Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection
Abstract
1. Introduction
2. Materials and Methods
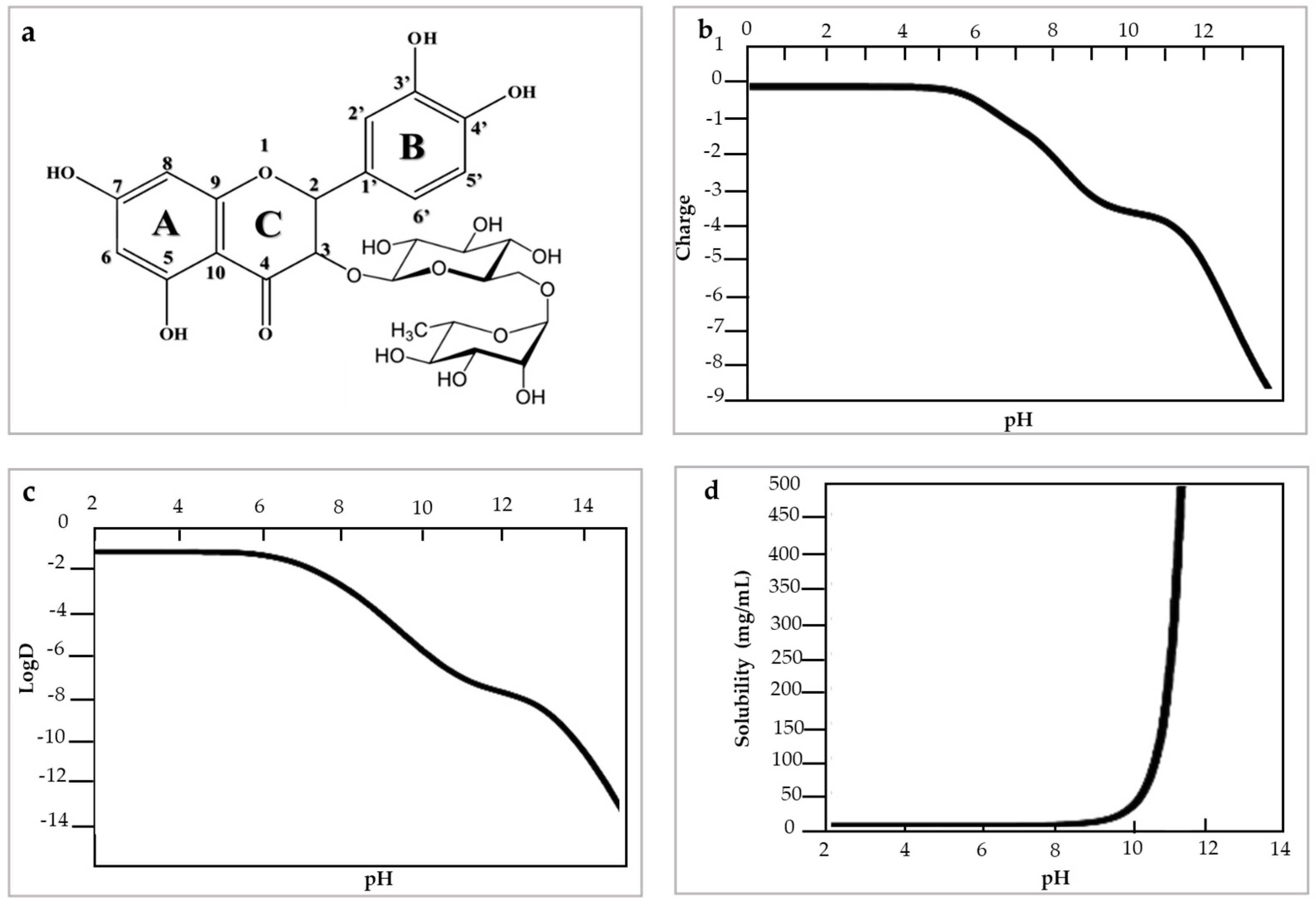
3. Structure and Physicochemical Properties of Rutin Flavonol
4. Sources of Rutin Obtained in Family Farming Products
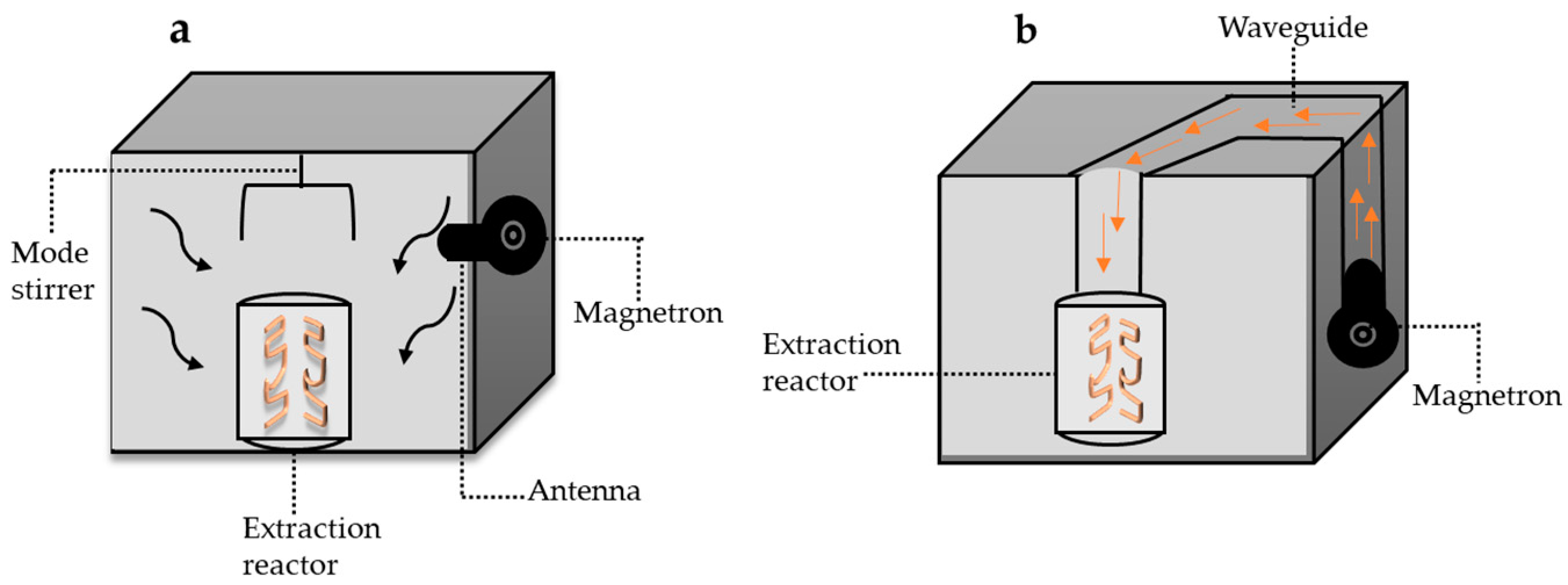
4.1. Microwave-Assisted Technology in Rutin Extraction
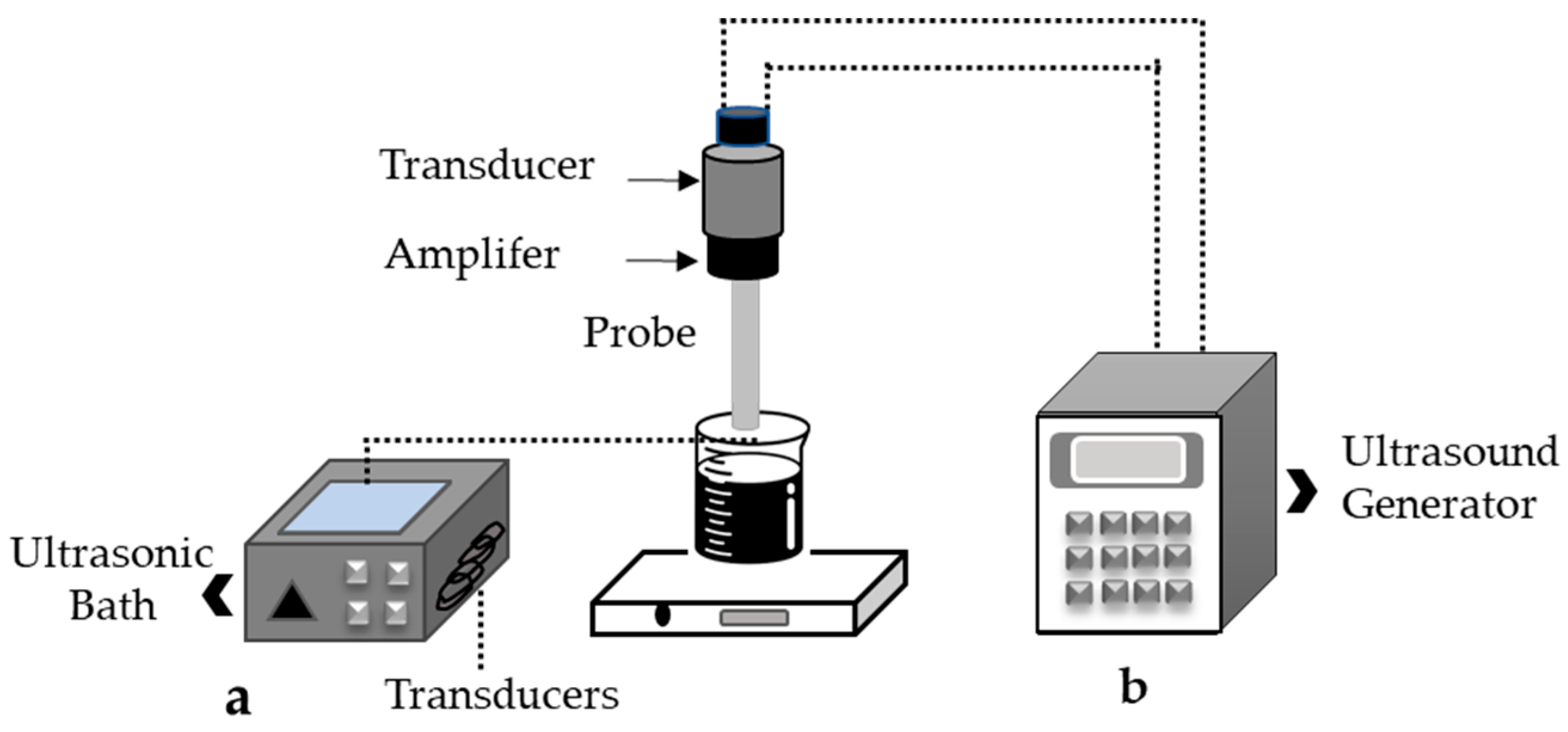
4.2. Ultrasound-Assisted Technology in Rutin Extraction
4.3. Supercritical Fluid Technology in Rutin Extraction
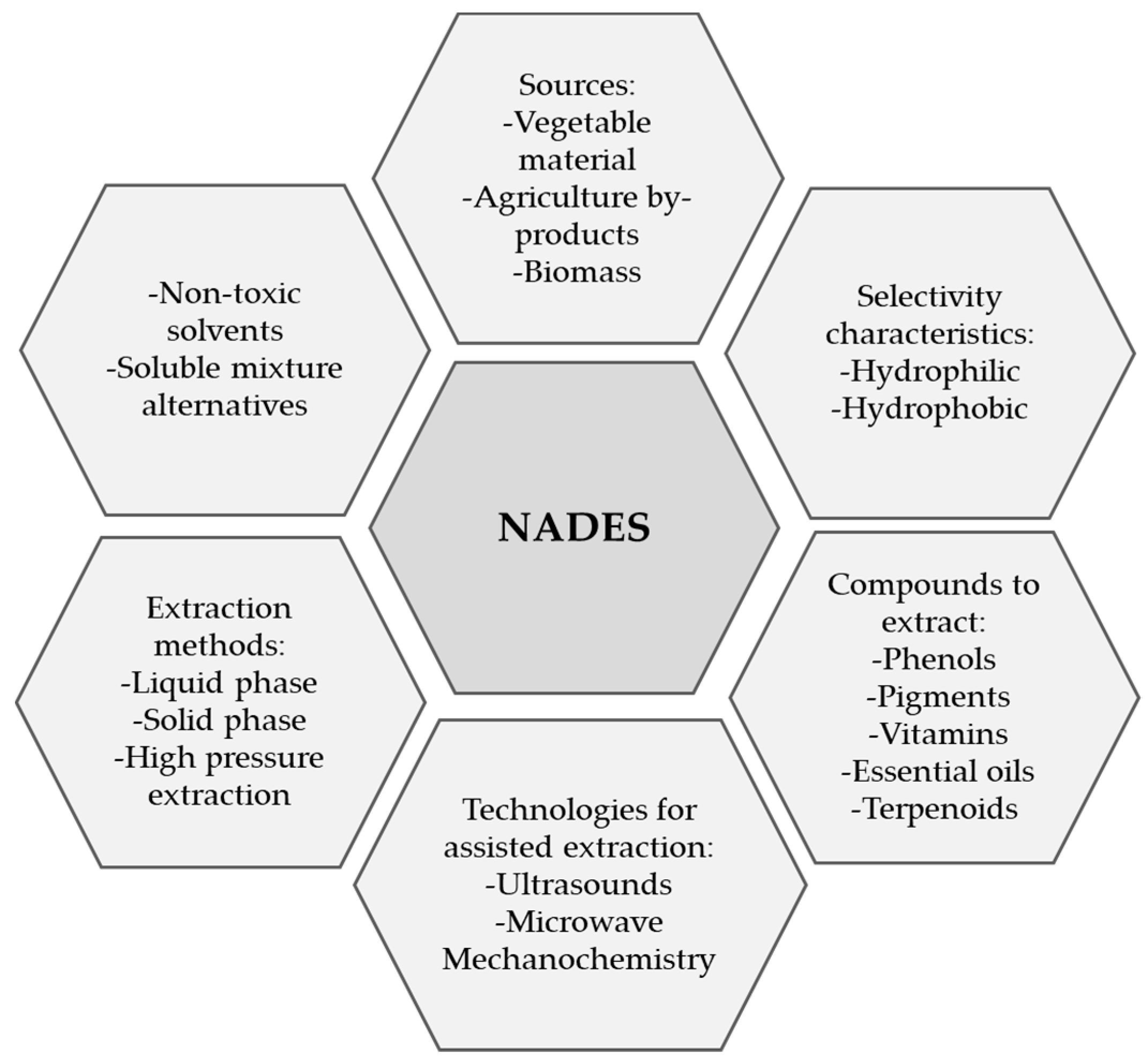
4.4. Alternative Solvents for Rutin Extraction
| Rutin Extraction Source | Extraction Method | Process Variables | Rutin Concentration | Ref. |
|---|---|---|---|---|
| Thyme (Thymus serpyllum L.) | Lixiviation | Methanol solvent, 5:50 (w/v), 24 h, 30 °C | 875 μg/g | [75] |
| Lettuce (Lactuca sativa) | Maceration | Methanol solvent, 1:5 (w/v), 3 h | 0.186 a mg/g | [76] |
| Lettuce (Lactuca sativa) | Maceration | Methanol solvent, 0.5:10 (w/v) | 50 mg/100 g | [77] |
| Cauliflower (Brassica oleracea var. botrytis) | Maceration, filtration | Methanol solvent, 25:250 (w/v) | 551 μg/g | [78] |
| Cucumber (Cucumis sativus L.) | Soxhlet | Ethanol solvent 70%, 30, 3 h | 647.08 µg/g | [79] |
| Thyme (Thymus vulgaris) | Soxhlet | Ethanol solvent 70%, 4 h, 50 °C | 10.05 mg/mL | [80] |
| Strawberry leaves | Microwave-assisted extraction | Ethanol solvent (51.1%), 6:61 (w/v) microwave power of 300 W | 8.08 mg/g | [81] |
| Broccoli (Brassica oleracea L.) | Maceration | Methanol solvent (80%), 2 h, pH:2 | 55.35 μg/mg | [82] |
| Eggplant | Lixiviation | Solvents: n-hexane, dichloromethane, ethanol, 95% (v/v) 1:10 (w/v), 30 °C | 32.4 mg/g | [23] |
| Tarragon (Artemisia dracunculus) | Soxhlet | Methanol solvent 200:10 (w/v), 48 h | 610 mg/100 g | [46] |
| Sophora (Sophora japonica) | NADES | Choline chloride and glycerol (1:1) | 284.81 mg/g | [83] |
| Cassava leaves (Manihot esculenta Crantz) | Ultrasonic-assisted extraction | Ethanol (60%), 50 °C, 2.5:50 (w/v), frequency 40 kHz 90 min | 24.49 g/kg | [60] |
| Rosemary | Ultrasonic-assisted extraction | Methanol solvent 80%, formic acid (1%), 2:20 (w/v), 50 °C, 10 min | 378 μg/mg | [84] |
| Cassava (Manihot esculenta Crantz) | Ultrasonic-assisted extraction | Ethanol solvent (50%), 1:40 (w/v), 50 °C, 4 h, power: 80 W | 622 mg/100 g | [85] |
| Golden berry leaves (Physalis peruviana L.) | Lixiviation | Ethanol solvent (50%), 1:10 (w/v), 60 °C, 5 h | 4996.37 μg/g | [86] |
| Golden berry (Physalis angulata) | Ultrasonic-assisted extraction | Solvents: 57% of water, 35% of ethanol and 8%, 0.6:15 (w/v), 10 min, power: 90 W, 30 °C | 88.2 μg/g | [29] |
| Chili pepper (Capsicum annuum L.) | Maceration | Ethanol solvent 70% v/v acidified by HCl (pH = 3), 2 h | 2.76 μg/mg | [87] |
| Broccoli (Brassica oleracea L.) | Decoction | Methanol solvent (60%), 500:20 (w/v), boiled for 5 min | 0.09 mg/kg | [88] |
| Black carrot (Daucus carota L.) | Maceration | Solvents: methanol, water, acetic acid (70/29.5/0.5, v/v/v), 35:150 (w/v), 2 h | 0.075 mg/100 g | [89] |
| Sideritis condensate | Maceration | Ethanol solvent 100 °C, 30 min | 879 μg/g | [90] |
