Neurological Applications of Celery (Apium graveolens): A Scoping Review
Abstract
1. Introduction
2. Results
2.1. Study Inclusion
2.2. Characteristics of Included Studies
2.3. In Vivo Studies
2.4. Risk of Bias Assessment of In Vivo Studies
2.5. In Vitro Studies
2.6. Clinical Trial
2.7. Safety Study
3. Discussion
3.1. Parkinson’s Disease
3.2. Alzheimer’s Disease
3.3. Stroke-Related Neurological Disorders
3.4. Other Neurological Disorders
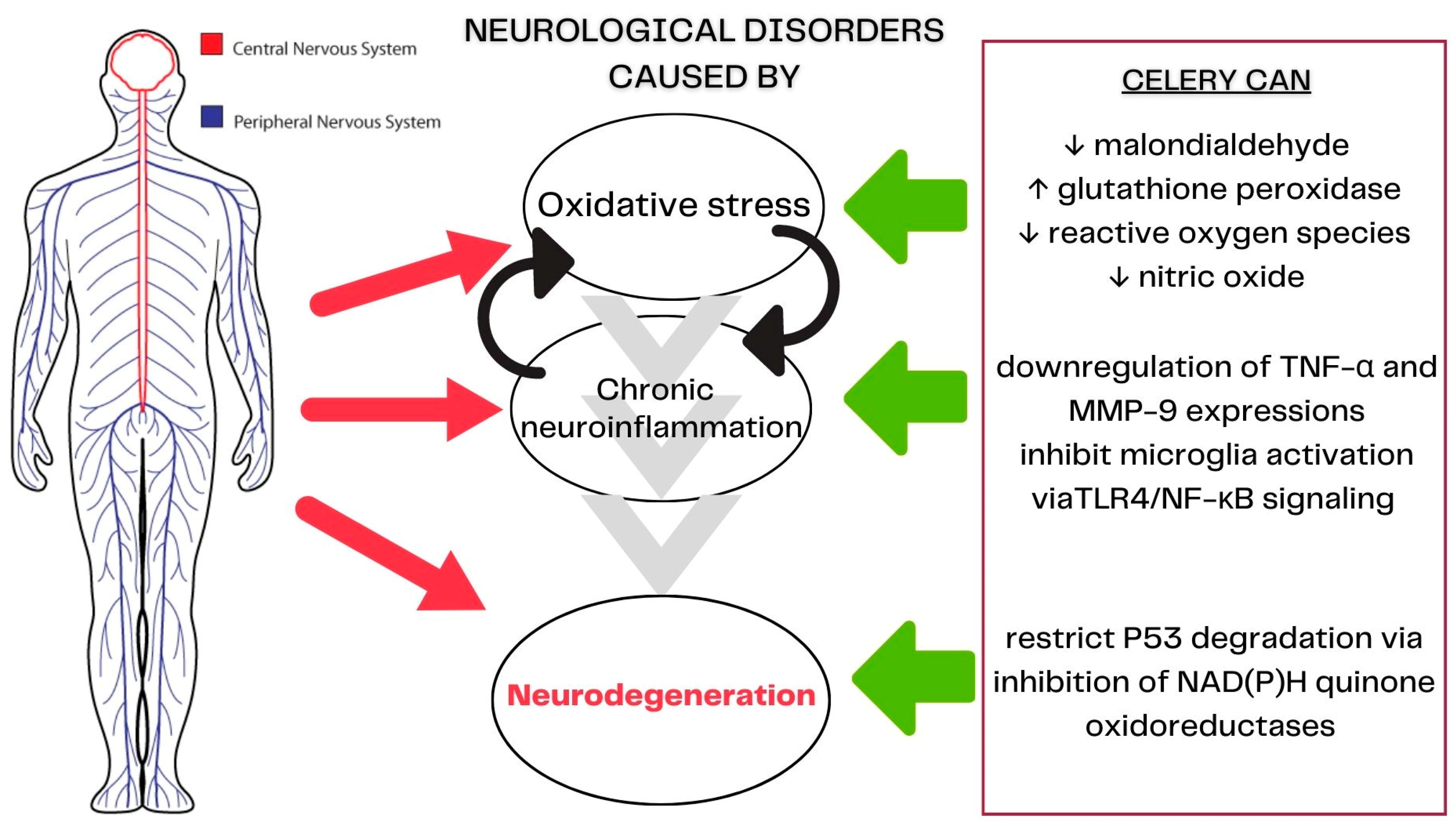
3.5. Celery’s Mechanisms of Action in Neurological Diseases
3.6. Limitations
4. Materials and Methods
4.1. Review Objective
4.2. Inclusion and Exclusion Criteria
4.2.1. Type of Study
4.2.2. Type of Participants
4.2.3. Type of Intervention
4.2.4. Type of Outcomes
Primary Outcomes
Secondary Outcomes
4.3. Search Strategy
4.4. Study Selection
4.5. Data Extraction and Management
4.6. Data Analysis
Risk of Bias Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- U.S. Department of Health and Human Services, National Institute of Environmental Health Sciences. Neurodegenerative Diseases. 2022. Available online: https://www.niehs.nih.gov/research/supported/health/neurodegenerative/index.cfm (accessed on 5 September 2022).
