Synthesis of Azuleno[2,1-b]quinolones and Quinolines via Brønsted Acid-Catalyzed Cyclization of 2-Arylaminoazulenes
Abstract
1. Introduction
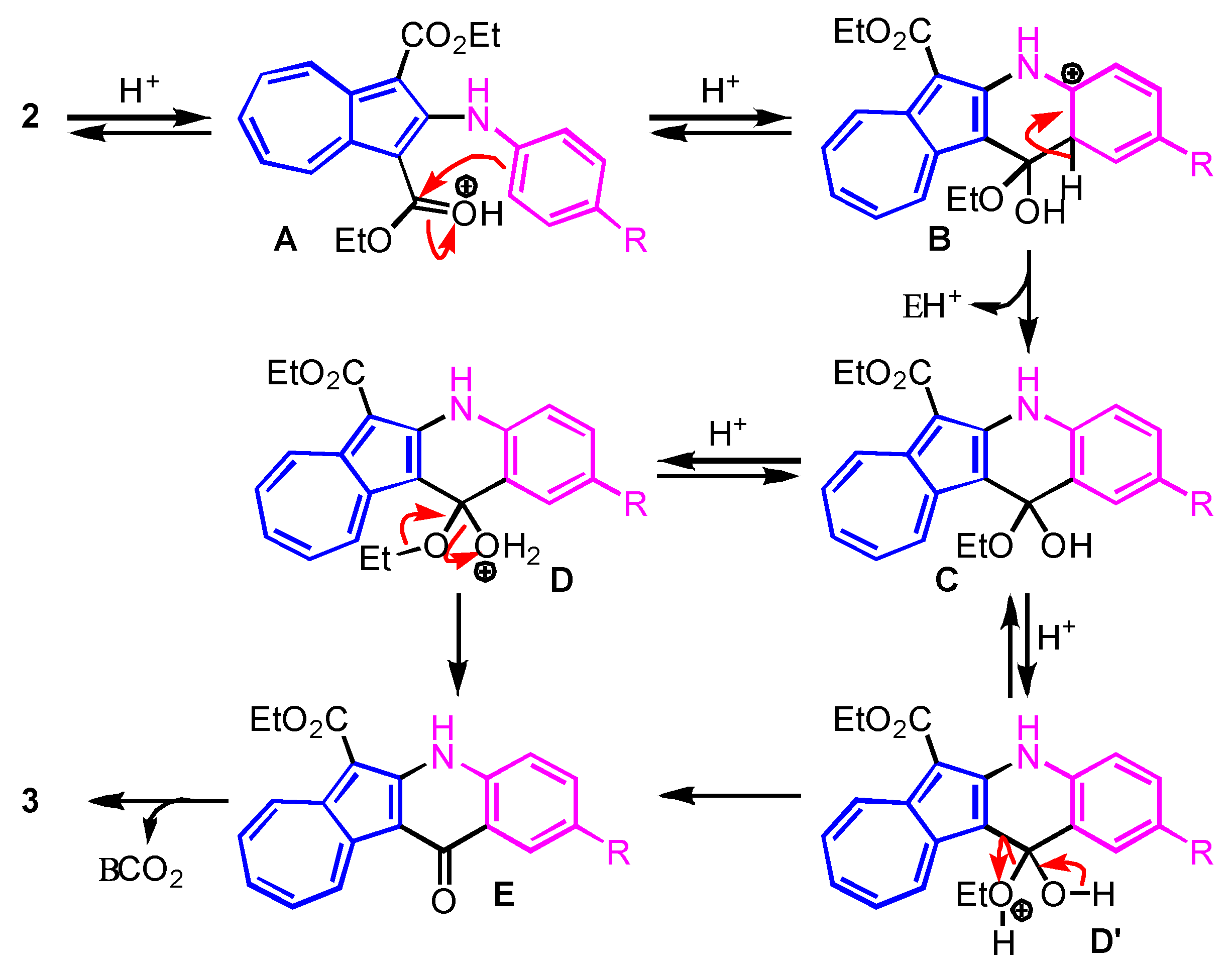
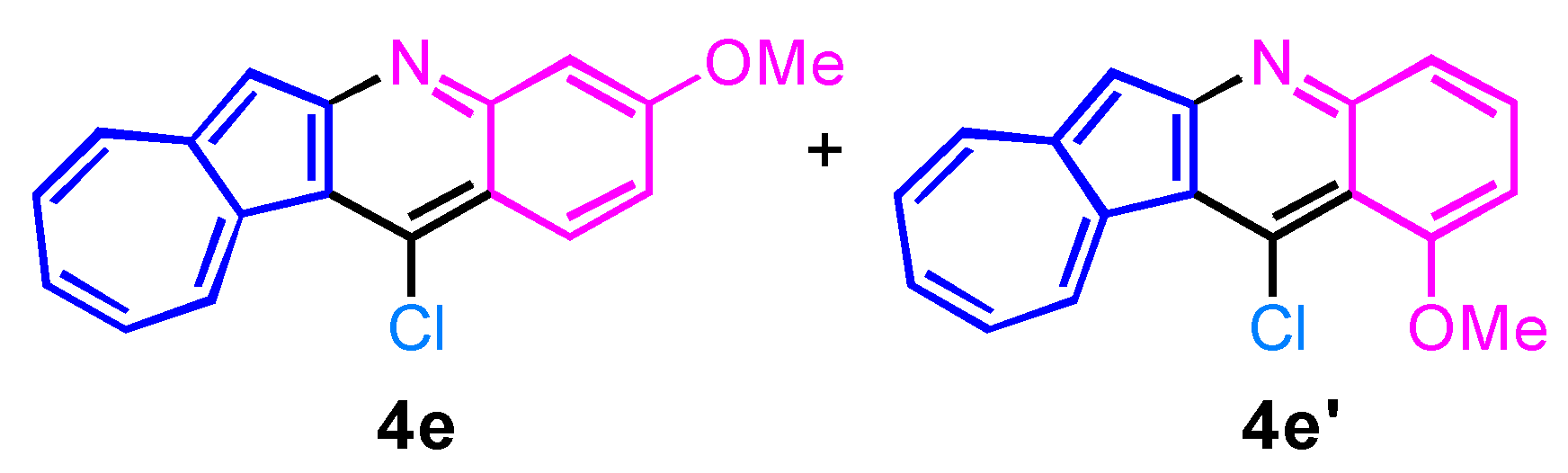
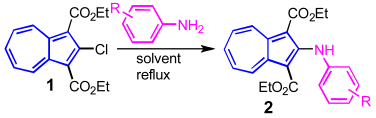
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jones, J.A.; Virga, K.G.; Gumina, G.; Hevener, K.E. Recent Advances in the Rational Design and Optimization of Antibacterial Agents. Med. Chem. Commun. 2016, 7, 1694–1715. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Med. Chem. Commun. 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Derbali, R.M.; Aoun, V.; Moussa, G.; Frei, G.; Tehrani, S.F.; Del’Orto, J.C.; Hildgen, P.; Roullin, V.G.; Chain, J.L. Tailored Nanocarriers for the Pulmonary Delivery of Levofloxacin against Pseudomonas Aeruginosa: A Comparative Study. Mol. Pharm. 2019, 16, 1906–1916. [Google Scholar] [CrossRef]
- Berry, L.; Domalaon, R.; Brizuela, M.; Zhanel, G.G.; Schweizer, F. Polybasic Peptide–Levofloxacin Conjugates Potentiate Fluoroquinolones and Other Classes of Antibiotics against Multidrug-Resistant Gram-Negative Bacteria. Med. Chem. Commun. 2019, 10, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Lee, B.-Y.; Plamthottam, S.; Tullius, M.V.; Wang, R.; Yu, C.-J.; Li, Z.; Dillon, B.J.; Zink, J.I.; Horwitz, M.A. Nanoparticle Formulation of Moxifloxacin and Intramuscular Route of Delivery Improve Antibiotic Pharmacokinetics and Treatment of Pneumonic Tularemia in a Mouse Model. ACS Infect. Dis. 2018, 5, 281–291. [Google Scholar] [CrossRef]
- Taha, M.O.; Bustanji, Y.; Al-Ghussein, M.A.S.; Mohammad, M.; Zalloum, H.; Al-Masri, I.M.; Atallah, N. Pharmacophore Modeling, Quantitative Structure–Activity Relationship Analysis, and in Silico Screening Reveal Potent Glycogen Synthase Kinase-3β Inhibitory Activities for Cimetidine, Hydroxychloroquine, and Gemifloxacin. J. Med. Chem. 2008, 51, 2062–2077. [Google Scholar] [CrossRef]
- Soltani, S.; Akhbari, K. Embedding an Extraordinary Amount of Gemifloxacin Antibiotic in ZIF-8 Framework with One-Step Synthesis and Measurement of Its H2O2-Sensitive Release and Potency against Infectious Bacteria. New J. Chem. 2022, 46, 19432–19441. [Google Scholar] [CrossRef]
- Dahiya, S.; Chuttani, K.; Khar, R.K.; Saluja, D.; Mishra, A.K.; Chopra, M. Synthesis and Evaluation of Ciprofloxacin Derivatives as Diagnostic Tools for Bacterial Infection by Staphylococcus Aureus. Metallomics 2009, 1, 409–417. [Google Scholar] [CrossRef]
