Self-Supported 3D PtPdCu Nanowires Networks for Superior Glucose Electro-Oxidation Performance
Abstract
1. Introduction
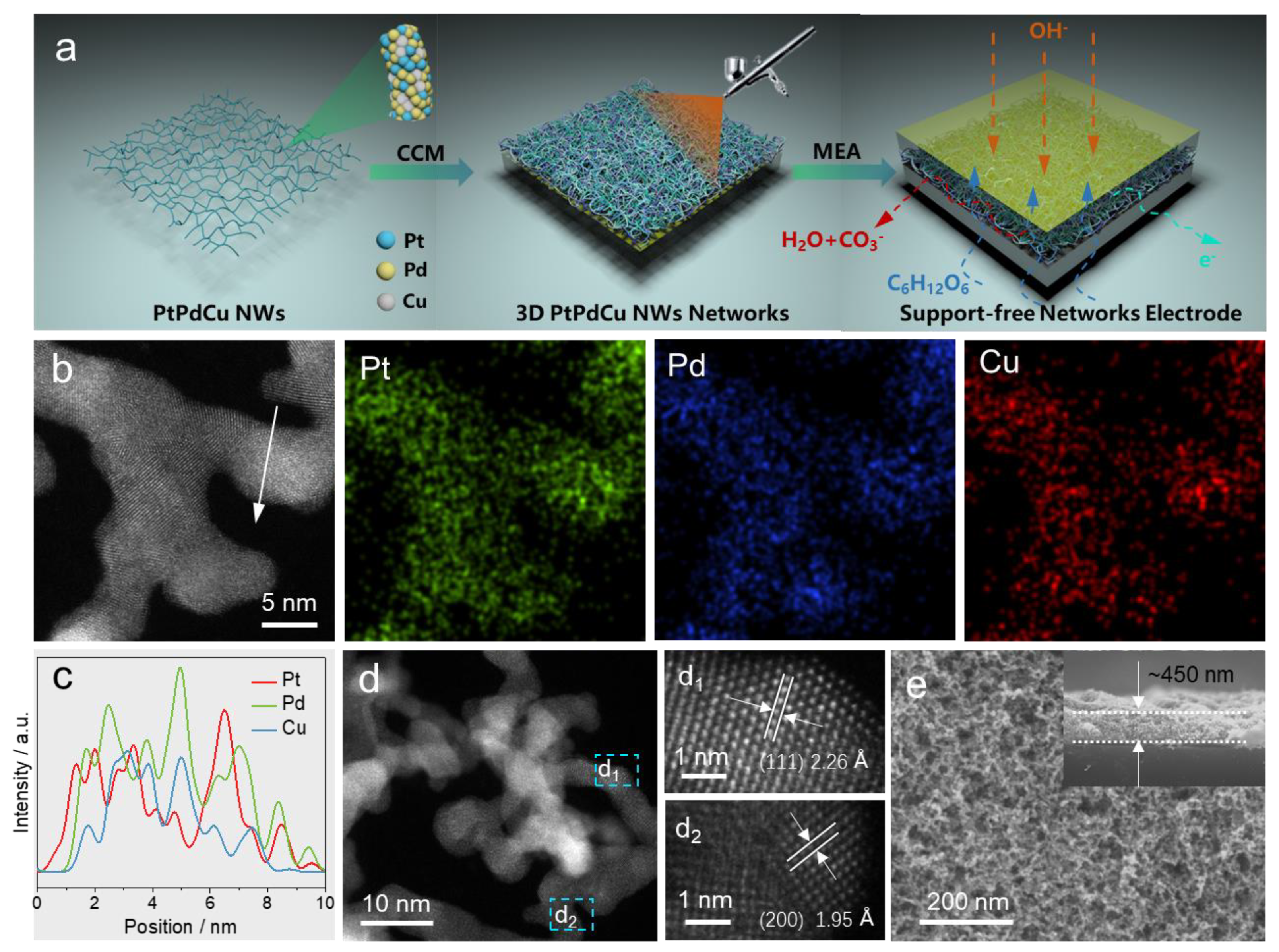
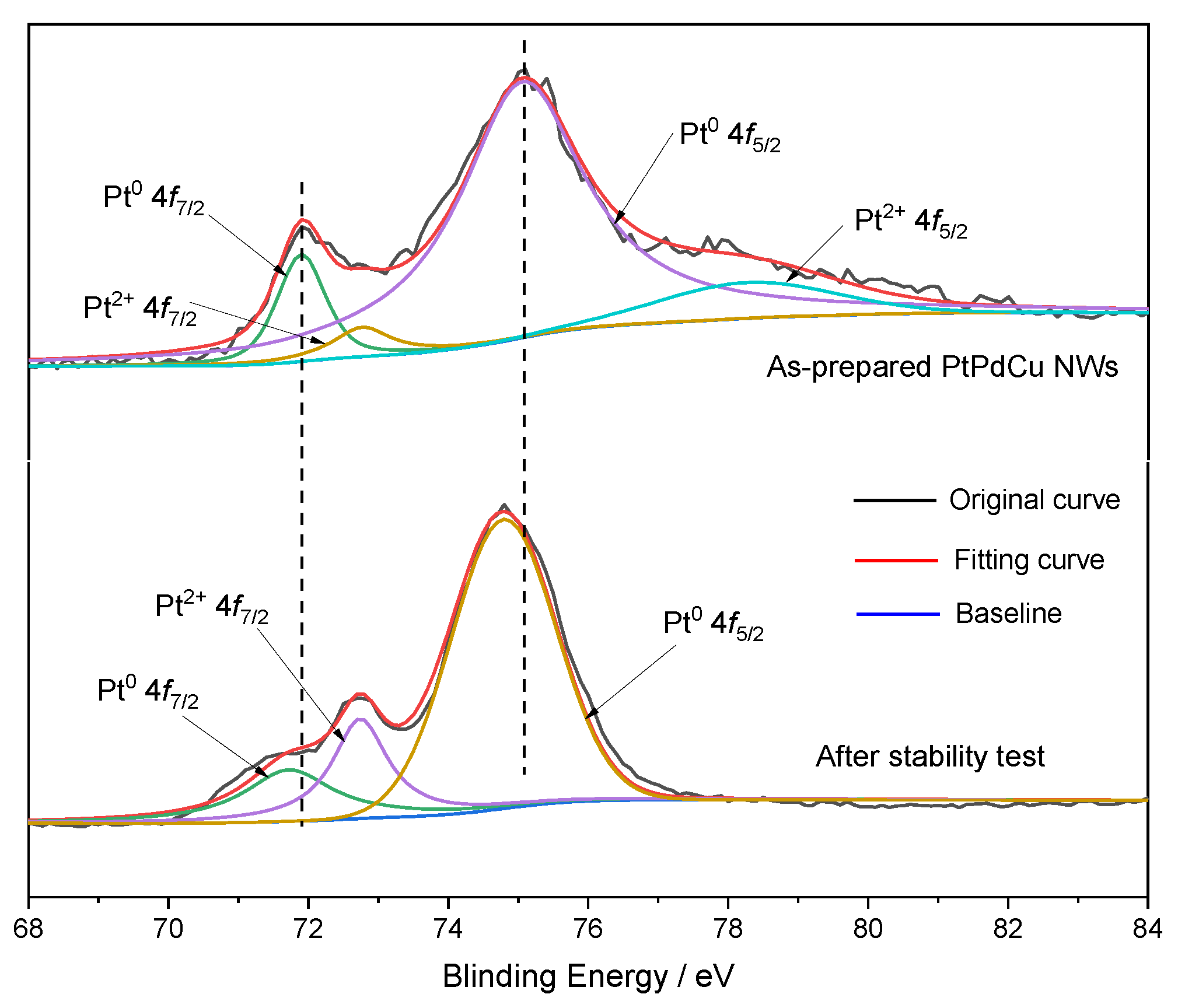
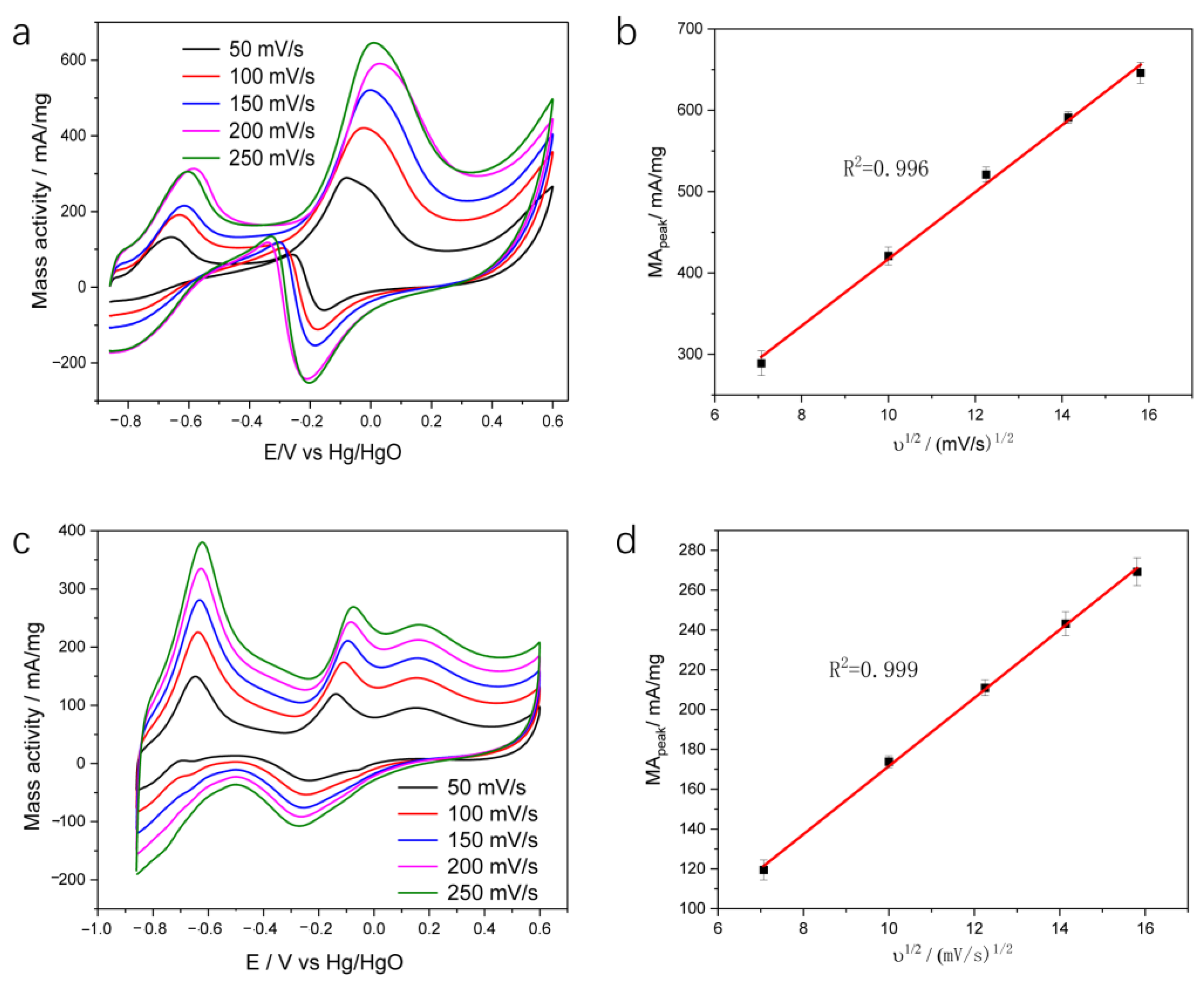
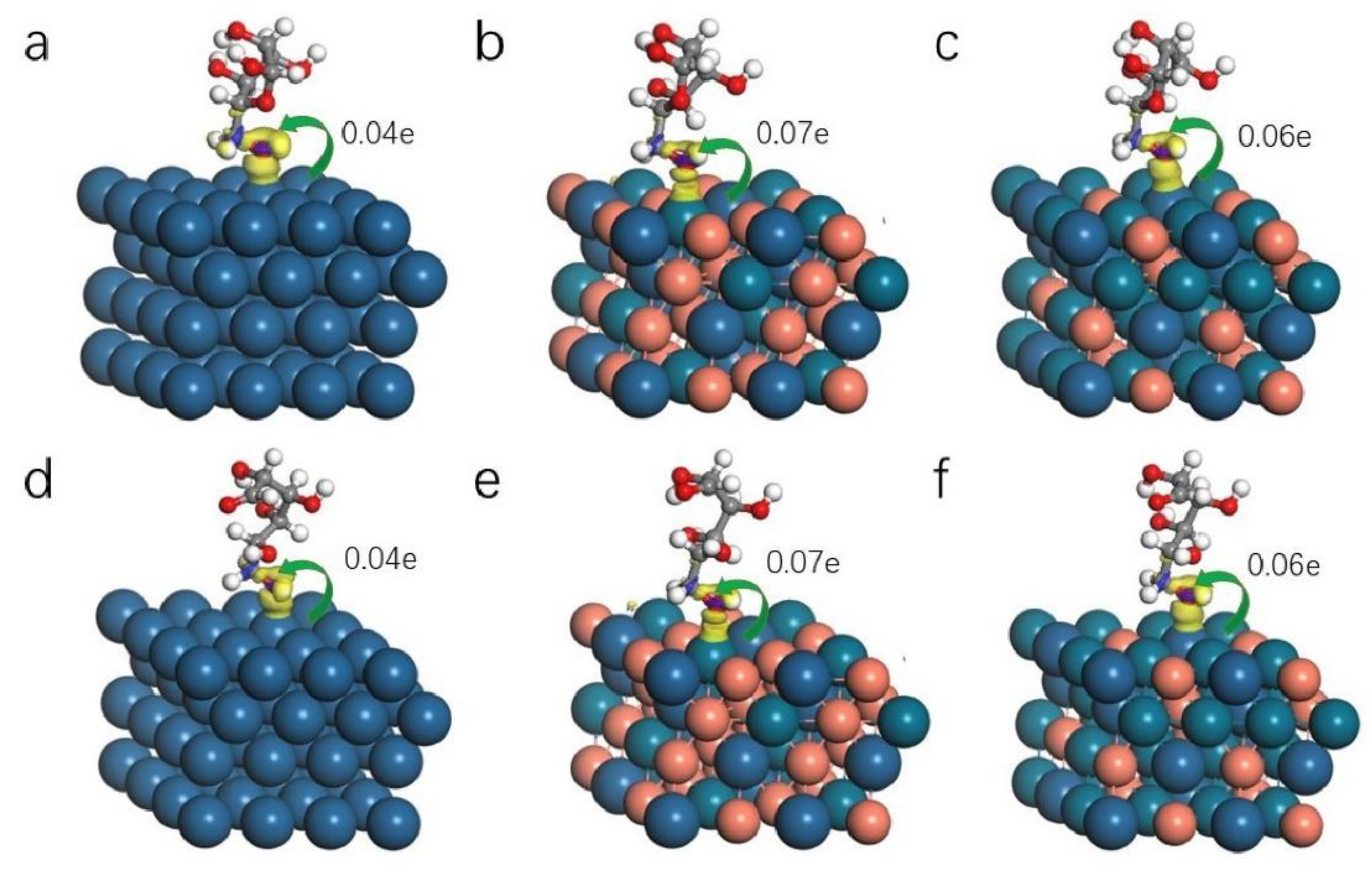
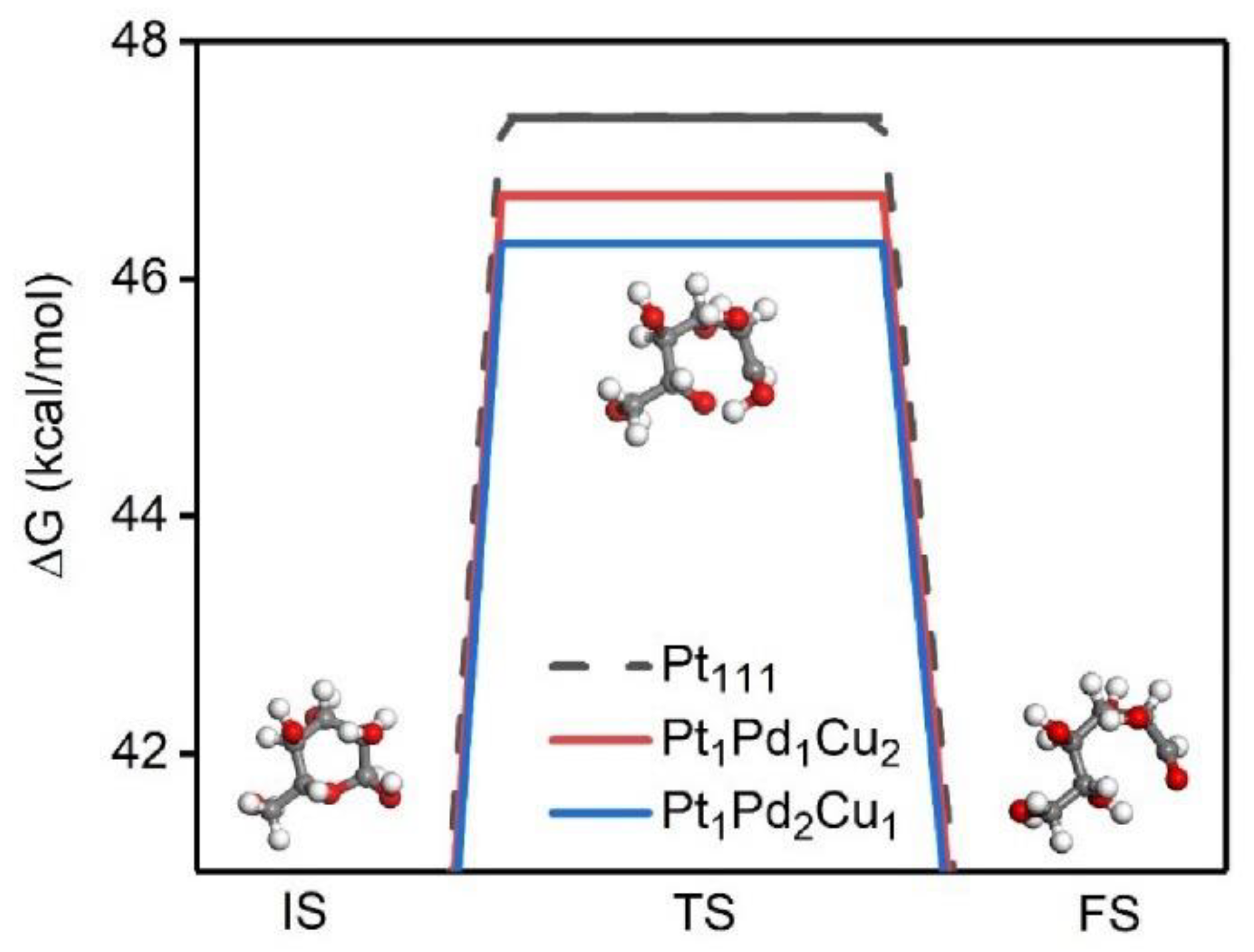
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of PtPdCu NWs Catalyst
3.3. Materials Characterization
3.4. Electrochemical Measurements
3.5. DFT Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Nag, S.; Banerjee, P. Electrochemical Nanosensors for Sensitization of Sweat Metabolites: From Concept Mapping to Personalized Health Monitoring. Molecules 2023, 28, 1259. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhou, X.; Heinig, N.F.; Thomas, J.P.; Zhang, L.; Leung, K.T. Nonenzymatic Saliva-Range Glucose Sensing Using Electrodeposited Cuprous Oxide Nanocubes on a Graphene Strip. ACS Appl. Nano Mater. 2021, 4, 4790–4799. [Google Scholar] [CrossRef]
- Bag, S.; Baksi, A.; Nandam, S.H.; Wang, D.; Ye, X.; Ghosh, J.; Pradeep, T.; Hahn, H. Nonenzymatic Glucose Sensing Using Ni(60)Nb(40) Nanoglass. ACS Nano 2020, 14, 5543–5552. [Google Scholar] [CrossRef] [PubMed]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1003, 1–34. [Google Scholar] [CrossRef]
- Newman, J.D.; Turner, A.P. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef]
