Abstract
Rhodium-catalyzed reactions of 2-ethynyl-3-pentamethyldisilanylpyridine derivatives (1 and 2) are reported. The reactions of compounds 1 and 2 in the presence of catalytic amounts of rhodium complexes at 110 °C gave the corresponding pyridine-fused siloles (3) and (4) through intramolecular trans-bis-silylation cyclization. The reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 3-phenyl-1-propyne in the presence of PdCl2(PPh3)2-CuI catalysts afforded 1:2 bis-silylation adduct 6. DFT calculations were also performed to understand the reaction mechanism for the production of compound 3 from compound 1.
1. Introduction
Various synthetic methods of organosilicon compounds have been reported so far [1]. Silicon-containing compounds, due to their unique physical and chemical properties, are attractive as candidates for optical and electronic materials such as organic thin film transistors, organic light-emitting diodes, and organic photovoltaics [2,3,4,5,6,7]. In particular, the synthesis and properties of silole derivatives with low-lying LUMO have been extensively studied. The bis-silylation of unsaturated carbon compounds, which provides two silicon-carbon bonds simultaneously, has been developed [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Bis-silylation reactions using various substrates have been reported so far, but most of them are cis-additions of a silicon–silicon bond to unsaturated compounds. This severely limits the practicality of the method. It seems to be very important that trans-bis-silylation of alkynes can be carried out easily and successfully. There are several reports on the trans-bis-silylation reactions of alkynes. In 2012, Matsuda and co-workers showed the possibility of a trans-selective bis-silylation reaction of the C–C triple bond in the Rh(I)-catalyzed intramolecular cyclization of specific (2-alkynylphenyl)disilanes [18]. The reaction mechanism of the rhodium-catalyzed reactions has not yet been clarified.
Recently, we reported that the reactions of 2-bromo-3-(pentamethyldisilanyl)pyridine with ethynylbenzene derivatives in the presence of PdCl2(PPh3)2-CuI as catalysts afforded the corresponding pyridine-fused siloles through intramolecular trans-bis-silylation [24]. DFT calculations for the above reaction were performed to rationalize the formation of trans-bis-silylation adducts via cis-bis-silylation adducts. We also demonstrated that the similar reactions of 2-bromo-3-(pentamethyldisilanyl)pyridine with alkynes having bulky substituents, such as ethynyltrimethylsilane, produced 2-ethynyl-3-pentamethyldisilanylpyridine derivatives arising from Sonogashira-coupling reactions [24]. Pyridine-containing materials have been examined for their optical and physical properties, as well as their medical potential [25].
It is of considerable interest to us to investigate the chemical behavior of 2-ethynyl-3-pentamethyldisilanylpyridine derivatives in the presence of rhodium catalysts to synthesize pyridine-fused silole derivatives. In this paper, we report the rhodium-catalyzed reactions of 2-ethynyl-3-pentamethyldisilanylpyridine derivatives, and DFT calculations to investigate the energy and structural changes in the synthesis route from 3-(1,1,2,2,2-pentamethyldisilanyl)-2-(trimethylsilylethynyl)pyridine (1) to trans-bis-silylation product 3.
2. Results and Discussion
2.1. Synthesis and Reactions
The starting compound, 3-(1,1,2,2,2-pentamethyldisilanyl)-2-(trimethylsilylethynyl)pyridine (1), was prepared by the Sonogashira coupling reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with ethynyltrimethylsilane in triethylamine [24]. It was shown that base desilylation can be accomplished in potassium carbonate/methanol to produce 2-ethynyl-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (2) (Scheme 1).
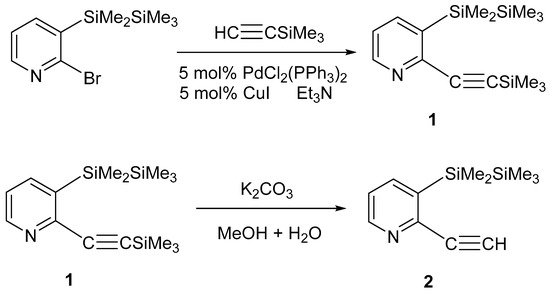
Scheme 1.
Synthesis of compounds 1 and 2.
We first examined the reaction of compound 1 in the presence of Di-μ-chloro-tetracarbonyldirhodium(I), [RhCl(CO)2]2. The treatment of compound 1 in the presence of a catalytic amount of [RhCl(CO)2]2 in refluxing toluene for 12 h gave 1,1-dimethyl-2,3-bis(trimethylsilyl)-1H-silolo(3,2-b)pyridine (3) in 46% yield (Scheme 2). Many unidentified products were detected in the reaction mixture by GLC and GPC. Compound 3 was obtained via the intramolecular trans-bis-silylation of compound 1. The structure of compound 3 was verified by spectroscopic analysis. The mass spectrum for compound 3 showed parent ions at m/z 305, corresponding to the calculated molecular weight of C15H27NSi3. The 1H NMR spectrum for compound 3 showed signals at 0.29, 0.34, and 0.38 ppm due to the methyl protons on the silicon atoms, and three doublets of doublet signals at 6.98, 7.73, and 8.46 ppm due to the pyridyl ring protons. The 29Si NMR spectrum for compound 3 showed signals at −9.8, −6.6, and 10.1 ppm.
