[IPr#–PEPPSI]: A Well-Defined, Highly Hindered and Broadly Applicable Pd(II)–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for Cross-Coupling Reactions
Abstract
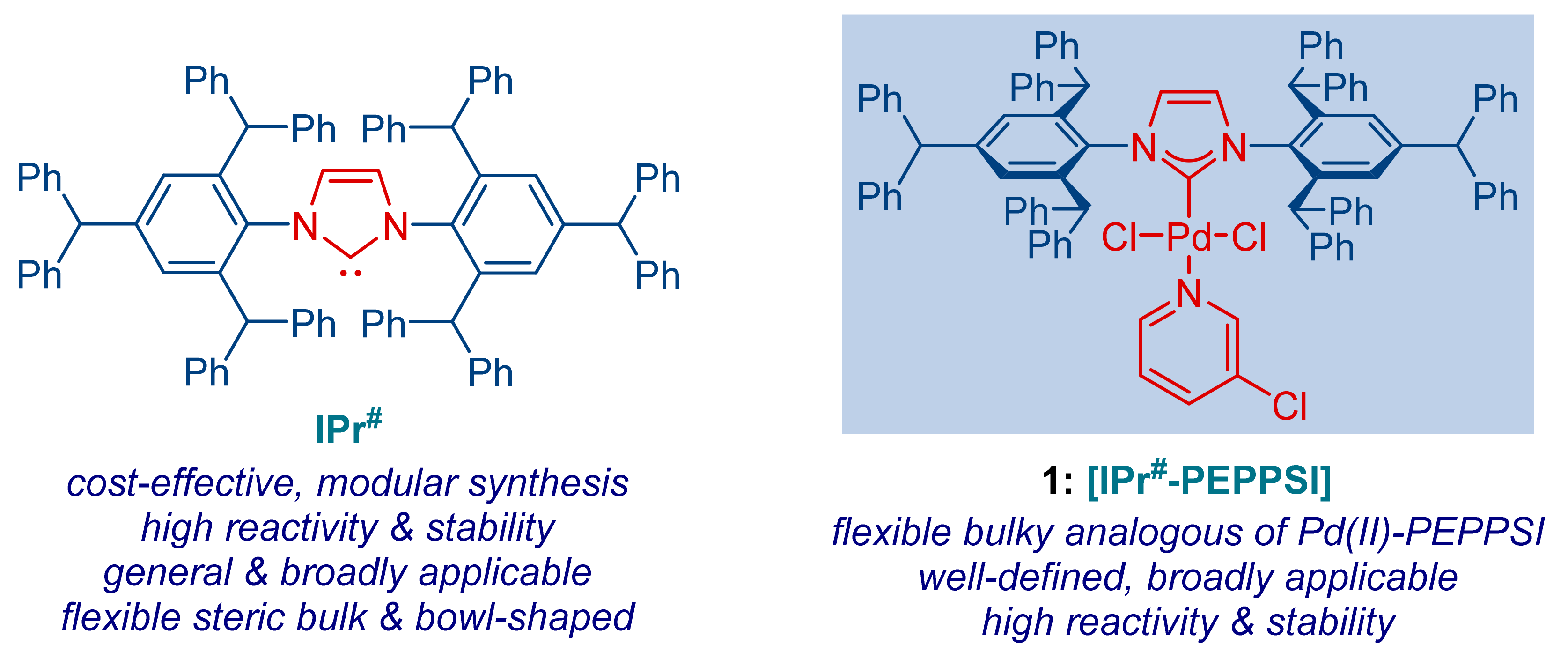
1. Introduction
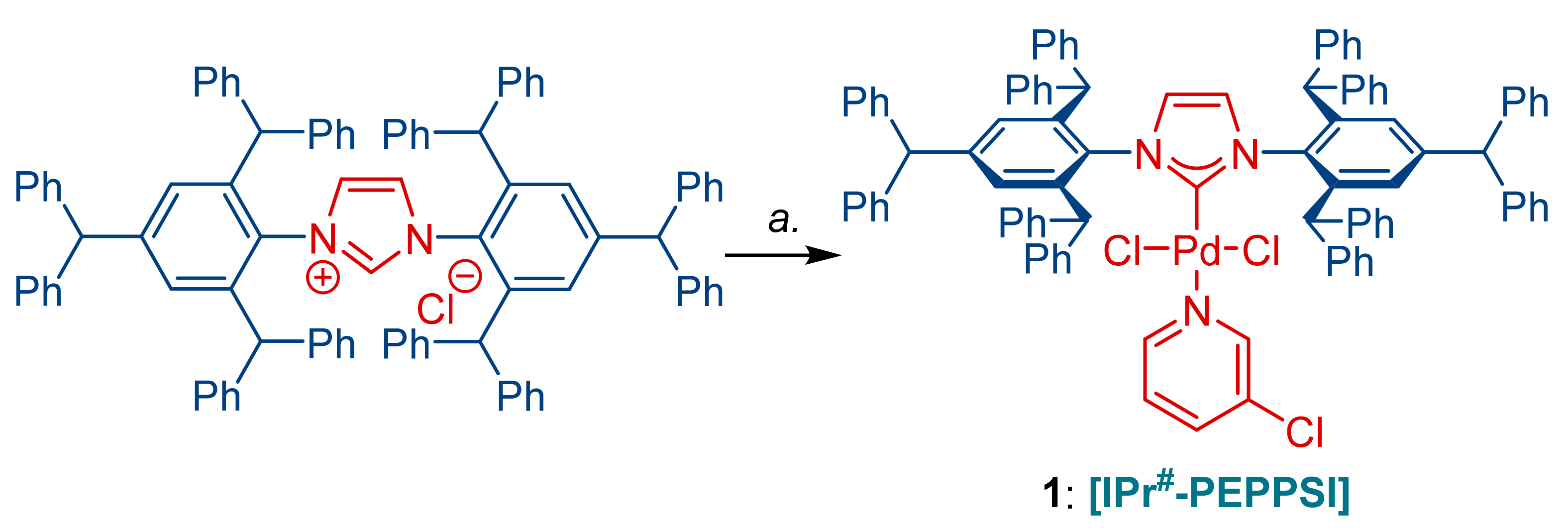
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Molander, G.A.; Wolfe, J.P.; Larhed, M. Science of Synthesis: Cross-Coupling and Heck-Type Reactions, 1st ed.; Thieme: Stuttgart, Germany, 2013. [Google Scholar]
- Molnar, A. Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Developments; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- Meijere, A.D.; Brase, S.; Oestreich, M. Metal Catalyzed Cross-Coupling Reactions and More, 3 Volume Set; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Colacot, T.J. New Trends in Cross-Coupling, 1st ed.; RSC: Cambridge, UK, 2015. [Google Scholar]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Vilar, R. Monoligated palladium species as catalysts in cross-coupling reactions. Angew. Chem. Int. Ed. 2005, 44, 366–374. [Google Scholar] [CrossRef]
- Wu, X.F.; Anbarasan, P.; Neumann, H.; Beller, M. From Noble Metal to Nobel Prize: Palladium-Catalyzed Coupling Reactions as Key Methods in Organic Synthesis. Angew. Chem. Int. Ed. 2010, 49, 9047–9050. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Johansson Seechurn, C.C.C.; Colacot, T.J. Development of Preformed Pd Catalysts for Cross-Coupling Reactions, Beyond the 2010 Nobel Prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef]
- Beller, M. Cross-Coupling Reactions in Organic Synthesis Themed Issue. Chem. Soc. Rev. 2011, 40, 4877–5208. [Google Scholar]
- Gildner, P.G.; Colacot, T.J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics 2015, 34, 5497–5508. [Google Scholar] [CrossRef]
- Campeau, L.C.; Hazari, N. Cross-Coupling and Related Reactions: Connecting Past Success to the Development of New Reactions for the Future. Organometallics 2019, 38, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Shi, Z.J. Upgrading Cross-Coupling Reactions for Biaryl Syntheses. Acc. Chem. Res. 2019, 52, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Cazin, C.S.J. (Eds.) Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis; Thieme: Stuttgart, Germany, 2017. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P. (Ed.) N-Heterocyclic Carbenes; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Huynh, H.V. The Organometallic Chemistry of N-Heterocyclic Carbenes; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Munz, D. Pushing Electrons–Which Carbene Ligand for Which Application? Organometallics 2018, 37, 275–289. [Google Scholar] [CrossRef]
