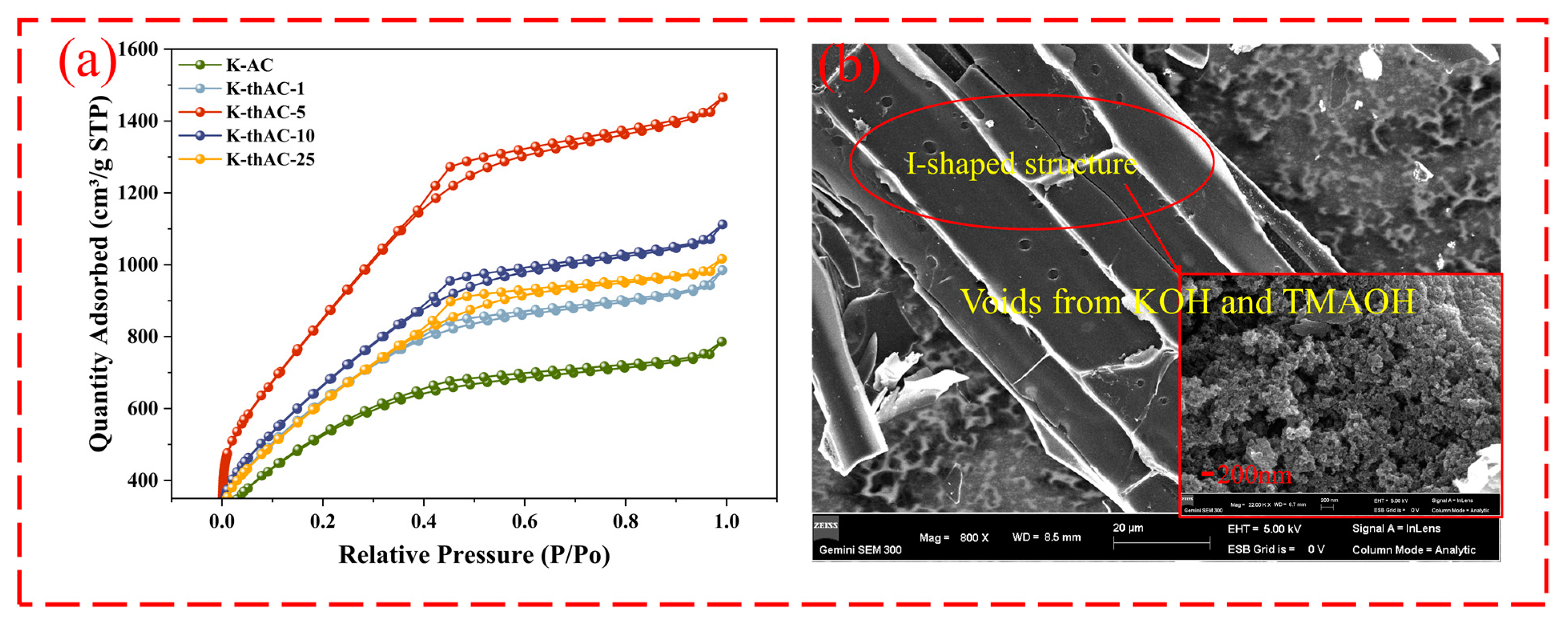
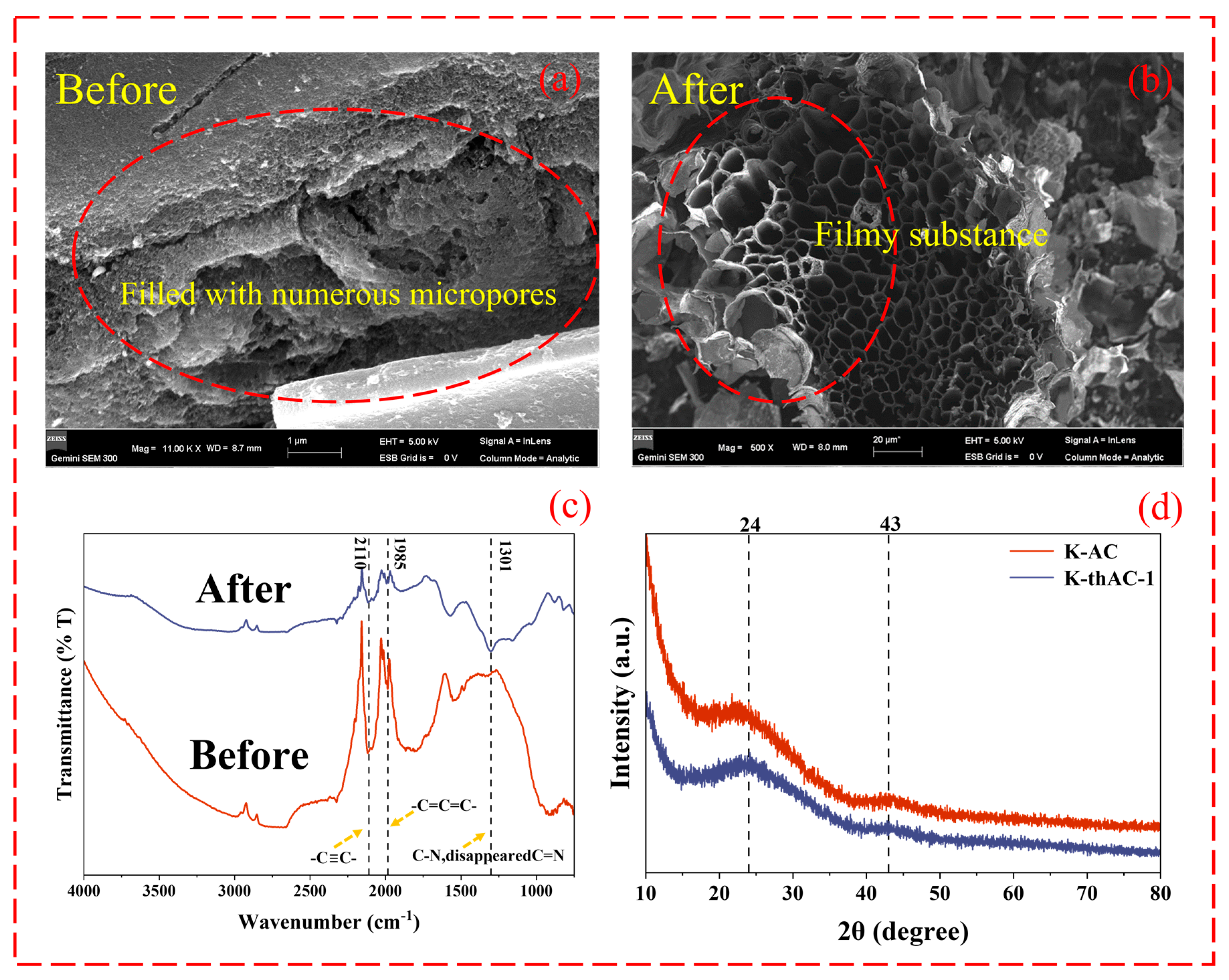
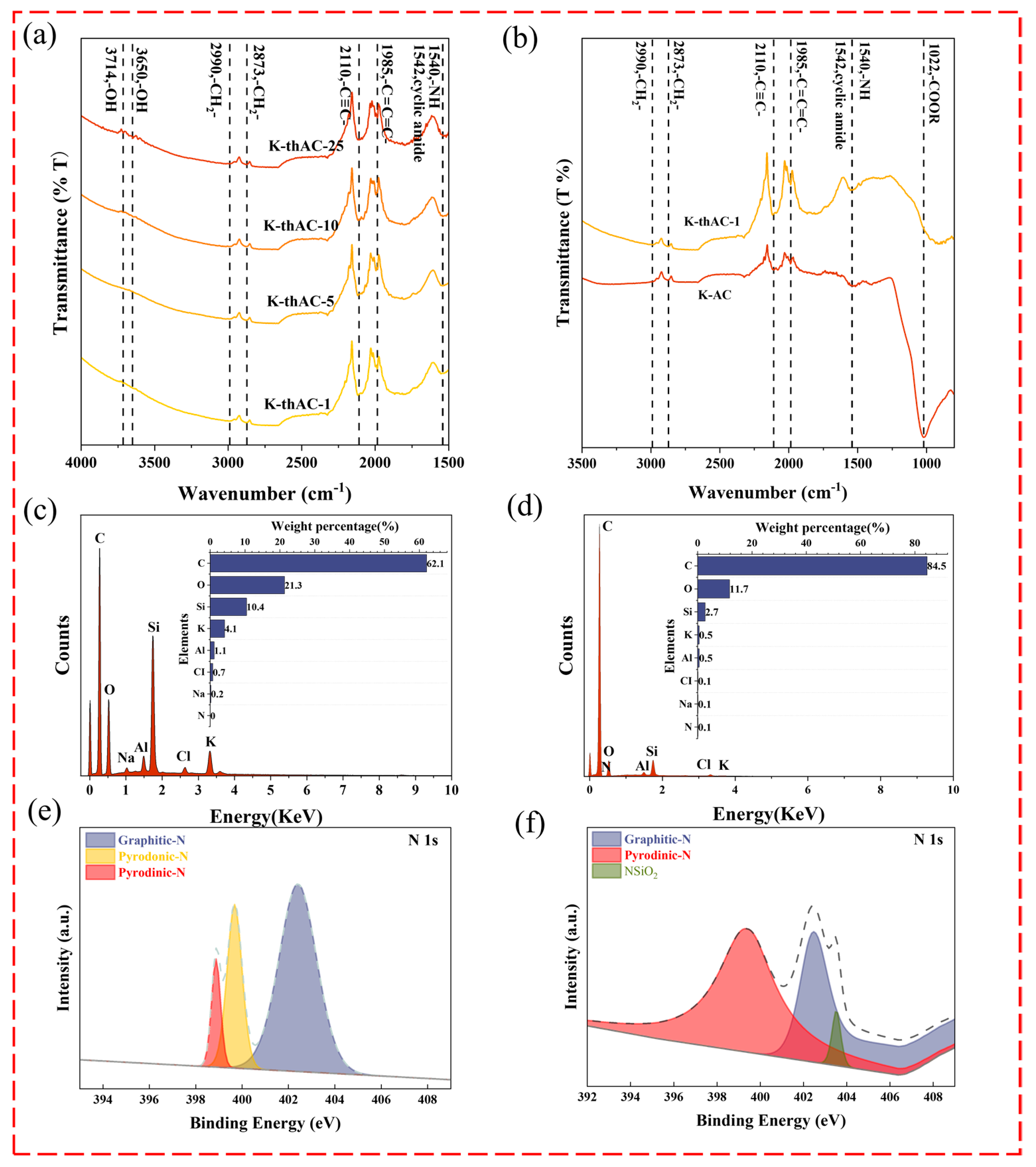
2.3. Characterization
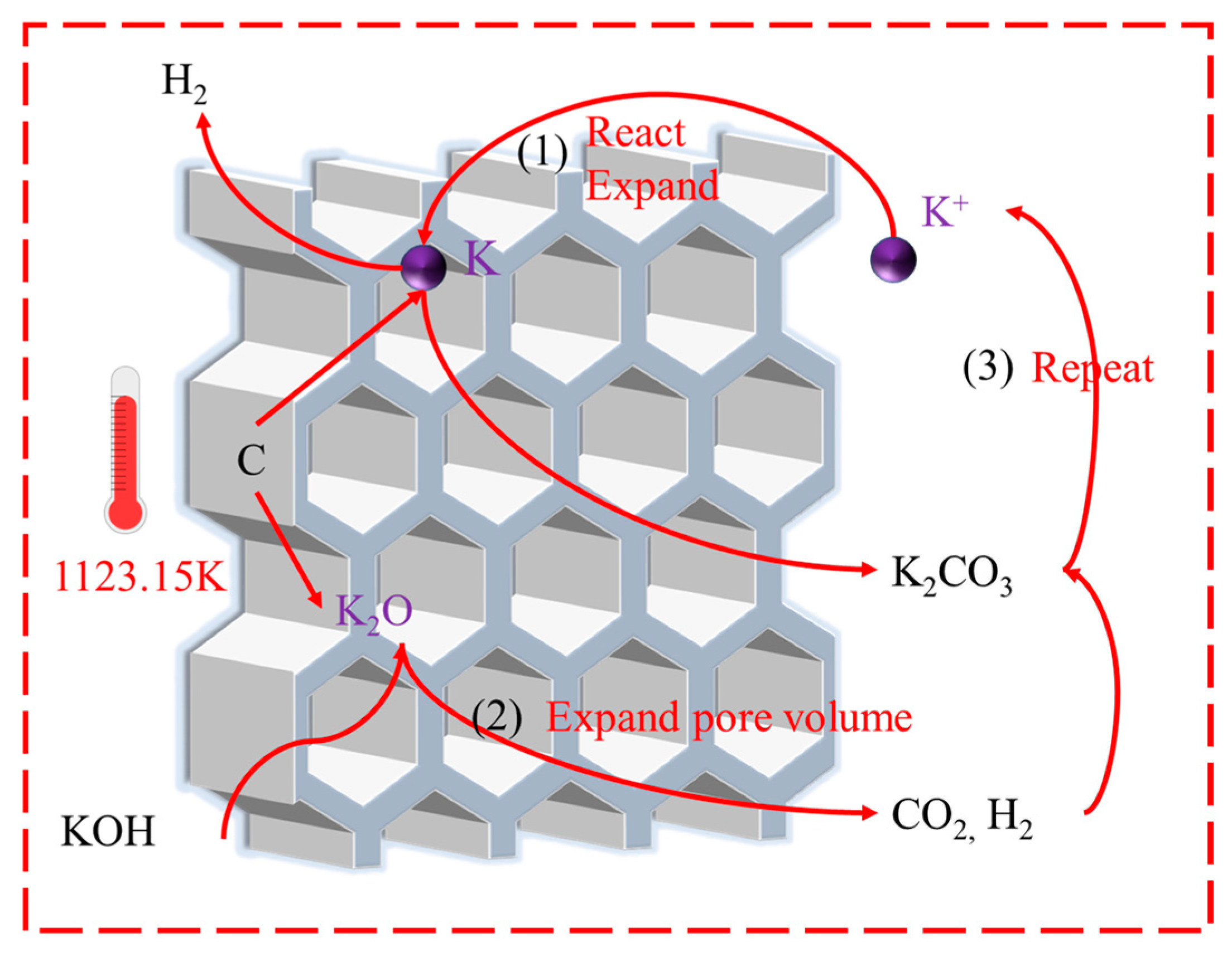
