Abstract
Neuropathic pain is a debilitating condition that affects millions of people worldwide. Numerous studies indicate that this type of pain is a chronic condition with a complex mechanism that tends to worsen over time, leading to a significant deterioration in patients’ quality of life and issues like depression, disability, and disturbed sleep. Presently used analgesics are not effective enough in neuropathy treatment and may cause many side effects due to the high doses needed. In recent years, many researchers have pointed to the important role of chemokines not only in the development and maintenance of neuropathy but also in the effectiveness of analgesic drugs. Currently, approximately 50 chemokines are known to act through 20 different seven-transmembrane G-protein-coupled receptors located on the surface of neuronal, glial, and immune cells. Data from recent years clearly indicate that more chemokines than initially thought (CCL1/2/3/5/7/8/9/11, CXCL3/9/10/12/13/14/17; XCL1, CX3CL1) have pronociceptive properties; therefore, blocking their action by using neutralizing antibodies, inhibiting their synthesis, or blocking their receptors brings neuropathic pain relief. Several of them (CCL1/2/3/7/9/XCL1) have been shown to be able to reduce opioid drug effectiveness in neuropathy, and neutralizing antibodies against them can restore morphine and/or buprenorphine analgesia. The latest research provides irrefutable evidence that chemokine receptors are promising targets for pharmacotherapy; chemokine receptor antagonists can relieve pain of different etiologies, and most of them are able to enhance opioid analgesia, for example, the blockade of CCR1 (J113863), CCR2 (RS504393), CCR3 (SB328437), CCR4 (C021), CCR5 (maraviroc/AZD5672/TAK-220), CXCR2 (NVPCXCR220/SB225002), CXCR3 (NBI-74330/AMG487), CXCR4 (AMD3100/AMD3465), and XCR1 (vMIP-II). Recent research has shown that multitarget antagonists of chemokine receptors, such as CCR2/5 (cenicriviroc), CXCR1/2 (reparixin), and CCR2/CCR5/CCR8 (RAP-103), are also very effective painkillers. A multidirectional strategy based on the modulation of neuronal–glial–immune interactions by changing the activity of the chemokine family can significantly improve the quality of life of patients suffering from neuropathic pain. However, members of the chemokine family are still underestimated pharmacological targets for pain treatment. In this article, we review the literature and provide new insights into the role of chemokines and their receptors in neuropathic pain.
1. Chemokines and Neuropathic Pain
Neuropathic pain is usually chronic, with an incidence ranging from 6.9% up to 10% of the general population, and is still an important clinical problem [1,2]. Diseases and injuries that involve the somatosensory nervous system may not only lead to a loss of its function but also increase hypersensitivity and evoke spontaneous pain [2]. Pain can result from etiologically diverse disorders affecting both peripheral and central nervous systems. The cause can be a metabolic, neurodegenerative, vascular, or autoimmune disease; a tumor; trauma; infection; or exposure to toxins. Neuropathic pain evokes severe suffering, disability, depression, and sleep disturbances [3,4,5], and its therapeutic management is challenging [6]. Recommended analgesics provide poor or unsatisfactory relief to patients [7]. The mechanism of neuropathic pain is complex, and its relationship with the pathological disease process often remains unclear [1,2]. The mechanism of neuropathic pain of various etiologies remains to be elucidated. Recently, an increasing number of studies have suggested the significant role of the development and maintenance of cytokines, especially chemokines. The knowledge of the function of individual chemokines will provide a better insight into the mechanisms of neuropathic pain and enable better therapy [8]. Today, approximately 50 chemokines are known (Table 1), which exert their effects via 20 different seven-transmembrane G-protein-coupled receptors localized on the surfaces of various immune and nervous cells [9,10]. Unfortunately, the chemokine family terminology is complicated since a double nomenclature is used, as a molecule’s name may refer to its biological function (e.g., Macrophage Inflammatory Protein-1 α (MIP-1α)) or to the setting of cysteine residues in the molecule plus a number (e.g., C-C motif chemokine ligand 2 (CCL2)) [11,12].

Table 1.
Chemokine family—ligands and their receptors, their pronociceptive properties and chemokine-neutralizing antibody effectiveness: evidence from mouse and/or rat studies.
In 2000, Zlotnik and Yoshie introduced terminology that divides the chemokine family into receptors (R) and ligands (L) [47]. Currently, chemokines are named with the prefix CCL-, CXCL-, XCL-, or CX3CL-, and their receptors are analogously divided into four subfamilies, CCR-, CXCR-, XCR-, and CX3CR-. Notably, many chemokine receptors can be activated by several different ligands, e.g., CCL2/3/4/5/6/7/8/9-CCR1, CXCL1/2/3/5/6/7/8-CXCR2, but some of them are activated by only one specific chemokine, e.g., CX3CL1-CX3CR1 or CXCL16-CXCR6—more details in Table 2 Importantly, chemokines are produced not only by immune cells but also by neurons and glial cells and are responsible for the chemotaxis of many immune cells, including granulocytes, lymphocytes, and monocytes [48]. Chemokine production is increased in many neurodegenerative and neuroimmunological diseases, often accompanied by neuropathic pain [48,49]. The majority of chemokines detected in the central nervous system (CNS) are only expressed under pathological conditions, in contrast to their receptors, which are often constitutively expressed, such as CCR1, CCR2, CCR3, CCR5, CXCR2, CXCR3, CXCR4, and CX3CR1.

Table 2.
The central and peripheral nervous system upregulation of chemokines at the mRNA and protein levels in neuropathic pain models: evidence from mouse and/or rat studies.
1.1. CC Chemokines in Neuropathic Pain
Numerous studies have indicated that there is a strong correlation between behavioral responses in various neuropathic pain models and changes in many chemokine receptors and their endogenous ligands. The largest group of chemokines is the CC subfamily, which consists of 28 members. These chemokines are produced by and attract many cells, including monocytes, neutrophils, eosinophils, basophils, T lymphocytes, natural killer cells, and dendritic cells [8,78]. In neuropathic pain caused by damage to the peripheral nervous system, especially by injury to the sciatic nerve, time-dependent changes in the levels of many endogenous chemokine receptor ligands have been shown in various anatomical structures in animals, including CCR1-CCL2, CCL3, CCL4, CCL5, CCL6, CCL7, CCL8, and CCL9 [16,53]; CCR2-CCL2 and CCL7 [15,52]; CCR3-CCL5, CCL7, CCL8, CCL11, CCL24, and CCL26 [16,23]; CCR4-CCL17 and CCL22 [27]; and CCR5-CCL3, CCL4, CCL5, CCL7, and CCL11 [56]. Some data unequivocally indicate that, in diabetic neuropathy, after nerve injury, time-dependent changes occur in some CC ligands: CCL2, CCL3, CCL4, and CCL9 [19,79]. Importantly, 11 chemokines from the CC group showed very strong pronociceptive properties after their intrathecal injection: CCL1 [13]; CCL2 [15,16]; CCL3 [16,19]; CCL4 [19]; CCL5 [16]; CCL7 [15,16]; CCL8 [16], CCL9 [16,19]; CCL17 [80]; CCL21 [28]; and CCL22 [80].
CCR1 seems to be one of the most important receptors involved in neuropathy because it is a target of several chemokines that have been revealed to possess strong pronociceptive properties in studies performed on rodents, such as CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, and CCL9 [15,16,17,19,20], and CCL6 is still poorly studied for its role in nociceptive transmission. Importantly, CCR1 is also the receptor for CCL13, CCL14, CCL15, CCL16, and CCL23, but these chemokines are not present in mice and rats.
Several studies have shown that CCL2 is one of the key factors upregulated during neuropathic pain development after nerve injury [16,51,53,81,82]. CCL2 acts as a ligand for CCR1, CCR2, and CCR4. Pharmacological studies have provided evidence that the intrathecal administration of CCL2 induces long-lasting pain-related behaviors in naive mice in a dose-dependent manner [15,17] and leads to microglial activation [51,83]. First, it was hypothesized that CCL2 is released by neurons and induces spinal microglia activation [51] and the phosphorylation of p38 MAPK [84], which leads to the production of numerous pronociceptive cytokines [85]. Later, it was discovered that activated astroglial cells are also a source of CCL2, which, after release, may activate CCR2 on spinal neurons and evoke sensitization by NMDA receptors through ERK pathway activation [15,17]. Currently, it is known that CCL2 can also be secreted by cells involved in neuropathy development, such as microglia, macrophages, and neutrophils [86,87,88]. Furthermore, the intrathecal injection of CCL2-neutralizing antibodies effectively reverses neuropathic pain-related behavior after nerve injury [15] and prevents microglial activation [51]. Moreover, CCL2 knockout by using siRNA may reduce pain-like behavior and macrophage density in DRGs after nerve crush [89]. Additionally, the inhibition of CCL2 in the spinal cord reduced mechanical hypersensitivity in a post-stroke animal model of neuropathy [90]. It is worth mentioning that the upregulation of this chemokine was also observed in neuropathy evoked by diabetes and chemotherapy [18,79]. These results highlight the pivotal role of CCL2 in nociception and suggest that it is an attractive target for the novel pharmacotherapy of neuropathic pain.
CCL3 also appears to be an important factor in neuropathic pain development, as its injection induces hypersensitivity [16,20], and its pronociceptive properties are diminished by a CCR1 antagonist (BX513) [20]; however, this chemokine also acts as a ligand of CCR5. It was shown that after nerve injury at the spinal cord level, CCL3 is enhanced in parallel with microglial/macrophage cell activation [55]. CCL3 is secreted by numerous cell types, such as microglia, neurons, neutrophils, and T lymphocytes [91,92,93,94]. Importantly, although after nerve damage caused by CCI, the spinal mRNA level of CCL3 is highly upregulated from the 2nd until the 28th day, its protein level is only changed between the 2nd and 7th days, suggesting the importance of CCL3 in the initial and middle phases of neuropathy [53,55]. Higher levels of this cytokine are also observed in diabetic neuropathy [19], chemotherapy-induced neuropathic pain [22,95], and a model of partial sciatic nerve ligation [21]. Notably, the neutralization of CCL3 reduces pain-like behavior development in STZ- [19], paclitaxel- [22], CCI- [57], and PSNL-induced [21] neuropathy in mice, which may indicate its significant role in this pathology. Importantly, it is postulated that autoantibodies against CCL3 are biomarkers of type 1 diabetes development [96]; therefore, research on the impact of the modulation of this chemokine on the development of neuropathy should undoubtedly be continued due to its potential therapeutic benefits.
CCL4 participation in the development of neuropathic pain does not seem to be crucial, although it acts as a ligand of three chemokine receptors—CCR1, CCR5, and CCR8. Recently, in vitro studies have suggested that microglia and astroglia might be CCL4-producing cells, suggesting that this chemokine may be involved in nociception [56]. To date, only the mRNA level of CCL4 has been shown to be increased after peripheral nerve injury in the spinal cord [16,19,53], while a protein increase has not been observed [16,57]. Similarly, studies conducted in streptozotocin- and chemotherapy-induced neuropathy models have suggested little, if any, participation of CCL4 in pathological nociception [19,97]. These data suggest that CCL4 may not be an appropriate target for future therapies for neuropathic pain.
CCL5 is the major ligand of CCR5; however, it also acts as a ligand for CCR1 and CCR3 and influences monocyte and T-lymphocyte migration [98,99]. In vitro studies suggest that at the spinal cord level, CCL5 can be produced by microglial and astroglial cells [56]. The pronociceptive properties of CCL5 have already been suggested since intrathecal injection of CCL5 induced strong mechanical and even stronger thermal hypersensitivity, which lasted until 48 h after chemokine administration [16]. To date, the upregulation of the CCL5 protein level after nerve injury and in bone cancer pain has been shown in rats [26,100] and mice [16]. Moreover, CCL5-knockout mice develop lower hypersensitivity after partial sciatic nerve ligation [101], and CCL5-neutralizing antibodies reduce pain-like behaviors [25,26]. An enhanced CCL5 level is also found in injured nerves, and its blockade by Met-RANTES indicates its potential role in peripheral sensitization [102,103]. Moreover, the intrathecal administration of an anti-CCL5 neutralizing antibody attenuates established hyperalgesia in rats with bone cancer pain [100]. Nevertheless, the relatively small and short-lasting spinal increase in the CCL5 level after nerve injury suggests that its contribution to central sensitization is less important than that of other chemokines, especially CCL2, CCL7, and CCL8.
CCL6 is the selective ligand of CCR1 produced by monocytes, macrophages, microglia, and eosinophils [104,105]; however, its role in nociceptive transmission is poorly understood. Long-lasting spinal CCL6 upregulation at the mRNA level was shown after CCI injury in mice [16] and rats [53]. However, in contrast, a slight spinal downregulation of the CCL6 protein level was observed [16], which suggests a less important role for this chemokine in nociception in rodents. Moreover, CCL6 is not present in humans; however, it is considered a human ortholog of CCL15 and CCL23 [106]. Importantly, in the cerebrospinal fluid of patients with neuropathic pain, increased CCL23 levels have also been observed [107]; therefore, it would certainly be worth checking the influence of CCL15 and CCL23 on nociceptive transmission and their involvement in the development of hypersensitivity in neurodegenerative and autoimmune diseases.
CCL7, known as a pleiotropic pronociceptive factor, can bind to CCR1, CCR2, CCR3, and CCR5. It was already shown that a single intrathecal injection of CCL7 into naive mice induces strong pain-related behavior, which lasts at least up to 48 h [15,16]. Moreover, after nerve injury, CCL7 may be released by macrophages, neurons, and astrocytes [108,109,110]. Importantly, this chemokine can evoke the activation and chemotaxis of many cells, e.g., microglia, macrophages, and neutrophils [59,111,112], which are known to be important in neuropathy. Moreover, nerve ligation sustainably increases the level of CCL7 in the spinal cord [23,59] and DRGs [23]. Furthermore, CCL7-neutralizing antibodies effectively decrease pain-related symptoms after nerve injury [15] and suppress microglial cell activation [59]. It was also shown that CCL7-knockout mice develop symptoms of neuropathic pain to a lesser extent [108]. Moreover, it was proven that neuron-derived CCL7 promotes glial activation during neuropathic pain [108]. Imai et al. suggested that increased CCL7 expression may serve to ease the interactions between astrocytes and microglia in the spinal cord and could play an essential role in neuropathic pain [59]. Later, in vitro studies revealed that both microglia and astrocytes may release CCL7 [15], indicating that these cells may be activated in a paracrine and autocrine manner by those chemokines. Interestingly, Li et al. reported that decreasing CCL7 levels is more effective for pain relief than decreasing CCL2 levels in a spinal nerve ligation model [113]. This finding agrees well with subsequent behavioral data, which demonstrated that intrathecal injection of a CCL7-neutralizing antibody effectively attenuates CCI-induced neuropathic pain in mice at lower doses than those required for a CCL2-neutralizing antibody [15]. Published data indicate that the long-lasting upregulation of CCL7 induced by nerve injury is associated with enhanced multidirectional spinal communication between neurons, microglia, and astrocytes, which leads to sensory neuron sensitization in the early and late phases of neuropathy.
CCL8, which binds to CCR1, CCR2, CCR3, and CCR5, is one of the most elevated chemokines in the spinal cord after CCI from the early to late phases of neuropathic pain [16]. Nevertheless, to the best of our knowledge, there are only a few studies on the role of CCL8 in painful neuropathy. The recent literature indicates that this chemokine is particularly important because CCL8 levels have already been reported to be increased in the CSF of patients with neuropathic pain [107]. It was previously shown that CCL8 injected intrathecally causes mechanical and thermal hypersensitivity with greater potency than other chemokines [16]. In the case of thermal stimulation, pain-related behaviors were observed even 48 h after chemokine administration [16]. Moreover, the secretion of this chemokine by neurons and macrophages has already been documented [114,115]. Based on the abovementioned data, we suggest that CCL8 may be one of the most important chemokines in nociceptive transmission, especially because it is also known that the inhibition of CCL8 may decrease visceral hyperalgesia [114]; however, experimental and clinical studies are still needed to investigate its exact role in neuropathy.
Similar to the aforementioned CCR1 ligands, CCL9 also seems to have an important role in nociceptive transmission. Intrathecal injection of CCL9 evokes mechanical and thermal hypersensitivity in naive mice [16,19]. This result is important, among other reasons, because CCL9 is a selective CCR1 ligand, and its pronociceptive effects clearly confirm the important role of CCR1 in the development of hypersensitivity. It was already shown that in a mouse model of diabetic neuropathic pain, strong spinal upregulation of CCL9 is in close association with the development of hypersensitivity [19] and that its neutralization by antibodies reduces pain-related symptoms [19]. Moreover, although in rats [53]/mice [16], increased levels of CCL9 mRNA in the spinal cord and/or DRGs are observed at many time points after injury, the protein level of CCL9 is enhanced between the 1st and 7th days post-CCI [53,57] and 7 days after streptozotocin injection [19]. Therefore, based on published results, we believe that CCL9 plays an important role in the early phase of neuropathic pain development. The immunohistochemical results provide evidence that CCL9 colocalizes with the neuronal marker NeuN, indicating that neurons are the main source of this chemokine [19]. Although CCL9 is not expressed in humans, this chemokine (similar to CCL6) has an ortholog, CCL23, which is known to be upregulated in the CSF of patients with neuropathic pain [107]. The literature results indicate that CCL9 (similar to CCL3) plays an important role in the development of neuropathy in rodents, unlike the previously mentioned CCR1 ligands CCL2, CCL7, and CCL8, which are responsible for both the development and maintenance of pain.
In the case of CCL13, CCL14, and CCL16, to our knowledge, there are no data showing their influence on neuropathy in humans or even in animal models (excluding mice and rats, which do not express these chemokines).
CCR2 is preferentially bound by CCL2 [9]; however, other chemokines that have pronociceptive properties, such as CCL7, CCL8, CCL12, and CCL13, are also able to bind to this receptor. The strong pronociceptive properties and role in neuropathy of CCL2, CCL7, and CCL8 have already been discussed above, as they are also CCR1 ligands. The remaining two chemokines still require thorough research; data on the role of CCL12 are ambiguous, while the possible involvement of CCL13 in nociception processes remains to be investigated.
CCL12 seems to be a selective ligand of CCR2. In vitro studies have provided evidence that CCL12 can be expressed by microglia and astrocytes [15]. Moreover, it was shown that CCL12 mRNA was slightly upregulated in the spinal cord 2 days after CCI [15] and then gradually decreased until Day 14 [15]; however, the protein level has not yet been studied. Its enhanced level is also observed in DRGs 7 days after CCI [60]. CCL12 is also one of the most upregulated genes after pSNL, as measured in FACS-sorted microglia from the spinal cord [116]. Considering the high homology between CCL2 and CCL12 and their similar mRNA changes after nerve injury [15], it would be expected that CCL12 also has strong pronociceptive properties. However, the intrathecal injection of CCL12 does not induce any pain-related behaviors in naive animals [15]. Therefore, in light of published data, it seems that spinal CCL12, in contrast to other CCR2 ligands, is not crucial for the development of pain-related behavior. To date, the upregulation of CCL12 has been demonstrated in the articular cartilage of osteoarthritic knees; therefore, its participation in joint pain has been proposed [116,117]. However, there is still a lack of data explaining the actual role of CCL12 in the CNS, which is why further studies in different animal models are needed.
CCR3 also seems to be an important receptor for nociceptive transmission since it is a target of several chemokines, including CCL5, CCL7, CCL8, CCL11, CCL13, CCL15, CCL24, CCL26, and CCL28. The strong pronociceptive properties of three of them, namely, CCL5, CCL7, and CCL8, have been described above; however, it remains unclear which of the chemokine receptors is most involved in their pleiotropic pronociceptive effects. Importantly, the role of other ligands of this receptor, which have been studied to a lesser extent thus far, remains to be clarified.
CCL11 also seems to be significant for nociceptive transmission; it has a very high affinity for CCR3 and has been shown to play a crucial role in the recruitment of eosinophils, basophils, neutrophils, and macrophages [118,119]. In the CNS, CCL11 is secreted by microglia and astrocytes [120,121]. In CCI-induced neuropathic pain, the long-term increase in CCL11 mRNA in the spinal cord and/or DRGs is known to occur in rats [23] but not in mice [16]. Nevertheless, 7 days after CCI, enhanced protein levels of CCL11 are observed in DRGs [23]. Notably, a high level of this chemokine has also been observed in the CSF of patients with neuropathic pain, indicating its significant role in neuropathy [107]. Moreover, CCL11 is currently suggested to be a biomarker of fibromyalgia [122]. In addition, it has been shown to be involved in pain development in osteoarthrosis [123]. Published data indicate that CCL11 acts via CCR3, CCR5, and CXCR3; therefore, CCL11 should be considered a potential target for pharmacotherapy. In our opinion, it would undoubtedly be worth using an anti-CCL11 monoclonal antibody in experimental studies to prove this hypothesis. In particular, recently, bertilimumab, a humanized monoclonal antibody against CCL11, has been used in clinical trials for treating severe allergic disorders, skin disorders, and inflammatory bowel disease [124,125].
Unlike the already-discussed CCR3 ligands, the other three CCLs, CCL24, CCL26, and CCL28, are still poorly studied. Experimental studies have shown only that after CCI, the spinal mRNA levels of CCL24 [16] and CCL28 [16] are unchanged in mice, and CCL28 is undetectable in rats [23]. Moreover, CCL26 levels are slightly elevated, but only in the late phase—on Day 28 in rats—while there is no detection of this chemokine in the spinal cords of mice [16,23]. Interestingly, a recent clinical study provided evidence that the serum level of CCL24 is higher in patients with fibromyalgia [126], which suggests its important role in peripheral sensitization. The available literature indicates that CCR3 and its ligands are potential targets for pain relief; however, more studies are needed.
Published data indicate that CCR4 has two selective ligands, CCL17 and CCL22 [127,128], and one nonselective ligand, CCL2. CCL17 is known to be secreted by lymphocytes, monocytes, dendritic cells, and neurons [129], while CCL22 is secreted by macrophages, dendritic cells, B and T lymphocytes, and monocytes [129,130]. Importantly, after nerve injury, the spinal levels of CCL17 and CCL22 mRNA remain unchanged, unlike the level of CCL2, which is highly upregulated [80]. Pharmacological studies have proven that intrathecally administered CCL2 evokes pain-related behavior in naive mice through CCR4 [80]. Subsequent results show that in the peripheral nervous system (PNS), the situation is different, and all three CCR4 ligands may be involved in nociceptive transmission. It has been shown that after CCI, the DRG levels of CCL17, CCL22, and CCL2 are strongly upregulated in rats [27,53]. It was also revealed that CCL17 levels are increased in patients suffering from fibromyalgia [122] and diabetic retinopathy [131]. Moreover, the importance of CCL17 and CCL22 during tissue repair in diabetes was recently proven [132]. Based on published data, we believe that in nociceptive transmission at the CNS and PNS levels, the CCL2/CCR4 and CCL2/CCL17/CCL22/CCR4 axes are involved, respectively.
CCR5 also seems to be an important receptor for nociceptive transmission because it is a target of several chemokines, CCL3, CCL4, CCL5, CCL7, CCL8, CCL11, and CCL13, all of which, except for CCL13, as described above, have strong pronociceptive properties, and most of them are implicated in the development and/or maintenance of neuropathy. CCL13 is not present in mice/rats; however, recently, in patients, it was identified as a potential biomarker for abdominal pain in irritable bowel syndrome [133] and osteoarthritis [134]; therefore, its role in neuropathic pain needs to be studied.
CCR6 is a target of CCL20; however, there are few data in the literature suggesting its role in neuropathy; therefore, further studies are necessary. This chemokine is expressed by the endothelium, macrophages, and Th17 lymphocytes [135]. In 2022, it was reported that in patients who developed persistent postoperative neuropathic pain (PPSNP) after breast cancer surgery, the level of CCL20 was increased [136]. Moreover, other recently published data have shown that a high level of CCL20 is strongly associated with thermal allodynia in patients after traumatic nerve injuries [137].