- Launch of First Who Position Paper on Optimizing Brain Health Across Life. 2023. Available online: https://www.who.int/news/item/09-08-2022-launch-of-first-who-position-paper-on-optimizing-brain-health-across-life (accessed on 12 March 2023).
- Carroll, W.M. The global burden of neurological disorders. Lancet Neurol. 2019, 18, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; Desai, R.; et al. Burden of Neurological Disorders Across the US From 1990–2017, A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [PubMed]
- Research, J. What? JPND. 2017. Available online: https://www.neurodegenerationresearch.eu/what/ (accessed on 5 September 2022).
- Kovacs, G.G. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef]
- Szigeti, K. Overcoming gaps in the treatment of neurodegenerative disease. EBioMedicine 2020, 60, 103088. [Google Scholar]
- Khazaei, H.; Pesce, M.; Patruno, A.; Aneva, I.Y.; Farzaei, M.H. Medicinal plants for diabetes associated neurodegenerative diseases: A systematic review of preclinical studies. Phytother. Res. 2021, 35, 1697–1718. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Revoori, A.; Vaibhav, K.; Ahluwalia, M.; Saxena, M. Herbal drugs an alternative medicine for the treatment of neurodegenerative diseases: Preclinical and clinical trial review. In Herbal Medicines: A Boon for Healthy Human Life; Medicines, I.H., Ed.; Academic Press: Cambridge, UK, 2022. [Google Scholar]
- Abdoulaye, I.A.; Guo, Y.J. A Review of Recent Advances in Neuroprotective Potential of 3-N-Butylphthalide and Its Derivatives. Biomed Res. Int. 2016, 2016, 5012341. [Google Scholar] [CrossRef]
- Chen, X.Q.; Qiu, K.; Liu, H.; He, Q.; Bai, J.H.; Lu, W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin. Med. J. 2019, 132, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Seidemann, J. World Spice Plants: Economic Usage, Botany, Taxonomy; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- United States Department of Agriculture. Apium graveolens L.—Wild Celery. 2023. Available online: http://plants.usda.gov/core/profile?symbol=APGR2 (accessed on 12 March 2023).
- Ovodova, R.G.; Golovchenko, V.V.; Popov, S.V.; Popova, G.Y.; Paderin, N.M.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of pectic polysaccharide isolated from celery stalks. Food Chem. 2009, 114, 610–615. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini. Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Momin, R.; Nair, M. Antioxidant, cyclooxygenase and topoisomerase inhibitory compounds from Apium graveolens Linn. seeds. Phytomedicine 2002, 9, 312–318. [Google Scholar] [CrossRef]
- Kitajima, J.; Ishikawa, T.; Satoh, M. Polar constituents of celery seed. Phytochemistry 2003, 64, 1003–1011. [Google Scholar] [CrossRef]
- Lombaert, G.A.; Siemens, K.H.; Pellaers, P.; Mankotia, M.; Ng, W. Furanocoumarins in celery and parsnips: Method and multiyear Canadian survey. J. AOAC Int. 2001, 84, 1135–1143. [Google Scholar] [CrossRef]
- Christensen, L.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.S.; Mohamed, S. Alpha-tocopherol content in 62 edible tropical plants. J. Agric. Food Chem. 2001, 49, 3101–3105. [Google Scholar] [CrossRef] [PubMed]
- Wynn, S.G.; Fougère, B. Veterinary Herbal Medicine; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006; p. 728. [Google Scholar]
- Schippers, R.R. Apium graveolens L. 2004. Available online: https://prota.prota4u.org/protav8.asp?h=M4&t=Apium,graveolens&p=Apium+graveolens#Synonyms (accessed on 12 March 2023).
