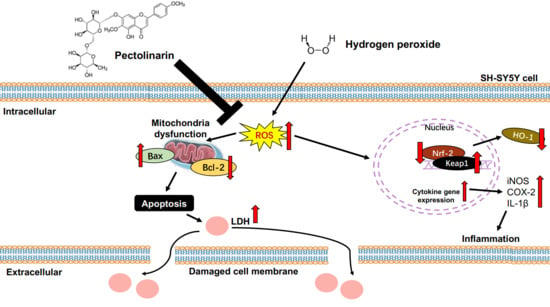
Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells
Abstract
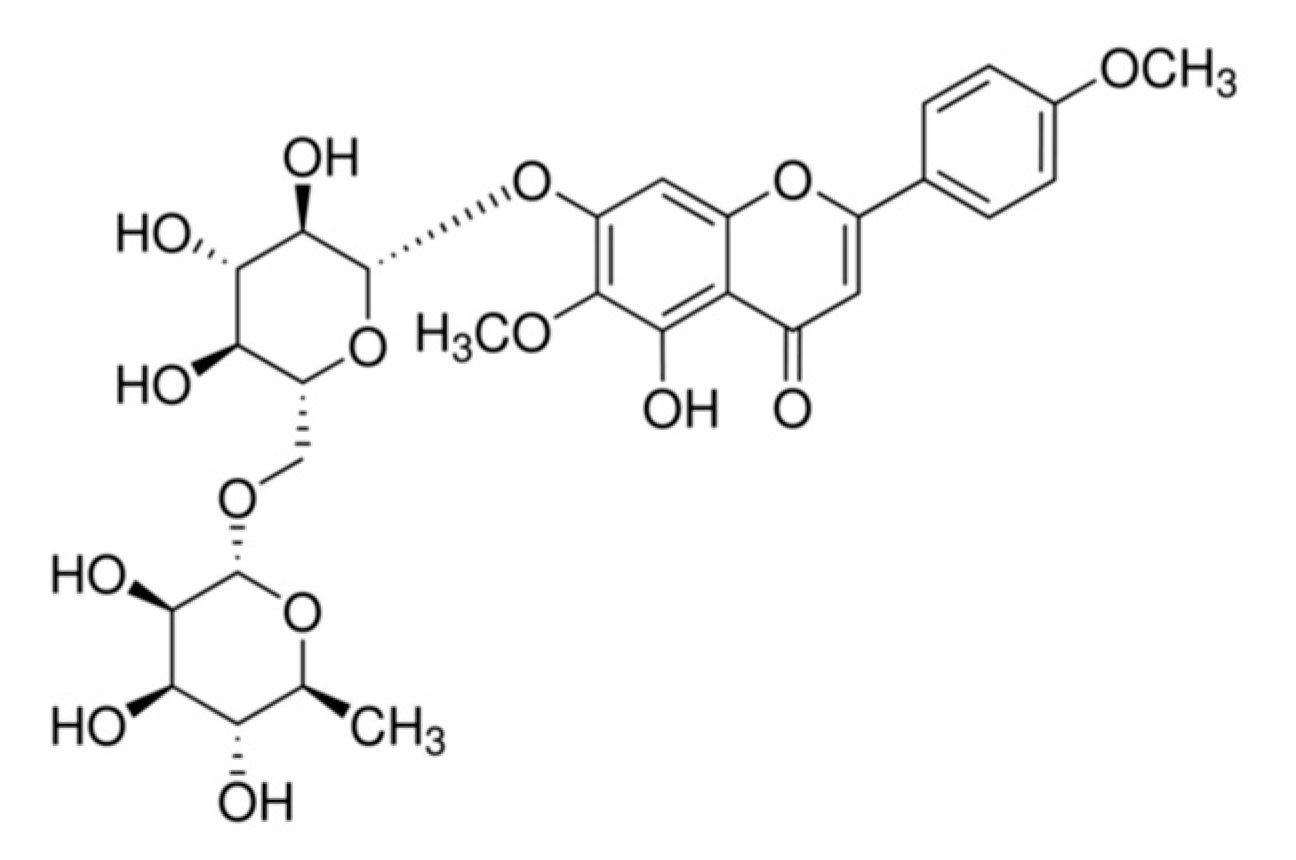
1. Introduction
2. Results
2.1. Hydroxyl Radical (OH) Scavenging Activity of Pectolinarin
2.2. NO Radical Scavenging Activity of Pectolinarin
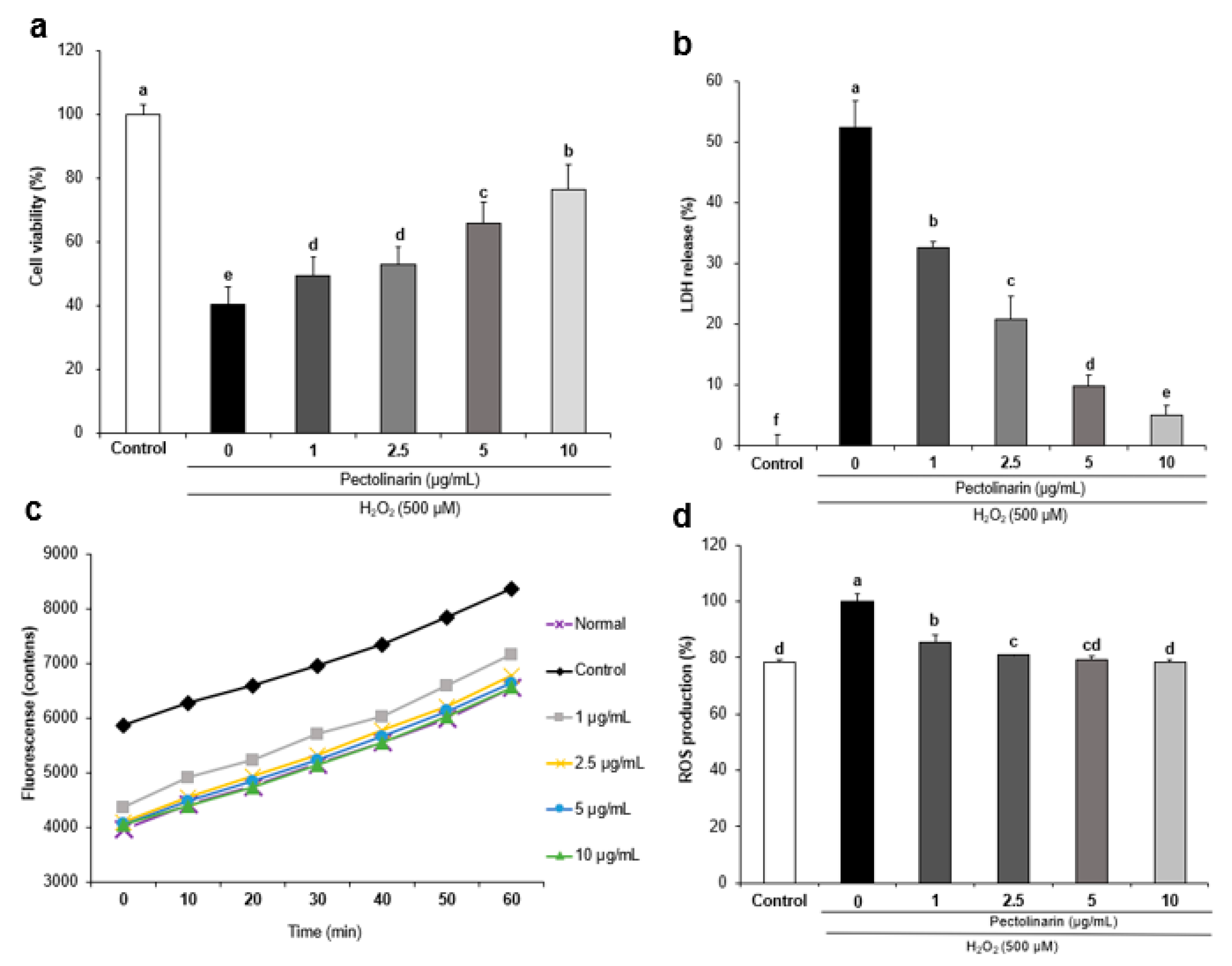
2.3. Effects of Pectolinarin against H2O2-Induced Cell Viability in SH-SY5Y Cells
2.4. Effects of Pectolinarin against H2O2-Induced Lactate Dehydrogenase Release in SH-SY5Y Cells
2.5. Effects of Pectolinarin against H2O2-Induced ROS Formation in SH-SY5Y Cells
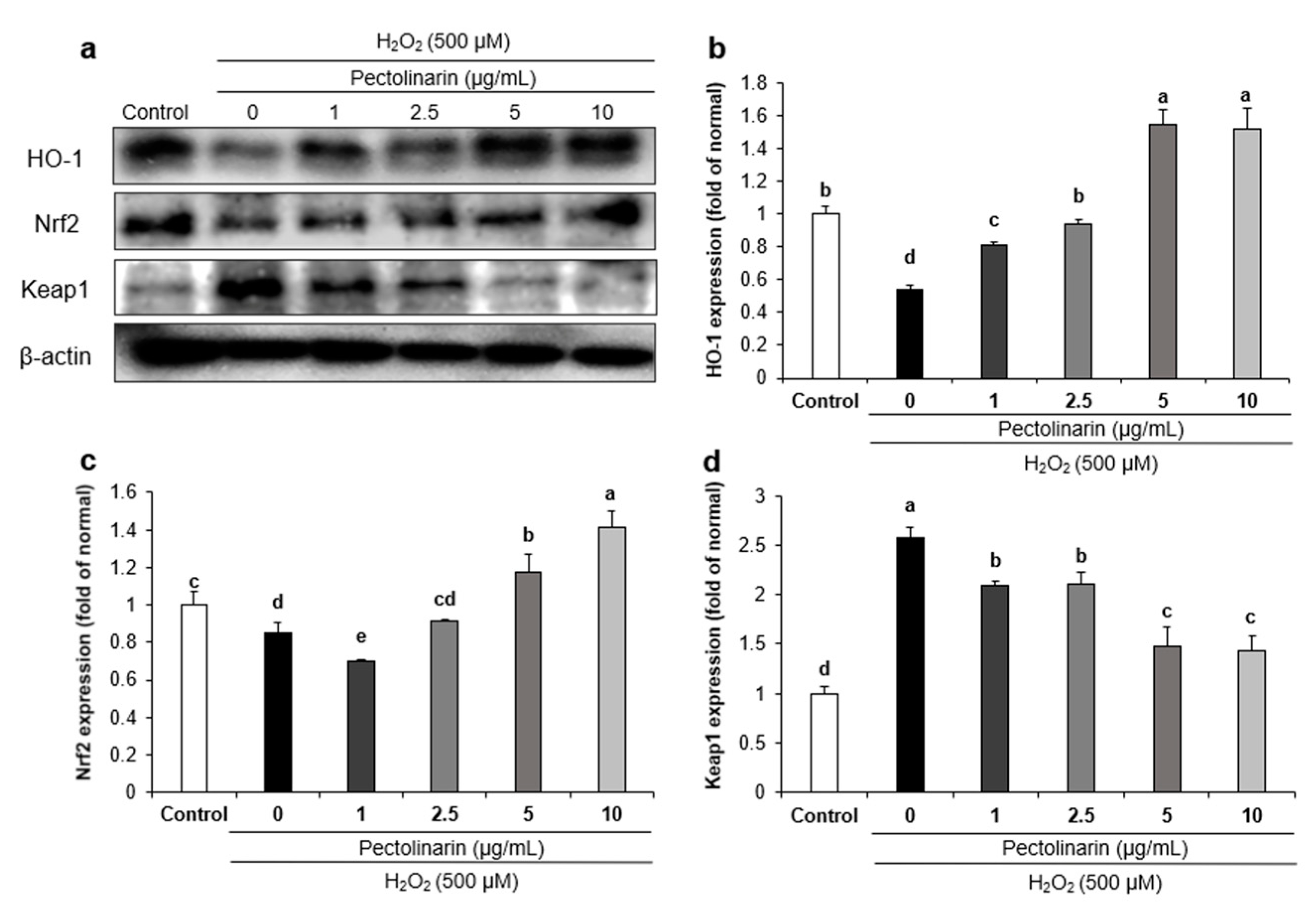
2.6. Effects of Pectolinarin on Oxidative Stress-Related Protein Expression in H2O2-Induced SH-SY5Y Cells
2.7. Effects of Pectolinarin on Inflammation-Related Protein Expression in H2O2-Induced SH-SY5Y Cells
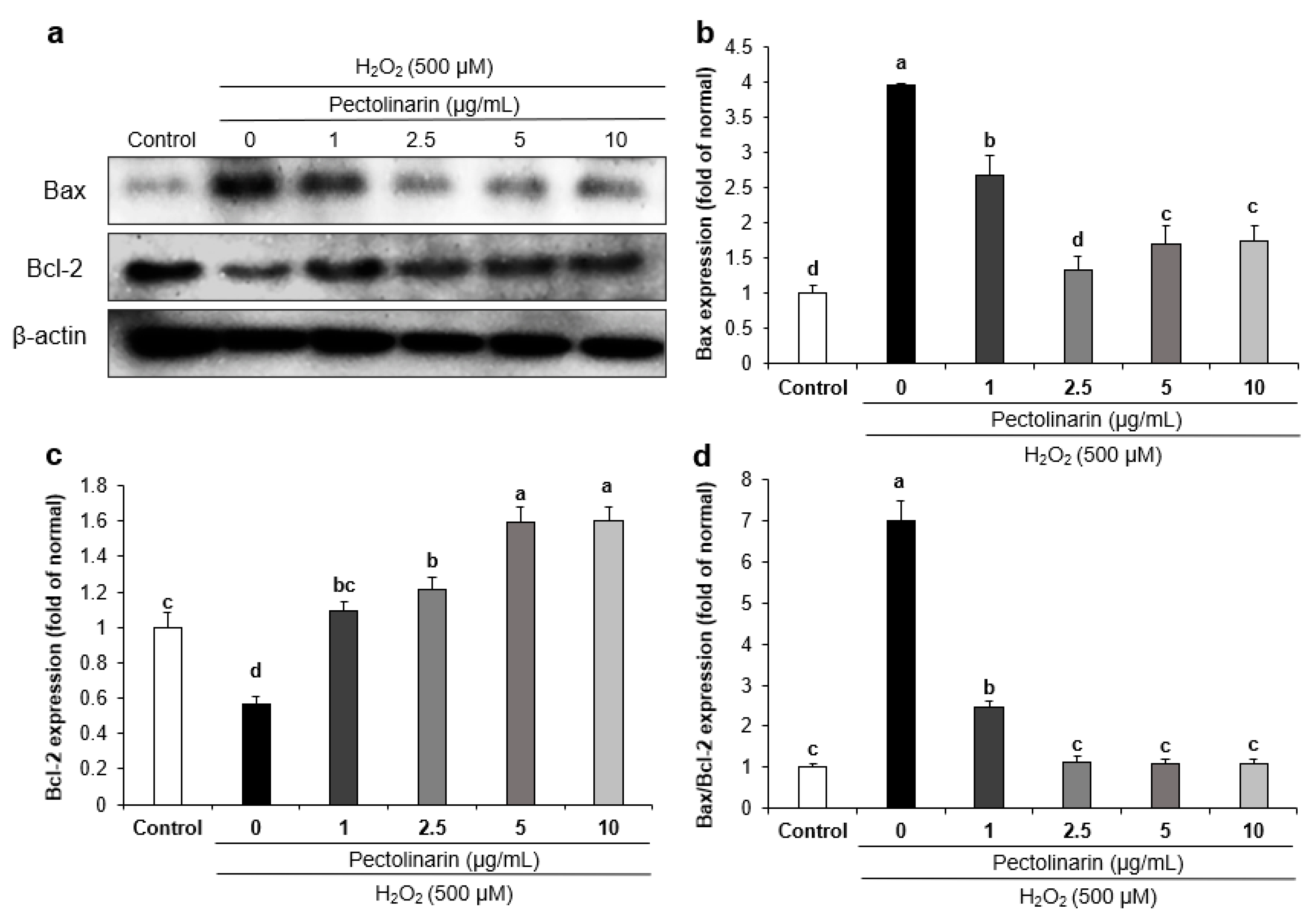
2.8. Effects of Pectolinarin on Apoptosis-Related Protein Expression in H2O2-Induced SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. OH Scavenging Assay
4.3. NO Radical Scavenging Assay
4.4. Cell Culture
4.5. Cell Viability
4.6. Measurement of ROS Production
4.7. Measurement of LDH Release Activity
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availabilty
References
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Li, C. Perspectives of new advances in the pathogenesis of vitiligo: From oxidative stress to autoimmunity. Med. Sci. Monit. 2019, 25, 1017–1023. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Wojtczyk-Miaskowska, A.; Schlichtholz, B. DNA damage and oxidative stress in long-lived aquatic organisms. DNA Repair 2018, 69, 14–23. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Singh, P.K.; Baxi, D.; Diwedi, R.; Ramachandran, A.V. Prior cadmium exposure improves glucoregulation in diabetic rats but exacerbates effects on metabolic dysregulation, oxidative stress, and hepatic and renal toxicity. Drug Chem. Toxicol. 2012, 35, 167–177. [Google Scholar] [CrossRef]
- Olajide, O.J.; Yawson, E.O.; Gbadamosi, I.T.; Arogundade, T.T.; Lambe, E.; Obasi, K.; Lawal, I.T.; Ibrahim, A.; Ogunrinola, K.Y. Ascorbic acid ameliorates behavioural deficits and neuropathological alterations in rat model of Alzheimer’s disease. Environ. Toxicol. Pharmacol. 2017, 50, 200–211. [Google Scholar] [CrossRef]
- Tang, K.S. The potential role of Nanoyttria in alleviating oxidative stress biomarkers: Implications for Alzheimer’s disease therapy. Life Sci. 2020, 259, 118287. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Serou, M.J.; DeCoster, M.A.; Bazan, N.G. Interleukin-1 beta activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: Platelet-activating factor as a preferential mediator of cyclooxygenase-2 expression. J. Neurosci. Res. 1999, 58, 593–598. [Google Scholar] [CrossRef]
- Li, C.Q.; Kim, M.Y.; Godoy, L.C.; Thiantanawat, A.; Trudel, L.J.; Wogan, G.N. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14547–14551. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Rojo, A.I.; Pajares, M.; Rada, P.; Nuñez, A.; Nevado-Holgado, A.J.; Killik, R.; Van Leuven, F.; Ribe, E.; Lovestone, S.; Yamamoto, M.; et al. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017, 13, 444–451. [Google Scholar] [CrossRef]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1–Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef]