- Melaimi, M.; Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)(amino)carbenes (CAACs): Recent Developments. Angew. Chem. Int. Ed. 2017, 56, 10046–10068. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic carbenes-palladium(II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef]
- Dröge, T.; Glorius, F. The Measure of All Rings: N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6952. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Albert, J.; D’Andrea, L.; Granell, J.; Zafrilla, J.; Font-Bardia, M.; Solans, X. The cyclopalladation reaction of 2-phenylaniline revisited. J. Organomet. Chem. 2005, 690, 422–429. [Google Scholar] [CrossRef]
- Chen, M.T.; Vicic, D.A.; Chain, W.J.; Turner, M.L.; Navarro, O. Inhibited Catalyst Activation in (N-Heterocyclic carbene)PdCl2(diethylamine) Complexes by Intramolecular Hydrogen Bonding. Organometallics 2011, 30, 6770–6773. [Google Scholar] [CrossRef]
- Bernhammer, J.C.; Singh, H.; Huynh, H.V. Amine-Functionalized Indazolin-3-Ylidene Complexes of Palladium (II) by Postmodification of a Single Precursor. Organometallics 2014, 33, 4295–4301. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Zhou, Y.; Bao, X.; Schaefer, H.F., III. Is Pd(II)-Promoted σ-Bond Metathesis Mechanism Operative for the Pd-PEPPSI Complex-Catalyzed Amination of Chlorobenzene with Aniline? Experiment and Theory. Chem.-Eur. J. 2015, 21, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Shi, S.; Gao, P.; Lalancette, R.; Szostak, R.; Szostak, M. [(NHC)PdCl2(Aniline)] Complexes: Easily Synthesized, Highly Active Pd(II)–NHC Precatalysts for Cross-Coupling Reactions. J. Org. Chem. 2021, 86, 15648–15657. [Google Scholar] [CrossRef] [PubMed]
- Navarro, O.; Kelly, R.A., III; Nolan, S.P. A General Method for the Suzuki–Miyaura Cross-Coupling of Sterically Hindered Aryl Chlorides: Synthesis of Di- and Tri-ortho-substituted Biaryls in 2-Propanol at Room Temperature. J. Am. Chem. Soc. 2003, 125, 16194–16195. [Google Scholar] [CrossRef]
- Bruno, N.C.; Tudge, M.T.; Buchwald, S.L. Design and Preparation of New Palladium Precatalysts for C-C and C-N Cross-Coupling Reactions. Chem. Sci. 2013, 4, 916–920. [Google Scholar] [CrossRef]