CCR7 is a target of two chemokines, CCL19 and CCL21. In light of the available literature, it remains unclear whether the CCL19/CCR7 and CCL21/CCR7 axes play a role in central sensitization. CCL19 may be secreted by mature dendritic cells [138]. It was already shown that the level of CCL19 increased in the plasma and cerebrospinal fluid of patients with neuropathic pain [107,139]. Furthermore, it was reported that CCL19 plays a crucial role in hypersensitivity, which develops during orofacial pain [140]. Undoubtedly, CCL21 plays an important role in central sensitization; however, despite belonging to the CC group, CCL21 shows an affinity for CXCR3-expressing microglia [141,142]. Published data provide evidence that the CCL21/CXCR3 axis is extremely important in the development of neuropathic pain symptoms [143], which will be discussed in the next section.
CCR8 in rodents is preferentially bound by CCL1, which is secreted by various cells, such as neurons, lymphocytes, monocytes, mast cells, epithelial cells, and endothelial cells [13,144]. Recently, it has been shown that recombinant CCL1 injected intrathecally into naive mice induces hypersensitivity [13,14] and, in parallel, activates glia to express higher levels of pronociceptive factors, such as interleukins (ILs): IL-1beta and IL-6 [14]. Moreover, CCL1/CCR8 signaling is suggested to be important for the development of neuropathic pain, since the spinal upregulation of CCL1 was demonstrated in STZ-, CCI- and PSNL-induced neuropathy [13,14,145]. Additionally, nerve-injury-induced hypersensitivity is reduced by CCL1-neutralizing antibody administration [13,14] and in CCR8-knockout mice [14]. Furthermore, current evidence shows that CCL1 is mainly produced in DRG neurons but secreted in the dorsal horn of the spinal cord [14]. It was proven that CCL1 injection evoked the activation of glial cells and the upregulation of proinflammatory cytokines [14]. Immunofluorescence staining proved that CCL1 colocalized with the neuronal marker NeuN [13]. Another ligand that binds to CCR8 is CCL18; however, it is not present in mice and rats, and thus far, its role in neuropathy in patients needs to be studied. Experimental data provide valuable insight into the CCL1/CCR8 signaling pathway as a novel therapeutic target for neuropathic pain.
CCR9 is a selective target for CCL25. The role of the CCL25/CCR9 axis in the development of neuropathy requires research; however, in 2022, it was shown that in the CSF of patients who developed postoperative neuropathic pain after breast cancer surgery, an enhanced level of CCL25 was observed [136].
CCR10 is a target for three chemokines, CCL26, CCL27, and CCL28. To date, none of these chemokines have been proven to play an essential role in nociceptive transmission, and some clinical studies are needed.
In summary, the literature provides evidence that many endogenous ligands of chemokine receptors, such as CCL1/2/3/5/7/8/9/11, are significant in the development (CCL2/3/5/7/8/9) and maintenance (CCL2/7/8) of neuropathic pain. Among them, CCL2/7/8 seem to be the most important because of the quick and long-lasting increase in their protein levels and strong pronociceptive properties. Importantly, it was shown that repeated injections of bindarit, an inhibitor of CCL2/7/8 synthesis, effectively attenuate pain-related behaviors in different phases of neuropathic pain development in mice [16]. Bindarit has analgesic effects even in fully developed neuropathy. The obtained results provide evidence that CCL2/7/8 may serve as potential therapeutic targets for neuropathic pain treatment, regardless of sex [16]. Moreover, in mice, bindarit suppresses cancer pain development [146] and autoimmune encephalomyelitis [147]. Importantly, bindarit was in the second phase of clinical trials for type 2 diabetic nephropathy (NCT01109212) [146]. The available literature clearly indicates that in the CNS, the CCL2/3/5/7/8/9/CCR1, CCL2/7/8/CCR2, CCL5/7/8/11/CCR3, CCL2/CCR4, CCL3/5/7/8/11/CCR5, and CCL1/CCR8 axes and, in the PNS, the CCL17/22/CCR4 axes are especially important in the development and/or maintenance of neuropathic pain. The results indicate that CC family members are promising targets in the search for neuropathic pain therapy; however, more studies are needed.
1.2. CXC Chemokines in Neuropathic Pain
The CXC subfamily is the second-largest group of chemokines in terms of the number of members (consisting of 17 members) (Table 1) and is characterized by a single amino acid between the first two cysteine residues. Neuropathic pain caused by sciatic nerve damage has also been shown to be associated with strong time-dependent changes in the levels of many endogenous chemokine receptor ligands, including CXCR2-CXCL1, CXCL2, and CXCL3 [31]; CXCR3-CXCL4, CXCL9, CXCL10, and CXCL11 [28]; and CXCR1/CXCR2-CXCL5 [34]. In addition, 10 chemokines acting through CXC receptors have a strong pronociceptive effect, namely, CXCL1 [31]; CXCL2 [31]; CXCL3 [31]; CXCL4 [28]; CXCL5 [34]; CXCL9 [28]; CXCL10 [28]; CXCL11 [28], CXCL17 [42], and CCL21 [28].
The role of CXCR1 in the processes of nociception remains unclear; however, recently, it was proven that this receptor is expressed by spinal cord neurons and upregulated during the development of bone cancer pain [148]. CXCR1 has four ligands: CXCL5, CXCL6, CXCL7, and CXCL8. The intrathecal administration of CXCL5 induces nociceptive hypersensitivity in naive rats [34]. Moreover, CCI-evoked pain is accompanied by an increase in spinal CXCL5; however, the involvement of CXCR1 in this phenomenon remains unclear since this chemokine also acts via CXCR2. Studies have also shown its protein upregulation in a diabetic neuropathy model [62]. Importantly, the intrathecal administration of a CXCL5-neutralizing antibody diminished neuropathic-pain-related behavior [34]. Importantly, an increased level of CXCL5 is also observed in the CSF of patients with neuropathy, similar to the level of CXCL6 [107], but in rats, after CCI, the CSF level of CXCL6 is not changed [64]. However, whether CXCL6 and CXCL7 are involved in nociceptive transmission still needs to be investigated. The most extensively studied ligand of CXCR1 is CXCL8, previously known as IL-8, which is also the ligand of CXCR2. This chemokine is produced by neutrophils, macrophages, T lymphocytes, endothelial cells, and epithelial cells [149]. The CXCL8 level increases in the injured sciatic nerve shortly after PSL [66]. In patients suffering from polyneuropathies, the level of CXCL8 was also enhanced in peripheral blood mononuclear cells [150] and in the serum of those with diabetic neuropathic pain [151].
CXCR2 seems to be one of the most significant CXC receptors involved in neuropathy. CXCR2 acts as a pronociceptive ligand belonging to the subfamily called cytokine-induced neutrophil chemoattractants (CINCs), which are closely related to each other; however, they have different tissue expression and regulation. The CINC family arose as a result of two rounds of gene duplication in the course of evolution [152] and currently includes CINC-1 (CXCL1), CINC-2 (CXCL3), and CINC-3 (CXCL2). CXCL1 has the highest efficacy against CXCR2 and intermediate efficacy against CXCL2 and CXCL3; however, all are expressed by macrophages and play important roles in neutrophil infiltration [153,154]. Although the intrathecal administration of CXCL1 and CXCL2 evoke mechanical and thermal hypersensitivity in naive mice [31], their spinal protein levels remain unchanged after a chronic constriction injury of the sciatic nerve [31]. In contrast, CXCL1 is highly upregulated in the spinal cord and/or DRGs following spinal nerve ligation [32,155]. Both chemokines are increased in a diabetic neuropathy model [62,63], but with CXCL1 in the spinal cord and CXCL2 in the sciatic nerve. Interestingly, the neutralization of spinal IL-17 alleviates neuropathic pain evoked by CCI by reducing the CXCL1 level [156]. Others have also shown that CXCL2 levels are enhanced in infiltrating neutrophils and macrophages in an injured sciatic nerve after its ligation [33]. Based on published data, we hypothesize that the CXCL1/CXCR2 and CXCL2/CXCR2 axes play an important role in nociception; however, their impact seems to be more significant in the PNS than in the CNS. Importantly, the latest data indicate that among CINCs, CXCL3 seems to play a crucial role in the CNS since it is highly upregulated at the spinal cord level after nerve injury [31]. Research on this chemokine has only recently begun, as its biophysical/structural characteristics were previously unavailable [152]. Recent studies have shown that although the overall oligomerization features of all CINCs are similar, prominent differences can be observed in their characteristic surface structures, thus indicating functional divergence [152]. CXCL3 exerts its effects via CXCR2 through signaling pathways, such as p38MAPK and ERK1/2 [157,158], which are known to be important factors in neuropathy [159]. Moreover, the behavioral results provide evidence for the pronociceptive properties of CXCL3, where, after its intrathecal administration, hypersensitivity appears quickly, which is connected to the fact that CXCR2 is present on neuronal cells [31]. Moreover, the CXCL3-neutralizing antibody diminishes pain-related behavior evoked by nerve injury [31]. Additionally, CXCL3 is responsible for neutrophil recruitment and acts as a mediator of macrophage chemotaxis, which is important since these cells are crucial in neuropathic pain pathogenesis [160]. The abovementioned results indicate the important role of CXCL3 in both the initiation and maintenance of neuropathic pain; therefore, in our opinion, both CXCL3 secretion and CXCR2 blockade can have beneficial effects, which may help relieve the symptoms of neuropathic pain.
The ligands of CXCR3 (CXCL4, CXCL9, CXCL10, CXCL11, and CCL21) may also evoke strong pain-like behaviors in naive mice after a single intrathecal administration [28]. Importantly, different time course changes are observed after nerve injury [28,30]. On Day 2 after injury, at the spinal cord level, increases in CXCL10 and CXCL11 were observed, indicating their role in triggering neuropathy [28]. Then, on Day 7, increases in CXCL4, CXCL9, and CXCL10 were also measured. However, long-lasting changes (until Day 28) were observed only for CXCL9, suggesting that this chemokine is responsible for persistent neuropathy [28]. Additionally, in DRGs, CXCL4 and CXCL9 levels were elevated 7–28 days after injury, which can be important for peripheral sensitivity development [28].
Changes in the levels of CXCL4 observed in the CNS and PNS indicate the important role of this chemokine in nociceptive transmission [28]. The in vivo and in vitro results are particularly interesting, as they indicate that CXCL4 is specifically expressed by microglia but not by astroglia or neurons [161]. It was also proven that microglial migration induced by CXCL4 is absent in CXCR3-deficient microglia [161]. The exact role of this chemokine in neuropathy, especially in DRGs, should undoubtedly be the subject of further research.
However, among the tested CXCR3 ligands, the role of CXCL9 seems to be the most important due to its strong pronociceptive effects [28] and long-lasting upregulation after nerve injury observed at the spinal cord and DRG levels [28,67]. Moreover, the spinal upregulation of CXCL9 has already been described in diabetic neuropathy [62] and in locally inflamed DRGs [68]. Published data indicate that CXCL9 is expressed by neurons, microglia, and astroglia [28,162]. Moreover, the administration of a CXCL9-neutralizing antibody diminishes the pain-related behavior observed after nerve injury [28]. Therefore, CXCL9 appears to be a very important nociceptive mediator in neuropathy and is particularly crucial for the persistence of neuropathy.
The role of CXCL10 in nociceptive transmission seems to be very important since numerous studies have described its role in this process: its spinal upregulation has already been proven in many animal models, e.g., SNL-, CCI-, CIBP-, and STZ-evoked neuropathy [28,35,36,68,163,164]. Moreover, it was already shown that the intrathecal administration of a CXCL10-neutralizing antibody reduces the development of CCI-evoked neuropathic pain [28] and cancer-induced bone pain [36]. It is known that at the spinal cord level, CXCL10 enhances the amplitude of spontaneous excitatory postsynaptic current and increases NMDA-/AMPA-induced currents via CXCR3; these results support a role for the CXCL10/CXCR3 axis in facilitating excitatory synaptic transmission in neuropathy [163]. Moreover, in the SNL model, increases in CXCL10 mRNA and the number of action potentials evoked by this chemokine were observed in DRGs [67]. Notably, the intrathecal administration of CXCL10 produces rapid and CXCR3-dependent pain hypersensitivity [28,163]. CXCL10 may also induce AKT and ERK activation in trigeminal ganglion neurons and contribute to the maintenance of neuropathic pain [165]. Interestingly, the levels of CXCL10 in blood samples were elevated in patients with diabetic polyneuropathy compared to patients without diabetes [166]. Therefore, in our opinion, the modulation of CXCR3/CXCL10 signaling can bring satisfactory pain relief in neuropathy.
Published data suggest that CXCL11 plays an important role in the first phase of the development of pain [28,167]. Intrathecal administration induces quick but short-term hypersensitivity in naive mice [28]. The upregulation of its mRNA is also observed after SNL [67] and in a diabetic model of neuropathy [62], while its protein level is slightly upregulated after CCI [28]. Moreover, in vitro results suggest that CXCL11 can be produced by microglial and astroglial cells [28,168]. However, based on available results, it is not possible to determine the exact role of CXCL11, and this issue requires future study.
Importantly, CCL21, which also binds to CXCR3, similarly has strong pronociceptive properties. It was already proven that this chemokine is not detected under physiological conditions in the CNS, but after injury, it is strongly upregulated [30,61,169]. Its increase is also observed after the use of anticancer drugs [29]. In neuropathy, CCL21 is expressed in the DRGs in injured small-diameter primary sensory neurons [30,170] and is immediately transported to axons within the dorsal horn of the spinal cord [161,171]. At the spinal cord level, CCL21 evoked strong microglial cell activation [141]. Therefore, the administration of a CCL21-neutralizing antibody attenuates the development of hypersensitivity in neuropathic pain models [28,30]. It has also been proven that in CCL21-knockout mice, pain-related behavior develops to a lesser extent after nerve injury [143]. Therefore, based on the literature, we think that CCL21-CXCR3 signaling is also strongly involved in the development and maintenance of neuropathic pain.
The involvement of CXCR4/CXCL12 in pathological neuropathic pain has been broadly studied [39]. Nerve injury evokes an increase in CXCL12 in DRGs [38,69] and in the spinal cord [39], suggesting that this chemokine may participate in hypersensitivity development both peripherally and centrally. Similarly, streptozotocin-evoked diabetic neuropathy leads to its protein upregulation in the spine [62] and in the area of the anterior cingulate cortex [70]. CXCL12/CXCR4 signaling is involved in pain development in a model of bone cancer [172] and SNL- [37], CCI- [173], and SNI-induced [38] neuropathy by sensitizing neurons or activating astrocytes and microglia. Moreover, it was proven that the intrathecal injection of CXCL12 affects the development of hypersensitivity in naive rats [39], while the intrathecal injection of a CXCL12-neutralizing antibody diminishes neuropathic pain development and maintenance [39]. In light of the obtained results, the authors agree that CXCL12/CXCR4 signaling may serve as a novel target that can be exploited for the treatment of neuropathic pain.
CXCR4 has a positive allosteric modulator, CXCL14 [174], that is constitutively expressed in the CNS [175] and PNS [176]. First, a microarray analysis of rat DRGs showed that local inflammation evokes the significant upregulation of CXCL14 in parallel with the development of hypersensitivity [68]. Later, increased spinal CXCL14 was described as involved in the development of paclitaxel-induced [76] neuropathic pain. Moreover, the use of CXCL14 siRNAs significantly attenuates hypersensitivity induced by paclitaxel [76]. Recently, it was proven that CXCL14 contributes to the modulation of hypersensitivity, together with somatostatin [177]. CXCL14 is currently suggested to be a crucial factor in the initial phase and maintenance of neuropathic pain.
CXCR5 is a selective target of CXCL13; however, under physiological conditions in the CNS, it is expressed at a very low level [40]. Nevertheless, CXCL13 mRNA and/or protein levels increase in the spinal cord and/or DRGs in SNL- [40,68,72], CCI- [75], SpNI- [73], DB- [71], and TNFα-induced [74] neuropathic pain models. The intrathecal injection of CXCL13 induces pain hypersensitivity and astrocyte activation via CXCR5 [40]. Moreover, CXCL13 promotes the production of cytokines to elicit hypersensitivity [71]. The shRNA-mediated spinal inhibition of CXCL13 diminishes SNL-induced neuropathic pain [40], and the spinal overexpression of miR-186-5p is able to reduce CXCL13 expression [40]. Moreover, the DRG microinjection of CXCL13 siRNA reduces SNL-induced hypersensitivities [72], while the neutralizing antibody diminishes CPIP-evoked neuropathic pain symptoms [41]. Moreover, it was shown that the neuronally enhanced production of CXCL13 after nerve injury contributes to neuropathic pain development through ERK-mediated nociceptive factor release [178,179]. Therefore, in our opinion, CXCL13 seems to also be a key player in neuropathic pain pathogenesis.
CXCR6 is a selective target of CXCL16; however, whether this chemokine is involved in nociceptive transmission still needs to be investigated. CXCL16 is composed of a mucin-like stalk, a transmembrane domain, and a cytoplasmic tail containing a potential tyrosine phosphorylation site that may bind to SH2 [180]. These are unusual features of CXCL16 and allow it to be expressed as a soluble form and a cell-surface-bound molecule [181]. Moreover, the expression of CXCL16 is induced by the inflammatory cytokines IFN-gamma and TNF-alpha [181]. The involvement of this chemokine in central sensitization has not been demonstrated, although, to the best of our knowledge, there are two papers that show, first, its changes after spinal cord stimulation [182] and, second, the slight upregulation of its gene 2 weeks after CCI [183]. More studies are needed.
CXCR8 is a target of CXCL17 [184,185]. This chemokine was one of the latest to be identified, that is, in 2006 [186]. To date, only one study has shown that the intrathecal administration of CXCL17 in naive mice induced strong tactile and thermal hypersensitivity [42]. Moreover, the author has shown that the effect is abolished by kynurenic acid and zaprinast administration [42]. The observed pronociceptive properties of CXCL17, which is a strong monocyte chemoattractant [184,187], are very important and may play a role in the development of neuropathy. Transcriptomic analysis has shown that CXCL17 is elevated after CCI in the anterior cingulate cortex and spinal cord [183]. However, more studies are needed since some studies have shown that CXCL17 has anti-inflammatory effects on LPS-activated macrophages by suppressing the production of proinflammatory cytokines [187]. Notably, it was recently shown that CXCL17 is a promising therapeutic target and is an independent biomarker of poor prognosis in patients with breast cancer [188]. The data in the literature need to be completed since those that already exist indicate that the modulation of the CXCL17/CXCR8 axis may become a potential strategy for the treatment of pain.
CXCL15 remains poorly understood in the context of nociception processes, and its receptor remains unknown. Moreover, CXCL15 is a typical murine chemokine that is not found in humans, and importantly, CXCL15 is mainly expressed in the lungs, digestive tract, and urogenital organs [189]; therefore, its role in nociceptive transmission seems unlikely.
In sum, the abovementioned results indicate that many endogenous ligands of chemokine receptors of the CXC subfamily are very important for nociceptive transmission. It seems that in the CNS, the axes CXCL3/CXCR2, CXCL9/CXCR3, CXCL10/CXCR3, CXCL12/CXCR4, CXCL13/CXCR5, CXCL14/CXCR4, CXCL17/CXCR8, and CCL21/CXCR3 and, in the PNS, the axes CXCL1/CXCR2 and CXCL2/CXCR2 are especially crucial during the development of neuropathic pain. The results indicate that these chemokines and their receptors may be promising targets in the search for pharmacotherapeutic agents to treat neuropathic pain; however, more studies are needed.
1.3. XC Chemokines in Neuropathic Pain
The XC subfamily consists of two members, XCL1 and XCL2, both of which are present in humans, but there is only XCL1 in the mouse and rat genome [190]. It was shown that both of these closely related chemokines act through XCR [191]. XCL1 is produced by immune cells and astrocytes [43,192]. First, in 2016, it was found that at the spinal cord level, XCL1 is highly upregulated in diabetic neuropathy [44]; later, in 2022, its time-dependent and long-lasting changes after sciatic nerve injury were described [43]. Moreover, in this study, using immunofluorescence, the authors showed that XCL1 is released at the spinal cord level, mainly by astroglial cells, but XCR1 is present mostly on neuronal cells [43]. Furthermore, it was proven that the intrathecal administration of XCL1 evokes hypersensitivity in naive mice [43,44]. Importantly, XCL1-neutralizing antibody administration attenuates hypersensitivity development in STZ- and CCI-evoked neuropathic pain in mice [43,44]. Recent research shows that XCL1 affects fibroblast migration through an atypical chemokine receptor, the heterodimeric (αβ) transmembrane receptor ITGA9 [193]. Pharmacological studies published in 2022 gave the first evidence that the blockade/neutralization of both receptors, XCR1 and ITGA9, reverses hypersensitivity evoked by intrathecal XCL1 administration in naive mice; however, the neutralization of ITGA9 is more effective. These data clearly indicate that XCL1 exhibits strong pronociceptive properties not only through XCR1 but also through ITGA9, which is also localized on neurons [43]. So far, only changes in the level of XCL2 have been studied in patients with neuropathy, and its downregulation has been observed; however, the authors indicated that more research is needed because this observation may be related to drug treatment [194]. Importantly, the neutralization of XCL1 improves morphine analgesia [43]. Moreover, the blockade of XCR1 positively influences buprenorphine effectiveness, and the neutralization of ITGA9 enhances not only buprenorphine but also morphine analgesia [43]. In summary, experimental studies suggest that both the XCL1/XCR1 and XCL1/ITGA9 axes play a role in neuropathic pain development; however, ITGA9 seems to be a more important neuronal target [43]. Therefore, it seems that the blockade of the XCL1/ITGA9 axis may serve as an innovative strategy for the polypharmacotherapy of neuropathic pain in combination with opioids [43]. Nevertheless, the role of XCL1 and XCL2 in neuropathic pain in humans needs to be elucidated.
1.4. CX3C Chemokine in Neuropathic Pain
Only one chemokine belongs to the CX3C subfamily, namely, CX3CL1, which occurs in two forms, membrane-bound or soluble [195]. CX3CL1 (also known as fractalkine) was one of the first described to be important for nociceptive transmission. It was shown that intrathecal CX3CL1 administration evokes strong mechanical and thermal hypersensitivity [45]. It was also shown that the mRNA level of this chemokine is elevated in the spinal cords of animals with neuropathy evoked by TNF-α injection [74]. Additionally, its upregulation is observed in the oxaliplatin model of neuropathic pain [46]. CX3CL1-induced pain hypersensitivity is abrogated in CX3CR1-knockout mice [196]. Moreover, neutralization of the chemokine diminishes pain-like behavior [46]. This chemokine is produced by neurons in the spinal cord and DRGs after nerve injury, while its receptor, CX3CR1, is present on the surface of microglial cells and is highly upregulated during neuropathic pain development [8]. It is also known that the binding of CX3CR1 by CX3CL1 increases microglial proliferation and migration [197,198]. There is also evidence that ERK5, which is expressed by microglia, is necessary for CX3CL1/CX3CR1-induced microglial activation and the induction of hyperalgesia [199]. CX3CR1 activation by CX3CL1 causes the phosphorylation of p38 MAPK, which results in the production of many pronociceptive factors, including TNFα, IL-1β, and IL-6 [9]. The link between CX3CL1/CX3CR1 signaling, microglial phenotypes, and neuronal damage/loss has long been proposed, with a growing body of data on the possible role of the CX3CL1/CX3CR1 axis in neurodegeneration. Molecules that target CX3CL1 may provide reduced side effects and stronger analgesia [200]. In sum, it seems that the modulation of the CX3CL1/CX3CR1 axis may bring some new benefits to neuropathic pain therapy. However, there is still a lack of selective safe substances that are able to cross the blood–brain barrier.
