Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects
Abstract
1. Introduction
2. Synthesis of Different Ferrocene-Based Compounds
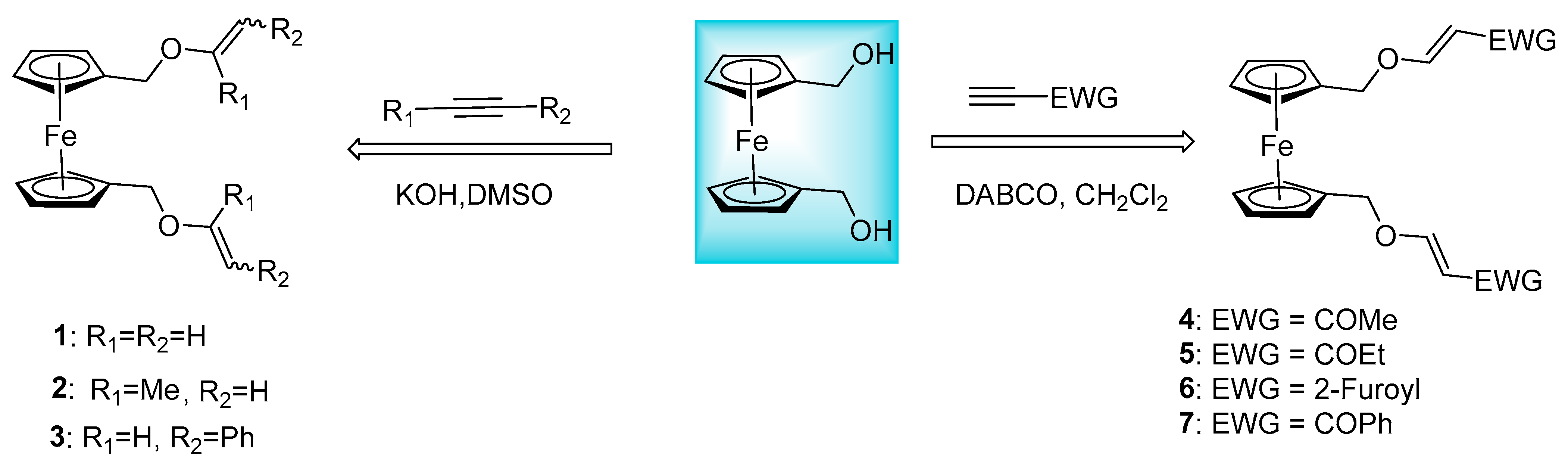
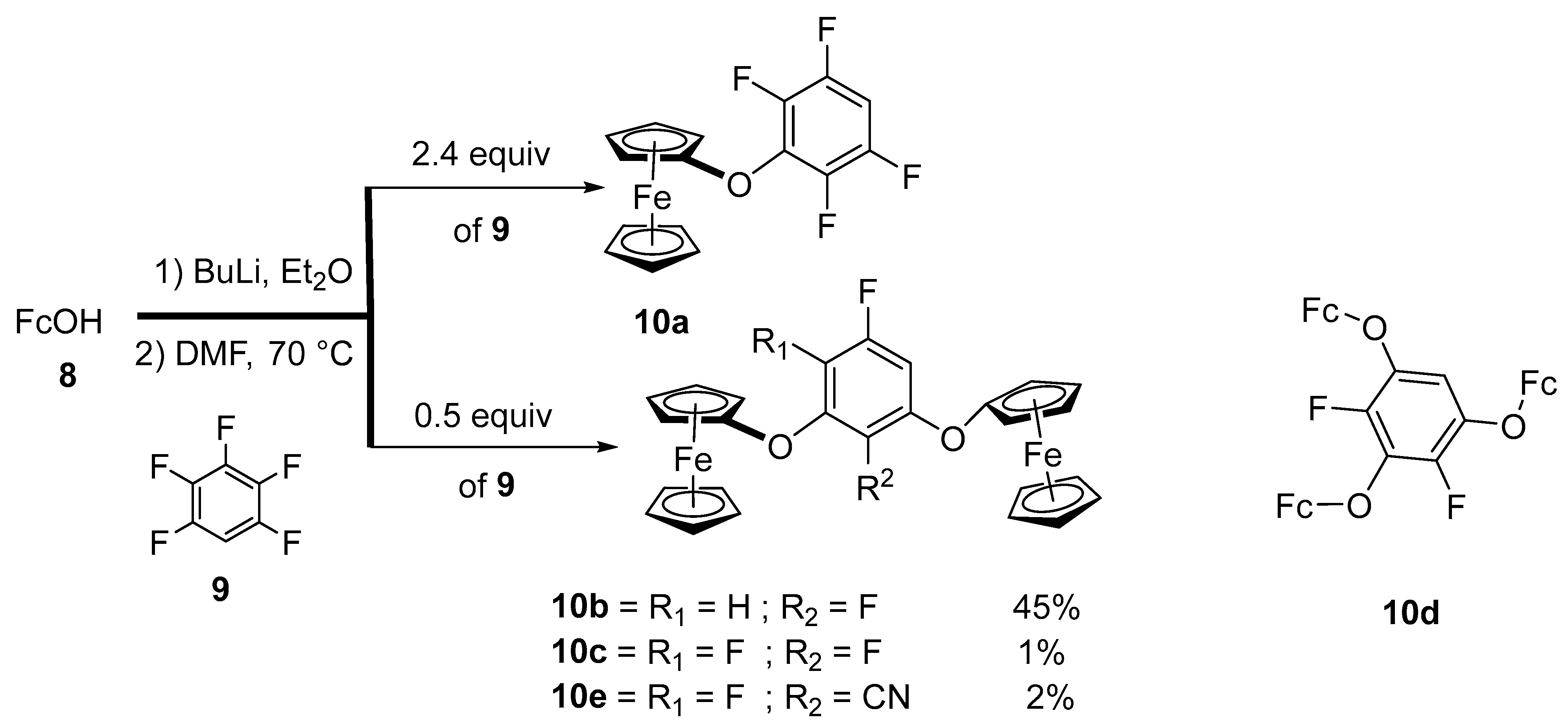
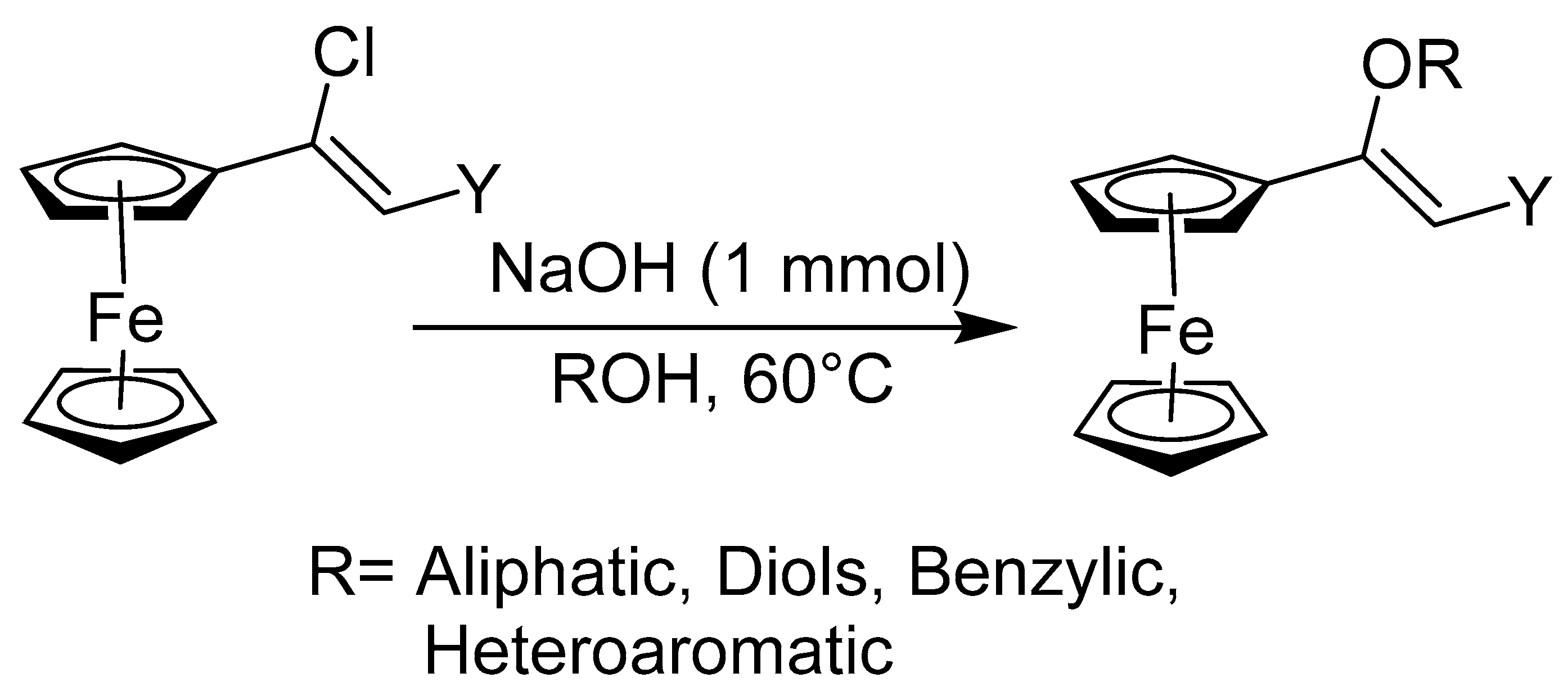
2.1. Synthesis of Ferrocenyl Ethers
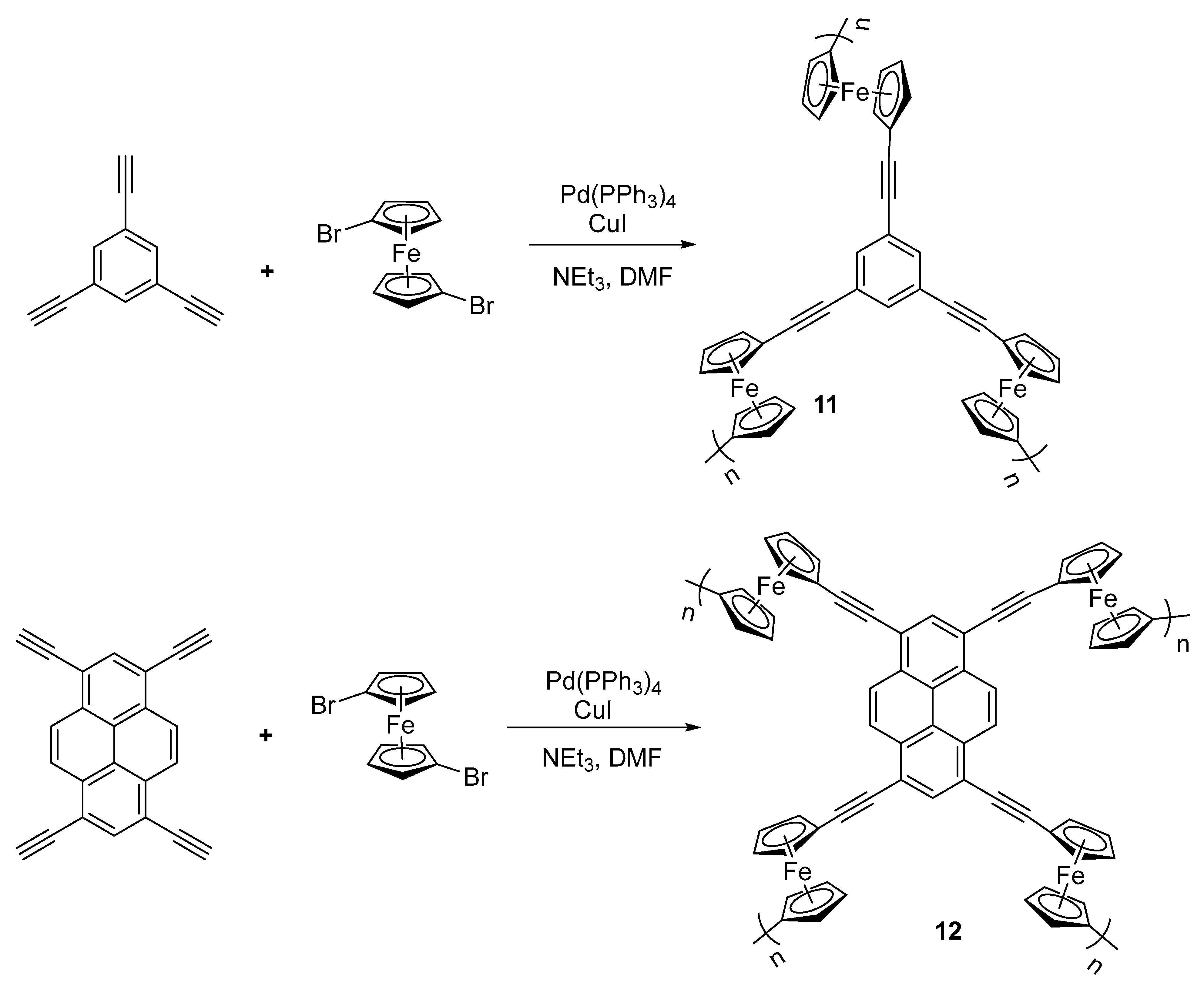
2.2. Synthesis of Alkynylated Ferrocenes
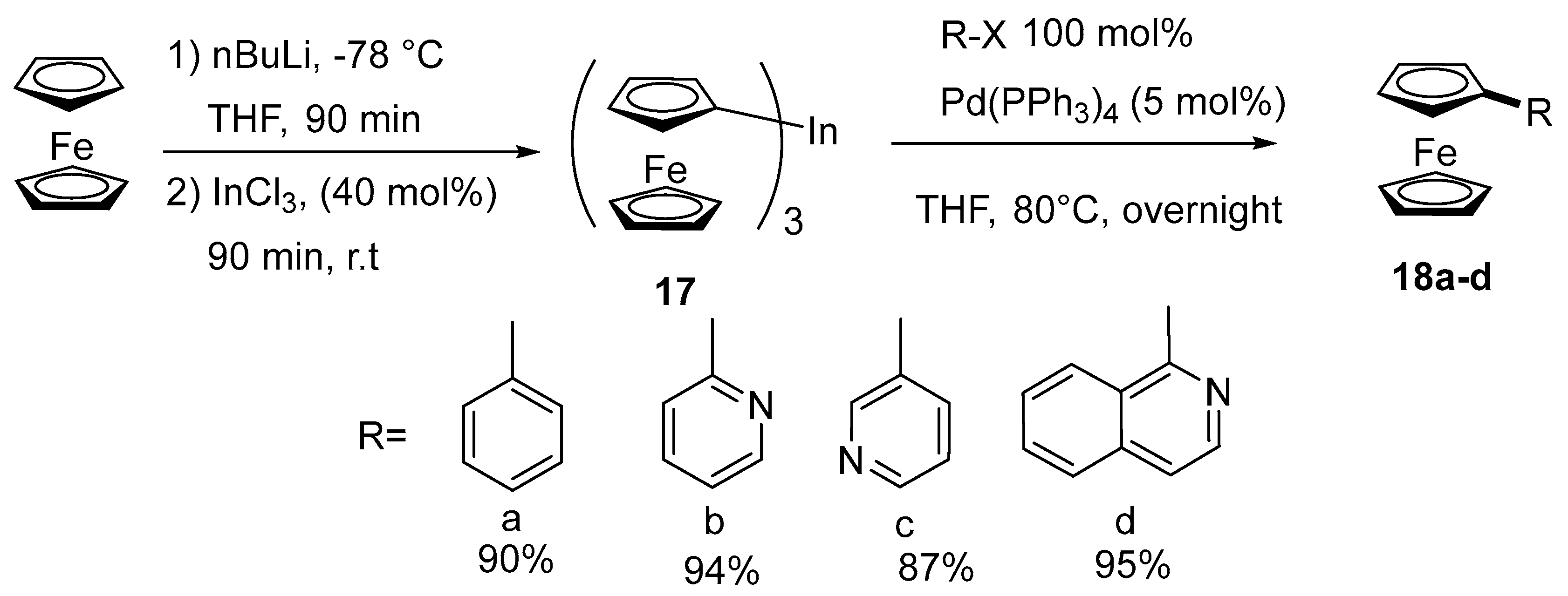
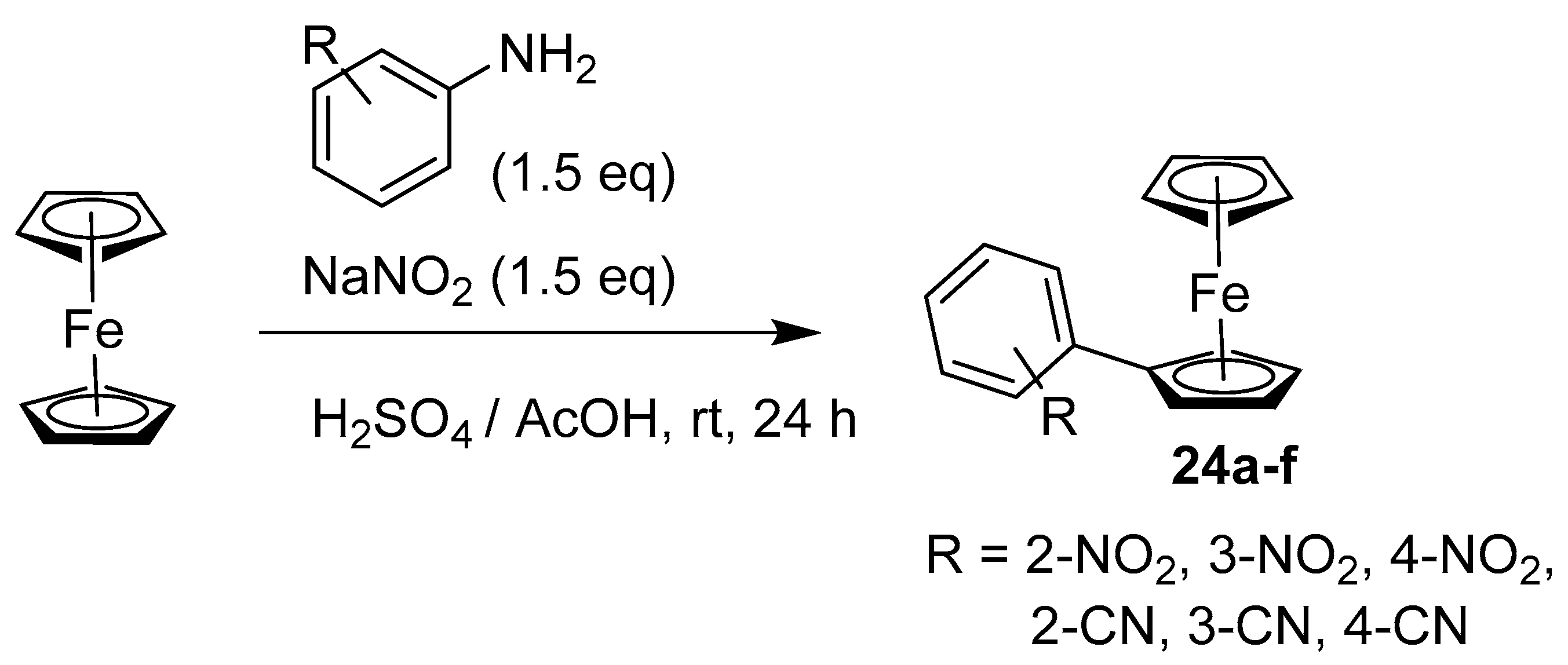
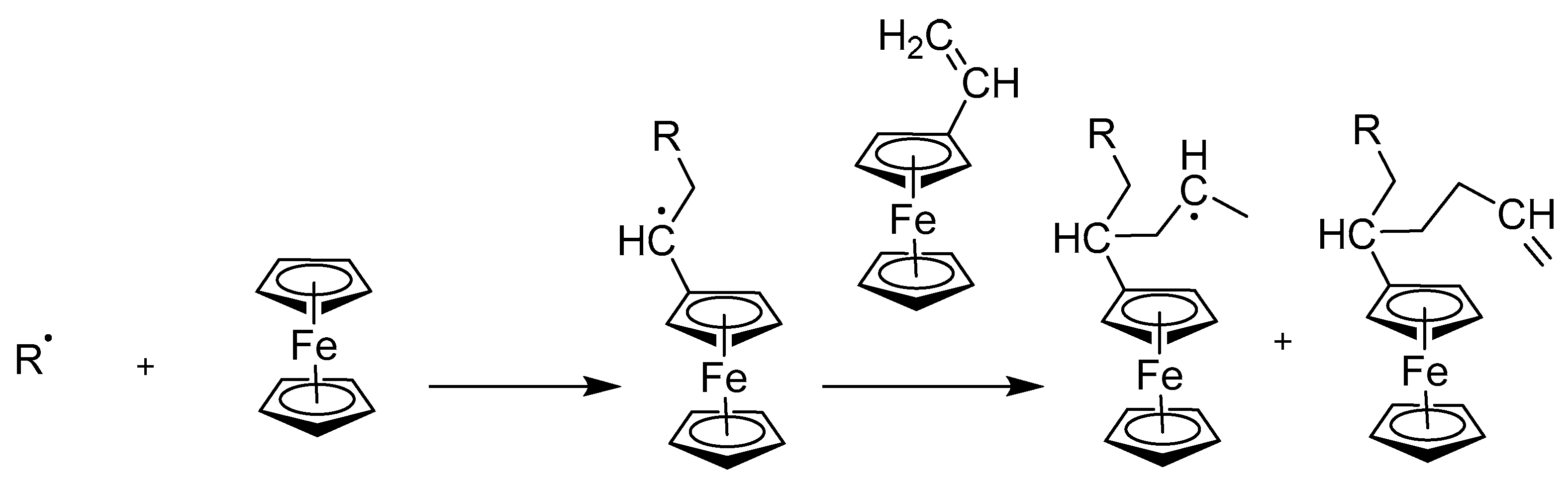
2.3. Synthesis of Vinyl and Aryl-Substituted Ferrocene
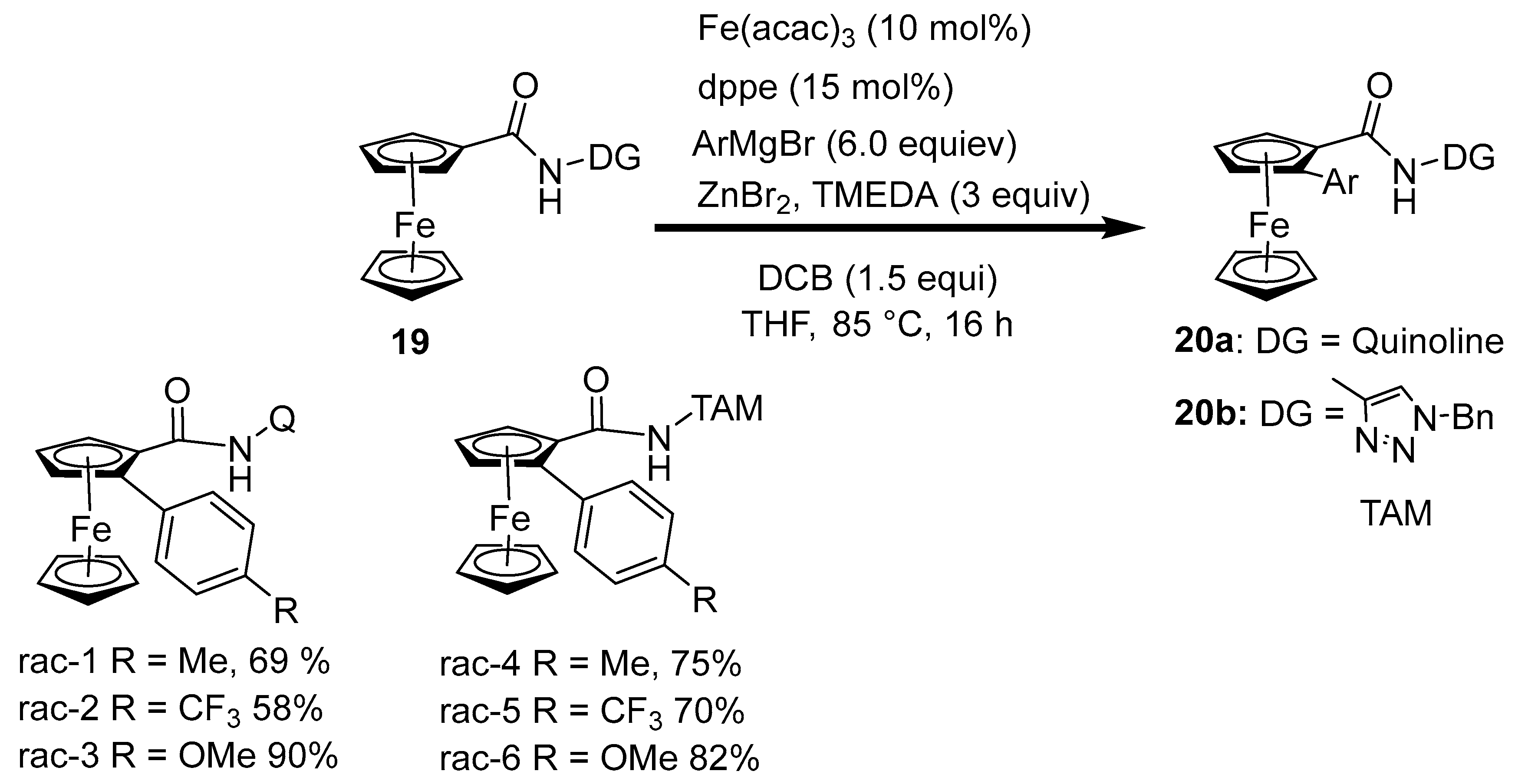
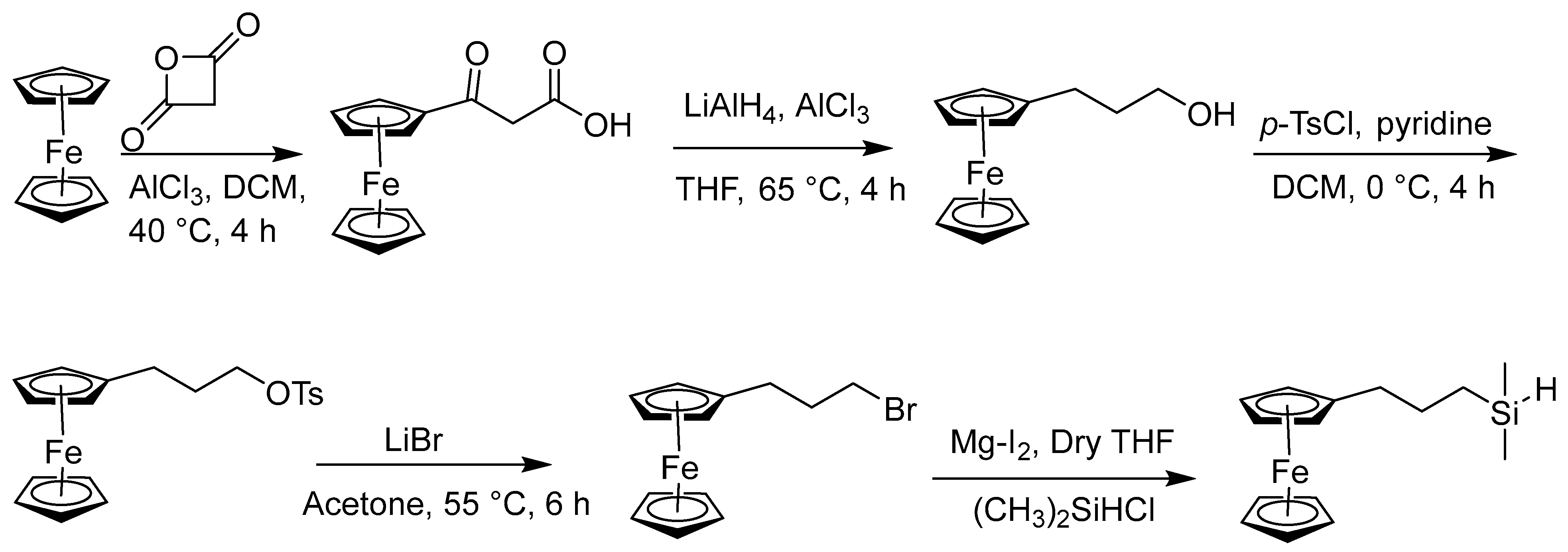
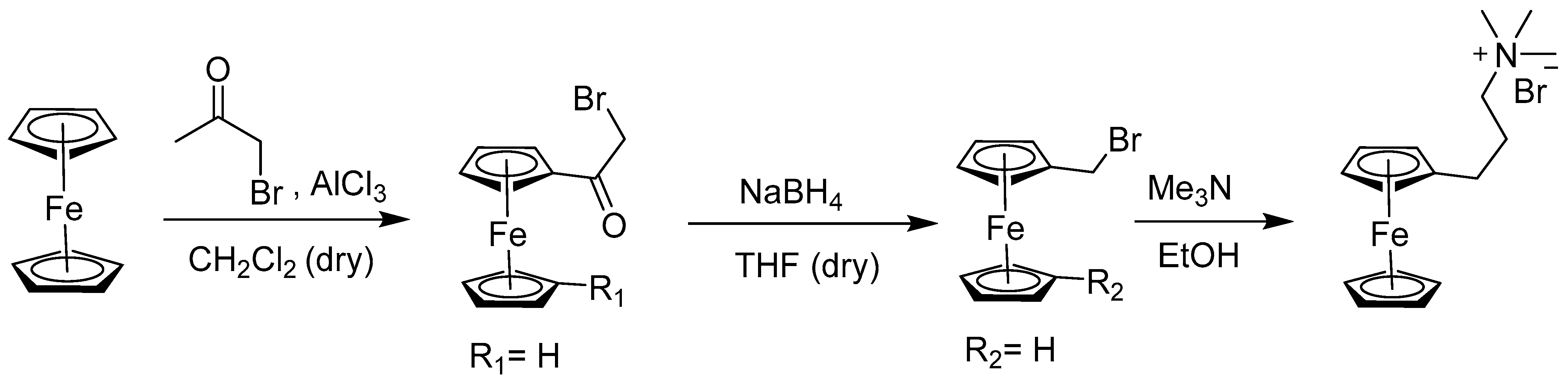
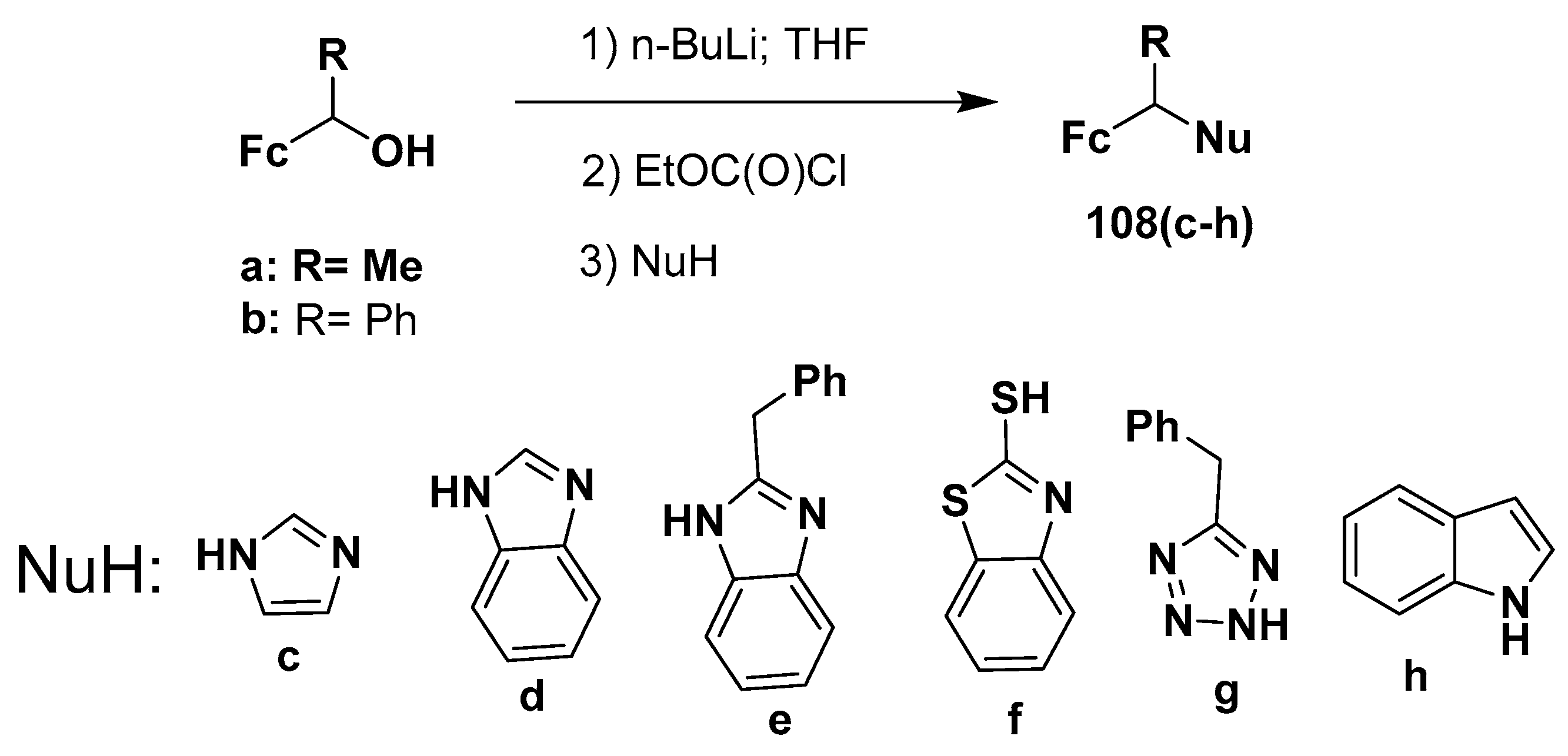
2.4. Synthesis of Alkyl and N-Heteroatom-Substituted Ferrocene Derivatives
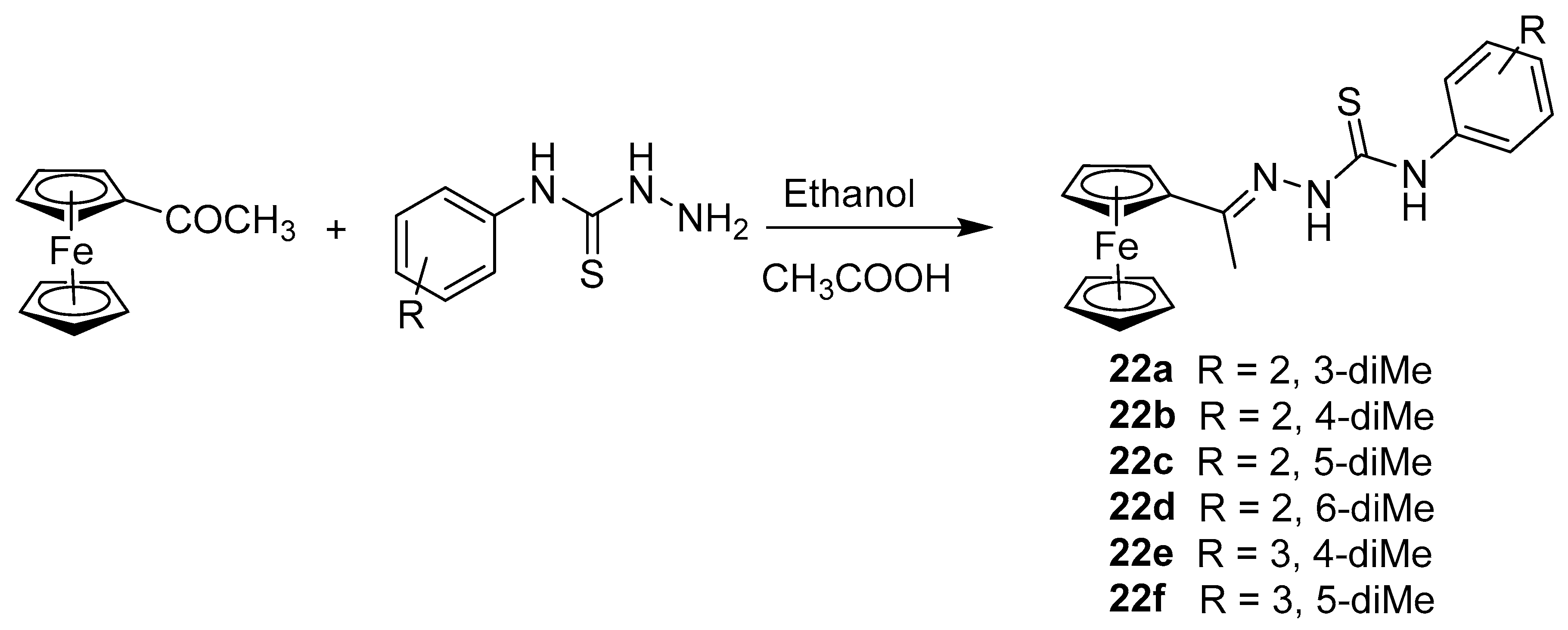
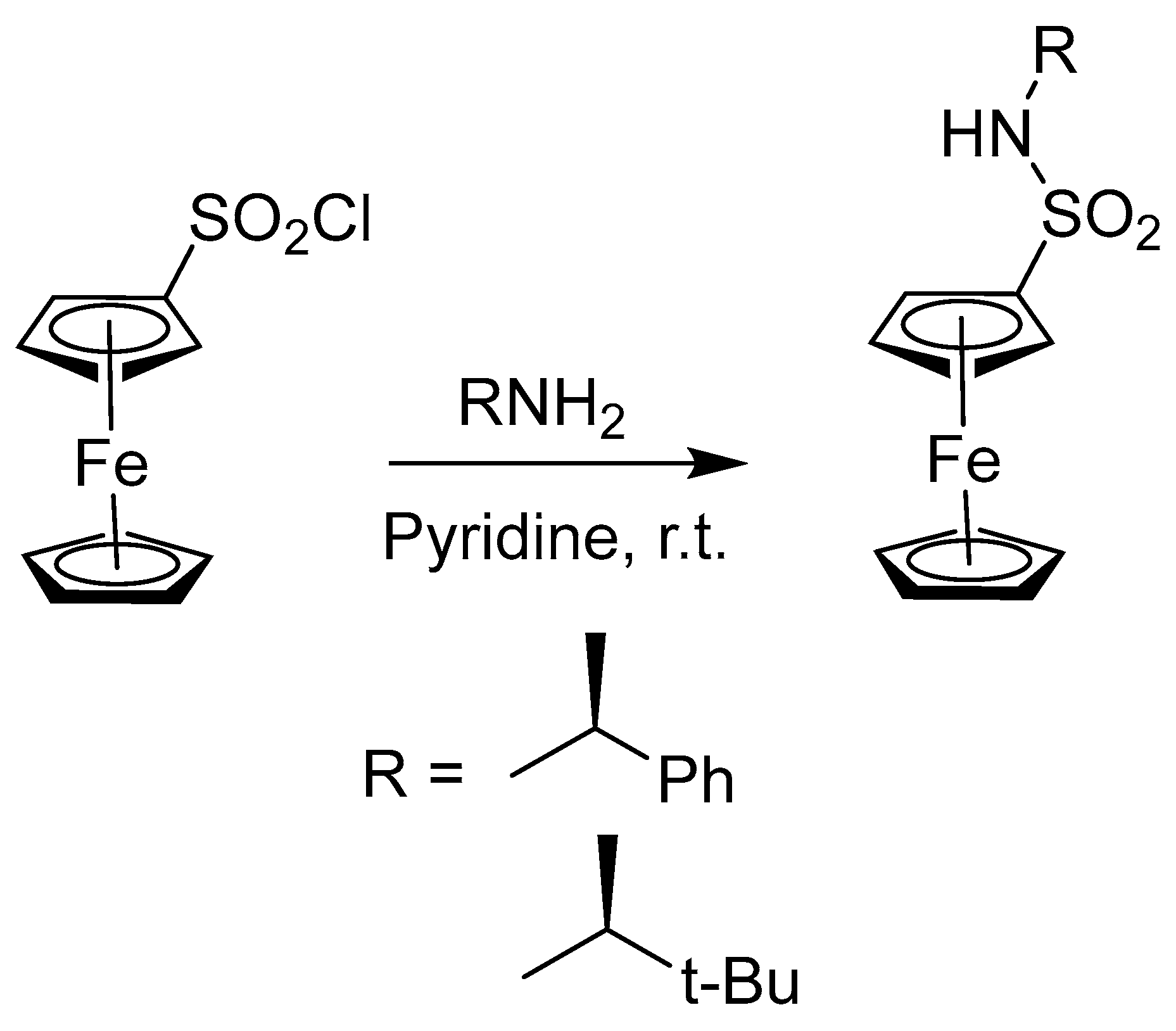
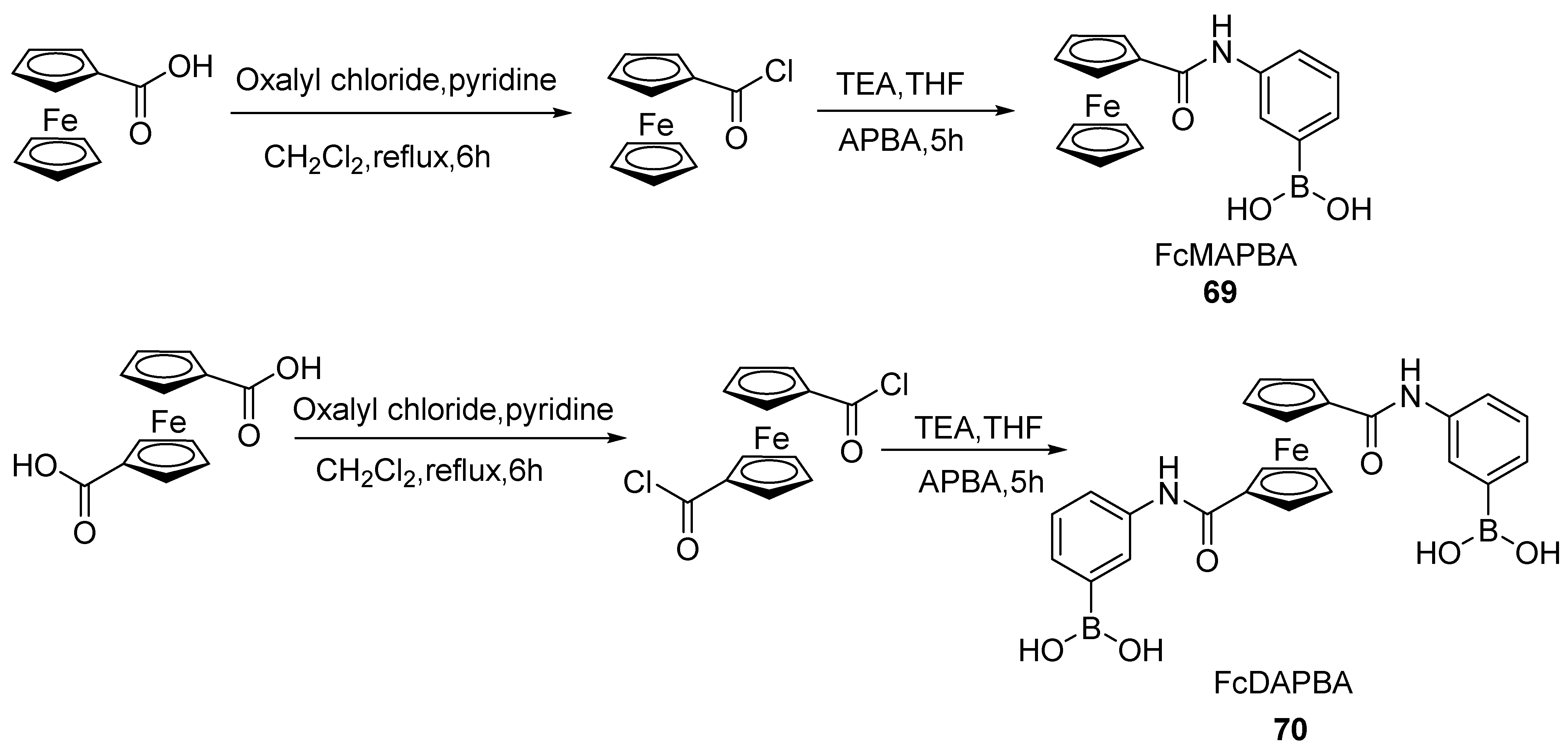
2.5. Synthesis of Ferrocenyl Derivative Directly Bonded with Heteroatom
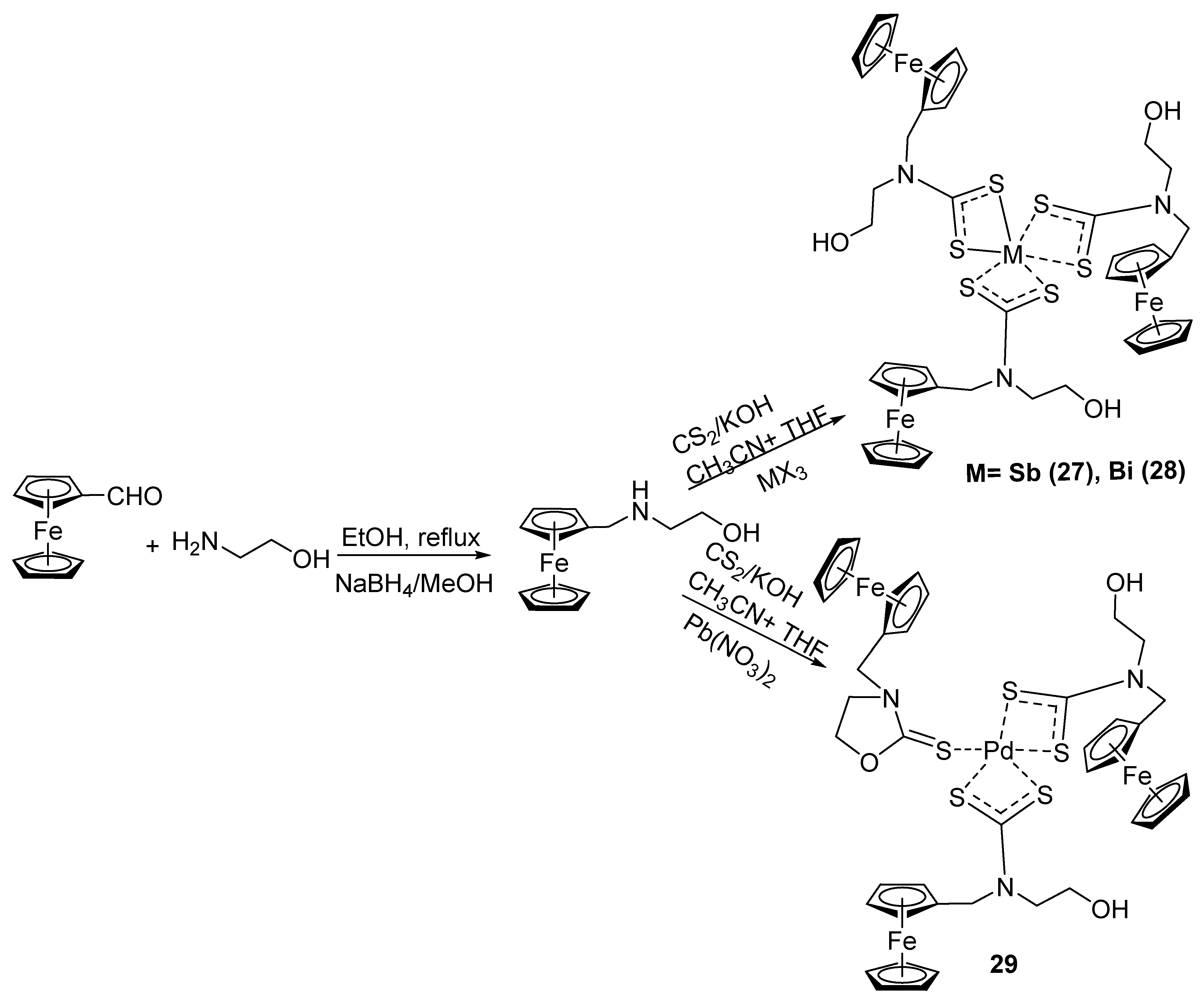
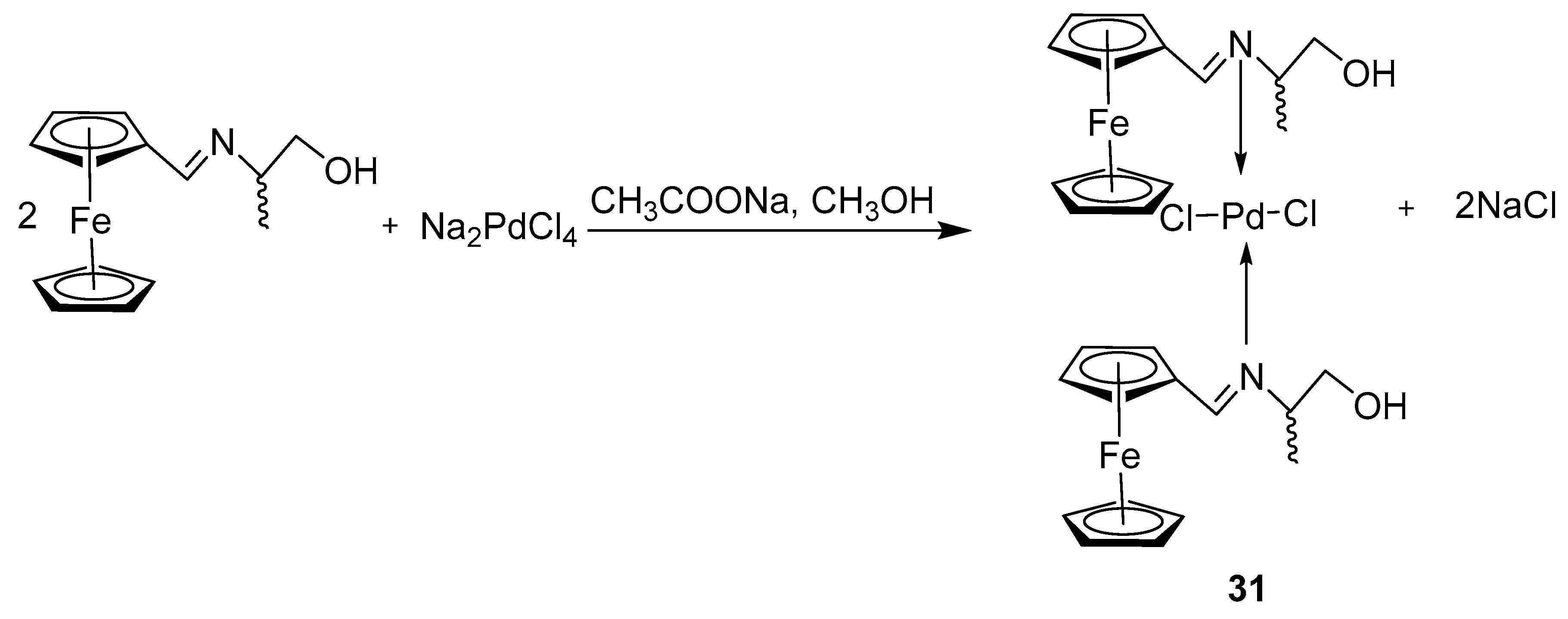
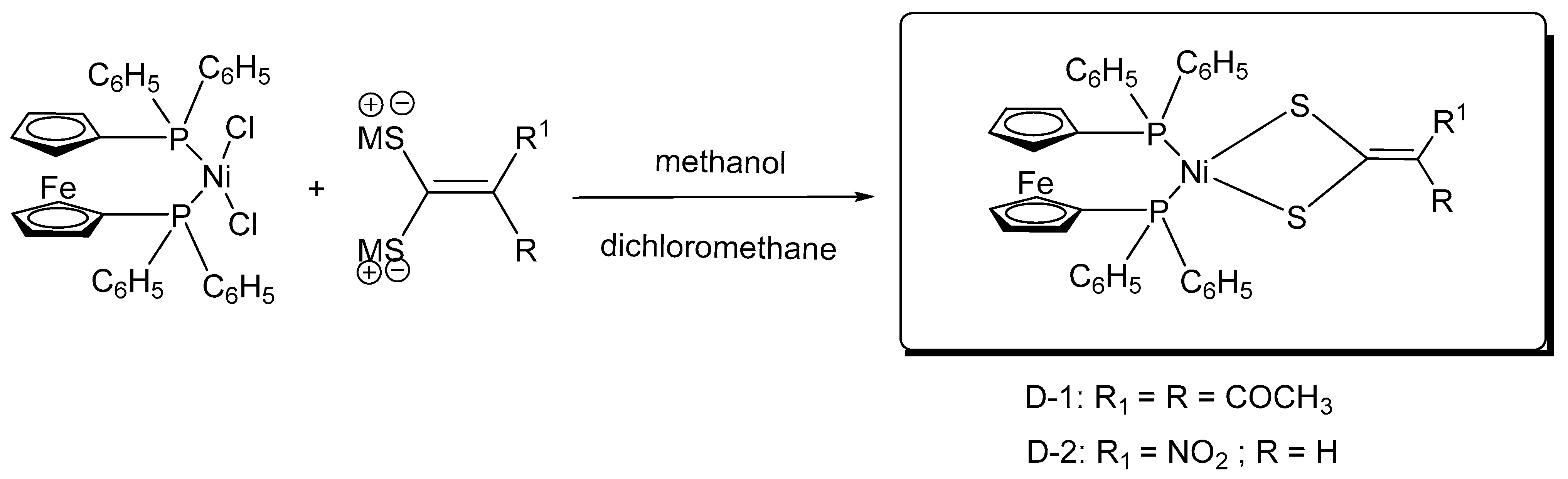
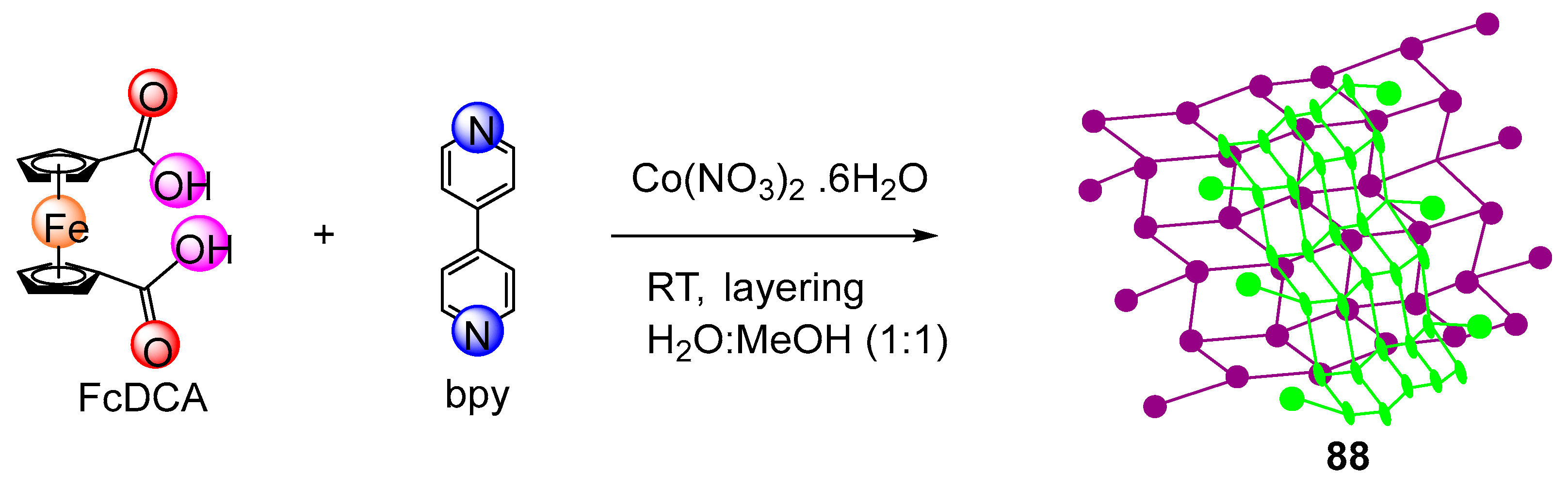
2.6. Synthesis of Ferrocene-Conjugated Metal Complexes
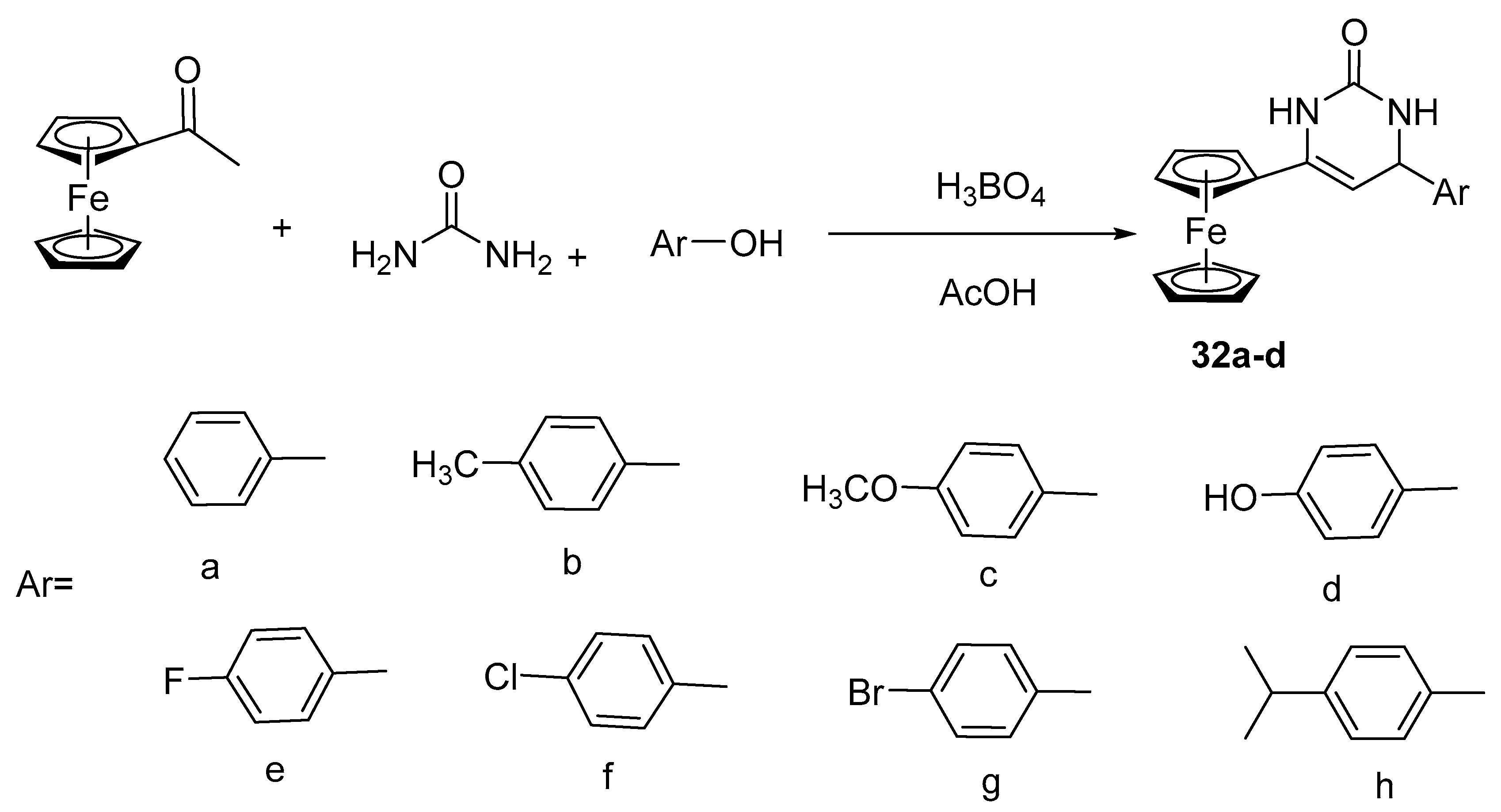
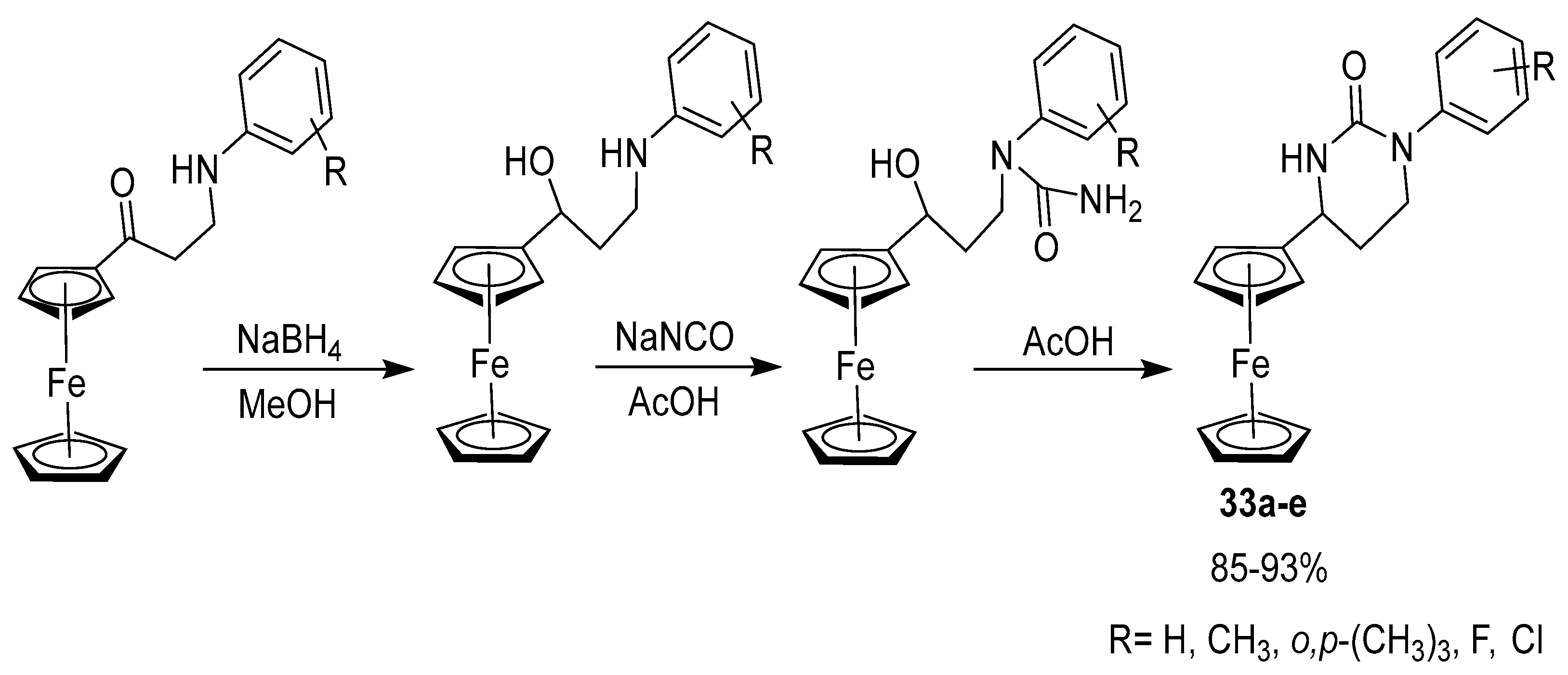
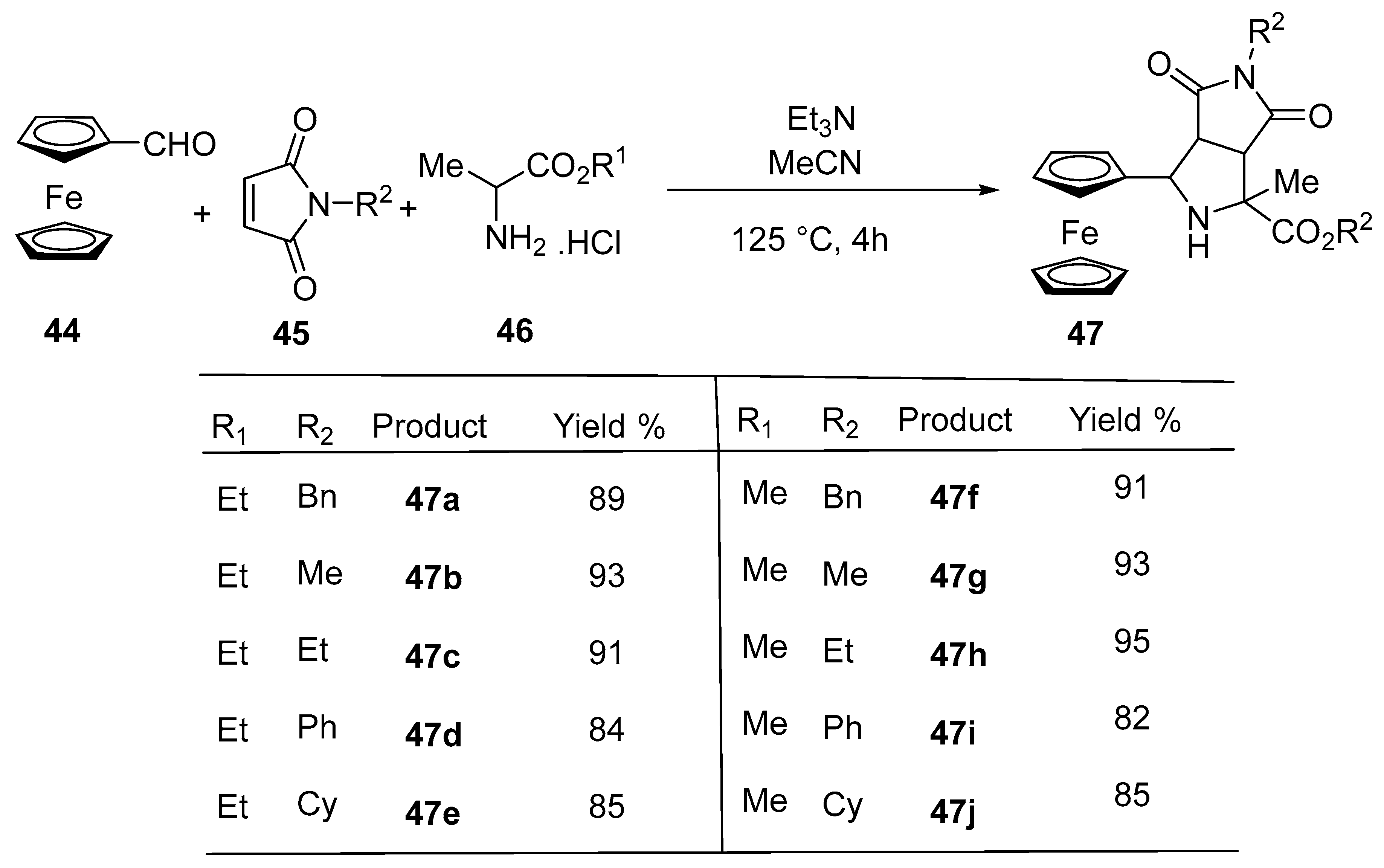
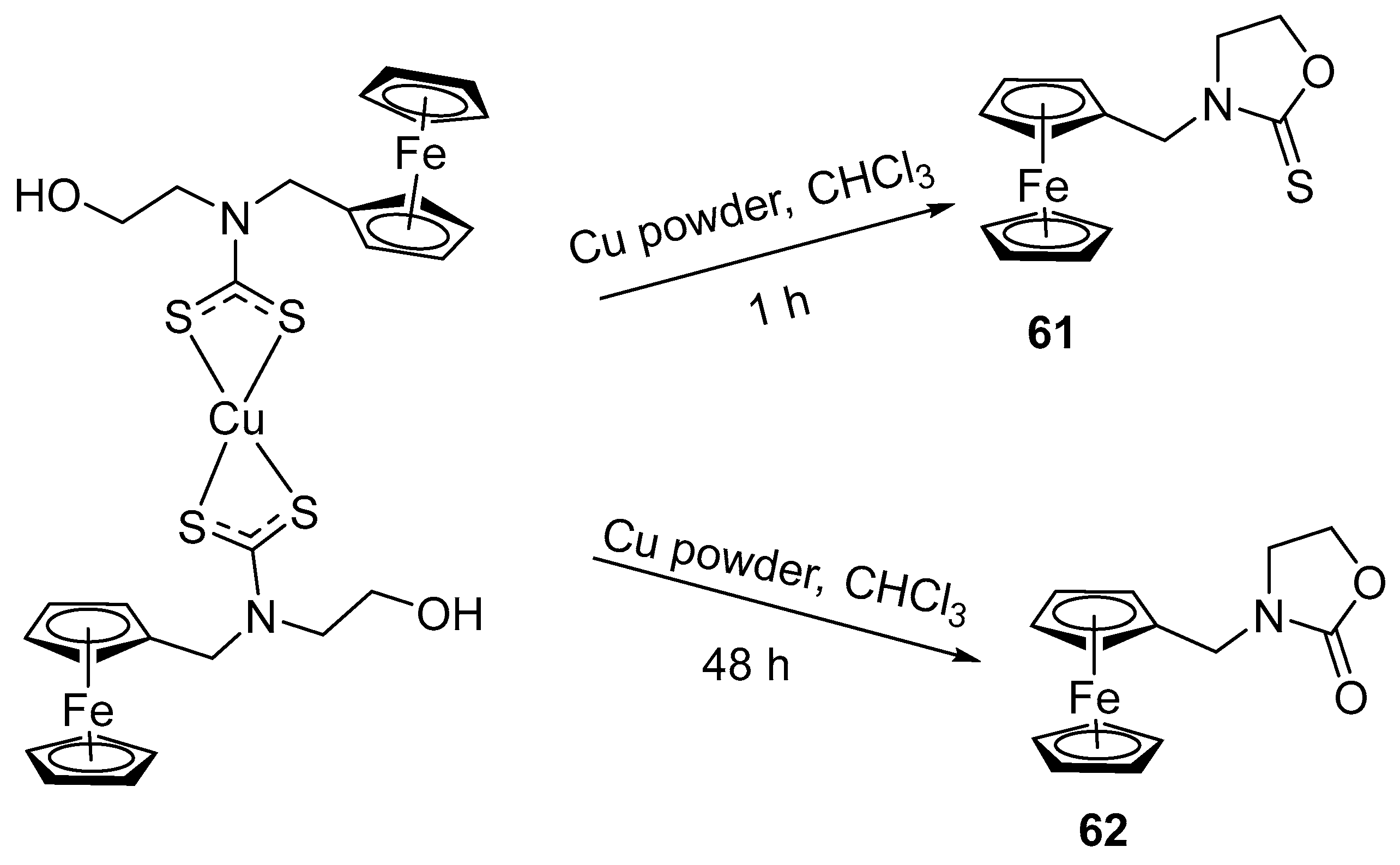
2.7. Synthesis of Ferrocene-Based Cyclic Urea
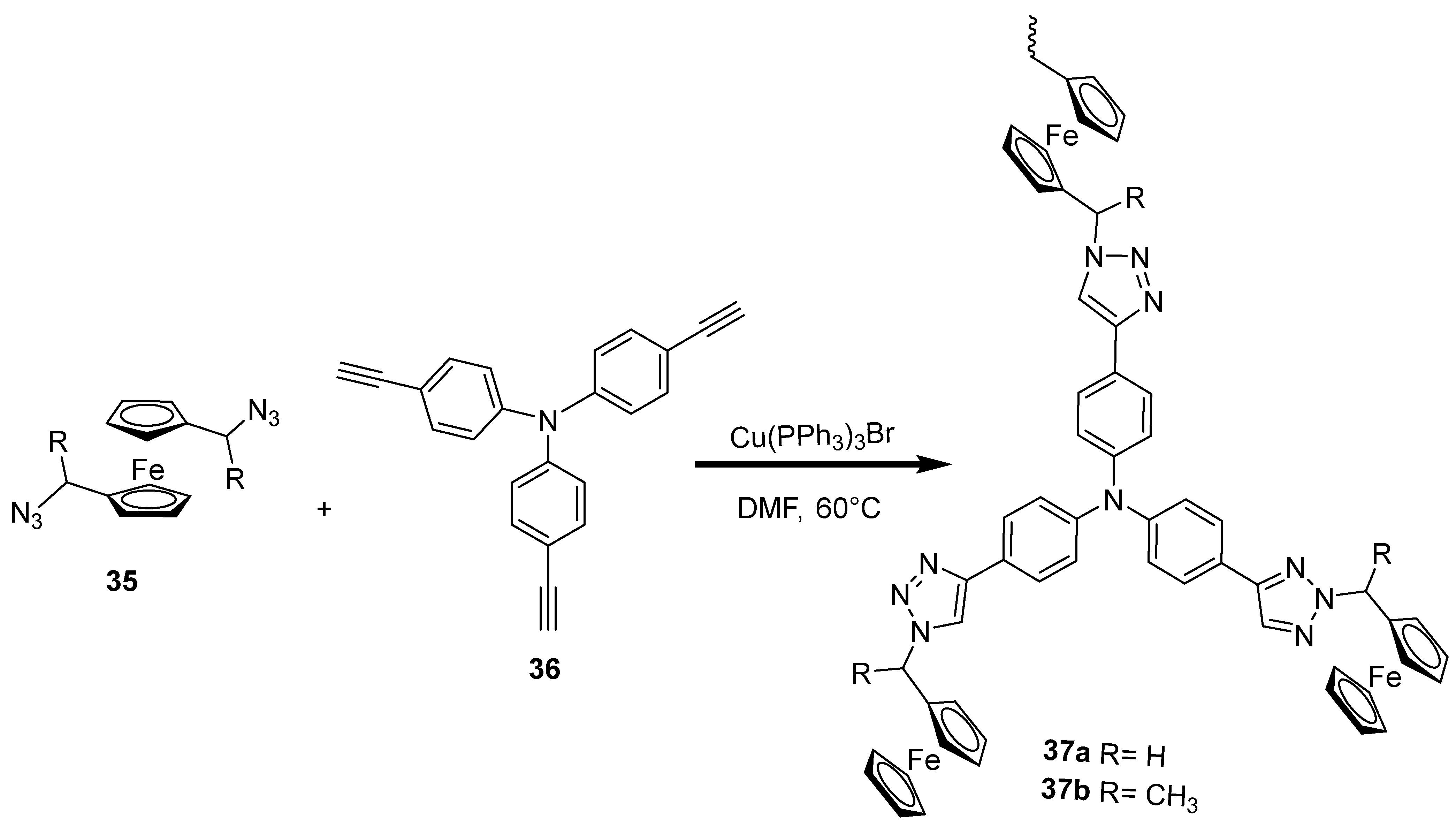
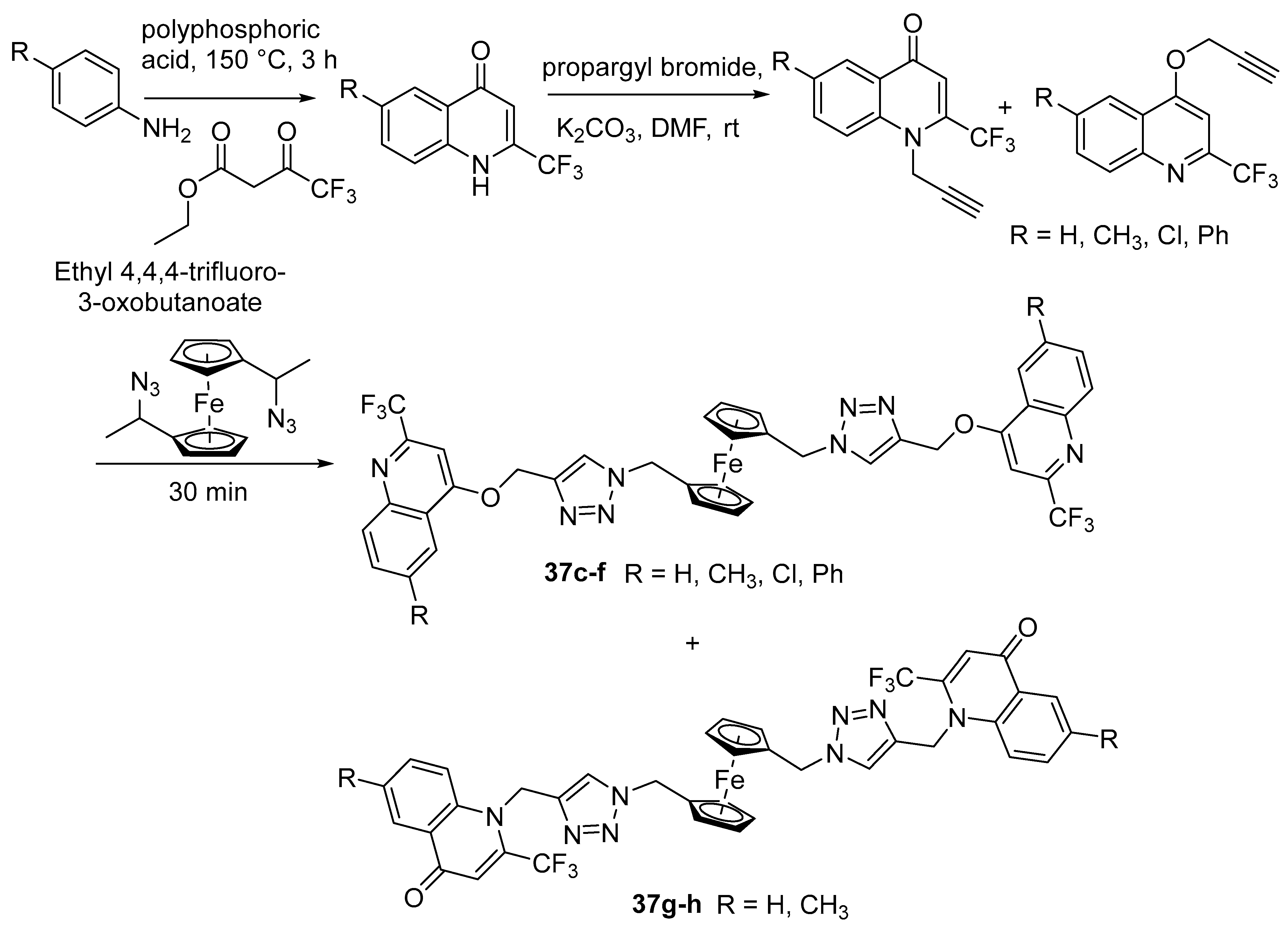
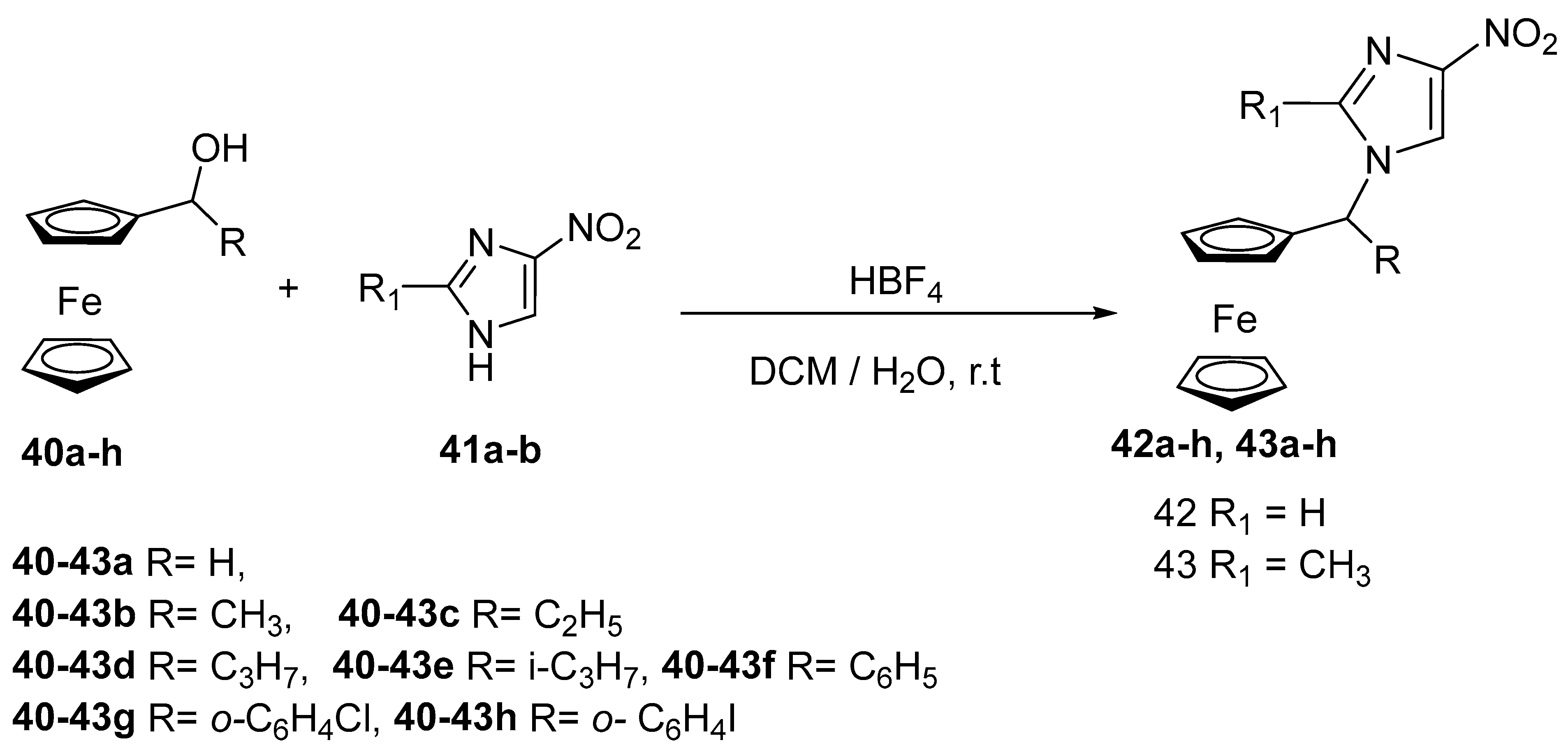
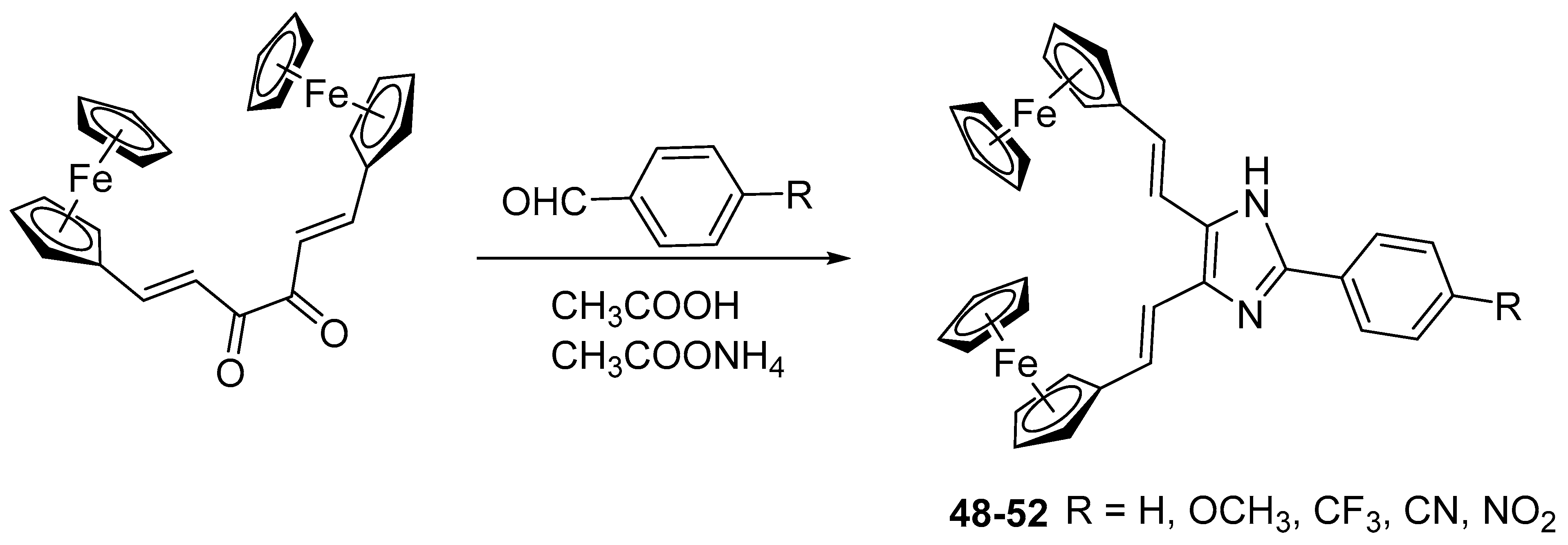
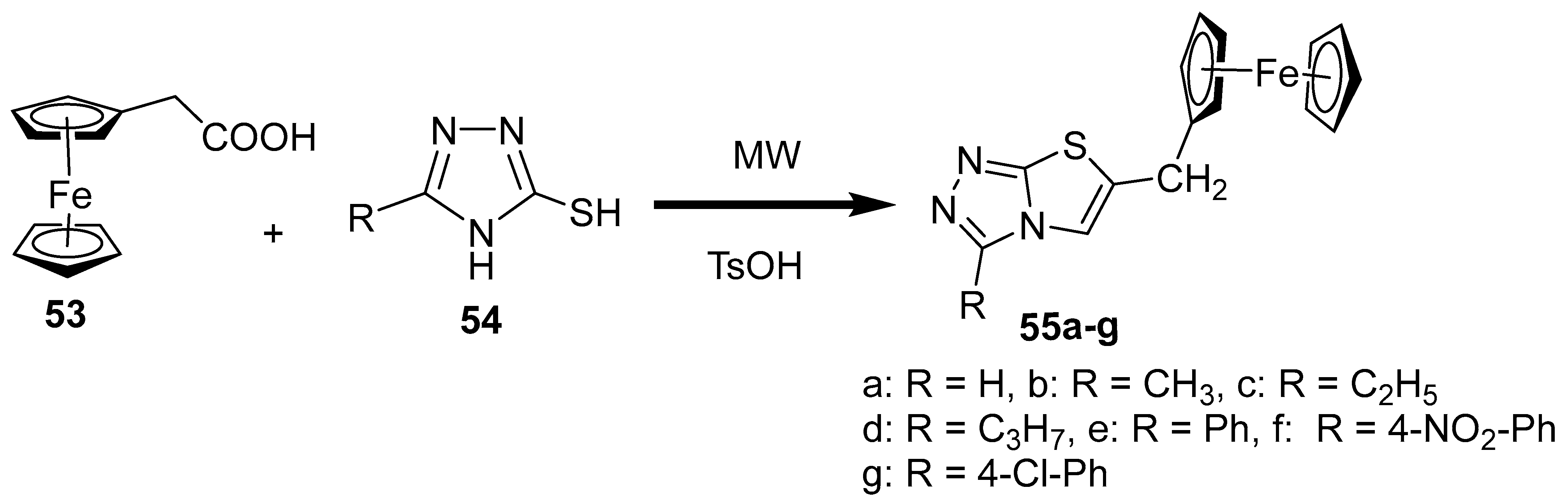
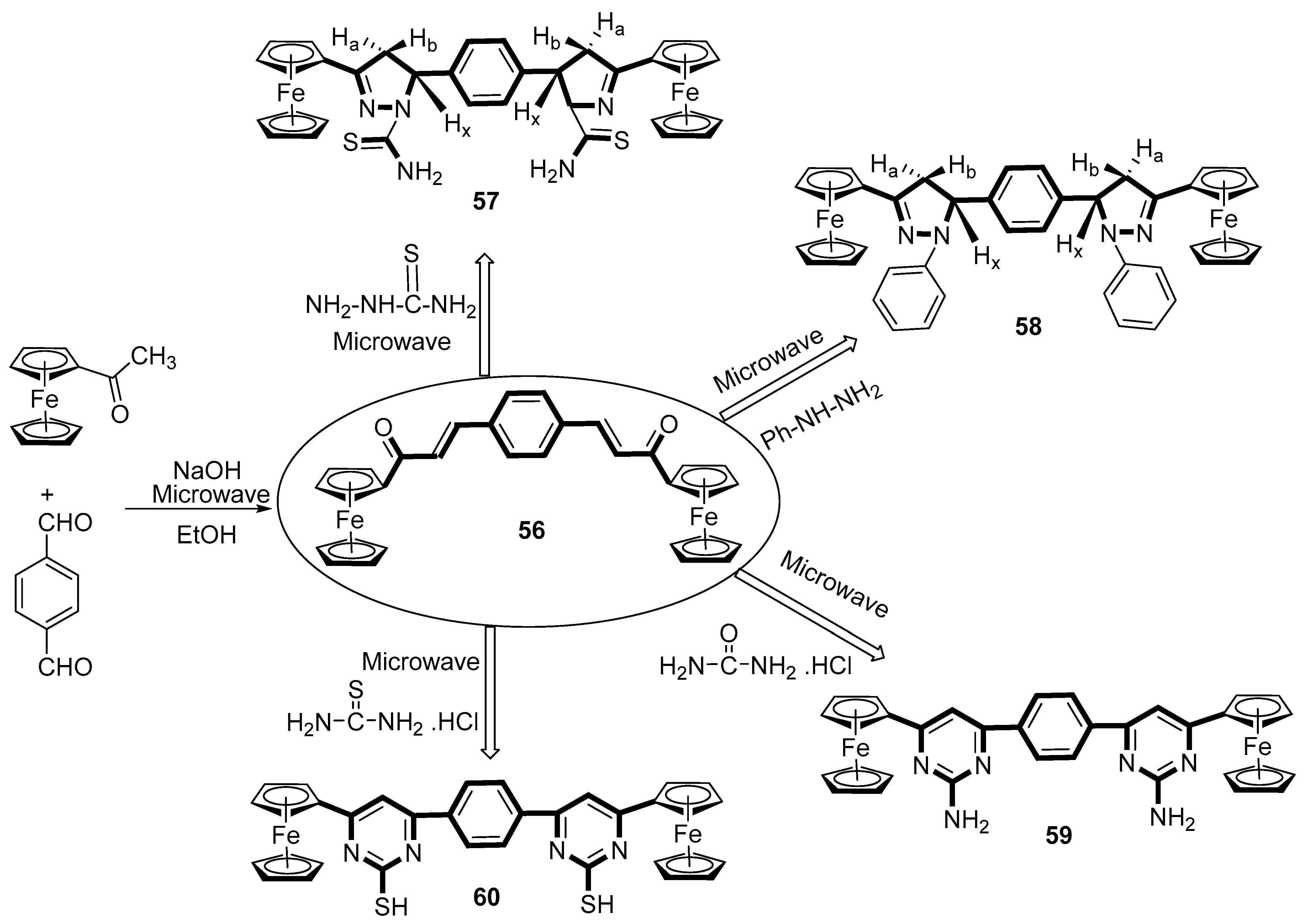
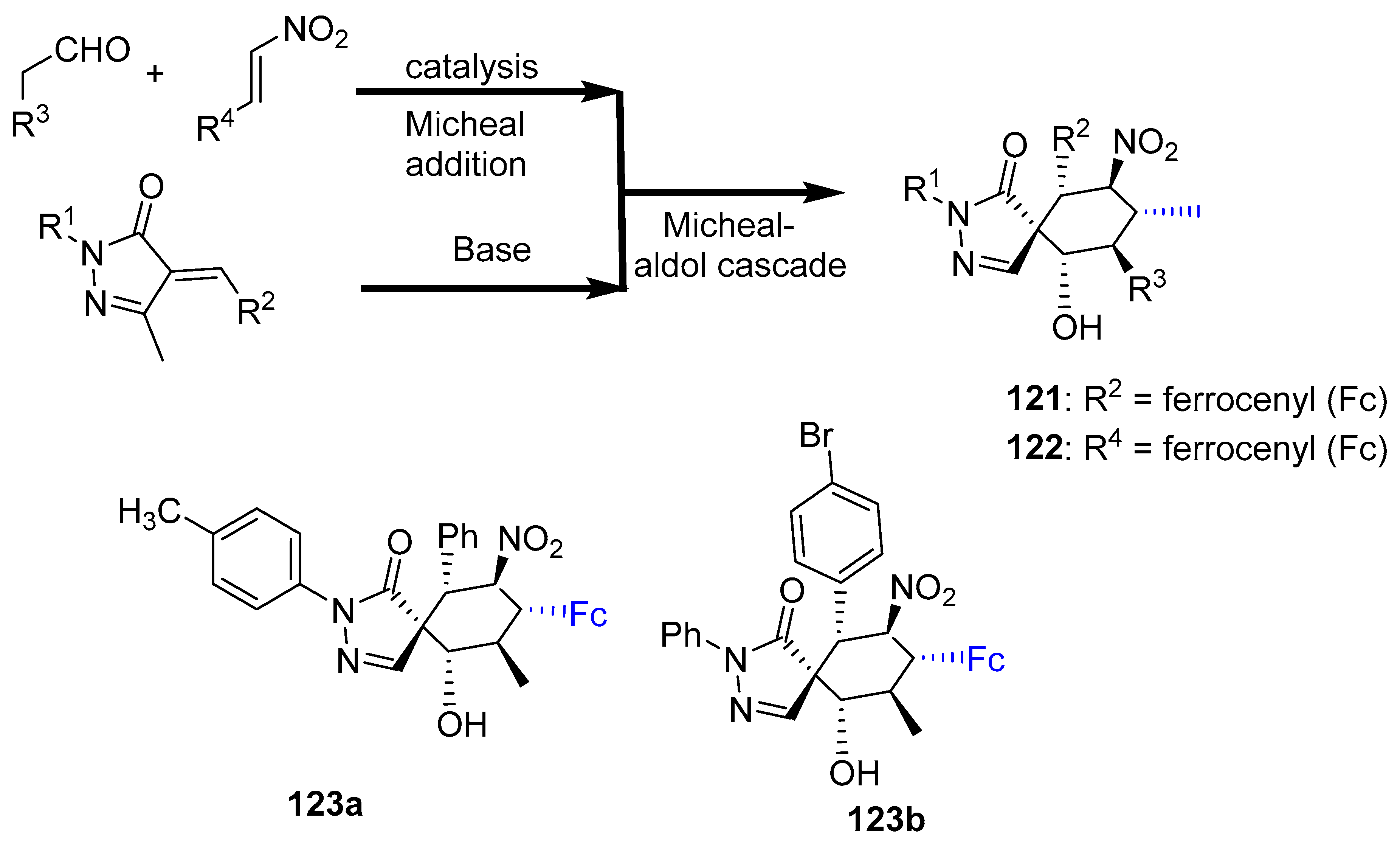
2.8. Synthesis of Ferrocene-Based Heterocyclic Derivatives
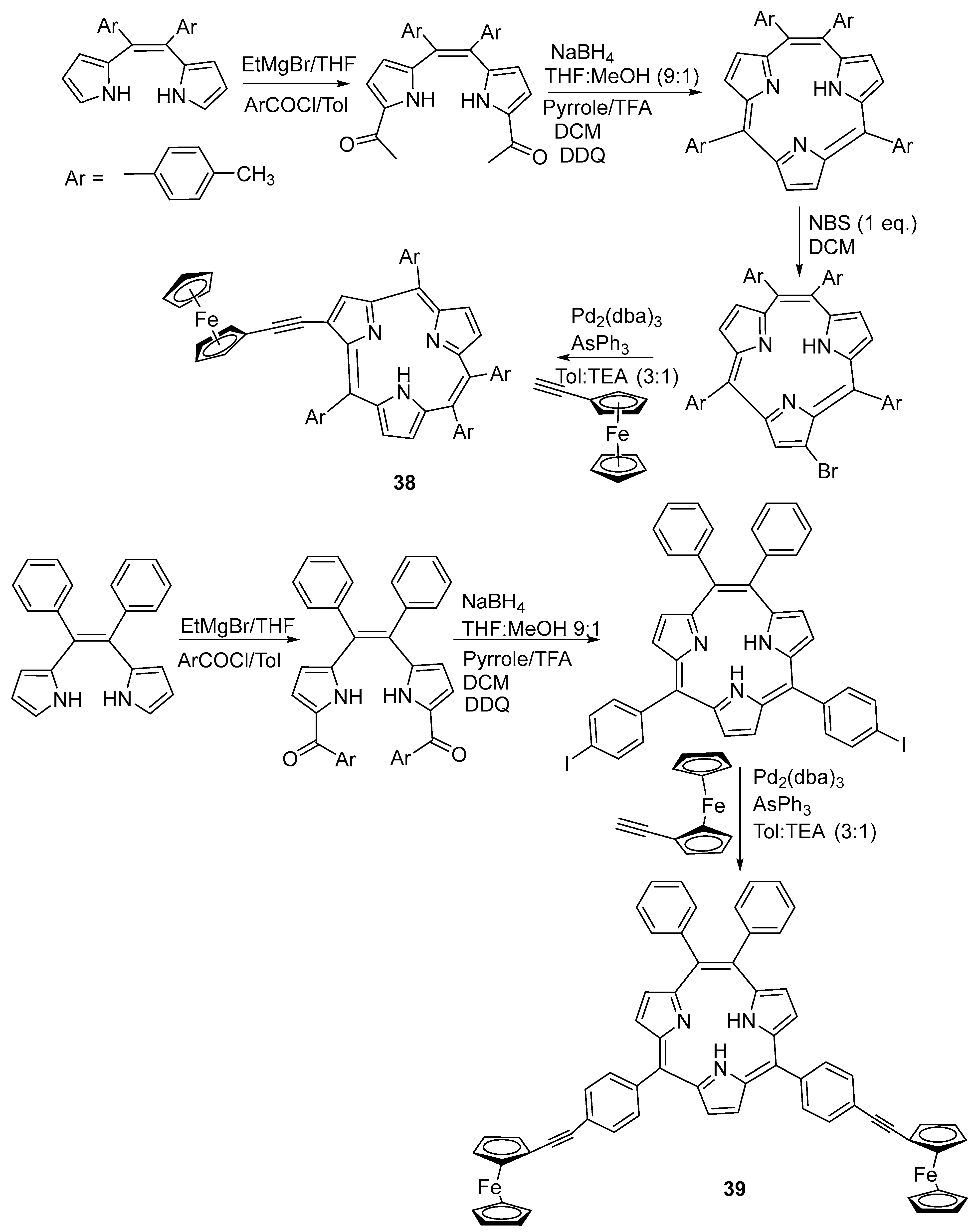
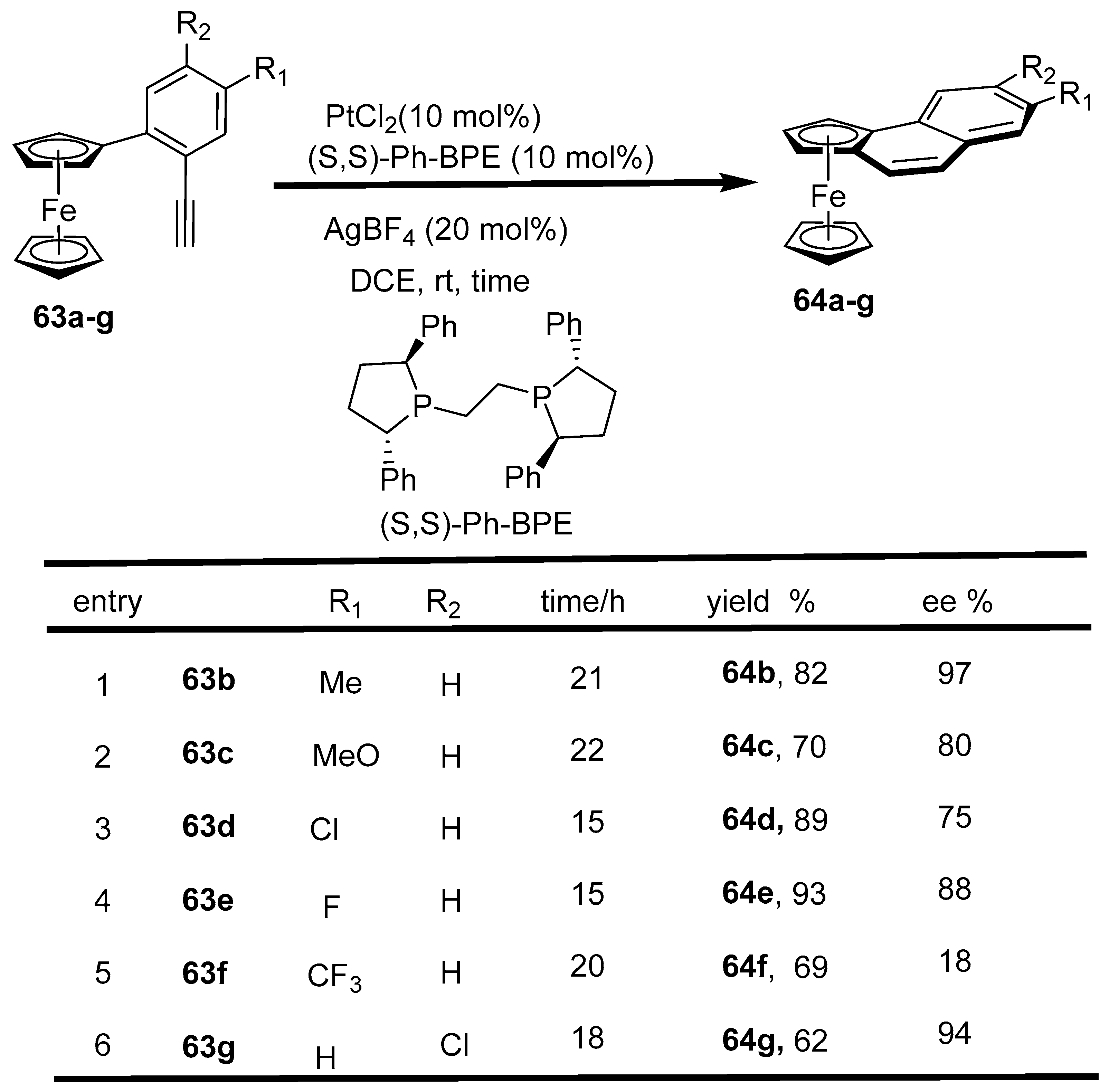
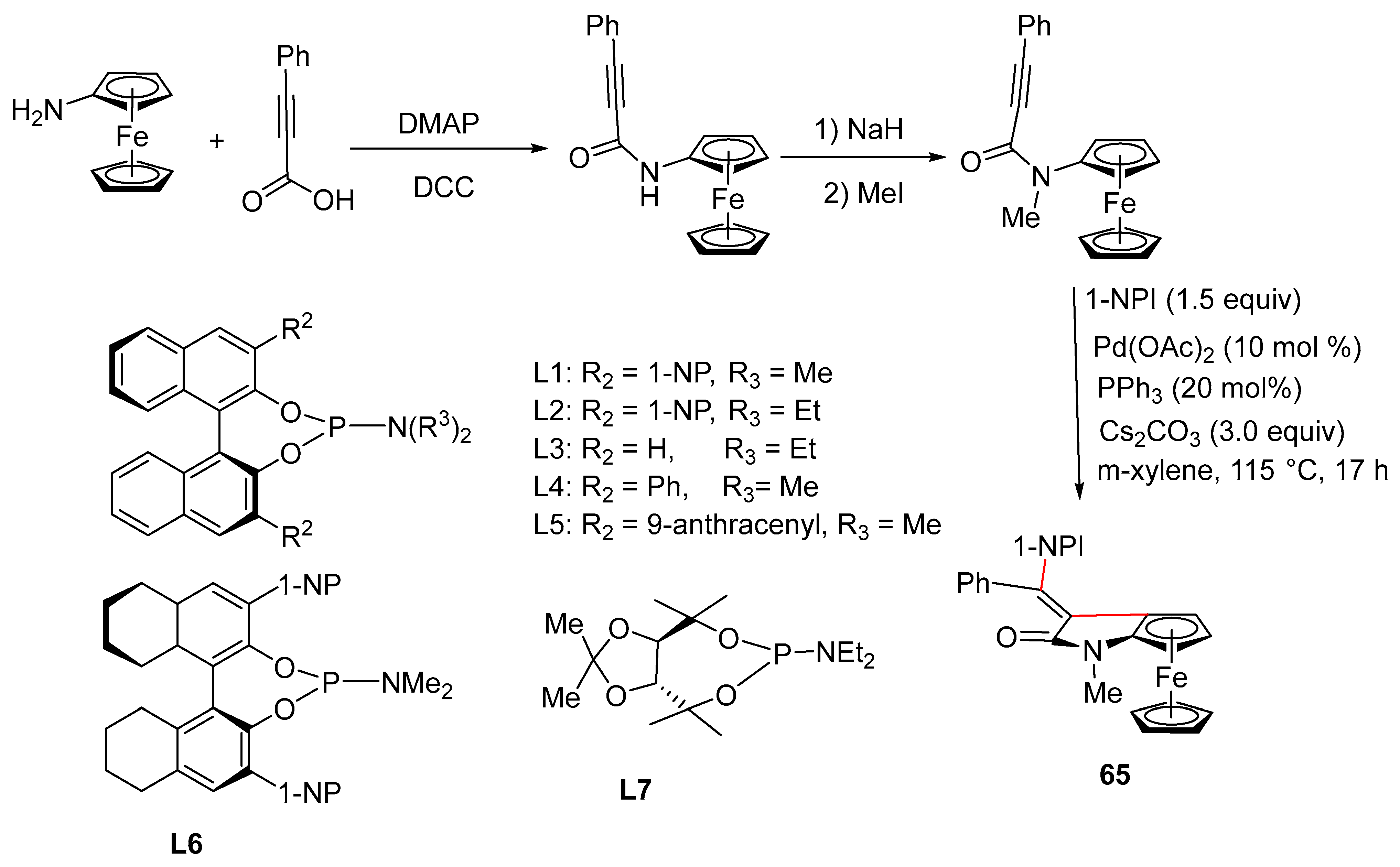
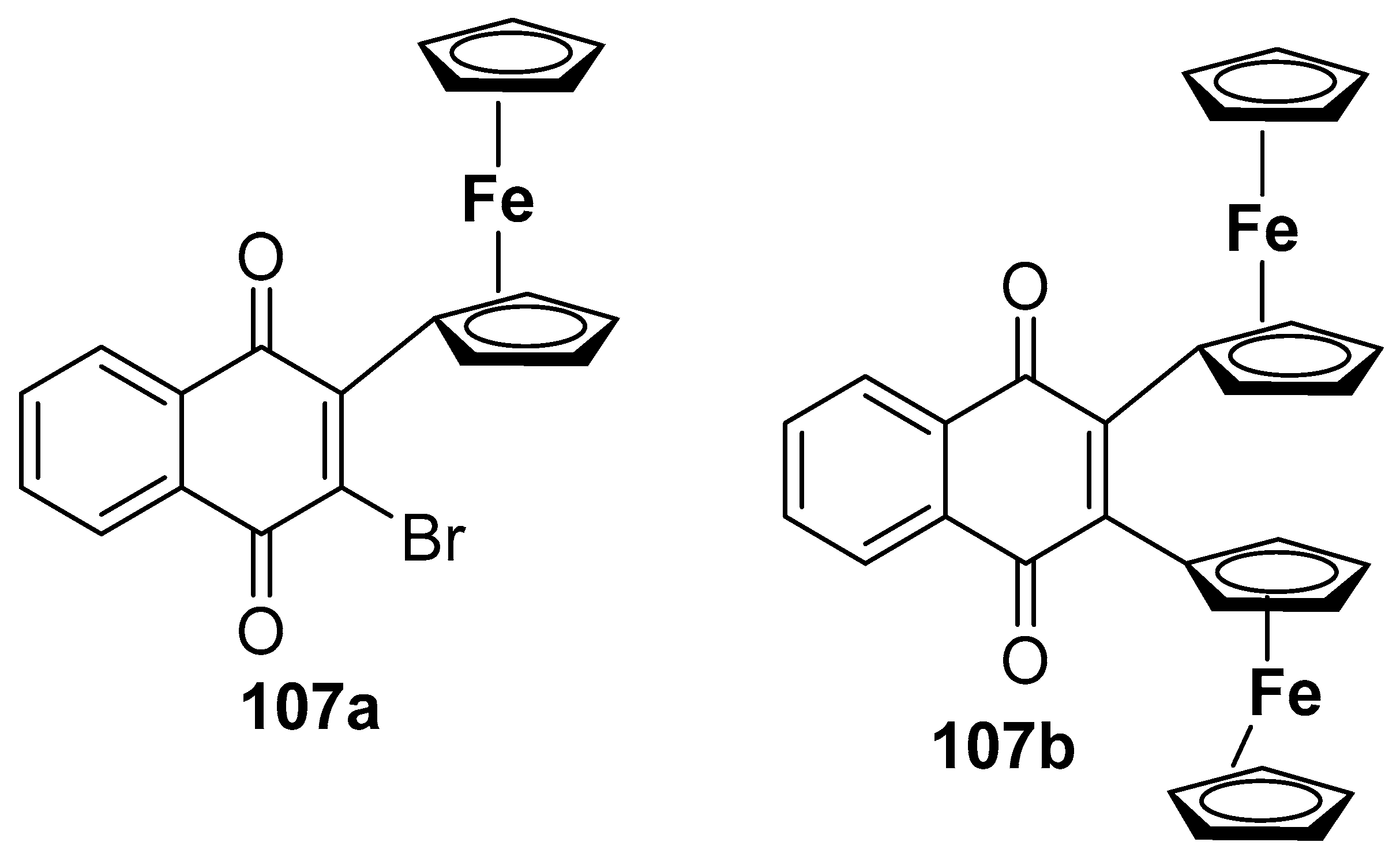
2.9. Synthesis of Ferrocene-Fused Cyclic Derivatives
3. Applications of Ferrocene Derivatives
3.1. Materials Science Applications
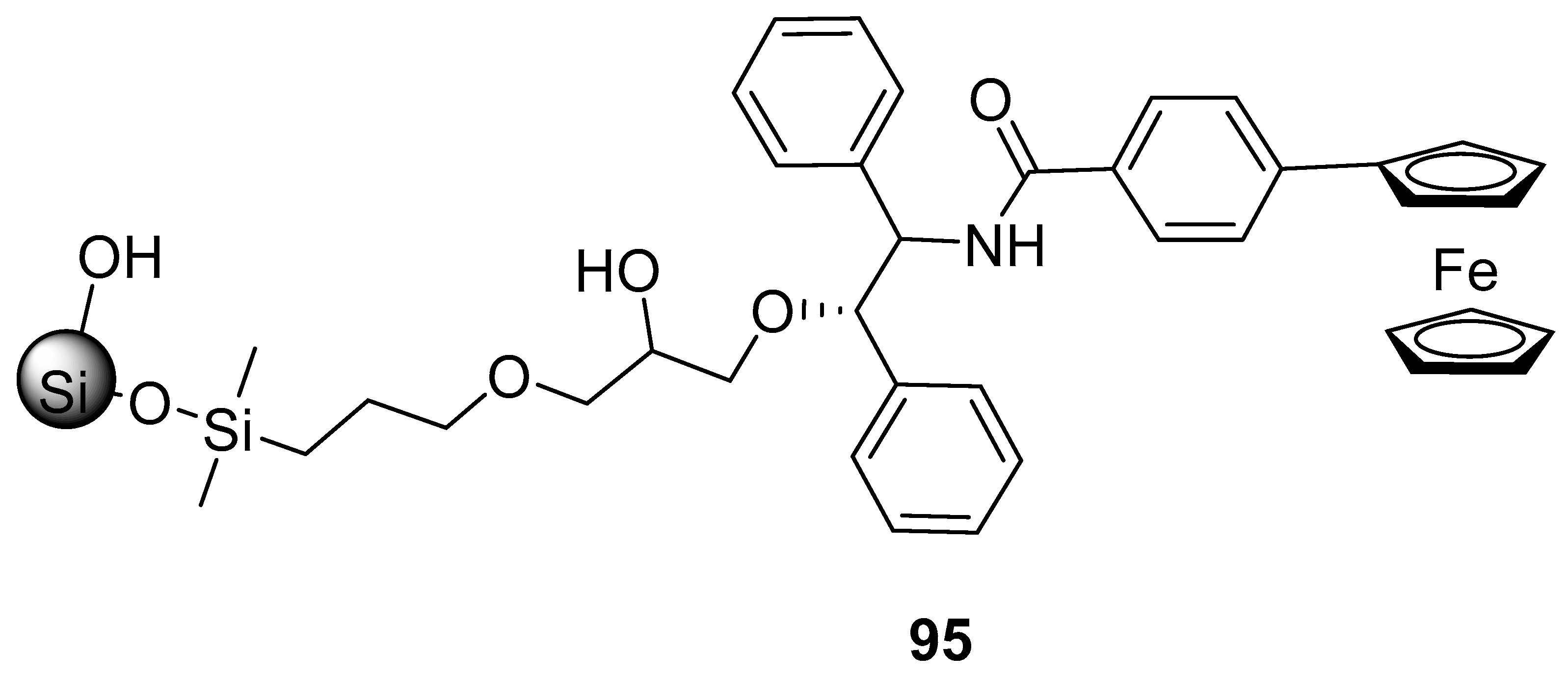
3.1.1. Biosensing Potential of Saccharides
3.1.2. Anion Biosensors
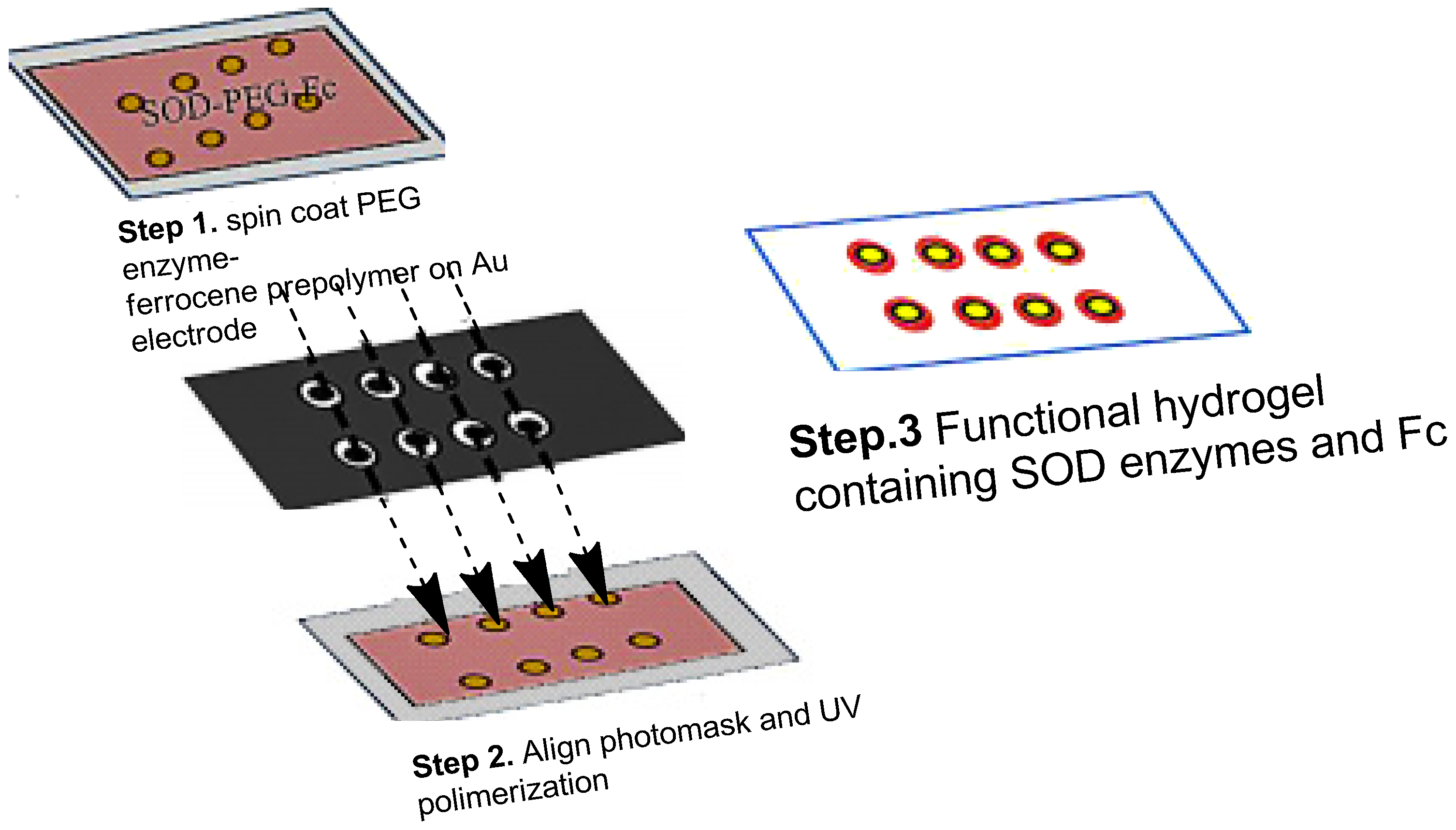
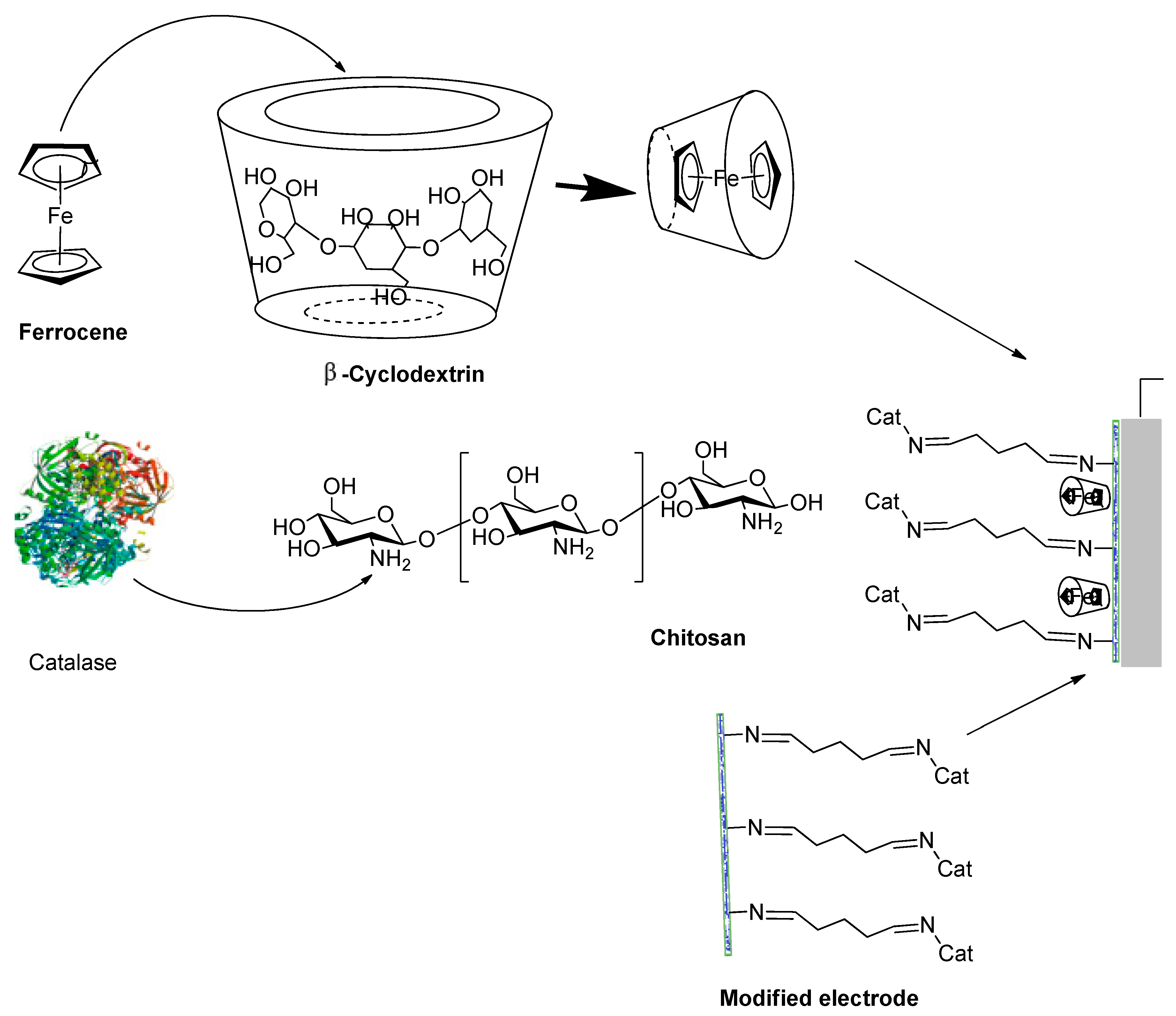
3.1.3. Hydrogen Peroxide Biosensor
3.1.4. Peptide Biosensors
3.1.5. Chemosensing Applications
3.1.6. Electron Beam Lithographic Application
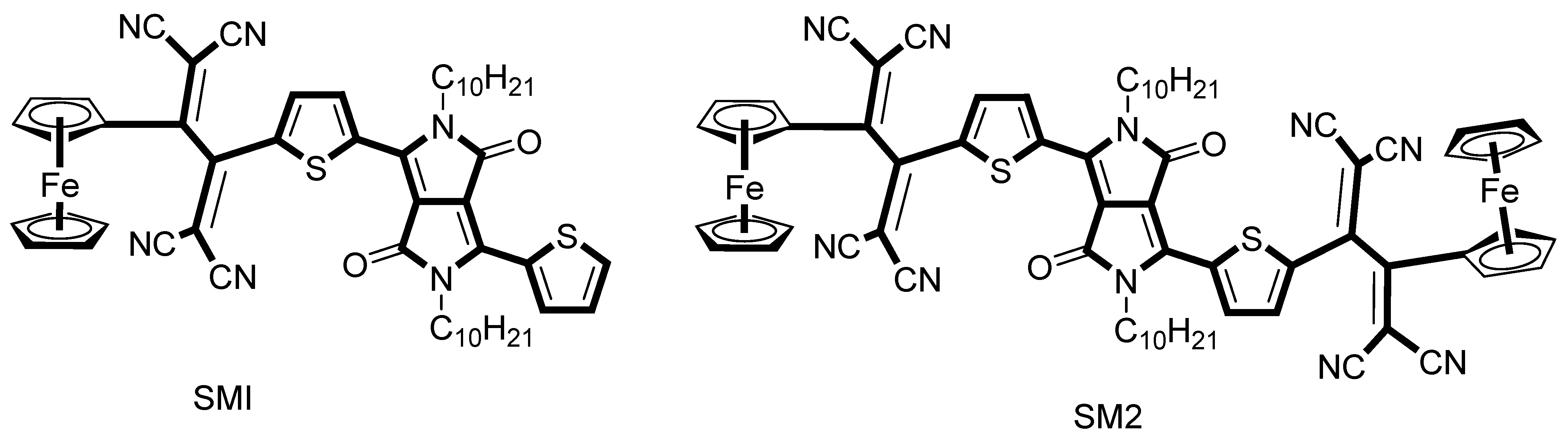
3.1.7. Nonlinear Optics Applications of Ferrocene-Based Conjugates
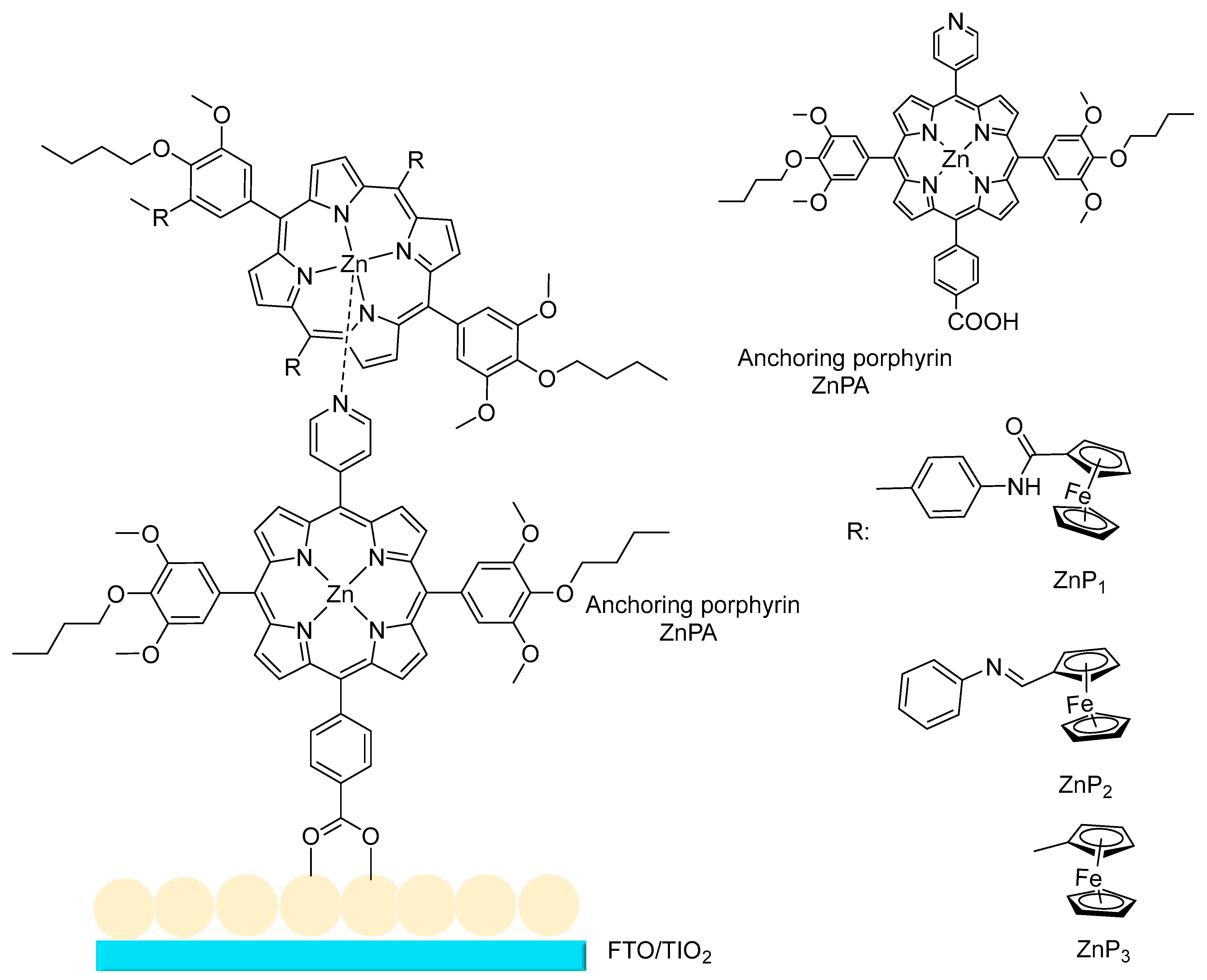
3.1.8. Solar Cell Applications
3.1.9. Supercapacitor Applications
3.1.10. Uses of Ferrocene in Modern Fuel Cells or Batteries
3.1.11. Redox-Mediating Potential of Ferrocene Derivatives
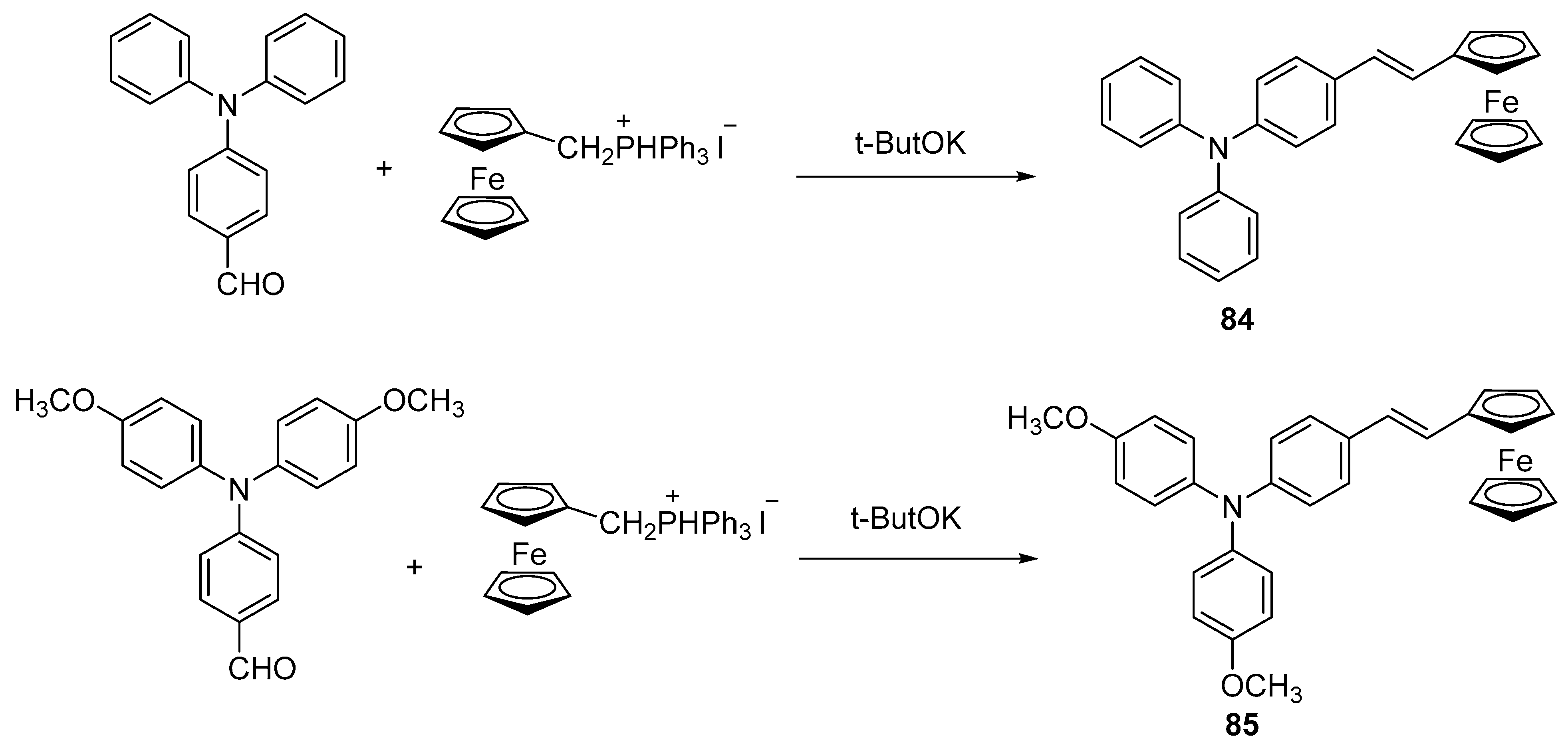
3.1.12. Application in Photo-Induced Electron Transfer: Cascaded Molecular Logic
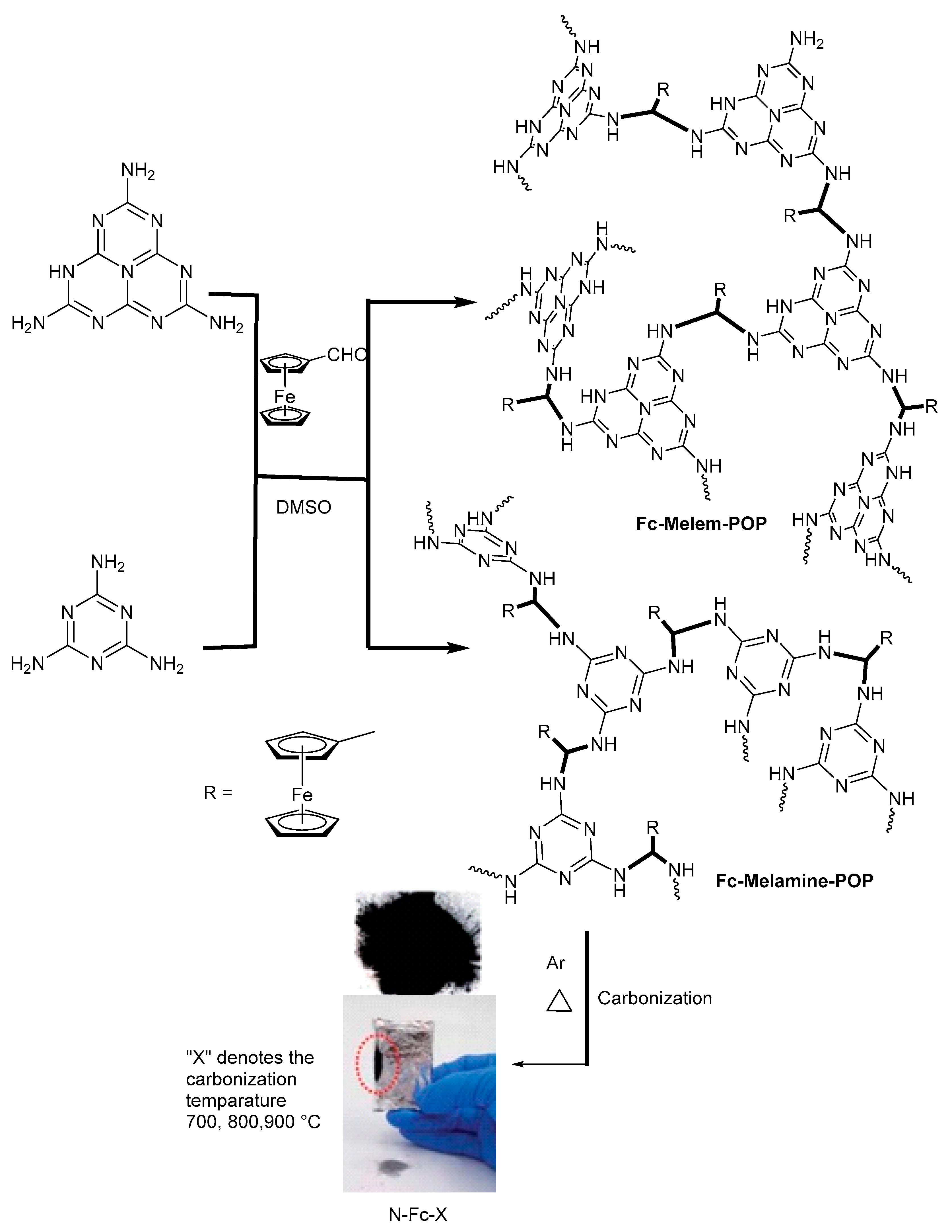
3.1.13. Role of Ferrocene in Catalytic Graphitization
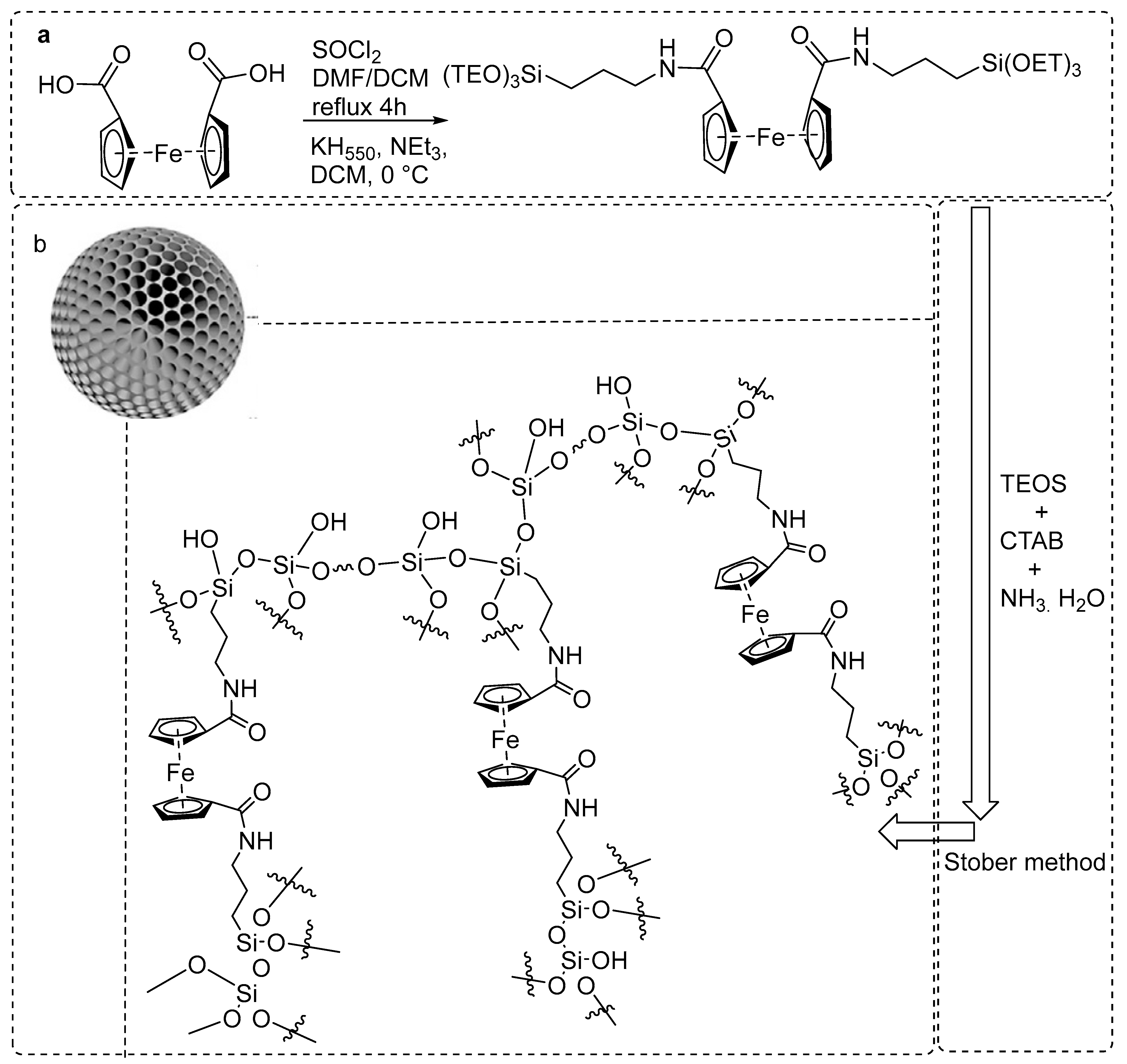
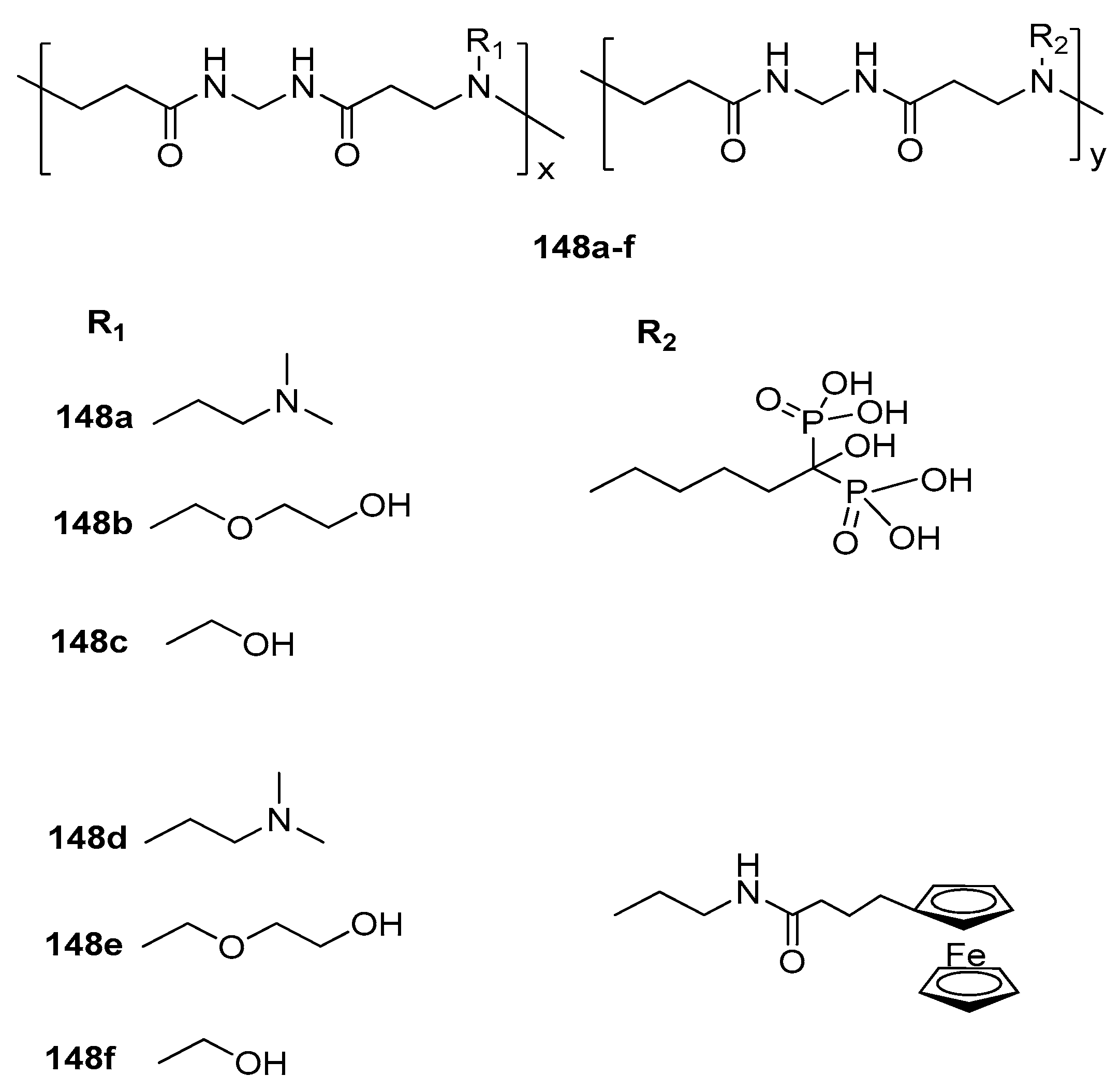
3.1.14. Ferrocene-Based Polymer as a Scavenger for Radioiodine
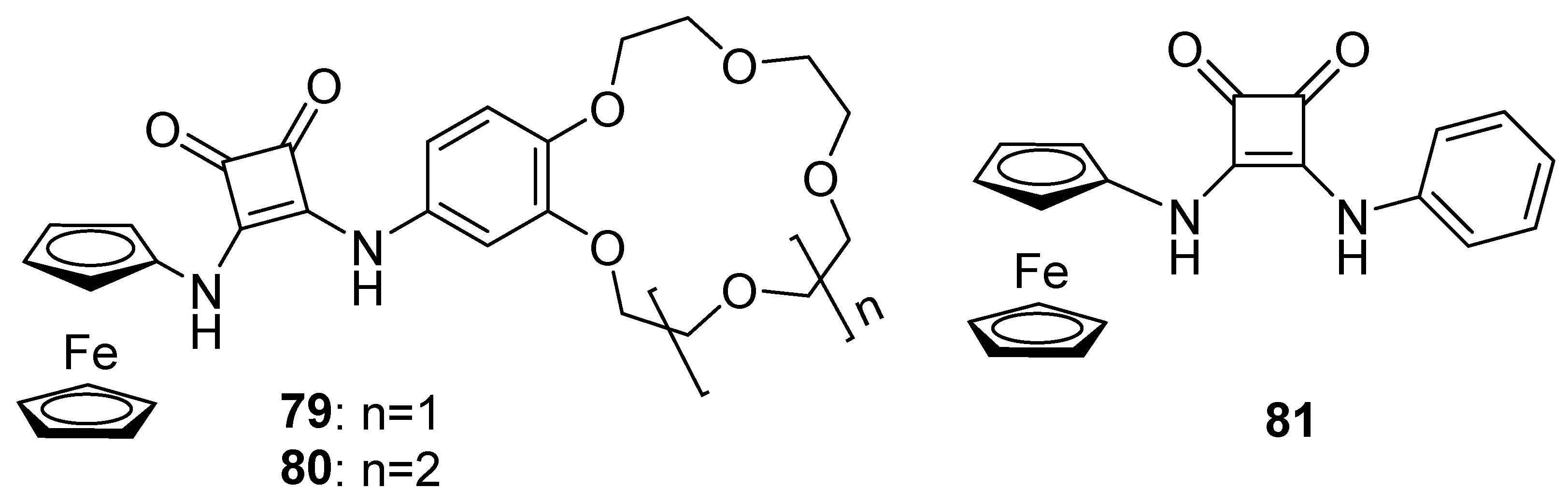
3.1.15. Ferrocene-Based Hyperbranched Polymers as Electroactive Materials
3.2. Applications in Catalysis
3.2.1. Ferrocene-Functionalized Phosphine Ligand
3.2.2. Ferrocene-Appended Thiazolidine Ligand Framework
3.2.3. Chiral Oxazolinyl Hydroxyl-Clubbed Ferrocene in Asymmetric Reactions
3.2.4. Ferrocene Ligand in sp2–sp3 Coupling Reactions
3.2.5. Applications of Ferrocene-Based Diols in Hetero Diels Alder Reactions
3.3. Industrial Applications
3.3.1. Chromatographic Applications
3.3.2. Corrosion Inhibition Potential
3.3.3. Water Treatment
3.3.4. Use in Rocket Propellants
3.3.5. Applications in BR Catalysis
3.3.6. Use in Electrochromic Appliances
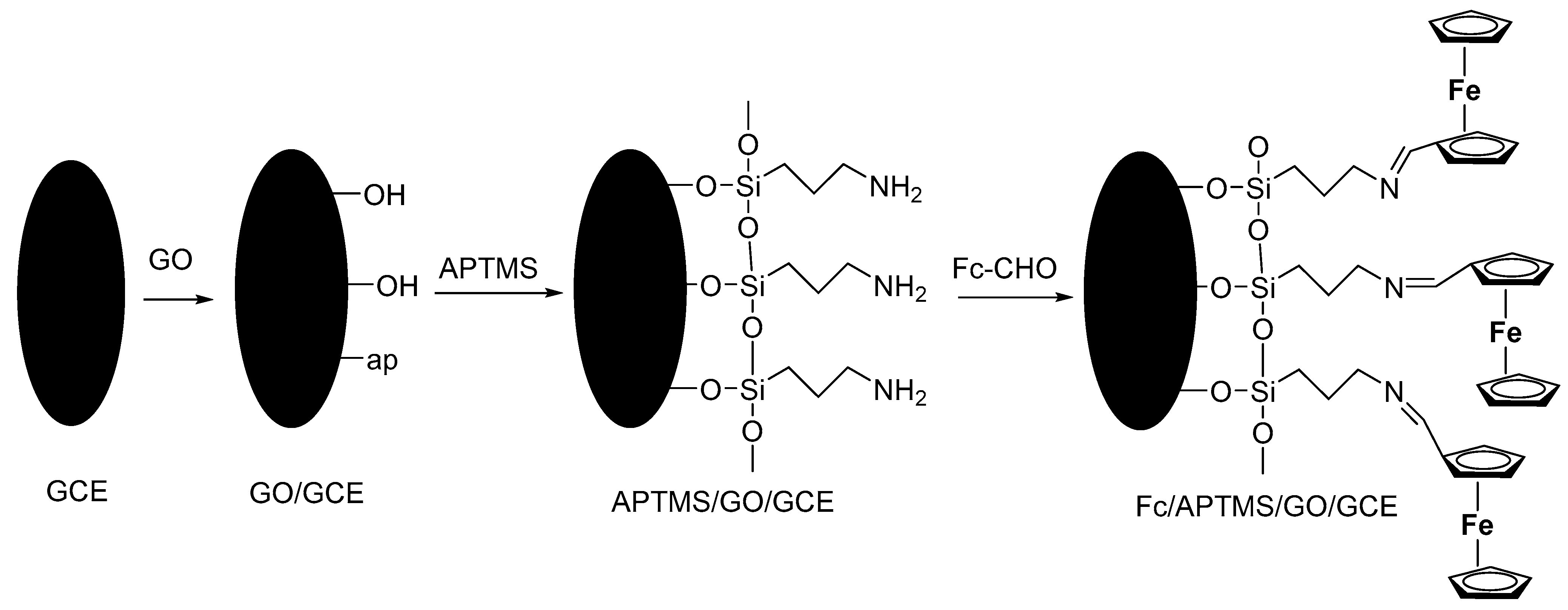
3.3.7. Modified Voltammetric Sensors with Ferrocene
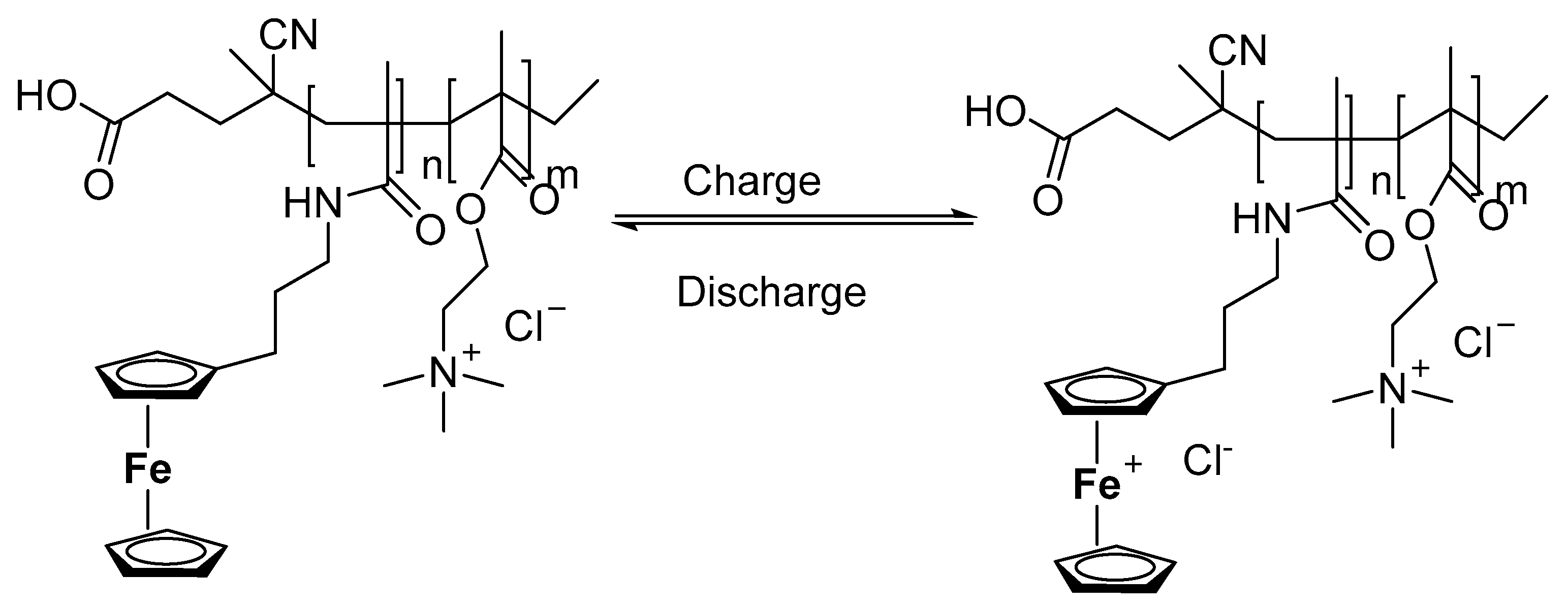
3.3.8. Application of Ferrocene in Aqueous Redox Flow Battery Suitable for High Temperatures
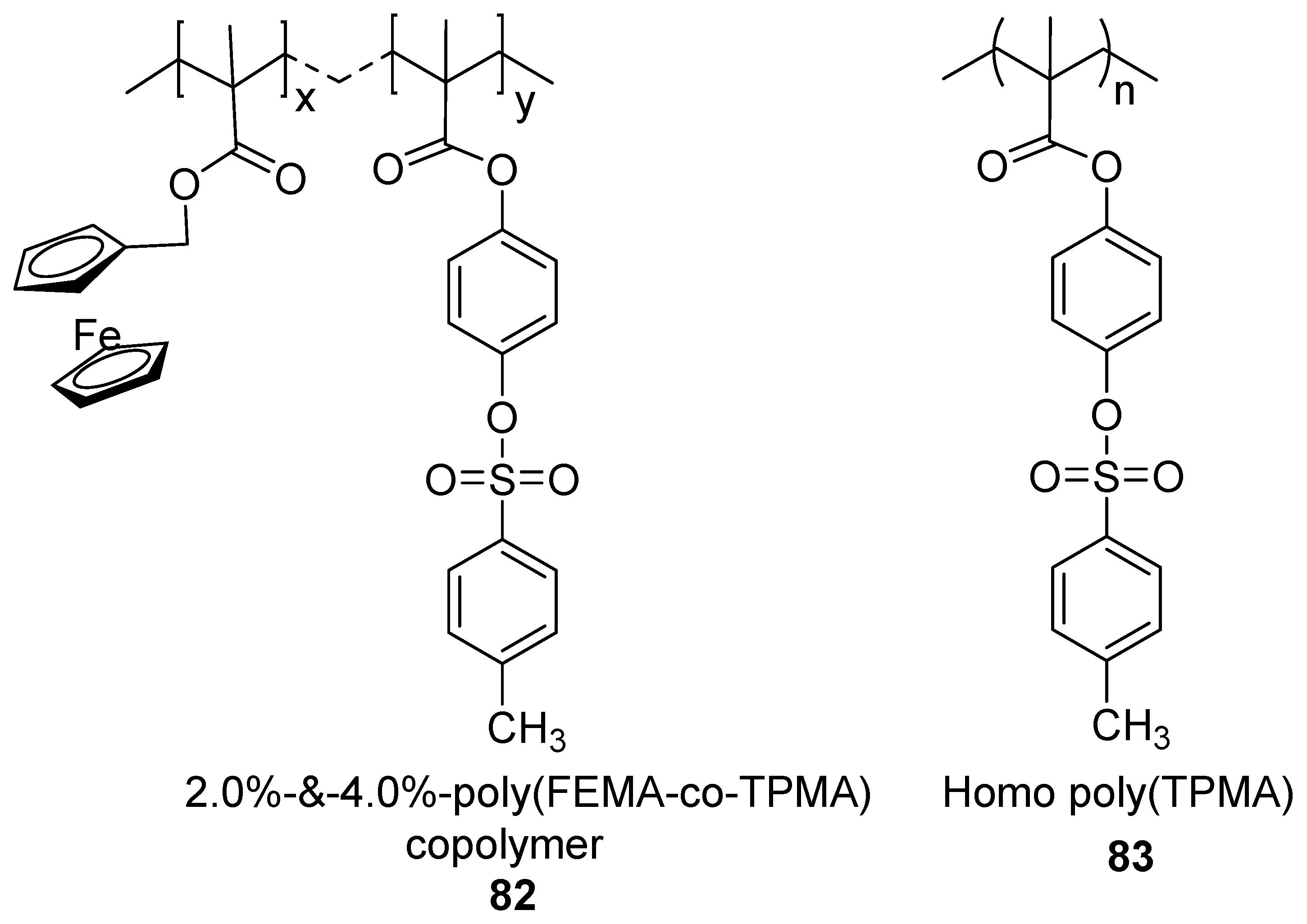
3.3.9. Ferrocene-Based Non-Enzymatic Hydrogen Peroxide Sensors
3.4. Agricultural Applications
3.4.1. Herbicide and Pesticide Safeners
3.4.2. Plant Growth Activators
3.4.3. Solid-Phase Microextraction for the Determination of Iron Organic Compounds in Seawater and Soil
3.4.4. A Probe-Free Electrochemical Immunosensor for Methyl Jasmonate
3.4.5. Ferrocene-Containing Nitrogen Fertilizers
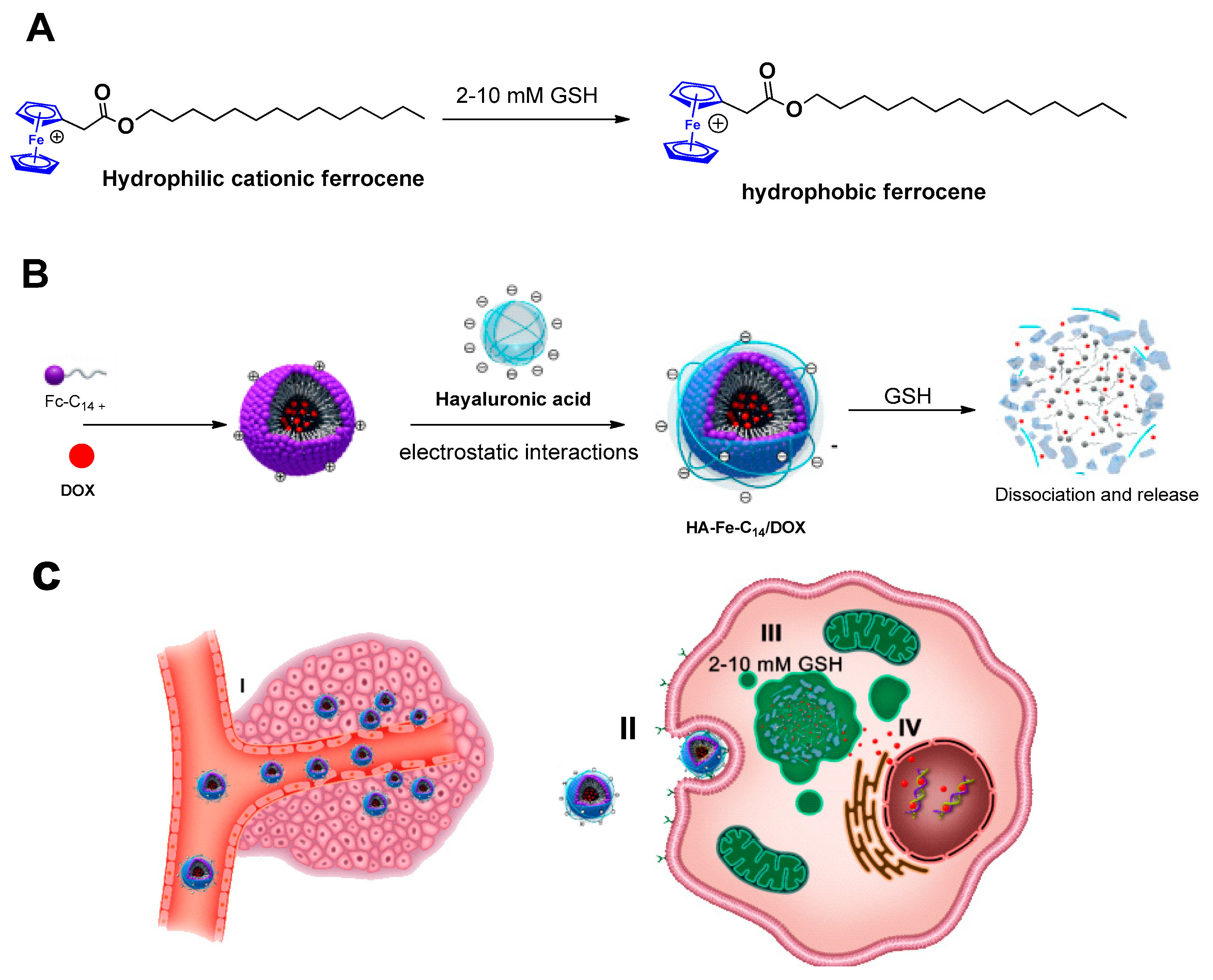
3.5. Biological Applications
3.6. Medicinal Applications
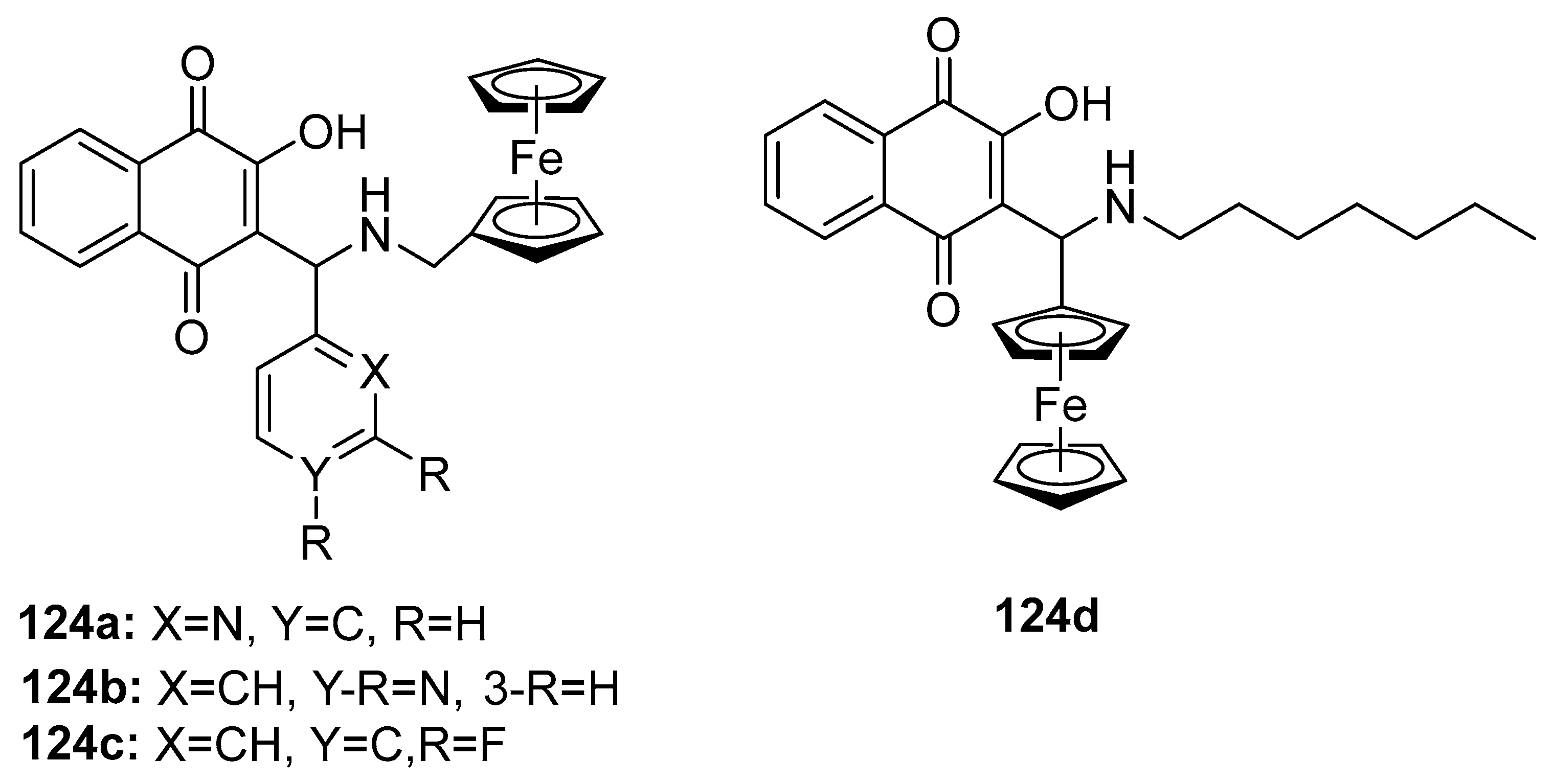
3.6.1. Anticancer Activity
3.6.2. Antimalarial Agents
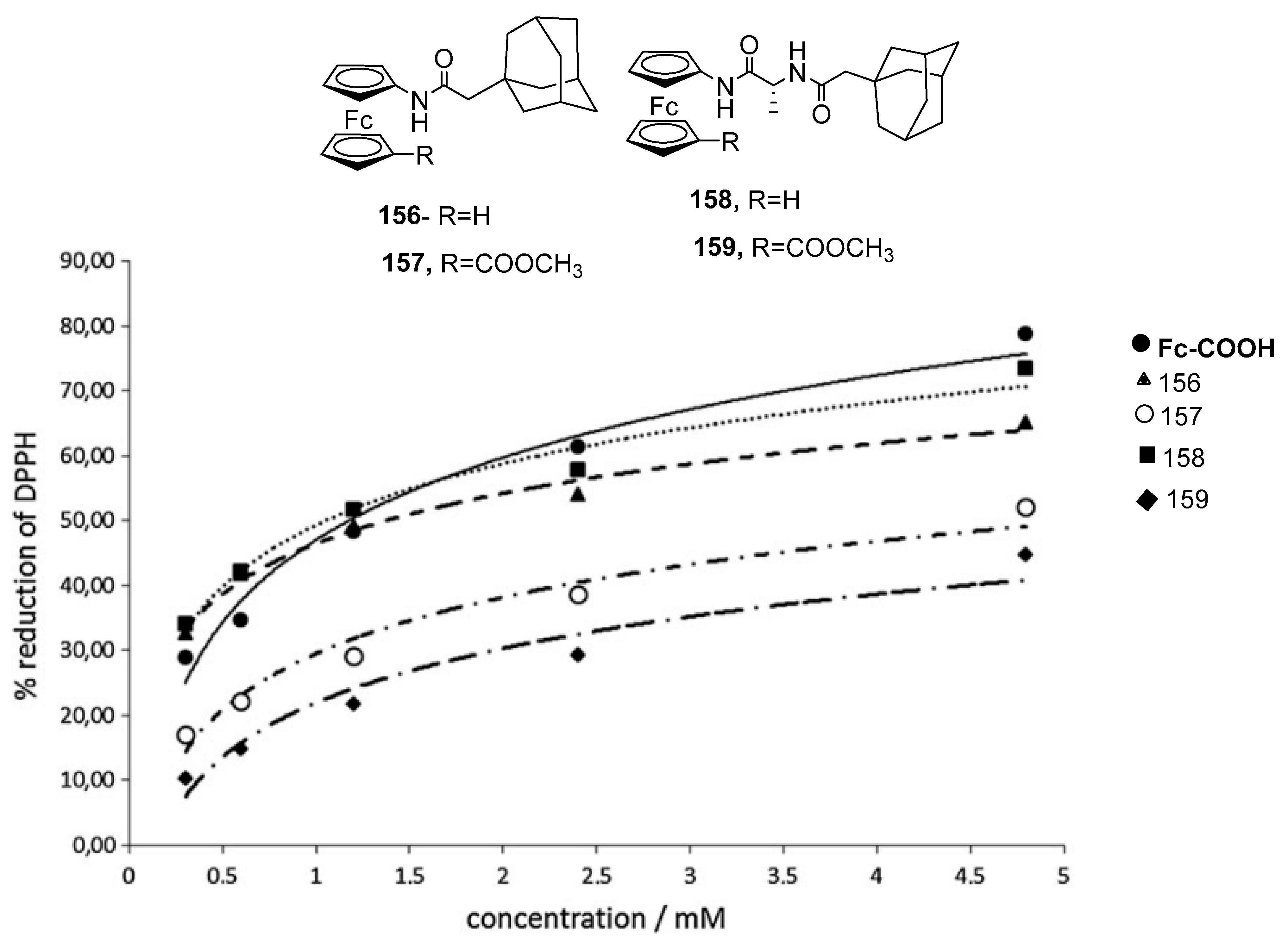
3.6.3. Antioxidant Activity
3.6.4. Antidiabetic Agents
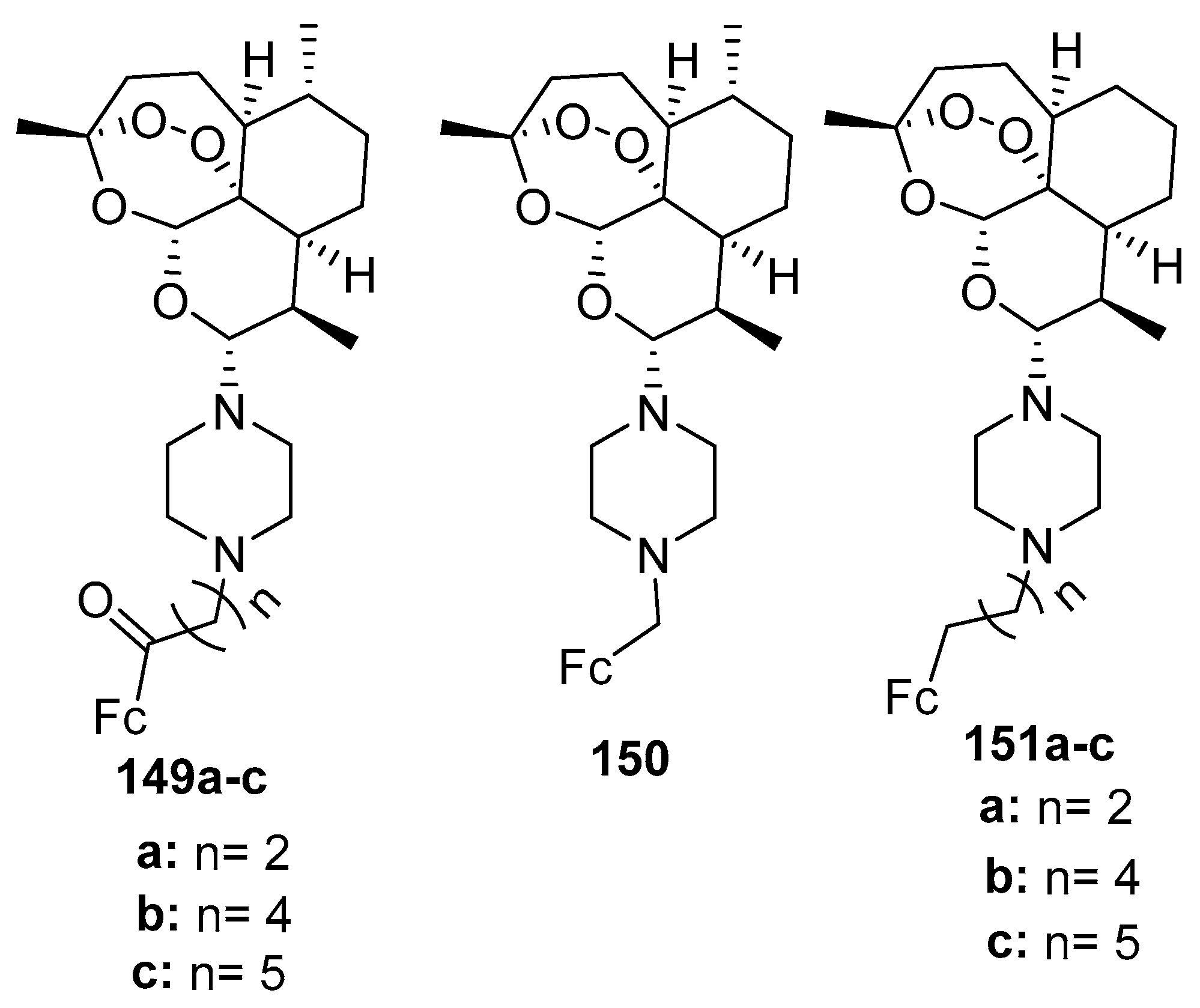
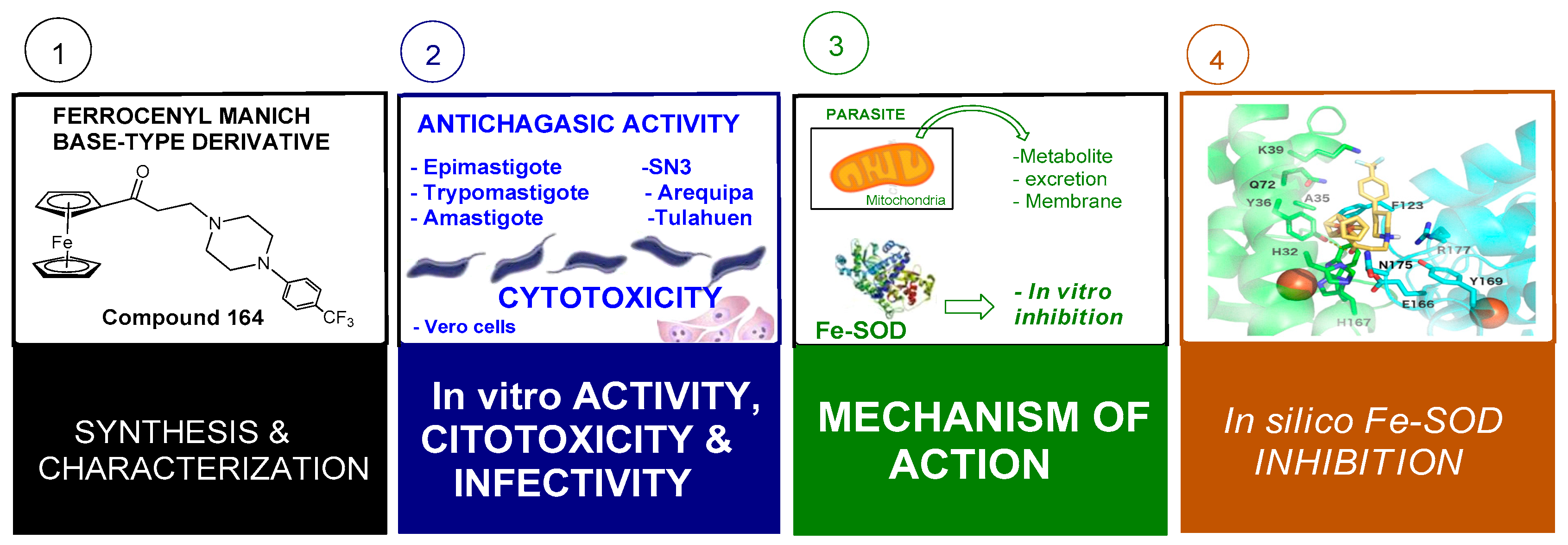
3.6.5. Ferrocene-Grafted Anti-Trypanosomal Agents
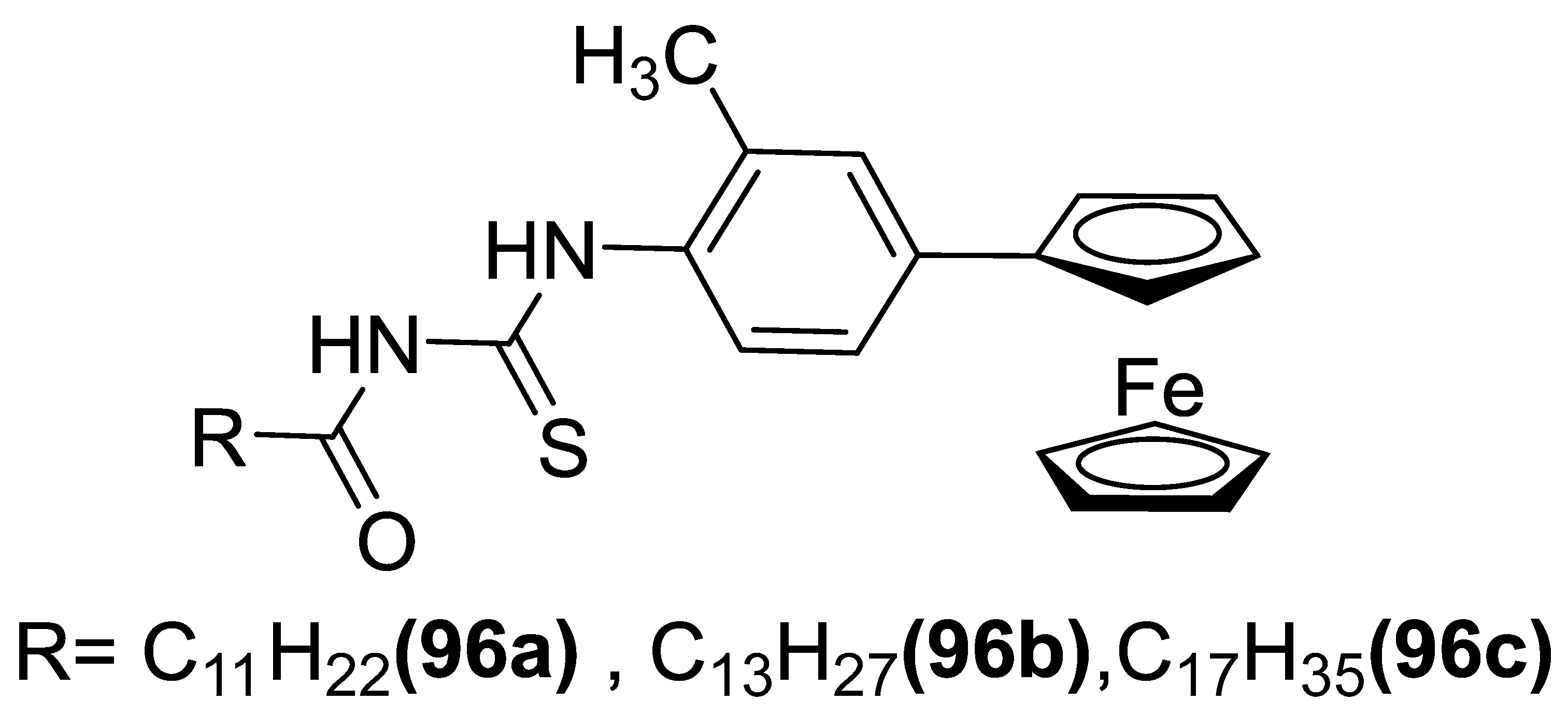
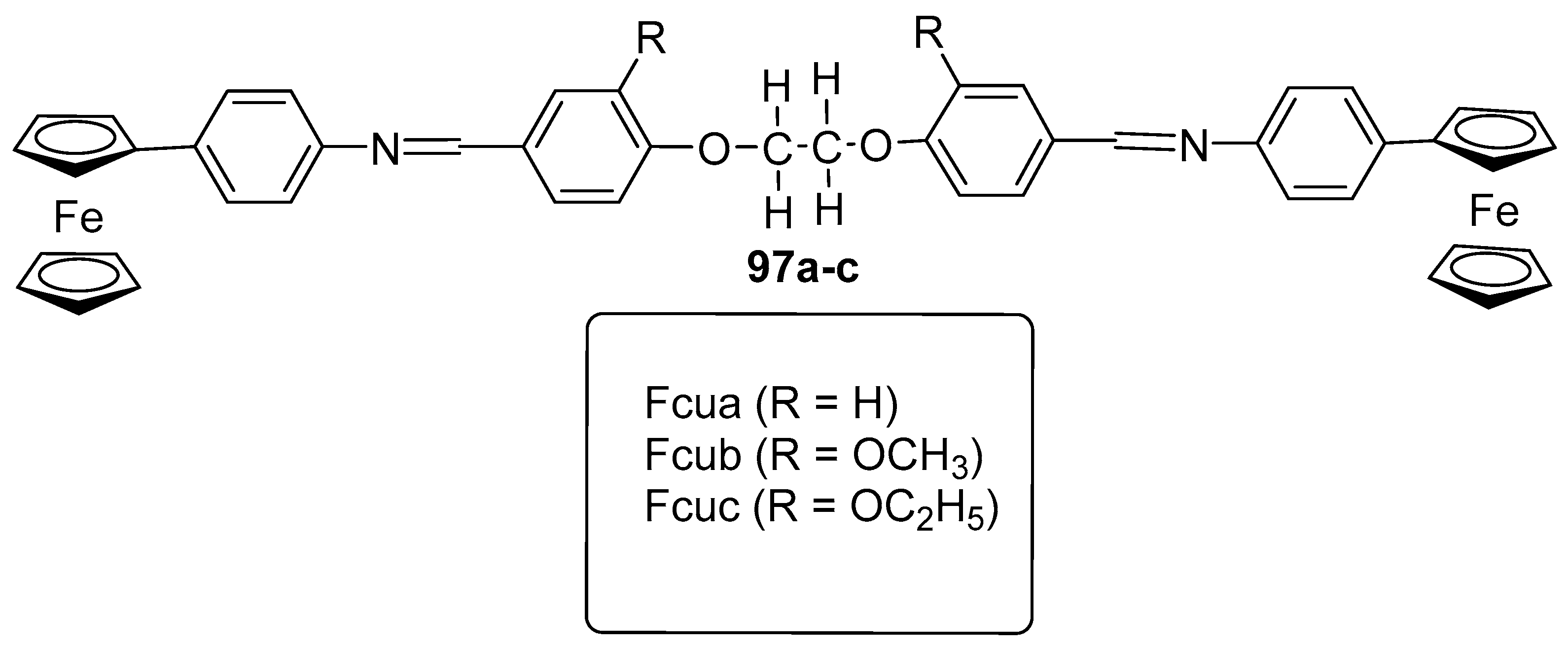
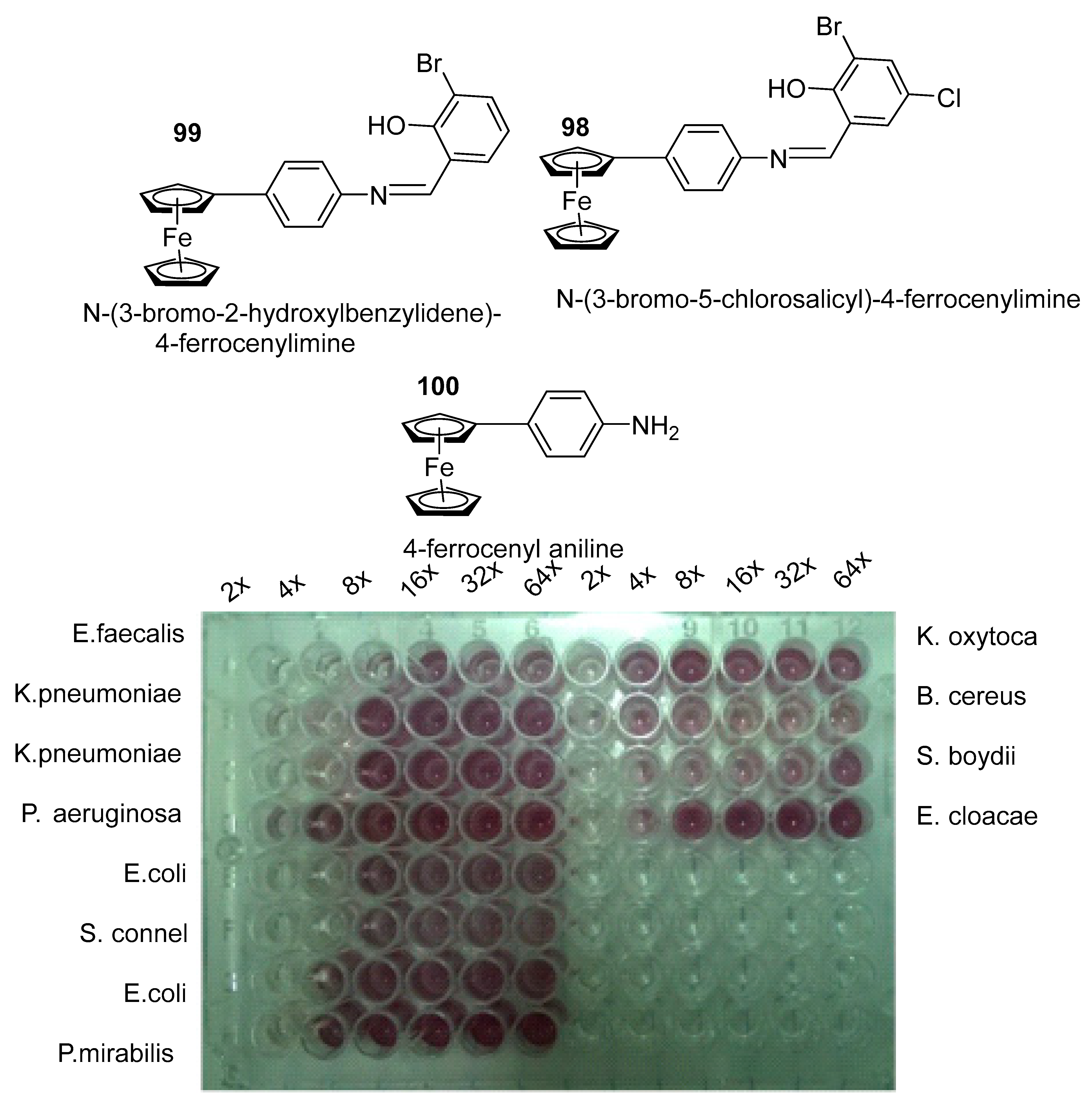
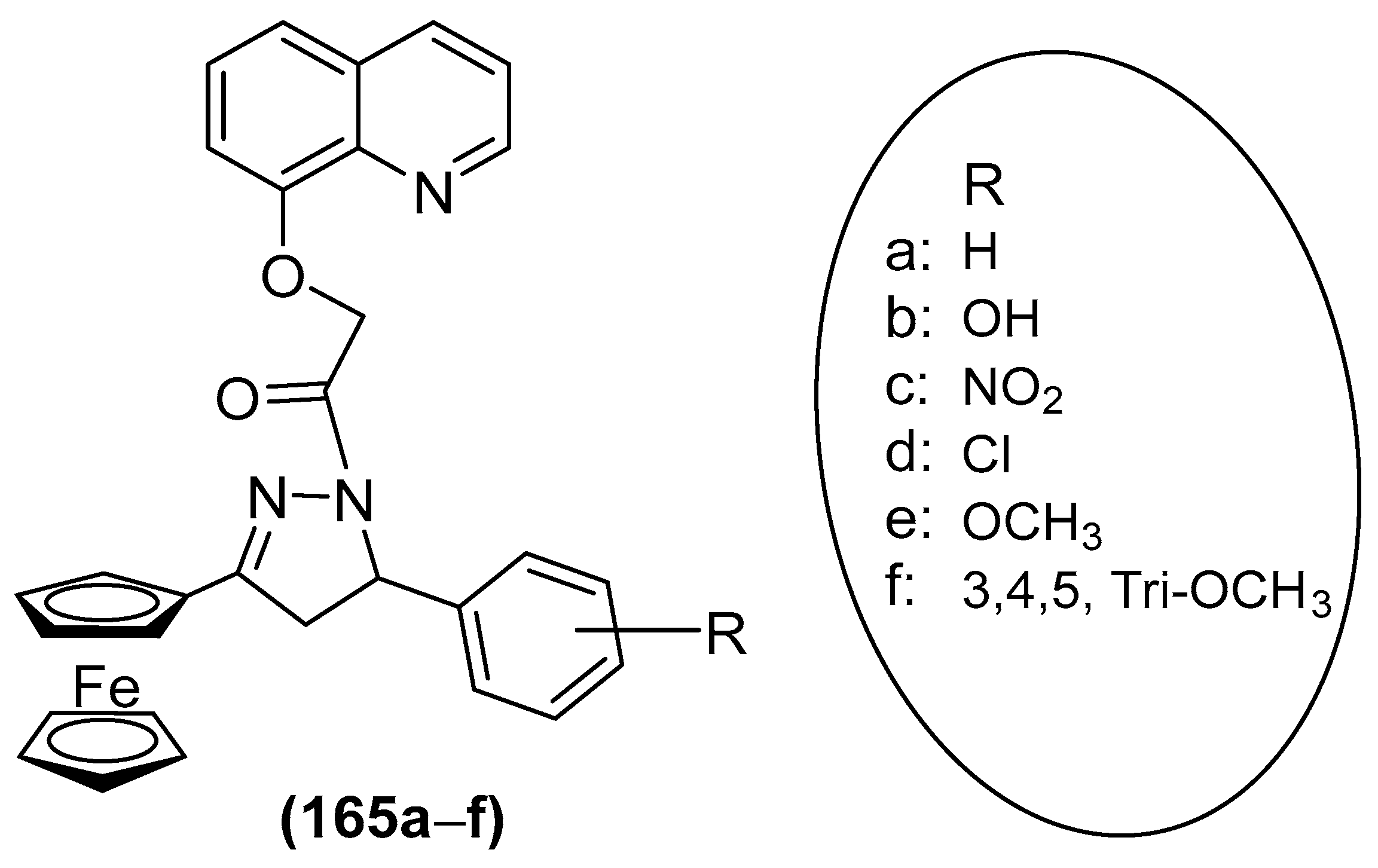
3.6.6. Antimicrobial Agent
3.6.7. Ferrocene Hybrids as BET Bromodomain Inhibitors
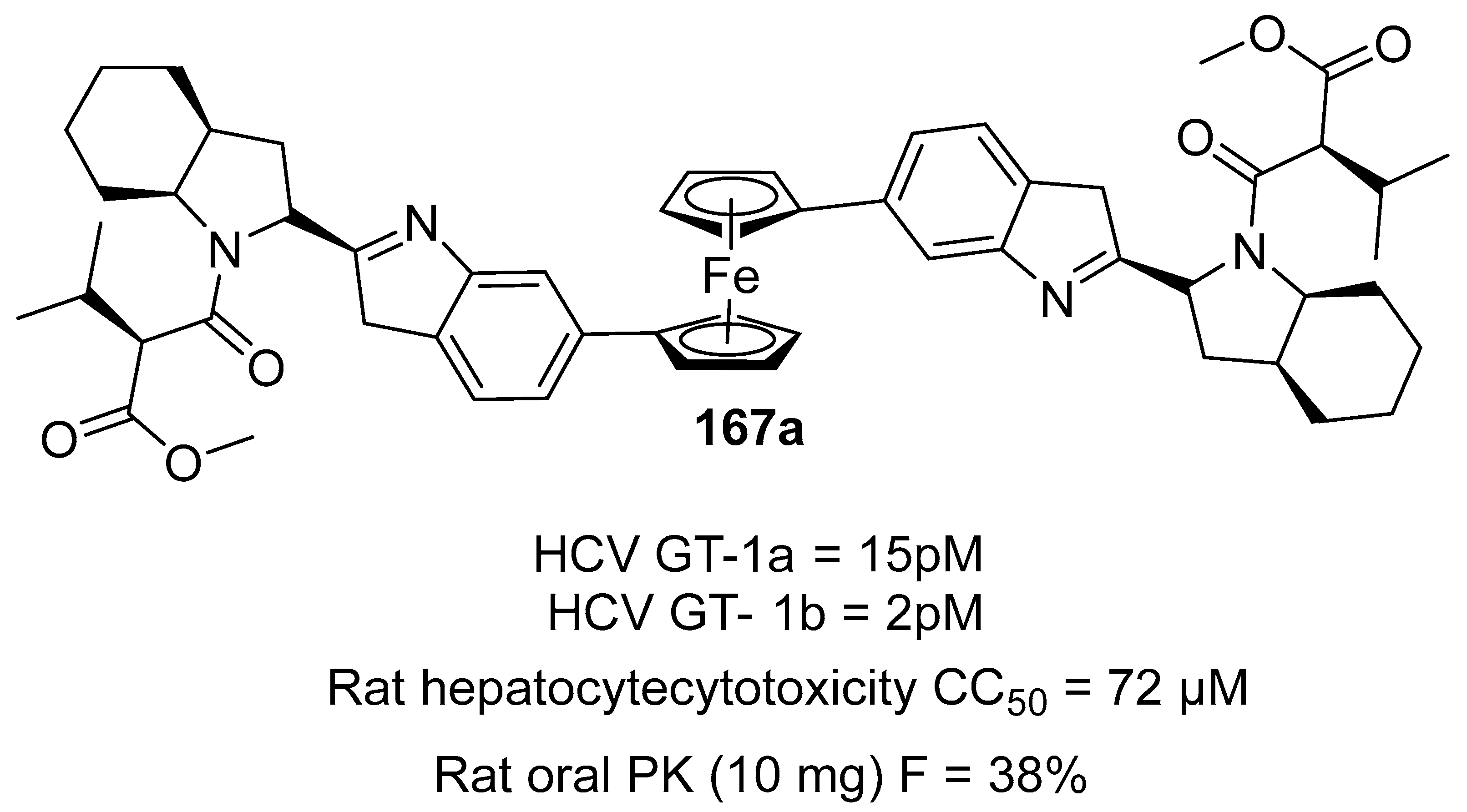
3.6.8. Antiviral Agent
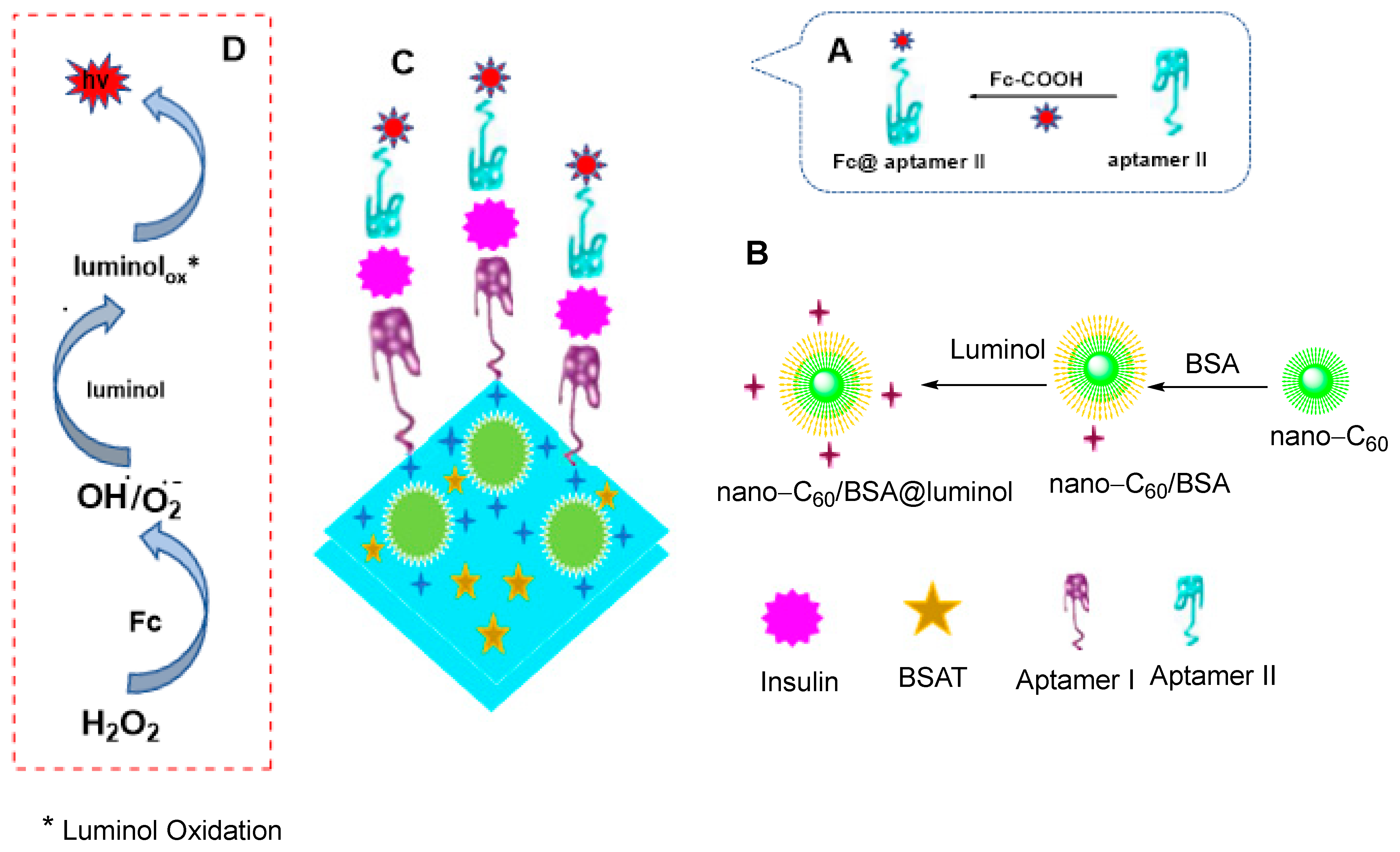
3.7. Electrochemical Insulin Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinze, K.; Lang, H. Ferrocene beauty and function. Organometallics 2013, 32, 5623–5625. [Google Scholar] [CrossRef]
- de Souza, A.; Pires, A.; Soldi, V. Thermal stability of ferrocene derivatives and ferrocene-containing polyamides. J. Therm. Anal. Calorim. 2002, 70, 405–414. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Delavaux-Nicot, B. Ferrocene and ferrocenyl derivatives in luminescent systems. J. Photochem. Photobiol. A 2000, 132, 137–159. [Google Scholar] [CrossRef]