- Marion, N.; Navarro, O.; Mei, J.; Stevens, E.D.; Scott, N.M.; Nolan, S.P. Modified (NHC)Pd(allyl)Cl (NHC = N-heterocyclic carbene) complexes for room-temperature Suzuki-Miyaura and Buchwald-Hartwig reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. [Google Scholar] [CrossRef]
- Li, G.; Lei, P.; Szostak, M.; Casals, E.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanistic Study of Suzuki-Miyaura Cross-Coupling Reactions of Amides Mediated by [Pd(NHC)(allyl)Cl] Precatalysts. ChemCatChem 2018, 10, 3096–3106. [Google Scholar] [CrossRef]
- Zhou, T.; Ma, S.; Nahra, F.; Obled, A.M.C.; Poater, A.; Cavallo, L.; Cazin, C.S.J.; Nolan, S.P.; Szostak, M. [Pd(NHC)(μ-Cl)Cl]2: Versatile and Highly Reactive Complexes for Cross-Coupling Reactions that Avoid Formation of Inactive Pd(I) Off-Cycle Products. iScience 2020, 23, 101377. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Tu, Y.; Li, Y.; Wu, J.; Zhao, J. Palladium-Catalyzed Oxidative Cross-Coupling of Arylhydrazines and Arenethiols with Molecular Oxygen as the Sole Oxidant. J. Org. Chem. 2018, 83, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Bryan, Z.J.; Smith, M.L.; McNeil, A.J. Chain-Growth Polymerization of Aryl Grignards Initiated by a Stabilized NHC-Pd Precatalyst. Macromol. Rapid Commun. 2012, 33, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-F.; Huang, Y.; Liu, H.; Gao, Z.-H.; Zhang, C.-L.; Ye, S. Photoredox cooperative N-heterocyclic carbene/palladium-catalysed alkylacylation of alkenes. Nat. Commun. 2022, 13, 5754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, G.; Li, G.; Flach, C.; Mendelsohn, R.; Lalancette, R.; Szostak, R.; Szostak, M. IPr#–Highly Hindered, Broadly Applicable N-Heterocyclic Carbenes. Chem. Sci. 2021, 12, 10583–10589. [Google Scholar] [PubMed]
- IPr# HCl. 1,3-Bis-(2,4,6-Tribenzhydrylphenyl)-1H-imidazol-3-ium chloride. Available online: www.sigmaaldrich.com/catalog/product/aldrich/915653 (accessed on 28 June 2023).
- [Pd(IPr#)(cin)Cl]. Available online: www.sigmaaldrich.com/US/en/product/aldrich/919616 (accessed on 28 June 2023).
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd-N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Calimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Pd(IPr#)(3-Cl-py)Cl2. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/925489 (accessed on 28 June 2023).