2. Chemokine Receptors and Neuropathic Pain
As mentioned in the previous section, chemokines activate specific receptors located on the surface of various immune, glial, and neural cells; twenty receptors have been characterized thus far [8,201]. Each is a seven-transmembrane G-protein-coupled receptor (Gαi) [8]. Published data indicate that the pharmacological blockade of some chemokine receptors from the CC group, as well as from the CXC, XC, and CX3C groups, relieves neuropathic pain of various etiologies in mice and/or rats, which we will discuss in detail in the following sections.
2.1. Analgesic Potential of Targeting Single CC Chemokine Receptors
Published data indicate that the blockade of five CC receptors causes analgesia and improves the effectiveness of opioids in different neuropathic pain models (Table 3).

Table 3.
Analgesic potential of targeting single CC chemokine receptors in animal models of neuropathic pain.
2.1.1. CCR1
The involvement of CCR1 in the development of hyperalgesia is still insufficiently investigated. However, it was recently shown that single and repeated intrathecal administration of the CCR1 antagonist J113863 significantly attenuates mechanical and thermal hypersensitivity in STZ [19], CCI [16,53], and cancer pain [207] models. Furthermore, the blockade of CCR1 by BI64 reduced hypersensitivity in a rat inflammatory pain model [208]. These beneficial analgesic effects of antagonists are undoubtedly related to both the cellular localization of CCR1 and spatiotemporal changes in its ligands. CCR1 is localized on neuronal, glial (astrocytes, microglia), and immune (neutrophils, basophils, monocytes, eosinophils, lymphocytes) cells [120,209,210,211,212,213]. CCR1 seems to be a very important receptor for nociceptive transmission since it has several ligands with strong pronociceptive properties, such as CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, and CCL9 [9,15,19,214,215,216,217,218,219]. It was already shown that in the early phase of neuropathic pain development, increases in CCL2, CCL3, CCL, CCL5, CCL7, CCL8, and CCL9 are observed, and in the maintenance phase, CCL2, CCL7, and CCL8 are observed [16]. Moreover, the neutralization of spinal CCL2, CCL3, CCL7, and CCL9 by their antibodies strongly attenuated neuropathic pain symptoms [15,19]. However, to date, it is still not known how and whether CCR1 blockade affects the biosynthesis of pronociceptive chemokines. Evidence shows that CCR1 is upregulated after nerve injury [220]. Importantly, CCR1 knockdown diminishes nerve pain in rats subjected to SNL and represses microglial activation [221]. Recent research indicates that the repeated intrathecal administration of J113863 reduces the activation and/or infiltration of microglia, macrophages, neutrophils, and lymphocytes into the spinal cord and/or DRGs and thus induces beneficial changes in the levels of factors with pronociceptive (IL-1 beta, IL-6, and IL-18) and antinociceptive (IL-1 receptor antagonist) properties [53]. Moreover, the intrathecal administration of a CCR1 antagonist not only relieves pain-related behavior but also improves the analgesic properties of opioids in STZ [19] and CCI [53] models. The authors suggest that CCR1 blockade probably restores the immune balance, which is one of the main proposed mechanisms by which it improves the effectiveness of opioids in the CCI model [53]. It is currently believed that the low effectiveness of opioid drugs in neuropathy is due to the strong production of pronociceptive factors with anti-opioid properties [222,223,224,225]. Recent studies have proven that a single administration of CCL2-, CCL3-, CCL7-, and CCL9-neutralizing antibodies can intensify morphine and/or buprenorphine analgesia [15,19,219]. The other reason for improved opioid analgesia might be the ability to form dimers between chemokine and opioid receptors, which was already shown for CCR5-MOR [226]. However, there is a lack of data showing that CCR1 can create heterodimers with opioid receptors, but it was shown that CCR1 is able to create dimers with CCR5 [227,228]. The available experimental studies indicate that CCR1 is involved in the pathogenesis of diseases with large neuroimmunological components, such as rheumatoid arthritis [208,210,229], disc inflammation [230], multiple sclerosis [231,232], and diabetes [19]. Importantly, several CCR1 antagonists have entered clinical trials, including MLN3897 and CP-481,715 for rheumatoid arthritis, BX471 for multiple sclerosis, AZD-4818 for chronic obstructive pulmonary disease [233], and BAY86-5047 for endometriosis [234]. In summary, based on available results from experimental and clinical studies, it can be hypothesized that pharmacological modulation via CCR1 may represent a novel strategy for effective polytherapy with opioids in patients suffering from neuropathic pain.
2.1.2. CCR2
Published data indicate that the repeated intrathecal administration of the single CCR2 antagonist RS504393 [52,60] reduces tactile and thermal hypersensitivity in neuropathic pain induced by sciatic nerve ligation in mice and/or rats. Moreover, RS504393 not only attenuates pain-related behavior but also enhances the analgesic properties of morphine and buprenorphine after CCI [52]. Similarly, intracisternal injections of the mentioned CCR2 antagonist reduce neuropathic pain symptoms induced by inferior alveolar nerve transection [204], mental nerve transection [204], and lumbar disc herniation [235]. Moreover, the subcutaneous (s.c.) or intrathecal administration of RS504393 diminishes paclitaxel-evoked pain [203]. Furthermore, the oral administration of another CCR2 antagonist, AZ889, may reverse mechanical and thermal hyperalgesia in CCI-subjected animals [205]. CCR2 is expressed by spinal microglia, astrocytes, and neurons [17,236,237], and it is already known that CCR2 is mainly bound by CCL2 [17]; however, not only CCL2 but also two of its ligands, CCL7 and CCL8, have strong pronociceptive properties. Many studies have shown that these ligands are mediators of spinal glial activation after nerve injury [8,9,51]. Interestingly, the abolition of the development of mechanical hypersensitivity after nerve injury is observed in CCR2-knockout mice [238]. It was already shown that RS504393 diminishes CCI-induced microglial activation at the spinal cord level after CCI [202] and, in parallel, prevents the upregulation of pronociceptive factors, such as IL-1beta, IL-18, IL-6, and inducible nitric oxide synthase, and increases the expression of antinociceptive IL-1alpha [52]. Moreover, it was demonstrated that repeated intrathecal administration of a single CCR2 antagonist (RS504393) significantly reduces the enhanced expression of CCL2, CCL3, CCL4, CCL5, CCL7, and CCL11 in DRGs but does not influence them at the spinal cord level [60]. However, a single intraperitoneal injection of RS504393 does not influence fully developed neuropathic-pain-related behaviors in mice [60]; therefore, further research is needed, which is now enabled by newly synthesized CCR2 antagonists (e.g., INCB 3284, RS 102895, MK-0812, PF-4136309). Additionally, BMS-741672 is in the second phase of clinical trials for patients with diabetic neuropathic pain; however, no study results have yet been posted [239]. Targeting CCR2 through siRNA, blocking antibodies, or small-molecule antagonists may provide new therapeutic possibilities for managing neuropathic pain [240]. The available literature suggests that the pharmacological modulation of spinal neuroimmunological interactions via CCR2 may represent a new strategy for effective polytherapy with opioids in patients suffering from neuropathic pain; however, better pharmacological tools are needed.
2.1.3. CCR3
To our knowledge, the role of CCR3 in nociceptive transmission has not been thoroughly investigated, and the first data were published in 2021. Its presence has been demonstrated in many types of pain-modulating cells, including neurons, microglia, astrocytes, neutrophils, lymphocytes, and satellite cells [23,241,242,243,244,245,246]. Moreover, data in the literature indicate the important role of CCR3 in disorders such as cancer, asthma, atopic skin inflammation, narcolepsy, and inflammatory bone resorption [247,248,249,250,251] and, recently, in neuropathic pain models [16,23]. It was shown that partial infraorbital nerve transection (pIONT) increases the expression of CCR3 [252]. The receptor has several endogenous ligands, such as CCL5, CCL7, CCL8, CCL11, CCL24, CCL26, and CCL28, and importantly, four of them, CCL5, CCL7, CCL8, and CCL11, appear to be important factors involved in nociception processes [15,16,26,56,97,102,107,108,251]. In addition, significant increases in CCL7, CCL5, CCL8, and CCL11 at the level of the spinal cord and/or DRGs have already been demonstrated in the CCI model in mice and rats [16,23]. It was also shown that the single or repeated intrathecal administration of the CCR3 antagonist SB328437 diminishes mechanical and thermal hypersensitivity [23]. Moreover, in parallel, it reduces the CCI-evoked increases in the levels of neutrophil, lymphocyte, and satellite cell protein markers in the spinal cord and/or DRGs, which are accompanied by the downregulation of IL-6, CCL7, and CCL11 [23]. Additionally, the repeated intrathecal administration of the CCR3 antagonist SB328437 enhances the analgesic properties of morphine and buprenorphine, although to a lesser extent for buprenorphine [23]. The mechanism by which SB328437 modulates opioid analgesia remains unclear. One of the reasons could be the heterologous desensitization of CCR3-MOR, as was shown for CCR5-MOR [253,254,255]; however, there are no data showing whether these dimers exist. Another explanation could be that SB328437, through its effects on immune and satellite cells, may participate in the potentiation of opioid analgesia; however, more studies are needed to explore the full mechanism. SB328437 also relieves mechanical hypersensitivity developed in the pIONT model of neuropathy and decreases upregulated pERK levels [252]. A few animal studies have already shown that SB328437 has beneficial effects on carcinoma [256], allergic inflammation [257], and osteoarthritis [258] models. Based on the published results, it can be concluded that CCR3-targeted therapy may be beneficial since its antagonist positively modulates immune cell activation and influx in neuropathic pain. Due to these promising results, we believe that the role of CCR3 in neuropathy should be further explored; moreover, novel pharmacological tools are needed.
2.1.4. CCR4
CCR4 has two selective ligands, CCL17 and CCL22, and one nonselective ligand, CCL2, which all have strong pronociceptive effects. Although the well-known pronociceptive properties of CCL2 are primarily associated with CCR2 [9,52,202], recent research provides evidence that CCL2/CCR4 signaling is also involved in these effects [80]. CCR4 is present on many immune cells, including T lymphocytes (Th2, Th17, and Tregs), platelets, natural killer (NK) cells, macrophages, dendritic cells [127,128,259], neurons [260], microglia [244], and astroglia [128,244]. Importantly, CCR4 is localized at different levels of the PNS (DRGs [103]) and CNS (spinal cord [261], brain [262]), which suggests its crucial role in nociceptive transmission. It was already shown that both of its selective ligands are enhanced in the serum of patients with fibromyalgia [122]. For pharmacological studies, several CCR4 antagonists are available [263,264]; however, the best studied thus far in neuropathic pain models is C021. It has been shown that single and repeated intrathecal and intraperitoneal injections of C021 diminish hypersensitivity in rats and/or mice with CCI- [27,80] and STZ-evoked [79] neuropathic pain. Importantly, C021 in the STZ model also improves locomotor activity [79], which is important from a clinical point of view since patients with diabetes mellitus often experience a decline in locomotor performance and neuropathic pain [265,266]. Surprisingly, in the CNS, the spinal levels of CCL17 and CCL22 in rats and mice after nerve injury are unchanged [27,80], similar to diabetic neuropathy [79]. Nevertheless, an increase in CCL2 in the spinal cord is observed in neuropathic pain models [53,79]. These results highlight the important role of the spinal CCL2/CCR4 axis in nociception. Additionally, it is worth noting that the repeated intrathecal administration of C021 reduces hypersensitivity and, in parallel, diminishes the spinal levels of macrophage/microglial activation, influx, and/or proliferation and, as a consequence, the expression of the pronociceptive IL-1beta and IL-18 [27]. In the PNS, all endogenous CCR4 ligands are elevated in DRGs. Therefore, CCR4 blockade is likely more effective when C021 is administered intraperitoneally, which is not similar to other chemokine antagonists, such as RS5043930 (antagonist of CCR2) [60] or maraviroc (antagonist of CCR5) [206]. Moreover, repeated intraperitoneal treatment with C021 diminished spinal macrophage/microglia levels during neuropathy development [80]. Therefore, targeting CCR4 is a promising strategy to provide a new basis for understanding neuropathic pain pathomechanisms with potentially new therapeutic utility. Importantly, in CCI-exposed rats and mice, the single, intrathecal, and intraperitoneal administration of C021 enhances the analgesic effect of morphine and buprenorphine [27,80] and diminishes the development of morphine tolerance in mice after nerve injury [80]. Overall, published data indicate that targeting CCR4 and its ligands is a promising strategy to provide neuropathic pain relief and enhance the analgesic effects of opioids, which represents a promising basis for the development of more effective combined therapy for pain treatment. Importantly, clinical trials have been studying the use of mogamulizumab, a monoclonal antibody against CCR4, and the results are promising for the treatment of lymphomas and leukemia [267,268] and advanced solid tumors [268], and perhaps one day, it may be explored for neuropathic pain relief.
2.1.5. CCR5
In 2013, Lee et al. [255] showed that CCR5-knockout mice develop hypersensitivity to a lesser extent, which, for the first time, drew attention to the possible involvement of this receptor in nociceptive transmission. It is now known that many endogenous CCR5 ligands exhibit strong pronociceptive effects. CCL3, CCL5, CCL7, CCL8, and CCL11 were described previously. CCR5 is expressed by a variety of immune cells (e.g., granulocytes, macrophages, and lymphocytes) but also in neurons and glia, including astroglia and microglia [269,270]. CCR5 is strongly upregulated in the ipsilateral dorsal spinal cord and DRGs after CCI, and the intrathecal administration of maraviroc (CCR5 antagonist) prevents these changes and, in parallel, the activation of microglia/macrophages and astroglial cells, which are known to be responsible for hypersensitivity development in neuropathy. The observed beneficial effect of maraviroc on CCI-evoked cell activation contributes to the reduction in secreted pronociceptive factors, such as IL-1β, IL-18, IL-6, nitric oxide synthase 2, CCL3, CCL4, and CCL5 [56,206], and in parallel, it is responsible for increasing the antinociceptive factors IL-1 receptor antagonist, IL-18 binding protein, and IL-10 [206]. It was also shown that another CCR5 antagonist, TAK-779, reduces microglial/macrophage activation/migration [271,272]. The obtained published data allow for the hypothesis that maraviroc attenuates neuropathy symptoms by promoting spinal glial “alternative” polarization and restoring the balance between pro- and antinociceptive factors. Despite numerous studies, the detailed mechanism of CCR5 action has not yet been fully ascertained, similar to the reason for the decreased analgesic potency of opioids in neuropathy. Interestingly, opioid receptor agonists, such as morphine, can increase CCR5 expression [273]. Therefore, it was suggested that the heterologous desensitization of opioid and chemokine receptors is possible, since it is already known that CCR5 and opioid receptors (MOR, KOR) are present on spinal glial and neuronal cells [101,273,274,275]. Currently, it is well established that MOR-CCR5 forms heterodimers, which contribute to cross-desensitization [276,277]. Therefore, it is not surprising that the observed maraviroc-evoked downregulation of CCR5, which is upregulated after CCI, beneficially influences opioid agonist effectiveness. Moreover, maraviroc diminishes microglial and astroglial cell activation, and in consequence, these cells secrete fewer factors whose anti-opioid role has already been documented, such as IL-1β [225], IL-18 [224], CCL3 [15,19,22], and CCL5 [25,26,100]. Importantly, data in the literature indicate that other CCR5 antagonists, i.e., AZD5672 and TAK-220, similar to maraviroc, diminish CCI-evoked neuropathic pain symptoms and enhance morphine-evoked analgesia [57]. Numerous studies clearly indicate that CCR5 is a potential target for drug development in the treatment of neuropathic pain, and maraviroc seems to be a substance worth future research in the clinic. Importantly, maraviroc has received accelerated approval from the Food and Drug Administration for clinical use and is currently used as a cure for HIV-infected patients who are infected by the R5-tropic virus [278,279], which indicates that the substance is safe for patients. In our opinion, maraviroc may, in the future, become a drug for the concomitant treatment of patients receiving opioid therapy for neuropathic pain.
2.1.6. CCR8
The role of CCR8 in nociception processes is poorly known thus far, while much is already known about the strong pronociceptive properties of its selective ligand, CCL1 [14]. Moreover, its increase has already been shown at the spinal cord and DRG levels in neuropathy of various etiologies. To date, it has not been demonstrated whether and to what extent the blockade of this receptor affects neuropathic pain. Four new pharmacological tools (AZ084; R243; type 1 and type 2 CCR8 antagonists) have recently been synthesized and are already enabling research. To date, it has been shown that nerve-ligation-induced hypersensitivity is attenuated in CCR8-knockout mice and that CCR8 siRNA blocks CCI-induced hypersensitivity [14]. Moreover, it was demonstrated by immunofluorescence techniques that at the spinal cord level, CCR8 is present on neurons [13,14]. However, after partial sciatic nerve ligation, the upregulation of CCR8 is observed in both neuronal and glial cells [14]. Later, an in vitro study performed in primary cultures of glial cells confirmed the possible localization of CCR8 in microglial and astroglial cells [13]. CCR8 contributes to neuropathic pain through the spinal release of nociceptive cytokines and is involved in the activation of NMDA receptors [14]. In light of recent evidence, it seems that the CCL1/CCR8 axis also plays an important role in opioid effectiveness during neuropathic pain, since neutralizing antibodies against CCL1 enhance the effects of morphine and buprenorphine [13]. Published data indicate that CCL1/CCR8 crosstalk contributes to the development of neuropathic pain of different etiologies [13,14] and, therefore, can be a potential target for drug development in the treatment of neuropathic pain; however, pharmacological studies with newly synthesized antagonists are still needed.
To sum up, clinical data clearly indicate the potential of already-created CC receptor blockers that are being used or currently tested for the treatment of various diseases—e.g., CCR1 antagonists (MLN3897, CP-481,715, AZD-4818, BAY86-5047) [233,234], a CCR2 antagonist (BMS-741672) [239], a monoclonal antibody against CCR4 [267,268], and a CCR5 antagonist (maraviroc) [278,279]. Experimental data suggest that in the future, they may also be used for neuropathic pain relief, but this requires clinical trials.
2.2. Analgesic Potential of Targeting Single CXC Chemokine Receptors
Literature data indicate that the blockade of four and activation of one of the CXC receptors cause analgesia, and two of them additionally improve the effectiveness of opioids in different neuropathic pain models (Table 4).

Table 4.
Analgesic potential of targeting the single CXC, XC, and CX3 receptors in animal models of neuropathic pain.
2.2.1. CXCR2
CXCR2 has three selective (CXCL1–3) and four unselective (CXCL5–8) endogenous ligands. In neuropathy, CXCR2 is upregulated at the spinal cord level in neuronal [31,32] and nonneuronal [32,287,288] cells and locally at the nerve injury site in macrophages and neutrophils [33]. The spinal neuronal expression of CXCR2 correlates well with the fast and strong pronociceptive effects of intrathecally injected CXCR2 ligands in naive mice [31]. In 2017, it was shown that the administration of the dual CXCR2/CXCR1 antagonist SCH527123 potently reverses central sensitization after brain injury [289]. Recently, in 2019, it was shown for the first time that a potent and selective CXCR2 receptor antagonist, NVP-CXCR2-20, administered intrathecally for seven days reduced the symptoms of neuropathic pain and the CCI-upregulated levels of CXCL3 in the spinal cord and DRGs. Moreover, in naive mice, this antagonist prevents CXCL3-induced hypersensitivity [31]. In addition, the repeated intrathecal administration of NVP-CXCR2-20 reduces the spinal secretion of pronociceptive interleukins (i.e., IL-1beta, IL-6, IL-18) and chemokines CCL2, CCL6, CCL7, and CXCL4 [58]. It was also shown that another CXCR2 antagonist, SB225002, decreases pain symptoms in many animal pain models, such as those induced by paclitaxel [280], SNL [32], L5-SNL [282], PSL [33], CCI [290], and inferior alveolar nerve transection [281]. Moreover, vincristine induces CXCR2 upregulation in spinal cord neurons, which is diminished by levo-corydalmine, consequently leading to a decrease in pain hypersensitivity [291]. However, the repeated intrathecal administration of NVP-CXCR2-20 does not improve the analgesic efficacy of morphine and buprenorphine in a CCI model [31]. According to the authors, this is because the blockade of CXCR2 does not affect microglial or astroglial cell activation [31], as has been observed with antagonists of other chemokine receptors and has already been described in our review. Based on published data, we suggest that the blockade of CXCR2 signaling may have effective analgesic effects in neuropathy [292]. Nevertheless, the lack of effect on opioid drug analgesia indicates that the blockade of other chemokine receptors may be more beneficial in the polytherapy of neuropathy.
2.2.2. CXCR3
Many published studies indicate that CXCR3 is an essential target for the development of neuropathic pain [28,58,143,293]. For the first time, in 2017, hypersensitivity was shown to be markedly reduced in CXCR3-knockout mice, and spinal inhibition of CXCR3 with shRNA attenuated fully developed neuropathic pain after SNI [163]. Moreover, CXCR3 knockout in animals alleviates trigeminal neuropathic pain [165]. To date, five CXCR3 ligands have been identified—CXCL4, CXCL9, CXCL10, CXCL11, and CCL21—and all of them have strong pronociceptive properties [28,294,295]. Recently, it was shown that after CCI [58,295] and/or SNI [143], the spinal level of CXCR3 is upregulated in parallel with its endogenous ligands [28]. Spinal CXCR3 is predominantly expressed on neuronal cells, rarely on microglia, and has not been detected in astrocytes [163]. The mainly neuronal location of CXCR3 confirms and explains why intrathecally administered CXCR3 ligands induce fast and strong pain-like behaviors in naive mice [28]. The binding of ligands to CXCR3 is associated with an increase in the intracellular Ca2+ concentration and the activation of MAPKs and NF-κB, which, as is well known, contributes to the development of hypersensitivity [296,297,298,299]. Therefore, the single and repeated intrathecal administration of a potent and selective CXCR3 antagonist ((±)-NBI-74330) attenuates neuropathic pain symptoms. Studies are needed to examine its molecular mechanisms of action. However, it is already known that the repeated administration of (±)-NBI-74330 significantly diminishes microglial cell activation and downregulates the expression of most of its ligands (CXCL4, CXCL9, CXCL10, and CCL21) in the spinal cord of rats with neuropathic pain symptoms. Importantly, behavioral experiments have shown that a CXCR3 antagonist is able to restore the analgesic properties of morphine. The exact role of CXCR3 ligands in opioid effectiveness requires more studies; however, it is already known that the inhibition of microglial activation and the secretion of nociceptive factors by pharmacological tools improve opioid analgesia [222,300]. Additionally, it was recently shown that CXCR3 colocalizes with neurons in the anterior cingulate cortex, and its CCI-evoked upregulation arises in parallel with hypersensitivity development. Importantly, the pharmacological blockade of CXCR3 using an injection of AMG487 directly into the anterior cingulate cortex reduces pain symptoms [283]. In sum, the available literature supports the theory that CXCR3 represents a novel strategy for effective neuropathic pain therapy, including polytherapy with opioids.