| Cabbage (Brassica oleracea sabauda) | Soxhlet | Ethanol solvent (50%), 25:50 (w/v), 24 h | 129.97 μg/mg | [91] |
| Tartary buckwheat (Fagopyrum tataricum) | NADES | Choline chloride and glycerol 40:1 (w/v), 40 °C, 1 h | 9.5 mg/g | [73] |
| Amaranth Amaranthus paniculatus | High-pressure extraction | Solvents: 70:30 (v/v) water/ethanol, 188 °C, 20 min, 10 MPa | 14.30 g/kg | [92] |
| Radish (Raphanus sativus L.) | Maceration | Methanol solvent (80%), 5:20 (w/v), 30 min | 9.15 mg/100 g | [93] |
| Cucumber (Cucumis anguria L.) | Ultrasound-assisted extraction | Methanol solvent, (80%), acetic acid (1%), 0.1:10 (w/v), sonication time: 30 min, 1200 rpm | 205.10 µg/g | [94] |
| Solanum tuberosum | Ultrasound-assisted extraction | Methanol solvent (40%), 3:25 (w/v), sonication time: 15 min | 10.76 mg/kg | [95] |
| Celery (Apium graveolens) | Soxhlet | Methanol solvent, 10:100 (w/v), 30 min, 40 °C | 417 mg/100 g | [96] |
| Lettuce (Lactuca sativa) | Soxhlet | Methanol solvent, 1:10 (w/v), 4 h | 78.43 μg/mL | [97] |
| Broccoli (Brassica oleracea L.) | Ultrasound-assisted extraction | Methanol solvent, 5:10 (w/v), sonication time: 15 min, 2700 rpm | 0.44 a mg/g | [98] |
5. Analytical Techniques for Qualitative and Quantitative Analysis of Rutin
5.1. HPLC and Liquid Chromatography Coupled to Mass Spectrometry
5.2. Electrochemical Methods
5.3. Fluorescence Methods
5.4. UV-VIS Spectrometry and FT-IR Spectroscopy
6. Current Trends to Preserve Therapeutic Properties and Improve the Efficiency of Rutin
6.1. Rutin Encapsulation in Colloidal and Heterodisperse Systems
6.1.1. Liposomal System
6.1.2. Nanoemulsions
6.1.3. Lipid and Biopolymeric Nanoparticles
| Encapsulation System | Wall Materials | Encapsulation Mechanism | Package Characteristics | In Vitro Release | Ref. |
|---|---|---|---|---|---|
| Anise extract nanoemulsion (extract with rutin content) | The extract (5 mL) was added into 10 mL H2O containing between 5% and 80 (with respect to extract weight) | Ultrasound 3 min, 50% amplitude, under ice cooling, agitation 24 h at room temperature | Droplet size: 400 nm, polydispersity index: 0.23, after 6 months the average droplet size increased to 649 nm | -- | [136] |
| Nanoemulsion | 7% of soybean oil (w/w), yarrow extract solution (1 mg/mL), 2% sodium caseinate as emulsifier | Homogenization 30,000 rpm 2 min, high pressure homogenization 450 kPa, five passes | Droplet size: 248 nm, Z potential: −37.9 ± 0.7, stability: two weeks | Nanoemulsion partially protected yarrow phenolic compounds during digestion; after digestion, phenolics in milk gels showed the highest antioxidant activity | [137] |
| Rutin emulsions stabilized by chitosan and lecithin | Continuous phase: lecithin (5% p/p), chitosan and water, the dispersed: rutin was dissolved in soybean oil (0.1% w/w). Chitosan solutions 2% (w/w) | Spontaneous emulsification followed by rotor-stator homogenization | Emulsions flow curves showed a near-Newtonian behavior, droplet size: 520 nm, stability: 30 days | Thermal degradation followed first-order reaction kinetics. The activation energy value for rutin degradation was 27.8 kJ mol−1. | [138] |