- Saleem, M.; Yu, H.; Wang, L.; Khalid, H.; Akram, M.; Abbasi, N.M.; Huang, J. Review on synthesis of ferrocene-based redox polymers and derivatives and their application in glucose sensing. Anal. Chim. Acta 2015, 876, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Why is ferrocene so exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Atkinson, R.C.; Gibson, V.C.; Long, N.J. The syntheses and catalytic applications of unsymmetrical ferrocene ligands. J. Chem. Soc. Rev. 2004, 33, 313–328. [Google Scholar] [CrossRef]
- Hudson, R.D. Ferrocene polymers: Current architectures, syntheses and utility. J. Organomet. Chem. 2001, 637, 47–69. [Google Scholar] [CrossRef]
- Herrmann, W.A. Ferrocene as a gasoline and fuel additive. In Applied Homogeneous Catalysis with Organometallic Compounds; Cornils, P., Mult., H.C., Herrmann, W.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 586–590. ISBN 9783527328970. [Google Scholar]
- Al-Khalidi, U.K.; Al-Lami, H.S. Ferrocene as accelerator for free radical copolymerization of methyl methacrylate/methacrylic acid monomers. Iraqi J. Polym. 2015, 18, 65–70. [Google Scholar]
- Alvarez, J.; Ren, T.; Kaifer, A.E. Redox potential selection in a new class of dendrimers containing multiple ferrocene centers. Organometallics 2001, 20, 3543–3549. [Google Scholar] [CrossRef]
- Gao, J.M.; Wang, L.; Yu, H.J.; Xiao, A.G.; Ding, W.B. Recent research progress in burning rate catalysts. Propellants Explos. Pyrotech. 2011, 36, 404–409. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Mathi, S.; Gupta, P.K.; Kumar, R.; Nagarale, R.K.; Sharma, A. Ferrocenium ion confinement in polyelectrolyte for electrochemical nitric oxide sensor. ChemistrySelect 2019, 4, 3833–3840. [Google Scholar] [CrossRef]
- Dakmouche, M.; Saidi, M.; Hadjadj, M.; Yousfi, M.; Rahmani, Z. Enhancement of the Inhibitor efficiency of 4-phenyl-1, 2-dithiol-3-thione on corrosion of mild steel for 20% sulphuric acid. Asian J. Chem. 2012, 24, 4887. [Google Scholar]
- Tan, J.; Li, H.; Hu, X.; Abdullah, R.; Xie, S.; Zhang, L.; Zhao, M.; Luo, Q.; Li, Y.; Sun, Z. Size-tunable assemblies based on ferrocene-containing DNA polymers for spatially uniform penetration. Chem 2019, 5, 1775–1792. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Mallik, B. Anomalous photoconductivity of ferrocene. Synth. Met. 1995, 73, 239–245. [Google Scholar] [CrossRef]
- Mamane, V. Metal-catalyzed cross-coupling reactions for ferrocene functionalization: Recent applications in synthesis, material science and asymmetric catalysis. Mini Rev. Org. Chem. 2008, 5, 303–312. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Qi, W.; Su, R.; He, Z. Bioorganometallic ferrocene-tripeptide nanoemulsions. Nanoscale 2017, 9, 15323–15331. [Google Scholar] [CrossRef]
- Falcone, N.; Kraatz, H.-B. Ferrocene peptide-based supramolecular gels: Current trends and applications. In Advances in Bioorganometallic Chemistry; Hirao, T., Moriuchi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–74. ISBN 9780128141977. [Google Scholar]
- Larik, F.A.; Saeed, A.; Fattah, T.A.; Muqadar, U.; Channar, P.A. Recent advances in the synthesis, biological activities and various applications of ferrocene derivatives. Appl. Organomet. Chem. 2017, 31, e3664. [Google Scholar] [CrossRef]
- Zhai, X.; Yu, H.; Wang, L.; Deng, Z.; Abdin, Z.U.; Tong, R.; Yang, X.; Chen, Y.; Saleem, M. Recent research progress in the synthesis, properties and applications of ferrocene-based derivatives and polymers with azobenzene. Appl. Organomet. Chem. 2016, 30, 62–72. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Yu, H.; Khan, R.U.; Haroon, M. Ferrocene-based redox-responsive polymer gels: Synthesis, structures and applications. J. Organomet. Chem. 2017, 828, 38–51. [Google Scholar] [CrossRef]
- Khan, A.; Wang, L.; Yu, H.; Haroon, M.; Ullah, R.S.; Nazir, A.; Elshaarani, T.; Usman, M.; Fahad, S.; Haq, F. Research advances in the synthesis and applications of ferrocene-based electro and photo responsive materials. Appl. Organomet. Chem. 2018, 32, e4575. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S. Antimicrobial activities of ferrocenyl complexes: A review. J. Appl. Pharm. Sci. 2018, 8, 156–165. [Google Scholar]
- Gao, C.; Chang, L.; Xu, Z.; Yan, X.-F.; Ding, C.; Zhao, F.; Wu, X.; Feng, L.-S. Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents. Eur. J. Med. Chem. 2019, 163, 404–412. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Correia, J.D.; Kühn, F.E. Ferrocene derivatives as anti-infective agents. Coord. Chem. Rev. 2019, 396, 22–48. [Google Scholar] [CrossRef]
- Oparina, L.A.; Kolyvanov, N.A.; Tarasova, O.A.; Albanov, A.I.; Tantsyrev, A.P.; Trofimov, B.A. Nucleophilic Addition of 1, 1′-Bis (hydroxymethyl) ferrocene to Alkynes: Synthesis of ferrocene diethenyl ethers. Synthesis 2020, 52, 320–326. [Google Scholar] [CrossRef]
- Korb, M.; Lang, H. Multi-ferrocenyl aryl ethers–applying nucleophilic aromatic substitution reactions to aryl fluorides. Eur. J. Inorg. Chem. 2017, 2017, 276–287. [Google Scholar] [CrossRef]
- Soni, A.; Upadhyay, Y.; Srivastava, A.K.; Sharma, C.; Joshi, R.K. A facile synthesis of ferrocene functionalized vinyl ethers and their application as optical sensors for Cu2+ ions detection. Inorganica Chim. Acta 2023, 548, 121371. [Google Scholar] [CrossRef]