2.4. Adsorption Mechanism
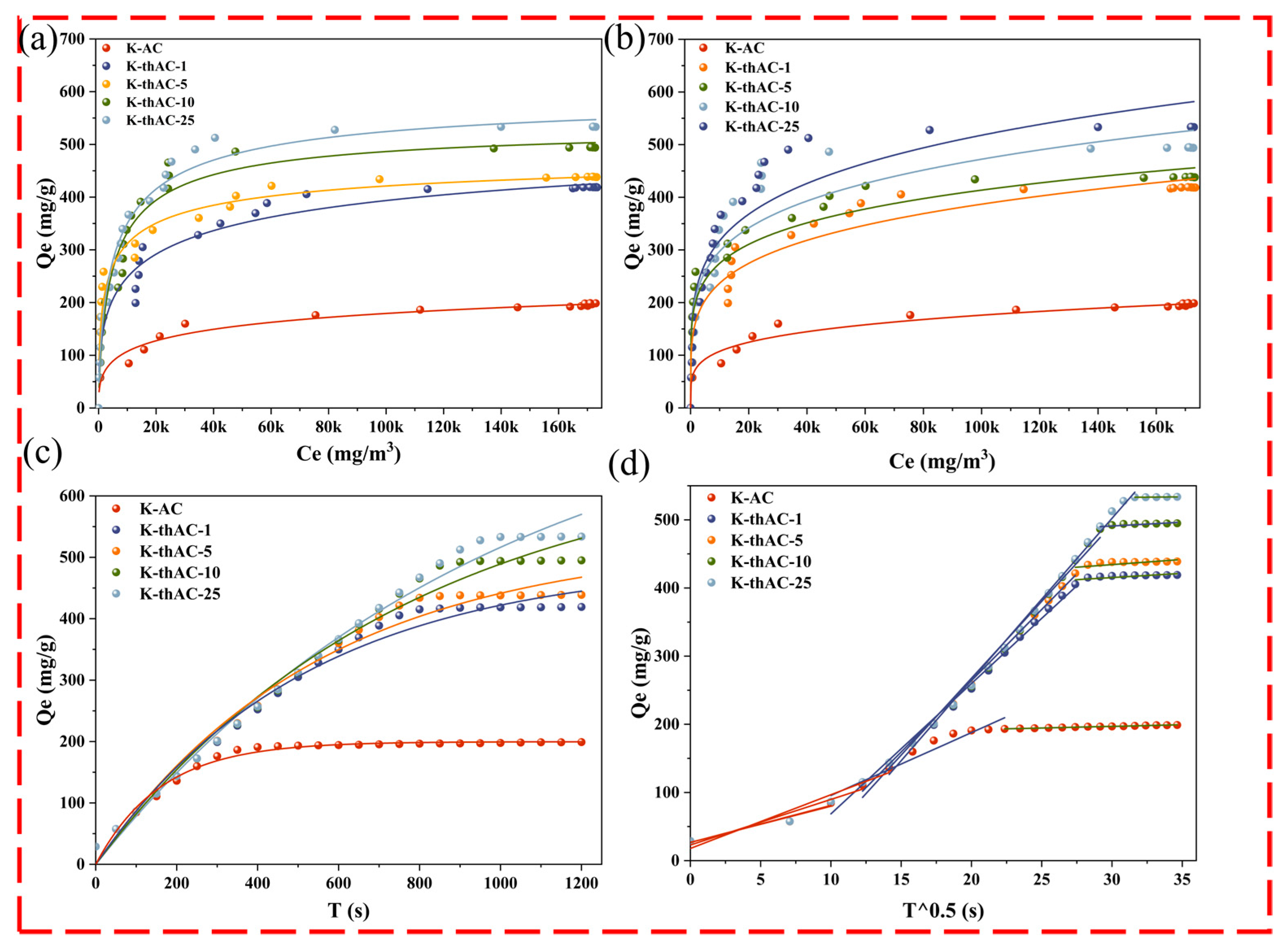
2.4.1. Adsorption Isotherm
2.4.2. Adsorption Kinetics Model
2.4.3. Adsorption Mechanism
3. Materials and Methods
3.1. Materials and Reagents