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC = N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(3-Cl-pyridinyl)Cl2]: A Novel and Efficient PEPPSI Precatalyst. Organometallics 2012, 31, 6947–6951. [Google Scholar] [CrossRef]
- Clavier, H.; Nolan, S.P. Percent buried volume for phosphine and N-heterocyclic carbene ligands: Steric properties in organometallic chemistry. Chem. Commun. 2010, 46, 841–861. [Google Scholar] [CrossRef]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the Online Computer-Aided Design of Catalytic Pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef]
- Lei, P.; Meng, G.; Shi, S.; Ling, Y.; An, J.; Szostak, R.; Szostak, M. Suzuki-Miyaura Cross-Coupling of Amides and Esters at Room Temperature: Correlation with Barriers to Rotation around C–N and C–O Bonds. Chem. Sci. 2017, 8, 6525–6530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Szostak, M. Palladium-catalyzed cross-couplings by C–O bond activation. Catal. Sci. Technol. 2020, 10, 5702–5739. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, T.; Poater, A.; Cavallo, L.; Nolan, S.P.; Szostak, M. Suzuki–Miyaura cross-coupling of esters by selective O–C(O) cleavage mediated by air- and moisture-stable [Pd(NHC)(μ-Cl)Cl]2 precatalysts: Catalyst evaluation and mechanism. Catal. Sci. Technol. 