- Ji, C.; Miller, P.A.; Miller, M.J. Syntheses and Antibacterial Activity of N-Acylated Ciprofloxacin Derivatives Based on the Trimethyl Lock. ACS Med. Chem. Lett. 2015, 6, 707–710. [Google Scholar] [CrossRef]
- Tremblay, T.; Bergeron, C.; Gagnon, D.; Bérubé, C.; Voyer, N.; Richard, D.; Giguère, D. Squaramide Tethered Clindamycin, Chloroquine, and Mortiamide Hybrids: Design, Synthesis, and Antimalarial Activity. ACS Med. Chem. Lett. 2023, 14, 217–222. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Ma, K.-Y.; Du, S.-S.; Zhang, Z.-J.; Wu, T.-L.; Sun, Y.; Liu, Y.-Q.; Yin, X.-D.; Zhou, R.; Yan, Y.-F.; et al. Antifungal Exploration of Quinoline Derivatives against Phytopathogenic Fungi Inspired by Quinine Alkaloids. J. Agric. Food Chem. 2021, 69, 12156–12170. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.J.; Bom, J.; Capela, R.; Casimiro, C.; Chambel, P.; Gomes, P.; Iley, J.; Lopes, F.; Morais, J.; Moreira, R.; et al. Imidazolidin-4-One Derivatives of Primaquine as Novel Transmission-Blocking Antimalarials. J. Med. Chem. 2005, 48, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Bowles, P.; Busch, F.R.; Leeman, K.R.; Palm, A.S.; Sutherland, K. Confirmation of Bosutinib Structure; Demonstration of Controls to Ensure Product Quality. Org. Process Res. Dev. 2015, 19, 1997–2005. [Google Scholar] [CrossRef]
- Okamoto, K.; Ikemori-Kawada, M.; Jestel, A.; von König, K.; Funahashi, Y.; Matsushima, T.; Tsuruoka, A.; Inoue, A.; Matsui, J. Distinct Binding Mode of Multikinase Inhibitor Lenvatinib Revealed by Biochemical Characterization. ACS Med. Chem. Lett. 2014, 6, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Veliks, J.; Videja, M.; Kinens, A.; Bobrovs, R.; Priede, M.; Kuka, J. Trans-Fluorine Effect in Cyclopropane: Diastereoselective Synthesis of Fluorocyclopropyl Cabozantinib Analogs. ACS Med. Chem. Lett. 2020, 11, 2146–2150. [Google Scholar] [CrossRef]
- Mitscher, L.A. Bacterial Topoisomerase Inhibitors: Quinolone and Pyridone Antibacterial Agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S. The Preparation and Properties of Heteroarylazulenes and Hetero-Fused Azulenes. Adv. Heterocyclic Chem. 2018, 126, 1–54. [Google Scholar]