- Zawia, N.H.; Lahiri, D.K.; Cardozo-Pelaez, F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic. Biol. Med. 2009, 46, 1241–1249. [Google Scholar] [CrossRef]
- Alamdary, S.Z.; Khodagholi, F.; Shaerzadeh, F.; Ansari, N.; Sonboli, A.; Tusi, S.K. S. choloroleuca, S. mirzayanii and S. santolinifolia protect PC12 cells from H2O2-induced apoptosis by blocking the intrinsic pathway. Cytotechnology 2012, 64, 403–419. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Lim, B.O. Quercetin is an active agent in berries against neurodegenerative diseases progression through modulation of Nrf2/HO1. Nutrients 2022, 14, 5132. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, R.; Yuan, Q.; Gao, Y.; Yang, M.Q.; Zhang, C.; Huang, J.; Sun, Y.; Yang, W.; Yang, J.Y.; et al. The emerging role of MicroRNA-4487/6845-3p in Alzheimer’s disease pathologies is induced by Aβ25–35 triggered in SH-SY5Y cell. BMC Syst. Biol. 2018, 12 (Suppl. S7), 119. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive biophenols reduces Alzheimer’s pathology in SH-SY5Y cells and APPswe mice. Int. J. Mol. Sci. 2018, 20, 125. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Zhang, Y.Q.; Zhang, Y.H.; Wei, X.Z.; Wang, H.; Zhang, M.; Yang, Z.J.; Zhang, C.H. The protective underlying mechanisms of schisandrin on SH-SY5Y cell model of Alzheimer’s disease. J. Toxicol. Environ. Health A 2019, 82, 1019–1026. [Google Scholar] [CrossRef]
- Akki, R.; Siracusa, R.; Morabito, R.; Remigante, A.; Campolo, M.; Errami, M.; La Spada, G.; Cuzzocrea, S.; Marino, A. Neuronal-like differentiated SH-SY5Y cells adaptation to a mild and transient H2O2 -Induced oxidative stress. Cell Biochem. Funct. 2018, 36, 56–64. [Google Scholar] [CrossRef]
