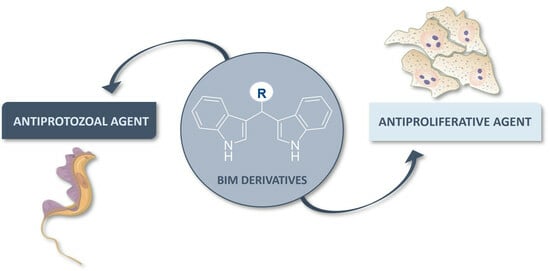
Polyaromatic Bis(indolyl)methane Derivatives with Antiproliferative and Antiparasitic Activity
Abstract
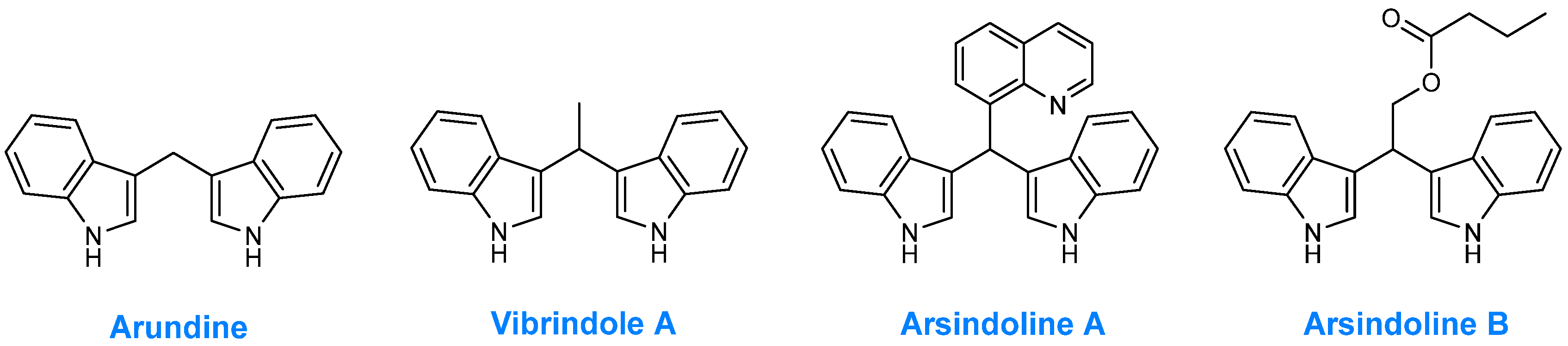
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Bis(indolyl)methane Derivatives
2.2. Biological Phenotypic Activity
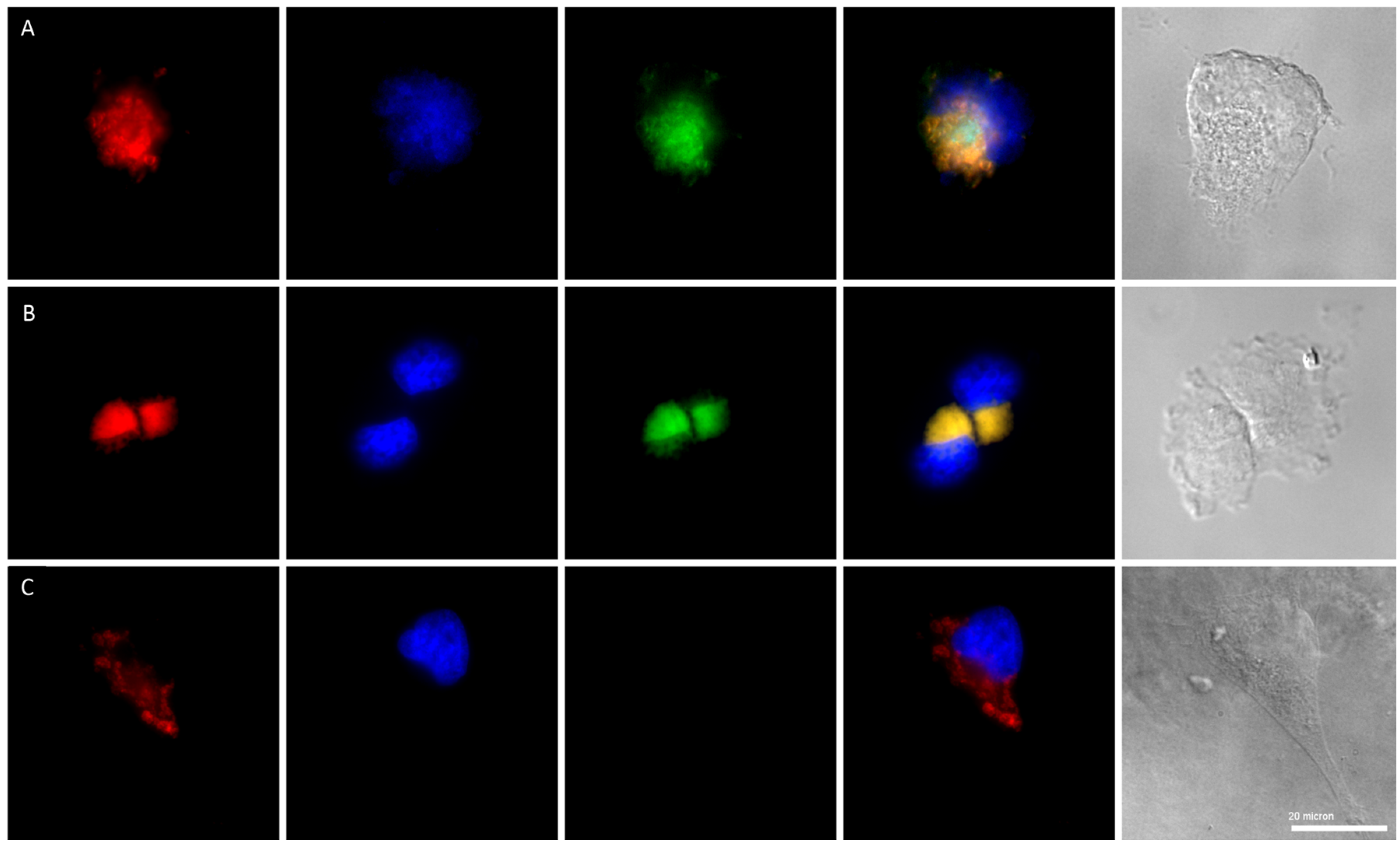
2.3. Fluorescence Microscopy of 2a
3. Experimental Section
3.1. General
3.2. Synthesis of Bis(indolyl)methanes Derivatives 2a–c
3.2.1. Bis(indolyl)methane 2a [39,40]
3.2.2. Bis(indolyl)methane 2b
3.2.3. Bis(indolyl)methane 2c
3.3. Biological Activity
3.3.1. Parasite and Cell Culturing
3.3.2. Anti-Parasitic Activity
3.4. Cytotoxicity
3.5. In Silico Evaluation of the Physicochemical and Pharmacokinetic Properties
3.6. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moafi, M.; Rezvan, H.; Sherkat, R.; Taleban, R. Leishmania Vaccines Entered in Clinical Trials: A Review of Literature. Int. J. Prev. Med. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Malvolti, S.; Malhame, M.; Mantel, C.F.; Le Rutte, E.A.; Kaye, P.M. Human Leishmaniasis Vaccines: Use Cases, Target Population and Potential Global Demand. PLoS Negl. Trop. Dis. 2021, 15, e0009742. [Google Scholar] [CrossRef] [PubMed]
- La Greca, F.; Magez, S. Vaccination against Trypanosomiasis: Can It Be Done or Is the Trypanosome Truly the Ultimate Immune Destroyer and Escape Artist? Hum. Vaccin. 2011, 7, 1225–1233. [Google Scholar] [CrossRef]
- Autheman, D.; Crosnier, C.; Clare, S.; Goulding, D.A.; Brandt, C.; Harcourt, K.; Tolley, C.; Galaway, F.; Khushu, M.; Ong, H.; et al. An Invariant Trypanosoma Vivax Vaccine Antigen Induces Protective Immunity. Nature 2021, 595, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.; Kaiser, M.; Burri, C.; Mäser, P. Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership. Diseases 2022, 10, 90. [Google Scholar] [CrossRef]
- Chavan, K.A.; Shukla, M.; Chauhan, A.N.S.; Maji, S.; Mali, G.; Bhattacharyya, S.; Erande, R.D. Effective Synthesis and Biological Evaluation of Natural and Designed Bis(Indolyl)Methanes via Taurine-Catalyzed Green Approach. ACS Omega 2022, 7, 10438–10446. [Google Scholar] [CrossRef]