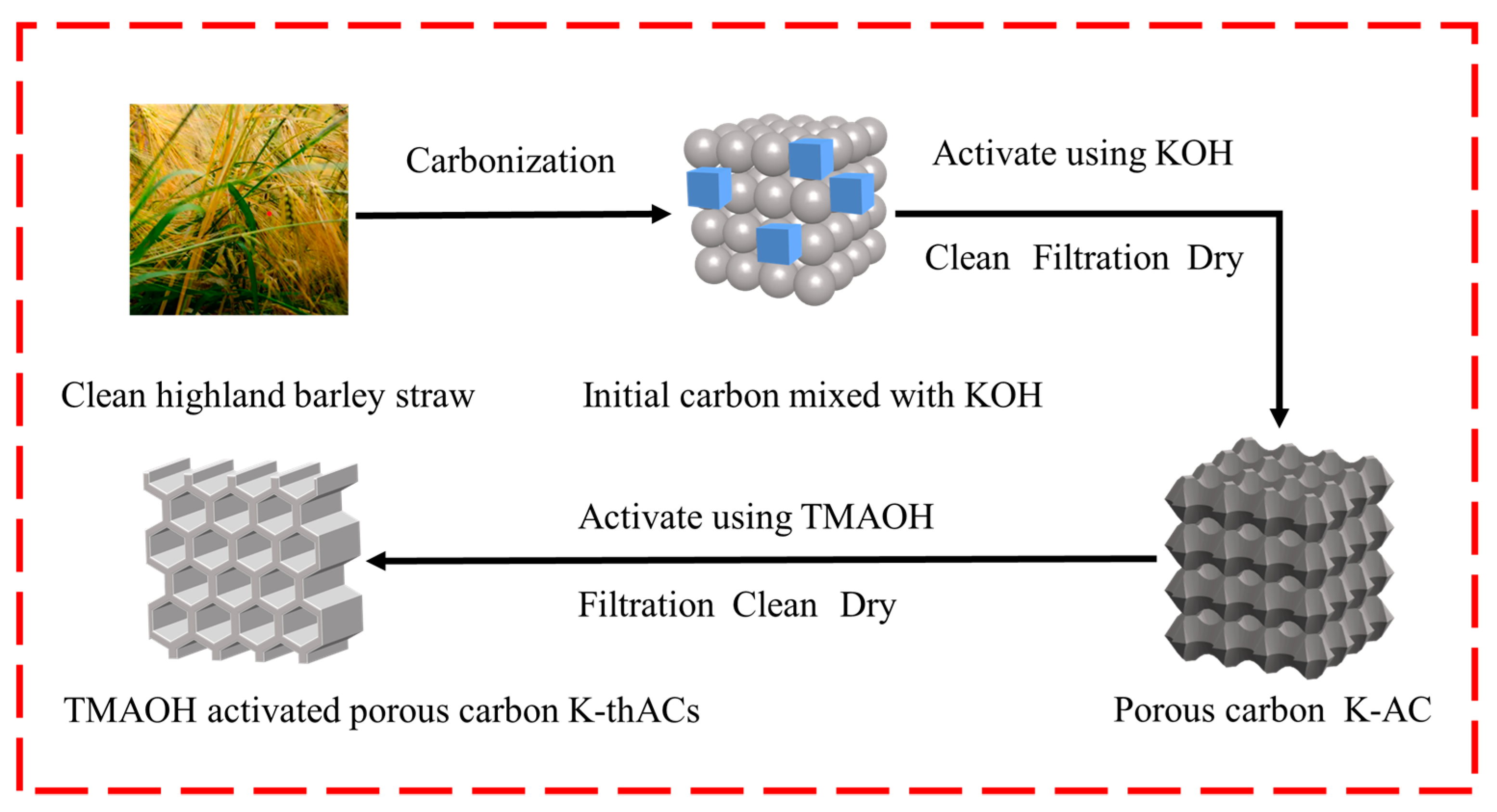
3.2. Synthesis of Materials
3.3. Chemical Surface Modification
3.4. Characterization of AC
3.5. Adsorption Studies
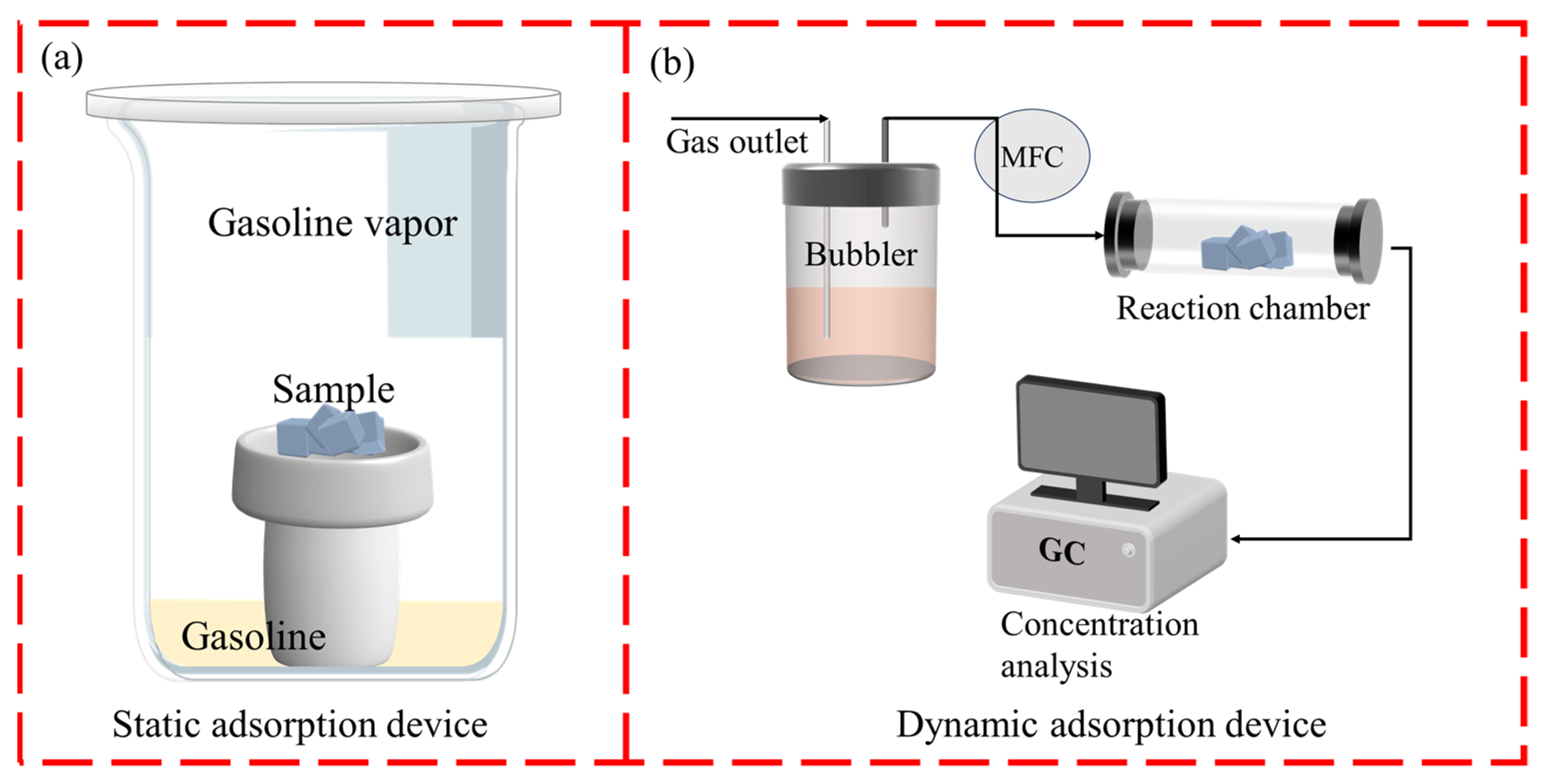
3.5.1. Static Adsorption Test
3.5.2. Dynamic Adsorption Test
3.5.3. Kinetics and Equilibrium Studies
4. Conclusions
- (1)
- The K-thACs had a maximum adsorption capacity of 501 mg/g for gasoline vapor, mainly owing to the specific surface area of 3119 m2/g. Moreover, the K-thACs showed great advantages compared with most industrial ACs materials for gasoline vapor adsorption.