- Abbasi, H.; Teimuri-Mofrad, R. Synthesis and characterization of novel ferrocenyl glycidyl ether polymer, ferrocenyl poly (epichlorohydrin) and ferrocenyl poly (glycidyl azide). Appl. Organomet. Chem. 2020, 34, e5270. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.; Liao, B.; Chen, L.; Zhou, H.; Zhou, Z.; Xia, B.; Huang, J.; Liu, B. Synthesis of novel ferrocene-based conjugated microporous polymers with intrinsic magnetism. Eur. Polym. J. 2017, 93, 556–560. [Google Scholar] [CrossRef]
- Mattoussi, M.; Matoussi, F.; Raouafi, N. Non-enzymatic amperometric sensor for hydrogen peroxide detection based on a ferrocene-containing cross-linked redox-active polymer. Sens. Actuators B Chem. 2018, 274, 412–418. [Google Scholar] [CrossRef]
- Mato, M.; Pérez-Caaveiro, C.; Sarandeses, L.A.; Perez Sestelo, J. Ferrocenylindium reagents in palladium-catalyzed cross-coupling reactions: Asymmetric synthesis of planar chiral 2-aryl oxazolyl and sulfinyl ferrocenes. Adv. Synth. Catal. 2017, 359, 1388–1393. [Google Scholar] [CrossRef]
- Schmiel, D.; Butenschön, H. Directed iron-catalyzed ortho-alkylation and arylation: Toward the stereoselective catalytic synthesis of 1, 2-disubstituted planar-chiral ferrocene derivatives. Organometallics 2017, 36, 4979–4989. [Google Scholar] [CrossRef]
- López, E.; Suárez, T.; Ballesteros, A.; López, L.A. Gold (I)-catalyzed reaction of ferrocene and propargylic esters: Synthesis of functionalized ferrocene derivatives. Eur. J. Inorg. Chem. 2017, 2017, 225–228. [Google Scholar] [CrossRef]
- Jawaria, R.; Khan, M.U.; Hussain, M.; Muhammad, S.; Sagir, M.; Hussain, A.; Al-Sehemi, A.G. Synthesis and characterization of ferrocene-based thiosemicarbazones along with their computational studies for potential as inhibitors for SARS-CoV-2. J. Iran. Chem. Soc. 2021, 19, 839–846. [Google Scholar] [CrossRef]
- Ochiai, K.; Fujii, S. Structure-property and structure–activity relationships of phenylferrocene derivatives as androgen receptor antagonists. Bioorganic Med. Chem. Lett. 2021, 46, 128141. [Google Scholar] [CrossRef]
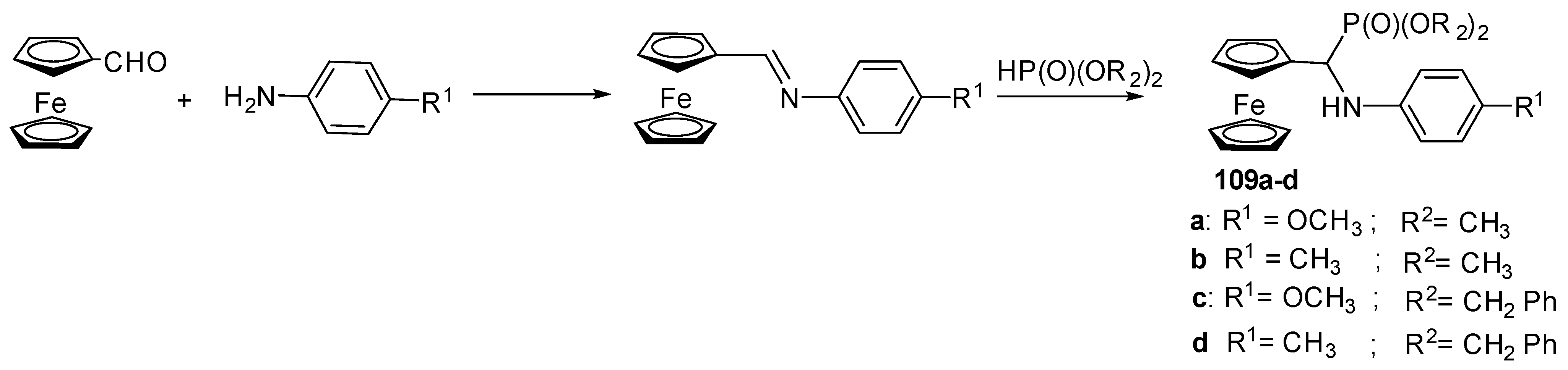
- Amin, B.U.; Yu, H.; Wang, L.; Nazir, A.; Fahad, S.; Haq, F.; Mahmood, S.; Liang, R.; Uddin, M.A.; Lin, T. Recent advances on ferrocene-based compounds and polymers as a burning rate catalysts for propellants. J. Organomet. Chem. 2020, 921, 121368. [Google Scholar] [CrossRef]
- Sattar, M.; Kumar, S. Palladium-catalyzed removable 8-aminoquinoline assisted chemo-and regioselective oxidative sp2-C–H/sp3-C–H cross-coupling of ferrocene with toluene derivatives. Org. Lett. 2017, 19, 5960–5963. [Google Scholar] [CrossRef]
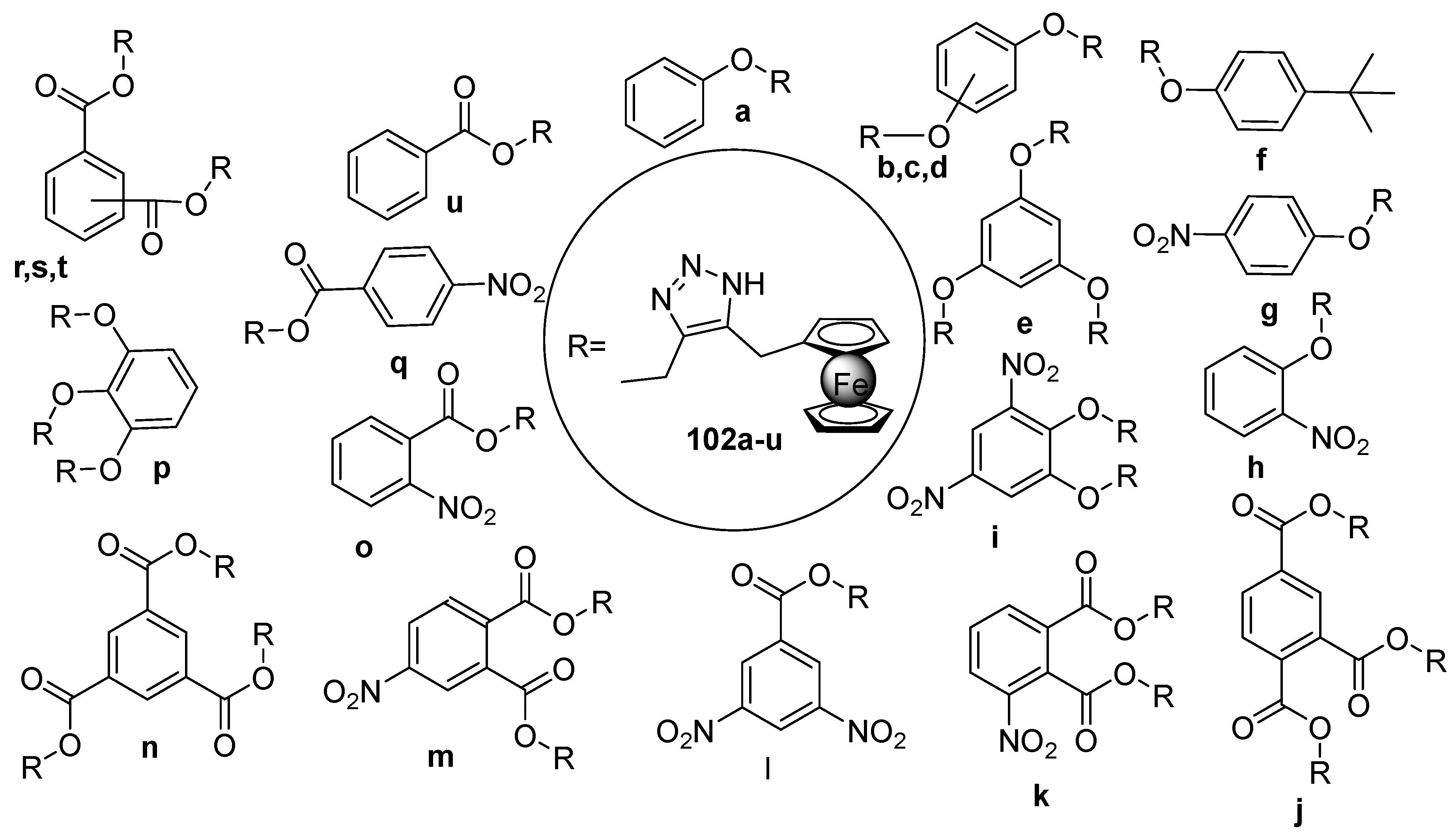
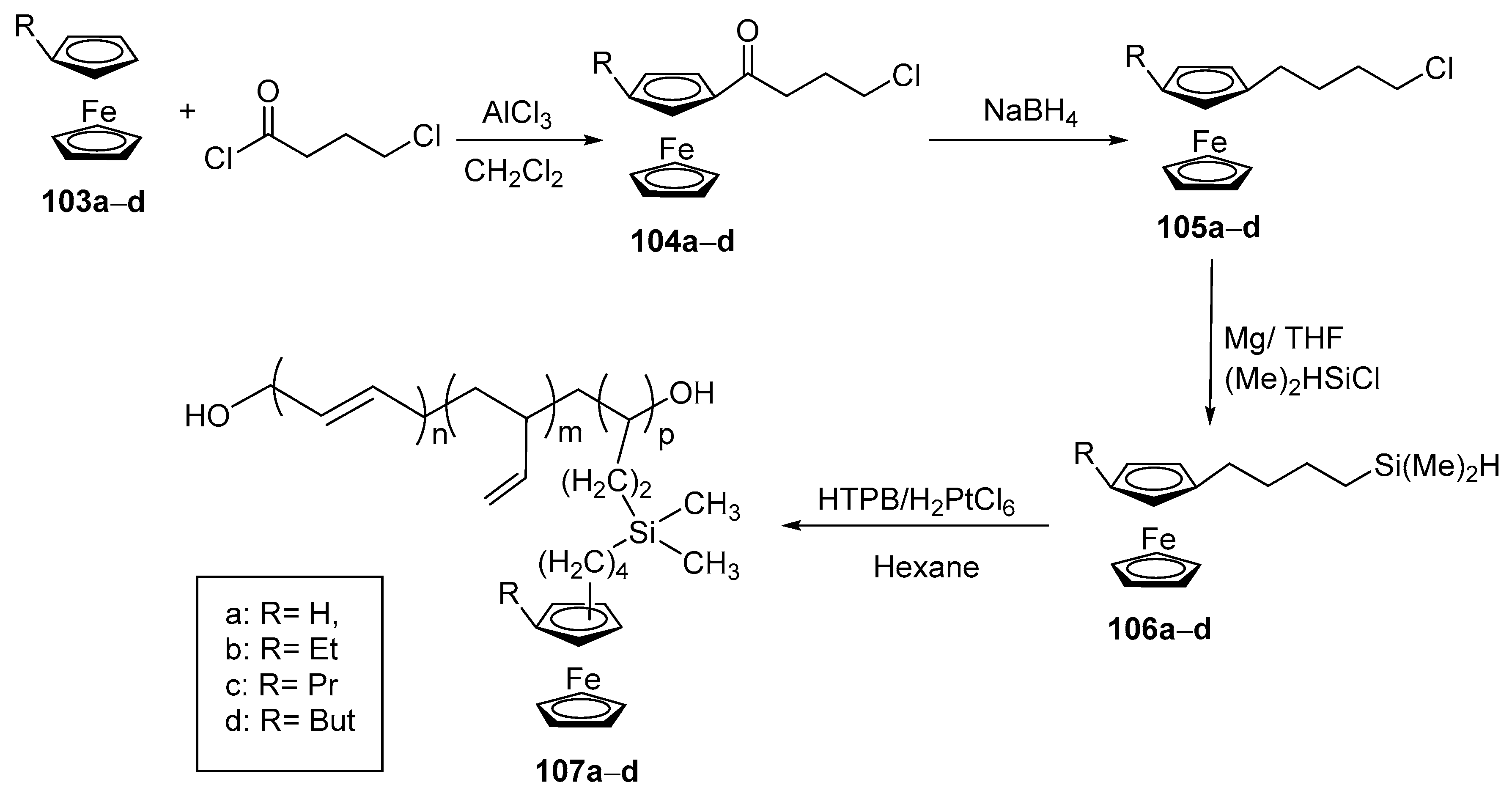
- Kumar Sikder, B.; Naidu Ganivada, M.; Jana, T. Functional alkyl-ferrocene grafted hydroxyl terminated polybutadiene. ChemistrySelect 2021, 6, 8058–8062. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Liu, Y.; Sun, P.; Yang, Z.; Xu, T. Designer ferrocene Catholyte for aqueous organic flow batteries. ChemSusChem 2021, 14, 1295–1301. [Google Scholar] [CrossRef]
- Wang, S.-B.; Gu, Q.; You, S.-L. Cp* Co (III)-catalyzed ortho C-H amidation of 2-pyridinyl ferrocenes with 1, 4, 2-dioxazol-5-ones. J. Catal. 2018, 361, 393–397. [Google Scholar] [CrossRef]
- Ravutsov, M.; Dobrikov, G.M.; Dangalov, M.; Nikolova, R.; Dimitrov, V.; Mazzeo, G.; Longhi, G.; Abbate, S.; Paoloni, L.; Fusè, M. 1, 2-Disubstituted planar chiral ferrocene derivatives from sulfonamide-directed ortho-lithiation: Synthesis, absolute configuration, and chiroptical properties. Organometallics 2021, 40, 578–590. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, H.; Tian, Z.; Zhang, J.; Zhan, F.; Yan, M.; Jia, C. Simple-synthesized sulfonated ferrocene ammonium for aqueous redox flow batteries. ACS Appl. Energy Mater. 2021, 4, 8052–8058. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, S.; Trivedi, M.; Kociok-Köhn, G.; Rath, N.P.; Köhn, R.D.; Muddassir, M.; Kumar, A. New main-group ferrocenyldithiocarbamates and conversion to ferrocene oxazolidine-2-thione and-2-one. New J. Chem. 2020, 44, 3268–3277. [Google Scholar] [CrossRef]
- Gao, X.; Gong, G.; Zhang, Z.; Du, G.; Cao, Y.; Zhao, G. A novel cyclopalladated ferrocene derivative: Synthesis, single crystal structure and evaluation of in vitro antitumor activity. J. Mol. Struct. 2020, 1200, 127077. [Google Scholar] [CrossRef]
- Sayed, F.N.; Mohamed, G.G. Newly synthesized lanthanides complexes of ferrocene-based Schiff base with high biological activities and improved molecular docking data. J. Organomet. Chem. 2022, 977, 122450. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, G.; Gong, G.; Sheng, Y.; Lu, X.; Cai, W.; Wang, F.; Zhao, G. A novel ferrocene-palladium metal complex: Synthesis, single crystal structure, in vitro cytotoxicity study and molecular docking. J. Mol. Struct. 2021, 1232, 130021. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Y.; Yin, D.; Yang, X.; Yang, L.; Zou, Q. One-pot, multi-component synthesis of 3, 4-dihydropyrimidin-2 (1H)-one derivatives containing ferrocenyl. Res. Chem. Intermed. 2020, 46, 547–555. [Google Scholar] [CrossRef]
- Minić, A.; Pešić, M.S.; Novaković, S.B.; Bogdanović, G.A.; Todosijević, A.; Komatina, D.I.; Stevanović, D. Synthesis, structural and electrochemical characterization of novel ferrocene-containing tetrahydropyrimidin-2 (1H)-ones. J. Organomet. Chem. 2020, 923, 121422. [Google Scholar] [CrossRef]
- Minić, A.; Novaković, S.B.; Bogdanović, G.A.; Bugarinović, J.P.; Pešić, M.S.; Todosijević, A.; Komatina, D.I.; Damljanović, I.; Stevanović, D. Synthesis and structural characterizations of novel atropoisomeric ferrocene-containing six-membered cyclic ureas. Polyhedron 2020, 177, 114316. [Google Scholar] [CrossRef]