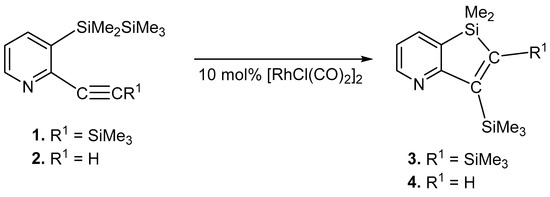
Scheme 2.
Reactions of 1 and 2 with Rh complex.
The intramolecular trans-bis-silylation of compound 1 proceeded in the presence of [RhCl(nbd)]2 (nbd = norborna-2,5-diene) to afford compound 3 in 6% yield. Many unidentified products were detected in the reaction mixture by GLC and GPC. Similar reactions of compound 1 in the presence of RhCl(PPh3)3 gave compound 3 in 5% yield. The starting compound 1 was recovered (87%) (Table 1).

Table 1.
Synthesis of silole derivatives 3 and 4. Compounds 1 and 2 were reacted in toluene at 110 °C for 12 h in the presence of rhodium catalysts (10 mol%).
The reactions of 2-ethynyl-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (2) bearing a terminal alkyne moiety in the presence of a catalytic amount of [RhCl(CO)2]2 afforded 1,1-dimethyl-3-(trimethylsilyl)-1H-silolo(3,2-b)pyridine (4) in 48% yield. The [RhCl(nbd)]2 catalyzed reaction of compound 2 afforded compound 4 in 5% yield. Many unidentified products were detected in the reaction mixture by GLC and GPC. When RhCl(PPh3)3 was used as the catalyst, compound 2 failed to yield compound 4. The starting compound 2 was recovered (80%) (Table 1).
We carried out the Sonogashira coupling reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 3-phenyl-1-propyne in triethylamine to obtain 2-(benzylethynyl)-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (5). When a mixture of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine and 1.9 equivalent of 3-phenyl-1-propyne in the presence of a catalytic amount of PdCl2(PPh3)2-CuI was heated to reflux in triethylamine, intermolecular bis-silylation product 6 produced from the reaction of compound 5 with 3-phenyl-1-propyne was obtained in 23% yield (Scheme 3). No compound 5, which is a 1:1 adduct of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine and 3-phenyl-1-propyne, was detected by spectroscopic analysis. The similar reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with slightly less (0.96 equivalent) 3-phenyl-1-propyne gave compound 6 in 12% yield based on 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine. All attempts to obtain compound 5 were unsuccessful. The 1H NMR spectrum for compound 6 revealed two signals at 0.10 and 0.45 ppm due to the methylsilyl protons and a signal at 6.18 ppm attributed to olefinic proton, as well as methylene protons, phenyl, and pyridyl ring protons. The 13C NMR spectrum of compound 6 showed two resonances at 26.3 and 37.6 ppm, attributed to methylene carbons, and two resonances at 85.7 and 106.4 ppm due to sp carbons, as well as methylsilyl carbons and phenyl, pyridyl ring, and olefinic carbons. Its 29Si NMR spectrum showed two signals at −22.2 and −3.3 ppm.
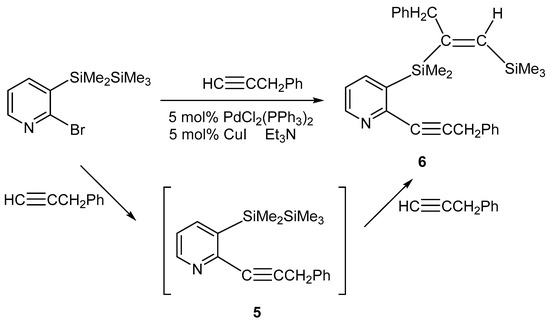
Scheme 3.
Reactions of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 3-phenyl-1-propyne.
We carried out the reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 1-hexyne in the presence of a PdCl2(PPh3)2-CuI catalyst. Many products were detected in the reaction mixture by GLC and GPC, and all attempts to isolate 2-(hex-1-yn-1-yl)-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine, analogous to compounds 1 and 2 were unsuccessful [24]. The reactions of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 1-octyne, 1-ethynylcyclohexene and ethynylcyclohexane did not afford 2-ethynyl-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine derivatives. Many products were also detected in the reaction mixture by GLC and GPC.