| Lipid carrier | Lipid phase: 100 mL olive oil, 15 mL oleic acid, 11.5 g rutin; the aqueous phase: 6.9 g of lecithin in 115 mL distilled water. | Homogenization at 1700 rpm 20 min followed by ultra-sonication 40 kHz, 30 min | Encapsulation efficiency 99.85%, particle size: 1.7 μm, lipid carrier significantly increased the ABTS radical scavenging ability, singlet oxygen-scavenging ability and lipoxygenase inhibition | Highest radical inhibition activity for all the digestive phases, controlled release (60 h) | [139] |
| System (SEDDS) for rutin fatty ester | The rutin fatty ester: (SEDDS to 7% w/w); polymer: [dimethylsiloxane-co-(3-(2-(2-hydroxyethoxy) ethoxy)propyl]methylsiloxane] (10%), Transcutol HP 40% w/w, Cremophor RH, 40% w, Labrafac PG 20% w/w | Self-emulsifying delivery system (SEDDS); enzymatic acylation rutin: lauric acid, catalyzed by lipase from Candida antarctica in acetone. | Droplet size: 48.4 nm, the incorporation of 10% of the polymer in SEDDS showed an almost 2-fold increase in mucus permeation | log D value of 3 indicating sustained release of the rutin ester | [140] |
| Rutin-NaCas co-precipitates | Sodium caseinate (10% w/v), rutin (10% w/v), trehalose (0, 2.5 or 5% w/v), the solution was acidified using a 4 M HCl | Lyophilization followed by co-precipitation | Entrapment efficiency: 98.1%, loading capacity: 48.6%, the addition trehalose improved the dispersibility and solubility of precipitate | -- | [135] |
| Polycaprolactone-based nanocarrier | Organic phase: Rutin, Polycaprolactone, acetone (solvent), aqueous phase: Poloxamer 407, Polysorbate 80 | Nanoprecipitation technique | Particle size: 173.63 nm, polydispersity index: 0.107, zeta potential: −22.63 mV, encapsulation efficiency: 72.64 ± 1.06% | Sustained in vitro release for 48 h (65.54–73.74%) | [141] |
| Rutin nanocomplexes | Rutin: Dimethyl sulphoxide (2.5% w/v), phosphatidylcholine: t-butyl alcohol (1.5% w/v). Rutin: phosphatidylcholine ratios (1:2 and 1% w/v) mannitol as a cryoprotectant | Solvent evaporation, salting out and lyophilization | The in vivo study showed better hepatoprotective activity of the formulation compared with pure rutin with improved oral bioavailability | Rutin nanocomplexes significantly improved the solubility and in vitro drug release, and kinetic studies confirmed the diffusion-controlled release | [22] |
| Rutin-loaded starch nanospheres | 10 mL of the rutin standard, 20 mg of the starch, Dimethyl sulphoxide (10 mL) | Dialysis embedding method, bag (MWO = 8000 to 15,000 g/mol) | The drug loading rate: 0.43 μg/mg, encapsulation efficiency: 85.7%, particle size: 70.16 nm | Controlled release, the rutin release rate was approximately 75.38% in pH 7.2 | [142] |
| Rutin Nanocarrier | 1. Rutin: ferritin (molar ratio of 28.2: 1). 2. Epigallocatechin gallate+Rutin/ferritin (binding number: 27.30, binding constant K: 2.65 × 10−4 M−1) | Homogenization, microfiltration, dialysis (MW 10 kDa cutoff) | Encapsulation efficiency: 18.80%, loading capacity: 2.98%, improved rutin stability | Prolonged release in simulated gastrointestinal tract fluid: Release rate of 47.1% | [143] |