- Urgunde, A.B.; Kumar, A.R.; Shejale, K.P.; Sharma, R.K.; Gupta, R. Metal Wire Networks Functionalized with Nickel Alkanethiolate for Transparent and Enzymeless Glucose Sensors. ACS Appl. Nano Mater. 2018, 1, 5571–5580. [Google Scholar]
- Li, L.-H.; Zhang, W.-D.; Ye, J.-S. Electrocatalytic Oxidation of Glucose at Carbon Nanotubes Supported PtRu Nanoparticles and Its Detection. Electroanaly 2008, 20, 2212–2216. [Google Scholar] [CrossRef]
- Zulfa, V.Z.; Nasori, N.; Farahdina, U.; Firdhaus, M.; Aziz, I.; Suprihatin, H.; Rhomadhoni, M.N.; Rubiyanto, A. Highly Sensitive ZnO/Au Nanosquare Arrays Electrode for Glucose Biosensing by Electrochemical and Optical Detection. Molecules 2023, 28, 617. [Google Scholar]
- Zhang, W.; Qin, X.; Wei, T.; Liu, Q.; Luo, J.; Liu, X. Single atomic cerium sites anchored on nitrogen-doped hollow carbon spheres for highly selective electroreduction of nitric oxide to ammonia. J. Colloid Interface Sci. 2023, 638, 650–657. [Google Scholar]
- Kang, Y.; Ren, X.; Li, Y.; Yu, Z. Ni-Coated Diamond-like Carbon-Modified TiO2 Nanotube Composite Electrode for Electrocatalytic Glucose Oxidation. Molecules 2022, 27, 5815. [Google Scholar]
- Mahmoud, M.E.A.; Omri, K.; Boudon, J.; Saviot, L.; Millot, N.; Chaabane, R.B. Cu-Doped ZnO Nanoparticles for Non-Enzymatic Glucose Sensing. Molecules 2021, 26, 929. [Google Scholar]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. One-step electrochemical synthesis of PtNi nanoparticle-graphene nanocomposites for nonenzymatic amperometric glucose detection. ACS Appl. Mater. Interfaces 2011, 3, 3049–3057. [Google Scholar]