- Shoji, T.; Sugiyama, S.; Kobayashi, Y.; Yamazaki, A.; Ariga, Y.; Katoh, R.; Wakui, H.; Yasunami, M.; Ito, S. Direct Synthesis of 2-Arylazulenes by [8+2] Cycloaddition of 2H-Cyclohepta[b]Furan-2-Ones with Silyl Enol Ethers. Chem. Commun. 2020, 56, 1485–1488. [Google Scholar] [CrossRef]
- Shoji, T.; Okujima, T.; Ito, S. Development of Heterocycle-Substituted and Fused Azulenes in the Last Decade (2010–2020). Int. J. Mol. Sci. 2020, 21, 7087. [Google Scholar] [CrossRef]
- Shoji, T.; Ito, S.; Yasunami, M. Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]Furan-2-Ones as Starting Materials: Their Reactivity and Properties. Int. J. Mol. Sci. 2021, 22, 10686. [Google Scholar] [CrossRef]
- Xin, H.; Hou, B.; Gao, X. Azulene-Based π-Functional Materials: Design, Synthesis, and Applications. Acc. Chem. Res. 2021, 54, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.R. Direct Access to Functionalized Azulenes and Pseudoazulenes via Unconventional Alkyne Cyclization Reactions. Chem Asian J. 2023, 18, e202300244. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Sakata, N.; Ariga, Y.; Yamazaki, A.; Katoh, R.; Okujima, T.; Sekiguchi, R.; Ito, S. Construction of a 2,2′-Biazulene Framework via Brønsted Acid-Promoted Annulation of 2,3-Di(1-Azulenyl)Benzofurans. Chem. Commun. 2023, 59, 3447–3450. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Kikuchi, K. The Synthesis of Azuleno[2,1-b]Pyridine Derivatives. Bull. Chem. Soc. Jpn. 1963, 36, 633–637. [Google Scholar] [CrossRef]
- Yamane, K.; Fujimori, K.; Takeuchi, T. A Convenient Synthesis of Azuleno[2,1-b]Thiophene. Bull. Chem. Soc. Jpn. 1981, 54, 2537–2538. [Google Scholar] [CrossRef]
- Shoji, T.; Miura, K.; Ohta, A.; Sekiguchi, R.; Ito, S.; Endo, Y.; Nagahata, T.; Mori, S.; Okujima, T. Synthesis of Azuleno[2,1-b]Thiophenes by Cycloaddition of Azulenylalkynes with Elemental Sulfur and Their Structural, Optical and Electrochemical Properties. Org. Chem. Front. 2019, 6, 2801–2811. [Google Scholar] [CrossRef]
- Fujimori, K.; Fukazawa, H.; Nezu, Y.; Yamane, K.; Yasunami, M.; Takase, K. Synthesis of azuleno[1,2-b]pyrrole and azuleno[1,2-b]furan. Chem. Lett. 1986, 15, 1021–1024. [Google Scholar] [CrossRef]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In Vitro and in Vivo Biological Activities of Azulene Derivatives with Potential Applications in Medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef]