| Liposomes | Phosphatidylcholine (10 mg/mL) and rutin (5 mg/mL) Glycerol (3%) | Heating/homogenization method | Particle size: around 419 nm, zeta potential: −40 mV, suspension stability: more than 30 days | Continuous process during the testing period (72 h). | [144] |
| Rutin-phospholipid nanoliposomes (Egg yolk phospholipid extracts) | (Lipid 25 μM + rutin 16.7 μM). Phospholipid content in the final fraction: 208.65 μmol/g fresh egg yolk, cholesterol (0.069–0.082 cholesterol/phospholipid molar ratio), lutein and zeaxanthin (89.24 and 14.9 mg/g, respectively). Saturated fatty acids: 50% of egg’s total yolk phospholipids, monounsaturated fatty acids: 20 to 25%, and polyunsaturated up to 35%. | Thin-film hydration method, followed by sonication cycles (2 min) alternated with hand shaking (2 min), for a total of 20 min at (37 °C) | Mean diameter < 140 nm, entrapment efficiency of rutin up to 87%, rutin-liposome attenuated glutamate-induced cytotoxicity | -- | [109] |
| Cationic liposomes | DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), DOPE (1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphoethanolamine), final ratio lipids and antioxidant 1:1 | Thin-film hydration method | Particle size: 129 nm, polydispersity index: 0.16, zeta potential: 35, stability: 3 months storage | Rutin liposomes interfere with the hydrogen peroxide-induced toxicity, showing a good ability as cell protector. | [145] |
| Rutin phytosomes | Rutin in methanol (40 mg/mL), 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (10 mg/mL), ratio of methanol in tetrahydrofuran (less than 10% v/v) | Thin-film hydration method followed by: bath sonication 40 min, extrusion through 200 nm and then 100 nm pore-size membrane filters | Mean diameter less than 200 nm neutral charge, the majority of the rutin is likely to be associated with lipid headgroups either in the core of the phytosome or on the outer surface of the phytosome (FT-IR and DSC analysis) | The release was less than 20% in all biorelevant media, release depends on membrane diffusion. Maximum release (over 2 h 10.4% and 15.9%) | [146] |
| Liposomes (carboxymethyl cellulose edible films) | Dipalmitoyl phosphatidylcholine (1 mg/mL), cholesterol 0.20 mg/mL), rutin (0.3 mg/mL), solvent: chloroform/methanol (3:1 v/v) | Thin-film hydration method followed by extrusion 10 times through polycarbonate filters 400 nm nominal pore size. Carboxymethyl cellulose edible films were prepared with casting method | Polydispersity index: 0.20, encapsulation efficiency: 74.1%, zeta potential 36.9–42.4 mV, stability: 21 days 4 °C | Controlled release (21 h) flavonol-loaded liposomes were incorporated into carboxymethyl cellulose edible films | [147] |
| Rutin nanophytosomes | Molar ratio rutin: phosphatidylcholine (1:3) | Thin-layer hydration method | Particle size < 100 nm and encapsulation efficiency: 99%, physical and chemical stability (30 days of storage) | -- | [148] |
7. Rutin, Industrial Applications
7.1. Rutin in the Food Industry
7.2. Active Packaging
7.3. Food Fortification
7.4. Cosmetic Applications
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO. Agricultura Familiar en América Latina y el Caribe 2015. Available online: www.fao.org/publications (accessed on 5 April 2023).