- Yeo, I.H.; Johnson, D.C. Anodic response of glucose at copper-based alloy electrodes. J. Electroanal. Chem. 2000, 484, 157–163. [Google Scholar]
- Asen, P.; Esfandiar, A.; Kazemi, M. Nonenzymatic Sweat-Based Glucose Sensing by Flower-like Au Nanostructures/Graphene Oxide. ACS Appl. Nano Mater. 2022, 5, 13361–13372. [Google Scholar]
- Mamleyev, E.R.; Weidler, P.G.; Nefedov, A.; Szabó, D.V.; Islam, M.; Mager, D.; Korvink, J.G. Nano- and Microstructured Copper/Copper Oxide Composites on Laser-Induced Carbon for Enzyme-Free Glucose Sensors. ACS Appl. Nano Mater. 2021, 4, 13747–13760. [Google Scholar]
- Wang, K.; Wang, F.; Zhao, Y.; Zhang, W. Surface-tailored PtPdCu ultrathin nanowires as advanced electrocatalysts for ethanol oxidation and oxygen reduction reaction in direct ethanol fuel cell. J. Energy Chem. 2021, 52, 251–261. [Google Scholar]
- Jiang, T.; Yan, L.; Meng, Y.; Xiao, M.; Wu, Z.; Tsiakaras, P.; Song, S. Glucose electrooxidation in alkaline medium: Performance enhancement of PdAu/C synthesized by NH3 modified pulse microwave assisted polyol method. Appl. Catal. B Environ. 2015, 162, 275–281. [Google Scholar]
- Yan, L.; Brouzgou, A.; Meng, Y.; Xiao, M.; Tsiakaras, P.; Song, S. Efficient and poison-tolerant PdxAuy/C binary electrocatalysts for glucose electrooxidation in alkaline medium. Appl. Catal. B Environ. 2014, 150–151, 268–274. [Google Scholar]