2.2.3. CXCR4
AMD3100 is a CXCR4 antagonist that has already been used in the clinic [301]. AMD3100 shows potential therapeutic effects on many inflammatory diseases [302,303,304] and is approved by the FDA for autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma and multiple myeloma [301]. Moreover, AMD3100 causes pain relief in AIDS-induced neuropathy [305]. Animal studies have shown that AMD3100 has analgesic effects on different pathological pain states in mice and rats, including bone cancer pain [172,306], diabetic neuropathy [307,308], peripheral neuropathic pain [37,284,309], and opioid-induced hyperalgesia [310]. Another CXCR4 antagonist, AMD3465, exhibits analgesic properties in animal models, such as pSNL and CPIP models [37]. In the SNL-induced rat neuropathic pain model, CXCR4 and its endogenous ligand CXCL12 are highly upregulated, mainly in astroglia and neuronal cells. Studies have also shown that CXCR4 colocalizes with microglia from the spinal dorsal horn. Moreover, the inhibition of CXCL12 and the blockade of CXCR4 relieve neuropathic pain symptoms [39]. AMD3100 alleviates hypersensitivity partially by augmenting leukocyte-derived endogenous opioid secretion in the spinal cord and downregulating pronociceptive factors (TNFα, IL-1β) [284]. Importantly, local naloxone methiodide injection inhibits AMD3100-related analgesia [284]. Moreover, the intraperitoneal administration of AMD3100 dose-dependently reverses opioid-induced hyperalgesia in rats [310]. Published papers provide evidence that analgesia via CXCR4 is mediated by the endogenous opioid system [284]. In summary, data from the literature suggest that the blockade of CXCR4 signaling represents a promising novel approach to the management of side effects associated with the use of opioids for chronic pain management [310]; however, studies in neuropathic pain models are needed.
2.2.4. CXCR8
In 2015, it was shown that CXCR8, also known as GPR35, is a receptor for CXCL17 [184]; however, the role of the CXCL17/CXCR8 axis in nociceptive transmission still needs to be studied. CXCR8 expression has been identified within the nervous system in both neuronal and nonneuronal cells, including glia, and is therefore suggested to be important for neuropathic pain development [311,312,313,314,315,316]. However, kynurenic acid is also an important agonist of CXCR8 [317,318,319]. It has already been shown that the intrathecal administration of CXCL17 in naive mice induces pain-related behaviors, which, however, are partially abolished by the administration of kynurenic acid. Moreover, in contrast to CXCL17, kynurenic acid diminishes pain-related behavior evoked by nerve injury and enhances the effectiveness of morphine [42,286]. However, kynurenic acid is not only an agonist of CXCR8 but also a noncompetitive antagonist at the glycine site of the NMDA receptor and an antagonist of ionotropic NMDA, AMPA, and kainate receptors [314,320,321,322,323]. Considering the abovementioned multitarget effects of kynurenic acid, it is not surprising that it has analgesic properties in a mouse/rat pain model [313,315,319,324,325]. However, similar effects were observed with zaprinast, an exogenous agonist of CXCR8 [316], which also partly diminished CXCL17-evoked hypersensitivity in naive mice [42]. Moreover, its analgesic properties have been proven in rat and mouse inflammatory [313,326] and neuropathic [42,286] pain models. To date, it has been shown that the activation of CXCR8 by zaprinast, kynurenic acid, or CXCL17 leads to the distinct modulation of Ca2+ levels: kynurenic acid and zaprinast reduce Ca2+ mobilization [327], while in contrast, CXCL17 increases Ca2+ mobilization [184]. Recently, two in vitro studies published by Binti et al. [185] and Park et al. [328] proposed that CXCL17 also acts through an unknown receptor. This is in line with published behavioral studies showing that the pronociceptive properties of CXCL17 are not completely blocked by zaprinast and kynurenic acid. Published data suggest that CXCR8 is a promising target for reducing neuropathic pain and enhancing opioid analgesia, but this hypothesis requires further in-depth research.
In sum, although pharmaceutical companies put a lot of effort into the production of CXC receptors antagonists, despite good results obtained in experimental studies in general only substances targeting CXCR4 are commonly used in clinical trials [329].
2.3. Analgesic Potential of Targeting the Single XCR Chemokine Receptor
Data in the literature indicate that the blockade of XCR1 causes analgesia and improves the effectiveness of opioids in a neuropathic pain model (Table 4). In 2016, it was shown for the first time that the XCL1/XCR1 axis plays an important role in hypersensitivity development in STZ-induced diabetes [44], and in 2022, it was also shown to play an important role in CCI-evoked neuropathy [43]. For a long time, XCR1 was suggested to be the only receptor for XCL1. Nevertheless, since 2017, it has been known that XCL1 also acts via fibroblast migration through the heterodimeric (αβ) transmembrane receptor ITGA9 [193]; thus, it was hypothesized that both of these receptors are involved in the development of neuropathic pain. XCR1 is expressed in neurons [44,330], oligodendrocytes [330], and many immune cells (e.g., T and B cells and neutrophils) [331]. Moreover, immunohistochemical studies have shown that XCR1 is expressed in nonpeptidergic and non-IB4 binding terminals of A-delta and C-fiber afferents and within excitatory interneurons [330]. However, in the spinal cord, the expression of XCR1 was not detected in microglia or astroglia [43]. Behavioral studies have shown that the blockade of XCR1 by vMIP-II may cause neuropathic pain relief [43], which may be due to the ability to reduce the p38MAPK and pERK activation observed after stimulation by XCL1 [330]. Published data indicate that the XCL1/XCR1 axis plays a key role in nociceptive transmission since it is able to participate in many aspects of neuronal–glial interactions.
2.4. Analgesic Potential of Targeting a Single CX3C Chemokine Receptor
JMS-17-2 is a new, potent, and selective small-molecule antagonist of CX3CR1 that is able to inhibit glial cell activation [332]; however, to our knowledge, it has not been studied in neuropathic pain models. Following nerve injury, the spinal enhancement of CX3CR1 levels in microglial cells is observed, together with hypersensitivity development [200,333,334]. Moreover, the intrathecal administration of CX3CL1 evokes both thermal and mechanical hypersensitivity [335]; however, both effects are abrogated in CX3CR1-knockout mice [196,336]. Importantly, it was already shown that a neutralizing antibody against CX3CR1 [337,338] diminishes neuropathic pain symptoms. Additionally, the intrathecal injection of PLGA-encapsulated CX3CR1 siRNA nanoparticles reduces mechanical hypersensitivity in SNL-induced animals [339]. Data in the literature strongly suggest that the CX3CL1/CX3CR1 axis is important for nociceptive transmission [200]; therefore, further studies with the use of novel selective CX3CR1 antagonists are necessary.
2.5. Analgesic Potential of Targeting Multiple Chemokine Receptors
Research in recent years has provided an increasing amount of data showing that multifunctional compounds also have good antinociceptive activity. Currently, studies are showing the significant advantages of compounds targeting more than one molecular target over a physical combination of individual substances in terms of their antinociceptive properties, which is the case for multitarget antagonists of chemokine receptors (Table 5), which we will review in the following sections.

Table 5.
Analgesic potential of targeting multiple multitarget chemokine receptors in animal models of neuropathic pain.
UCB35625 is a recently synthesized dual antagonist of CCR1 and CCR3 [344]. The importance of these receptors in nociceptive transmission has already been proven [16,23,53]. It was shown that the blockade of CCR1 by J113863 and CCR3 by SB328437 diminishes pain-like behavior in a CCI model [23,53]. Moreover, it was proven that several pronociceptive chemokines can act through CCR1 and CCR3 [16]. Among them, CCL7 and CCL8 are the common ligands of both receptors with well-known long-lasting increases and strong pronociceptive properties in neuropathy [16]. In 2022, it was shown that UCB35625 diminishes pain-like behavior [16]. However, surprisingly, the reduction in hypersensitivity by this dual agonist of CCR1/3 is similar to or even less than that produced by the single antagonists J113863 and SB328437 administered alone [16]. Nevertheless, we still believe that CCR1 and CCR3 may serve as potential therapeutic targets for the treatment of neuropathic pain, but new and better pharmacological tools that can target several chemokine receptors are needed.
Three dual antagonists of CCR2 and CCR5 are currently available: cenicriviroc [55,60], BMS-813160 [345], and, recently, PF-04634817 [346]. To date, only the effectiveness of cenicriviroc has been tested in neuropathic pain models. Many studies highlight the importance of CCR2 and CCR5 in nociceptive transmission [52,56,202,206]. Interestingly, these two receptors are structurally similar: they share 70% sequence homology [8]. Importantly, it has been proven that CCR2 and CCR5 undergo heterodimerization, specifically after costimulation with their ligands [347]. In the CNS, the receptors are coexpressed on infiltrating and/or resident immune (monocytes, lymphocytes) and glial (microglia, astrocytes) cells [8,201,348] and are present on neurons [201]. The common feature of CCR2 and CCR5 ligands is pleiotropy; therefore, some of them have the ability to act through both receptors, and moreover, importantly, most of them evoke pain-related behavior [8,15,19,349]. Recently, it was shown that the repeated intrathecal administration of selective CCR2 (RS504393) [52] and CCR5 (maraviroc) [56] antagonists and a dual CCR2/CCR5 antagonist (cenicriviroc) caused analgesia in a rat neuropathic pain model [55,60]. The authors have shown that both single and dual antagonists similarly diminish the activation and infiltration of microglia and macrophages in the spinal cord and/or DRGs and lower the levels of pronociceptive interleukins, such as IL-1beta, IL-6, and IL-18 [52,55,56]. However, the dual antagonist is a more effective agent in silencing a broad spectrum of chemokines. It was shown that the administration of cenicriviroc results in the downregulation of many pronociceptive chemokines, including CCL2, CCL3, CCL4, CCL5, CCL7, and CCL12, in DRGs and/or the spinal cord. In contrast, single antagonists affect the biosynthesis of pronociceptive chemokines to a lesser extent; RS504393 reduces the expression of CCL2, CCL3, CCL4, CCL5, CCL7, and CCL11 only in DRGs, whereas maraviroc diminishes only the level of CCL5 in DRGs and CCL4 in the spinal cord. Undoubtedly, the dual antagonist cenicriviroc is the most effective drug for silencing pronociceptive chemokine signaling pathways. Moreover, importantly, from a clinical point of view, the intraperitoneal injection of cenicriviroc has greater analgesic properties than RS504393 or maraviroc against neuropathic pain [60]. In addition, it is also extremely significant that repeated intrathecal and intraperitoneal injections of cenicriviroc strongly enhance the analgesic effects of morphine and buprenorphine [55,60]. The mechanism underlying this phenomenon is not clear; however, it may be related to heterologous desensitization between opioid/chemokine receptors or a beneficial influence on neuroimmune interactions [60], or probably both processes. This is clinically relevant, particularly since cenicriviroc is a drug candidate for the treatment of HIV infection, nonalcoholic steatohepatitis, and liver fibrosis (CENTAUR, phase 2b, and AURORA, phase 3) [350]. Based on data in the literature, it seems that targeting CCR2 and CCR5 simultaneously is an interesting alternative for neuropathic pain pharmacotherapy, especially in combination with opioids. However, further behavioral and clinical research is needed.
In 2004, reparixin (also called repertaxin), a potent CXCR1 and CXCR2 allosteric inhibitor, was presented for the first time [351]. Later, it was shown that this substance diminishes the development of paclitaxel-induced nociception in rats [342]. In 2023, it was shown that reparixin blocks oxaliplatin-induced hypersensitivity but not vincristine-induced hypersensitivity [343]. Considering that CXCR1 and CXCR2 are upregulated in several animal models of neuropathic pain [282,352], it is undoubtedly important to conduct further research with reparixin.
Another interesting pharmacological tool is RAP-103, which blocks four chemokine receptors, CCR2, CCR5, CCR8 [353], and CXCR4, as the latest research shows [354]. It was already shown that RAP-103 prevents and/or diminishes established neuropathic pain hypersensitivity in rats after partial sciatic nerve injury [341] and in a diabetic peripheral neuropathy model [340]. Moreover, RAP-103 evokes analgesia by reducing spinal cord microglial/macrophage activation and monocyte infiltration and by reducing the mRNA expression of IL-1β and IL-6 in the sciatic nerve and spinal cord [341]. Moreover, RAP-103 administered together with morphine enhances its analgesic effect in the DPN model, which is related to the fact that it increases the sensitivity of MOR and reduces the levels of the pronociceptive cytokines TNFα and CCL3, and it also slightly reduced CCL2 [340]. Importantly, in 2022, Bongiovanni et al. reported that RAP-103 diminishes opioid-derived respiratory depression and physical dependence in rats [354]. The authors proposed that this multitarget chemokine receptor antagonist may lessen the risk of opioid abuse [354], which indicates that polytherapy may be useful for the management of both pain and opioid addiction. Therefore, in light of the current literature, it can be said with certainty that by blocking several chemokine receptors that are significantly involved in nociceptive transmission, pain can be relieved; such therapeutic approaches may be extremely helpful in wide range of clinical applications, including opioid pharmacotherapy.
3. Conclusions
A deeper understanding of the role of chemokines and their temporal fluctuations is crucial for the development of novel therapeutic strategies for neuropathy. Data in the literature clearly indicate that numerous chemokines possess pronociceptive effects; therefore, blocking their action by using neutralizing antibodies or blocking their biosynthesis relieves pain. For example, from the CC family, CCL1/2/3/5/7/8/9/11 are significant in the development (CCL2/3/5/7/8/9) and maintenance (CCL2/7/8) of neuropathic pain.
Among them, CCL2/7/8 seem to be the most important because of the quick and long-lasting increase in their protein levels and their strong pronociceptive properties. Additionally, chemokines from the CXC family, such as CXCL3/9/10/12/13/14/17, from the XC family, such as XCL1, and from the CX3C family, such as CX3CL1, are already known to be crucial during neuropathic pain. The literature data indicate that these chemokines and their receptors are promising targets in the search for novel therapies for neuropathic pain; however, more studies are needed. Many studies, including ours, have shown that the use of single chemokine receptor antagonists (Scheme 1) can relieve pain of different etiologies, for example, the blockade of CCR1 (J113863) [7,8,17], CCR2 (RS504393) [6,187,188,220], CCR3 (SB328437) [7,21], CCR4 (C021) [28,64,65], CCR5 (maraviroc, AZD5672, TAK-220) [15,16,27,190], CXCR2 (NVP CXCR2 20, SB225002) [25,32,34,35,267,268,270], CXCR3 (NBI-74330, AMG487) [25,31,272], CXCR4 (AMD3100, AMD 3465) [43,273,274], and XCR1 (vMIP-II) [52,276]. It has been shown that some chemokines, e.g., CCL1 [5], CCL2 [12], CCL3 [17], CCL7 [12], CCL9 [17], and XCL1 [52], reduce the analgesic effect of opioids, and the use of neutralizing antibodies restores morphine and/or buprenorphine analgesia.
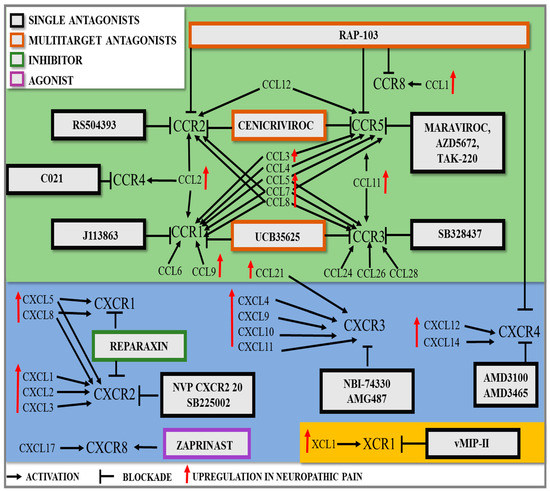
Scheme 1.
Changes and targets in chemokine systems in animal models of neuropathic pain, prepared based on the following references: [16,19,23,27,28,31,32,33,37,43,53,55,56,57,58,60,79,80,206,280,281,282,283,284,285,330,340].
Similarly, single antagonists of chemokine receptors, e.g., J113863 [19,53], RS504393 [52], C021 [27,79,80], maraviroc [56,60], vMIP-II [43,330], and NBI-74330 [28,31,58], enhance opioid analgesia due to their ability to inhibit the activation of microglia/macrophages (Scheme 2). The exception is SB328437 [23], which reduces hypersensitivity and enhances opioid analgesia; however, SB328437 does not affect the level of spinal microglial/macrophage activation or infiltration but strongly inhibits the activation/influx of neutrophils in neuropathy [23].
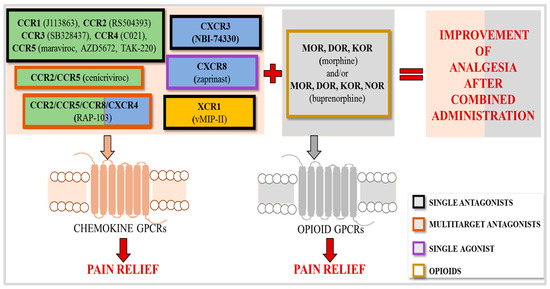
Scheme 2.
Targeting chemokine systems enhances opioid effectiveness, prepared based on the following references: [19,23,27,28,31,43,52,53,55,56,58,60,79,80,330,340,342].
Taking into account the pleiotropy of many chemokines and the different mechanisms of action of single antagonists of chemokine receptors, it seems that pharmacological modulation based on simultaneously blocking several chemokine receptors can be used as an innovative method of treating neuropathic pain, with great effectiveness, and can also be used in combination with opioid drugs. Multitarget chemokine receptor antagonists/inhibitors, such as cenicriviroc (CCR2/5) [55,60], reparixin (CXCR1/2) [342], and RAP-103 [340] (CCR2/5/8/CXCR4) (Scheme 1), seem to be interesting painkillers. Such a multidirectional treatment strategy based on the modulation of neuronal–glial–immune interactions by changing the activity of the chemokine family can significantly improve the quality of life of patients suffering from neuropathic pain.
Author Contributions
Conceptualization, K.P. and J.M.; writing—original draft preparation, K.P. and J.M.; writing—review and editing, K.P. and J.M.; visualization, K.P. and J.M.; supervision, J.M.; project administration, J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland grants, OPUS 22 2021/43/B/NZ7/00230, and statutory funds from the Maj Institute of Pharmacology Polish Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Sample Availability
Not applicable.