Scheme 4 illustrates a possible mechanistic interpretation of the reaction course. Due to the steric hindrance, the [RhCl(CO)2]2 binuclear complex decomposed into monomer complex RhCl(CO)x. A neutral RhCl(CO)x is the real catalytic species. The coordination of RhCl(CO)x to compound 1 resulted in model 0, where the Si-Si bond was only slightly stretched from 1 (2.393 Å from 2.389 Å when x = 1). Therefore, model 0 is not shown in Scheme 4, but was included in the DFT calculations, as shown in Supplementary Materials as Figures S15 and S23. By way of TS 0–1, the Si-Si bond was cleaved and Model 1 was formed. From Model 1, a trimethylsilyl group on the rhodium atom migrated to the carbon atom of the C=C bond (Model 2) via TS 1–2. The final change was a crossing of Me3Si and RhCl(CO)x groups over the C=C bond via TS 2–3. This structure took a skew configuration, and counted the highest energy along the reaction coordinate (when x = 1). Model 3 was generated from TS 2–3, and the elimination of rhodium species from Model 3 afforded compound 3 (Model 3 is not shown in Scheme 4, but is shown in the Supplementary Materials as Figures S21 and S29).
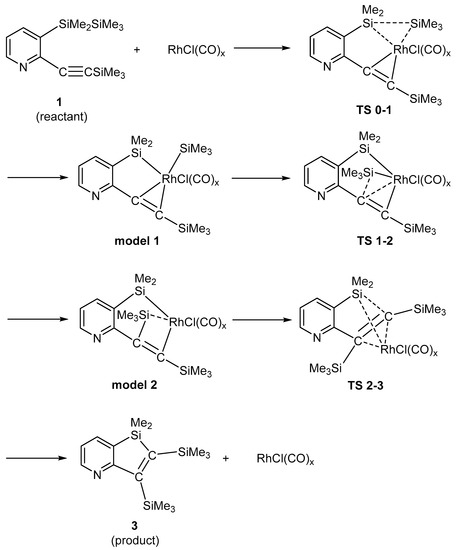
Scheme 4.
Proposed Mechanism for the Production of Compound 3.
2.2. Theoretical Study
DFT calculations were conducted for the reaction mechanisms from compound 1 to compound 3 (trans-bis-silylation product), and also for the corresponding cis-bis-silylation product, 8-[bis(trimethylsilyl)methylene]-7,7-dimethyl-2-aza-7-silabicyclo [4.2.0]octa-1,3,5-triene, for comparison. The Gaussian09 program package [26] was employed along with the Becke’s three-parameter Lee–Yang–Parr hybrid functional [27]. Los Alamos effective core potentials [28] and the Dunning/Huzinaga full double basis sets [29] were used for the Rh atom. The 6-311G(d) basis sets were used for H, C, N, O, Si, and Cl atoms.
Firstly, transition states (TSs) were searched based on Scheme 4. Then, for each TS, the intrinsic reaction coordinate (IRC) [30] was evaluated for both directions (reactant and product). At the end of IRC, normal optimization was followed until the two local minima (LMs) were reached.
Two catalyst models were used: RhCl(CO)2 and RhCl(CO) in the DFT calculations. The former is formed by the decomposition of [RhCl(CO)2]2, and the latter is formed by the disproportionation of the former, i.e., 2RhCl(CO)2 → RhCl(CO) + RhCl(CO)3.
Model 0 is a combined system of compound 1 and RhCl(CO)x. The reaction proceeded in the order of Model 0 → TS 0–1 → Model 1 → TS 1–2 → Model 2 → TS 2–3 → Model 3 (compound 3 + RhCl(CO)x). Model 3 corresponds to compound 3 with Rh complex. All the optimized structures for LMs and TSs on the trans route are shown in the Supplementary Materials (Figures S13–S29). The energy change, along the reaction coordinate, are shown in Figure 1.
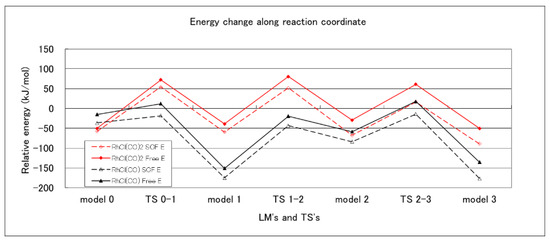
Figure 1.
Energy diagrams for Model 0 [compound 1 + RhCl(CO)x] to Model 3 [compound 3 + RhCl(CO)x] at the B3LYP/6-311G(d) level of theory. SCF energies (broken line) and free energies (solid line) are plotted for the RhCl(CO) model (black color) and the RhCl(CO)2 model (red color).
For comparison, the reaction mechanisms from compound 1 to compound 3b, 8-[bis(trimethylsilyl)methylene]-7,7-dimethyl-2-aza-7-silabicyclo [4.2.0]octa-1,3,5-triene (cis-bis-silylation product), were also investigated based on Scheme S1 in the Supplementary Materials. All the LMs and TSs on the cis route are shown in the Supplementary Materials as Figures S30–S37. The energy change, along the reaction coordinate, are shown in Figure 2.
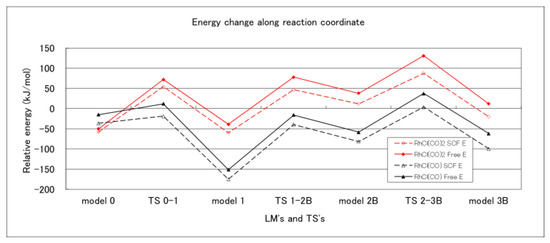
Figure 2.