- (2)
- The mechanism of the adsorption of gasoline vapor by K-thACs could be attributed to the substitution of intermediate carbon or oxygen atoms by nitrogen atoms in TMAOH, which is similar to NH3 doping.
- (3)
- The porous structure of the K-thACs could be effectively etched owing to the basicity of TMAOH under mild conditions. This study provided proof of the superiority of TMAOH as a nitrogen-doping agent.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, Z.; Wu, J.; He, T.; Wu, J. Corn stalks char from fast pyrolysis as precursor material for preparation of activated carbon in fluidized bed reactor. Bioresour. Technol. 2014, 167, 551–554. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, B.; Xiao, S.; Yan, M.; Cai, Y.; Liu, B.; Zheng, X.; Wu, Y. Species profiles, in-situ photochemistry and health risk of volatile organic compounds in the gasoline service station in China. Sci. Total Environ. 2022, 842, 156813. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, M.; Zhang, J.; Fu, L.; Lu, S. Distributions and source apportionment of ambient volatile organic compounds in Beijing city, China. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2005, 40, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Yenisoy-Karakas, S.; Dorter, M.; Odabasi, M. Intraday and interday variations of 69 volatile organic compounds (BVOCs and AVOCs) and their source profiles at a semi-urban site. Sci. Total Environ. 2020, 723, 138028. [Google Scholar] [CrossRef]
- Srivastava, A.; Joseph, A.E.; More, A.; Patil, S. Emissions of VOCs at urban petrol retail distribution centres in India (Delhi and Mumbai). Environ. Monit. Assess. 2005, 109, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo Sosa, E.; Humberto Bravo, A.; Violeta Mugica, A.; Pablo Sanchez, A.; Emma Bueno, L.; Krupa, S. Levels and source apportionment of volatile organic compounds in southwestern area of Mexico City. Environ. Pollut. 2009, 157, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wu, S.; Zhou, S.; Tong, G.; Li, F.; Wang, Y.; Li, B. Characteristics, sources and health risk assessment of airborne particulate PAHs in Chinese cities: A review. Environ. Pollut. 2019, 248, 804–814. [Google Scholar] [CrossRef]
- Xie, Z. Hazard of Industrial VOC and Treatment Technology. Chem. Eng. Manag. 2021, 35, 31–32. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.-J. Role of heteroatoms (nitrogen and sulfur)-dual doped corn-starch based porous carbons for selective CO2 adsorption and separation. J. CO2 Util. 2021, 51, 101641. [Google Scholar] [CrossRef]
- Rakic, V.; Rac, V.; Krmar, M.; Otman, O.; Auroux, A. The adsorption of pharmaceutically active compounds from aqueous solutions onto activated carbons. J. Hazard. Mater. 2015, 282, 141–149. [Google Scholar] [CrossRef]
- Liu, Z.-F.; Yao, Z.-J.; Yu, C.-Q.; Zhong, Z.-M. Assessing Crop Water Demand and Deficit for the Growth of Spring Highland Barley in Tibet, China. J. Integr. Agric. 2013, 12, 541–551. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, U.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J. Clean. Prod. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Y.; Wu, F.; Peng, S.; Li, J.; Liu, Z. Tubular activated carbons made from cotton stalk for dynamic adsorption of airborne toluene. J. Taiwan Inst. Chem. Eng. 2017, 80, 399–405. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Tang, M.; Peng, Y.; Wang, S.; Makwarimba, C.P. Synthesis of N-doped hierarchical porous carbon with excellent toluene adsorption properties and its activation mechanism. Environ. Pollut. 2021, 284, 117113. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhou, D.; Hashisho, Z.; Wang, S.; Chen, H.; Wang, H. Preparation of Pinewood- and Wheat Straw-based Activated Carbon via a Microwave-assisted Potassium Hydroxide Treatment and an Analysis of the Effects of the Microwave Activation Conditions. BioResources 2015, 10, 809–821. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Bailon-Garcia, E.; Perez-Cadenas, A.F.; Carrasco-Marin, F.; Maldonado-Hodar, F.J. Tailoring activated carbons for the development of specific adsorbents of gasoline vapors. J. Hazard. Mater. 2013, 263 Pt 2, 533–540. [Google Scholar] [CrossRef]