- Jella, R.R.; Nagarajan, R. Synthesis of Indole Alkaloids Arsindoline A, Arsindoline B and Their Analogues in Low Melting Mixture. Tetrahedron 2013, 69, 10249–10253. [Google Scholar] [CrossRef]
- Praveen, P.; Parameswaran, P.; Majik, M. Bis(Indolyl)Methane Alkaloids: Isolation, Bioactivity, and Syntheses. Synthesis 2015, 47, 1827–1837. [Google Scholar] [CrossRef]
- Marrelli, M.; Cachet, X.; Conforti, F.; Sirianni, R.; Chimento, A.; Pezzi, V.; Michel, S.; Statti, G.A.; Menichini, F. Synthesis of a New Bis(Indolyl)Methane That Inhibits Growth and Induces Apoptosis in Human Prostate Cancer Cells. Nat. Prod. Res. 2013, 27, 2039–2045. [Google Scholar] [CrossRef]
- Tian, X.; Liu, K.; Zu, X.; Ma, F.; Li, Z.; Lee, M.; Chen, H.; Li, Y.; Zhao, Y.; Liu, F.; et al. 3,3’-Diindolylmethane Inhibits Patient-Derived Xenograft Colon Tumor Growth by Targeting COX1/2 and ERK1/2. Cancer Lett. 2019, 448, 20–30. [Google Scholar] [CrossRef]
- Lee, J. 3,3′-Diindolylmethane Inhibits TNF-α- and TGF-β-Induced Epithelial–Mesenchymal Transition in Breast Cancer Cells. Nutr. Cancer 2019, 71, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Singh, A.; Kumar, P.; Dhasmana, D.; Krishnan, V.; Garg, N. Bisindolemethane Derivatives as Highly Potent Anticancer Agents: Synthesis, Medicinal Activity Evaluation, Cell-Based Compound Discovery, and Computational Target Predictions. Comput. Biol. Med. 2020, 116, 103574. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Taha, M.; Ismail, N.; Khan, K.; Naz, F.; Hussain, M.; Tauseef, S. Synthesis of Novel Bisindolylmethane Schiff Bases and Their Antibacterial Activity. Molecules 2014, 19, 11722–11740. [Google Scholar] [CrossRef]
- Roy, S.; Gajbhiye, R.; Mandal, M.; Pal, C.; Meyyapan, A.; Mukherjee, J.; Jaisankar, P. Synthesis and Antibacterial Evaluation of 3,3′-Diindolylmethane Derivatives. Med. Chem. Res. 2014, 23, 1371–1377. [Google Scholar] [CrossRef]
- Fan, S.; Meng, Q.; Saha, T.; Sarkar, F.H.; Rosen, E.M. Low Concentrations of Diindolylmethane, a Metabolite of Indole-3-Carbinol, Protect against Oxidative Stress in a BRCA1-Dependent Manner. Cancer Res. 2009, 69, 6083–6091. [Google Scholar] [CrossRef]