- Van der Vliet, D.F.; Wang, C.; Li, D.; Paulikas, A.P.; Greeley, J.; Rankin, R.B.; Strmcnik, D.; Tripkovic, D.; Markovic, N.M.; Stamenkovic, V.R. Unique Electrochemical Adsorption Properties of Pt-Skin Surfaces. Angew. Chem. Int. Ed. 2012, 51, 3139–3142. [Google Scholar]
- Fan, X.; Tang, M.; Wu, X.; Luo, S.; Chen, W.; Song, X.; Quan, Z. SnO2 patched ultrathin PtRh nanowires as efficient catalysts for ethanol electrooxidation. J. Mater. Chem. A 2019, 7, 27377–27382. [Google Scholar]
- Ge, M.; Mengmeng, J.; Tianran, W.; Qian, L.; Shusheng, Z.; Xianyun, P.; Jun, L.; Xijun, L. MoC nanocrystals confined in N-doped carbon nanosheets toward highly selective electrocatalytic nitric oxide reduction to ammonia. Nano Res. 2022, 15, 8890–8896. [Google Scholar]
- Gao, S.; Wei, T.; Sun, J.; Liu, Q.; Ma, D.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Atomically Dispersed Metal-Based Catalysts for Zn–CO2 Batteries. Small Struct. 2022, 3, 2200086. [Google Scholar]
- Liu, W.; Feng, J.; Wei, T.; Liu, Q.; Zhang, S.; Luo, Y.; Luo, J.; Liu, X. Active-site and interface engineering of cathode materials for aqueous Zn—Gas batteries. Nano Res. 2023, 16, 2325–2346. [Google Scholar]
- Chai, D.; Wang, W.; Wang, F.; Kang, Y.; Yang, Y.; Lei, Z. A facile precipitation procedure for synthesis of binary Sn-Co oxide promoting Pd catalyst towards glucose electrooxidation. Electrochim. Acta 2016, 189, 295–302. [Google Scholar]
- Wang, W.; Dong, Y.; Xu, L.; Dong, W.; Niu, X.; Lei, Z. Combining Bimetallic-Alloy with Selenium Functionalized Carbon to Enhance Electrocatalytic Activity towards Glucose Oxidation. Electrochim. Acta 2017, 24, 16–25. [Google Scholar]