- Li, H.; Chi, W.; Liu, Y.; Yuan, W.; Li, Y.; Li, Y.; Tang, B.Z. Ferrocene-based hyperbranched polytriazoles: Synthesis by click polymerization and application as precursors to nanostructured magnetoceramics. Macromol. Rapid Commun. 2017, 38, 1700075. [Google Scholar] [CrossRef]
- Maračić, S.; Jakopec, S.; Piškor, M.; Leventić, M.; Lapić, J.; Djaković, S.; Raić-Malić, S. Mechanochemical synthesis and antiproliferative activity of novel ferrocene quinoline/quinolone hybrids. J. Organomet. Chem. 2023, 37, e7124. [Google Scholar] [CrossRef]
- Panda, K.N.; Thorat, K.G.; Ravikanth, M. Covalently linked meso-tetraaryl triphyrin (2.1. 1)-ferrocene (s) conjugates: Synthesis and properties. Org. Biomol. Chem. 2019, 17, 5066–5074. [Google Scholar] [CrossRef]
- Snegur, L.V.; Lyapunova, M.V.; Verina, D.D.; Kachala, V.V.; Korlyukov, A.A.; Ilyin, M.M., Jr.; Davankov, V.A.; Ostrovskaya, L.A.; Bluchterova, N.V.; Fomina, M.M. Nitro-imidazoles in ferrocenyl alkylation reaction. Synthesis, enantiomeric resolution and in vitro and in vivo bioeffects. J. Organomet. Chem. 2018, 871, 10–20. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Ma, C.; Li, Y. Synthesis of ferrocene-based polysubstituted pyrrolidines. Chem. Heterocycl. Compd. 2019, 55, 939–942. [Google Scholar] [CrossRef]
- Prabu, S.; David, E.; Viswanathan, T.; Thirumoorthy, K.; Panda, T.; Dragonetti, C.; Colombo, A.; Marinotto, D.; Righetto, S.; Roberto, D. NLO-active Y-shaped ferrocene conjugated imidazole chromophores as precursors for SHG polymeric films. Dalton Trans. 2020, 49, 1854–1863. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Yin, J.; Yin, D. Microwave-assisted synthesis of 3-substituted-6-ferrocene methylene-1, 2, 4-triazolo [3, 4-b]-1, 3, 4-thiadiazoles. Transit. Met. Chem. 2018, 43, 381–385. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M.; Zayed, M.E.; Parveen, H.; Aqlan, F.M.; Sharma, K. Microwave-assisted synthesis, characterization, and density functional theory study of biologically active ferrocenyl bis-pyrazoline and bis-pyrimidine as organometallic macromolecules. J. Heterocycl. Chem. 2019, 56, 312–318. [Google Scholar] [CrossRef]
- Shibata, T.; Uno, N.; Sasaki, T.; Kanyiva, K.S. Pt-catalyzed enantioselective cycloisomerization for the synthesis of planar-chiral ferrocene derivatives. J. Org. Chem. 2016, 81, 6266–6272. [Google Scholar] [CrossRef]
- Jia, L.; Liu, X.; Zhang, A.-A.; Wang, T.; Hua, Y.; Li, H.; Liu, L. Synthesis of planar chiral ferrocenes via a Pd (0)-catalyzed syn-carbopalladation/asymmetric C–H alkenylation process. Chem. Commun. 2020, 56, 1737–1740. [Google Scholar] [CrossRef]
- Rozhkova, Y.S.; Plekhanova, I.V.; Gorbunov, A.A.; Stryapunina, O.G.; Chulakov, E.N.; Krasnov, V.P.; Ezhikova, M.A.; Kodess, M.I.; Slepukhin, P.A.; Shklyaev, Y.V. Synthesis of novel racemic 3, 4-dihydroferroceno [c] pyridines via the Ritter reaction. Tetrahedron Lett. 2019, 60, 768–772. [Google Scholar] [CrossRef]
- Kameoka, A.; Tsuchiya, K. Influence of ferrocene on engine and vehicle performance. SAE Int. J. Engines 2006, 1, 34–48. [Google Scholar]
- Janecki, H. Ferrocene derivatives in boundary lubrication. Tribol. Lett. 2006, 24, 99–103. [Google Scholar] [CrossRef]
- Apreutesei, D.; Lisa, G.; Scutaru, D.; Hurduc, N. Investigations on thermal stability of some ferrocene liquid crystals bearing azo, ferrocenyl and cholesteryl units. J. Optoelectron. Adv. Mater. 2006, 8, 737–740. [Google Scholar]
- Yousefinejad, S.; Honarasa, F.; Solhjoo, A. On the solubility of ferrocene in nonaqueous solvents. J. Chem. Eng. Data 2016, 61, 614–621. [Google Scholar] [CrossRef]
- Saleem, M.; Yu, H.; Wang, L.; Khalid, H.; Akram, M.; Abbasi, N.M.; Chen, Y. Study on synthesis of ferrocene-based boronic acid derivatives and their saccharides sensing properties. J. Electroanal. Chem. 2016, 763, 71–78. [Google Scholar] [CrossRef]
- Rabti, A.; Mayorga-Martinez, C.C.; Baptista-Pires, L.; Raouafi, N.; Merkoçi, A. Ferrocene-functionalized graphene electrode for biosensing applications. Anal. Chim. Acta 2016, 926, 28–35. [Google Scholar] [CrossRef]
- Dong, W.; Wang, K.; Chen, Y.; Li, W.; Ye, Y.; Jin, S. Construction and characterization of a chitosan-immobilized-enzyme and β-cyclodextrin-included-ferrocene-based electrochemical biosensor for H2O2 detection. Materials 2017, 10, 868. [Google Scholar] [CrossRef]
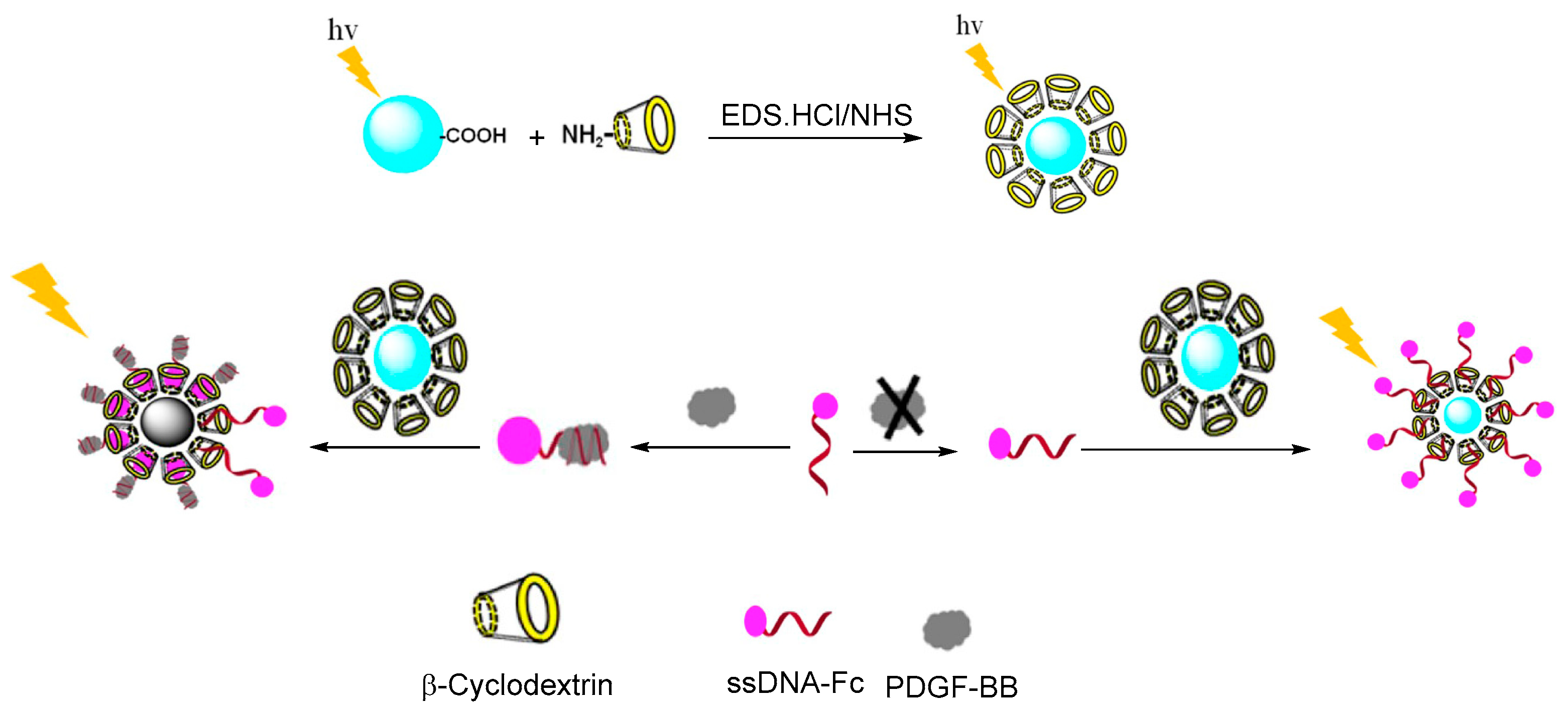
- Lu, X.; Wen, X.; Fan, Z. A sensitive biosensor based on a ferrocene-marked adapter for the fluorescence detection of platelet-derived growth factor BB. J. Lumin. 2020, 221, 117042. [Google Scholar] [CrossRef]
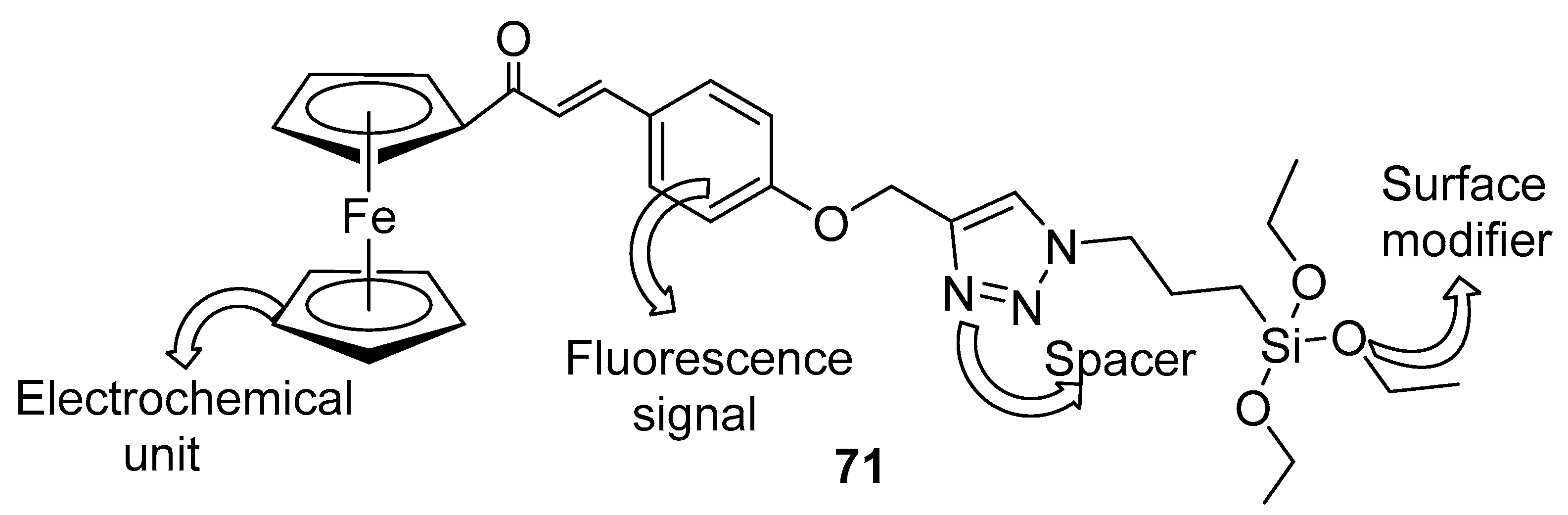
- Singh, G.; Arora, A.; Rani, S.; Kalra, P.; Kumar, M. A click-generated triethoxysilane tethered ferrocene-chalcone-triazole triad for selective and colorimetric detection of Cu2+ ions. ChemistrySelect 2017, 2, 3637–3647. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Teng, Q.; Zhang, H. Ferrocenyl-based thioethers and sulphones as optical, and electrochemical sensors for the differential detection of Hg2+ and Cu2+ ions. Sens. Actuators B Chem. 2017, 238, 166–174. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, Y.; Gong, G.; Zhang, Y.; Zhao, H.; Gao, X.; Zhao, G. A simple but effective ferrocene derivative as a redox, and fluorescent receptor for highly selective recognition of Al3+ ions. Inorg. Chem. Commun. 2018, 96, 170–174. [Google Scholar] [CrossRef]
- Atasen, S.K.; Alcay, Y.; Yavuz, O.; Yucel, B.; Yilmaz, I. Beryllium ion sensing through the ion pair formation between the electrochemically reduced ferrocenyl naphthoquinone radicals and Be2+ ions. J. Chem. Sci. 2019, 131, 41. [Google Scholar] [CrossRef]