- Benabadji, S.H.; Wen, R.; Zheng, J.; Dong, X.; Yuan, S. Anticarcinogenic and Antioxidant Activity of Diindolylmethane Derivatives. Acta Pharmacol. Sin. 2004, 25, 666–671. [Google Scholar]
- De Miranda, B.R.; Miller, J.A.; Hansen, R.J.; Lunghofer, P.J.; Safe, S.; Gustafson, D.L.; Colagiovanni, D.; Tjalkens, R.B. Neuroprotective Efficacy and Pharmacokinetic Behavior of Novel Anti-Inflammatory Para -Phenyl Substituted Diindolylmethanes in a Mouse Model of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2013, 345, 125–138. [Google Scholar] [CrossRef]
- Sujatha, K.; Perumal, P.T.; Muralidharan, D.; Rajendran, M. Synthesis, Analgesic and Antiinflammatory Activities of Bis(Indolyl)Methanes. ChemInform 2009, 40, 267–272. [Google Scholar] [CrossRef]
- Sivaprasad, G.; Perumal, P.T.; Prabavathy, V.R.; Mathivanan, N. Synthesis and Anti-Microbial Activity of Pyrazolylbisindoles—Promising Anti-Fungal Compounds. Bioorganic Med. Chem. Lett. 2006, 16, 6302–6305. [Google Scholar] [CrossRef]
- Kang, J.; Gao, Y.; Zhang, M.; Ding, X.; Wang, Z.; Ma, D.; Wang, Q. Streptindole and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 7839–7849. [Google Scholar] [CrossRef]
- Imran, S.; Taha, M.; Ismail, N. A Review of Bisindolylmethane as an Important Scaffold for Drug Discovery. Curr. Med. Chem. 2015, 22, 4412–4433. [Google Scholar] [CrossRef] [PubMed]
- Koli, P.; Reena; Indurthi, H.K.; Sharma, D.K. Anticancer Activity of 3,3′-Diindolylmethane and the Molecular Mechanism Involved in Various Cancer Cell Lines. ChemistrySelect 2020, 5, 11540–11548. [Google Scholar] [CrossRef]
- Biersack, B. 3,3’-Diindolylmethane and Its Derivatives: Nature-Inspired Strategies Tackling Drug Resistant Tumors by Regulation of Signal Transduction, Transcription Factors and microRNAs. Cancer Drug Resist. 2020, 3, 867–878. [Google Scholar] [CrossRef]
- Choi, H.J.; Lim, D.Y.; Park, J.H.Y. Induction of G1 and G2/M Cell Cycle Arrests by the Dietary Compound 3,3’-Diindolylmethane in HT-29 Human Colon Cancer Cells. BMC Gastroenterol. 2009, 9, 39. [Google Scholar] [CrossRef]
- Kim, S. 3,3’-Diindolylmethane Suppresses Growth of Human Esophageal Squamous Cancer Cells by G1 Cell Cycle Arrest. Oncol. Rep. 2012, 27, 1669. [Google Scholar] [CrossRef]