- Ahmed, M.S.; Jeon, S. Highly Active Graphene-Supported NixPd100–x Binary Alloyed Catalysts for Electro-Oxidation of Ethanol in an Alkaline Media. ACS Catal. 2014, 4, 1830–1837. [Google Scholar]
- Mayrhofer, K.; Hartl, K.; Juhart, V.; Arenz, M. Degradation of Carbon-Supported Pt Bimetallic Nanoparticles by Surface Segregation. J. Am. Chem. Soc. 2009, 131, 16348–16349. [Google Scholar]
- Chattot, R.; Martens, I.; Scohy, M.; Herranz, J.; Drnec, J.; Maillard, F.; Dubau, L. Disclosing Pt-Bimetallic Alloy Nanoparticle Surface Lattice Distortion with Electrochemical Probes. ACS Energ. Lett. 2019, 5, 162–169. [Google Scholar]
- Meng, G.; Cao, H.; Wei, T.; Liu, Q.; Fu, J.; Zhang, S.; Luo, J.; Liu, X. Highly dispersed Ru clusters toward an efficient and durable hydrogen oxidation reaction. Chem. Commun. 2022, 58, 11839–11842. [Google Scholar]
- Wang, H.; Zhang, F.; Jin, M.; Zhao, D.; Fan, X.; Li, Z.; Luo, Y.; Zheng, D.; Li, T.; Wang, Y.; et al. V-doped TiO2 nanobelt array for high-efficiency electrocatalytic nitrite reduction to ammonia. Mater. Today Phys. 2023, 30, 100944. [Google Scholar]
- Li, G.G.; Wang, Z.; Blom, D.A.; Wang, H. Tweaking the Interplay among Galvanic Exchange, Oxidative Etching, and Seed-Mediated Deposition toward Architectural Control of Multimetallic Nanoelectrocatalysts. ACS Appl. Mater. Interfaces 2019, 11, 23482–23494. [Google Scholar]