- Leino, T.O.; Sieger, P.; Yli-Kauhaluoma, J.; Wallén, E.A.A.; Kley, J.T. The Azulene Scaffold from a Medicinal Chemist’s Perspective: Physicochemical and in Vitro Parameters Relevant for Drug Discovery. Eur. J. Med. Chem. 2022, 237, 114374. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, G. Synthesis, Structure and Properties of Fused π-Extended Acridone Derivatives. Eur. J. Org. Chem. 2020, 2020, 5455–5463. [Google Scholar] [CrossRef]
- Shoji, T.; Sugiyama, S.; Araki, T.; Ohta, A.; Sekiguchi, R.; Ito, S.; Mori, S.; Okujima, T.; Yasunami, M. Synthesis of 2-Amino- and 2-Arylazoazulenes via Nucleophilic Aromatic Substitution of 2-Chloroazulenes with Amines and Arylhydrazines. Org. Biomol. Chem. 2017, 15, 3917–3923. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Sugiyama, S.; Takeuchi, M.; Ohta, A.; Sekiguchi, R.; Ito, S.; Yatsu, T.; Okujima, T.; Yasunami, M. Synthesis of 6-Amino- and 6-Arylazoazulenes via Nucleophilic Aromatic Substitution and Their Reactivity and Properties. J. Org. Chem. 2019, 84, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Seto, S.; Matsumura, S. Synthesis of 2-Substituted Azulenes by Nucleophilic Substitution Reactions of 2-Haloazulene Derivatives. Bull. Chem. Soc. Jpn. 1962, 35, 1990–1998. [Google Scholar] [CrossRef]
- Ito, S.; Kubo, T.; Morita, N.; Ikoma, T.; Tero-Kubota, S.; Kawakami, J.; Tajiri, A. Azulene-Substituted Aromatic Amines. Synthesis and Amphoteric Redox Behavior of N, N-Di (6-azulenyl)-p-toluidine and N, N, N ‘, N ‘-Tetra (6-azulenyl)-p-phenylenediamine and Their Derivatives. J. Org. Chem. 2005, 70, 2285–2293. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Corbet, J.-P.; Mignani, G. Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Anderson, J.M.; Measom, N.D.; Murphy, J.A.; Poole, D.L. Bridge Heteroarylation of Bicyclo[1.1.1]Pentane Derivatives. Org. Lett. 2023, 25, 2053–2057. [Google Scholar] [CrossRef]
- Cirujano, F.G.; López-Maya, E.; Almora-Barrios, N.; Rubio-Gaspar, A.; Martín, N.; Navarro, J.A.R.; Martí-Gastaldo, C. Diffusion Control in Single-Site Zinc Reticular Amination Catalysts. Inorg. Chem. 2020, 59, 18168–18173. [Google Scholar] [CrossRef]
- Kanzian, T.; Nigst, T.A.; Maier, A.; Pichl, S.; Mayr, H. Nucleophilic Reactivities of Primary and Secondary Amines in Acetonitrile. Eur. J. Org. Chem. 2009, 2009, 6379–6385. [Google Scholar] [CrossRef]



 | ||||
|---|---|---|---|---|
| Entry | Aniline Derivative | Solvent | Product | Yield a |
| 1 | aniline | EtOH |  | 2a, 96% |