- Khairullah, A.R.; Solikhah, T.I.; Ansori, A.N.M.; Hidayatullah, A.R.; Hartadi, E.B.; Ram, S.C.; Fadholly, A. Review on the pharmacological and health aspects of Apium graveolens or celery: An update. Syst. Rev. Pharm. 2021, 12, 595–601. [Google Scholar]
- Abu-Taweel, G.M. Celery ameliorating against neurobehavioral and neurochemical disorders of perinatal lipopolysaccharides exposure in mice offspring. J. King Saud Univ. Sci. 2020, 32, 1764–1771. [Google Scholar] [CrossRef]
- Boonruamkaew, P.; Sukketsiri, W.; Panichayupakaranant, P.; Kaewnam, W.; Tanasawet, S.; Tipmanee, V.; Hutamekalin, P.; Chonpathompikunlert, P. Apium graveolens extract influences mood and cognition in healthy mice. J. Nat. Med. 2017, 71, 492–505. [Google Scholar] [CrossRef]
- Chen, C.; Ma, H.; Fu, Z. Antidepressant-like Effect of 3-n-Butylphthalide in Rats Exposed to Chronic Unpredictable Mild Stress: Modulation of Brain-Derived Neurotrophic Factor Level and mTOR Activation in Cortex. Neurochem. Res. 2021, 46, 3075–3084. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Boonruamkaew, P.; Sukketsiri, W.; Hutamekalin, P.; Sroyraya, M. The antioxidant and neurochemical activity of Apium graveolens L. and its ameliorative effect on MPTP-induced Parkinson-like symptoms in mice. BMC Complement. Altern. Med. 2018, 18, 103. [Google Scholar] [CrossRef]
- Choupankareh, S.; Beheshti, F.; Karimi, S.; Sadeghnia, H.R.; Rakhshandeh, H.; Rezaeipour, M.; Hosseini, M. The effects of aqueous extract of Apium Graveolens on brain tissues oxidative damage in pentylenetetrazole-induced seizures model in rat. Curr. Nutr. Food Sci. 2018, 14, 47–53. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, X.; Chen, R.; Zhang, L.; Xue, J.; Gao, X. Dl-3-N-Butylphthalide Alleviates the Blood-Brain Barrier Permeability of Focal Cerebral Ischemia Reperfusion in Mice. Neuroscience 2019, 413, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, D.; Zhu, Z.; Xie, X.; Zhan, S.; Zhang, R.; Zhang, G.; Huang, L. Dl-3-n-Butylphthalide Alleviates Hippocampal Neuron Damage in Chronic Cerebral Hypoperfusion via Regulation of the CNTF/CNTFRα/JAK2/STAT3 Signaling Pathways. Front. Aging Neurosci. 2020, 12, 587403. [Google Scholar] [CrossRef] [PubMed]
- Min, J.J.; Huo, X.L.; Xiang, L.Y.; Qin, Y.Q.; Chai, K.Q.; Wu, B.; Jin, L.; Wang, X.T. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci. Rep. 2014, 4, 5555. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, J.; Hon, S.; Nylander, A.N.; Xia, W.; Feng, Y.; Wang, X. Lemere CA L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer’s disease. J. Neurosci. 2010, 30, 8180–8189. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, Y.; Xu, S.; Li, P.; Li, J.; Lu, L.; Yang, H.; Feng, N.; Wang, L.; Wang, X. L-3-n-butylphthalide reduces tau phosphorylation and improves cognitive deficits in AβPP/PS1-Alzheimer’s transgenic mice. J. Alzheimers Dis. 2012, 29, 379–391. [Google Scholar] [CrossRef]