- Xu, H.; Yan, B.; Zhang, K.; Wang, J.; Li, S.; Wang, C.; Du, Y.; Yang, P. Sub-5nm monodispersed PdCu nanosphere with enhanced catalytic activity towards ethylene glycol electrooxidation. Electrochim. Acta 2018, 261, 521–529. [Google Scholar]
- Zhai, Q.; Liu, Y.; Wang, R.; Wang, Y.; Lyu, Q.; Gong, S.; Wang, J.; Simon, G.P.; Cheng, W. Intrinsically Stretchable Fuel Cell Based on Enokitake-Like Standing Gold Nanowires. Adv. Energy Mater. 2019, 10, 1903512. [Google Scholar]
- Do, U.P.; Seland, F.; Wang, K.; Johannessen, E.A. Raney-platinum thin film electrodes for the catalysis of glucose in abiotically catalyzed micro-glucose fuel cells. J. Mater. Sci. 2019, 54, 14143–14156. [Google Scholar]
- Ding, J.; Yang, H.; Zhang, S.; Liu, Q.; Cao, H.; Luo, J.; Liu, X. Advances in the Electrocatalytic Hydrogen Evolution Reaction by Metal Nanoclusters-based Materials. Small 2022, 18, 2204524. [Google Scholar]
- Cao, R.D.F.; Rui, Z.; Wang, X.; Liu, J.; Jiang, X. Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells. Molecules 2023, 28, 773. [Google Scholar]
- Hou, X.; Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. Asymmetric Coordination Environment Engineering of Atomic Catalysts for CO2 Reduction. Nanomaterials 2023, 13, 309. [Google Scholar]
- Gao, S.; Wang, T.; Jin, M.; Zhang, S.; Liu, Q.; Hu, G.; Yang, H.; Luo, J.; Liu, X. Bifunctional Nb-N-C atomic catalyst for aqueous Zn-air battery driving CO2 electrolysis. Sci. China Mater. 