- Chang, X. 3,3’-Diindolylmethane Inhibits Angiogenesis and the Growth of Transplantable Human Breast Carcinoma in Athymic Mice. Carcinogenesis 2005, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Munakarmi, S.; Shrestha, J.; Shin, H.-B.; Lee, G.-H.; Jeong, Y.-J. 3,3′-Diindolylmethane Suppresses the Growth of Hepatocellular Carcinoma by Regulating Its Invasion, Migration, and ER Stress-Mediated Mitochondrial Apoptosis. Cells 2021, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Uddin, I.; Gollapalli, M.; Almandil, N.B.; Rahim, F.; Farooq, R.K.; Nawaz, M.; Ibrahim, M.; Alqahtani, M.A.; Bamarouf, Y.A.; et al. Synthesis, Anti-Leishmanial and Molecular Docking Study of Bis-Indole Derivatives. BMC Chem. 2019, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Bharate, J.B.; Khan, S.I.; Tekwani, B.L.; Jacob, M.R.; Mudududdla, R.; Yadav, R.R.; Singh, B.; Sharma, P.R.; Maity, S.; et al. Discovery of 3,3′-Diindolylmethanes as Potent Antileishmanial Agents. Eur. J. Med. Chem. 2013, 63, 435–443. [Google Scholar] [CrossRef]
- Roy, A.; Ganguly, A.; BoseDasgupta, S.; Das, B.B.; Pal, C.; Jaisankar, P.; Majumder, H.K. Mitochondria-Dependent Reactive Oxygen Species-Mediated Programmed Cell Death Induced by 3,3′-Diindolylmethane through Inhibition of F0F1-ATP Synthase in Unicellular Protozoan Parasite Leishmania donovani. Mol. Pharmacol. 2008, 74, 1292–1307. [Google Scholar] [CrossRef]
- Roy, A.; Das, B.B.; Ganguly, A.; Bose Dasgupta, S.; Khalkho, N.V.M.; Pal, C.; Dey, S.; Giri, V.S.; Jaisankar, P.; Dey, S.; et al. An Insight into the Mechanism of Inhibition of Unusual Bi-Subunit Topoisomerase I from Leishmania Donovani by 3,3′-Di-Indolylmethane, a Novel DNA Topoisomerase I Poison with a Strong Binding Affinity to the Enzyme. Biochem. J. 2008, 409, 611–622. [Google Scholar] [CrossRef]
- Roy, A.; Chowdhury, S.; Sengupta, S.; Mandal, M.; Jaisankar, P.; D’Annessa, I.; Desideri, A.; Majumder, H.K. Development of Derivatives of 3, 3′-Diindolylmethane as Potent Leishmania Donovani Bi-Subunit Topoisomerase IB Poisons. PLoS ONE 2011, 6, e28493. [Google Scholar] [CrossRef]
- Zeligs, M.A. Anti-Parasitic Methods and Compositions Utilizing Diindolylmethane-Related Indoles. US20100055201A1, 19 November 2013. [Google Scholar]