- Roghani, M.; Amin, A.A.; Amirtouri, R. The effect of chronic administration of Apium graveolens aqueous extract on learning and memory in normal and diabetic rats. Basic Clin. Neurosci. 2009, 1, 26. [Google Scholar]
- Wang, B.N.; Wu, C.B.; Chen, Z.M.; Zheng, P.P.; Liu, Y.Q.; Xiong, J.; Xu, J.Y.; Li, P.F.; Mamun, A.A.; Ye, L.B.; et al. DL-3-n-butylphthalide ameliorates diabetes-associated cognitive decline by enhancing PI3K/Akt signaling and suppressing oxidative stress. Acta Pharmacol. Sin. 2021, 42, 347–360. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Chen, D.; Lee, M.J.H.; Zhao, Y.; Gu, X.; Yu, S.P.; Wei, L. DL-3-n-butylphthalide Increases Collateriogenesis and Functional Recovery after Focal Ischemic Stroke in Mice. Aging Dis. 2021, 12, 1835–1849. [Google Scholar] [CrossRef]
- Wongtawatchai, T.; Sarsutham, K.; Sukketsiri, W.; Tipmanee, V.; Chonpathompikunlert, P. Anti-stress effects of Apium graveolens on rats subjected to immobilization. Int. Food Res. J. 2017, 24, 1490. [Google Scholar]
- Xiang, J.; Pan, J.; Chen, F.; Zheng, L.; Chen, Y.; Zhang, S.; Feng, W. L-3-n-butylphthalide improves cognitive impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int. J. Clin. Exp. Med. 2014, 7, 1706–1713. [Google Scholar] [PubMed]
- Yang, M.; Dang, R.; Xu, P.; Guo, Y.; Han, W.; Liao, D.; Jiang, P. Dl-3-n-Butylphthalide improves lipopolysaccharide-induced depressive-like behavior in rats: Involvement of Nrf2 and NF-κB pathways. Psychopharmacology 2018, 235, 2573–2585. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Rong, Z.; Li, Y.; Wang, X.; Cheng, B.; Cheng, Y.; Luo, H.; Ti, Y.; Huang, X.; Liu, Z.; et al. Protective Role of L-3-n-Butylphthalide in Cognitive Function and Dysthymic Disorders in Mouse With Chronic Epilepsy. Front. Pharmacol. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zheng, L.; Zhao, Y. Protective effect of 3-n-butylphthalide against intrastriatal injection of malonic acid-induced neurotoxicity and biochemical alteration in rats. Biomed Pharmacother. 2022, 155, 113664. [Google Scholar] [CrossRef]
- Zeng, Z.; Gong, X.; Hu, Z. L-3-n-butylphthalide attenuates inflammation response and brain edema in rat intracerebral hemorrhage model. Aging 2020, 12, 11768–11780. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, W.H.; Wang, Y.X.; Wang, C.; Zhao, F.; Qi, W.; Chan, W.M.; Huang, Y.; Wai, M.S.; Dong, J.; et al. DL-3-n-Butylphthalide, an antioxidant agent, prevents neurological deficits and cerebral injury following stroke per functional analysis, magnetic resonance imaging and histological assessment. Curr. Neurovasc. Res. 2012, 9, 167–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.J.; Shi, S.; Xu, S.F.; Wang, X.L.; Peng, Y. L-3-n-butylphthalide Rescues Hippocampal Synaptic Failure and Attenuates Neuropathology in Aged APP/PS1 Mouse Model of Alzheimer’s Disease. CNS Neurosci. Ther. 2016, 22, 979–987. [Google Scholar] [CrossRef]
- Huang, J.Z.; Chen, Y.Z.; Su, M.; Zheng, H.F.; Yang, Y.P.; Chen, J.; Liu, C.F. dl-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP(+)-induced cellular model of Parkinson’s disease. Neurosci. Lett. 2010, 475, 89–94. [Google Scholar] [CrossRef]