- FAO. Frutas y Hortalizas Oportunidades y Desafios Para la Agricultura Sostenible a Pequeña Escala. Frutas y Hortalizas; FAO-CIRAD: Rome, Italy, 2021. [Google Scholar]
- OMS. OMS 2008 Microbiological Hazards in Fresh Leafy Vegetables and Herbs Meeting Report; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- FAO (Food and Agriculture Organisation). Urban and Peri-Urban Agriculture Sourcebook; FAO: Rome, Italy, 2020. [Google Scholar]
- Gawlik-Dziki, U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J. Funct. Foods 2012, 4, 872–882. [Google Scholar] [CrossRef]
- Li, X.; Park, N.I.; Xu, H.; Woo, S.-H.; Park, C.H.; Park, S.U. Differential Expression of Flavonoid Biosynthesis Genes and Accumulation of Phenolic Compounds in Common Buckwheat (Fagopyrum esculentum). J. Agric. Food Chem. 2010, 58, 12176–12181. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2016, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Patel, S.; Muzaffar, R.; Bawazeer, S.S. Rutin: Exploitation of the flavonol for health and homeostasis. Biomed. Pharmacother. 2017, 96, 1559–1561. [Google Scholar] [CrossRef]
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Roles of rutin in cardiac remodeling. J. Funct. Foods 2020, 64, 103606. [Google Scholar] [CrossRef]
- Gullón, B.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Chang, C.; Song, M.; Ma, M.; Song, J.; Cao, F.; Qin, Q. Preparation, Characterization and Molecular Dynamics Simulation of Rutin–Cyclodextrin Inclusion Complexes. Molecules 2023, 28, 955. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Qiu, C.; Long, J.; Ji, H.; Zhao, J.; Zhou, X.; Jin, Z. Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles. Foods 2022, 11, 2827. [Google Scholar] [CrossRef] [PubMed]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2020, 592, 120028. [Google Scholar] [CrossRef] [PubMed]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin in Pickering emulsions stabilized using octenyl succinic anhydride (OSA) modified quinoa, maize, and potato starch nanoparticles. Food Chem. 2023, 405, 134790. [Google Scholar] [CrossRef]
- Amjadi, S.; Shahnaz, F.; Shokouhi, B.; Azarmi, Y.; Siahi-Shadbad, M.; Ghanbarzadeh, S.; Kouhsoltani, M.; Ebrahimi, A.; Hamishehkar, H. Nanophytosomes for enhancement of rutin efficacy in oral administration for diabetes treatment in streptozotocin-induced diabetic rats. Int. J. Pharm. 2021, 610, 121208. [Google Scholar] [CrossRef]
- Sengupta, P.; Das, D.; Bhattacharya, S.; Sur, R.; Bose, A.; Sen, K. A pH-driven method for liposomal encapsulation of dietary flavonoid rutin: Sustained release and enhanced bioefficacy. Food Biosci. 2023, 52, 102392. [Google Scholar] [CrossRef]
- Safarbalou, A.; Haghipanah, M.; Moradi-Kor, N.; Ramezani, E.; Hosseini, S.M.F.; Roudsari, S.S.T.; Sadat Afraz, E. Physicochemical properties of rutin loaded into nanoliposomes and its uses for the treatment of oral ulcers. Eurasian Chem. Commun. 2022, 4, 202–208. [Google Scholar]
- Huang, A.; McClements, D.J.; Luo, S.; Chen, T.; Ye, J.; Liu, C. Fabrication of rutin-protein complexes to form and stabilize bilayer emulsions: Impact of concentration and pretreatment. Food Hydrocoll. 2021, 122, 107056. [Google Scholar] [CrossRef]
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid Complex of Rutin: Development, Characterisation and In Vivo Investigation of Hepatoprotective, Antioxidant Activity and Bioavailability Study in Rats. AAPS PharmSciTech 2018, 19, 3631–3649. [Google Scholar] [CrossRef]
- Dong, R.; Yu, B.; Yan, S.; Qiu, Z.; Lei, J.; Chen, C.; Li, Y.; Cao, B. Analysis of Vitamin P Content and Inheritance Models in Eggplant. Hortic. Plant J. 2020, 6, 240–246. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT -Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Martin, C. The interface between plant metabolic engineering and human health. Curr. Opin. Biotechnol. 2013, 24, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lim, S.B. Subcritical water extraction of rutin from the aerial parts of common buckwheat. J. Supercrit. Fluids 2019, 152, 104561. [Google Scholar] [CrossRef]
- Liao, J.; Qu, B.; Liu, D.; Zheng, N. New method to enhance the extraction yield of rutin from Sophora japonica using a novel ultrasonic extraction system by determining optimum ultrasonic frequency. Ultrason. Sonochemistry 2015, 27, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.C.; Dias, F.D.S. Mixture design and Doehlert matrix for optimization of the ultrasonic assisted extraction of caffeic acid, rutin, catechin and trans-cinnamic acid in Physalis angulata L. and determination by HPLC DAD. Microchem. J. 2018, 141, 247–252. [Google Scholar] [CrossRef]
- Habtemariam, S.; Varghese, G.K. Extractability of Rutin in Herbal Tea Preparations of Moringa stenopetala Leaves. Beverages 2015, 1, 169–182. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorisation. Part 2: Characterization of bioactive compound. J. Chromatogr. A 2019, 1584, 144–154. [Google Scholar] [CrossRef]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.-M.; Gil-Izquierdo, Á. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic compounds. J. Sci. Food Agric. 2017, 99, 2194–2204. [Google Scholar] [CrossRef]
- Toro, R.M.; Aragón, D.M.; Ospina, L.F.; Ramos, F.A.; Castellanos, L. Phytochemical analysis, antioxidant and anti-inflammatory activity of calyces from physalis peruviana. Nat. Prod. Commun. 2014, 9, 1573–1575. [Google Scholar] [CrossRef]
- de Souza, A.S.N.; Schmidt, H.d.O.; Pagno, C.; Rodrigues, E.; da Silva, M.A.S.; Flôres, S.H.; Rios, A.d.O. Influence of cultivar and season on carotenoids and phenolic compounds from red lettuce influence of cultivar and season on lettuce. Food Res. Int. 2022, 155, 111110. [Google Scholar] [CrossRef]
- Omer, H. Radiobiological effects and medical applications of non-ionizing radiation. Saudi J. Biol. Sci. 2021, 28, 5585–5592. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef]
- Upadhyay, R.; Sehwag, S.; Singh, S.P. Antioxidant Activity and Polyphenol Content of Brassica oleracea Varieties. Int. J. Veg. Sci. 2015, 22, 353–363. [Google Scholar] [CrossRef]
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Raslan, M.; Salama, M.M.; Menze, E.T.; El Hawary, S.S. In vivo anti-inflammatory activity and UPLC-MS/MS profiling of the peels and pulps of Cucumis melo var. cantalupensis and Cucumis melo var. reticulatus. J. Ethnopharmacol. 2019, 237, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Stoner, G.D. Red beetroot and betalains as cancer chemopreventative agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Sharma, N.; Sanwal, N.; Lorenzo, J.M.; Sahu, J. Bioactive potential of beetroot (Beta vulgaris). Food Res. Int. 2022, 158, 111556. [Google Scholar] [CrossRef]
- Tacherfiout, M.; Kherbachi, S.; Kheniche, M.; Mattonai, M.; Degano, I.; Ribechini, E.; Khettal, B. HPLC-DAD and HPLC-ESI-MS-MS profiles of hydroalcoholic extracts of Chamaemelum nobile and Mentha pulegium, and study of their antihemolytic activity against AAPH-induced hemolysis. S. Afr. J. Bot. 2022, 150, 678–690. [Google Scholar] [CrossRef]
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–18. [Google Scholar]