- Dorosti, Z.; Yousefi, M.; Sharafi, S.M.; Darani, H.Y. Mutual Action of Anticancer and Antiparasitic Drugs: Are There Any Shared Targets? Future Oncol. 2014, 10, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Zheng, Z.; Liu, Q.-X.; Lu, X.; Zhou, D.; Zhang, J.; Zheng, H.; Dai, J.-G. Repositioning of Antiparasitic Drugs for Tumor Treatment. Front. Oncol. 2021, 11, 670804. [Google Scholar] [CrossRef] [PubMed]
- Belmonte-Reche, E.; Benassi, A.; Peñalver, P.; Cucchiarini, A.; Guédin, A.; Mergny, J.L.; Rosu, F.; Gabelica, V.; Freccero, M.; Doria, F.; et al. Thiosugar Naphthalene Diimide Conjugates: G-Quadruplex Ligands with Antiparasitic and Anticancer Activity. Eur. J. Med. Chem. 2022, 232, 114183. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, M.P.; Peñalver, P.; Gibson, R.S.L.; Morales, J.C.; Galan, M.C. Stiff-Stilbene Ligands Target G-Quadruplex DNA and Exhibit Selective Anticancer and Antiparasitic Activity. Chem. Eur. J. 2020, 26, 6224–6233. [Google Scholar] [CrossRef]
- Nagarajan, R.; Perumal, P.T. Potassium Hydrogen Sulfate-Catalyzed Reactions of Indoles: A Mild, Expedient Synthesis of Bis-Indolylmethanes. Chem. Lett. 2004, 33, 288–289. [Google Scholar] [CrossRef]
- Xu, J.; Shang, W.; Zhu, J.; Cheng, Z.; Zhou, N.; Zhu, X. Synthesis and Characterization of Triphenylamine and Bis(Indolyl)Methane Center-Functionalized Polymer via Reversible Addition-Fragmentation Chain Transfer Polymerization. e-Polymers 2008, 8, 024. [Google Scholar] [CrossRef][Green Version]
- Zarandi, M.; Salimi Beni, A. Synthesis and DFT Calculation on Novel Derivatives of Bis-(Indolyl) Methanes. J. Mol. Struct. 2016, 1119, 404–412. [Google Scholar] [CrossRef]
- Ahmad, A.; Dandawate, P.; Schruefer, S.; Padhye, S.; Sarkar, F.H.; Schobert, R.; Biersack, B. Pentafluorophenyl Substitution of Natural Di(Indol-3-yl)Methane Strongly Enhances Growth Inhibition and Apoptosis Induction in Various Cancer Cell Lines. Chem. Biodivers. 2019, 16, e1900028. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-11538-7. [Google Scholar]
