Synthesis and Properties of Carbon Microspheres from Waste Office Paper
Abstract
1. Introduction
2. Results and Discussion
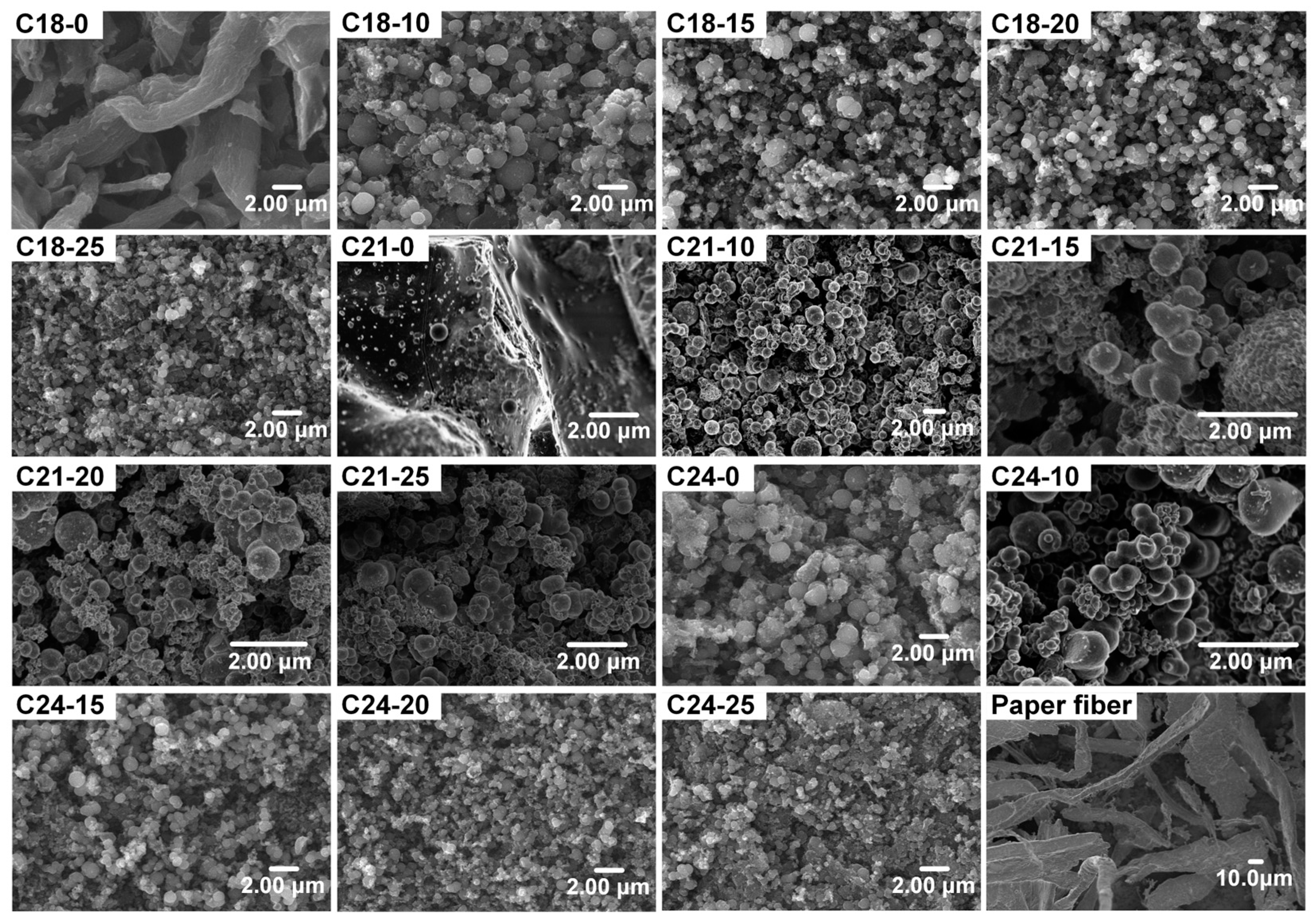
2.1. Morphology and Microstructure of Carbon Microspheres
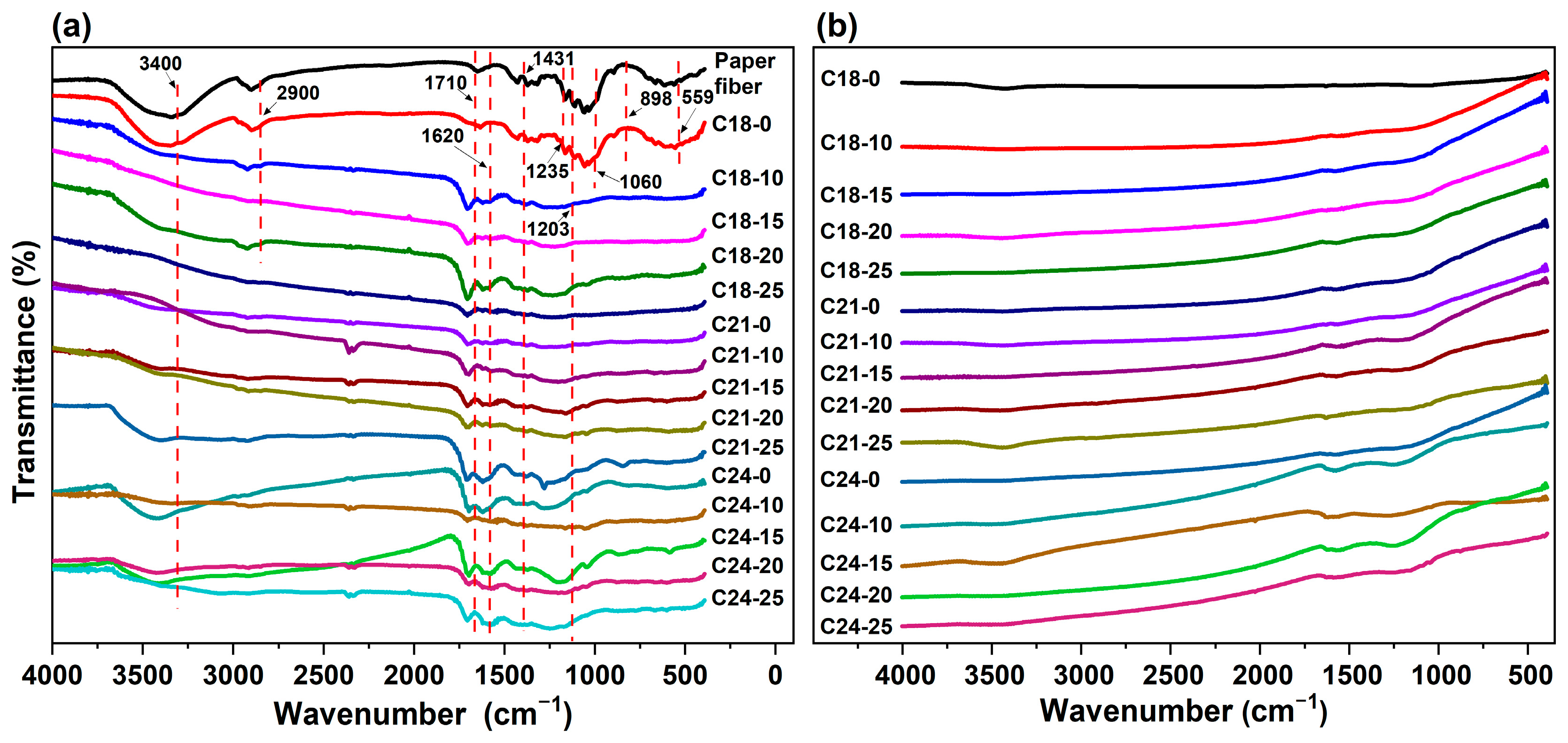
2.2. FT-IR Analysis
2.3. X-ray Photoelectron Spectroscopy Analysis
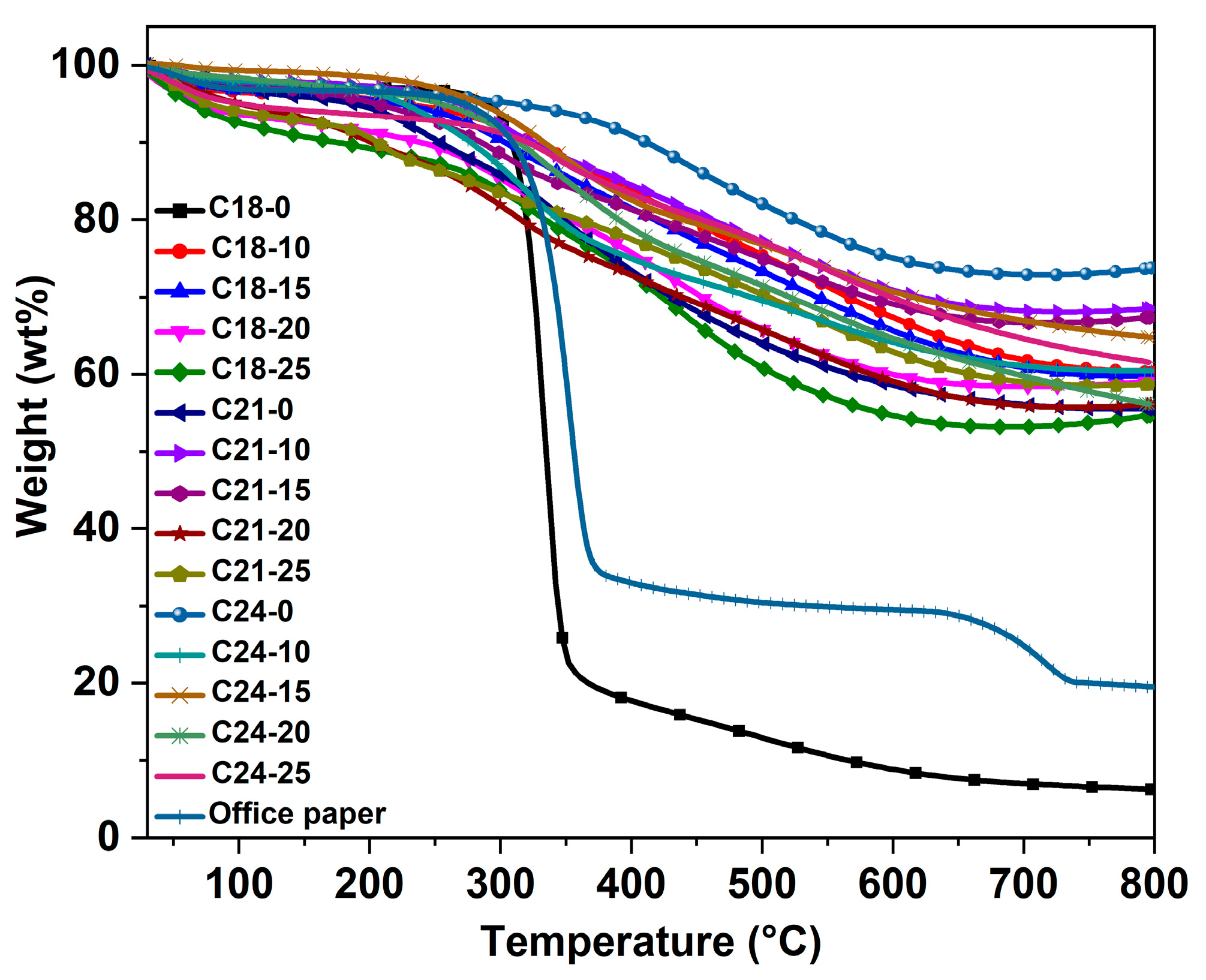
2.4. Thermogravimetric Analysis
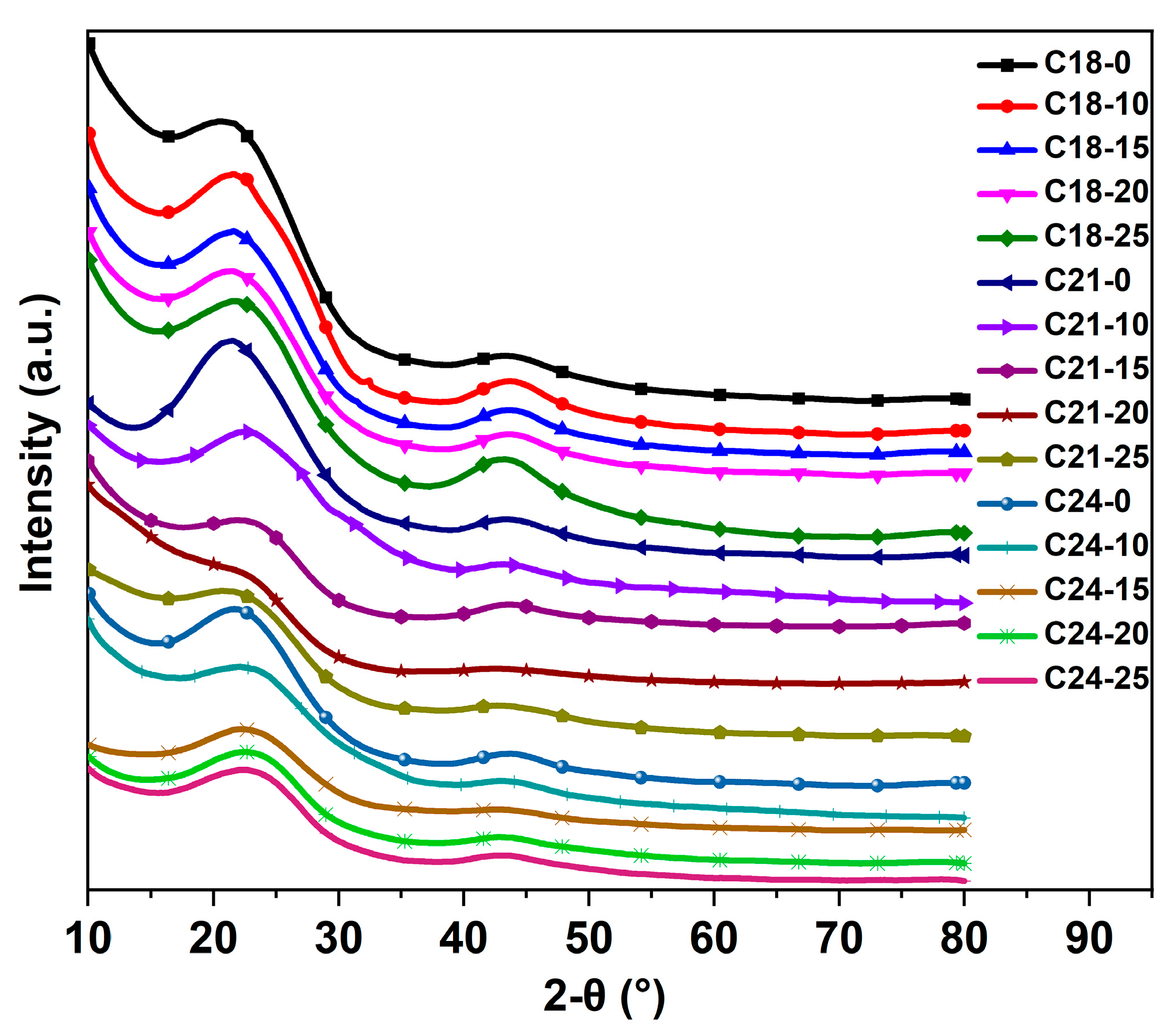
2.5. XRD Analysis
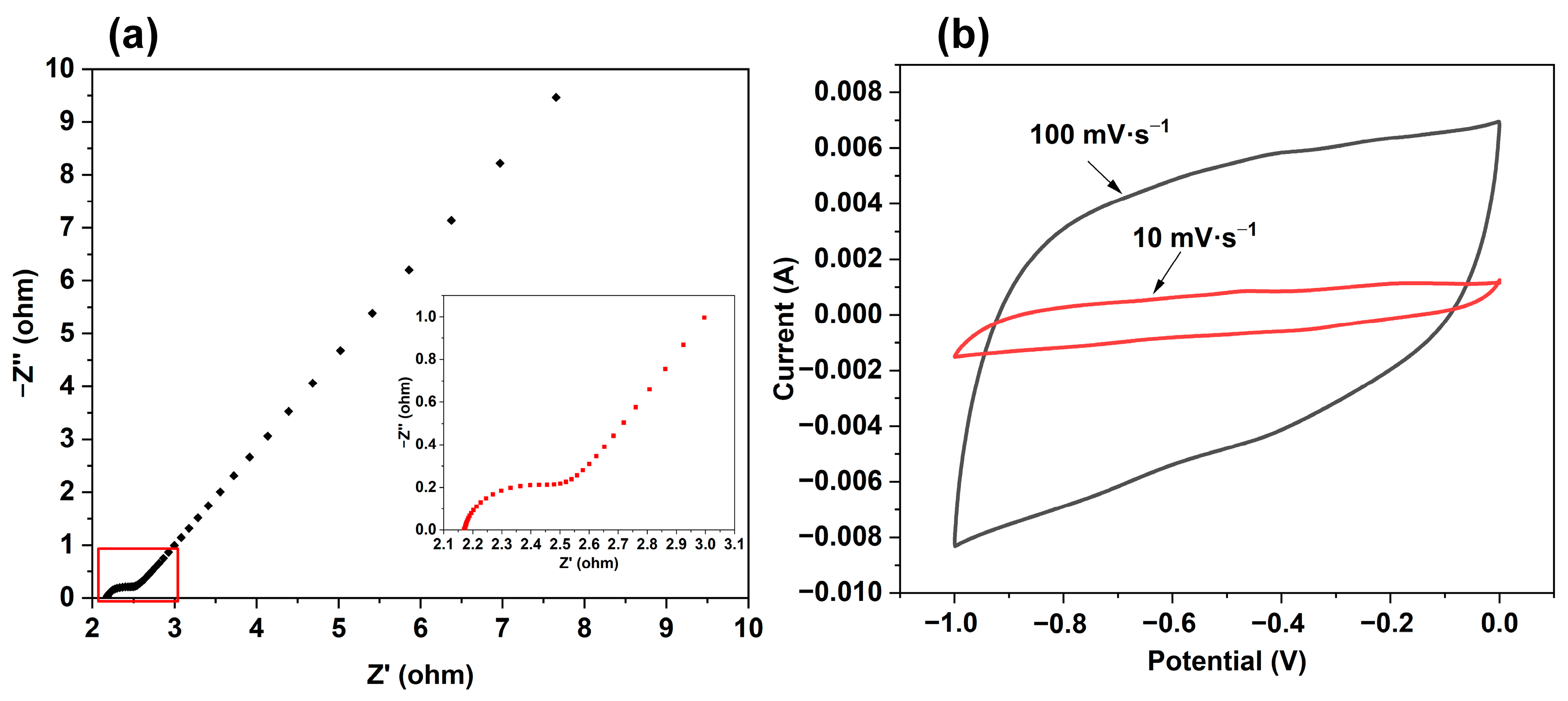
2.6. Electrochemical Performance
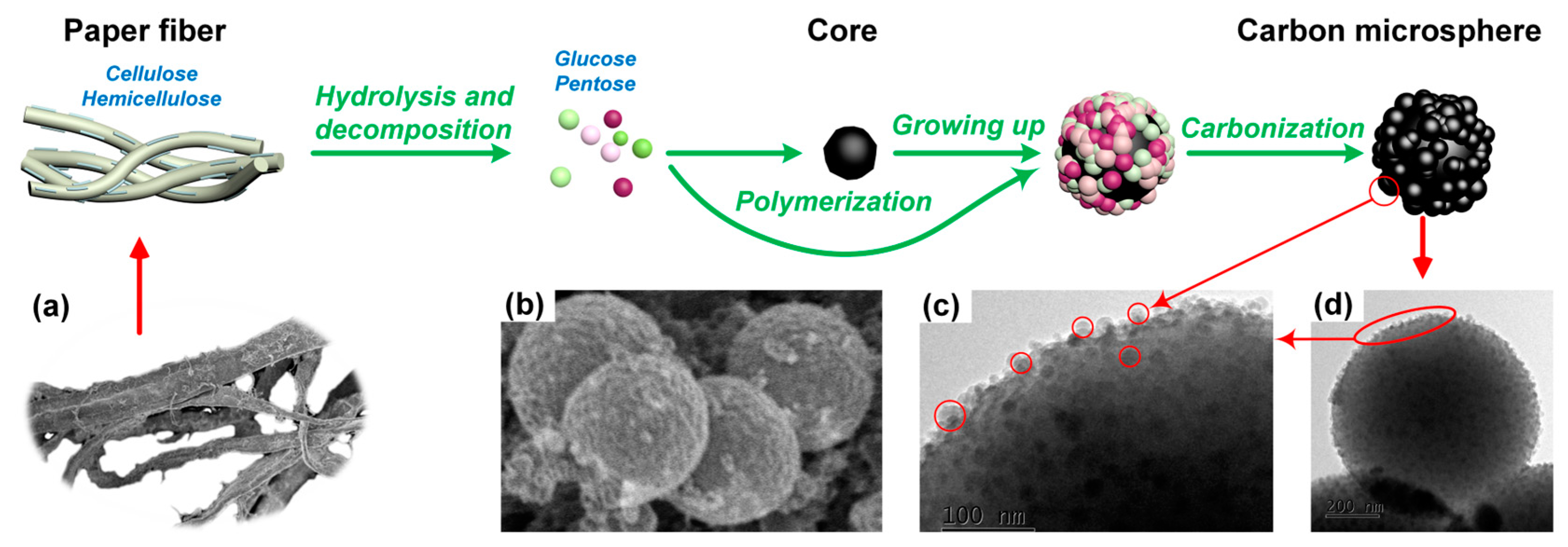
3. Formation Mechanism of Carbon Microsphere
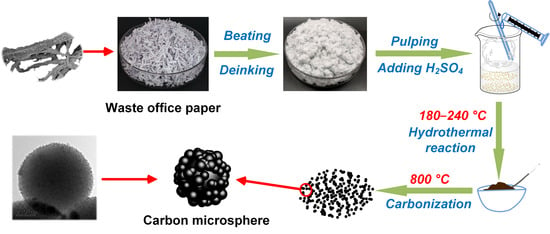
4. Materials and Methods
4.1. Materials
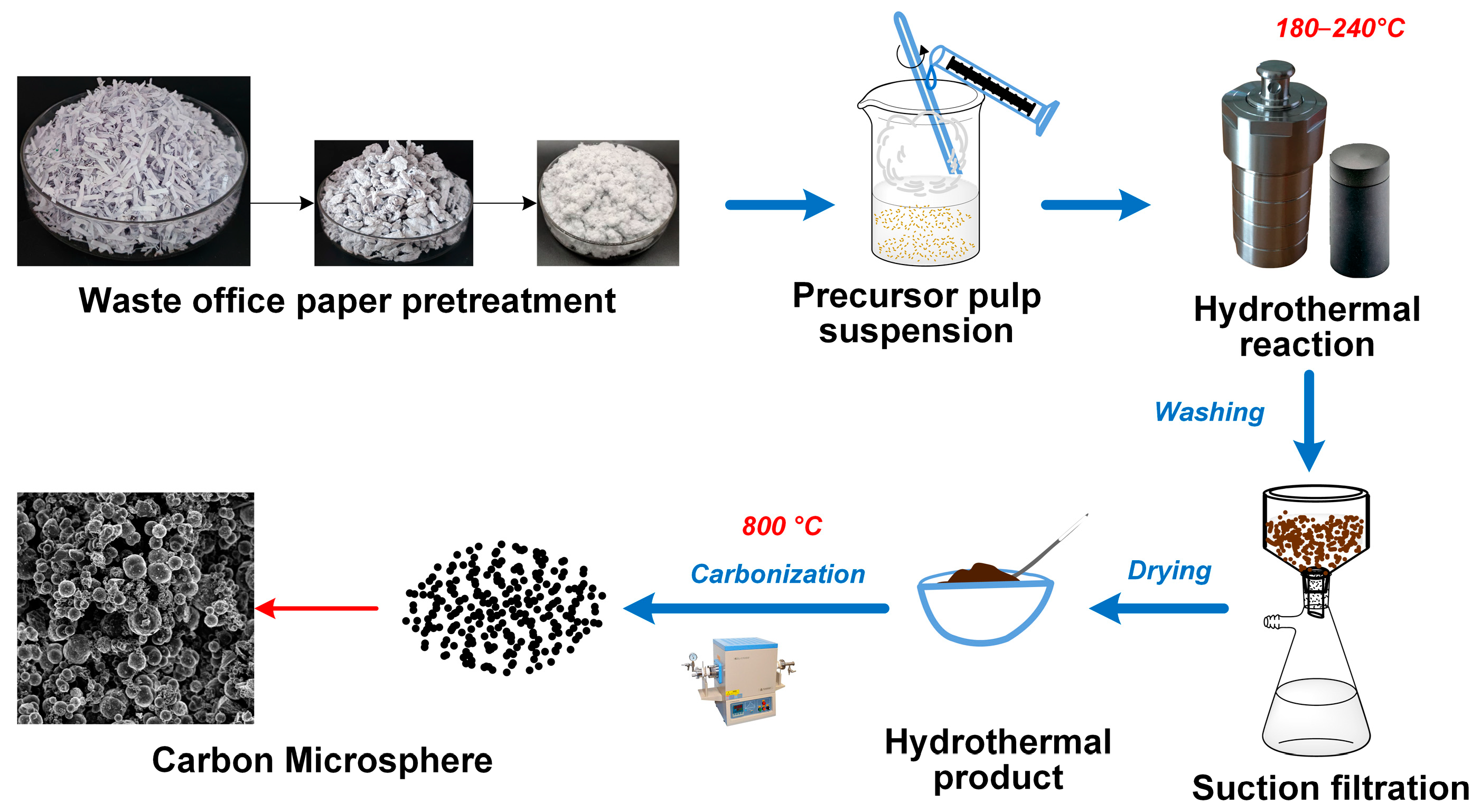
4.2. Synthesis of Carbon Microspheres