- Martins, F.S.; da Conceição, E.C.; Bandeira, E.S.; Silva, J.; Costa, R.M.R. The effects of extraction method on recovery rutin from Calendula officinalis L. (Asteraceae). Pharmacogn. Mag. 2014, 10, S569–S573. [Google Scholar] [CrossRef]
- Méabed, E.M.; El Sayed, N.M.; Abou-Sreea, A.I.; Roby, M.H. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Brede, C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2019, 309, 125678. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2020, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Mills, J. Concurrent and sequential networks—Implications for prwecrp management. Eng. Manag. Int. 1986, 3, 279–282. [Google Scholar] [CrossRef]
- Bondam, A.F.; Diolinda da Silveira, D.; Pozzada dos Santos, J.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Trends in Analytical Chemistry Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Yu, L.; Meng, Y.; Wang, Z.L.; Cao, L.; Liu, C.; Gao, M.Z. Sustainable and efficient surfactant-based microwave-assisted extraction of target polyphenols and furanocoumarins from fig (Ficus carica L.). J. Mol. Liq. 2020, 318, 114196. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; da Machado, A.P.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Thring, R.W.; Arocena, J. Ultrasonic oil recovery and salt removal from refinery tank bottom sludge. J. Env. Sci. Health A Tox. Hazard Subst. Env. Eng. 2014, 49, 1425–1435. [Google Scholar] [CrossRef]
- Romanini, E.B.; Rodrigues, L.M.; Finger, A.; Chierrito, T.P.C.; Scapim, M.R.D.S.; Madrona, G.S. Ultrasound assisted extraction of bioactive compounds from BRS Violet grape pomace followed by alginate-Ca2+ encapsulation. Food Chem. 2020, 338, 128101. [Google Scholar] [CrossRef]
- Zhu, J.; Kou, X.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Shen, D. Enhanced extraction of bioactive natural products using ultrasound-assisted aqueous two-phase system: Application to flavonoids extraction from jujube peels. Food Chem. 2022, 395, 133530. [Google Scholar] [CrossRef]
- Chitrakar, B.; Hou, Y.; Devahastin, S.; Zhang, M.; Sang, Y. Protocols for extraction and purification of rutin from leafy by-products of asparagus (Asparagus officinalis) and characterization of the purified product. Food Chem. 2023, 418, 136014. [Google Scholar] [CrossRef]
- Chahyadi, A. Elfahmi The influence of extraction methods on rutin yield of cassava leaves (Manihot esculenta Crantz). Saudi Pharm. J. 2020, 28, 1466–1473. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice, 2nd ed.; Butterworth-Heinemann: Stoneham, MA, USA, 2013. [Google Scholar]
- de Castro, M.D.L.; Valcarcel, M.; Tena, M.T. Analytical Supercritical Fluid Extraction; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils—A short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Solana, M.; Boschiero, I.; Dall’acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.; Silvestre, A.J.; Freire, M.G. Solvatochromic parameters of deep eutectic solvents formed by ammonium-based salts and carboxylic acids. Fluid Phase Equilibria 2017, 448, 15–21. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Shahar, B.; Indira, A.; Santosh, O.; Dolma, N.; Chongtham, N. Nutritional composition, antioxidant activity and characterization of bioactive compounds from Thymus serpyllum L.: An underexploited wild aromatic plant. Meas. Food 2023, 10, 100092. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Rasiukevičiūtė, N.; Viršilė, A.; Miliauskienė, J.; Laužikė, K.; Valiuškaitė, A.; Dėnė, L.; Chrapačienė, S.; et al. Phenolic Compounds Content Evaluation of Lettuce Grown under Short-Term Preharvest Daytime or Nighttime Supplemental LEDs. Plants 2022, 11, 1123. [Google Scholar] [CrossRef]
- Endo, M.; Fukuda, N.; Yoshida, H.; Fujiuchi, N.; Yano, R.; Kusano, M. Effects of light quality, photoperiod, CO2 concentration, and air temperature on chlorogenic acid and rutin accumulation in young lettuce plants. Plant Physiol. Biochem. 2022, 186, 290–298. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abdullah, A.T.M.; Sharif, M.; Jahan, S.; Kabir, A.; Motalab; Khan, T.A. Relative evaluation of in-vitro antioxidant potential and phenolic constituents by HPLC-DAD of Brassica vegetables extracted in different solvents. Heliyon 2022, 8, e10838. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Saqib, F.; Chicea, L.; Ahmedah, H.T.; Sajer, B.H.; Vlaic, R.A.M.; Pop, O.L.; Moga, M.; Gavris, C. Metabolomics analysis delineates the therapeutic effects of hydroethanolic extract of Cucumis sativus L. seeds on hypertension and isoproterenol-induced myocardial infarction. Biomed. Pharmacother. 2022, 148, 112704. [Google Scholar] [CrossRef]
- Dyshlyuk, L.S.; Fedorova, A.M.; Loseva, A.I.; Eremeeva, N.I. Callus cultures of Thymus vulgaris and Trifolium pratense as a source of geroprotec. Food Process. Tech. Technol. 2021, 51, 423–432. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic compounds with antioxidant activity from strawberry leaves: A study on micro-wave-assisted extraction optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Zhao, M.; Yu, J.; Ji, Y.; Sarengaowa; Yang, X.; Feng, K. The effect of cutting style on the biosynthesis of phenolics and cellular antioxidant capacity in wounded broccoli. Food Res. Int. 2020, 137, 109565. [Google Scholar] [CrossRef]
- Zang, Y.-Y.; Yang, X.; Chen, Z.-G.; Wu, T. One-pot preparation of quercetin using natural deep eutectic solvents. Process. Biochem. 2019, 89, 193–198. [Google Scholar] [CrossRef]
- Moyo, S.; Serem, J.; Bester, M.; Mavumengwana, V.; Kayitesi, E. Influence of boiling and subsequent phases of digestion on the phenolic content, bioaccessibility, and bioactivity of Bidens pilosa (Blackjack) leafy vegetable. Food Chem. 2019, 311, 126023. [Google Scholar] [CrossRef]
- Tao, H.; Cui, B.; Zhang, H.; Bekhit, A.E.-D.; Lu, F. Identification and characterization of flavonoids compounds in cassava leaves (Manihot esculenta Crantz) by HPLC/FTICR-MS. Int. J. Food Prop. 2019, 22, 1134–1145. [Google Scholar] [CrossRef]
- Ivanova, T.; Popova, V.; Mazova, N.; Stoyanova, A.; Damyanova, S. Extracts from physalis leaves (Physalis peruviana L.) for prospective application in medicine and cosmetics. Food Technol. Ukr. Food J. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vecchiato, M.; Abete, L.; Toniolo, C.; Giusti, A.M.; Mannina, L.; Locatelli, M.; Nicoletti, M.; Di Giacomo, S. Capsicum annuum L. var. Cornetto di Pontecorvo PDO: Polyphenolic profile and in vitro biological activities. J. Funct. Foods 2018, 40, 679–691. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Orlando, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; et al. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018, 107, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; Barreca, D.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia 2018, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Ombra, M.N.; Cozzolino, A.; Nazzaro, F.; D’acierno, A.; Tremonte, P.; Coppola, R.; Fratianni, F. Biochemical and biological characterization of two Brassicaceae after their commercial expiry date. Food Chem. 2017, 218, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kraujalis, P.; Venskutonis, P.R.; Ibáñez, E.; Herrero, M. Optimization of rutin isolation from Amaranthus paniculatus leaves by high pressure extraction and fractionation techniques. J. Supercrit. Fluids 2015, 104, 234–242. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.-M. Phenolic compound production and biological activities from in vitro regenerated plants of gherkin (Cucumis anguria L.). Electron. J. Biotechnol. 2015, 18, 295–301. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J. Food Compos. Anal. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Pandey, M.M.; Vijayakumar, M.; Rastogi, S.; Rawat, A.K.S. Phenolic content and antioxidant properties of selected Indian spices of apiaceae. J. Herbs Spices Med. Plants 2012, 18, 246–256. [Google Scholar] [CrossRef]