- Wirtz, E.; Leal, S.; Ochatt, C.; Cross, G.A.M. A Tightly Regulated Inducible Expression System for Conditional Gene Knock-Outs and Dominant-Negative Genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 1999, 99, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Donayre, M.; Malagarie-Cazenave, S.; Campos-Salinas, J.; Gálvez, F.J.; Rodríguez-Martínez, A.; Pineda-Molina, E.; Orrego, L.M.; Martínez-García, M.; Sánchez-Cañete, M.P.; Estévez, A.M.; et al. Trypanosomatid Parasites Rescue Heme from Endocytosed Hemoglobin through Lysosomal HRG Transporters: Trypanosomatid HRG Proteins Rescue Heme from Hb. Mol. Microbiol. 2016, 101, 895–908. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Martínez-García, M.; Guédin, A.; Zuffo, M.; Arévalo-Ruiz, M.; Doria, F.; Campos-Salinas, J.; Maynadier, M.; López-Rubio, J.J.; Freccero, M.; et al. G-Quadruplex Identification in the Genome of Protozoan Parasites Points to Naphthalene Diimide Ligands as New Antiparasitic Agents. J. Med. Chem. 2018, 61, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.M.; Doughman, D.J.; Gregerson, D.S.; Obritsch, W.F. A New, Simple, Nonradioactive, Nontoxic in Vitro Assay to Monitor Corneal Endothelial Cell Viability. Invest. Ophtalmol. Vis. Sci. 1997, 38, 1929–1933. [Google Scholar]
- Pérez-Victoria, J.M.; Bavchvarov, B.I.; Torrecillas, I.R.; Martínez-García, M.; López-Martín, C.; Campillo, M.; Castanys, S.; Gamarro, F. Sitamaquine Overcomes ABC-Mediated Resistance to Miltefosine and Antimony in Leishmania. Antimicrob. Agents Chemother. 2011, 55, 3838–3844. [Google Scholar] [CrossRef] [PubMed]
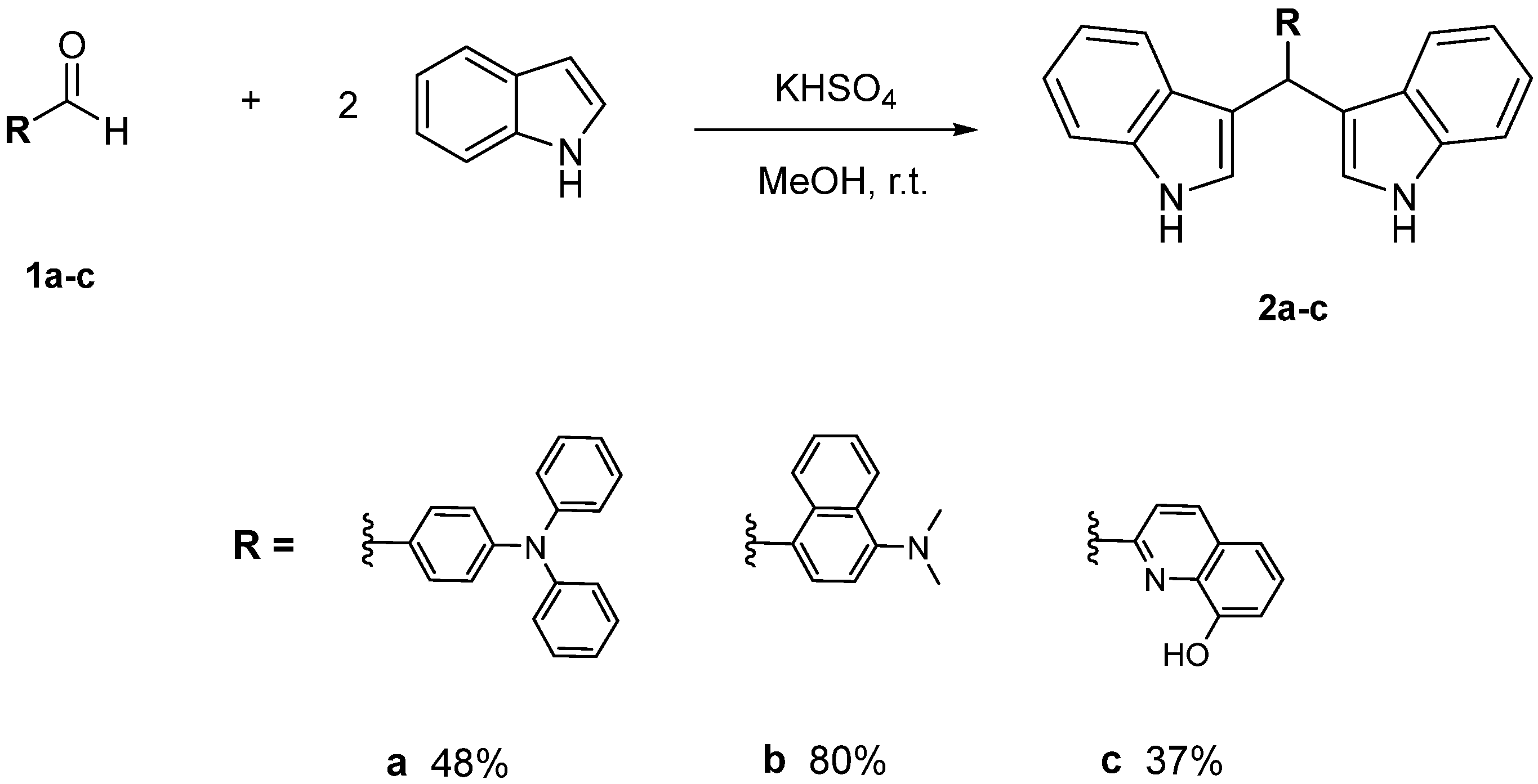

| Compound | Yield (%) | λabs (nm) | λfluo (nm) | ΔSS(cm−1) | ϕF |
|---|---|---|---|---|---|
| 2a | 48 | 292 | 372 | 7365 | 0.28 |
| 2b | 80 | 218 | - | - | - |
| 2c | 37 | 241 | 483 | 20790 | 0.01 |
| Compound | IC50 μM | Selectivity Index (SI) * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T. brucei | L. major | MRC-5 | HeLa | HT-29 | T. brucei | L. major | HeLa | HT-29 | |
| 2a | 3.21 ± 0.04 | 3.30 ± 0.25 | 33.69 ± 3.19 | 37.53 ± 1.86 | 3.93 ± 0.49 | 10.50 | 10.21 | 0.90 | 8.57 |
| 2b | 15.99 ± 4.44 | >100 | 94.86 ± 7.27 | 89.56 ± 0.15 | 25.44 ± 5.23 | 5.93 | <0.95 | 1.06 | 3.73 |
| 2c | 14.66 ± 0.05 | 20.67 ± 1.71 | 3.25 ± 0.29 | 28.27 ± 5.01 | 71.36 ± 3.99 | 0.22 | 0.16 | 0.11 | 0.05 |