4.3. Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, Y.; Ma, Y.Y.; Dang, Z.; Hu, R.R.; Liu, C.J.; Chen, M.; Gao, L.; Lin, Y.; Wang, T.; Chen, G.; et al. The efficient absorption of electromagnetic waves by tunable N-doped multi-cavity mesoporous carbon microspheres. Carbon 2023, 201, 1115–1125. [Google Scholar] [CrossRef]
- Liu, P.; Cai, W.; Wei, J.; Cai, Z.; Zhu, M.; Han, B.; Yang, Z.; Chen, J.; Jaroniec, M. Ultrafast preparation of saccharide-derived carbon microspheres with excellent dispersibility via ammonium persulfate-assisted hydrothermal carbonization. J. Mater. Chem. A 2019, 7, 18840–18845. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Pan, Z.; Tian, J.; Lin, N.; Qian, Y. Hydrothermal “disproportionation” of biomass into oriented carbon microsphere anode and 3D porous carbon cathode for potassium ion hybrid capacitor. Adv. Funct. Mater. 2021, 31, 2103115. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Liu, Y.N.; Sun, X.D.; Zhang, D.K.; Xue, D.F. Hydrophobic precipitation of carbonaceous spheres from fructose by a hydrothermal process. Carbon 2012, 50, 2155–2161. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Shan, T.; Miao, X.Y.; Zhu, Y. A green, rapid, scalable and versatile hydrothermal strategy to fabricate monodisperse carbon spheres with tunable micrometer size and hierarchical porosity. Chem. Eng. J. 2019, 372, 1164–1173. [Google Scholar] [CrossRef]
- Yang, M.N.; Fang, C.Q.; Su, J.; Cheng, Y.L.; Zhang, Q.; Liu, M. Synthesis mechanism of carbon microsphere from waste office paper via hydrothermal method. BioResources 2022, 17, 5568–5577. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Chen, L.Q.; Huang, X.J. Monodispersed hard carbon spherules with uniform nanopores. Carbon 2001, 39, 2211–2214. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Fang, C.Q.; Yang, M.N.; Cheng, Y.L.; Wang, Z.; Huang, Z.G.; You, C.Y. A controllable soft-templating approach to synthesize mesoporous carbon microspheres derived from d-xylose via hydrothermal method. J. Mater. Sci. Technol. 2020, 38, 183–188. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Ab Rahim, M.H.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.J.; Hao, X.; Peng, P.; Shi, J.Y.; Peng, F.; Sun, R.C. Hydrothermal synthesis and applications of advanced carbonaceous materials from biomass: A review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Yu, H.; Li, J.; Liu, S. Wood-derived carbon materials and light-emitting materials. Adv. Mater. 2021, 33, 2000596. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Suh, Y.W.; Suh, D.J.; Ahn, D.J. Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds. Carbon 2010, 48, 1990–1998. [Google Scholar] [CrossRef]
- Ni, J.; Tan, Z.N.; Sai, Q.J.; Zhu, J.; Wang, X.Y.; Lin, B.Y.; Lin, J.X.; Au, C.T.; Jiang, L.L. Target-oriented confinement of Ru-Co nanoparticles inside N-doped carbon spheres via a benzoic acid guided process for high-efficient low-temperature ammonia synthesis. J. Energy Chem. 2021, 57, 140–146. [Google Scholar] [CrossRef]