- Liu, K.; Huang, J.; Chen, R.; Zhang, T.; Shen, L.; Yang, J.; Sun, X. Protection against neurotoxicity by an autophagic mechanism. Braz. J. Med. Biol. Res. 2012, 45, 401–407. [Google Scholar] [CrossRef]
- Yang, X.D.; Cen, Z.D.; Cheng, H.P.; Shi, K.; Bai, J.; Xie, F.; Wu, H.W.; Li, B.B.; Luo, W. L-3-n-Butylphthalide Protects HSPB8 K141N Mutation-Induced Oxidative Stress by Modulating the Mitochondrial Apoptotic and Nrf2 Pathways. Front. Neurosci. 2017, 11, 402. [Google Scholar] [CrossRef]
- Peng, Y.; Xing, C.; Lemere, C.A.; Chen, G.; Wang, L.; Feng, Y.; Wang, X. l-3-n-Butylphthalide ameliorates beta-amyloid-induced neuronal toxicity in cultured neuronal cells. Neurosci. Lett. 2008, 434, 224–229. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, M.; Xu, W.; Yu, M.; Liu, X.; Chen, Y. DL-3-n-butylphthalide therapy for Parkinson’s disease: A randomized controlled trial. Exp. Ther. Med. 2019, 17, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Burden of Neurological Conditions. 2023. Available online: https://www.paho.org/en/enlace/burden-neurological-conditions#:~:text=with%20disability%20(YLDs)-,Regionwide%20in%202019%2C%20neurological%20disorders%20account%20for%3A,per%20100%2C000%20population%20for%20women (accessed on 9 March 2023).
- Niu, F.; Sharma, A.; Wang, Z.; Feng, L.; Muresanu, D.F.; Sahib, S.; Tian, Z.R.; Lafuente, J.V.; Buzoianu, A.D.; Castellani, R.J.; et al. Co-administration of TiO2-nanowired dl-3-n-butylphthalide (dl-NBP) and mesenchymal stem cells enhanced neuroprotection in Parkinson’s disease exacerbated by concussive head injury. Prog. Brain Res. 2020, 258, 101–155. [Google Scholar]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Winship, I.R. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation 2015, 22, 228–236. [Google Scholar] [CrossRef]
- Kurzepa, J.; Kurzepa, J.; Kurzepa, J.; Golab, P.; Czerska, S.; Bielewicz, J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int. J. Neurosci. 2014, 124, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Han, Y.W.; Han, X.H.; Zhang, K.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol. Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef]
- Peng, Y.; Zeng, X.; Feng, Y.; Wang, X. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J. Cardiovasc. Pharmacol. 2004, 43, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Liu, R.Z.; Fan, C.X.; Zhang, Z.L.; Zhao, X.; Sun, Y.; Liu, H.H.; Nie, Z.X.; Pu, X.P. Effects of Dl-3-n-butylphthalide on Cerebral Ischemia Infarction in Rat Model by Mass Spectrometry Imaging. Int. J. Mol. Sci. 2017, 18, 2451. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, Y.; Wang, Y.; Huang, Z.; Zhuang, Z.; Yang, D.; Liang, G.; Zhang, X.; Chen, G. Dl-3-n-Butylphthalide Ameliorates Concanavalin-Induced Autoimmune Hepatitis in Mice via Inhibiting Oxidative Stress and Inflammation. SSRN. 2022. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4020102 (accessed on 12 March 2023).