2021, 11, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Lei, P.; Szostak, M. A General Method for Two-Step Transamidation of Secondary Amides Using Commercially Available, Air- and Moisture-Stable Palladium/NHC (N-Heterocyclic Carbene) Complexes. Org. Lett. 2017, 19, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Vicic, D.A.; Turner, M.L.; Navarro, O. (N-Heterocyclic Carbene)PdCl2(TEA) Complexes: Studies on the Effect of the “Throw-Away” Ligand in Catalytic Activity. Organometallics 2011, 30, 5052–5056. [Google Scholar] [CrossRef]
- Huang, J.; Grasa, G.; Nolan, S.P. General and Efficient Catalytic Amination of Aryl Chlorides Using a Palladium/Bulky Nucleophilic Carbene System. Org. Lett. 1999, 1, 1307–1309. [Google Scholar] [CrossRef]
- Giannerini, M.; Fananas-Mastral, M.; Feringa, B.L. Direct catalytic cross-coupling of organolithiums compounds. Nat. Chem. 2013, 5, 667–672. [Google Scholar] [CrossRef]
- Pinxterhuis, E.B.; Giannerini, M.; Hornillos, V.; Feringa, B.L. Fast, greener and scalable direct coupling of organolithium compounds with no additional solvents. Nat. Commun. 2016, 7, 11698. [Google Scholar] [CrossRef]