Energy diagrams for Model 0 [compound 1 + RhCl(CO)x] to Model 3B [cis-bis-silylation product + RhCl(CO)x] at the B3LYP/6-311G(d) level of theory. SCF energies (broken line) and free energies (solid line) are plotted for the RhCl(CO) model (black color) and the RhCl(CO)2 model (red color).
Figure 1 and Figure 2 show the SCF energy and the free energy, referring to the sum of energies of [RhCl(CO)2]2 and compound 1, where the destabilization due to decomposition or disproportionation was taken into consideration. Both of the energies changed in parallel, and the location of the rate determining step did not depend on the two criteria.
Adopting the free energy, the rate determining steps were TS 2–3 and TS 2–3B for the trans and cis routes, and their activation energies were 169 and 189 kJ mol−1, respectively, with the RhCl(CO) model. With the RhCl(CO)2 model, the rate determining step for the cis route was TS 2–3B with an activation energy of 170 kJ mol−1; however, the rate determining step for the trans route was TS 0–1 with an activation energy of 123 kJ mol−1. Thus, both of the calculation models afforded the same result: that the trans-bis-silylation is the more stable product with respect to the activation energy, in accordance with our experimental result. Although the reactions with the RhCl(CO) model seem to proceed on the lower potential energy surfaces, the RhCl(CO)2 mode is a more promising catalyst for two reasons. (1) The activation energies for the rate determining step were 169 and 123 kJ mol−1 with RhCl(CO) and RhCl(CO)2 modes for the trans route. (2) The destabilization by disproportionation ([RhCl(CO)2]2 → RhCl(CO) + RhCl(CO)3) was 117 and 59 kJ mol−1 for the SCF and free energies. On the other hand, the decomposition energies ([RhCl(CO)2]2 → 2RhCl(CO)2) were 30 and −36 kJ mol−1, respectively. At the free energy level, the decomposition became stabilization. The present work is the first to investigate the two catalyst models intensively.
3. Conclusions
We have described here the rhodium-catalyzed reactions of 2-ethynyl-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine derivatives. The reactions of 3-(1,1,2,2,2-pentamethyldisilanyl)-2-(trimethylsilylethynyl)pyridine (1) in the presence of a catalytic amount of rhodium complexes proceeded to give the pyridine-fused silole, 1,1-dimethyl-2,3-bis(trimethylsilyl)-1H-silolo(3,2-b)pyridine (3). Similar treatment of 2-ethynyl-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (2) afforded 1,1-dimethyl-3-(trimethylsilyl)-1H-silolo(3,2-b)pyridine (4). DFT calculations were performed to rationalize the formation of compound 3 via the intramolecular trans-bis-silylation of compound 1. The synthesis of 2-(alkyl-substituted ethynyl)-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine was unsuccessful.
4. Materials and Methods
4.1. General Procedure
All reactions were carried out under an inert atmosphere using dry nitrogen. NMR spectra were recorded on a JMN–ECS400 spectrometer using a deuteriochloroform solution. Low-resolution mass spectrometry was performed on a JEOL JMS-700 mass spectrometer. High-resolution mass spectrometry (HR-MS) was performed on a JEOL JMS-700 mass spectrometer. Gas chromatographic separations were carried out using a column (3 m × 10 mm) packed with 30% silicone on chromosorb W AM DMCS 80/100. Gel permeation chromatographic analysis was performed with a Model LC-908 Recycling Preparative HPLC (Japan Analytical Industry Co., Ltd., Tokyo, Japan). Column chromatography was performed using a silica gel column (Wakogel C–300; Wako Pure Chemical Industries, Osaka, Japan). Bis(triphenylphosphine)palladium(II) dichloride [PdCl2(PPh3)2], copper(I) iodide (CuI), Di-μ-chloro-tetracarbonyldirhodium(I) [RhCl(CO)2], Bis(norbornadiene-μ-chlororhodium) [RhCl(nbd)]2, and tris(triphenylphosphine)rhodium(I) chloride [RhCl(PPh3)3] were purchased from Sigma-Aldrich, St. Louis, MO, USA. Potassium carbonate, triethylamine, methanol, and toluene were purchased from Tokyo Kasei Kogyo, Tokyo, Japan. Triethylamine was distilled over potassium hydroxide under nitrogen just before use, and toluene was distilled from sodium benzophenone ketyl under nitrogen just before use. 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine was prepared as reported in the literature [24].