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Huang, L.; Zhang, Y.; Peng, Y.; Xing, C.; Feng, Y.; Wang, X.; Peng, Y. Dl-3-n-Butylphthalide (NBP): A Promising Therapeutic Agent for Ischemic Stroke. CNS Neurol. Disord. Drug Targets 2018, 17, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, M.; Niu, X.; Li, M.; Xu, L.; Xiao, Y.; Zhang, J.; Wang, H.; Li, L.; Chu, B.; et al. Dl-3-n-Butylphthalide Improves Neuroinflammation in Mice with Repeated Cerebral Ischemia-Reperfusion Injury through the Nrf2-Mediated Antioxidant Response and TLR4/MyD88/NF-κB Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 8652741. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhou, Y.; Lin, L.; Wang, Q.; Khor, S.; Mao, Y.; Li, J.; Zhen, Z.; Chen, J.; Gao, Z.; et al. Dl-3-n-butylphthalide attenuates acute inflammatory activation in rats with spinal cord injury by inhibiting microglial TLR4/NF-κB signalling. J. Cell. Mol. Med. 2017, 21, 3010–3022. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Zhang, L.; Ying, Z.; Sha, O.; Li, C.; Lü, L.; Chen, X.; Li, Z.; Niu, F.; et al. The novel targets of DL-3-n-butylphthalide predicted by similarity ensemble approach in combination with molecular docking study. Quant. Imaging Med. Surg. 2017, 7, 532–536. [Google Scholar] [CrossRef]
- Hou, D.R.; Xue, L.; Tang, J.C.; Zhou, J.; Sun, J.J. Butylphthalide improves learning and memory abilities of rats with Alzheimer’s disease possibly by enhancing protein disulfide isomerase and inhibiting P53 expressions. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 2104–2107. [Google Scholar]
- Tian, J.; Lei, P.; He, Y.; Zhang, N.; Ge, X.; Luo, L.; Yan, S.; Diao, X. Absorption, distribution, metabolism, and excretion of [(14)C]NBP (3-n-butylphthalide) in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1181, 122915. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Periyasamy, P.; Thangaraj, A.; Chivero, E.T.; Buch, S. PDGF/PDGFR axis in the neural systems. Mol. Asp. Med. 2018, 62, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020, 5, 354–365. [Google Scholar] [CrossRef]
- Paladino, S.; Conte, A.; Caggiano, R.; Pierantoni, G.M.; Faraonio, R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell. Physiol. Biochem. 2018, 47, 1951–1976. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Qin, D.; Chen, H.; Wang, J.; Wang, J.; Song, S.; Wang, C.; Wang, Y.; Liu, S.; et al. The role of brain derived neurotrophic factor in central nervous system. Front. Aging Neurosci. 2022, 14, 986443. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Metalloproteinases and neurodegenerative diseases: Pathophysiological and therapeutic perspectives. Met. Med. 2015, 2, 39–50. [Google Scholar] [CrossRef]
- Review Manager 5 (RevMan 5) (Computer Program), Version 5.4; Nordic Cochrane Centre: Copenhagen, Denmark, 2014.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]



| Animal | Intervention Details | Disease Model | Dosage, Duration, Route | Effect/Mechanism | Reference |
|---|---|---|---|---|---|
| Rat | Details of celery not mentioned | Perinatal effect | 300 or 600 mg/kg, 15 days, oral | Protective effects of celery against various lipopolysaccharide-induced oxidative stresses | Abu-Taweel, 2020 [25] |
| Mice | Crude 70% methanol extract of A. graveolens | Depression | 65, 125, 250, 375 and 500 mg/kg, 4 weeks, oral | Antidepressant-like effects of A. graveolens in the forced swimming and tail suspension tests, and the cognitive-enhancing effect validated in the Morris water maze and object recognition tests | Boonruamkaew, 2017 [26] |
| Rat and mice | DL-NBP | Depression | Rats: 10, 30, 100 mg/kg Mice: 30 mg/kg 6 weeks, oral | Antidepressant effect of DL-NBP via activation of BDNF/ERK/ mTOR cascade in the cortex and involvement of serotonergic system | Chen, 2021 [27] |