| 2 | p-toluidine | EtOH | 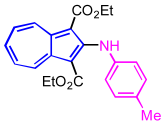 | 2b, 89% |
| 3 | p-n-butylaniline | EtOH | 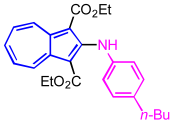 | 2c, 89% |
| 4 | p-methoxyaniline | EtOH |  | 2d, 96% |
| 5 | m-methoxyaniline | EtOH |  | 2e, 92% |
| 6 | p-iodoaniline | EtOH | 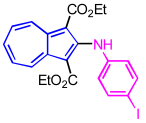 | 2f, 72% |
| 7 | p-nitroaniline | NMP | No reaction | |
| 8 | α-naphthylamine | EtOH |  | 2g, 61% |
| 9 | N-methylaniline | without solvent | 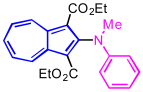 | 2h, 33% |
 | |||
|---|---|---|---|
| Entry | Substrate | Product | Yield a |
| 1 | 2a | 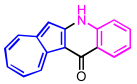 | 3a, 96% |
| 2 | 2b | 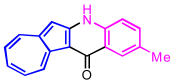 | 3b, 85% |
| 3 | 2c | 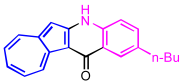 | 3c, 86% |
| 4 | 2d |  | 3d, 85% |
| 5 | 2e | 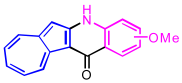 | 3e + 3e′, 93% b |
| 6 | 2f |  | 3f, 89% |
| 7 | 2g |  | 3g, 100% |
 | |||
|---|---|---|---|
| Entry | Substrate | Product | Yield a |
| 1 | 3a |  | 4a, 89% |
| 2 | 3b | 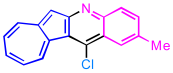 | 4b, 94% |
| 3 | 3c |  | 4c, 93% |
| 4 | 3d |  | 4d, 83% |
| 5 | 3e + 3e′ |  | 4e + 4e′, 72% b |
| 6 | 3f | 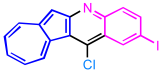 | 4f, 61% |
| 7 | 3g |  | 4g, 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoji, T.; Takeuchi, M.; Uda, M.; Ariga, Y.; Yamazaki, A.; Sekiguchi, R.; Ito, S. Synthesis of Azuleno[2,1-b]quinolones and Quinolines via Brønsted Acid-Catalyzed Cyclization of 2-Arylaminoazulenes. Molecules 2023, 28, 5785. https://doi.org/10.3390/molecules28155785
Shoji T, Takeuchi M, Uda M, Ariga Y, Yamazaki A, Sekiguchi R, Ito S. Synthesis of Azuleno[2,1-b]quinolones and Quinolines via Brønsted Acid-Catalyzed Cyclization of 2-Arylaminoazulenes. Molecules. 2023; 28(15):5785. https://doi.org/10.3390/molecules28155785
Chicago/Turabian StyleShoji, Taku, Mutsumi Takeuchi, Mayumi Uda, Yukino Ariga, Akari Yamazaki, Ryuta Sekiguchi, and Shunji Ito. 2023. "Synthesis of Azuleno[2,1-b]quinolones and Quinolines via Brønsted Acid-Catalyzed Cyclization of 2-Arylaminoazulenes" Molecules 28, no. 15: 5785. https://doi.org/10.3390/molecules28155785
APA StyleShoji, T., Takeuchi, M., Uda, M., Ariga, Y., Yamazaki, A., Sekiguchi, R., & Ito, S. (2023). Synthesis of Azuleno[2,1-b]quinolones and Quinolines via Brønsted Acid-Catalyzed Cyclization of 2-Arylaminoazulenes. Molecules, 28(15), 5785. https://doi.org/10.3390/molecules28155785