- Edziri, H.; Smach, M.; Ammar, S.; Mahjoub, M.; Mighri, Z.; Aouni, M.; Mastouri, M. Antioxidant, antibacterial, and antiviral effects of Lactuca sativa extracts. Ind. Crops Prod. 2011, 34, 1182–1185. [Google Scholar] [CrossRef]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and Quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, S.; Xu, H.; Wang, L.; Zhu, X.; Chen, X.; Xu, W.; Xu, W.; Zhang, H.; Lin, Y. Masking quercetin: A simple strategy for selective detection of rutin by combination of bovine serum albumin and fluorescent silicon nanoparticles. Anal. Chim. Acta 2020, 1126, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Naveen, P.; Lingaraju, H.; Anitha; Prasad, K.S. Simultaneous determination of rutin, isoquercetin, and quercetin flavonoids in Nelumbo nucifera by high-performance liquid chromatography method. Int. J. Pharm. Investig. 2017, 7, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.-L.; Duan, L.; Guo, L.; Liu, L.-L.; Zhang, Y.-D.; Li, P.; Liu, E.-H. An UHPLC-MS/MS method for simultaneous determination of quercetin 3-O-rutinoside, kaempferol 3-O-rutinoside, isorhamnetin 3-O-rutinoside, bilobalide and ligustrazine in rat plasma, and its application to pharmacokinetic study of Xingxiong injection. Chin. J. Nat. Med. 2017, 15, 710–720. [Google Scholar] [CrossRef]
- Ivaska, A.; Bobacka, J. Process Analysis—Electroanalytical Techniques. In Encyclopedia of Analytical Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 309–316. [Google Scholar]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; Deng, P.; Chen, D. Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef]
- Sivam, T.; Gowthaman, N.S.K.; Lim, H.N.; Andou, Y.; Arul, P.; Narayanamoorthi, E.; John, S.A. Tunable electrochemical behavior of dicarboxylic acids anchored Co-MOF: Sensitive determination of rutin in pharmaceutical samples. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126667. [Google Scholar] [CrossRef]
- Sun, W.; Yang, M.; Li, Y.; Jiang, Q.; Liu, S.; Jiao, K. Electrochemical behavior and determination of rutin on a pyridinium-based ionic liquid modified carbon paste electrode. J. Pharm. Biomed. Anal. 2008, 48, 1326–1331. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tian, F.-S. Highly sensitive spectrofluorimetic determination of rutin based on its activated effect on a multi-enzyme redox system. Luminescence 2011, 27, 59–62. [Google Scholar] [CrossRef]
- Paczkowska, M.; Lewandowska, K.; Bednarski, W.; Mizera, M.; Podborska, A.; Krause, A.; Cielecka-Piontek, J. Application of spectroscopic methods for identification (FT-IR, Raman spectroscopy) and determination (UV, EPR) of quercetin-3-O-rutinoside. Experimental and DFT based approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. J. 2015, 140, 132–139. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2023–2050. [Google Scholar] [CrossRef]
- Bernardo, J.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. Extraction of phospholipid-rich fractions from egg yolk and development of liposomes entrapping a dietary polyphenol with neuroactive potential. Food Chem. Toxicol. 2019, 133, 110749. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, S.M.; Valderrama, I.H.; Costa, G.M.; Ospina-Giraldo, L.F.; Aragón, D.M. Development and optimization of microparticles containing a hypoglycemic fraction of calyces from Physalis peruviana. J. Appl. Pharm. Sci. 2018, 8, 10–18. [Google Scholar]
- Dammak, I.; do Amaral Sobral, P.J. Formulation and Stability Characterization of Rutin-Loaded Oil-in-Water Emulsions. Food Bioprocess Technol. 2017, 10, 926–939. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, V. Ultrasonication assisted formation and stability of water-in-oil nanoemulsions: Optimization and ternary diagram analysis. Ultrason. Sonochem. 2018, 49, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; McClements, D.J. Theoretical stability maps for guiding preparation of emulsions stabilized by protein−polysaccharide interfacial complexes. Langmuir 2009, 25, 6649–6657. [Google Scholar] [CrossRef]
- Lane, L.A.; Qian, X.; Smith, A.M.; Nie, S. Physical chemistry of nanomedicine: Understanding the complex behaviors of nanoparticles in vivo. Annu. Rev. Phys. Chem. 2015, 66, 521–547. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Carvalho, E.L.S.; von Poser, G.L.; Teixeira, H.F. On the use of nanotechnology-based strategies for association of complex matrices from plant extracts. Rev. Bras. De Farm. 2015, 25, 426–436. [Google Scholar] [CrossRef]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Current demands for food-approved liposome nanoparticles in food and safety sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome Technology for Industrial Purposes. J. Drug Deliv. 2010, 2011, 591325. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Popova, A.V.; Hincha, D.K. Effects of flavonol glycosides on liposome stability during freezing and drying. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2016, 1858, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Dziubińska, H.; Król, E.; Trębacz, K.; Jarosz-Wilkołazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2014, 1838, 254–265. [Google Scholar] [CrossRef]
- Košinová, P.; Berka, K.; Wykes, M.; Otyepka, M.; Trouillas, P. Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: Implication for their lipid-peroxidation inhibition. J. Phys. Chem. B 2012, 116, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Uekusa, Y.; Kamihira-Ishijima, M.; Sugimoto, O.; Ishii, T.; Kumazawa, S.; Nakamura, K.; Tanji, K.-I.; Naito, A.; Nakayama, T. Interaction of epicatechin gallate with phospholipid membranes as revealed by solid-state NMR spectroscopy. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2011, 1808, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Krstonošić, V.S.; Kalić, M.D.; Dapčević-Hadnađev, T.R.; Lončarević, I.S.; Hadnađev, M.S. Physico-chemical characterization of protein stabilized oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125045. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Montoliu-Boneu, J.; Salvia-Trujillo, L.; Martín-Belloso, O. Factors affecting the formation of highly concentrated emulsions and nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123577. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2020, 288, 102344. [Google Scholar] [CrossRef]