- Akyuva, Y.; Nazıroğlu, M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020, 10, 6449. [Google Scholar] [CrossRef]
- Islam, M.d.I.; Shanta, M.A.; Mondal, M.; Hoque, N.; Majumder, S.; Ahmed, T.; Rana, M.S. Protective effect of chloroform extract of Stereospermum Chelonoides Bark against amyloid Beta42 induced cell death in SH-SY5Y cells and against inflammation in Swiss albino mice. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 621–630. [Google Scholar] [CrossRef]
- Tian, X.; Gao, L.; An, L.; Jiang, X.; Bai, J.; Huang, J.; Meng, W.; Zhao, Q. Pretreatment of MQA, a caffeoylquinic acid derivative compound, protects against H2O2-induced oxidative stress in SH-SY5Y cells. Neurol. Res. 2016, 38, 1079–1087. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Öz, A.; Nazıroğlu, M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J. Recept. Signal Transduct. Res. 2016, 36, 395–401. [Google Scholar] [CrossRef]
- Martins, G.R.; da Fonseca, T.S.; Martínez-Fructuoso, L.; Simas, R.C.; Silva, F.T.; Salimena, F.R.G.; Alviano, D.S.; Alviano, C.S.; Leitão, G.G.; Pereda-Miranda, R.; et al. Antifungal phenylpropanoid glycosides from Lippia rubella. J. Nat. Prod. 2019, 82, 566–572. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Cuong, T.D.; Hung, T.M.; Lee, J.H.; Na, M.; Son, J.K.; Jung, H.J.; Fang, Z.; Woo, M.H.; Choi, J.S.; et al. Simultaneous determination of bioactive flavonoids in some selected Korean thistles by high-performance liquid chromatography. Arch. Pharm. Res. 2011, 34, 455–461. [Google Scholar] [CrossRef]
- Cheriet, T.; Ben-Bachir, B.; Thamri, O.; Seghiri, R.; Mancini, I. Isolation and biological properties of the natural flavonoids Pectolinarin and Pectolinarigenin—A review. Antibiotics 2020, 9, 417. [Google Scholar] [CrossRef]