- Viciu, M.S.; Germaneau, R.F.; Nolan, S.P. Well-Defined, Air-Stable (NHC)Pd(Allyl)Cl (NHC = N-Heterocyclic Carbene) Catalysts for the Arylation of Ketones. Org. Lett. 2002, 4, 4053–4056. [Google Scholar] [CrossRef]
- Lian, Z.; Bhwal, B.N.; Yu, P.; Morandi, B. Palladium-catalyzed carbon-sulfur or carbon-phosphorus bond metathesis by reversible arylation. Science 2017, 356, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Chartoire, A.; Slawin, A.M.Z.; Nolan, S.P. Extending the utility of [Pd(NHC)(cinnamyl)Cl] precatalysts: Direct arylation of heterocycles. Beilstein J. Org. Chem. 2012, 8, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef]
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the Medicinal Chemistry Synthetic Toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Dunsford, J.J.; Cavell, K.J. Pd–PEPPSI-Type Expanded Ring N-Heterocyclic Carbene Complexes: Synthesis, Characterization, and Catalytic Activity in Suzuki–Miyaura Cross Coupling. Organometallics 2014, 33, 2902–2905. [Google Scholar] [CrossRef]
- Sizova, O.V.; Skripnikov, L.V.; Sokolov, A.Y. Symmetry decomposition of quantum chemical bond orders. J. Mol. Struc. Theochem. 2008, 870, 1–9. [Google Scholar] [CrossRef]
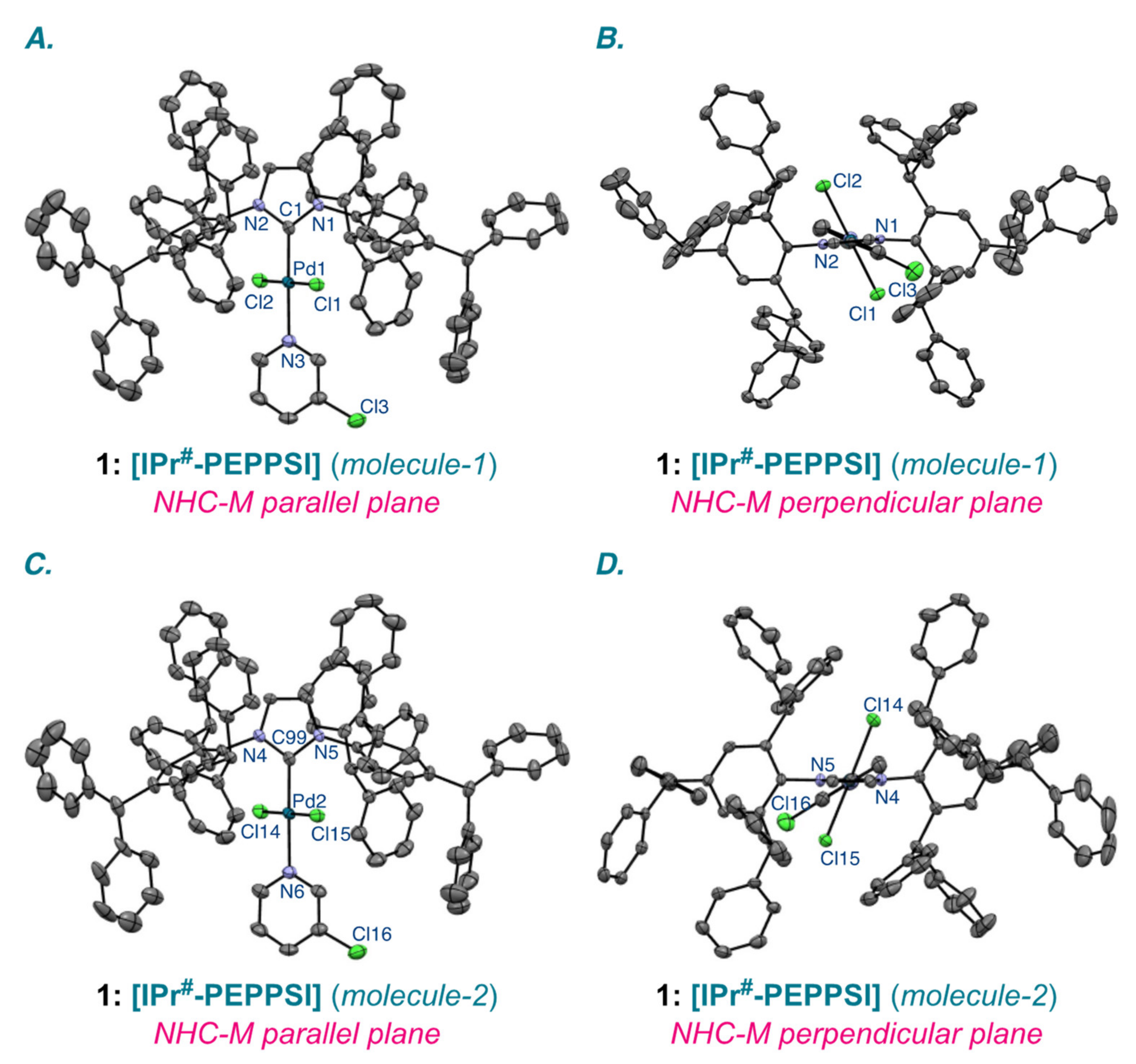
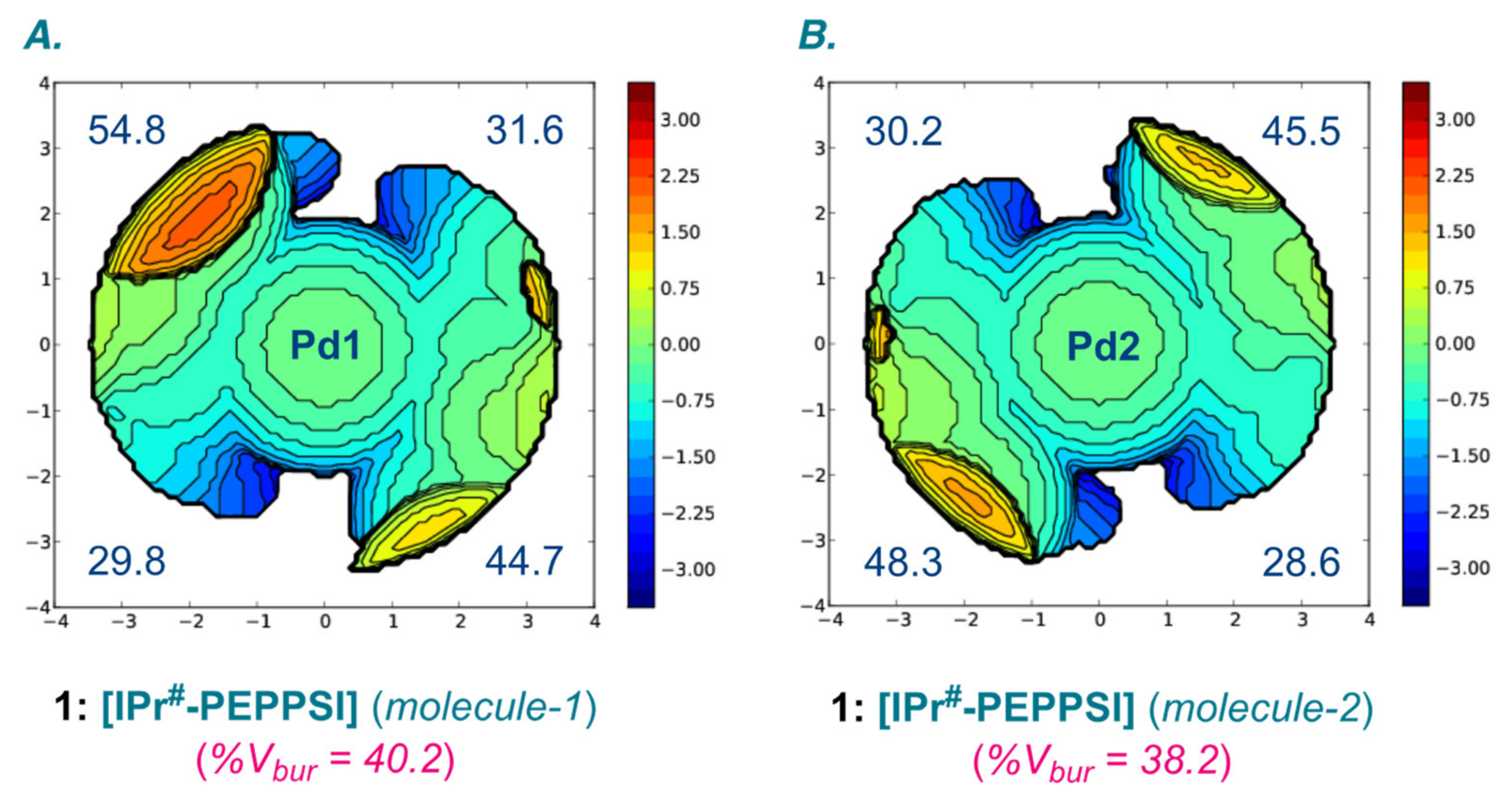
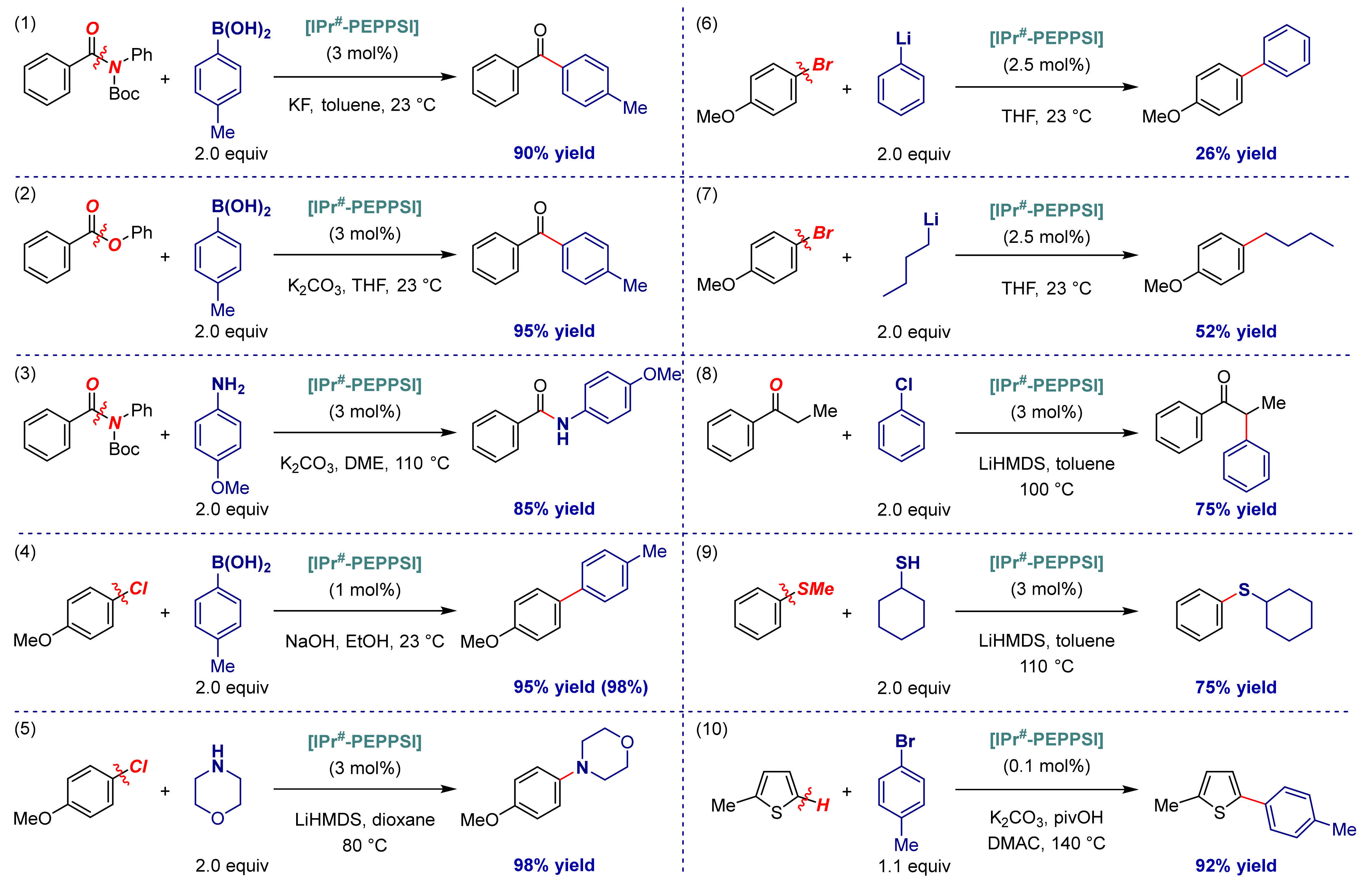
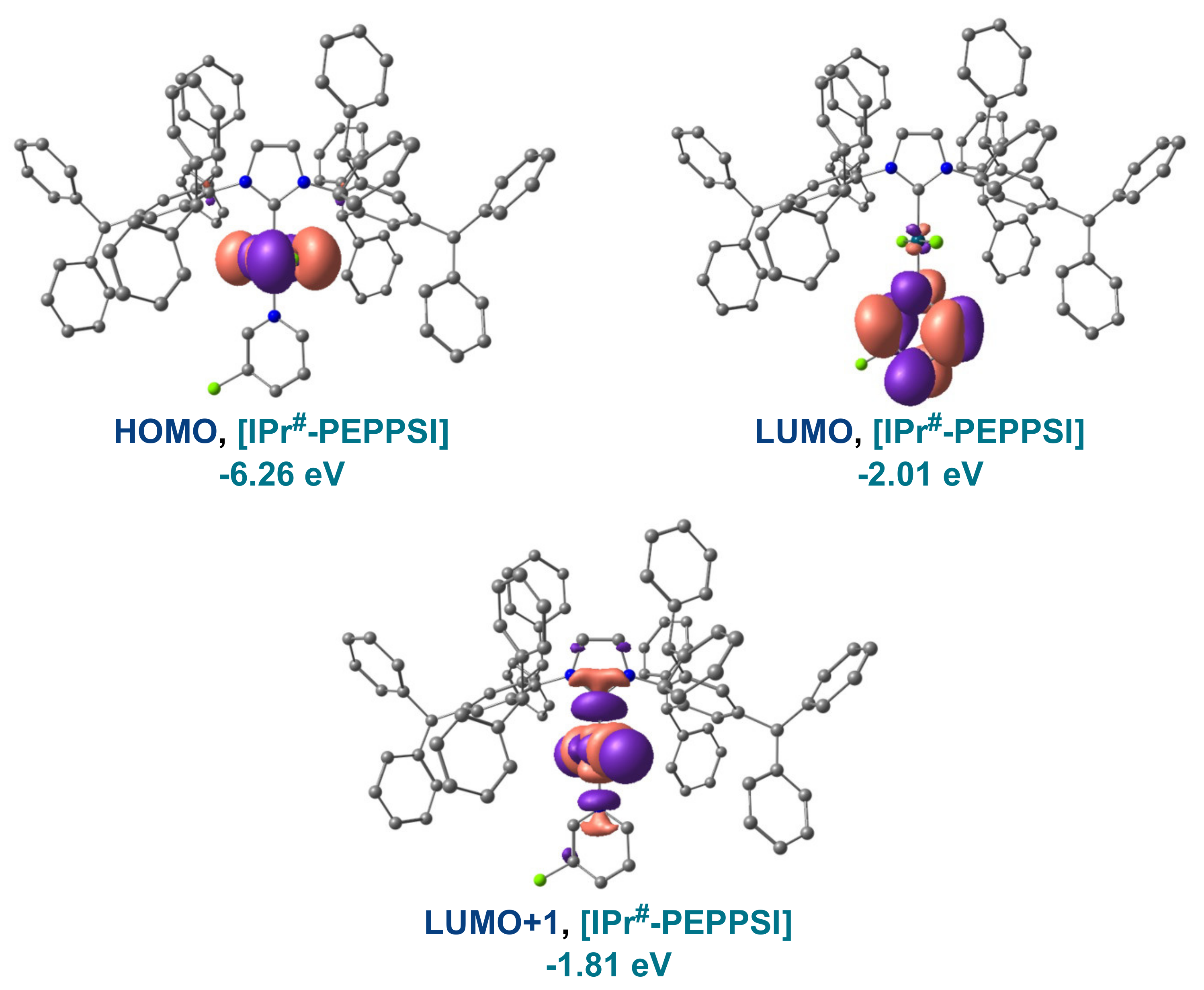
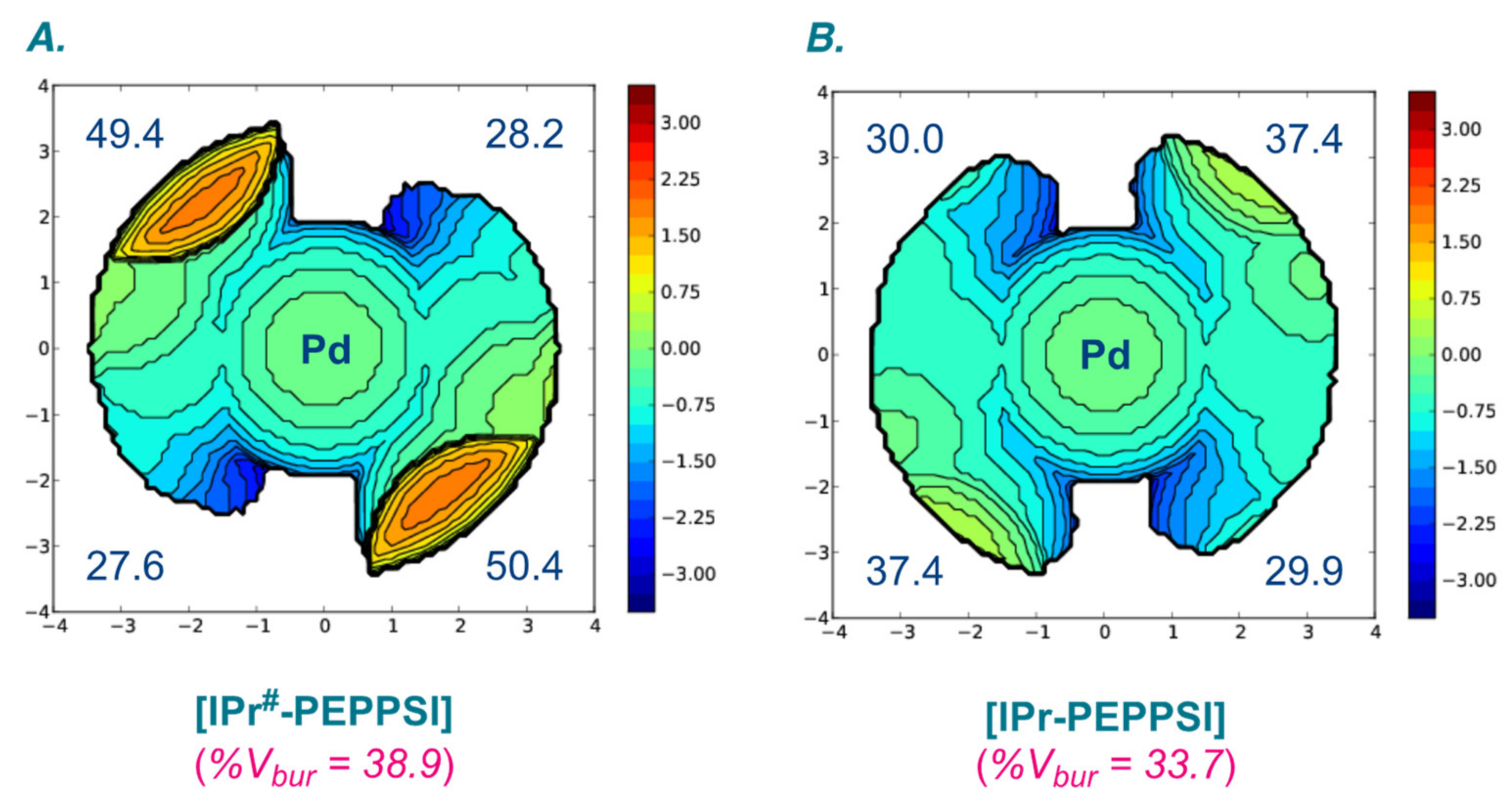
| Entry | Complexes | %Vbur | Pd–CNHC (Å) | Pd–Npy (Å) | Pd–Cl (Å) |
|---|---|---|---|---|---|
| 1 | [IPr#–PEPPSI] (molecule-1) | 40.2 | 1.978(4) | 2.119(4) | 2.285(1), 2.300(1) |
| 2 | [IPr#–PEPPSI] (molecule-2) | 38.2 | 1.965(4) | 2.114(4) | 2.301(1), 2.302(1) |
| 3 | [IPr–PEPPSI] | 34.8 | 1.969(3) | 2.137(3) | 2.290(9), 2.298(7) |
| 4 | [IPent–PEPPSI] | 38.3 | 1.975(3) | 2.097(2) | 2.2868(9), 2.3033(9) |
| 5 | [Pd(IPr#)(cin)Cl] | 44.7 | 2.046(4) | 2.117(5), 2.133(6), 2.216(7) a | 2.374(1) |
| Entry | Complexes | Pd–CNHC | Pd–Npy | Pd–Cl |
|---|---|---|---|---|
| 1 | [IPr#–PEPPSI] | 0.6801 | 0.3099 | 0.6062, 0.6032 |
| 2 | [IPr–PEPPSI] | 0.6871 | 0.3267 | 0.6278, 0.6302 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Zhao, Q.; Meng, G.; Lalancette, R.; Szostak, R.; Szostak, M. [IPr#–PEPPSI]: A Well-Defined, Highly