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Neuropathic Pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mitsikostas, D.-D.; Moka, E.; Orrillo, E.; Aurilio, C.; Vadalouca, A.; Paladini, A.; Varrassi, G. Neuropathic Pain in Neurologic Disorders: A Narrative Review. Cureus 2022, 14, e22419. [Google Scholar] [CrossRef]
- Cherif, F.; Zouari, H.G.; Cherif, W.; Hadded, M.; Cheour, M.; Damak, R. Depression Prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Res. Manag. 2020, 2020, 7408508. [Google Scholar] [CrossRef] [PubMed]
- Duo, L.; Yu, X.; Hu, R.; Duan, X.; Zhou, J.; Wang, K. Sleep Disorders in Chronic Pain and Its Neurochemical Mechanisms: A Narrative Review. Front. Psychiatry 2023, 14, 1157790. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and Challenges in Neuropathic Pain: A Narrative Review and Future Directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Mika, J. The Importance of Chemokines in Neuropathic Pain Development and Opioid Analgesic Potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, R. Chemokines, Neuronal-Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. Chemokines and Chemokine Receptors: Standing at the Crossroads of Immunobiology and Neurobiology. Immunity 2009, 31, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Spinal CCL1/CCR8 Signaling Interplay as a Potential Therapeutic Target—Evidence from a Mouse Diabetic Neuropathy Model. Int. Immunopharmacol. 2017, 52, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, N.; Honda, K.; Uta, D.; Beppu, K.; Ushijima, Y.; Matsuzaki, Y.; Nakashima, S.; Kido, M.A.; Imoto, K.; Takano, Y.; et al. CCL-1 in the Spinal Cord Contributes to Neuropathic Pain Induced by Nerve Injury. Cell Death Dis. 2013, 4, e679. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but Not CCL12, Play a Significant Role in the Development of Pain-Related Behavior and Opioid-Induced Analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef]
- Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Kwiatkowski, K.; Mika, J. Pharmacological Evidence of the Important Roles of CCR1 and CCR3 and Their Endogenous Ligands CCL2/7/8 in Hypersensitivity Based on a Murine Model of Neuropathic Pain. Cells 2023, 12, 98. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.; Park, J.-Y.; Lind, A.-L.; Ma, Q.; Ji, R.-R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef]
- Illias, A.; Gist, A.C.; Zhang, H.; Kosturakis, A.K.; Dougherty, P.M. Chemokine CCL2 and Its Receptor CCR2 in the Dorsal Root Ganglion Contribute to Oxaliplatin-Induced Mechanical Hypersensitivity. Pain 2018, 159, 1308–1316. [Google Scholar] [CrossRef]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C Motif) Receptor 5 Is an Important Pathological Regulator in the Development and Maintenance of Neuropathic Pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Saika, F.; Kishioka, S. CC-Chemokine MIP-1α in the Spinal Cord Contributes to Nerve Injury-Induced Neuropathic Pain. Neurosci. Lett. 2010, 484, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ochi-Ishi, R.; Nagata, K.; Inoue, T.; Tozaki-Saitoh, H.; Tsuda, M.; Inoue, K. Involvement of the Chemokine CCL3 and the Purinoceptor P2×7 in the Spinal Cord in Paclitaxel-Induced Mechanical Allodynia. Mol. Pain 2014, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Ciechanowska, A.; Ciapała, K.; Rojewska, E. Blockade of CC Chemokine Receptor Type 3 Diminishes Pain and Enhances Opioid Analgesic Potency in a Model of Neuropathic Pain. Front. Immunol. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. CC-Chemokine Ligand 4/Macrophage Inflammatory Protein-1beta Participates in the Induction of Neuropathic Pain after Peripheral Nerve Injury. Eur. J. Pain 2012, 16, 1271–1280. [Google Scholar] [CrossRef]
- Malon, J.T.; Cao, L. Calcitonin Gene-Related Peptide Contributes to Peripheral Nerve Injury-Induced Mechanical Hypersensitivity through CCL5 and P38 Pathways. J. Neuroimmunol. 2016, 297, 68–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, Q.; Fan, Q.; Zhao, Y.; Cheng, M.-Y.; Liu, H.; Li, J.; Lu, F.-F.; Jia, J.-T.; Cheng, W.; Yan, C.-D. Spinal NF-ΚB and Chemokine Ligand 5 Expression during Spinal Glial Cell Activation in a Neuropathic Pain Model. PLoS ONE 2015, 10, e0115120. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Popiolek-Barczyk, K.; Pawlik, K.; Ciechanowska, A.; Makuch, W.; Rojewska, E.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Influences the Level of Nociceptive Factors and Enhances the Analgesic Potency of Morphine in a Rat Model of Neuropathic Pain. Eur. J. Pharmacol. 2020, 880, 173166. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Zychowska, M.; Mika, J. Pharmacological Blockade of CXCR3 by (±)-NBI-74330 Reduces Neuropathic Pain and Enhances Opioid Effectiveness—Evidence from in Vivo and in Vitro Studies. BBA-Mol. Basis Dis. 2018, 1864, 3418–3437. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Y.; Yang, Y.; Zhang, S.; Xu, T.; Xin, W.; Wu, S.; Zhang, X. GATA3-Dependent Epigenetic Upregulation of CCL21 Is Involved in the Development of Neuropathic Pain Induced by Bortezomib. Mol. Pain 2019, 15, 1744806919863292. [Google Scholar] [CrossRef]
- Biber, K.; Tsuda, M.; Tozaki-Saitoh, H.; Tsukamoto, K.; Toyomitsu, E.; Masuda, T.; Boddeke, H.; Inoue, K. Neuronal CCL21 Up-Regulates Microglia P2X4 Expression and Initiates Neuropathic Pain Development. EMBO J. 2011, 30, 1864–1873. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Nalepa, I.; Mika, J. Pharmacological Blockade of Spinal CXCL3/CXCR2 Signaling by NVP CXCR2 20, a Selective CXCR2 Antagonist, Reduces Neuropathic Pain Following Peripheral Nerve Injury. Front. Immunol. 2019, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Cao, D.L.; Zhang, X.; Ji, R.R.; Gao, Y.J. Chemokine Contribution to Neuropathic Pain: Respective Induction of CXCL1 and CXCR2 in Spinal Cord Astrocytes and Neurons. Pain 2013, 154, 2185–2197. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Fukazawa, Y.; Tohya, K.; Kimura, M.; Kishioka, S. Epigenetic Augmentation of the Macrophage Inflammatory Protein 2/C-X-C Chemokine Receptor Type 2 Axis through Histone H3 Acetylation in Injured Peripheral Nerves Elicits Neuropathic Pain. J. Pharmacol. Exp. Ther. 2012, 340, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, M.; Yuan, S.; Yu, W. Spinal CXCL5 Contributes to Nerve Injury-Induced Neuropathic Pain via Modulating GSK-3β Phosphorylation and Activity in Rats. Neurosci. Lett. 2016, 634, 52–59. [Google Scholar] [CrossRef]
- Li, H.L.; Huang, Y.; Zhou, Y.L.; Teng, R.H.; Zhou, S.Z.; Lin, J.P.; Yang, Y.; Zhu, S.M.; Xu, H.; Yao, Y.X. C-X-C Motif Chemokine 10 Contributes to the Development of Neuropathic Pain by Increasing the Permeability of the Blood–Spinal Cord Barrier. Front. Immunol. 2020, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Shu, B.; Gao, F.; Liu, C.; Guan, X.; Ke, C.; Cao, F.; Hinton, A.O.; Xiang, H.; Yang, H.; et al. Spinal IFN-γ-Induced Protein-10 (CXCL10) Mediates Metastatic Breast Cancer-Induced Bone Pain by Activation of Microglia in Rat Models. Breast Cancer Res. Treat. 2014, 143, 255–263. [Google Scholar] [CrossRef]
- Luo, X.; Tai, W.L.; Sun, L.; Pan, Z.; Xia, Z.; Chung, S.K.; Cheung, C.W. Crosstalk between Astrocytic CXCL12 and Microglial CXCR4 Contributes to the Development of Neuropathic Pain. Mol. Pain 2016, 12, 1744806916636385. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Li, Z.; Kong, C.; Zhao, Y.; Qian, J.-L.; Kan, Q.; Zhang, W.; Xu, J.-T. Upregulation of Chemokine CXCL12 in the Dorsal Root Ganglia and Spinal Cord Contributes to the Development and Maintenance of Neuropathic Pain Following Spared Nerve Injury in Rats. Neurosci. Bull. 2016, 32, 27–40. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Song, Z.W.; Guo, S.W.; He, J.S.; Wang, S.Y.; Zhu, J.G.; Yang, H.L.; Liu, J.B. CXCL12/CXCR4 Signaling Contributes to Neuropathic Pain via Central Sensitization Mechanisms in a Rat Spinal Nerve Ligation Model. CNS Neurosci. Ther. 2019, 25, 922–936. [Google Scholar] [CrossRef]
- Jiang, B.C.; Cao, D.L.; Zhang, X.; Zhang, Z.J.; He, L.N.; Li, C.H.; Zhang, W.W.; Wu, X.B.; Berta, T.; Ji, R.R.; et al. CXCL13 Drives Spinal Astrocyte Activation and Neuropathic Pain via CXCR5. J. Clin. Investig. 2016, 126, 745–761. [Google Scholar] [CrossRef]
- Wang, J.; Yin, C.; Pan, Y.; Yang, Y.; Li, W.; Ni, H.; Liu, B.; Nie, H.; Xu, R.; Wei, H.; et al. CXCL13 Contributes to Chronic Pain of a Mouse Model of CRPS-I via CXCR5-Mediated NF-ΚB Activation and pro-Inflammatory Cytokine Production in Spinal Cord Dorsal Horn. J. Neuroinflamm. 2023, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Ciapała, K.; Mika, J. Kynurenic Acid and Zaprinast Diminished CXCL17-Evoked Pain-Related Behaviour and Enhanced Morphine Analgesia in a Mouse Neuropathic Pain Model. Pharmacol Rep. 2019, 71, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New Insights into the Analgesic Properties of the XCL1/XCR1 and XCL1/ITGA9 Axes Modulation under Neuropathic Pain Conditions—Evidence from Animal Studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Mika, J. Microglial Inhibition Influences XCL1/XCR1 Expression and Causes Analgesic Effects in a Mouse Model of Diabetic Neuropathy. Anesthesiology 2016, 125, 573–589. [Google Scholar] [CrossRef]
- Milligan, E.D.; Zapata, V.; Chacur, M.; Schoeniger, D.; Biedenkapp, J.; O’Connor, K.A.; Verge, G.M.; Chapman, G.; Green, P.; Foster, A.C.; et al. Evidence That Exogenous and Endogenous Fractalkine Can Induce Spinal Nociceptive Facilitation in Rats. Eur. J. Neurosci. 2004, 20, 2294–2302. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.S.; Tao, R.; Zhang, J.; Liu, L.; Jiang, Y.H.; Ma, S.H.; Song, L.X.; Xia, L.J. Upregulation of CX3CL1 Mediated by NF-ΚB Activation in Dorsal Root Ganglion Contributes to Peripheral Sensitization and Chronic Pain Induced by Oxaliplatin Administration. Mol. Pain 2017, 13, 1744806917726256. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A New Classification System and Their Role in Immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Moser, B.; Wolf, M.; Walz, A.; Loetscher, P. Chemokines: Multiple Levels of Leukocyte Migration Control. Trends Immunol. 2004, 25, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.H. Chemokine Receptors in the Central Nervous System: Role in Brain Inflammation and Neurodegenerative Diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kwiatkowski, K.; Pawlik, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Wordliczek, J.; Mika, J. Metamizole Relieves Pain by Influencing Cytokine Levels in Dorsal Root Ganglia in a Rat Model of Neuropathic Pain. Pharmacol. Rep. 2020, 72, 1310–1322. [Google Scholar] [CrossRef]
- Michael, T.; Clark, A.K.; Bishop, T.; Grist, J.; Yip, P.K.; Moon, L.D.F.; Thompson, S.W.N.; Marchand, F.; McMahon, S.B. CCL2 Is a Key Mediator of Microglia Activation in Neuropathic Pain States. Eur. J. Pain 2009, 13, 263–272. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Mika, J. The RS504393 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in Neuropathic Rats. J. Neuroimmune Pharmacol. 2017, 12, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The Blockade of CC Chemokine Receptor Type 1 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in a Rat Model of Neuropathic Pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Stammers, A.T.; Liu, J.; Kwon, B.K. Expression of Inflammatory Cytokines Following Acute Spinal Cord Injury in a Rodent Model. J. Neurosci. Res. 2012, 90, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Bidirectional Action of Cenicriviroc, a CCR2/CCR5 Antagonist, Results in Alleviation of Pain-Related Behaviors and Potentiation of Opioid Analgesia in Rats With Peripheral Neuropathy. Front. Immunol. 2020, 11, 615327. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial Properties of Maraviroc on Neuropathic Pain Development and Opioid Effectiveness in Rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Pawlik, K.; Ciapała, K.; Mika, J. Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model. Brain Sci. 2023, 13, 579. [Google Scholar] [CrossRef]
- Piotrowska, A.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Rojewska, E.; Mika, J. Comparison of the Effects of Chemokine Receptors CXCR2 and CXCR3 Pharmacological Modulation in Neuropathic Pain Model—In Vivo and In Vitro Study. Int. J. Mol. Sci. 2021, 22, 11074. [Google Scholar] [CrossRef]
- Imai, S.; Narita, M.M.; Ikegami, D.; Yamashita, A.; Shimizu, T.; Narita, M.M.; Niikura, K.; Furuya, M.; Kobayashi, Y.; Miyashita, K.; et al. Epigenetic Transcriptional Activation of Monocyte Chemotactic Protein 3 Contributes to Long-Lasting Neuropathic Pain. Brain 2013, 136, 828–843. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Comparison of the Beneficial Effects of RS504393, Maraviroc and Cenicriviroc on Neuropathic Pain-Related Symptoms in Rodents: Behavioral and Biochemical Analyses. Int. Immunopharmacol. 2020, 84, 106540. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Zhao, L.; Wang, L. Expression Levels of CX3CL1 and CCL21 in the Spinal Cords of Rats with Neuropathic Pain and Correlation Levels with JNK/MCP-1 Signaling Pathways. Int. J. Clin. Exp. Med. 2020, 13, 3572–3579. [Google Scholar]
- Zychowska, M.; Rojewska, E.; Pilat, D.; Mika, J. The Role of Some Chemokines from the CXC Subfamily in a Mouse Model of Diabetic Neuropathy. J. Diabetes Res. 2015, 2015, 750182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Wang, Z.; Wang, T.; Zhou, Y.; Zheng, L. Blocking CXC Motif Chemokine Ligand 2 Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Apoptosis and NLRP3 Inflammasome Activation. Biol. Pharm. Bull. 2023, 46, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xue, Z.; Yang, B.; Liu, L.; Zhang, P.; Shi, J.; Fu, X.; Xue, Y.; Hao, Y.; Ji, G. Effects of Intrathecally Administered Interferon α on Chronic Constriction Injury Model Rats’ Mechanical Pain Threshold and G Protein Expression in the Spinal Cord. Folia Neuropathol. 2023, 61, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.M.; Cho, Y.W.; Jung, Y.J.; Lee, D.G.; Jang, S.H.; Park, H.W.; Hwang, S.J.; Ahn, S.H. Changes in Expression of MRNA for Interleukin-8 and Effects of Interleukin-8 Receptor Inhibitor in the Spinal Dorsal Horn in a Rat Model of Lumbar Disc Herniation. Spine 2011, 36, 2139–2146. [Google Scholar] [CrossRef]
- Khan, J.; Hassun, H.; Zusman, T.; Korczeniewska, O.; Eliav, E. Interleukin-8 Levels in Rat Models of Nerve Damage and Neuropathic Pain. Neurosci. Lett. 2017, 657, 106–112. [Google Scholar] [CrossRef]
- Kong, Y.F.; Sha, W.L.; Wu, X.B.; Zhao, L.X.; Ma, L.J.; Gao, Y.J. CXCL10/CXCR3 Signaling in the DRG Exacerbates Neuropathic Pain in Mice. Neurosci. Bull. 2021, 37, 339–352. [Google Scholar] [CrossRef]
- Strong, J.A.; Xie, W.; Coyle, D.E.; Zhang, J.M. Microarray Analysis of Rat Sensory Ganglia after Local Inflammation Implicates Novel Cytokines in Pain. PLoS ONE 2012, 7, e40779. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, X.; Di, Y.; Qu, L.; Fan, N. Effect of CXCL12/CXCR4 Signaling on Neuropathic Pain after Chronic Compression of Dorsal Root Ganglion. Sci. Rep. 2017, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.-H.; Song, X.-J.; Yang, C.-L.; Cao, P.; Mao, Y.; Jin, Y.; Xu, M.-Y.; Wang, H.-T.; Zhu, X.; Wang, W.; et al. Up-Regulation of Microglial Chemokine CXCL12 in Anterior Cingulate Cortex Mediates Neuropathic Pain in Diabetic Mice. Acta Pharmacol. Sin. 2023, 44, 1337–1349. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Xiong, H.; Wang, W.; Liu, Y.; Yin, L.; Tu, C.; Wang, H.; Xiang, X.; Xu, J.; et al. CXCL13/CXCR5 Signaling Contributes to Diabetes-Induced Tactile Allodynia via Activating PERK, PSTAT3, PAKT Pathways and pro-Inflammatory Cytokines Production in the Spinal Cord of Male Mice. Brain. Behav. Immun. 2019, 80, 711–724. [Google Scholar] [CrossRef]
- Ma, L.; Yu, L.; Jiang, B.C.; Wang, J.; Guo, X.; Huang, Y.; Ren, J.; Sun, N.; Gao, D.S.; Ding, H.; et al. Znf382 Controls Mouse Neuropathic Pain via Silencer-Based Epigenetic Inhibition of Cxcl13 in Drg Neurons. J. Exp. Med. 2021, 218, e20210920. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yi, D.; Yu, Z.; Zhu, B.; Li, S.; Liu, X. Identification of the Hub Genes Related to Nerve Injury-Induced Neuropathic Pain. Front. Neurosci. 2020, 14, 488. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, W.; Zhang, M.; Cui, S.; Huang, X.; Ou, W.; Huang, R.; Gao, J.; Jia, Z.; Zhang, S. Daphnetin Improves Neuropathic Pain by Inhibiting the Expression of Chemokines and Inflammatory Factors in the Spinal Cord and Interfering with Glial Cell Polarization. Pharmaceuticals 2023, 16, 243. [Google Scholar] [CrossRef]
- Bu, H.; Jiao, P.; Fan, X.; Gao, Y.; Zhang, L.; Guo, H. The Role of Botulinum Toxin Type A Related Axon Transport in Neuropathic Pain Induced by Chronic Constriction Injury. Korean J. Pain 2022, 35, 391–402. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.B.; Luo, Y.X.; Yang, Y.L.; Zhang, X.Z.; Li, B.; Meng, Y.; Chen, Y.J.; Guo, R.X.; Xiong, Y.C.; et al. NFATc2-Dependent Epigenetic Upregulation of CXCL14 Is Involved in the Development of Neuropathic Pain Induced by Paclitaxel. J. Neuroinflamm. 2020, 17, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Song, C.; Huang, Y.; Lei, W.; Sun, J. MMP-9 Regulates CX3CL1/CX3CR1 in the Early Phase of Neuropathic Pain in Chronic Sciatic Nerve Constriction Injury (CCI) Rats. Ann. Palliat. Med. 2020, 9, 2020027. [Google Scholar] [CrossRef]
- Savarin-Vuaillat, C.; Ransohoff, R.M. Chemokines and Chemokine Receptors in Neurological Disease: Raise, Retain, or Reduce? Neurotherapeutics 2007, 4, 590–601. [Google Scholar] [CrossRef]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. Blockade of CCR4 Diminishes Hypersensitivity and Enhances Opioid Analgesia—Evidence from a Mouse Model of Diabetic Neuropathy. Neuroscience 2020, 441, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain. Front. Immunol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.Y.; Cui, Y.; Kim, H.; Le Thi, A.H.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 Mediates Neuron–Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Saika, F.; Kishioka, S. Epigenetic Upregulation of CCL2 and CCL3 via Histone Modifications in Infiltrating Macrophages after Peripheral Nerve Injury. Cytokine 2013, 64, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, Q.S.; Echeverry, S.; Mogil, J.S.; De Koninck, Y.; Rivest, S. Expression of CCR2 in Both Resident and Bone Marrow-Derived Microglia Plays a Critical Role in Neuropathic Pain. J. Neurosci. 2007, 27, 12396–12406. [Google Scholar] [CrossRef]
- Cho, J.; Gruol, D.L. The Chemokine CCL2 Activates P38 Mitogen-Activated Protein Kinase Pathway in Cultured Rat Hippocampal Cells. J. Neuroimmunol. 2008, 199, 94. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Suter, M.R. P38 MAPK, Microglial Signaling, and Neuropathic Pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kotani, T.; Kuwabara, H.; Suzuka, T.; Kiboshi, T.; Fukui, K.; Ishida, T.; Fujiki, Y.; Shiba, H.; Hata, K.; et al. CCL2 Produced by CD68+/CD163+ Macrophages as a Promising Clinical Biomarker of Microscopic Polyangiitis-Interstitial Lung Disease. Rheumatology 2021, 60, 4643–4653. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-Derived Chemokines on the Road to Immunity. Semin. Immunol. 2016, 28, 119. [Google Scholar] [CrossRef] [PubMed]
- Errede, M.; Annese, T.; Petrosino, V.; Longo, G.; Girolamo, F.; de Trizio, I.; d’Amati, A.; Uccelli, A.; Kerlero de Rosbo, N.; Virgintino, D. Microglia-Derived CCL2 Has a Prime Role in Neocortex Neuroinflammation. Fluids Barriers CNS 2022, 19, 68. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, W.; Zhang, J.; Strong, J.A.; Zhang, J.M. Sympathectomy Decreases Pain Behaviors and Nerve Regeneration by Downregulating Monocyte Chemokine CCL2 in Dorsal Root Ganglia in the Rat Tibial Nerve Crush Model. Pain 2022, 163, E106–E120. [Google Scholar] [CrossRef]
- Yang, F.; Jing, J.J.; Fu, S.Y.; Su, X.Z.; Zhong, Y.L.; Chen, D.S.; Wu, X.Z.; Zou, Y.Q. Spinal MCP-1 Contributes to Central Post-Stroke Pain by Inducing Central Sensitization in Rats. Mol. Neurobiol. 2023, 60, 2086–2098. [Google Scholar] [CrossRef]
- De Haas, A.H.; Van Weering, H.R.J.; De Jong, E.K.; Boddeke, H.W.G.M.; Biber, K.P.H. Neuronal Chemokines: Versatile Messengers in Central Nervous System Cell Interaction. Mol. Neurobiol. 2007, 36, 137–151. [Google Scholar] [CrossRef]
- Hanisch, U.K. Microglia as a Source and Target of Cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Wallace, C.A.; Moir, G.; Malone, D.F.G.; Duncan, L.; Devarajan, G.; Crane, I.J. Regulation of T-Lymphocyte CCL3 and CCL4 Production by Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 722–730. [Google Scholar] [CrossRef][Green Version]
- Blidberg, K.; Palmberg, L.; Dahlén, B.; Lantz, A.S.; Larsson, K. Chemokine Release by Neutrophils in Chronic Obstructive Pulmonary Disease. Innate Immun. 2012, 18, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K. Chemotherapy-Induced Peripheral Neuropathy: Part 1—Current State of Knowledge and Perspectives for Pharmacotherapy. Pharmacol. Rep. 2020, 72, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Pollack, S.; Wildbaum, G.; Zohar, Y.; Shafat, I.; Makhoul, R.; Daod, E.; Hakim, F.; Perlman, R.; Karin, N. Selective Autoantibody Production against CCL3 Is Associated with Human Type 1 Diabetes Mellitus and Serves As a Novel Biomarker for Its Diagnosis. J. Immunol. 2009, 182, 8104–8109. [Google Scholar] [CrossRef]
- Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.N.; Hogaboam, C.M.; Kunkel, S.L.; Proudfoot, A.E.I.; Wallace, J.L. The Chemokine RANTES Is a Crucial Mediator of the Progression from Acute to Chronic Colitis in the Rat. J. Immunol. 2001, 166, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacon, K.; Toy, K.J.; Goeddel, D.V. Selective Attraction of Monocytes and T Lymphocytes of the Memory Phenotype by Cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef]
- Hang, L.; Shao, D.-H.; Chen, Z.; Chen, Y.-F.; Shu, W.-W.; Zhao, Z.-G. Involvement of Spinal CC Chemokine Ligand 5 in the Development of Bone Cancer Pain in Rats. Basic Clin. Pharmacol. Toxicol. 2013, 113, 325–328. [Google Scholar] [CrossRef]
- Liou, J.T.; Yuan, H.B.; Mao, C.C.; Lai, Y.S.; Day, Y.J. Absence of C-C Motif Chemokine Ligand 5 in Mice Leads to Decreased Local Macrophage Recruitment and Behavioral Hypersensitivity in a Murine Neuropathic Pain Model. Pain 2012, 153, 1283–1291. [Google Scholar] [CrossRef]
- Liou, J.; Mao, C.-C.; Ching-Wah Sum, D.; Liu, F.-C.; Lai, Y.-S.; Li, J.-C.; Day, Y.-J. Peritoneal Administration of Met-RANTES Attenuates Inflammatory and Nociceptive Responses in a Murine Neuropathic Pain Model. J. Pain 2013, 14, 24–35. [Google Scholar] [CrossRef]
- Oh, S.; Tran, P.B.; Gillard, S.E.; Hurley, R.W.; Hammond, D.L.; Miller, R.J. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J. Neurosci. 2001, 21, 5027–5035. [Google Scholar] [CrossRef]
- Li, F.; Du, X.; Lan, F.; Li, N.; Zhang, C.; Zhu, C.; Wang, X.; He, Y.; Shao, Z.; Chen, H.; et al. Eosinophilic Inflammation Promotes CCL6-Dependent Metastatic Tumor Growth. Sci. Adv. 2021, 7, 5943–5969. [Google Scholar] [CrossRef]
- Kanno, M.; Suzuki, S.; Fujiwara, T.; Yokoyama, A.; Sakamoto, A.; Takahashi, H.; Imai, Y.; Tanaka, J. Functional Expression of CCL6 by Rat Microglia: A Possible Role of CCL6 in Cell–cell Communication. J. Neuroimmunol. 2005, 167, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Zhang, C.; Li, N.; Huang, H.; Shao, Z.; Zhang, M.; Zhan, X.; He, Y.; Ju, Z.; et al. Eosinophil-Derived Chemokine (HCCL15/23, MCCL6) Interacts with CCR1 to Promote Eosinophilic Airway Inflammation. Signal Transduct. Target. Ther. 2021, 6, 91. [Google Scholar] [CrossRef]
- Bäckryd, E.; Lind, A.-L.; Thulin, M.; Larsson, A.; Gerdle, B.; Gordh, T. High Levels of Cerebrospinal Fluid Chemokines Point to the Presence of Neuroinflammation in Peripheral Neuropathic Pain: A Cross-Sectional Study of 2 Cohorts of Patients Compared with Healthy Controls. Pain 2017, 158, 2487–2495. [Google Scholar] [CrossRef]
- Ke, B.; Huang, X.X.; Li, Y.; Li, L.Y.; Xu, Q.X.; Gao, Y.; Liu, Y.; Luo, J. Neuronal-Derived Ccl7 Drives Neuropathic Pain by Promoting Astrocyte Proliferation. Neuroreport 2016, 27, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Thirion, S.; Nys, G.; Fiten, P.; Masure, S.; Van Damme, J.; Opdenakker, G. Mouse Macrophage Derived Monocyte Chemotactic Protein-3: CDNA Cloning and Identification as MARC/FIC. Biochem. Biophys. Res. Commun. 1994, 201, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; Lv, Q.; Su, J. Astrocyte-Derived CCL7 Promotes Microglia-Mediated Inflammation Following Traumatic Brain Injury. Int. Immunopharmacol. 2021, 99, 107975. [Google Scholar] [CrossRef]
- Ali, S.; Robertson, H.; Wain, J.H.; Isaacs, J.D.; Malik, G.; Kirby, J.A. A Non-Glycosaminoglycan-Binding Variant of CC Chemokine Ligand 7 (Monocyte Chemoattractant Protein-3) Antagonizes Chemokine-Mediated Inflammation. J. Immunol. 2005, 175, 1257–1266. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The Chemotaxis of M1 and M2 Macrophages Is Regulated by Different Chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Li, J.; Deng, G.; Wang, H.; Yang, M.; Yang, R.; Li, X.; Zhang, X.; Yuan, H. Interleukin-1β Pre-Treated Bone Marrow Stromal Cells Alleviate Neuropathic Pain through CCL7-Mediated Inhibition of Microglial Activation in the Spinal Cord. Sci. Rep. 2017, 7, 42260. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.C.; Cao, D.L.; Zhao, L.X.; Zhang, Y.L. Chemokine CCL8 and Its Receptor CCR5 in the Spinal Cord Are Involved in Visceral Pain Induced by Experimental Colitis in Mice. Brain Res. Bull. 2017, 135, 170–178. [Google Scholar] [CrossRef]
- Yang, P.; Chen, W.; Xu, H.; Yang, J.; Jiang, J.; Jiang, Y.; Xu, G. Correlation of CCL8 Expression with Immune Cell Infiltration of Skin Cutaneous Melanoma: Potential as a Prognostic Indicator and Therapeutic Pathway. Cancer Cell Int. 2021, 21, 635. [Google Scholar] [CrossRef]
- Denk, F.; Crow, M.; Didangelos, A.; Lopes, D.M.; McMahon, S.B. Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep. 2016, 15, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, L.; Temple, J.D.; Tagliafierro, L.; Willcockson, H.; Esposito, A.; D’Onofrio, N.; Stein, E.; Li, T.; Myers, T.J.; Ozkan, H.; et al. Role of the C-C Chemokine Receptor-2 in a Murine Model of Injury-Induced Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Provost, V.; Larose, M.-C.; Langlois, A.; Rola-Pleszczynski, M.; Flamand, N.; Laviolette, M. CCL26/Eotaxin-3 Is More Effective to Induce the Migration of Eosinophils of Asthmatics than CCL11/Eotaxin-1 and CCL24/Eotaxin-2. J. Leukoc. Biol. 2013, 94, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Ying, S.; Sabroe, I.; Stubbs, V.L.; Soler, D.; Williams, T.J.; Kay, A.B. Eotaxin (CCL11) and Eotaxin-2 (CCL24) Induce Recruitment of Eosinophils, Basophils, Neutrophils, and Macrophages as Well as Features of Early- and Late-Phase Allergic Reactions Following Cutaneous Injection in Human Atopic and Nonatopic Volunteers. J. Immunol. 2002, 169, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An Emerging Role for Eotaxins in Neurodegenerative Disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef]
- Nazarinia, D.; Behzadifard, M.; Gholampour, J.; Karimi, R.; Gholampour, M. Eotaxin-1 (CCL11) in Neuroinflammatory Disorders and Possible Role in COVID-19 Neurologic Complications. Acta Neurol. Belg. 2022, 122, 865–869. [Google Scholar] [CrossRef]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered Profile of Chemokines in Fibromyalgia Patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.L.; Yu, S.Y. Synovial Fluid Eotaxin-1 Levels May Reflect Disease Progression in Primary Knee Osteoarthritis Among Elderly Han Chinese: A Cross-Sectional Study. Cartilage 2019, 10, 408. [Google Scholar] [CrossRef]
- Izumi, K.; Bieber, K.; Ludwig, R.J. Current Clinical Trials in Pemphigus and Pemphigoid. Front. Immunol. 2019, 10, 978. [Google Scholar] [CrossRef]
- Pelletier, J.P.R.; Mukhtar, F. Passive Monoclonal and Polyclonal Antibody Therapies. In Immunologic Concepts in Transfusion Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–348. [Google Scholar] [CrossRef]
- Furer, V.; Hazan, E.; Mor, A.; Segal, M.; Katav, A.; Aloush, V.; Elkayam, O.; George, J.; Ablin, J.N. Elevated Levels of Eotaxin-2 in Serum of Fibromyalgia Patients. Pain Res. Manag. 2018, 2018, 7257681. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and Its Ligands: From Bench to Bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Wintergerst, M.W.M.; Kunz, W.G.; Vetter, V.K.; Knott, M.M.L.; Lisowski, D.; Haubner, S.; Moder, S.; Thaler, R.; Eiber, S.; et al. CCL22 Controls Immunity by Promoting Regulatory T Cell Communication with Dendritic Cells in Lymph Nodes. J. Exp. Med. 2019, 216, 1170. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Z.; Wang, F.; Zhang, Z.; Yu, M. Identification of Chemokines and Growth Factors in Proliferative Diabetic Retinopathy Vitreous. Biomed Res. Int. 2014, 2014, 486386. [Google Scholar] [CrossRef]
- Barros, J.F.; Waclawiak, I.; Pecli, C.; Borges, P.A.; Georgii, J.L.; Ramos-Junior, E.S.; Canetti, C.; Courau, T.; Klatzmann, D.; Kunkel, S.L.; et al. Role of Chemokine Receptor CCR4 and Regulatory T Cells in Wound Healing of Diabetic Mice. J. Investig. Dermatol. 2019, 139, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, Y.; Lin, S.; Liu, D.; Mo, G.; Zhang, H.; Dou, Y. Identification of Potential Biomarkers for Abdominal Pain in IBS Patients by Bioinformatics Approach. BMC Gastroenterol. 2021, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Paish, H.L.; Baldock, T.E.; Gillespie, C.S.; del Carpio Pons, A.; Mann, D.A.; Deehan, D.J.; Borthwick, L.A.; Kalson, N.S. Chronic, Active Inflammation in Patients With Failed Total Knee Replacements Undergoing Revision Surgery. J. Orthop. Res. 2019, 37, 2316–2324. [Google Scholar] [CrossRef]
- Lee, A.Y.S.; Reimer, D.; Zehrer, A.; Lu, M.; Mielenz, D.; Körner, H. Expression of Membrane-Bound CC Chemokine Ligand 20 on Follicular T Helper Cells in T-B-Cell Conjugates. Front. Immunol. 2017, 8, 1871. [Google Scholar] [CrossRef]
- Lötsch, J.; Mustonen, L.; Harno, H.; Kalso, E. Machine-Learning Analysis of Serum Proteomics in Neuropathic Pain after Nerve Injury in Breast Cancer Surgery Points at Chemokine Signaling via SIRT2 Regulation. Int. J. Mol. Sci. 2022, 23, 3488. [Google Scholar] [CrossRef] [PubMed]
- Miclescu, A.A.; Granlund, P.; Butler, S.; Gordh, T. Association between Systemic Inflammation and Experimental Pain Sensitivity in Subjects with Pain and Painless Neuropathy after Traumatic Nerve Injuries. Scand. J. Pain 2023, 23, 184–199. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, R.; Wang, X.; Hu, K.; Huang, L.; Lu, M.; Hu, Q. CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front. Cell Dev. Biol. 2019, 7, 212. [Google Scholar] [CrossRef]
- Jönsson, M.; Gerdle, B.; Ghafouri, B.; Bäckryd, E. The Inflammatory Profile of Cerebrospinal Fluid, Plasma, and Saliva from Patients with Severe Neuropathic Pain and Healthy Controls-a Pilot Study. BMC Neurosci. 2021, 22, 6. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Liu, L.; Wen, J.; Yang, H.; Zhu, Y.; Gao, M.; Liang, H.; Lai, W.; Long, H. Nerve Growth Factor Enhances Tooth Mechanical Hyperalgesia Through C-C Chemokine Ligand 19 in Rats. Front. Neurol. 2021, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Rappert, A.; Biber, K.; Nolte, C.; Lipp, M.; Schubel, A.; Lu, B.; Gerard, N.P.; Gerard, C.; Boddeke, H.W.G.M.; Kettenmann, H. Secondary Lymphoid Tissue Chemokine (CCL21) Activates CXCR3 to Trigger a Cl− Current and Chemotaxis in Murine Microglia. J. Immunol. 2002, 168, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Waxman, S.G.; Hains, B.C. Modulation of Thalamic Nociceptive Processing after Spinal Cord Injury through Remote Activation of Thalamic Microglia by Cysteine–Cysteine Chemokine Ligand 21. J. Neurosci. 2007, 27, 8893–8902. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.; Pickert, G.; Wijnvoord, N.; Häussler, A.; Tegeder, I. Dichotomy of CCL21 and CXCR3 in Nerve Injury-Evoked and Autoimmunity-Evoked Hyperalgesia. Brain. Behav. Immun. 2013, 32, 186–200. [Google Scholar] [CrossRef]
- Jun, K.J.; Lee, M.J.; Shin, D.C.; Woo, M.Y.; Kim, K.; Park, S. Identification of CCL1 as a Gene Differentially Expressed in CD4+ T Cells Expressing TIM-3. Immune Netw. 2011, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xiao, W.; Wang, F.; Liu, J.; Zhi, L.J. MiR-21-5p Inhibits Neuropathic Pain Development via Directly Targeting C-C Motif Ligand 1 and Tissue Inhibitor of Metalloproteinase-3. J. Cell. Biochem. 2019, 120, 16614–16623. [Google Scholar] [CrossRef]
- The Effects of Bindarit in Diabetic Nephropathy. Available online: https://clinicaltrials.gov/ct2/show/NCT01109212 (accessed on 1 June 2023).
- Ge, S.; Shrestha, B.; Paul, D.; Keating, C.; Cone, R.; Guglielmotti, A.; Pachter, J.S. The CCL2 Synthesis Inhibitor Bindarit Targets Cells of the Neurovascular Unit, and Suppresses Experimental Autoimmune Encephalomyelitis. J. Neuroinflamm. 2012, 9, 171. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, B.; Xu, L.; Wang, Y.; Liu, B.; Xu, M.; He, Q.; Ni, C.; Fu, J.; Kong, M.; et al. CXCR1 Participates in Bone Cancer Pain Induced by Walker 256 Breast Cancer Cells in Female Rats. Mol. Pain 2022, 18, 17448069221135743. [Google Scholar] [CrossRef] [PubMed]
- Bie, Y.; Ge, W.; Yang, Z.; Cheng, X.; Zhao, Z.; Li, S.; Wang, W.; Wang, Y.; Zhao, X.; Yin, Z.; et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis. Markers 2019, 2019, 8023460. [Google Scholar] [CrossRef]
- Langjahr, M.; Schubert, A.L.; Sommer, C.; Üçeyler, N. Increased Pro-Inflammatory Cytokine Gene Expression in Peripheral Blood Mononuclear Cells of Patients with Polyneuropathies. J. Neurol. 2018, 265, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Staats Pires, A.; Heng, B.; Tan, V.X.; Latini, A.; Russo, M.A.; Santarelli, D.M.; Bailey, D.; Wynne, K.; O’Brien, J.A.; Guillemin, G.J.; et al. Kynurenine, Tetrahydrobiopterin, and Cytokine Inflammatory Biomarkers in Individuals Affected by Diabetic Neuropathic Pain. Front. Neurosci. 2020, 14, 890. [Google Scholar] [CrossRef]
- Gulati, K.; Gangele, K.; Agarwal, N.; Jamsandekar, M.; Kumar, D.; Poluri, K.M. Molecular Cloning and Biophysical Characterization of CXCL3 Chemokine. Int. J. Biol. Macromol. 2018, 107, 575–584. [Google Scholar] [CrossRef]
- Shibata, F. The Role of Rat Cytokine-Induced Neutrophil Chemoattractants (CINCs) in Inflammation. Yakugaku Zasshi 2002, 122, 263–268. [Google Scholar] [CrossRef]
- Lv, Q.-Y.; Zou, H.-Z.; Xu, Y.-Y.; Shao, Z.-Y.; Wu, R.-Q.; Li, K.-J.; Deng, X.; Gu, D.-N.; Jiang, H.-X.; Su, M.; et al. Expression Levels of Chemokine (C-X-C Motif) Ligands CXCL1 and CXCL3 as Prognostic Biomarkers in Rectal Adenocarcinoma: Evidence from Gene Expression Omnibus (GEO) Analyses. Bioengineered 2021, 12, 3711. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Strong, J.A.; Zhang, J.M. Systemic Antiinflammatory Corticosteroid Reduces Mechanical Pain Behavior, Sympathetic Sprouting, and Elevation of Proinflammatory Cytokines in a Rat Model of Neuropathic Pain. Anesthesiology 2007, 107, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xin, P.; Kong, Y.; Wang, Z.; Wang, X. Resolvin D2 Reduces Chronic Neuropathic Pain and Bone Cancer Pain via Spinal Inhibition of IL-17 Secretion, CXCL1 Release and Astrocyte Activation in Mice. Brain Sci. 2023, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential Roles of CXCL2 and CXCL3 and Their Receptors in Regulating Normal and Asthmatic Airway Smooth Muscle Cell Migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Li, H.; Ge, C.; Zhao, F.; Tian, H.; Chen, T.; Jiang, G.; Xie, H.; Cui, Y.; et al. CXCL3 Contributes to CD133+CSCs Maintenance and Forms a Positive Feedback Regulation Loop with CD133 in HCC via Erk1/2 Phosphorylation. Sci. Rep. 2016, 6, 27426. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef]
- Furuichi, K.; Wada, T.; Yokoyama, H.; Kobayashi, K. ichi Role of Cytokines and Chemokines in Renal Ischemia-Reperfusion Injury. Drug News Perspect. 2002, 15, 477–482. [Google Scholar] [CrossRef]
- De Jong, E.K.; De Haas, A.H.; Brouwer, N.; Van Weering, H.R.J.; Hensens, M.; Bechmann, I.; Pratley, P.; Wesseling, E.; Boddeke, H.W.G.M.; Biber, K. Expression of CXCL4 in Microglia in Vitro and in Vivo and Its Possible Signaling through CXCR3. J. Neurochem. 2008, 105, 1726–1736. [Google Scholar] [CrossRef]
- Turbic, A.; Leong, S.Y.; Turnley, A.M. Chemokines and Inflammatory Mediators Interact to Regulate Adult Murine Neural Precursor Cell Proliferation, Survival and Differentiation. PLoS ONE 2011, 6, e25406. [Google Scholar] [CrossRef]
- Jiang, B.C.; He, L.N.; Wu, X.B.; Shi, H.; Zhang, W.W.; Zhang, Z.J.; Cao, D.L.; Li, C.H.; Gu, J.; Gao, Y.J. Promoted Interaction of C/EBPα with Demethylated Cxcr3 Gene Promoter Contributes to Neuropathic Pain in Mice. J. Neurosci. 2017, 37, 685–700. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Song, C.Y.; Yuan, Y.; Eber, A.; Rodriguez, Y.; Levitt, R.C.; Takacs, P.; Yang, Z.; Goldberg, R.; Candiotti, K.A. Diabetic Neuropathic Pain Development in Type 2 Diabetic Mouse Model and the Prophylactic and Therapeutic Effects of Coenzyme Q10. Neurobiol. Dis. 2013, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.Y.; Jiang, M.; Xu, F.; Wang, D.; Ding, B.; Ma, L.J.; Wu, H. CXCL10 and CXCR3 in the Trigeminal Ganglion Contribute to Trigeminal Neuropathic Pain in Mice. J. Pain Res. 2021, 14, 41–51. [Google Scholar] [CrossRef]
- Ascaso, P.; Palanca, A.; Martinez-Hervás, S.; Sanz, M.J.; Ascaso, J.F.; Piqueras, L.; Real, J.T. Peripheral Blood Levels of CXCL10 Are a Useful Marker for Diabetic Polyneuropathy in Subjects with Type 2 Diabetes. Int. J. Clin. Pract. 2021, 75, e14302. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tan, Y.H.; Feng, S.Y.; Fu, K.Y. CXCR3 Signalling Partially Contributes to the Pathogenesis of Neuropathic Pain in Male Rodents. J. Oral Rehabil. 2022, 49, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kim, S.H.; Arifuzzaman, S.; Yoon, T.; Chai, J.C.; Lee, Y.S.; Park, K.S.; Jung, K.H.; Chai, Y.G. Transcriptome Sequencing Reveals That LPS-Triggered Transcriptional Responses in Established Microglia BV2 Cell Lines Are Poorly Representative of Primary Microglia. J. Neuroinflamm. 2016, 13, 182. [Google Scholar] [CrossRef]
- Biber, K.; Boddeke, E. Neuronal CC Chemokines: The Distinct Roles of CCL21 and CCL2 in Neuropathic Pain. Front. Cell. Neurosci. 2014, 8, 210. [Google Scholar] [CrossRef]
- Biber, K.; Sauter, A.; Brouwer, N.; Copray, S.C.V.M.; Boddeke, H.W.G.M. Ischemia-Induced Neuronal Expression of the Microglia Attracting Chemokine Secondary Lymphoid-Tissue Chemokine (SLC). Glia 2001, 34, 121–133. [Google Scholar] [CrossRef]
- Jong, E.K.; Vinet, J.; Stanulovic, V.S.; Meijer, M.; Wesseling, E.; Sjollema, K.; Boddeke, H.W.G.M.; Biber, K.; de Jong, E.K. Expression, Transport, and Axonal Sorting of Neuronal CCL21 in Large Dense-Core Vesicles. FASEB J. 2008, 22, 4136–4145. [Google Scholar] [CrossRef]
- Shen, W.; Hu, X.-M.; Liu, Y.-N.; Han, Y.; Chen, L.-P.; Wang, C.-C.; Song, C. CXCL12 in Astrocytes Contributes to Bone Cancer Pain through CXCR4-Mediated Neuronal Sensitization and Glial Activation in Rat Spinal Cord. J. Neuroinflamm. 2014, 11, 75. [Google Scholar] [CrossRef]
- Cheng, K.I.; Chen, S.L.; Hsu, J.H.; Cheng, Y.C.; Chang, Y.C.; Lee, C.H.; Yeh, J.L.; Dai, Z.K.; Wu, B.N. Loganin Prevents CXCL12/CXCR4-Regulated Neuropathic Pain via the NLRP3 Inflammasome Axis in Nerve-Injured Rats. Phytomedicine 2021, 92, 153734. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; McCully, M.L.; Martínez-Muñoz, L.; Santiago, C.; Wheeldon, J.; Caucheteux, S.; Thelen, S.; Cecchinato, V.; Laufer, J.M.; Purvanov, V.; et al. Epithelial Chemokine CXCL14 Synergizes with CXCL12 via Allosteric Modulation of CXCR4. FASEB J. 2017, 31, 3084–3097. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Holman, D.W.; Klein, R.S. Chemokines in the Balance: Maintenance of Homeostasis and Protection at CNS Barriers. Front. Cell. Neurosci. 2014, 8, 154. [Google Scholar] [CrossRef]
- Barbaria, E.M.; Kohl, B.; Buhren, B.A.; Hasenpusch-Theil, K.; Kruse, F.; Küry, P.; Martini, R.; Müller, H.W. The α-Chemokine CXCL14 Is up-Regulated in the Sciatic Nerve of a Mouse Model of Charcot–Marie–Tooth Disease Type 1A and Alters Myelin Gene Expression in Cultured Schwann Cells. Neurobiol. Dis. 2009, 33, 448–458. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sasaguri, K.; Mizumoto, N.; Suzuki, H. The Chemokine CXCL14-like Immunoreactivity Co-Exists with Somatostatin, but Not NPY in the Rat Dorsal Horn and Has Intimate Association with GABAergic Neurons in the Lateral Spinal Nucleus. Acta Histochem. Cytochem. 2020, 53, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Cao, D.L.; Zhang, X.; Jiang, B.C.; Zhao, L.X.; Qian, B.; Gao, Y.J. CXCL13/CXCR5 Enhances Sodium Channel Nav1.8 Current Density via P38 MAP Kinase in Primary Sensory Neurons Following Inflammatory Pain. Sci. Rep. 2016, 6, 34836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, D.L.; Zhang, Z.J.; Jiang, B.C.; Gao, Y.J. Chemokine CXCL13 Mediates Orofacial Neuropathic Pain via CXCR5/ERK Pathway in the Trigeminal Ganglion of Mice. J. Neuroinflamm. 2016, 13, 183. [Google Scholar] [CrossRef]
- Matloubian, M.; David, A.; Engel, S.; Ryan, J.E.; Cyster, J.G. A Transmembrane CXC Chemokine Is a Ligand for HIV-Coreceptor Bonzo. Nat. Immunol. 2000, 1, 298–304. [Google Scholar] [CrossRef]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The Transmembrane CXC-Chemokine Ligand 16 Is Induced by IFN-γ and TNF-α and Shed by the Activity of the Disintegrin-Like Metalloproteinase ADAM10. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef]
- Vallejo, R.; Tilley, D.M.; Cedeño, D.L.; Kelley, C.A.; DeMaegd, M.; Benyamin, R. Genomics of the Effect of Spinal Cord Stimulation on an Animal Model of Neuropathic Pain. Neuromodulation 2016, 19, 576–586. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Liao, F.; Huang, Z.; Yang, X.; Zou, Y.; He, X.; Guo, Q.; Huang, C. A Transcriptomic Analysis of Neuropathic Pain in the Anterior Cingulate Cortex after Nerve Injury. Bioengineered 2022, 13, 2058–2075. [Google Scholar] [CrossRef] [PubMed]