- Ahmed-Chaouch, M.; Cheriet, T.; Beretta, G.; Sarri, D.; Bensouici, C.; Ouelbani, R.; Mancini, I.; Sekhara, I.; Seghiri, R. Chemical composition, in vitro antioxidant, anticholinesterase and antibacterial activities of Linaria scariosa Desf. Nat. Prod. Res. 2021, 35, 1722–1726. [Google Scholar] [CrossRef]
- Lim, H.; Son, K.H.; Chang, H.W.; Bae, K.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of Pectolinarigenin and Pectolinarin isolated from Cirsium chanroenicum. Biol. Pharm. Bull. 2008, 31, 2063–2067. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Li, D.; Zhang, J.; Qiu, D.; Liu, W.; She, L.; Yang, Z. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int. Immunopharmacol. 2006, 6, 1387–1393. [Google Scholar] [CrossRef]
- Mehta, P.; Dhapte, V. A comprehensive review on pharmacokinetic profile of some traditional Chinese medicines. New J. Sci. 2016, 2016, 7830367. [Google Scholar] [CrossRef]
- Chen, Q.; Gong, F.; Liu, Z.; Zhong, L.; Gong, Q. Comparison on intestinal absorption of pectolinarin and pectolinarigenin by everted rat intestinal sac method. World J. Tradit. Chin. Med. 2014, 12, 352–357. [Google Scholar]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Peltier, J.; Stone, D.; Schaffer, D.V.; Chang, C.J. Nox2 redox signaling maintains essential cell populations in the brain. Nat. Chem. Biol. 2011, 7, 106–112. [Google Scholar] [CrossRef]
- Lee, Y.M.; He, W.; Liou, Y.-C. The Redox Language in Neurodegenerative Diseases: Oxidative Post-Translational Modifications by Hydrogen Peroxide. Cell Death Dis. 2021, 12, 58. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, P53 and puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A.J. Dementia of the Eye: The role of amyloid beta in retinal degeneration. Eye 2015, 29, 1013–1026. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef]