2023, 66, 1013–1023. [Google Scholar]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, S.; Dong, S. PdM (M = Pt, Au) bimetallic alloy nanowires with enhanced electrocatalytic activity for electro-oxidation of small molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Brouzgou, A.; Song, S.; Tsiakaras, P. Carbon-supported PdSn and Pd3Sn2 anodes for glucose electrooxidation in alkaline media. Appl. Catal. B Environ. 2014, 158–159, 209–216. [Google Scholar] [CrossRef]
- Qazzazie, D.; Yurchenko, O.; Urban, S.; Kieninger, J.; Urban, G. Platinum nanowires anchored on graphene-supported platinum nanoparticles as a highly active electrocatalyst towards glucose oxidation for fuel cell applications. Nanoscale 2017, 9, 6436–6447. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.I.; Maeng, B.; Lee, S.; Kwon, Y.; Kang, M.S.; Park, J.; Kim, J. Safe, Durable, and Sustainable Self-Powered Smart Contact Lenses. ACS Nano 2022, 16, 15827–15836. [Google Scholar] [CrossRef]
- Deng, X.; Huang, C.; Pei, X.; Hu, B.; Zhou, W. Recent progresses and remaining issues on the ultrathin catalyst layer design strategy for highperformance proton exchange membrane fuel cell with further reduced Pt loadings: A review. Int. J. Hydrogen Energy 2021, 47, 1529–1542. [Google Scholar] [CrossRef]
- Naik, K.K.; Gangan, A.; Chakraborty, B.; Nayak, S.K.; Rout, C.S. Enhanced Nonenzymatic Glucose-Sensing Properties of Electrodeposited NiCo(2)O(4)-Pd Nanosheets: Experimental and DFT Investigations. ACS Appl. Mater. Interfaces 2017, 9, 23894–23903. [Google Scholar] [CrossRef]