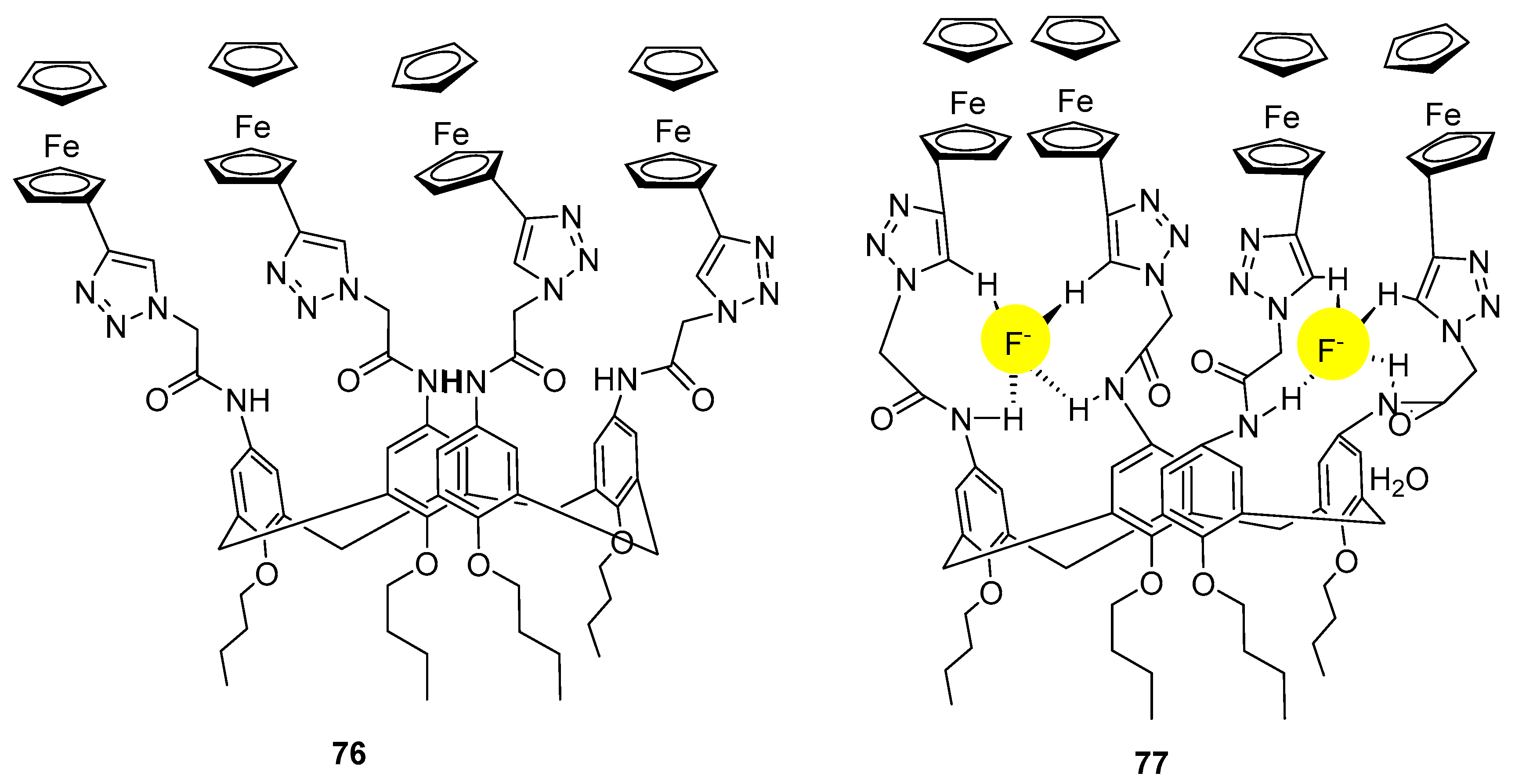
- Hosseinzadeh, R.; Maliji, F.; Golchoubian, H.; Bekhradnia, A. A novel ferrocene-based calix [4] arene as an efficient optical and electrochemical sensor for highly selective fluoride recognition. ChemistrySelect 2019, 4, 3914–3920. [Google Scholar] [CrossRef]
- Wu, P.; Wang, G.; Zhou, L.; Lu, J.; Wang, J. The first colorimetric receptor for the B4O72− anion based on nitro substituted phenanthro imidazole ferrocene derivatives. RSC Adv. 2018, 8, 3782–3788. [Google Scholar] [CrossRef]
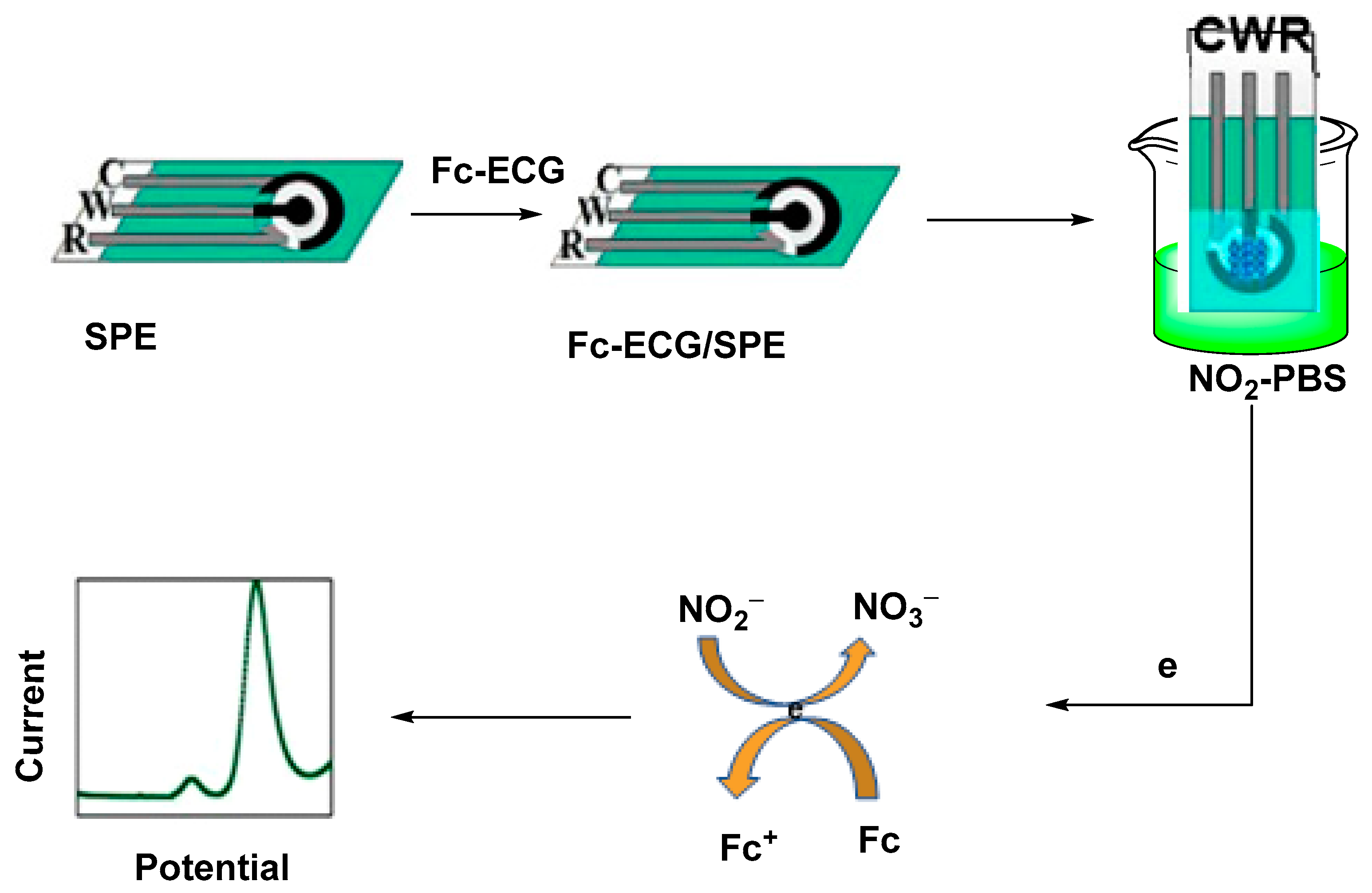
- Feng, X.-Z.; Ferranco, A.; Su, X.; Chen, Z.; Jiang, Z.; Han, G.-C. A facile electrochemical sensor labeled by ferrocenoyl cysteine conjugate for the detection of nitrite in pickle juice. Sensors 2019, 19, 268. [Google Scholar] [CrossRef]
- Boink, A.; Speijers, G. Health effects of nitrates and nitrites, a review. Acta Hortic. 1999, 563, 29–36. [Google Scholar] [CrossRef]
- Zaleskaya, M.; Jagleniec, D.; Karbarz, M.; Dobrzycki, Ł.; Romański, J. Squaramide based ion pair receptors possessing ferrocene as a signaling unit. Inorg. Chem. Front. 2020, 7, 972–983. [Google Scholar] [CrossRef]
- Reddy, P.G.; MG, M.; Joseph, A.M.; Nandi, S.; Ghosh, S.; Pradeep, C.P.; Sharma, S.K.; Gonsalves, K.E. Ferrocene bearing non-ionic poly-aryl tosylates: Synthesis, characterization and electron beam lithography applications. J. Photopolym. Sci. Technol. 2018, 31, 669–678. [Google Scholar] [CrossRef]
- Shi, Y.; Xiao, L.; Wu, D.; Li, F.; Li, D.; Zhang, J.; Li, S.; Zhou, H.; Wu, J.; Tian, Y. Synthesis, crystal structure, electrochemistry and third-order nonlinear optical properties of two novel ferrocene derivatives. J. Organomet. Chem. 2016, 817, 36–42. [Google Scholar] [CrossRef]
- Pielak, K.; Bondu, F.; Sanguinet, L.; Rodriguez, V.; Castet, F.; Champagne, B. Acid-triggered switching of the second-order nonlinear optical properties of a ferrocenyl-containing indolino-oxazolidine derivative. Dyes Pigm. 2019, 160, 641–646. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.; Kociok-Köhn, G.; Trivedi, M.; Kumar, A.; Chauhan, R.; Rane, S.B.; Terashima, C.; Gosavi, S.W.; Fujishima, A. 1, 1′-Bis (diphenylphosphino) ferrocene-appended nickel (ii) dithiolates as sensitizers in dye-sensitized solar cells. New J. Chem. 2018, 42, 9306–9316. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Wu, F.-Y.; Liu, J.-C. New type of ferrocene group substituted porphyrin axial coordinate self-assembly for dye-sensitized solar cells. Org. Electron. 2019, 71, 290–295. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Mohammad, A.; Mobin, S.M. Design and construction of a ferrocene based inclined polycatenated Co-MOF for supercapacitor and dye adsorption applications. J. Mater. Chem. A 2017, 5, 17998–18011. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Abbasi, H.; Hadi, R. Graphene oxide-grafted ferrocene moiety via ring opening polymerization (ROP) as a supercapacitor electrode material. Polymer 2019, 167, 138–145. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, L.; Cai, P.; Zeng, G.; Li, X.; Wen, Z.; Chen, L. Ferrocene-based porous organic polymer derived high-performance electrocatalysts for oxygen reduction. J. Mater. Chem. A 2017, 5, 22163–22169. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, L.; Su, X.; Qin, C.; Zhao, K.; Hu, F.; Zhou, M.; Xia, Y. Micro-sized organometallic compound of ferrocene as high-performance anode material for advanced lithium-ion batteries. J. Power Sources 2018, 375, 102–105. [Google Scholar] [CrossRef]
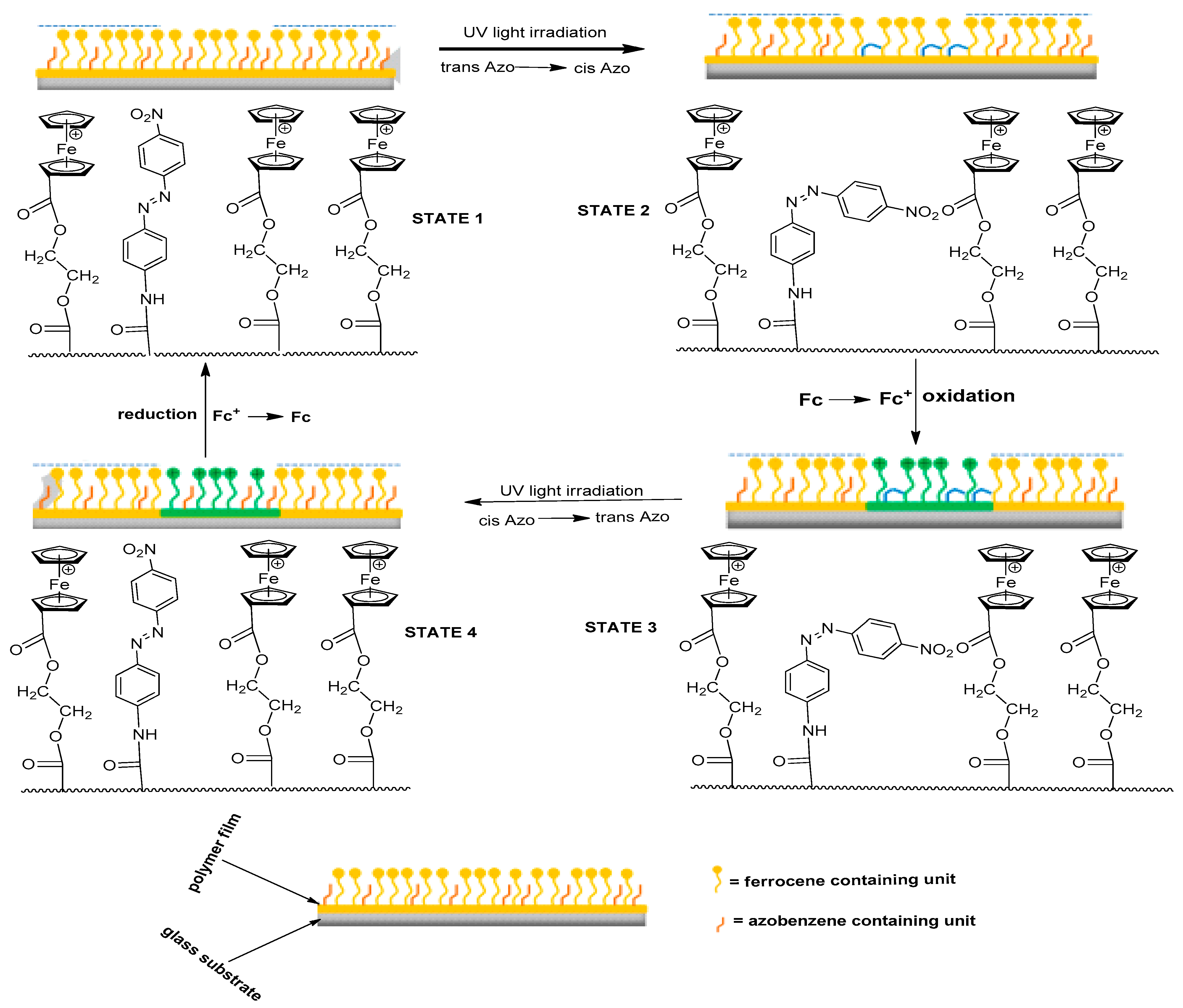
- Xia, X.; Yu, H.; Wang, L.; Deng, Z.; Shea, K.J. Preparation of redox-and photo-responsive ferrocene-and azobenzene-based polymer films and their properties. Eur. Polym. J. 2018, 100, 103–110. [Google Scholar] [CrossRef]
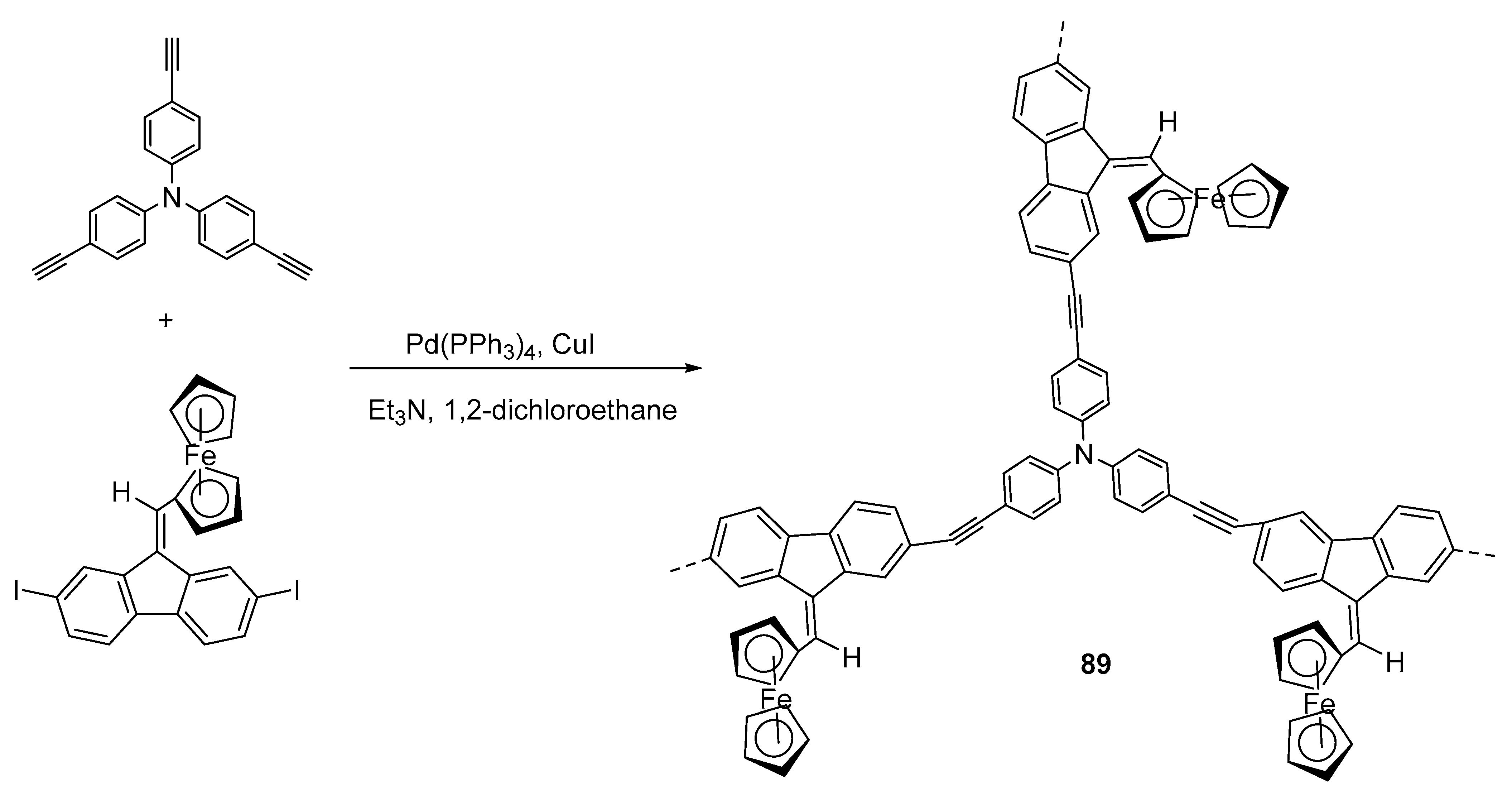
- Karmakar, M.; Bhatta, S.R.; Giri, S.; Thakur, A. Oxidation-induced differentially selective turn-on fluorescence via photoinduced electron transfer based on a ferrocene-appended coumarin–quinoline platform: Application in cascaded molecular logic. Inorg. Chem. 2020, 59, 4493–4507. [Google Scholar] [CrossRef]
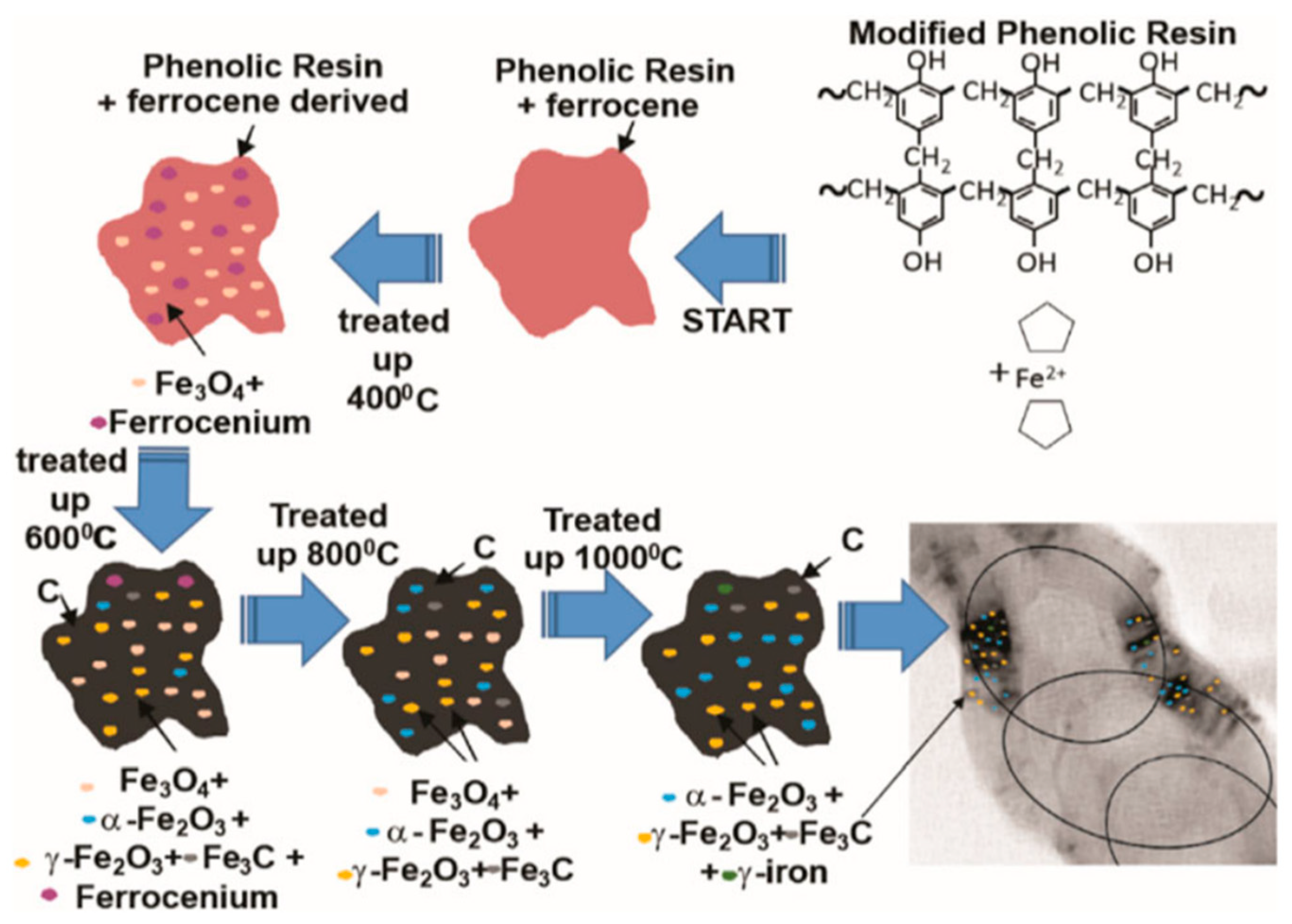
- Renda, C.G.; Contreras Medrano, C.P.; Costa, L.J.D.; Litterst, F.J.; Saitovitch, E.M.B.; Magon, C.J.; Gualdi, A.J.; Venâncio, T.; Bertholdo, R.; Moreira, A.J. Role of ferrocene-derived iron species in the catalytic graphitization of novolak resins. J. Mater. Sci. 2021, 56, 1298–1311. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Xiong, S.; Lu, P.; Tang, J.; He, J.; Javaid, M.U.; Pan, C.; Yu, G. Ferrocene-based porous organic polymers for high-affinity iodine capture. Chem. Eng. J. 2020, 380, 122420. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, D.; Liu, Y.; Guo, X.; Zhu, Y.; Meng, Z.; Yu, Z.-Q.; Wong, W.-Y. Ferrocene-based hyperbranched polymers: A synthetic strategy for shape control and applications as electroactive materials and precursor-derived magnetic ceramics. J. Mater. Chem. C 2020, 8, 10774–10780. [Google Scholar] [CrossRef]
- Wang, H.; Lu, W.; Zhang, J. Ferrocene derived bifunctional phosphine-catalyzed asymmetric Oxa[4 + 2] cycloaddition of α-substituted allenones with enones. Chem. Eur. J. 2018, 23, 13587–13590. [Google Scholar] [CrossRef]
- Mo, Y.Z.; Wang, Q.J.; Nie, H.F.; Wang, Q.F. The Application of New Chiral Ferrocene Ligands in Asymmetric Transfer Hydrogenation of Ketones. Int. J. Org. Chem. 2018, 8, 54–83. [Google Scholar]