4.2. Procedures
Synthesis of Compound 1. In a 300-mL three-necked flask fitted with a stirrer, reflux condenser, and dropping funnel, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (6.108 g, 21.2 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.760 g, 1.08 mmol), and copper(I) iodide (0.214 g, 1.12 mmol) were added to 50 mL of dry triethylamine. To this mixture, ethynyltrimethylsilane (2.553 g, 26.0 mmol) was added dropwise at room temperature. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column and eluted with hexane-ethyl acetate (10:1) to obtain 1.571 g (24% yield) of compound 1: 1H NMR δ(CDCl3) 0.09 (s, 9H, Me3Si), 0.27 (s, 9H, Me3Si), 0.45 (s, 6H, Me2Si), 7.17 (dd, 1H, pyridyl-ring proton, J = 7.6, 5.2 Hz), 7.70 (dd, 1H, pyridyl-ring proton, J = 7.6, 2.0 Hz), 8.51 (dd, 1H, pyridyl-ring proton, J = 5.2, 2.0 Hz). All spectral data for compound 1 were identical to those of an authentic sample [24].
Synthesis of Compound 2. A mixture of compound 1 (1.571 g, 5.14 mmol), potassium carbonate (0.982 g, 7.11 mmol), and methanol (50 mL) was stirred at room temperature for 2 h. The reaction mixture was concentrated under reduced pressure, and hexane (15 mL) and water (30 mL) were added to the residue. The layers were separated and the aqueous layer was extracted with hexane (4 × 15 mL). The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluted with hexane-ethyl acetate (10:1), to obtain 0.995 g (83% yield) of compound 2: HR-MS: calcd. for C12H19NSi2 (M+): 233.1056, found: 233.1052. MS m/z 233 (M+); 1H NMR δ(CDCl3) 0.10 (s, 9H, Me3Si), 0.44 (s, 6H, Me2Si), 3.24 (s, 1H, HC), 7.21 (dd, 1H, pyridyl ring proton, J = 7.8 Hz, 4.8 Hz), 7.72 (dd, 1H, pyridyl ring proton, J = 7.8 Hz, 2.0 Hz), 8.52 (dd, 1H, pyridyl ring proton, J = 4.8 Hz, 2.0 Hz); 13C NMR δ(CDCl3) −3.7 (Me2Si), 1.3 (Me3Si), 79.5, 85.0 (sp carbons), 122.8, 138.1, 142.0, 146.7, 149.5 (pyridyl ring and olefinic carbons); 29Si NMR δ(CDCl3) −20.7, −17.2.
Reaction of Compound 1 in the Presence of [RhCl(CO)2]2 Catalyst. In a 30 mL two-necked flask fitted with a reflux condenser were placed 0.375 g (1.23 mmol) of compound 1 and 0.047 g (0.121 mmol) of [RhCl(CO)2]2 in 5 mL of dry toluene. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1), to obtain 0.172 g (46% yield) of compound 3: HR-MS: calcd. for C15H27NSi3 (M+): 305.1451, found: 305.1455. MS m/z 305 (M+); 1H NMR δ(CDCl3) 0.29 (s, 9H, Me3Si), 0.34 (s, 6H, Me2Si), 0.38 (s, 9H, Me3Si), 6.98 (dd, 1H, pyridyl ring proton, J = 7.2 Hz, 5.2 Hz), 7.73 (dd, 1H, pyridyl ring proton, J = 7.2 Hz, 1.6 Hz), 8.46 (dd, 1H, pyridyl ring proton, J = 5.2 Hz, 1.6 Hz); 13C NMR δ(CDCl3) −2.7 (Me2Si), 2.5, 2.7 (Me3Si), 120.5, 132.6, 138.1, 149.0, 165.0, 172.0, 176.6 (pyridyl ring and olefinic carbons); 29Si NMR δ(CDCl3) −9.8, −6.6, 10.1.
Reaction of Compound 1 in the Presence of [RhCl(nbd)]2 Catalyst. In a 30 mL two-necked flask fitted with a reflux condenser were placed 0.300 g (0.98 mmol) of compound 1 and 0.045 g (0.098 mmol) of [RhCl(nbd)]2 in 5 mL of dry toluene. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1), to obtain 0.018 g (6% yield) of compound 3. All spectral data for compound 3 were identical to those of an authentic sample.
Reaction of Compound 1 in the Presence of RhCl(PPh3)3 Catalyst. In a 30 mL two-necked flask fitted with a reflux condenser were placed 0.575 g (1.88 mmol) of 1 and 0.176 g (0.190 mmol) of RhCl(PPh3)3 in 5 mL of dry toluene. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1), to obtain 0.030 g (5% yield) of compound 3. The starting compound 1 was almost recovered (0.502 g). All spectral data for compound 3 were identical to those of an authentic sample.