- Maravillas-Montero, J.L.; Burkhardt, A.M.; Hevezi, P.A.; Carnevale, C.D.; Smit, M.J.; Zlotnik, A. Cutting Edge: GPR35/CXCR8 Is the Receptor of the Mucosal Chemokine CXCL17. J. Immunol. 2015, 194, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Binti Mohd Amir, N.A.S.; Mackenzie, A.E.; Jenkins, L.; Boustani, K.; Hillier, M.C.; Tsuchiya, T.; Milligan, G.; Pease, J.E. Evidence for the Existence of a CXCL17 Receptor Distinct from GPR35. J. Immunol. 2018, 201, 714–724. [Google Scholar] [CrossRef]
- Pisabarro, M.T.; Leung, B.; Kwong, M.; Corpuz, R.; Frantz, G.D.; Chiang, N.; Vandlen, R.; Diehl, L.J.; Skelton, N.; Kim, H.S.; et al. Cutting Edge: Novel Human Dendritic Cell- and Monocyte-Attracting Chemokine-Like Protein Identified by Fold Recognition Methods. J. Immunol. 2006, 176, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Wang, C.J.; Lin, T.Y.; Hsiao, C.L.; Luo, C.W. CXCL17, an Orphan Chemokine, Acts as a Novel Angiogenic and Anti-Inflammatory Factor. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 32–40. [Google Scholar] [CrossRef]
- Guo, Y.J.; Zhou, Y.J.; Yang, X.L.; Shao, Z.M.; Ou, Z.L. The Role and Clinical Significance of the CXCL17-CXCR8 (GPR35) Axis in Breast Cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.M.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G. Expression of CXCL15 (Lungkine) in Murine Gastrointestinal, Urogenital and Endocrine Organs. J. Histochem. Cytochem. 2007, 55, 515–524. [Google Scholar] [CrossRef]
- Fox, J.C.; Nakayama, T.; Tyler, R.C.; Sander, T.L.; Yoshie, O.; Volkman, B.F. Structural and Agonist Properties of XCL2, the Other Member of the C-Chemokine Subfamily. Cytokine 2015, 71, 302–311. [Google Scholar] [CrossRef]
- Ni, L.Y.; Zhou, L.; Wang, H.Q.; Luo, X.C.; Dan, X.M.; Li, Y.W. Identification and Expression Analysis of Three XCR1-like Receptors from Epinephelus Coioides after Cryptocaryon Irritans Infection. Fish Shellfish Immunol. 2017, 67, 95–102. [Google Scholar] [CrossRef]
- Lei, Y.; Takahama, Y. XCL1 and XCR1 in the Immune System. Microbes Infect. 2012, 14, 262–267. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kon, S.; Nakatsuru, T.; Miyashita, T.; Inui, K.; Saitoh, K.; Kitai, Y.; Muromoto, R.; Kashiwakura, J.-I.; Uede, T.; et al. A Novel A9 Integrin Ligand, XCL1/Lymphotactin, Is Involved in the Development of Murine Models of Autoimmune Diseases. J. Immunol. 2017, 199, 82–90. [Google Scholar] [CrossRef]
- Islam, B.; Stephenson, J.; Young, B.; Manca, M.; Buckley, D.A.; Radford, H.; Zis, P.; Johnson, M.I.; Finn, D.P.; McHugh, P.C. The Identification of Blood Biomarkers of Chronic Neuropathic Pain by Comparative Transcriptomics. NeuroMolecular Med. 2021, 24, 320–338. [Google Scholar] [CrossRef]
- Winter, A.N.; Subbarayan, M.S.; Grimmig, B.; Weesner, J.A.; Moss, L.; Peters, M.; Weeber, E.; Nash, K.; Bickford, P.C. Two Forms of CX3CL1 Display Differential Activity and Rescue Cognitive Deficits in CX3CL1 Knockout Mice. J. Neuroinflamm. 2020, 17, 157. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of Spinal Microglial Cathepsin S for the Reversal of Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar] [CrossRef]
- Paul, D.; Basavan, D. Implications of Fractalkine on Glial Function, Ablation and Glial Proteins/Receptors/Markers—Understanding Its Therapeutic Usefulness in Neurological Settings: A Narrative Review. Futur. J. Pharm. Sci. 2022, 8, 56. [Google Scholar] [CrossRef]
- Atta, A.A.; Ibrahim, W.W.; Mohamed, A.F.; Abdelkader, N.F. Microglia Polarization in Nociplastic Pain: Mechanisms and Perspectives. Inflammopharmacology 2023, 31, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Xiao, C.; Lu, B.; Zhang, J.; Yuan, X.Z.; Chen, W.; Yu, L.N.; Zhang, F.J.; Chen, G.; Yan, M. CX3CL1/CX3CR1 Regulates Nerve Injury-Induced Pain Hypersensitivity through the ERK5 Signaling Pathway. J. Neurosci. Res. 2013, 91, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Malcangio, M. Fractalkine/CX3CR1 Pathway in Neuropathic Pain: An Update. Front. Pain Res. 2021, 2, 35. [Google Scholar] [CrossRef]
- White, F.; Feldman, P.; Miller, R.J. Chemokine Signaling and the Management of Neuropathic Pain. Mol. Interv. 2009, 9, 188–195. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Slusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Direct and Indirect Pharmacological Modulation of CCL2/CCR2 Pathway Results in Attenuation of Neuropathic Pain—In Vivo and in Vitro Evidence. J. Neuroimmunol. 2016, 297, 9–19. [Google Scholar] [CrossRef]
- Pevida, M.; Lastra, A.; Hidalgo, A.; Baamonde, A.; Menéndez, L. Spinal CCL2 and Microglial Activation Are Involved in Paclitaxel-Evoked Cold Hyperalgesia. Brain Res. Bull. 2013, 95, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Dong, Y.L.; Lu, Y.; Cao, S.; Zhao, Z.Q.; Gao, Y.J. Chemokine CCL2 and Its Receptor CCR2 in the Medullary Dorsal Horn Are Involved in Trigeminal Neuropathic Pain. J. Neuroinflamm. 2012, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Paré, M.; Mcintosh, F.; Jr Elmes, S.; Martino, G.; Jomphe, C.; Lessard, E.; Lembo, P.M.; Vaillancourt, F.; Perkins, M.N.; et al. Blocking Spinal CCR2 with AZ889 Reversed Hyperalgesia in a Model of Neuropathic Pain. Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc Reduces Neuropathic Pain through Polarization of Microglia and Astroglia—Evidence from in Vivo and in Vitro Studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef]
- Pevida, M.; Lastra, A.; Meana, Á.; Hidalgo, A.; Baamonde, A.; Menéndez, L. The Chemokine CCL5 Induces CCR1-Mediated Hyperalgesia in Mice Inoculated with NCTC 2472 Tumoral Cells. Neuroscience 2014, 259, 113–125. [Google Scholar] [CrossRef]
- Lewis, N.D.; Muthukumarana, A.; Fogal, S.E.; Corradini, L.; Stefanopoulos, D.E.; Adusumalli, P.; Pelletier, J.; Panzenbeck, M.; Berg, K.; Canfield, M.; et al. CCR1 Plays a Critical Role in Modulating Pain through Hematopoietic and Non-Hematopoietic Cells. PLoS ONE 2014, 9, e105883. [Google Scholar] [CrossRef]
- Ambrosini, E.; Aloisi, F. Chemokines and Glial Cells: A Complex Network in the Central Nervous System. Neurochem. Res. 2004, 29, 1017–1038. [Google Scholar] [CrossRef]
- Amat, M.; Benjamim, C.F.; Williams, L.M.; Prats, N.; Terricabras, E.; Beleta, J.; Kunkel, S.L.; Godessart, N. Pharmacological Blockade of CCR1 Ameliorates Murine Arthritis and Alters Cytokine Networks in Vivo. Br. J. Pharmacol. 2006, 149, 666–675. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil Cell Surface Receptors and Their Intracellular Signal Transduction Pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Henc, I.; Rodzinnej, E.B.-F.M. Chemokiny Jako Ważne Mediatory Stanu Zapalnego. Forum Med. Rodz. 2013, 7, 251–262. [Google Scholar]
- Strazza, M.; Mor, A. Consider the Chemokines: A Review of the Interplay between Chemokines and T Cell Subset Function. Discov. Med. 2017, 24, 31–39. [Google Scholar] [PubMed]
- White, F.; Bhangoo, S.K.; Miller, R.J. Chemokines: Integrators of Pain and Inflammation. Nat. Rev. Drug Discov. 2005, 4, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Lee, D.H.; Lee, J.; Choi, C.; Kim, J.-Y.; Nam, J.-S.; Lim, Y.; Lee, Y.H. C-C Motif Chemokine Receptor 1 (CCR1) Is a Target of the EGF-AKT-MTOR-STAT3 Signaling Axis in Breast Cancer Cells. Oncotarget 2017, 8, 94591–94605. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Mika, J. Chemokines under Neuropathic Pain. Ból 2014, 15, 19–35. [Google Scholar] [CrossRef]
- Kan, A.A.; van der Hel, W.S.; Kolk, S.M.; Bos, I.W.M.; Verlinde, S.A.M.W.; van Nieuwenhuizen, O.; de Graan, P.N.E. Prolonged Increase in Rat Hippocampal Chemokine Signalling after Status Epilepticus. J. Neuroimmunol. 2012, 245, 15–22. [Google Scholar] [CrossRef]
- Lu, P.; Nakamoto, Y.; Nemoto-Sasaki, Y.; Fujii, C.; Wang, H.; Hashii, M.; Ohmoto, Y.; Kaneko, S.; Kobayashi, K.; Mukaida, N. Potential Interaction between CCR1 and Its Ligand, CCL3, Induced by Endogenously Produced Interleukin-1 in Human Hepatomas. Am. J. Pathol. 2003, 162, 1249–1258. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, X.-Y.; Yang, J.-P.; Wang, L.-N.; Ji, F.-H. Involvement of Spinal Monocyte Chemoattractant Protein-1 (MCP-1) in Cancer-Induced Bone Pain in Rats. Neurosci. Lett. 2012, 517, 60–63. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Gu, K.; Sun, X.; Gu, J.; Li, C.; Wang, G. Lidocaine Alleviates Neuropathic Pain and Neuroinflammation by Inhibiting HMGB1 Expression to Mediate MIP-1α/CCR1 Pathway. J. Neuroimmune Pharmacol. 2021, 16, 318–333. [Google Scholar] [CrossRef]
- Shi, C.; Jin, J.; Xu, H.; Ma, J.; Li, T.; Xie, Y.; Li, Z. CCR1 Enhances SUMOylation of DGCR8 by Up-Regulating ERK Phosphorylation to Promote Spinal Nerve Ligation-Induced Neuropathic Pain. Gene Ther. 2021, 29, 379–389. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of Glial Activation in Neuropathic Pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Mika, J. Modulation of Microglia Can Attenuate Neuropathic Pain Symptoms and Enhance Morphine Effectiveness. Pharmacol Rep. 2008, 60, 297–307. [Google Scholar] [PubMed]
- Pilat, D.; Piotrowska, A.; Rojewska, E.; Jurga, A.; Ślusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Blockade of IL-18 Signaling Diminished Neuropathic Pain and Enhanced the Efficacy of Morphine and Buprenorphine. Mol. Cell. Neurosci. 2016, 71, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Pilat, D.; Rojewska, E.; Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. IL-1 Receptor Antagonist Improves Morphine and Buprenorphine Efficacy in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 2015, 764, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Arnatt, C.K.; Falls, B.A.; Yuan, Y.; Raborg, T.J.; Masvekar, R.R.; El-Hage, N.; Selley, D.E.; Nicola, A.V.; Knapp, P.E.; Hauser, K.F.; et al. Exploration of Bivalent Ligands Targeting Putative Mu Opioid Receptor and Chemokine Receptor CCR5 Dimerization. Bioorg. Med. Chem. 2016, 24, 5969–5987. [Google Scholar] [CrossRef]
- Kramp, B.K.; Megens, R.T.A.; Sarabi, A.; Winkler, S.; Projahn, D.; Weber, C.; Koenen, R.R.; von Hundelshausen, P. Exchange of Extracellular Domains of CCR1 and CCR5 Reveals Confined Functions in CCL5-Mediated Cell Recruitment. Thromb. Haemost. 2013, 110, 795–806. [Google Scholar] [CrossRef]
- Di Prisco, S.; Summa, M.; Chellakudam, V.; Rossi, P.I.A.; Pittaluga, A. RANTES-Mediated Control of Excitatory Amino Acid Release in Mouse Spinal Cord. J. Neurochem. 2012, 121, 428–437. [Google Scholar] [CrossRef]
- Liu, J.; Robert Merritt, J. CC Chemokine Receptor Small Molecule Antagonists in the Treatment of Rheumatoid Arthritis and Other Diseases: A Current View. Curr. Top. Med. Chem. 2010, 10, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.H.; Chee, A.; Shi, P.; Lin, C.L.; Zhao, Y.; Zhang, L.; An, H.S. Small Molecule Antagonist of C-C Chemokine Receptor 1 (CCR1) Reduces Disc Inflammation in the Rabbit Model. Spine J. 2020, 20, 2025–2036. [Google Scholar] [CrossRef]
- Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Shahid, M.; Rehman, M.U.; Alanazi, M.M.; Alhamed, A.S.; Ibrahim, K.E.; Albekairi, N.A.; et al. CCR1 Antagonist J-113863 Corrects the Imbalance of pro- and Anti-Inflammatory Cytokines in a SJL/J Mouse Model of Relapsing-Remitting Multiple Sclerosis. Immunobiology 2022, 227, 152245. [Google Scholar] [CrossRef]
- Al-Mazroua, H.A.; Nadeem, A.; Ansari, M.A.; Attia, S.M.; Bakheet, S.A.; Albekairi, T.H.; Ali, N.; Alasmari, F.; Algahtani, M.; Alsaad, A.M.S.; et al. CCR1 Antagonist Ameliorates Experimental Autoimmune Encephalomyelitis by Inhibition of Th9/Th22-Related Markers in the Brain and Periphery. Mol. Immunol. 2022, 144, 127–137. [Google Scholar] [CrossRef]
- Gladue, R.P.; Brown, M.F.; Zwillich, S.H. CCR1 Antagonists: What Have We Learned From Clinical Trials. Curr. Top. Med. Chem. 2010, 10, 1268–1277. [Google Scholar] [CrossRef]
- Trummer, D.; Walzer, A.; Groettrup-Wolfers, E.; Schmitz, H. Efficacy, Safety and Tolerability of the CCR1 Antagonist BAY 86-5047 for the Treatment of Endometriosis-Associated Pelvic Pain: A Randomized Controlled Trial. Acta Obstet. Gynecol. Scand. 2017, 96, 694–701. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, S.; Zhu, M.-D.; Liu, J.-Q.; Chen, J.-J.; Gao, Y.-J. Contribution of Chemokine CCL2/CCR2 Signaling in the Dorsal Root Ganglion and Spinal Cord to the Maintenance of Neuropathic Pain in a Rat Model of Lumbar Disc Herniation. J. Pain 2014, 15, 516–526. [Google Scholar] [CrossRef]
- Kurihara, T.; Bravo, R. Cloning and Functional Expression of MCCR2, a Murine Receptor for the C-C Chemokines JE and FIC. J. Biol. Chem. 1996, 271, 11603–11606. [Google Scholar] [CrossRef]
- Jung, H.; Bhangoo, S.; Banisadr, G.; Freitag, C.; Ren, D.; White, F.A.; Miller, R.J. Visualization of Chemokine Receptor Activation in Transgenic Mice Reveals Peripheral Activation of CCR2 Receptors in States of Neuropathic Pain. J. Neurosci. 2009, 29, 8051–8062. [Google Scholar] [CrossRef]
- Abbadie, C.; Lindia, J.A.; Cumiskey, A.M.; Peterson, L.B.; Mudgett, J.S.; Bayne, E.K.; DeMartino, J.A.; MacIntyre, D.E.; Forrest, M.J. Impaired Neuropathic Pain Responses in Mice Lacking the Chemokine Receptor CCR2. Proc. Natl. Acad. Sci. USA 2003, 100, 7947–7952. [Google Scholar] [CrossRef]
- BMS-741672 for Diabetic Neuropathic Pain—No Study Results Posted—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00683423 (accessed on 25 May 2023).
- Liu, S.; Lan, X.B.; Tian, M.M.; Zhu, C.H.; Ma, L.; Yang, J.M.; Du, J.; Zheng, P.; Yu, J.Q.; Liu, N. Targeting the Chemokine Ligand 2–chemokine Receptor 2 Axis Provides the Possibility of Immunotherapy in Chronic Pain. Eur. J. Pharmacol. 2023, 947, 175646. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Delarasse, C. Complex Role of Chemokine Mediators in Animal Models of Alzheimer’s Disease. Biomed. J. 2018, 41, 34–40. [Google Scholar] [CrossRef]
- Albright, A.V.; Shieh, J.T.C.; Itoh, T.; Lee, B.; Pleasure, D.; O’Connor, M.J.; Doms, R.W.; González-Scarano, F. Microglia Express CCR5, CXCR4, and CCR3, but of These, CCR5 Is the Principal Coreceptor for Human Immunodeficiency Virus Type 1 Dementia Isolates. J. Virol. 1999, 73, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.N.; Lloyd, C.M.; Sabroe, I.; Durham, S.R.; Till, S.J. T Lymphocytes Expressing CCR3 Are Increased in Allergic Rhinitis Compared with Non-Allergic Controls and Following Allergen Immunotherapy. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.; Maru, S.; Loughlin, J.; Romero, I.A.; Male, D. Regulation of Chemokine Receptor Expression in Human Microglia and Astrocytes. J. Neuroimmunol. 2003, 136, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huaux, F.; Gharaee-Kermani, M.; Liu, T.; Morel, V.; McGarry, B.; Ullenbruch, M.; Kunkel, S.L.; Wang, J.; Xing, Z.; Phan, S.H. Role of Eotaxin-1 (CCL11) and CC Chemokine Receptor 3 (CCR3) in Bleomycin-Induced Lung Injury and Fibrosis. Am. J. Pathol. 2005, 167, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Humbles, A.A.; Lu, B.; Friend, D.S.; Okinaga, S.; Lora, J.; Al-garawi, A.; Martin, T.R.; Gerard, N.P.; Gerard, C. The Murine CCR3 Receptor Regulates Both the Role of Eosinophils and Mast Cells in Allergen-Induced Airway Inflammation and Hyperresponsiveness. Proc. Natl. Acad. Sci. USA 2002, 99, 1479–1484. [Google Scholar] [CrossRef]
- Bertrand, C.P.; Ponath, P.D. CCR3 Blockade as a New Therapy for Asthma. Expert Opin. Investig. Drugs 2000, 9, 43–52. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.Y.; Song, S.J.; Hong, H.K.; Lee, Y.; Oh, B.Y.; Lee, W.Y.; Cho, Y.B. Crosstalk between CCL7 and CCR3 Promotes Metastasis of Colon Cancer Cells via ERK-JNK Signaling Pathways. Oncotarget 2016, 7, 36842–36853. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, K.; Kukova, G.; Bunemann, E.; Buhren, B.A.; Sonkoly, E.; Szollosi, A.G.; Muller, A.; Savinko, T.; Lauerma, A.I.; Alenius, H.; et al. The Chemokine Receptor CCR3 Participates in Tissue Remodeling during Atopic Skin Inflammation. J. Dermatol. Sci. 2013, 71, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Honda, Y.; Tanaka, S.; Miyagawa, T.; Honda, M.; Honda, K.; Tokunaga, K.; Kodama, T. Narcolepsy Susceptibility Gene CCR3 Modulates Sleep-Wake Patterns in Mice. PLoS ONE 2017, 12, e0187888. [Google Scholar] [CrossRef]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a Novel Mediator of Inflammatory Bone Resorption. Sci. Rep. 2017, 7, 5334. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Xu, M.-L.; Yuan, B.-T.; Ma, L.-J.; Gao, Y.-J. Chemokine CCL7 Mediates Trigeminal Neuropathic Pain via CCR2/CCR3-ERK Pathway in the Trigeminal Ganglion of Mice. Mol. Pain 2023, 174480692311693. [Google Scholar] [CrossRef]
- Ugur, M.; Derouiche, L.; Massotte, D. Heteromerization Modulates Mu Opioid Receptor Functional Properties in Vivo. Front. Pharmacol. 2018, 9, 1240. [Google Scholar] [CrossRef]
- TJ, R. Bidirectional Regulation of Opioid and Chemokine Function. Front. Immunol. 2020, 11, 94. [Google Scholar] [CrossRef]
- Lee, Y.K.; Choi, D.Y.; Jung, Y.Y.; Yun, Y.W.; Lee, B.J.; Han, S.B.; Hong, J.T. Decreased Pain Responses of C-C Chemokine Receptor 5 Knockout Mice to Chemical or Inflammatory Stimuli. Neuropharmacology 2013, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Hong, T.; Cheng, B.; Gan, S.; Chen, L.; Zhang, J.; Zuo, L.; Li, J.; Cui, X. Blocking the Autocrine Regulatory Loop of Gankyrin/STAT3/CCL24/CCR3 Impairs the Progression and Pazopanib Resistance of Clear Cell Renal Cell Carcinoma. Cell Death Dis. 2020, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Kanabar, V.; Riffo-Vasquez, Y.; Mohamed, Z.; Cleary, S.J.; Corrigan, C.; James, A.L.; Elliot, J.G.; Shute, J.K.; Page, C.P.; et al. Platelets Independently Recruit into Asthmatic Lungs and Models of Allergic Inflammation via CCR3. Am. J. Respir. Cell Mol. Biol. 2021, 64, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Shen, J.; Yang, H.; Xu, Y.; Gao, W.; Wang, J.; Zhang, H.; He, S. Upregulated Expression of CCR3 in Osteoarthritis and CCR3 Mediated Activation of Fibroblast-like Synoviocytes. Cytokine 2016, 77, 211–219. [Google Scholar] [CrossRef]
- Salanga, C.L.; Handel, T.M. Chemokine Oligomerization and Interactions with Receptors and Glycosaminoglycans: The Role of Structural Dynamics in Function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Bushell, T.J.; Gray, P.W.; Miller, R.J. Chemokines Regulate Hippocampal Neuronal Signaling and Gp120 Neurotoxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 14500–14505. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Arabi, Z.; Ahangar-Parvin, R.; Mohammadi-Kordkhayli, M.; Nemati, M. Ginger Extract Modulates the Expression of Chemokines CCL20 and CCL22 and Their Receptors (CCR6 and CCR4) in the Central Nervous System of Mice with Experimental Autoimmune Encephalomyelitis. Drug Res. 2017, 67, 632–639. [Google Scholar] [CrossRef]
- Bajetto, A.; Bonavia, R.; Barbero, S.; Schettini, G. Characterization of Chemokines and Their Receptors in the Central Nervous System: Physiopathological Implications. J. Neurochem. 2002, 82, 1311–1329. [Google Scholar] [CrossRef]
- Purandare, A.; Somerville, J. Antagonists of CCR4 as Immunomodulatory Agents. Curr. Top. Med. Chem. 2012, 6, 1335–1344. [Google Scholar] [CrossRef]
- Purandare, A.V.; Gao, A.; Wan, H.; Somerville, J.; Burke, C.; Seachord, C.; Vaccaro, W.; Wityak, J.; Poss, M.A. Identification of Chemokine Receptor CCR4 Antagonist. Bioorganic Med. Chem. Lett. 2005, 15, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, J.; Hellhammer, J.; Musholt, P.; Pfützner, A.H.; Böhnke, J.; Hero, T.; Amann-Zalan, I.; Ganz, M.; Forst, T.; Pfützner, A. Evaluation of Dexterity in Insulin-Treated Patients with Type 1 and Type 2 Diabetes Mellitus. J. Diabetes Sci. Technol. 2011, 5, 158–165. [Google Scholar] [CrossRef]
- Mueller, M.J.; Minor, S.D.; Sahrmann, S.A.; Schaaf, J.A.; Strube, M.J. Differences in the Gait Characteristics of Patients with Diabetes and Peripheral Neuropathy Compared with Age-Matched Controls. Phys. Ther. 1994, 74, 299–313. [Google Scholar] [CrossRef]
- Remer, M.; Al-Shamkhani, A.; Glennie, M.; Johnson, P. Mogamulizumab and the Treatment of CCR4-Positive T-Cell Lymphomas. Immunotherapy 2014, 6, 1187–1206. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef]
- Kaul, M.; Ma, Q.; Medders, K.E.; Desai, M.K.; Lipton, S.A. HIV-1 Coreceptors CCR5 and CXCR4 Both Mediate Neuronal Cell Death but CCR5 Paradoxically Can Also Contribute to Protection. Cell Death Differ. 2007, 14, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Rottman, J.B.; Ganley, K.P.; Williams, K.; Wu, L.; Mackay, C.R.; Ringler, D.J. Cellular Localization of the Chemokine Receptor CCR5: Correlation to Cellular Targets of HIV-1 Infection. Am. J. Pathol. 1997, 151, 1341–1351. [Google Scholar]
- Carbonell, W.S.; Murase, S.I.; Horwitz, A.F.; Mandell, J.W. Migration of Perilesional Microglia after Focal Brain Injury and Modulation by CC Chemokine Receptor 5: An in Situ Time-Lapse Confocal Imaging Study. J. Neurosci. 2005, 25, 7040–7047. [Google Scholar] [CrossRef]
- Marella, M.; Chabry, J. Neurons and Astrocytes Respond to Prion Infection by Inducing Microglia Recruitment. J. Neurosci. 2004, 24, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Chuang, L.F.; Doi, R.H.; Carlos, M.P.; Torres, J.V.; Chuang, R.Y. Morphine Induces Gene Expression of CCR5 in Human CEM X174 Lymphocytes. J. Biol. Chem. 2000, 275, 31305–31310. [Google Scholar] [CrossRef]