- Sánchez-Rodríguez, E.; Hochberger-Roa, F.; Corona-Sánchez, R.; Barquera-Lozada, J.; Toscano, R.; Urrutigoïty, M.; Gouygou, M.; Ortega-Alfaro, M.; López-Cortés, J. Chiral bidentate [N, S]-ferrocene ligands based on a thiazoline framework. Synthesis and use in palladium-catalyzed asymmetric allylic alkylation. Dalton Trans. 2017, 46, 1510–1519. [Google Scholar] [CrossRef]
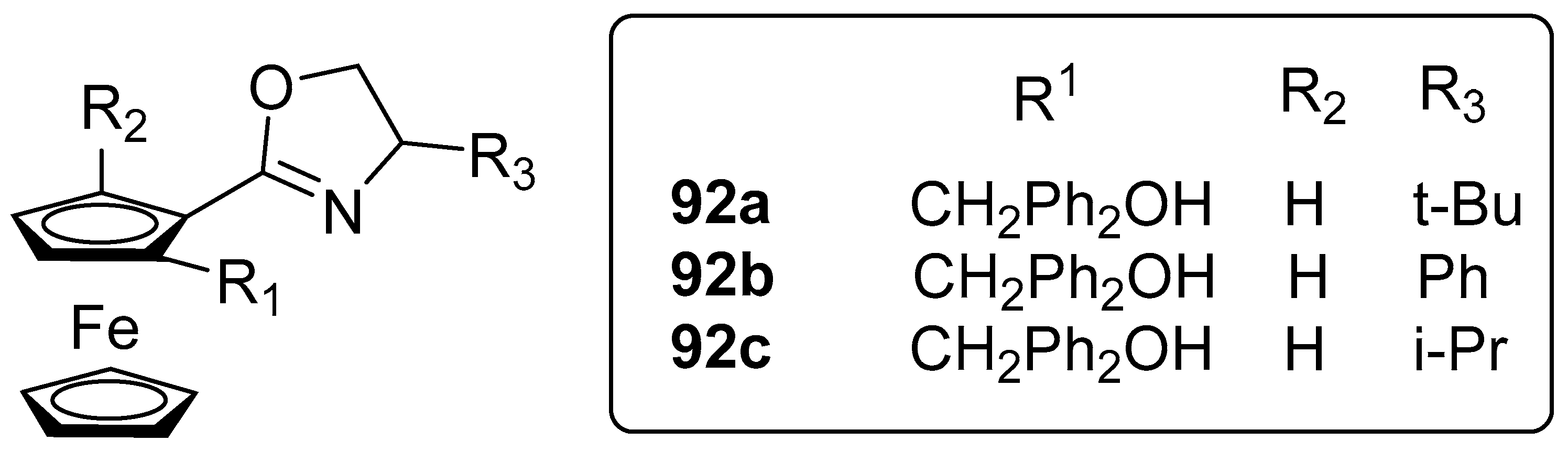
- Xu, D.; Zhang, J.; Dai, L. Chiral oxazolinyl hydroxyl ferrocene catalysts: Synthesis and applications in asymmetric reactions. ChemistrySelect 2020, 5, 9443–9456. [Google Scholar] [CrossRef]
- Xu, G.; Gao, P.; Colacot, T.J. Tunable unsymmetrical ferrocene ligands bearing a bulky di-1-adamantylphosphino motif for many kinds of csp2–csp3 couplings. ACS Catal. 2022, 12, 5123–5135. [Google Scholar] [CrossRef]
- Cunningham, L.; Guiry, P.J. Design and synthesis of a ferrocene-based diol library and application in the hetero-Diels-Alder reaction. Chem. Eur. J. 2023, 29, e202203006. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, X.; Li, X.; Du, W.; Yu, A.; Zhang, S.; Wu, Y. Synthesis and performance of chiral ferrocene modified silica gel for mixed-mode chromatography. Talanta 2017, 163, 94–101. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Li, Q.; Zhao, P.; Ren, Y.; Jiang, T.; Shen, S. Fabrication of a ferrocene-based monolithic column with a network structure and its application in separation of protein and small molecules. J. Chromatogr. B 2019, 1114, 71–75. [Google Scholar] [CrossRef]
- Fatima, S.; Sharma, R.; Asghar, F.; Kamal, A.; Badshah, A.; Kraatz, H.-B. Study of new amphiphiles based on ferrocene containing thioureas as efficient corrosion inhibitors: Gravimetric, electrochemical, SEM and DFT studies. J. Ind. Eng. Chem. 2019, 76, 374–387. [Google Scholar] [CrossRef]
- Nazir, U.; Akhter, Z.; Janjua, N.K.; Asghar, M.A.; Kanwal, S.; Butt, T.M.; Sani, A.; Liaqat, F.; Hussain, R.; Shah, F.U. Biferrocenyl Schiff bases as efficient corrosion inhibitors for an aluminium alloy in HCl solution: A combined experimental and theoretical study. RSC Adv. 2020, 10, 7585–7599. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, M.J.; Toledano, M.B.; Eaton, N.E.; Fawell, J.; Elliott, P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: A review. Occup. Environ. Med. 2000, 57, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, S.; Fan, J.; Shang, T.; Huang, D.; Li, G. Novel mesoporous organosilica nanoparticles with ferrocene group for efficient removal of contaminants from wastewater. J. Colloid Interface Sci. 2019, 554, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, H. Diphenylphosphine-substituted ferrocene/silsesquioxane-based hybrid porous polymers as highly efficient adsorbents for water treatment. ACS Appl. Mater. Interfaces 2019, 11, 26474–26482. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, L.; Yu, H.; Yang, X.; Chen, Q.; Wang, J. Synthesis of a novel ferrocene-based epoxy compound and its burning rate catalytic property. RSC Adv. 2016, 6, 53679–53687. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Weng, X.; Li, R.; Zhou, X.; Lin, H.; Yu, H.; Liao, B.-Q. Novel indicators for thermodynamic prediction of interfacial interactions related with adhesive fouling in a membrane bioreactor. J. Colloid Interface Sci. 2017, 487, 320–329. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Saleem, M.; Akram, M.; Khalid, H.; Abbasi, N.M.; Yang, X. Synthesis of ethylene diamine-based ferrocene terminated dendrimers and their application as burning rate catalysts. J. Colloid Interface Sci. 2017, 487, 38–51. [Google Scholar]
- Cheng, W.; Shi, X.; Zhang, Y.; Jian, Y.; Zhang, G. Novel ferrocene-based 1, 2, 3-triazolyl compounds: Synthesis, anti-migration properties and catalytic effects on oxidizers during combustion. Inorganica Chim. Acta 2020, 502, 119374. [Google Scholar] [CrossRef]
- Rahimpour, K.; Teimuri-Mofrad, R.; Abbasi, H.; Parchehbaf, M.; Abedinpour, S.; Soleimani, S. Synthesis, characterization and properties investigation of [4–(dimethylsilyl) butyl] alkylferrocene grafted HTPB as novel ferrocene based burning rate accelerator catalysts for composite gas generators. Polym-Plast. Techn. Mat. 2019, 58, 2056–2065. [Google Scholar] [CrossRef]
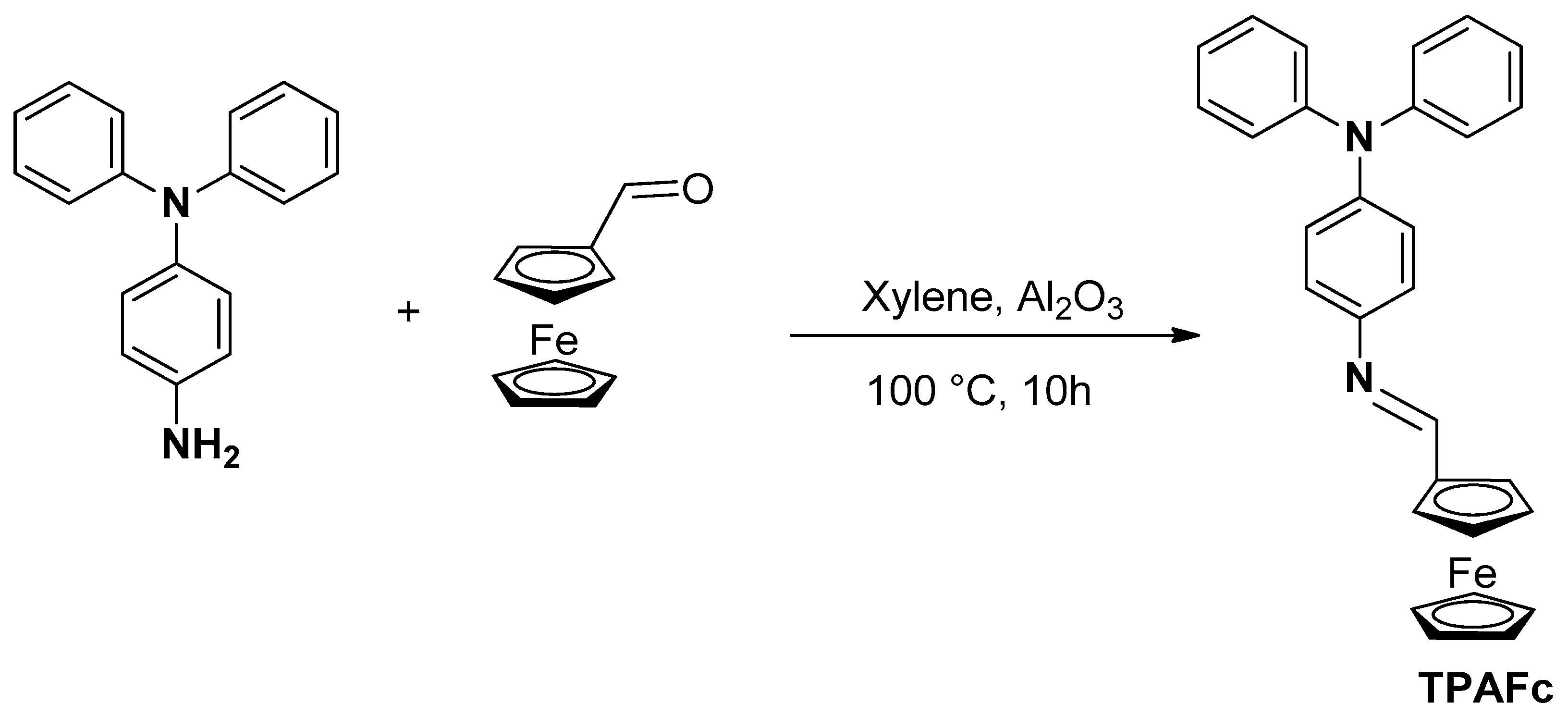
- Lv, X.; Huang, C.; Tameev, A.; Qian, L.; Zhu, R.; Katin, K.; Maslov, M.; Nekrasov, A.; Zhang, C. Electrochemical polymerization process and excellent electrochromic properties of ferrocene-functionalized polytriphenylamine derivative. Dyes Pigm. 2019, 163, 433–440. [Google Scholar] [CrossRef]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef]
- Borchers, P.S.; Strumpf, M.; Friebe, C.; Nischang, I.; Hager, M.D.; Elbert, J.; Schubert, U.S. Aqueous redox flow battery suitable for high temperature applications based on a tailor-made ferrocene copolymer. Adv. Energy Mater. 2020, 10, 2001825. [Google Scholar] [CrossRef]
- Ertas, N.A.; Kavak, E.; Salman, F.; Kazici, H.C.; Kivrak, H.; Kivrak, A. Synthesis of ferrocene based naphthoquinones and its application as novel non-enzymatic hydrogen peroxide. Electroanalysis 2020, 32, 1178–1185. [Google Scholar] [CrossRef]
- Shevaldina, E.V.; Opredelennova, K.A.; Chichvarina, O.A.; Spiridonov, Y.Y.; Smol’yakov, A.F.; Dorovatovskii, P.V.; Moiseev, S.K. One-pot acid-free ferrocenylalkylation of azoles with α-ferrocenyl alcohols: Ferrocene-based plant growth regulators and herbicide safeners. Appl. Organomet. Chem. 2019, 33, e5228. [Google Scholar] [CrossRef]
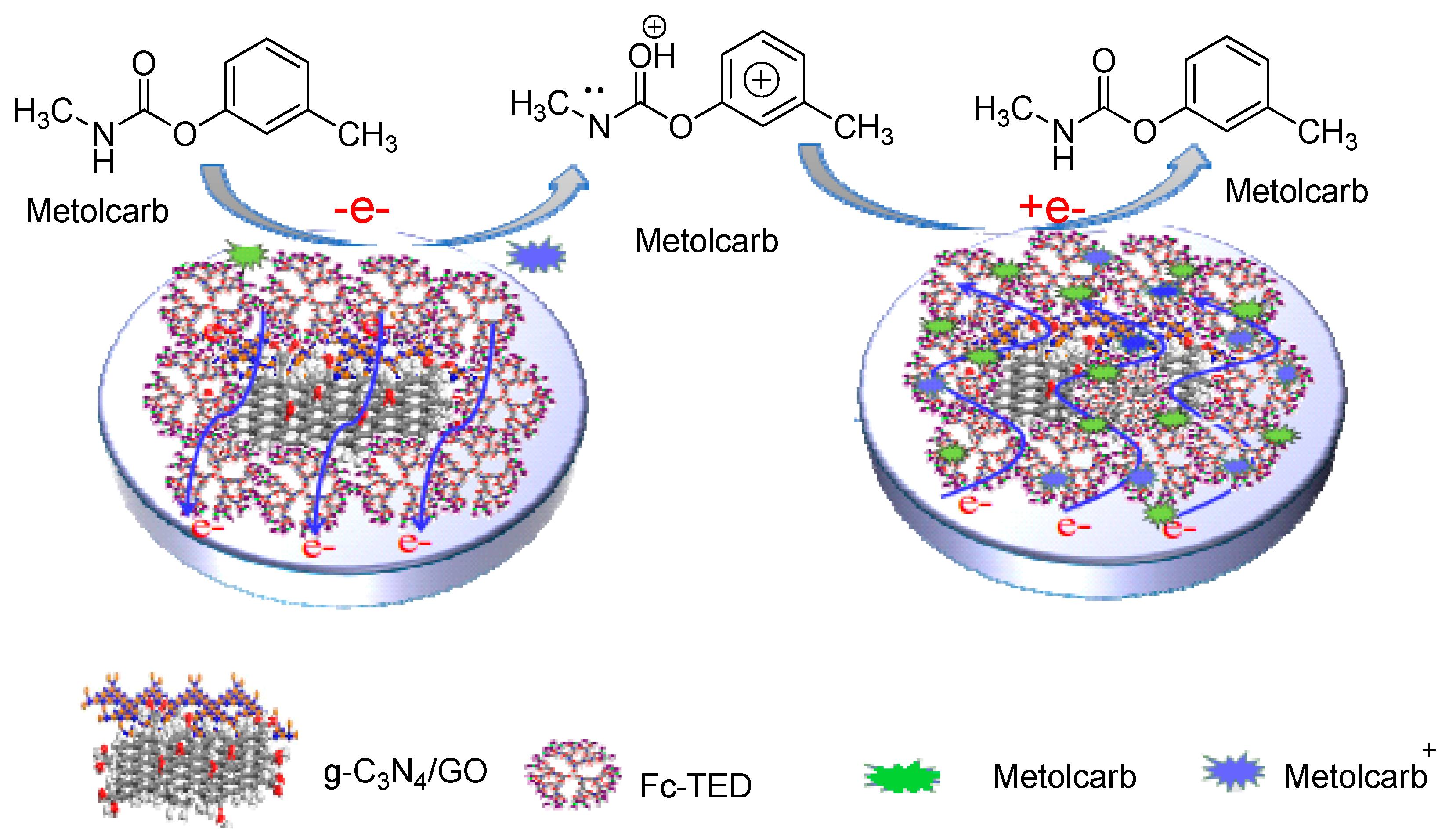