- Bazana, M.T.; da Silva, S.S.; Codevilla, C.F.; de Deus, C.; Lucas, B.N.; Ugalde, G.A.; Mazutti, M.A.; Flores, E.M.M.; Barin, J.S.; Silva, C.D.B.D.; et al. Development of nanoemulsions containing Physalis peruviana calyx extract: A study on stability and antioxidant capacity. Food Res. Int. 2019, 125, 108645. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Carvalho, I.; Miranda, M.; Silva, L.; Chrysostomo-Massaro, T.; Paschoal, J.; Bastos, J.; Marcato, P. In Vitro Anticancer activity and physicochemical properties of solanum lycocarpum Alkaloidic Extract Loaded in Natural Lipid-Based Nanoparticles. Colloid Interface Sci. Commun. 2019, 28, 5–14. [Google Scholar] [CrossRef]
- Razavi, M.S.; Ebrahimnejad, P.; Fatahi, Y.; D’emanuele, A.; Dinarvand, R. Recent Developments of Nanostructures for the Ocular Delivery of Natural Compounds. Front. Chem. 2022, 10, 850757. [Google Scholar] [CrossRef]
- Attama, A.; Mumuni, A.; Philip, B. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; InTech: London, UK, 2012. [Google Scholar]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Loveday, S.; Jameson, G.B.; Hindmarsh, J.P.; Singh, H. Rutin-casein co-precipitates as potential delivery vehicles for flavonoid rutin. Food Hydrocoll. 2019, 96, 451–462. [Google Scholar] [CrossRef]
- Ghazy, O.; Fouad, M.; Saleh, H.; Kholif, A.; Morsy, T. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2020, 341, 128259. [Google Scholar] [CrossRef]
- Villalva, M.; Jaime, L.; Arranz, E.; Zhao, Z.; Corredig, M.; Reglero, G.; Santoyo, S. Nanoemulsions and acidified milk gels as a strategy for improving stability and antioxidant activity of yarrow phenolic compounds after gastrointestinal digestion. Food Res. Int. 2020, 130, 108922. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.J.D.A. Investigation into the physicochemical stability and rheological properties of rutin emulsions stabilized by chitosan and lecithin. J. Food Eng. 2018, 229, 12–20. [Google Scholar] [CrossRef]
- Mel, M.; Gunathilake, K.; Fernando, C. Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int. J. Biol. Macromol. 2020, 159, 316–323. [Google Scholar] [CrossRef]
- Cardona, M.I.; Le, N.-M.N.; Zaichik, S.; Aragón, D.M.; Schnürch, A.B. Development and in vitro characterization of an oral self-emulsifying delivery system (SEDDS) for rutin fatty ester with high mucus permeating properties. Int. J. Pharm. 2019, 562, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Sharma, A.; Verma, R.K.; Shukla, R. Polycaprolactone based nano-carrier for co-administration of moxifloxacin and rutin and its In-vitro evaluation for sepsis. J. Drug Deliv. Sci. Technol. 2019, 54, 101286. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, G.; Zhang, M.; Zhou, Z.; Li, Q.; Strappe, P.; Blanchard, C. Epigallocatechin Gallate (EGCG) Decorating Soybean Seed Ferritin as a Rutin Nanocarrier with Prolonged Release Property in the Gastrointestinal Tract. Plant Foods Hum. Nutr. 2016, 71, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-Lopez, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2019, 130, 108967. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Vu, H.T.H.; Hook, S.M.; Siqueira, S.D.; Müllertz, A.; Rades, T.; McDowell, A. Are phytosomes a superior nanodelivery system for the antioxidant rutin? Int. J. Pharm. 2018, 548, 82–91. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Quilaqueo, M.; Venegas, O.; Ahumada, M.; Silva, W.; Osorio, F.; Giménez, B. Design of dipalmitoyl lecithin liposomes loaded with quercetin and rutin and their release kinetics from carboxymethyl cellulose edible films. J. Food Eng. 2018, 224, 165–173. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Phosphatidylcholine-rutin complex as a potential nanocarrier for food applications. J. Funct. Foods 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Drinkwater, J.M.; Tsao, R.; Liu, R.; Defelice, C.; Wolyn, D.J. Effects of cooking on rutin and glutathione concentrations and antioxidant activity of green asparagus (Asparagus officinalis) spears. J. Funct. Foods 2015, 12, 342–353. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannova, A.; Tóth, T.; Golob, A.; Luthar, Z.; Kreft, I. The temperature threshold for the transformation of rutin to quercetin in Tartary buckwheat dough. Food Chem. 2019, 283, 28–31. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, Y.; Yoo, S.-H.; Inglett, G.E.; Lee, S. Reduction of rutin loss in buckwheat noodles and their physicochemical characterisation. Food Chem. 2012, 132, 2107–2111. [Google Scholar] [CrossRef]
- Capitani, C.D.; Hatano, M.K.; Marques, M.F.; Castro, I.A. Effects of optimized mixtures containing phenolic compounds on the oxidative stability of sausages. Food Sci. Technol. Int. 2013, 19, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Szwengiel, A.; Dziedzic, K.; Le Thanh-Blicharz, J.; Kmiecik, D.; Górecka, D. The Effect of Buckwheat Hull Extract on Lipid Oxidation in Frozen-Stored Meat Products. J. Food Sci. 2017, 82, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, H. Preparation and properties of zein–rutin composite nanoparticle/corn starch films. Carbohydr. Polym. 2017, 169, 385–392. [Google Scholar] [CrossRef]
- Přikryl, J.; Hájek, T.; Švecová, B.; Salek, R.N.; Černíková, M.; Červenka, L.; Buňka, F. Antioxidant properties and textural characteristics of processed cheese spreads enriched with rutin or quercetin: The effect of processing conditions. LWT—Food Sci. Technol. 2018, 87, 266–271. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Novel nanostructured lipid carriers as a promising food grade delivery system for rutin. J. Funct. Foods 2016, 26, 167–175. [Google Scholar] [CrossRef]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pintoa, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017, 12, 733–744. [Google Scholar] [CrossRef]
- Peres, D.A.; de Oliveira, C.A.; Da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.F.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Ski. Res. Technol. 2016, 22, 325–333. [Google Scholar] [CrossRef]
- de Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; de Araújo, G.L.B.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- FAO. Summary for Policymakers. In Special Report: Special Report on Climate Change and Land; Cambridge University Press: Cambridge, UK, 2019; pp. 1–36. Available online: https://www.cambridge.org/core/product/identifier/9781009157988%23prf2/type/book_part (accessed on 29 June 2021).
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for plant-based diets: Challenges and innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Giaconia, M.A.; dos Passos Ramos, S.; Pereira, C.F.; Lemes, A.C.; de Rosso, V.V.; Braga, A.R.C. Overcoming restrictions of bioactive compounds biological effects in food using nanometer-sized structures. Food Hydrocoll. 2020, 107, 105939. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. https://doi.org/10.3390/molecules28155864
Tobar-Delgado E, Mejía-España D, Osorio-Mora O, Serna-Cock L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules. 2023; 28(15):5864. https://doi.org/10.3390/molecules28155864
Chicago/Turabian StyleTobar-Delgado, Elizabeth, Diego Mejía-España, Oswaldo Osorio-Mora, and Liliana Serna-Cock. 2023. "Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection" Molecules 28, no. 15: 5864. https://doi.org/10.3390/molecules28155864
APA StyleTobar-Delgado, E., Mejía-España, D., Osorio-Mora, O., & Serna-Cock, L. (2023). Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules, 28(15), 5864. https://doi.org/10.3390/molecules28155864