- Bidlack, J.M. Detection and Function of Opioid Receptors on Cells from the Immune System. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef]
- Mika, J.; Popiolek-Barczyk, K.; Rojewska, E.; Makuch, W.; Starowicz, K.; Przewlocka, B. Delta-Opioid Receptor Analgesia Is Independent of Microglial Activation in a Rat Model of Neuropathic Pain. PLoS ONE 2014, 9, e104420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J.; Bot, G.; Szabo, I.; Rogers, T.J.; Liu-Chen, L.-Y. Heterodimerization and Cross-Desensitization between the μ-Opioid Receptor and the Chemokine CCR5 Receptor. Eur. J. Pharmacol. 2004, 483, 175–186. [Google Scholar] [CrossRef]
- Szabo, I.; Chen, X.-H.; Xin, L.; Adler, M.W.; Howard, O.M.Z.; Oppenheim, J.J.; Rogers, T.J. Heterologous Desensitization of Opioid Receptors by Chemokines Inhibits Chemotaxis and Enhances the Perception of Pain. Proc. Natl. Acad. Sci. USA 2002, 99, 10276–10281. [Google Scholar] [CrossRef]
- Lisi, L.; Tramutola, A.; De Luca, A.; Navarra, P.; Dello Russo, C. Modulatory Effects of the CCR5 Antagonist Maraviroc on Microglial Pro-Inflammatory Activation Elicited by Gp120. J. Neurochem. 2012, 120, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pease, J.E.; Horuk, R. Chemokine Receptor Antagonists: Part 2. Expert Opin. Ther. Pat. 2009, 19, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Manjavachi, M.N.; Passos, G.F.; Trevisan, G.; Araújo, S.B.; Pontes, J.P.; Fernandes, E.S.; Costa, R.; Calixto, J.B. Spinal Blockage of CXCL1 and Its Receptor CXCR2 Inhibits Paclitaxel-Induced Peripheral Neuropathy in Mice. Neuropharmacology 2019, 151, 136–143. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.; Zhang, Y.Y.; Lin, J.; Li, Y.L.; Zhou, C.; Li, C.J.; Shen, J.F. C-X-C Motif Chemokine Ligand 1 and Its Receptor C-X-C Motif Chemokine Receptor 2 in Trigeminal Ganglion Contribute to Nerve Injury-Induced Orofacial Mechanical Allodynia. J. Oral Rehabil. 2022, 49, 195–206. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Wang, M.; Qian, C.; Wang, C.; Tang, J.; Cai, Z.; Dai, W.; Zhu, X. Pharmacological Inhibition of CXCR2 Alleviates Neuropathic Pain by Inactivating Microglia in a Rat L5 Spinal Nerve Ligation Model. Am. J. Transl. Res. 2020, 12, 3803–3812. [Google Scholar]
- Qin, J.; Li, A.; Huang, Y.; Teng, R.H.; Yang, Y.; Yao, Y.X. CXCR3 Contributes to Neuropathic Pain via ERK Activation in the Anterior Cingulate Cortex. Biochem. Biophys. Res. Commun. 2020, 531, 166–171. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Li, X.; Chao, Y.-C.; Yue, Y. Early Repeated Administration of CXCR4 Antagonist AMD3100 Dose-Dependently Improves Neuropathic Pain in Rats After L5 Spinal Nerve Ligation. Neurochem. Res. 2016, 41, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zou, Y.Q.; Li, M.; Luo, W.J.; Chen, G.Z.; Wu, X.Z. Intervertebral Foramen Injection of Plerixafor Attenuates Neuropathic Pain after Chronic Compression of the Dorsal Root Ganglion: Possible Involvement of the down-Regulation of Nav1.8 and Nav1.9. Eur. J. Pharmacol. 2021, 908, 174322. [Google Scholar] [CrossRef]
- Rojewska, E.; Piotrowska, A.; Jurga, A.; Makuch, W.; Mika, J. Zaprinast Diminished Pain and Enhanced Opioid Analgesia in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 2018, 839, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Horuk, R.; Martin, A.W.; Wang, Z.; Schweitzer, L.; Gerassimides, A.; Guo, H.; Lu, Z.; Hesselgesser, J.; Perez, H.D.; Kim, J.; et al. Expression of Chemokine Receptors by Subsets of Neurons in the Central Nervous System. J. Immunol. 1997, 158, 2882–2890. [Google Scholar] [CrossRef]
- Sun, Y.; Sahbaie, P.; Liang, D.Y.; Li, W.W.; Li, X.Q.; Shi, X.Y.; Clark, J.D. Epigenetic Regulation of Spinal Cxcr2 Signaling in Incisional Hypersensitivity in Mice. Anesthesiology 2013, 119, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Y.; Shi, X.; Liu, P.; Sun, Y.; Sahbaie, P.; Li, W.W.; Yeomans, D.C.; Clark, J.D. The Chemokine Receptor CXCR2 Supports Nociceptive Sensitization after Traumatic Brain Injury. Mol. Pain 2017, 13, 1744806917730212. [Google Scholar] [CrossRef]
- Moraes, T.R.; Elisei, L.S.; Malta, I.H.; Galdino, G. Participation of CXCL1 in the Glial Cells during Neuropathic Pain. Eur. J. Pharmacol. 2020, 875, 173039. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Y.; Li, C.; Yan, Y.; Ao, L.; Yu, B.; Fang, W.; Liu, J.; Li, Y. Levo-Corydalmine Alleviates Vincristine-Induced Neuropathic Pain in Mice by Inhibiting an NF-Kappa B-Dependent CXCL1/CXCR2 Signaling Pathway. Neuropharmacology 2018, 135, 34–47. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, J.; Li, W.; Wang, L.; Song, M.; Xu, S.; Liu, G.; Du, Q.; Zhai, D.; Tang, L.; et al. The Role of CXCL1/CXCR2 Axis in Neurological Diseases. Int. Immunopharmacol. 2023, 120, 110330. [Google Scholar] [CrossRef]
- Old, E.A.; Malcangio, M. Chemokine Mediated Neuron-Glia Communication and Aberrant Signalling in Neuropathic Pain States. Curr. Opin. Pharmacol. 2012, 12, 67–73. [Google Scholar] [CrossRef]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E.; et al. An Alternatively Spliced Variant of CXCR3 Mediates the Inhibition of Endothelial Cell Growth Induced by IP-10, Mig, and I-TAC, and Acts as Functional Receptor for Platelet Factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Clark-Lewis, I.; Mattioli, I.; Gong, J.H.; Loetscher, P. Structure-Function Relationship between the Human Chemokine Receptor CXCR3 and Its Ligands. J. Biol. Chem. 2003, 278, 289–295. [Google Scholar] [CrossRef]
- Smit, M.J.; Verdijk, P.; Van der Raaij-Helmer, E.M.H.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-Mediated Chemotaxis of Human T Cells Is Regulated by a G i-and Phospholipase C-Dependent Pathway and Not via Activation of MEK/P44/P42 MAPK nor Akt/PI-3 Kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef]
- Green, M.V.; Thayer, S.A. HIV Gp120 Upregulates Tonic Inhibition through A5-Containing GABA A Rs. Neuropharmacology 2019, 149, 161–168. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Man, K.; Li, X.; Du, J.; Chu, E.S.H.; Go, M.Y.Y.; Sung, J.J.Y.; Yu, J. CXC Chemokine Receptor 3 Promotes Steatohepatitis in Mice through Mediating Inflammatory Cytokines, Macrophages and Autophagy. J. Hepatol. 2016, 64, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Berchiche, Y.A.; Sakmar, T.P. CXC Chemokine Receptor 3 Alternative Splice Variants Selectively Activate Different Signaling Pathways. Mol. Pharmacol. 2016, 90, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Northcutt, A.L.; Chao, L.W.; Kearney, J.J.; Zhang, Y.; Berkelhammer, D.L.; Loram, L.C.; Rozeske, R.R.; Bland, S.T.; Maier, S.F.; et al. Minocycline Suppresses Morphine-Induced Respiratory Depression, Suppresses Morphine-Induced Reward, and Enhances Systemic Morphine-Induced Analgesia. Brain. Behav. Immun. 2008, 22, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Mozobil® (Plerixafor, AMD3100), 10 Years after Its Approval by the US Food and Drug Administration. Chem. Chemother. 2019, 27, 2040206619829382. [Google Scholar] [CrossRef]
- Zuk, A.; Gershenovich, M.; Ivanova, Y.; MacFarland, R.T.; Fricker, S.P.; Ledbetter, S. CXCR4 Antagonism as a Therapeutic Approach to Prevent Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2014, 307, F783–F797. [Google Scholar] [CrossRef][Green Version]
- Jujo, K.; Ii, M.; Sekiguchi, H.; Klyachko, E.; Misener, S.; Tanaka, T.; Tongers, J.; Roncalli, J.; Renault, M.A.; Thorne, T.; et al. CXC-Chemokine Receptor 4 Antagonist AMD3100 Promotes Cardiac Functional Recovery after Ischemia/Reperfusion Injury via Endothelial Nitric Oxide Synthase-Dependent Mechanism. Circulation 2013, 127, 63–73. [Google Scholar] [CrossRef]
- Hendrix, C.W.; Flexner, C.; Macfarland, R.T.; Giandomenico, C.; Fuchs, E.J.; Redpath, E.; Bridger, G.; Henson, G.W. Pharmacokinetics and Safety of AMD-3100, a Novel Antagonist of the CXCR- 4 Chemokine Receptor, in Human Volunteers. Antimicrob. Agents Chemother. 2000, 44, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Bhangoo, S.K.; Ripsch, M.S.; Buchanan, D.J.; Miller, R.J.; White, F.A. Increased Chemokine Signaling in a Model of HIV1-Associated Peripheral Neuropathy. Mol. Pain 2009, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Liu, Y.-N.; Zhang, H.-L.; Cao, S.-B.; Zhang, T.; Chen, L.-P.; Shen, W. CXCL12/CXCR4 Chemokine Signaling in Spinal Glia Induces Pain Hypersensitivity through MAPKs-Mediated Neuroinflammation in Bone Cancer Rats. J. Neurochem. 2015, 132, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Menichella, D.M.; Abdelhak, B.; Ren, D.; Shum, A.; Frietag, C.; Miller, R.J. CXCR4 Chemokine Receptor Signaling Mediates Pain in Diabetic Neuropathy. Mol. Pain 2014, 10, 42. [Google Scholar] [CrossRef]
- Ashjari, D.; Karamali, N.; Rajabinejad, M.; Hassani, S.S.; Afshar Hezarkhani, L.; Afshari, D.; Gorgin Karaji, A.; Salari, F.; Rezaiemanesh, A. The Axis of Long Non-Coding RNA MALAT1/MiR-1-3p/CXCR4 Is Dysregulated in Patients with Diabetic Neuropathy. Heliyon 2022, 8, e09178. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tai, W.L.; Sun, L.; Qiu, Q.; Xia, Z.; Chung, S.K.; Cheung, C.W. Central Administration of C-X-C Chemokine Receptor Type 4 Antagonist Alleviates the Development and Maintenance of Peripheral Neuropathic Pain in Mice. PLoS ONE 2014, 9, e104860. [Google Scholar] [CrossRef]
- Wilson, N.M.; Jung, H.; Ripsch, M.S.; Miller, R.J.; White, F.A. CXCR4 Signaling Mediates Morphine-Induced Tactile Hyperalgesia. Brain. Behav. Immun. 2011, 25, 565–573. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.R.; Todd, S.W.; Randall, W.R.; Lane, M.V.; Albuquerque, E.X. Functional G-Protein-Coupled Receptor 35 Is Expressed by Neurons in the CA1 Field of the Hippocampus. Biochem. Pharmacol. 2015, 93, 506–518. [Google Scholar] [CrossRef]
- Berlinguer-Palmini, R.; Masi, A.; Narducci, R.; Cavone, L.; Maratea, D.; Cozzi, A.; Sili, M.; Moroni, F.; Mannaioni, G. GPR35 Activation Reduces Ca2+ Transients and Contributes to the Kynurenic Acid-Dependent Reduction of Synaptic Activity at CA3-CA1 Synapses. PLoS ONE 2013, 8, e82180. [Google Scholar] [CrossRef]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carl, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-Protein Coupled Receptor 35 (GPR35) Activation and Inflammatory Pain: Studies on the Antinociceptive Effects of Kynurenic Acid and Zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic Acid as a Ligand for Orphan G Protein-Coupled Receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- Ohshiro, H.; Tonai-Kachi, H.; Ichikawa, K. GPR35 Is a Functional Receptor in Rat Dorsal Root Ganglion Neurons. Biochem. Biophys. Res. Commun. 2008, 365, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Tonai-Kachi, H.; Shinjo, K. Zaprinast, a Well-Known Cyclic Guanosine Monophosphate-Specific Phosphodiesterase Inhibitor, Is an Agonist for GPR35. FEBS Lett. 2006, 580, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.E.; Milligan, G. The Emerging Pharmacology and Function of GPR35 in the Nervous System. Neuropharmacology 2017, 113, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Lane, T.R.; Gao, H.G.L.; Hurst, D.P.; Kotsikorou, E.; Le, L.; Brailoiu, E.; Reggio, P.H.; Abood, M.E. Crucial Positively Charged Residues for Ligand Activation of the GPR35 Receptor. J. Biol. Chem. 2014, 289, 3625–3638. [Google Scholar] [CrossRef] [PubMed]
- Resta, F.; Masi, A.; Sili, M.; Laurino, A.; Moroni, F.; Mannaioni, G. Kynurenic Acid and Zaprinast Induce Analgesia by Modulating HCN Channels through GPR35 Activation. Neuropharmacology 2016, 108, 136–143. [Google Scholar] [CrossRef]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of Endogenous Kynurenic Acid Produce Spatial Working Memory Deficits. Schizophr. Bull. 2007, 33, 797–804. [Google Scholar] [CrossRef]
- Koola, M.M. Galantamine-Memantine Combination for Cognitive Impairments Due to Electroconvulsive Therapy, Traumatic Brain Injury, and Neurologic and Psychiatric Disorders: Kynurenic Acid and Mismatch Negativity Target Engagement. Prim. Care Companion J. Clin. Psychiatry 2018, 20, 27296. [Google Scholar] [CrossRef]
- Banerjee, J.; Alkondon, M.; Pereira, E.F.R.; Albuquerque, E.X. Regulation of GABAergic Inputs to CA1 Pyramidal Neurons by Nicotinic Receptors and Kynurenic Acid. J. Pharmacol. Exp. Ther. 2012, 341, 500–509. [Google Scholar] [CrossRef]
- Elmslie, K.S.; Yoshikami, D. Effects of Kynurenate on Root Potentials Evoked by Synaptic Activity and Amino Acids in the Frog Spinal Cord. Brain Res. 1985, 330, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Näsström, J.; Karlsson, U.; Post, C. Antinociceptive Actions of Different Classes of Excitatory Amino Acid Receptor Antagonists in Mice. Eur. J. Pharmacol. 1992, 212, 21–29. [Google Scholar] [CrossRef]
- Chapman, V.; Dickenson, A.H. Time-Related Roles of Excitatory Amino Acid Receptors during Persistent Noxiously Evoked Responses of Rat Dorsal Horn Neurones. Brain Res. 1995, 703, 45–50. [Google Scholar] [CrossRef]
- Yoon, M.H.; Jeong, I.C.; Hong, B.B.; Seong, W.J.; Sung, S.C.; Kyung, Y.Y.; Chang, Y.J.; Seok, J.K.; Sung, T.C.; Chang, M.K. Lack of the Nitric Oxide-Cyclic GMP-Potassium Channel Pathway for the Antinociceptive Effect of Intrathecal Zaprinast in a Rat Formalin Test. Neurosci. Lett. 2005, 390, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Southern, C.; Cook, J.M.; Neetoo-Isseljee, Z.; Taylor, D.L.; Kettleborough, C.A.; Merritt, A.; Bassoni, D.L.; Raab, W.J.; Quinn, E.; Wehrman, T.S.; et al. Screening β-Arrestin Recruitment for the Identification of Natural Ligands for Orphan G-Protein-Coupled Receptors. J. Biomol. Screen. 2013, 18, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, S.J.; Nam, S.Y.; Im, D.S. GPR35 Mediates Lodoxamide-Induced Migration Inhibitory Response but Not CXCL17-Induced Migration Stimulatory Response in THP-1 Cells; Is GPR35 a Receptor for CXCL17? Br. J. Pharmacol. 2018, 175, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, J.; Li, Z.; Zhang, W.; Wang, F.; Zhang, B. Recent Advances in CXCL12/CXCR4 Antagonists and Nano-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Bird, E.; Iannitti, T.; Christmas, C.R.; Obara, I.; Andreev, V.I.; King, A.E.; Boissonade, F.M. A Novel Role for Lymphotactin (XCL1) Signaling in the Nervous System: XCL1 Acts via Its Receptor XCR1 to Increase Trigeminal Neuronal Excitability. Neuroscience 2018, 379, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, F.; Cairns, C.M.; Gordon, J.R.; Xiang, J. Neutrophils and B Cells Express XCR1 Receptor and Chemotactically Respond to Lymphotactin. Biochem. Biophys. Res. Commun. 2001, 281, 378–382. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Wang, N.; Yu, D.; Yu, S.; Wang, X.; Liu, T.; Lv, L.; Guan, Q. Total Saponins of Panax Ginseng via the CX3CL1/CX3CR1 Axis Attenuates Neuroinflammation and Exerted Antidepressant-like Effects in Chronic Unpredictable Mild Stress in Rats. Phyther. Res. 2022, 37, 1823–1838. [Google Scholar] [CrossRef]
- Verge, G.M.; Milligan, E.D.; Maier, S.F.; Watkins, L.R.; Naeve, G.S.; Foster, A.C. Fractalkine (CX3CL1) and Fractalkine Receptor (CX3CR1) Distribution in Spinal Cord and Dorsal Root Ganglia under Basal and Neuropathic Pain Conditions. Eur. J. Neurosci. 2004, 20, 1150–1160. [Google Scholar] [CrossRef]
- Zhuang, Z.; Kawasaki, Y.; Tan, P.H.; Wen, Y.R.; Huang, J.; Ji, R.R. Role of the CX3CR1/P38 MAPK Pathway in Spinal Microglia for the Development of Neuropathic Pain Following Nerve Injury-Induced Cleavage of Fractalkine. Brain Behav. Immun. 2007, 21, 642–651. [Google Scholar] [CrossRef]
- Milligan, E.; Zapata, V.; Schoeniger, D.; Chacur, M.; Green, P.; Poole, S.; Martin, D.; Maier, S.F.; Watkins, L.R. An Initial Investigation of Spinal Mechanisms Underlying Pain Enhancement Induced by Fractalkine, a Neuronally Released Chemokine. Eur. J. Neurosci. 2005, 22, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Staniland, A.A.; Clark, A.K.; Wodarski, R.; Sasso, O.; Maione, F.; D’Acquisto, F.; Malcangio, M. Reduced Inflammatory and Neuropathic Pain and Decreased Spinal Microglial Response in Fractalkine Receptor (CX3CR1) Knockout Mice. J. Neurochem. 2010, 114, 1143–1157. [Google Scholar] [CrossRef]
- Sessler, K.; Blechschmidt, V.; Hoheisel, U.; Mense, S.; Schirmer, L.; Treede, R.D. Spinal Cord Fractalkine (CX3CL1) Signaling Is Critical for Neuronal Sensitization in Experimental Nonspecific, Myofascial Low Back Pain. J. Neurophysiol. 2021, 125, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Holmes, F.E.; Arnott, N.; Vanderplank, P.; Kerr, N.C.H.; Longbrake, E.E.; Popovich, P.G.; Imai, T.; Combadière, C.; Murphy, P.M.; Wynick, D. Intra-Neural Administration of Fractalkine Attenuates Neuropathic Pain-Related Behaviour. J. Neurochem. 2008, 106, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.; Shin, H.J.; Lee, S.; Kim, S.I.; Kim, Y.H.; Lee, W.H.; Kim, D.W.; Lee, S.Y.; Ko, Y.K. CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation. Int. J. Mol. Sci. 2020, 21, 3469. [Google Scholar] [CrossRef]
- Ruff, M.R.; Inan, S.; Shi, X.Q.; Meissler, J.J.; Adler, M.W.; Eisenstein, T.K.; Zhang, J. Potentiation of Morphine Antinociception and Inhibition of Diabetic Neuropathic Pain by the Multi-Chemokine Receptor Antagonist Peptide RAP-103. Life Sci. 2022, 306, 120788. [Google Scholar] [CrossRef]
- Padi, S.S.V.; Shi, X.Q.; Zhao, Y.Q.; Ruff, M.R.; Baichoo, N.; Pert, C.B.; Zhang, J. Attenuation of Rodent Neuropathic Pain by an Orally Active Peptide, RAP-103, Which Potently Blocks CCR2- and CCR5-Mediated Monocyte Chemotaxis and Inflammation. Pain 2012, 153, 95–106. [Google Scholar] [CrossRef]
- Laura, B.; Elisabetta, B.; Adelchi, R.P.; Roberto, R.; Loredana, C.; Andrea, A.; d’Angelo Michele; Vanessa, C.; Antonio, G.; Marcello, A.; et al. CXCR1/2 Pathways in Paclitaxel-Induced Neuropathic Pain. Oncotarget 2017, 8, 23188–23201. [Google Scholar] [CrossRef]
- Cho, H.S.; Choi, Y.I.; Park, S.U.; Han, Y.S.; Kwon, J.; Jung, S.J. Prevention of Chemotherapy-Induced Peripheral Neuropathy by Inhibiting C-X-C Motif Chemokine Receptor 2. Int. J. Mol. Sci. 2023, 24, 1855. [Google Scholar] [CrossRef]
- Wise, E.L.; Duchesnes, C.; Da Fonseca, P.C.A.; Allen, R.A.; Williams, T.J.; Pease, J.E. Small Molecule Receptor Agonists and Antagonists of CCR3 Provide Insight into Mechanisms of Chemokine Receptor Activation. J. Biol. Chem. 2007, 282, 27935–27943. [Google Scholar] [CrossRef]
- Cherney, R.J.; Anjanappa, P.; Selvakumar, K.; Batt, D.G.; Brown, G.D.; Rose, A.V.; Vuppugalla, R.; Chen, J.; Pang, J.; Xu, S.; et al. BMS-813160: A Potent CCR2 and CCR5 Dual Antagonist Selected as a Clinical Candidate. ACS Med. Chem. Lett. 2021, 12, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Gale, J.D.; Gilbert, S.; Blumenthal, S.; Elliott, T.; Pergola, P.E.; Goteti, K.; Scheele, W.; Perros-Huguet, C. Effect of PF-04634817, an Oral CCR2/5 Chemokine Receptor Antagonist, on Albuminuria in Adults with Overt Diabetic Nephropathy. Kidney Int. Rep. 2018, 3, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.L.; O’Hayre, M.; Handel, T. Modulation of Chemokine Receptor Activity through Dimerization and Crosstalk. Cell. Mol. Life Sci. 2009, 66, 1370–1386. [Google Scholar] [CrossRef]
- Pranzatelli, M.R. Advances in Biomarker-Guided Therapy for Pediatric- and Adult-Onset Neuroinflammatory Disorders: Targeting Chemokines/Cytokines. Front. Immunol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. Macrophage Inflammatory Protein-1alpha Mediates the Development of Neuropathic Pain Following Peripheral Nerve Injury through Interleukin-1beta up-Regulation. Pain 2010, 149, 305–315. [Google Scholar] [CrossRef]
- Thompson, M.; Saag, M.; Dejesus, E.; Gathe, J.; Lalezari, J.; Landay, A.L.; Cade, J.; Enejosa, J.; Lefebvre, E.; Feinberg, J. A 48-Week Randomized Phase 2b Study Evaluating Cenicriviroc versus Efavirenz in Treatment-Naive HIV-Infected Adults with C-C Chemokine Receptor Type 5-Tropic Virus. AIDS 2016, 30, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Bertini, R.; Allegretti, M.; Bizzarri, C.; Moriconi, A.; Locati, M.; Zampella, G.; Cervellera, M.N.; Di Cioccio, V.; Cesta, M.C.; Galliera, E.; et al. Noncompetitive Allosteric Inhibitors of the Inflammatory Chemokine Receptors CXCR1 and CXCR2: Prevention of Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2004, 101, 11791–11796. [Google Scholar] [CrossRef]
- Meesawatsom, P.; Hathway, G.; Bennett, A.; Constantin-Teodosiu, D.; Chapman, V. Spinal Neuronal Excitability and Neuroinflammation in a Model of Chemotherapeutic Neuropathic Pain: Targeting the Resolution Pathways. J. Neuroinflamm. 2020, 17, 316. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Tomonaga, D.; Kitazono, K.; Yoshioka, Y.; Liu, J.; Rousseau, J.P.; Kinkead, R.; Ruff, M.R.; Pert, C.B. Neuropathic Pain Inhibitor, RAP-103, Is a Potent Inhibitor of Microglial CCL1/CCR8. Neurochem. Int. 2018, 119, 184–189. [Google Scholar] [CrossRef]
- Bongiovanni, A.R.; Zhao, P.; Inan, S.; Wiah, S.; Shekarabi, A.; Farkas, D.J.; Watson, M.N.; Wimmer, M.E.; Ruff, M.R.; Rawls, S.M. Multi-Chemokine Receptor Antagonist RAP-103 Inhibits Opioid-Derived Respiratory Depression, Reduces Opioid Reinforcement and Physical Dependence, and Normalizes Opioid-Induced Dysregulation of Mesolimbic Chemokine Receptors in Rats. Drug Alcohol Depend. 2022, 238, 109556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).