| Mice | 70% methanol crude extract of A. graveolens whole plant | Parkinson’s disease | 125, 250, 375 mg/kg, 21 days, oral | Amelioration of behavioral impairments, improvement in oxidative stress parameters, decrease in the activity of MAO-A and B, and protection of dopaminergic neurons by celery extract | Chonpathompikunlert, 2018 [28] |
| Rat | Aqueous extract of A. graveolens aerial part | Epilepsy | 100, 500, and 1000 mg/kg, 30 min, i.p | A. graveolens extract possesses anticonvulsant activity and is accompanied by an antioxidant effect in the brain | Choupankareh, 2018 [29] |
| Mice | DL-NBP | Ischemic stroke | 40 mg/kg, duration and route not mentioned | DL-NBP NBP exerts its neuroprotective effects through attenuating the cerebral infarct size and neurological deficit score, reducing cerebral edema and BBB permeability | Li, 2019 [30] |
| Rats | DL-NBP | Chronic cerebral hypoperfusion (CCH) | 5 mg/kg, once daily, 21 days, intravenous | DL-NBP administration markedly rescues memory deficits and hippocampal neuronal death/ apoptosis by upregulating the CNTF/CNTFRa/JAK2/STAT3 signaling pathway in CCH rats | Li, 2020 [31] |
| Rats | DL-NBP | Chronic intermittent hypoxia hypercapnia (CIHH) | 80 mg/kg, 2 weeks, oral | Neuroprotective effects of DL-NBP under CIHH condition possibly occurring through the inhibition of apoptosis, promotion of hypoxia-induced autophagy, and activation of the SIRT1/PGC-1a signalling pathway | Min, 2014 [32] |
| Mice | L-NBP | Alzheimer’s disease | 15 mg/kg, 5 days/week, 18 weeks, oral | L-NBP reduces cerebral Aβ levels, glial activation, oxidative stress, cognitive impairment; regulates APP processing toward the nonamyloidogenic pathway; and promotes APP release, thereby precluding Aβ generation. | Peng, 2010 [33] |
| Mice | L-NBP | Alzheimer’s disease | 15 mg/kg, 5 days/week, 12 weeks, oral | L-NBP is able to inhibit tau abnormal hyperphosphorylation and improve cognitive impairment in an APP/PS1 transgenic | Peng, 2012 [34] |
| Rat | Aqueous extract of A. graveolens | Diabetes (learning memory) | Dosage, and route of administration not mentioned, 4 weeks | Chronic oral administration of celery could enhance the consolidation and recall capability of stored information and does not affect spatial memory | Roghani, 2009 [35] |
| Mice | DL-NBP | Diabetes (cognitive decline) | 20, 60, 120 mg/kg, 8 weeks, oral | DL-NBP shows neuroprotective effects and inhibits cognitive impairment in diabetes by normalizing hippocampal morphology, improving synaptic plasticity, and reducing neuronal apoptosis | Wang, 2021 [36] |
| Mice | DL-NBP | Stroke | 5 µL (total 80 mg/kg in 400 μL vegetable oil), 1 h after the stroke onset and once daily, 14 days, intranasal | DL-NBP has potential arteriogenic effects for stroke treatment through restoration of local cerebral blood flow and other sustainable positive outcomes | Wei, 2021 [37] |
| Rat | Methanol extract of A. graveolens whole plant | Anxiety | 125 and 250 mg/kg, 3 weeks, oral | Methanol extract of A. graveolens has protective effect against immobilization (stress-induced anxiety-like behavior) without memory loss. | Wongtawatchai, 2017 [38] |
| Mice | L-NBP | Alzheimer’s disease | 10 and 30 mg/kg, 4 weeks, route of administration not reported | L-NBP significantly increases the expression of BDNF/TrkB/PI3K/AKT, in the brain improving cognitive impairment | Xiang, 2014 [39] |
| Rat | DL-NBP | Depression | 30 mg/kg, 14 days, oral | DL-NBP has antidepressive effects involving the Nrf2 and NF-κB pathways responsible for neuroinflammation and oxidative stress | Yang, 2018 [40] |