Reaction of Compound 2 in the Presence of [RhCl(CO)2]2 Catalyst. In a 30 mL two-necked flask fitted with a reflux condenser were placed 0.134 g (0.574 mmol) of compound 1 and 0.023 g (0.059 mmol) of [RhCl(CO)2]2 in 5 mL of dry toluene. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1), to obtain 0.064 g (48% yield) of compound 4: HR-MS: calcd. for C12H19NSi2 (M+): 233.1056, found: 233.1060. MS m/z 233 (M+); 1H NMR δ(CDCl3) 0.30 (s, 9H, Me3Si), 0.31 (s, 6H, Me2Si), 6.99 (s, 1H, olefinic proton), 7.00 (dd, 1H, pyridyl ring proton, J = 7.0 Hz, 5.2 Hz), 7.75 (dd, 1H, pyridyl ring proton, J = 7.0 Hz, 2.0 Hz), 8.48 (dd, 1H, pyridyl ring proton, J = 5.2 Hz, 2.0 Hz); 13C NMR δ(CDCl3) −4.3 (Me2Si), −1.0 (Me3Si), 120.6, 132.3, 138.7, 148.4, 149.6, 168.7, 170.7 (pyridyl ring and olefinic carbons); 29Si NMR δ(CDCl3) −6.4, 2.9.
Reaction of Compound 2 in the Presence of [RhCl(nbd)]2 Catalyst. In a 30 mL two-necked flask fitted with a reflux condenser were placed 0.130 g (0.557 mmol) of compound 1 and 0.025 g (0.054 mmol) of [RhCl(nbd)]2 in 5 mL of dry toluene. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1), to obtain 0.007 g (5% yield) of compound 4. All spectral data for compound 4 were identical to those of an authentic sample.
Reaction of Compound 2 in the Presence of RhCl(PPh3)3 Catalyst. In a 30 mL two-necked flask fitted with a reflux condenser were placed 0.094 g (0.403 mmol) of compound 2 and 0.037 g (0.040 mmol) of RhCl(PPh3)3 in 5 mL of dry toluene. The mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1). The starting compound 2 was recovered (0.075 g).
Reaction of 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 1.9 equivalent of 3-Phenyl-1-propyne in the Presence of Palladium and Copper Catalysts. In a 300 mL two-necked flask fitted with a reflux condenser, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (2.675 g, 9.28 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.316 g, 0.450 mmol), and copper(I) iodide (0.086 g, 0.452 mmol) were added to 50 mL of dry triethylamine. To this mixture, 3-phenyl-1-propyne (2.091 g, 18.0 mmol) was added dropwise at room temperature, after which the mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (50:1) to obtain 0.933 g (23% yield) of compound 6: HR-MS: calcd. for C28H33NSi2 (M+): 439.2152, found: 439.2150. MS m/z 439 (M+); 1H NMR δ(CDCl3) 0.10 (s, 9H, Me3Si), 0.45 (s, 6H, Me2Si), 3.14-3.27 (m, 2H, methylene), 3.66 (s, 2H, methylene), 6.18 (br s, 1H, olefinic proton), 7.12−7.35 (m, 11H, phenyl and pyridyl ring protons), 8.10 (s, 1H, pyridyl ring proton), 8.62 (br d, 1H, pyridyl ring proton, J = 3.2 Hz); 13C NMR δ(CDCl3) −0.9 (Me3Si), 1.0, 1.3 (MeSi), 26.3, 37.6 (CH2), 85.7, 106.4 (sp carbons), 120.0, 125.9, 126.6, 127.8, 128.3, 128.5, 128.7, 129.5, 136.0, 140.1, 140.6, 143.9, 147.5, 149.7, 165.8 (phenyl, pyridyl ring, and olefinic carbons); 29Si NMR δ(CDCl3) −22.2, −3.3.
Reaction of 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 0.96 equivalent of 3-Phenyl-1-propyne in the Presence of Palladium and Copper Catalysts. In a 300 mL two-necked flask fitted with a reflux condenser, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (1.800 g, 6.24 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.211 g, 0.301 mmol), and copper(I) iodide (0.057 g, 0.299 mmol) were added to 50 mL of dry triethylamine. To this mixture, 3-phenyl-1-propyne (0.695 g, 5.98 mmol) was added dropwise at room temperature, after which the mixture was heated to reflux for 12 h. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column eluting with hexane-ethyl acetate (50:1) to obtain 0.334 g (12% yield based on 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine) of compound 6. All spectral data for compound 6 were identical to those of an authentic sample.
Reaction of 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 1-Hexyne in the Presence of Palladium and Copper Catalysts. In a 300 mL two-necked flask fitted with a reflux condenser, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (5.474 g, 19.0 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.667 g, 0.950 mmol), and copper(I) iodide (0.181 g, 0.950 mmol) were added to 50 mL of dry triethylamine. To this mixture, 1-hexyne (3.194 g, 38.9 mmol) was added dropwise at room temperature, after which the mixture was heated to reflux for 12 h. Many products were detected in the reaction mixture by GLC and GPC. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1). Although the 1H NMR spectrum showed the existence of 2-(hex-1-yn-1-yl)-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine produced from a Sonogashira coupling reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine and 1-hexyne, analogous to compounds 1 and 2, all attempts to isolate the compound were unsuccessful.