- Yu, H.; Lin, F.; Li, K.; Wang, W.; Yan, B.; Song, Y.; Chen, G. Triple combination of natural microbial action, etching, and gas foaming to synthesize hierarchical porous carbon for efficient adsorption of VOCs. Environ. Res. 2021, 202, 111687. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, H.; Niu, Q.; Fu, M.; Huang, H.; Ye, D. Microbial Targeted Degradation Pretreatment: A Novel Approach to Preparation of Activated Carbon with Specific Hierarchical Porous Structures, High Surface Areas, and Satisfactory Toluene Adsorption Performance. Environ. Sci. Technol. 2019, 53, 7632–7640. [Google Scholar] [CrossRef]
- Tian, R.; Zhu, B.; Liu, Q.; Hu, Y.; Yang, Z.; Rao, J.; Wu, Y.; Lu, B.; Bian, J.; Peng, F. Rapid and massive fractionation of hemicelluloses for purifying cellulose at room temperature by tetramethylammonium hydroxide. Bioresour. Technol. 2023, 369, 128490. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations. Carbon 2005, 43, 1758–1767. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Montes-Morán, M.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Concheso, A.; Rojas-Mayorga, C.K.; González, J. Importance of iron oxides on the carbons surface vs the specific surface for VOC’s adsorption. Ecol. Eng. 2017, 106, 400–408. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Lin, B.; Huang, Q. Surface modified and activated waste bone char for rapid and efficient VOCs adsorption. Chemosphere 2020, 256, 127054. [Google Scholar] [CrossRef]
- Ma, X.; Cao, M.; Hu, C. MgO modified nanoporous carbon composites for methanol separation. RSC Adv. 2013, 3, 10396–10402. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Mohammed, J.; Nasri, N.S.; Ahmad Zaini, M.A.; Hamza, U.D.; Ani, F.N. Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int. Biodeterior. Biodegrad. 2015, 102, 245–255. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, X.; Qin, Y.; Li, X.; Zhu, T.; Tang, X. An experimental and theoretical study of the adsorption removal of toluene and chlorobenzene on coconut shell derived carbon. Chemosphere 2018, 206, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Kim, O.H.; Cho, Y.H.; Chung, D.Y.; Kim, M.J.; Yoo, J.M.; Park, J.E.; Choe, H.; Sung, Y.E. Facile and gram-scale synthesis of metal-free catalysts: Toward realistic applications for fuel cells. Sci. Rep. 2015, 5, 8376. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Xu, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Adsorption of VOCs on reduced graphene oxide. J. Environ. Sci. 2018, 67, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhu, J.; Wei, B.; Hu, H.; Jin, L. Catalytic upgrading of ex-situ heavy coal tar over modified activated carbon. Fuel 2022, 312, 122912. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N. Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Bioresour. Technol. 2019, 282, 294–300. [Google Scholar] [CrossRef]
- Shaarani, F.W.; Hameed, B.H. Ammonia-modified activated carbon for the adsorption of 2,4-dichlorophenol. Chem. Eng. J. 2011, 169, 180–185. [Google Scholar] [CrossRef]
- Tazibet, S.; Boucheffa, Y.; Lodewyckx, P. Heat treatment effect on the textural, hydrophobic and adsorptive properties of activated carbons obtained from olive waste. Microporous Mesoporous Mater. 2013, 170, 293–298. [Google Scholar] [CrossRef]
- Eren, O.; Ucar, N.; Onen, A.; Kizildag, N.; Karacan, I. Synergistic effect of polyaniline, nanosilver, and carbon nanotube mixtures on the structure and properties of polyacrylonitrile composite nanofiber. J. Compos. Mater. 2015, 50, 2073–2086. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Acetic acid-mediated cellulose-based carbons: Influence of activation conditions on textural features and carbon dioxide uptakes. J. Colloid Interface Sci. 2021, 594, 745–758. [Google Scholar] [CrossRef]
- Wen, J.; Liu, Z.; Xi, H.; Huang, B. Synthesis of hierarchical porous carbon with high surface area by chemical activation of (NH4)2C2O4 modified hydrochar for chlorobenzene adsorption. J. Environ. Sci. 2023, 126, 123–137. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Lei, B.; Xie, H.; Chen, S.; Liu, B.; Zhou, G. Control of pore structure and surface chemistry of activated carbon derived from waste Zanthoxylum bungeanum branches for toluene removal in air. Environ. Sci. Pollut. Res. Int. 2020, 27, 27072–27092. [Google Scholar] [CrossRef]