- Collier, A.C.; Pritsos, C.A. The mitochondrial uncoupler dicumarol disrupts the MTT assay. Biochem. Pharmacol. 2003, 66, 281–287. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef]
- Esgalhado, M.; Stenvinkel, P.; Mafra, D. Nonpharmacologic strategies to modulate nuclear factor erythroid 2–related Factor 2 pathway in chronic kidney disease. J. Ren. Nutr. 2017, 27, 282–291. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Villeneuve, N.F.; Lau, A.; Zhang, D.D. Regulation of the Nrf2–Keap1 antioxidant response by the ubiquitin proteasome system: An insight into Cullin-Ring ubiquitin ligases. Antioxid. Redox Signal. 2010, 13, 1699–1712. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, S.R.; Park, Y.J.; Ra, M.; Lee, Y.; Pang, C.; Kim, K.H. Suppression of 6-hydroxydopamine-induced oxidative stress by hyperoside via activation of Nrf2/HO-1 signaling in dopaminergic neurons. Int. J. Mol. Sci. 2019, 20, 5832. [Google Scholar] [CrossRef]
- Wen, C.; Huang, C.; Yang, M.; Fan, C.; Li, Q.; Zhao, J.; Gan, D.; Li, A.; Zhu, L.; Lu, D. The secretion from bone marrow mesenchymal stem cells pretreated with berberine rescues neurons with oxidative damage through activation of the Keap1-Nrf2-HO-1 signaling pathway. Neurotox. Res. 2020, 38, 59–73. [Google Scholar] [CrossRef]
- Xia, Q.; Gao, S.; Rapael Gnanamuthu, S.R.; Zhuang, K.; Song, Z.; Zhang, Y.; Wang, X.; Tu, P.; Li, J.; Liu, K. Involvement of Nrf2-HO-1/JNK-Erk signaling pathways in aconitine-induced developmental toxicity, oxidative stress, and ROS-mitochondrial apoptosis in zebrafish embryos. Front. Pharmacol. 