Reaction of 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 1-Octyne in the Presence of Palladium and Copper Catalysts. In a 300 mL two-necked flask fitted with a reflux condenser, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (4.285 g, 14.9 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.529 g, 0.754 mmol), and copper(I) iodide (0.144 g, 0.756 mmol) were added to 50 mL of dry triethylamine. To this mixture, 1-octyne (3.270 g, 29.7 mmol) was added dropwise at room temperature, after which the mixture was heated to reflux for 12 h. Many products were detected in the reaction mixture by GLC and GPC. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1). No 2-(oct-1-yn-1-yl)-3-(1,1,2,2,2-pentamethyldisilaneyl)pyridine would be produced from a Sonogashira coupling reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine, and 1-octyne, analogous to compounds 1 and 2, was not detected.
Reaction of 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with 1-Ethynylcyclohexene in the Presence of Palladium and Copper Catalysts. In a 300 mL two-necked flask fitted with a reflux condenser, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (1.899 g, 6.59 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.667 g, 0.349 mmol), and copper(I) iodide (0.068 g, 0.357 mmol) were added to 50 mL of dry triethylamine. To this mixture, 1-ethynylcyclohexene (1.513 g, 14.3 mmol) was added dropwise at room temperature, after which the mixture was heated to reflux for 12 h. Many products were detected in the reaction mixture by GLC and GPC. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1). No 2-(cyclohex-1-en-1-ylethynyl)-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine would be produced from a Sonogashira coupling reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine, and 1-ethynylcyclohexene, analogous to compounds 1 and 2, was not detected.
Reaction of 2-Bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine with Ethynylcyclohexane in the Presence of Palladium and Copper Catalysts. In a 300 mL two-necked flask fitted with a reflux condenser, 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine (1.558 g, 5.40 mmol), bis(triphenylphosphine)palladium(II) dichloride (0.175 g, 0.249 mmol), and copper(I) iodide (0.047 g, 0.247 mmol) were added to 50 mL of dry triethylamine. To this mixture, ethynylcyclohaxane (1.064 g, 9.84 mmol) was added dropwise at room temperature, after which the mixture was heated to reflux for 12 h. Many products were detected in the reaction mixture by GLC and GPC. The solution was then hydrolyzed, and the organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The solvent was then evaporated, and the residue was chromatographed on a silica gel column, eluting with hexane-ethyl acetate (10:1). No 2-(cyclohexylethynyl)-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine would be produced from a Sonogashira coupling reaction of 2-bromo-3-(1,1,2,2,2-pentamethyldisilanyl)pyridine, and ethynylcyclohexane, analogous to compounds 1 and 2, was not detected.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083284/s1, all NMR spectra for compounds 2–4 and 6, and optimized structures for all local minima (LMs) and transition states (TSs) for trans-bis-silylation product and cis-bis-silylation product.
Author Contributions
Initial conceptualization, A.N.; methodology, A.N.; software, H.K.; validation, A.N. and H.K.; formal analysis, A.N.; investigation, A.N. and H.K.; writing—original draft preparation, A.N. and H.K.; writing—review and editing, A.N. and H.K.; supervision, A.N.; project administration, A.N.; funding acquisition, A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Yakumo Foundation for Environmental Science and the WESCO Science Promotion Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
We are grateful for grants to A.N. from the Yakumo Foundation for Environmental Science and the WESCO Science Promotion Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples are not available from the authors.
References
- Hiyama, T.; Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions; Wiley-VCH Press: Weinheim, Germany, 2019. [Google Scholar]
- Yamaguchi, S.; Tamao, K. The Chemistry of Organic Silicon Compounds; Chapter 1; Rappoport, Z., Apeloig, Y., Eds.; Wiley: Chichester, UK, 2001; Volume 3. [Google Scholar]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission of Silole Molecules and Polymers: Fundamental and Applications. J. Inorg. Organomet. Polym. Mater. 2009, 19, 249–285. [Google Scholar] [CrossRef]
- Sołoducho, J.; Zając, D.; Spychalska, K.; Baluta, S.; Cabaj, J. Conducting Silicone-Based Polymers and Their Application. Molecules 2021, 26, 2012. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Nguyen, H.M.T.; Nguyen, H.; Dung, T.N.; Nguyen, M.T.; Dehaen, W. Advances in Synthesis of π-Extended Benzosilole Derivatives and Their Analogs. Molecules 2020, 25, 548. [Google Scholar] [CrossRef]