| Mice | L-NBP | Epilepsy | 80 mg/kg, 14 days, intraperitoneal | L-NBP reduces seizure severity and aberrant electroencephalogram | Ye, 2018 [41] |
| Rat | NBP | Neurotoxicity | 40 and 80 mg/kg, 22 days, oral | NBP administration could mitigate the motor and cognitive impairment caused by neurotoxicity and mitochondrial damage | Yuan, 2022 [42] |
| Rat | L-NBP | Stroke | 50 mg/kg, duration not clear, intraperitoneal | L-NBP inhibits the expression of TNF-α and MMP-9 reducing inflammation, BBB damage and intracerebral hemorrhage | Zeng, 2020 [43] |
| Rat | DL-NBP | Stroke | 60 mg/kg (pre-treatment); 80 mg/kg (post treatment), 2 months before stroke-induced (pre-treatment), 1 week post (post-treatment), intragastric | DL-NBP exerts both preventive and therapeutic effects on ischemic stroke in hypertensive rats, but only exerts therapeutic effects in normotensive rats | Zhang, 2012 [44] |
| Mice | L-NBP | Alzheimer’s disease | 15 mg/kg, 12 weeks, oral | L-NBP enhances synaptic performance, decreases Aβ plaque load, and inhibits microglia activation | Zhang, 2016 [45] |
| Cell | Intervention Details | Disease Model | Dosage, Duration | Effect/Mechanism | Reference |
|---|---|---|---|---|---|
| PC12 | DL-NBP | Parkinson’s disease | 0.01, 0.1, 1.0, 10 or 100 µM, 4 h | Accumulation of alpha-synuclein was diminished by L-NBP, which also decreased the formation of ROS and NO which indicate cytoprotection through inhibition of oxidative stress | Huang, 2010 [46] |
| PC12 | NBP | Parkinson’s disease | 0.1 M, 72 h | Groups that received NBP have a majority of their dendritic processes around maintained, indicating neuronal cells protection | Liu, 2012 [47] |
| Rat hippocampal neurons and SH-SY5Y human neuroblastoma | L-NBP | Parkinson’s disease | 0.1, 1, 10 μM, 4 h | L-NBP guard neurons from harm brought on by Aβ -induced damage, possibly through preventing tau protein hyperphosphorylation. | Peng, 2008 [49] |
| PC12 | DL-NBP | Diabetes (cognitive decline) | 10 μM, 24 h | DL-NBP possibly acts on Nrf2 signaling pathway to alleviates oxidative stress and PI3K/Akt pathways, which are essential to enhance brain-derived neurotrophic factor expression levels | Wang, 2021 [36] |
| iPSC-VPC | DL-NBP | Stroke | 10 μM, 48 h | DL-NBP significantly increased the expression of newly formed vascular marker PDGFR, SERCA2 and GLUT-1 | Wei, 2021 [37] |
| Spinal motor neuron and SH-SY5Y human neuroblastoma | L-NBP | Charcot–Marie–Tooth disease | 10 and 100 μmol/L, pre-treatment and treatment | Protective effects of L-NBP against mutation of HSPB8 caused by mitochondrial dysfunction | Yang, 2017 [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, T.Y.C.; Lim, X.Y.; Norahmad, N.A.; Chanthira Kumar, H.; Teh, B.P.; Lai, N.M.; Syed Mohamed, A.F. Neurological Applications of Celery (Apium graveolens): A Scoping Review. Molecules 2023, 28, 5824. https://doi.org/10.3390/molecules28155824
Tan TYC, Lim XY, Norahmad NA, Chanthira Kumar H, Teh BP, Lai NM, Syed Mohamed AF. Neurological Applications of Celery (Apium graveolens): A Scoping Review. Molecules. 2023; 28(15):5824. https://doi.org/10.3390/molecules28155824
Chicago/Turabian StyleTan, Terence Yew Chin, Xin Yi Lim, Nor Azrina Norahmad, Hemahwathy Chanthira Kumar, Bee Ping Teh, Nai Ming Lai, and Ami Fazlin Syed Mohamed. 2023. "Neurological Applications of Celery (Apium graveolens): A Scoping Review" Molecules 28, no. 15: 5824. https://doi.org/10.3390/molecules28155824
APA StyleTan, T. Y. C., Lim, X. Y., Norahmad, N. A., Chanthira Kumar, H., Teh, B. P., Lai, N. M., & Syed Mohamed, A. F. (2023). Neurological Applications of Celery (Apium graveolens): A Scoping Review. Molecules, 28(15), 5824. https://doi.org/10.3390/molecules28155824