2021, 12, 642480. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Luo, C.; Urgard, E.; Vooder, T.; Metspalu, A. The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: Aging and anti-aging. Med. Hypotheses 2011, 77, 174–178. [Google Scholar] [CrossRef]
- Green, H.F.; Nolan, Y.M. GSK-3 mediates the release of IL-1β, TNF-α and IL-10 from cortical glia. Neurochem. Int. 2012, 61, 666–671. [Google Scholar] [CrossRef]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Tripathi, R.; Gupta, R.; Sahu, M.; Srivastava, D.; Das, A.; Ambasta, R.K.; Kumar, P. Free radical biology in neurological manifestations: Mechanisms to therapeutics interventions. Environ. Sci. Pollut. Res. Int. 2022, 29, 62160–62207. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin attenuated hydrogen peroxide (H2O2)-induced oxidative injury in SH-SY5Y and hippocampal neurons via the activation of AMPK pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, J.; Lee, J.-H.; Akanda, M.R.; Cho, J.-H.; Kim, S.-K.; Choi, Y.-J.; Park, B.-Y. Neuroprotective effects of Cornus officinalis on stress-induced hippocampal deficits in rats and H2O2-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Antioxidants 2019, 9, 27. [Google Scholar] [CrossRef]
- Aminzadeh, A.; Salarinejad, A. Citicoline protects against lead-induced oxidative injury in neuronal PC12 Cells. Biochem. Cell Biol. 2019, 97, 715–721. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, G.; Wang, H.; He, S.; Zhu, Y. A preparation of Ginkgo biloba L. leaves extract inhibits the apoptosis of hippocampal neurons in post-stroke mice via regulating the expression of Bax/Bcl-2 and Caspase-3. J. Ethnopharmacol. 2021, 280, 114481. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Liu, C.; Zhu, X.; Zhang, P.; Yu, H.; Li, Y.; Li, Z.; Li, M. Inhibiting c-Jun N-terminal kinase (JNK)-mediated apoptotic signaling pathway in PC12 cells by a polysaccharide (CCP) from Coptis chinensis against Amyloid-β (Aβ)-induced neurotoxicity. Int. J. Biol. Macromol. 2019, 134, 565–574. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, R.G.; Zhu, X.Z. Subtoxic concentration of manganese synergistically potentiates 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Brain Res. 2003, 961, 131–138. [Google Scholar] [CrossRef]

| Treatment (μg/mL) | Scavenging Activity (%) |
|---|---|
| 1 | 63.09 ± 1.97 c |
| 2.5 | 80.43 ± 1.37 b |
| 5 | 83.60 ± 0.75 a |
| 10 | 84.77 ± 0.95 a |
| Treatment (μg/mL) | Scavenging Activity (%) |
|---|---|
| 1 | 23.21 ± 1.09 d |
| 2.5 | 24.50 ± 1.37 c |
| 5 | 30.40 ± 0.59 b |
| 10 | 35.19 ± 0.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Q.Q.; Kim, J.H.; Kim, H.Y.; Kim, J.-H.; Cho, E.J. Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells. Molecules 2023, 28, 5826. https://doi.org/10.3390/molecules28155826
Pang QQ, Kim JH, Kim HY, Kim J-H, Cho EJ. Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells. Molecules. 2023; 28(15):5826. https://doi.org/10.3390/molecules28155826
Chicago/Turabian StylePang, Qi Qi, Ji Hyun Kim, Hyun Young Kim, Ji-Hyun Kim, and Eun Ju Cho. 2023. "Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells" Molecules 28, no. 15: 5826. https://doi.org/10.3390/molecules28155826
APA StylePang, Q. Q., Kim, J. H., Kim, H. Y., Kim, J.-H., & Cho, E. J. (2023). Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells. Molecules, 28(15), 5826. https://doi.org/10.3390/molecules28155826