- Chen, J.W.; Cao, Y. Silole-Containing Polymers: Chemistry and Optoelectronic Properties. Macromol. Rapid Commun. 2007, 28, 1714–1742. [Google Scholar] [CrossRef]
- Lu, G.; Usta, H.; Risko, C.; Wang, L.; Facchetti, A.; Ratner, M.A.; Marks, T.J. Synthesis, Characterization, and Transistor Response of Semiconducting Silole Polymers with Substantial Hole Mobility and Air Stability. Experiment and Theory. J. Am. Chem. Soc. 2008, 130, 7670–7685. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.C.; Ju, C.W.; Zhao, D. Bis-Silylation of Internal Alkynes Enabled by Ni(0) Catalysis. Nat. Commun. 2021, 12, 68. [Google Scholar] [CrossRef]
- Suginome, M.; Ito, Y. Activation of Silicon–Silicon σ Bonds by Transition-Metal Complexes: Synthesis and Catalysis of New Organosilyl Transition-Metal Complexes. J. Chem. Soc. Dalton Trans. 1998, 1925–1934. [Google Scholar] [CrossRef]
- Suginome, M.; Ito, Y. Activation of Si–Si Bonds by Transition-Metal Complexes. Organomet. Chem. 1999, 3, 131–159. [Google Scholar]
- Beletskaya, I.; Moberg, C. Element-Element Addition to Alkynes Catalyzed by the Group 10 Metals. Chem. Rev. 1999, 99, 3435–3462. [Google Scholar] [CrossRef]
- Suginome, M.; Ito, Y. Transition-Metal-Catalyzed Additions of Silicon–Silicon and Silicon-Heteroatom Bonds to Unsaturated Organic Molecules. Chem. Rev. 2000, 100, 3221–3256. [Google Scholar] [CrossRef]
- Beletskaya, I.; Moberg, C. Element-Element Additions to Unsaturated Carbon–Carbon Bonds Catalyzed by Transition Metal Complexes. Chem. Rev. 2006, 106, 2320–2354. [Google Scholar] [CrossRef]
- Suginome, M.; Matsuda, T.; Ohmura, T.; Seki, A.; Murakami, M. Comprehensive Organometallic Chemistry, 10th ed.; Crabtree, R.H., Mingos, D.M.P., Eds.; Elsevier: London, UK, 2007; Volume III, pp. 725–787. [Google Scholar]
- Ansell, M.B.; Navarro, O.; Spencer, J. Transition Metal Catalyzed Element–Element’ Additions to Alkynes. Coord. Chem. Rev. 2017, 336, 54–77. [Google Scholar] [CrossRef]
- Ozawa, F.; Sugawara, M.; Hayashi, T. A New Reactive System for Catalytic Bis-Silylation of Acetylenes and Olefins. Organometallics 1994, 13, 3237–3243. [Google Scholar] [CrossRef]
- Ansell, M.B.; Roberts, D.E.; Cloke, F.G.N.; Navarro, O.; Spencer, J. Synthesis of an [(NHC)2Pd(SiMe3)2] Complex and Catalytic cis-Bis(Silyl)ations of Alkynes with Unactivated Disilanes. Angew. Chem. Int. Ed. 2015, 54, 5578–5582. [Google Scholar] [CrossRef]
- Matsuda, T.; Ichioka, Y. Rhodium-Catalysed Intramolecular Trans-Bis-Silylation of Alkynes to Synthesise 3-Silyl-1-Benzosiloles. Org. Biomol. Chem. 2012, 10, 3175–3177. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Matsuura, T.; Murakami, M. Palladium-catalyzed regular insertion of isonitriles into silicon-silicon linkage of polysilane. J. Am. Chem. Soc. 1988, 110, 3692–3693. [Google Scholar] [CrossRef]
- Hayashi, T.; Kobayashi, T.; Kawamoto, A.; Yamashita, H.; Tanaka, M. Platinum complex catalyzed double silylation of ethylene and norbornene with disilanes. Organometallics 1990, 9, 280–281. [Google Scholar] [CrossRef]
- Tamao, K.; Hayashi, T.; Kumada, M. Fluorinated polysilanes. palladium-catalyzed disilane metathesis, double silylation of acetylenes, and the stereo chemical course. J. Organomet. Chem. 1976, 114, C19–C22. [Google Scholar] [CrossRef]
- Sakurai, H.; Kamiyama, Y.; Nakadaira, Y. Chemistry of organosilicon compounds. 79. Novel [s + p] reactions of hexaorganodisilanes with acetylenes catalyzed by palladium complexes. J. Am. Chem. Soc. 1975, 97, 931–932. [Google Scholar] [CrossRef]
- Watanabe, H.; Kobayashi, M.; Higuchi, K.; Nagai, Y. Reaction of disilanes with acetylenes: I. Stereoselective addition of methoxymethyldisilanes to phenylacetylene catalyzed by group-VIII metal phosphine complexes. J. Organomet. Chem. 1980, 186, 51–62. [Google Scholar] [CrossRef]
- Naka, A.; Shimomura, N.; Kobayashi, H. Synthesis of Pyridine-Fused Siloles by Palladium-Catalyzed Intramolecular Bis-Silylation. ACS Omega 2022, 7, 30369–30375. [Google Scholar] [CrossRef] [PubMed]
- Kishbaugh, T.L.S. Six-membered ring systems: Pyridine and benzo derivatives. Prog. Heterocycl. Chem. 2012, 24, 343–391. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennuci, B.; Petersson, G.A.; et al. Gaussian09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum Press: New York, NY, USA, 1976; pp. 1–28. [Google Scholar]
- Fukui, K.; Kato, S.; Fujimoto, H. Constituent analysis of the potential gradient along a reaction coordinate. Method and an application to CH4 + T reaction. J. Am. Chem. Soc. 1975, 97, 1–7. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).