- Xiao, F.; Li, H.; Yan, X.; Yan, L.; Zhang, X.; Wang, M.; Qian, C.; Wang, Y. Graphitic carbon nitride/graphene oxide (g-C3N4/GO) nanocomposites covalently linked with ferrocene containing dendrimer for ultrasensitive detection of pesticide. Anal. Chim. Acta 2020, 1103, 84–96. [Google Scholar] [CrossRef]
- Lewkowski, J.; Karpowicz, R.; Morawska, M.; Rychter, P.; Rogacz, D.; Lewicka, K.; Dobrzyński, P. Synthesis and ecotoxicological impact of ferrocene-derived amino-phosphonates using a battery of bioassays. RSC Adv. 2017, 7, 38399–38409. [Google Scholar] [CrossRef]
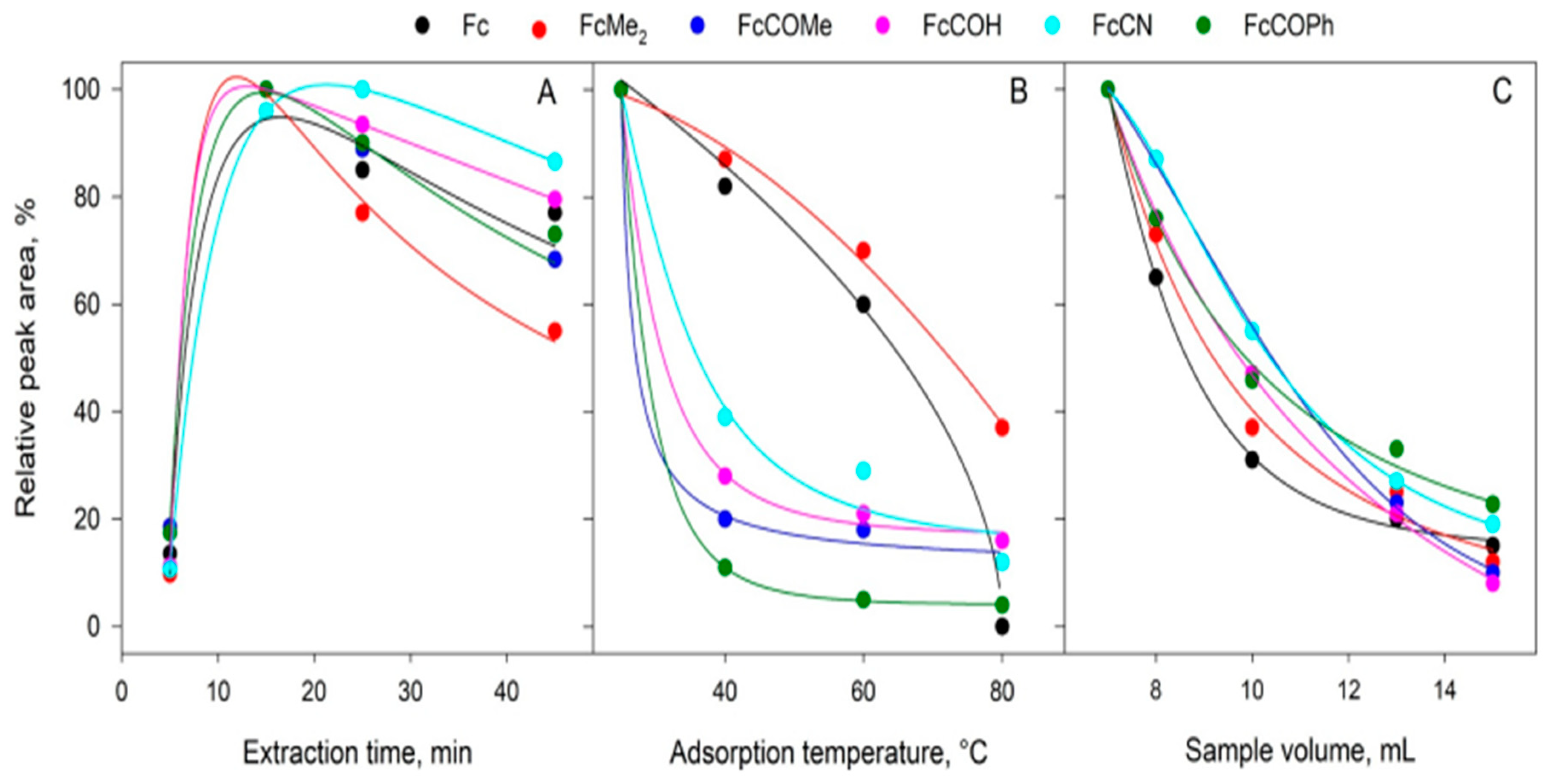
- Peñalver, R.; Campillo, N.; López-García, I.; Hernández-Córdoba, M. Solid-phase microextraction for the determination of iron organic compounds in seawaters and soils by gas chromatography coupled to microwave-induced plasma with atomic emission detection spectrometry. Microchem. J. 2020, 154, 104630. [Google Scholar] [CrossRef]
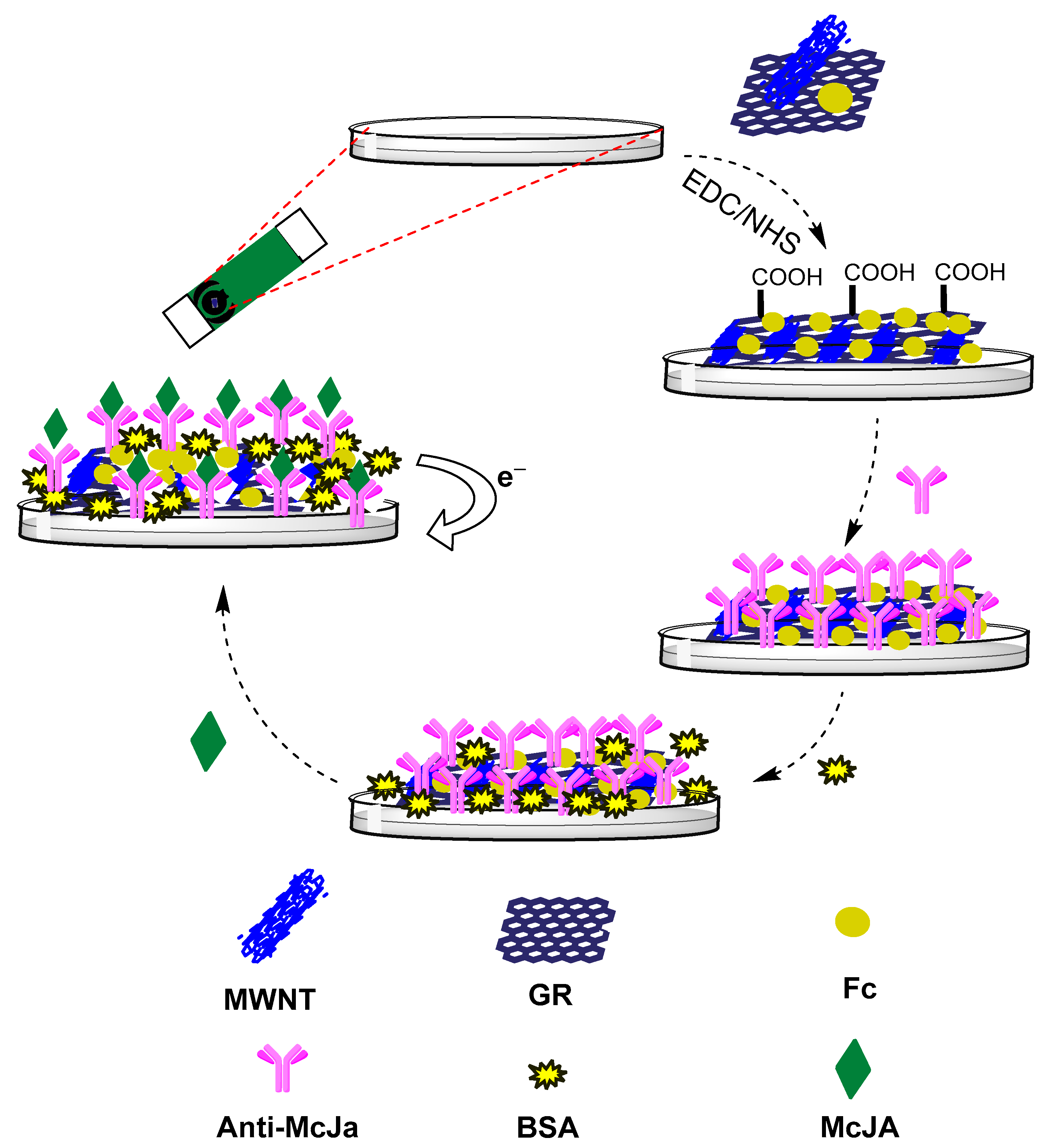
- Xing, G.; Luo, B.; Qin, J.; Wang, X.; Hou, P.; Zhang, H.; Wang, C.; Wang, J.; Li, A. A probe-free electrochemical immunosensor for methyl jasmonate based on ferrocene functionalized-carboxylated graphene-multi-walled carbon nanotube nanocomposites. Talanta 2021, 232, 122477. [Google Scholar] [CrossRef]
- Ibrokhim, A.; Dilnoza, K. Synthesis of ferrocene-containing liquid nitrogen fertilizers and study of their biological activity. Univers. Химия И Биoлoгия 2021, 9, 91–94. [Google Scholar]
- Jolly, P.; Batistuti, M.R.; Miodek, A.; Zhurauski, P.; Mulato, M.; Lindsay, M.A.; Estrela, P. Highly sensitive dual mode electrochemical platform for microRNA detection. Sci. Rep. 2016, 6, 36719. [Google Scholar] [CrossRef]
- Ning, S.; Zhou, M.; Liu, C.; Waterhouse, G.I.; Dong, J.; Ai, S. Ultrasensitive electrochemical immunosensor for avian leukosis virus detection based on a β-cyclodextrin-nanogold-ferrocene host-guest label for signal amplification. Anal. Chim. Acta 2019, 1062, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kaneyoshi, S.; Zou, T.; Ozaki, S.; Takeuchi, R.; Udou, A.; Nakahara, T.; Fujimoto, K.; Fujii, S.; Sato, S.; Takenaka, S. Cyclic naphthalene diimide with a ferrocene moiety as a redox-active tetraplex-dna ligand. Chem. Eur. J. 2020, 26, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.-G.; Zheng, J.-A.; Fan, Y.-R.; Yu, J.-Q.; Wu, X.-L.; Wang, B.-J.; Yang, X.-B.; Huang, Y. Ferrocene appended naphthalimide derivatives: Synthesis, DNA binding, and in vitro cytotoxic activity. J. Organomet. Chem. 2019, 888, 16–23. [Google Scholar] [CrossRef]
- Konkoľová, E.; Janočková, J.; Perjési, P.; Vašková, J.; Kožurková, M. Selected ferrocenyl chalcones as DNA/BSA-interacting agents and inhibitors of DNA topoisomerase I and II activity. J. Organomet. Chem. 2018, 861, 1–9. [Google Scholar] [CrossRef]
- De La Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef]
- Mao, H.-L.; Qian, F.; Li, S.; Shen, J.-W.; Ye, C.-K.; Hua, L.; Zhang, L.-Z.; Wu, D.-M.; Lu, J.; Yu, R.-T. Delivery of doxorubicin from hyaluronic acid-modified glutathione-responsive ferrocene micelles for combination cancer therapy. Mol. Pharm. 2019, 16, 987–994. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Huang, W.; Haruehanroengra, P.; Peng, C.; Sheng, J.; Han, B.; He, G. Application of organocatalysis in bioorganometallic chemistry: Asymmetric synthesis of multifunctionalized spirocyclic pyrazolone–ferrocene hybrids as novel RalA inhibitors. Org. Chem. Front. 2018, 5, 2229–2233. [Google Scholar] [CrossRef]
- Messina, P.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Hamels, D.; Top, S.; Jaouen, G.; Frapart, Y.M.; Mansuy, D. Deciphering the activation sequence of ferrociphenol anticancer drug candidates. Chem. Eur. J. 2012, 18, 6581–6587. [Google Scholar] [CrossRef]
- Gulam Farooq, M.; Lokhande, M.V. Synthesis of ferrocene based organometallic compounds & antimicrobial activity. J. Appl. Chem. 2014, 7, 2278–5736. [Google Scholar]
- Ahmad, A.; Mahal, K.; Padhye, S.; Sarkar, F.H.; Schobert, R.; Biersack, B. New ferrocene modified lawsone Mannich bases with anti-proliferative activity against tumor cells. J. Saudi Chem. Soc. 2017, 21, 105–110. [Google Scholar] [CrossRef]
- Realista, S.; Quintal, S.; Martinho, P.N.; Melato, A.I.; Gil, A.; Esteves, T.; Carvalho, M.D.D.; Ferreira, L.P.; Vaz, P.D.; Calhorda, M.J. Electrochemical studies and potential anticancer activity in ferrocene derivatives. J. Coord. Chem. 2017, 70, 314–327. [Google Scholar] [CrossRef]
- Pedotti, S.; Ussia, M.; Patti, A.; Musso, N.; Barresi, V.; Condorelli, D.F. Synthesis of the ferrocenyl analogue of clotrimazole drug. J. Organomet. Chem. 2017, 830, 56–61. [Google Scholar] [CrossRef]
- Ren, S.-Z.; Wang, Z.-C.; Zhu, D.; Zhu, X.-H.; Shen, F.-Q.; Wu, S.-Y.; Chen, J.-J.; Xu, C.; Zhu, H.-L. Design, synthesis and biological evaluation of novel ferrocene-pyrazole derivatives containing nitric oxide donors as COX-2 inhibitors for cancer therapy. Eur. J. Med. Chem. 2018, 157, 909–924. [Google Scholar] [CrossRef]
- Banerjee, R.; Kumar, H.; Banerjee, M. Medicinal significance of furan derivatives: A review. Int. J. Res. Phytochem. Pharmacol. 2015, 5, 48–57. [Google Scholar]
- Fouda, M.F.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625. [Google Scholar] [CrossRef]
- Tombul, M.; Bulut, A.; Türk, M.; Uçar, B.; Işılar, Ö. Synthesis and biological activity of ferrocenyl furoyl derivatives. Inorg. Nano-Met. Chem. 2017, 47, 865–869. [Google Scholar] [CrossRef]
- Sansook, S.; Tuo, W.; Lemaire, L.; Tourteau, A.; Barczyk, A.; Dezitter, X.; Klupsch, F.; Leleu-Chavain, N.; Tizzard, G.J.; Coles, S.J. Synthesis of bioorganometallic nanomolar-potent CB2 agonists containing a ferrocene unit. Organometallics 2016, 35, 3361–3368. [Google Scholar] [CrossRef]