| Properties | Compound 2a | Compound 2b | Compound 2c | Arundine | |
|---|---|---|---|---|---|
| Physicochemical | MW | 489.22 | 415.2 | 389.15 | 246.12 |
| logP | 7.363 | 6.249 | 5.281 | 4.185 | |
| TPSA (Å2) | 34.82 | 34.82 | 64.7 | 31.58 | |
| nHA | 3 | 3 | 4 | 2 | |
| nHD | 2 | 2 | 3 | 2 | |
| nRot | 6 | 4 | 3 | 2 | |
| Medicinal chemistry | Lipinski | Accepted | Accepted | Accepted | Accepted |
| PAINS | 0 alerts | 0 alerts | 0 alerts | 0 alerts | |
| Absorption | Caco-2 Permeability (log cm/s) | −5.14 (Yes) | −5.14 (Yes) | −5.323 (No) | −4.852 (Yes) |
| MDCK Permeability (cm/s) | 4 × 10−6 (Yes) | 7 × 10−6 (Yes) | 5 × 10−6 (Yes) | 9 × 10−6 (Yes) | |
| Distribution | VD (L/Kg) | 3.16 (Yes) | 3.068 (Yes) | 1.843 (Yes) | 1.857 (Yes) |
| Metabolism | CYP2C9 Substrate * | 0.94 (Yes) | 0.96 (Yes) | 0.96 (Yes) | 0.97 (Yes) |
| Excretion | CL (mL/min/kg) | 4.598 | 5.843 | 4.475 | 7.19 |
| Toxicity | AMES Toxicity * | 0.053 (Low) | 0.984 (High) | 0.683 (Medium) | 0.609 (Medium) |
| Carcinogenicity * | 0.159 (Low) | 0.092 (Low) | 0.053 (Low) | 0.044 (Low) | |
| Rat Acute Oral Toxicity * | 0.086(Low) | 0.355 (Medium) | 0.2 (Low) | 0.202 (Low) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.C.R.; Peñalver, P.; Costa, S.P.G.; Morales, J.C.; Raposo, M.M.M. Polyaromatic Bis(indolyl)methane Derivatives with Antiproliferative and Antiparasitic Activity. Molecules 2023, 28, 7728. https://doi.org/10.3390/molecules28237728
Gonçalves RCR, Peñalver P, Costa SPG, Morales JC, Raposo MMM. Polyaromatic Bis(indolyl)methane Derivatives with Antiproliferative and Antiparasitic Activity. Molecules. 2023; 28(23):7728. https://doi.org/10.3390/molecules28237728
Chicago/Turabian StyleGonçalves, Raquel C. R., Pablo Peñalver, Susana P. G. Costa, Juan C. Morales, and Maria Manuela M. Raposo. 2023. "Polyaromatic Bis(indolyl)methane Derivatives with Antiproliferative and Antiparasitic Activity" Molecules 28, no. 23: 7728. https://doi.org/10.3390/molecules28237728
APA StyleGonçalves, R. C. R., Peñalver, P., Costa, S. P. G., Morales, J. C., & Raposo, M. M. M. (2023). Polyaromatic Bis(indolyl)methane Derivatives with Antiproliferative and Antiparasitic Activity. Molecules, 28(23), 7728. https://doi.org/10.3390/molecules28237728