- Rahbar-Shamskar, K.; Aberoomand Azar, P.; Rashidi, A.; Baniyaghoob, S.; Yousefi, M. Synthesis of micro/mesoporous carbon adsorbents by in-situ fast pyrolysis of reed for recovering gasoline vapor. J. Clean. Prod. 2020, 259, 120832. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Qiao, J.; Kołodyńska, D.; Ju, Y.; Zhang, M.; Cai, M.; Deng, D.; Dionysiou, D.D. Malic acid-enhanced chitosan hydrogel beads (mCHBs) for the removal of Cr(VI) and Cu(II) from aqueous solution. Chem. Eng. J. 2018, 353, 225–236. [Google Scholar] [CrossRef]
- Xuan, L.; Ma, Y.; Xing, Y.; Meng, Q.; Song, J.; Chen, T.; Wang, H.; Wang, P.; Zhang, Y.; Gao, P. Source, temporal variation and health risk of volatile organic compounds (VOCs) from urban traffic in harbin, China. Environ. Pollut. 2021, 270, 116074. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Hu, D.; Li, Z.; Hao, A.; Li, R.; Wang, C.; Xu, Y.; Cao, J.; Liu, Z.; et al. Source apportionment of atmospheric volatile aromatic compounds (BTEX) by stable carbon isotope analysis: A case study during heating period in Taiyuan, northern China. Atmos. Environ. 2020, 225, 117369. [Google Scholar] [CrossRef]
- Yun, L.; Li, C.L.; Zhang, M.D.; He, L.; Guo, J.F. Pollution Characteristics and Source Analysis of Atmospheric VOCs in the Coastal Background of the Pearl River Delta. Environ. Sci. 2021, 9, 4191–4201. [Google Scholar] [CrossRef]
- Na, K.; Pyo Kim, Y. Chemical mass balance receptor model applied to ambient C2–C9 VOC concentration in Seoul, Korea: Effect of chemical reaction losses. Atmos. Environ. 2007, 41, 6715–6728. [Google Scholar] [CrossRef]
- Yamada, H. Contribution of evaporative emissions from gasoline vehicles toward total VOC emissions in Japan. Sci. Total Environ. 2013, 449, 143–149. [Google Scholar] [CrossRef]
- Gentner, D.R.; Harley, R.A.; Miller, A.M.; Goldstein, A.H. Diurnal and Seasonal Variability of Gasoline-Related Volatile Organic Compound Emissions in Riverside, California. Environ. Sci. Technol. 2009, 43, 4247–4252. [Google Scholar] [CrossRef]
- Morino, Y.; Ohara, T.; Yokouchi, Y.; Ooki, A. Comprehensive source apportionment of volatile organic compounds using observational data, two receptor models, and an emission inventory in Tokyo metropolitan area. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]

| T (s) | α | k | Carbon Yield | (s−1) |
|---|---|---|---|---|
| 750 | 1:2 | 0.2370 | 0.4956 | 0.16 |
| 1:5 | 0.5997 | 0.4746 | 0.16 | |
| 1:7 | 0.7069 | 0.1993 | 0.13 | |
| 850 | 1:2 | 0.2899 | 0.4229 | 0.17 |
| 1:5 | 0.9366 | 0.5043 | 0.22 | |
| 1:7 | 1.1968 | 0.0698 | 0.20 | |
| 900 | 1:2 | 0.2192 | 0.6268 | 0.12 |
| 1:5 | 0.7288 | 0.2225 | 0.16 | |
| 1:7 | 0.9741 | 0.0521 | 0.15 |
| Samples | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Median Pore Width (nm) |
|---|---|---|---|---|
| K-AC | 1871.21 | 1.219472 | 2.6068 | 0.4271 |
| K-thAC-1 | 2246.07 | 1.527990 | 2.7212 | 0.4367 |
| K-thAC-5 | 3119.43 | 2.273983 | 2.9159 | 0.4400 |
| K-thAC-10 | 2435.61 | 1.725368 | 2.8336 | 0.4389 |
| K-thAC-25 | 2243.78 | 1.577563 | 2.8123 | 0.4354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Yang, J.; Gong, Y.; Ju, Y.; Tao, J.; Jiang, X. Synthesis of Porous Activated Carbon Doped with Tetramethylammonium Hydroxide: Evaluation of Excellent Gasoline Vapor Adsorption Performance and Activation Mechanism. Molecules 2023, 28, 5868. https://doi.org/10.3390/molecules28155868
Wu C, Yang J, Gong Y, Ju Y, Tao J, Jiang X. Synthesis of Porous Activated Carbon Doped with Tetramethylammonium Hydroxide: Evaluation of Excellent Gasoline Vapor Adsorption Performance and Activation Mechanism. Molecules. 2023; 28(15):5868. https://doi.org/10.3390/molecules28155868
Chicago/Turabian StyleWu, Chenyu, Jing Yang, Yu Gong, Yongming Ju, Jiahui Tao, and Xinmeng Jiang. 2023. "Synthesis of Porous Activated Carbon Doped with Tetramethylammonium Hydroxide: Evaluation of Excellent Gasoline Vapor Adsorption Performance and Activation Mechanism" Molecules 28, no. 15: 5868. https://doi.org/10.3390/molecules28155868
APA StyleWu, C., Yang, J., Gong, Y., Ju, Y., Tao, J., & Jiang, X. (2023). Synthesis of Porous Activated Carbon Doped with Tetramethylammonium Hydroxide: Evaluation of Excellent Gasoline Vapor Adsorption Performance and Activation Mechanism. Molecules, 28(15), 5868. https://doi.org/10.3390/molecules28155868