- Souissi, M.; Sahara, R.; Darvishi, S.; Ahadian, S. Responses to comments on “Ni nanoparticle-decorated reduced graphene oxide for non-enzymatic glucose sensing: An experimental and modeling study [Electrochim. Acta 240 (2017) 388–398]”. Electrochim. Acta 2019, 300, 145–149. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, Y.; Zhao, T.; Xiao, W.; Yao, X. Mechanism of Glucose–Fructose Isomerization over Aluminum-Based Catalysts in Methanol Media. ACS Sustain. Chem. Eng. 2019, 7, 14962–14972. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; He, S.; Zhang, B.; Cao, Z.; Zhou, T.; He, J.; Chu, G. Self-Supported 3D PtPdCu Nanowires Networks for Superior Glucose Electro-Oxidation Performance. Molecules 2023, 28, 5834. https://doi.org/10.3390/molecules28155834
Wang K, He S, Zhang B, Cao Z, Zhou T, He J, Chu G. Self-Supported 3D PtPdCu Nanowires Networks for Superior Glucose Electro-Oxidation Performance. Molecules. 2023; 28(15):5834. https://doi.org/10.3390/molecules28155834
Chicago/Turabian StyleWang, Kaili, Shuang He, Bowen Zhang, Zhen Cao, Tingting Zhou, Jia He, and Ganghui Chu. 2023. "Self-Supported 3D PtPdCu Nanowires Networks for Superior Glucose Electro-Oxidation Performance" Molecules 28, no. 15: 5834. https://doi.org/10.3390/molecules28155834
APA StyleWang, K., He, S., Zhang, B., Cao, Z., Zhou, T., He, J., & Chu, G. (2023). Self-Supported 3D PtPdCu Nanowires Networks for Superior Glucose Electro-Oxidation Performance. Molecules, 28(15), 5834. https://doi.org/10.3390/molecules28155834