- Maračić, S.; Lapić, J.; Djaković, S.; Opačak-Bernardi, T.; Glavaš-Obrovac, L.; Vrček, V.; Raić-Malić, S. Quinoline and ferrocene conjugates: Synthesis, computational study and biological evaluations. Appl. Organomet. Chem. 2019, 33, e4628. [Google Scholar] [CrossRef]
- Wei, J.-N.; Jia, Z.-D.; Zhou, Y.-Q.; Chen, P.-H.; Li, B.; Zhang, N.; Hao, X.-Q.; Xu, Y.; Zhang, B. Synthesis, characterization and antitumor activity of novel ferrocene-coumarin conjugates. J. Organomet. Chem. 2019, 902, 120968. [Google Scholar] [CrossRef]
- Mukaya, E.H.; Van Zyl, R.; Van Vuuren, N.J.; Yangkou Mbianda, X. Polymeric prodrugs containing neridronate and ferrocene: Synthesis, characterization, and antimalarial activity. Int. J. Polym. Mater. 2018, 67, 401–409. [Google Scholar] [CrossRef]
- De Lange, C.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; David, D.D. Synthesis, in vitro antimalarial activities and cytotoxicities of amino-artemisinin-ferrocene derivatives. Bioorganic Med. Chem. Lett. 2018, 28, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Mbaba, M.; de la Mare, J.-A.; Sterrenberg, J.N.; Kajewole, D.; Maharaj, S.; Edkins, A.L.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Novobiocin–ferrocene conjugates possessing anticancer and antiplasmodial activity independent of HSP90 inhibition. JBIC J. Biol. Inorg. Chem. 2019, 24, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Minić, A.; Van de Walle, T.; Van Hecke, K.; Combrinck, J.; Smith, P.J.; Chibale, K.; D’hooghe, M. Design and synthesis of novel ferrocene-quinoline conjugates and evaluation of their electrochemical and antiplasmodium properties. Eur. J. Med. Chem. 2020, 187, 111963. [Google Scholar] [CrossRef] [PubMed]
- Štimac, A.; Lapić, J.; Blasina, V.; Lukinac, M.; Djaković, S.; Crnolatac, I.; Frkanec, L.; Frkanec, R. Adamantyl ferrocene derivatives: Antioxidant abilities and effects on model lipid membranes. Appl. Organomet. Chem. 2018, 32, e4042. [Google Scholar] [CrossRef]
- Bano, S.; Khan, A.-U.; Asghar, F.; Usman, M.; Badshah, A.; Ali, S. Computational and pharmacological evaluation of Ferrocene-based acyl ureas and homoleptic cadmium carboxylate derivatives for anti-diabetic potential. Front. Pharmacol. 2018, 8, 1001. [Google Scholar] [CrossRef]
- Mbaba, M.; Dingle, L.M.; Cash, D.; de la Mare, J.-A.; Laming, D.; Taylor, D.; Hoppe, H.C.; Edkins, A.L.; Khanye, S.D. Repurposing a polymer precursor: Synthesis and in vitro medicinal potential of ferrocenyl 1, 3-benzoxazine derivatives. Eur. J. Med. Chem. 2020, 187, 111924. [Google Scholar] [CrossRef]
- Paucar, R.; Martín-Escolano, R.; Moreno-Viguri, E.; Cirauqui, N.; Rodrigues, C.R.; Marín, C.; Sánchez-Moreno, M.; Pérez-Silanes, S.; Ravera, M.; Gabano, E. A step towards development of promising trypanocidal agents: Synthesis, characterization and in vitro biological evaluation of ferrocenyl Mannich base-type derivatives. Eur. J. Med. Chem. 2019, 163, 569–582. [Google Scholar] [CrossRef]
- Parveen, H.; Alatawi, R.A.S.; Alsharif, M.A.; Alahmdi, M.I.; Mukhtar, S.; Khan, S.A.; Hasan, S.; Khan, A.U. Novel pyrazoline-based organometallic compounds containing ferrocenyl and quinoline units: Synthesis, characterization and microbial susceptibilities. Appl. Organomet. Chem. 2018, 32, e4257. [Google Scholar] [CrossRef]
- Hassell-Hart, S.; Runcie, A.; Krojer, T.; Doyle, J.; Lineham, E.; Ocasio, C.A.; Neto, B.A.; Fedorov, O.; Marsh, G.; Maple, H. Synthesis and biological investigation of (+)-JD1, an organometallic BET bromodomain inhibitor. Organometallics 2019, 39, 408–416. [Google Scholar] [CrossRef]
- Gadhachanda, V.R.; Eastman, K.J.; Wang, Q.; Phadke, A.S.; Patel, D.; Yang, W.; Marlor, C.W.; Deshpande, M.; Huang, M.; Wiles, J.A. Ferrocene-based inhibitors of hepatitis C virus replication that target NS5A with low picomolar in vitro antiviral activity. Bioorganic Med. Chem. Lett. 2018, 28, 3463–3471. [Google Scholar] [CrossRef]
- Wang, Y.; Sha, H.; Ke, H.; Xiong, X.; Jia, N. A sandwich-type electrochemiluminescence aptasensor for insulin detection based on the nano-C60/BSA@ luminol nanocomposite and ferrocene derivative. Electrochim. Acta 2018, 290, 90–97. [Google Scholar] [CrossRef]
- Huang, X.; Miao, J.; Fang, J.; Xu, X.; Wei, Q.; Cao, W. Ratiometric electrochemical immunosensor based on L-cysteine grafted ferrocene for detection of neuron specific enolase. Talanta 2022, 239, 123075. [Google Scholar] [CrossRef]

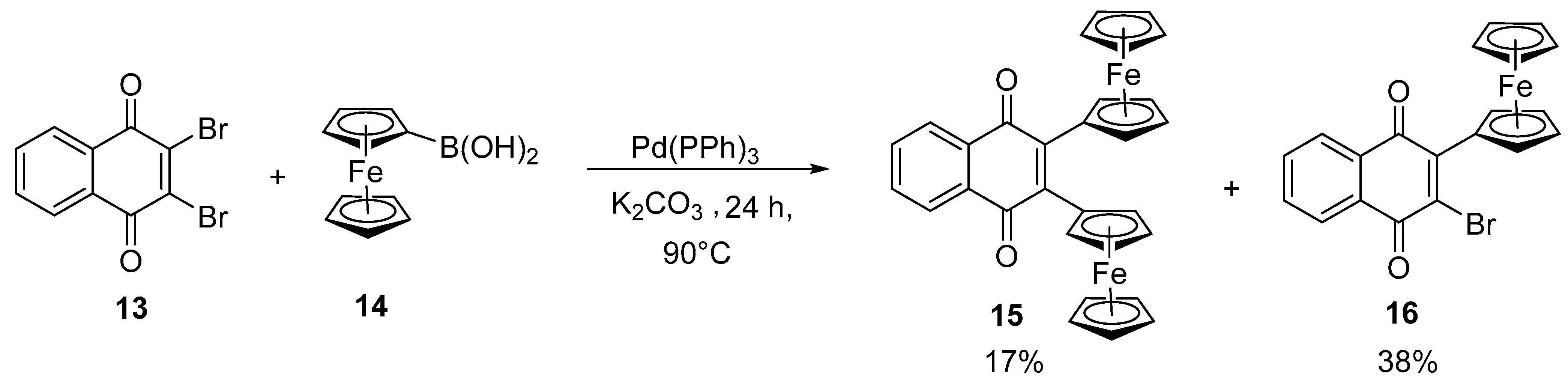



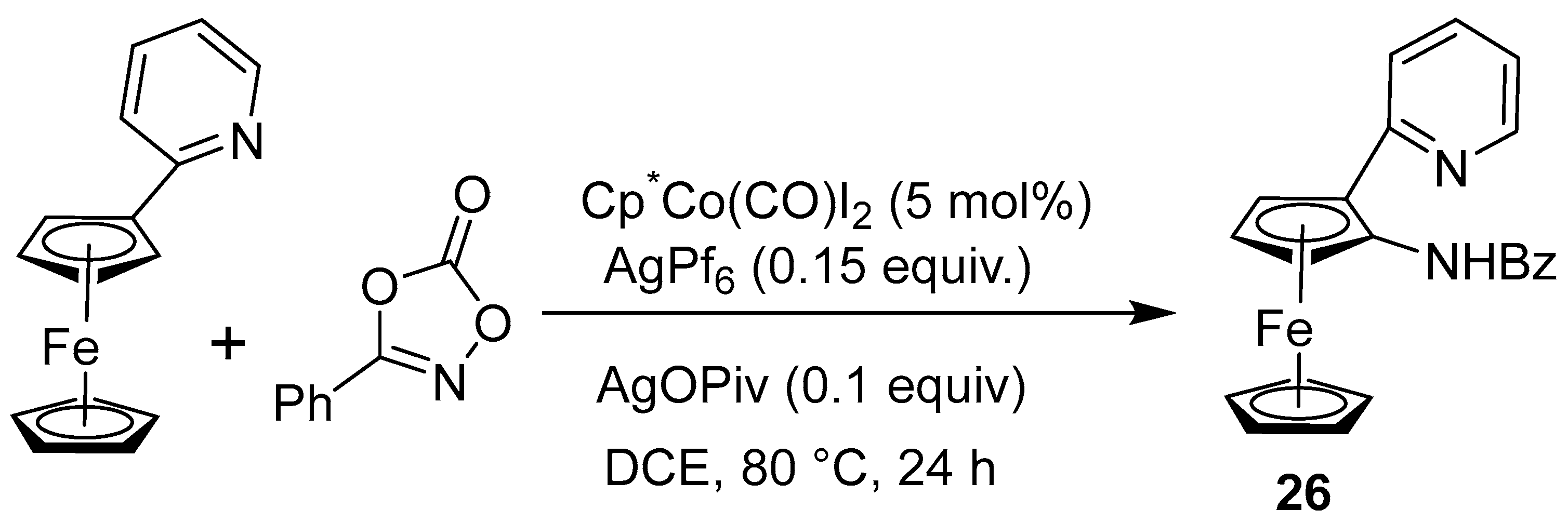





















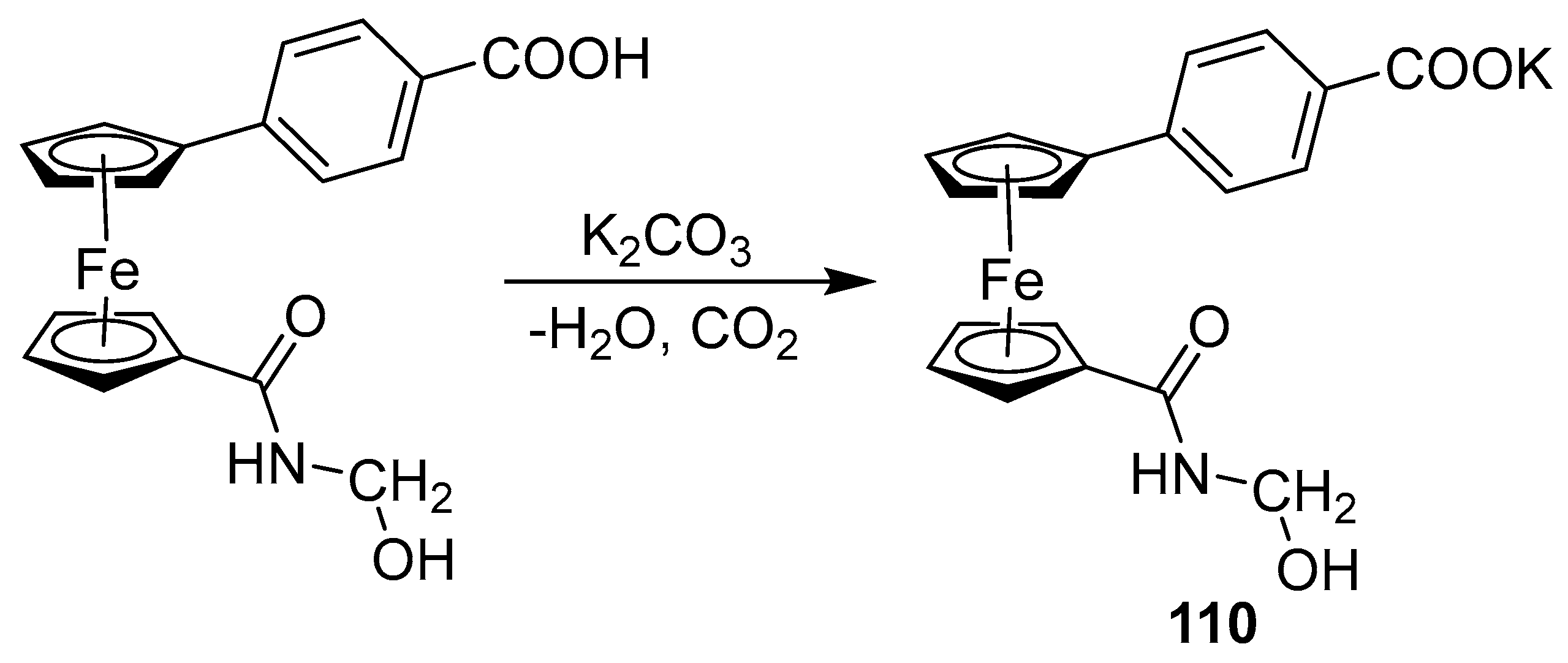






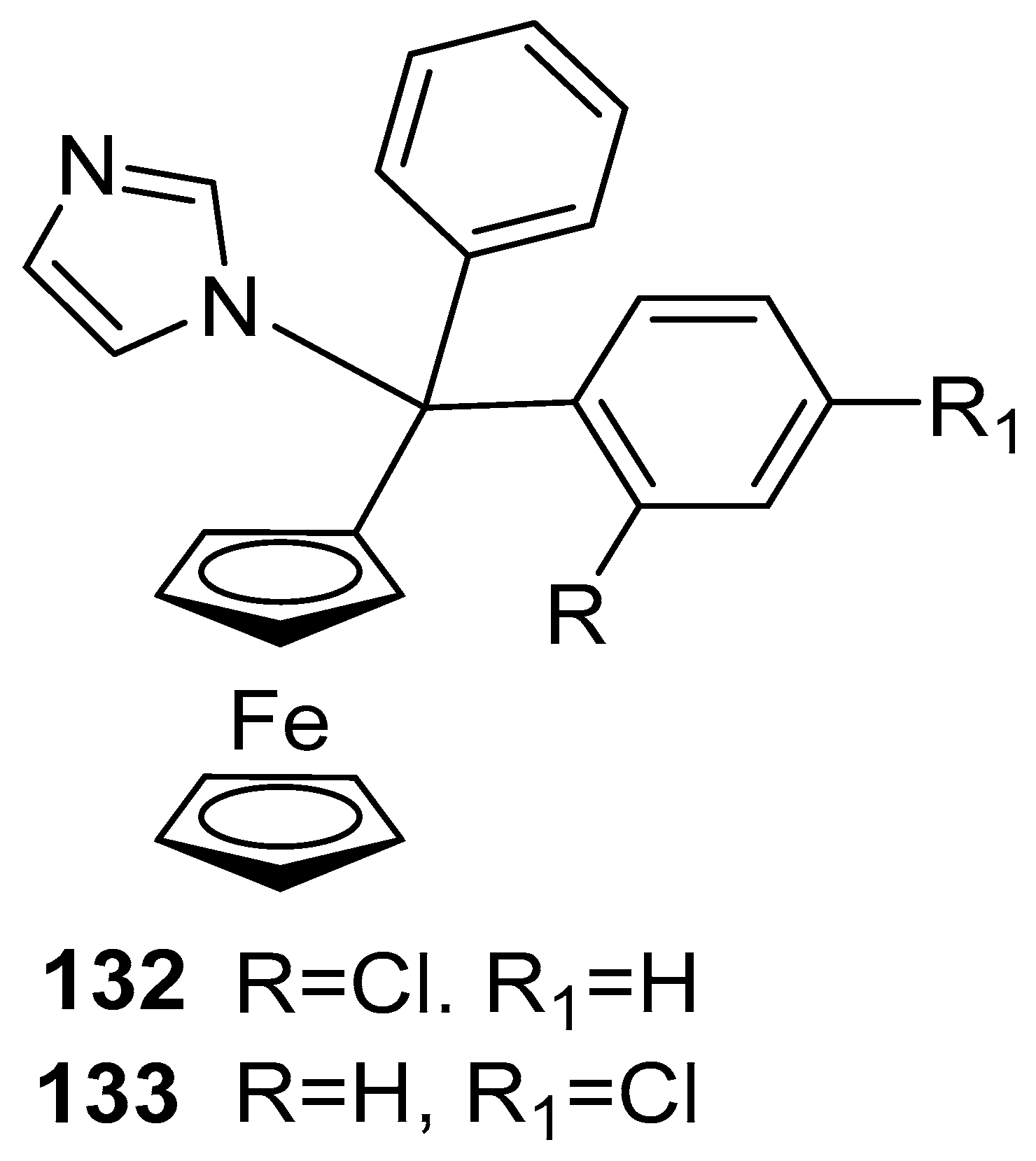
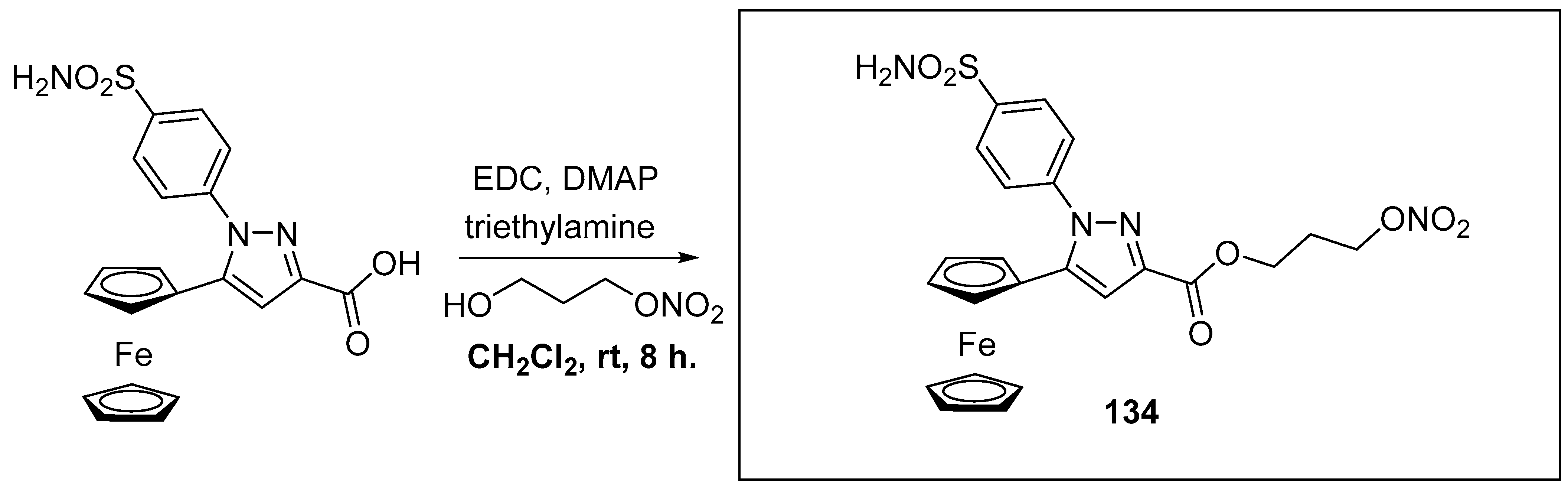
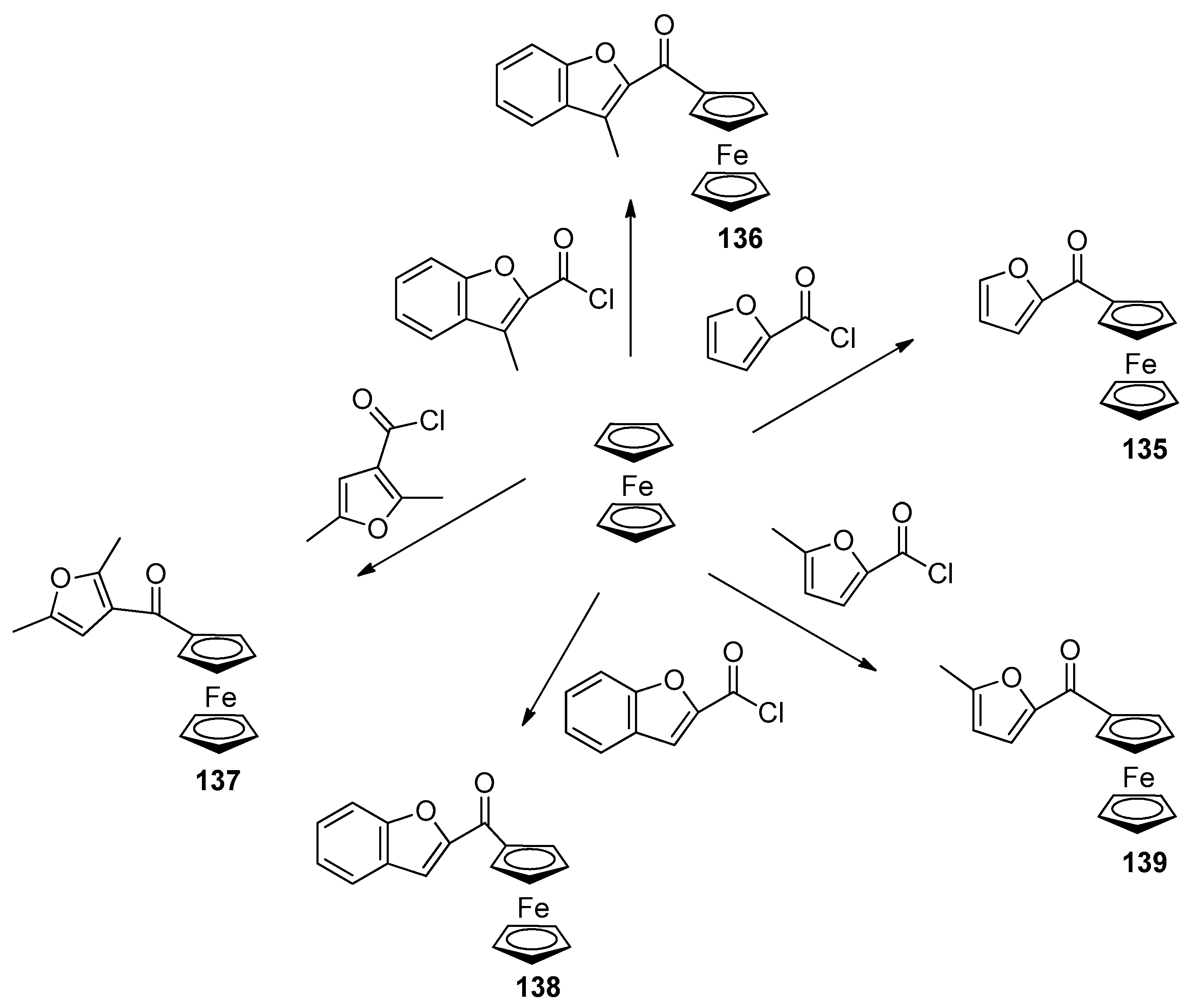


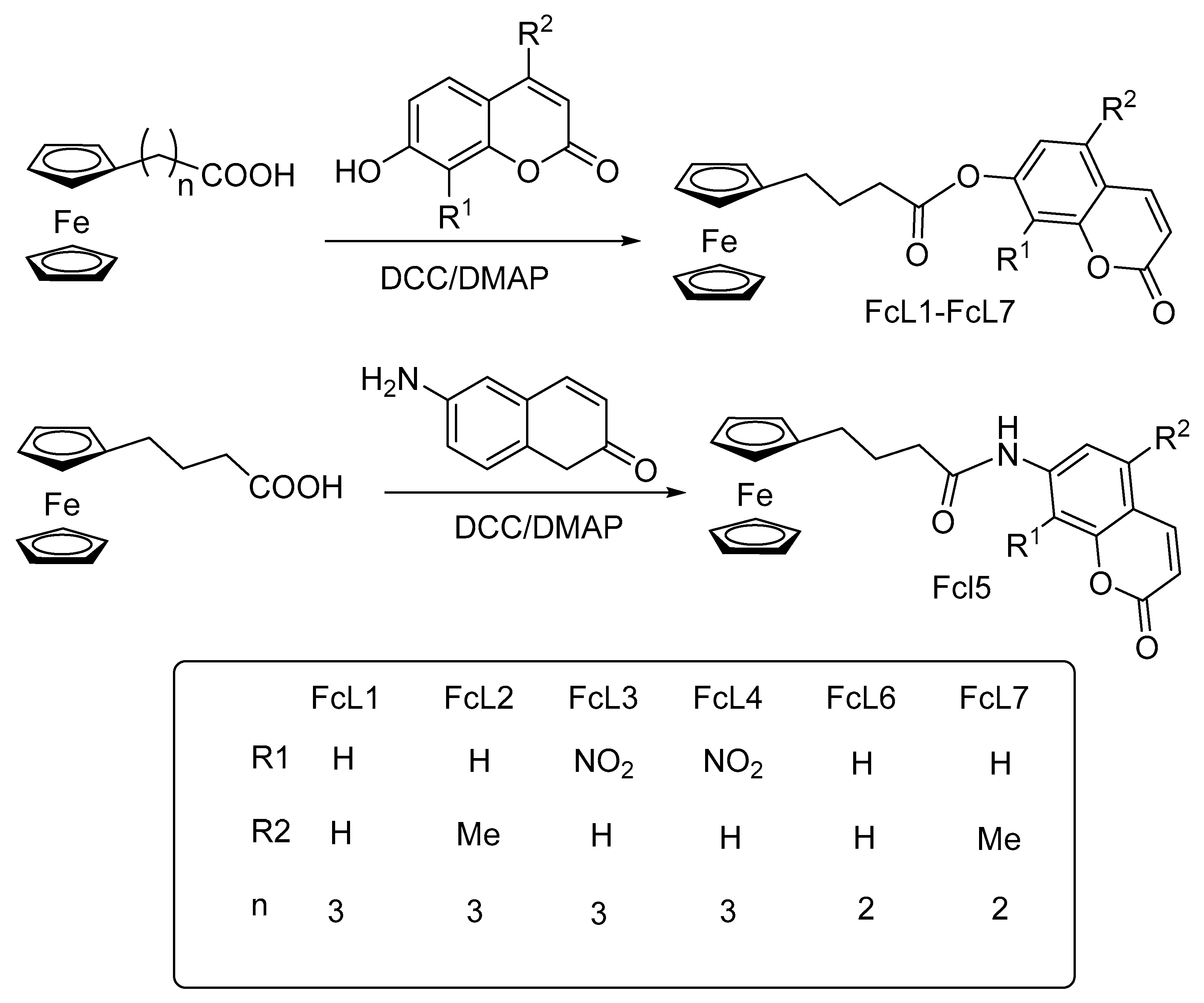





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauf, U.; Shabir, G.; Bukhari, S.; Albericio, F.; Saeed, A. Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules 2023, 28, 5765. https://doi.org/10.3390/molecules28155765
Rauf U, Shabir G, Bukhari S, Albericio F, Saeed A. Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules. 2023; 28(15):5765. https://doi.org/10.3390/molecules28155765
Chicago/Turabian StyleRauf, Umair, Ghulam Shabir, Saba Bukhari, Fernando Albericio, and Aamer Saeed. 2023. "Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects" Molecules 28, no. 15: 5765. https://doi.org/10.3390/molecules28155765
APA StyleRauf, U., Shabir, G., Bukhari, S., Albericio, F., & Saeed, A. (2023). Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules, 28(15), 5765. https://doi.org/10.3390/molecules28155765