Hindered and Broadly Applicable Pd(II)–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for Cross-Coupling Reactions. Molecules 2023, 28, 5833. https://doi.org/10.3390/molecules28155833
Rahman MM, Zhao Q, Meng G, Lalancette R, Szostak R, Szostak M. [IPr#–PEPPSI]: A Well-Defined, Highly Hindered and Broadly Applicable Pd(II)–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for Cross-Coupling Reactions. Molecules. 2023; 28(15):5833. https://doi.org/10.3390/molecules28155833
Chicago/Turabian StyleRahman, Md. Mahbubur, Qun Zhao, Guangrong Meng, Roger Lalancette, Roman Szostak, and Michal Szostak. 2023. "[IPr#–PEPPSI]: A Well-Defined, Highly Hindered and Broadly Applicable Pd(II)–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for Cross-Coupling Reactions" Molecules 28, no. 15: 5833. https://doi.org/10.3390/molecules28155833
APA StyleRahman, M. M., Zhao, Q., Meng, G., Lalancette, R., Szostak, R., & Szostak, M. (2023). [IPr#–PEPPSI]: A Well-Defined, Highly Hindered and Broadly Applicable Pd(II)–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for Cross-Coupling Reactions. Molecules, 28(15), 5833. https://doi.org/10.3390/molecules28155833