- Zheng, Q.; Morimoto, M.; Fouquet, T.; Sato, H.; Takanohashi, T. Effect of hydrothermal conditions on production of coal organic microspheres. Fuel 2018, 234, 1301–1312. [Google Scholar] [CrossRef]
- Wu, T.H.; Sun, L.X.; Xu, F.; Cai, D. Nitrogen-doped hierarchical porous carbon materials derived from diethylenetriaminepentaacetic acid (DTPA) for supercapacitors. J. Mater. Sci. Technol. 2018, 34, 2384–2391. [Google Scholar] [CrossRef]
- Sun, X.M.; Li, Y.D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Sevilla, M.; Macia-Agullo, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, Y.L.; Yang, M. Preparation of corn stover hydrothermal carbon sphere-CdS/g-C3N4 composite and evaluation of its performance in the photocatalytic co-reduction of CO2 and decomposition of water for hydrogen production. J. Alloys Compd. 2023, 933, 167871. [Google Scholar] [CrossRef]
- Yang, M.N.; Fang, C.Q.; Zeng, H.L.; Su, J.; Cheng, Y.L.; Pei, L.; Liu, M. Porous carbon derived from waste corrugated paper board using different activators. J. Mater. Cycles Waste Manag. 2022, 24, 1893–1901. [Google Scholar] [CrossRef]
- Yang, M.N.; Fang, C.Q.; Su, J.; Zeng, H.L.; Lin, Q.L. Porous carbon derived from waste corrugated paper with KOH-NaOH mixture and its adsorption property for methylene blue. Carbon Lett. 2023, 33, 1205–1215. [Google Scholar] [CrossRef]
- Yang, G.Z.; Song, S.; Li, J.; Tang, Z.H.; Ye, J.Y.; Yang, J.H. Preparation and CO2 adsorption properties of porous carbon by hydrothermal carbonization of tree leaves. J. Mater. Sci. Technol. 2019, 35, 875–884. [Google Scholar] [CrossRef]
- Ji, C.; Liu, Y.; Li, Y.Y.; Su, X.L.; Xu, J.; Lu, L.L. Facile preparation and excellent microwave absorption properties of cobalt-iron/porous carbon composite materials. J. Magn. Magn. Mater. 2021, 527, 167776. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zhang, G.X.; Zhang, J.Y.; Guo, H.B.; Feng, X.; Chen, Y.G. Synthesis of porous carbon spheres derived from lignin through a facile method for high performance supercapacitors. J. Mater. Sci. Technol. 2018, 34, 2189–2196. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Liu, Z.D.; Zhang, Y.H.; Li, B.M.; Lu, H.F.; Duan, N.; Si, B.C.; Shen, R.X.; Lu, J.W. Recovery of reducing sugars and volatile fatty acids from cornstalk at different hydrothermal treatment severity. Bioresour. Technol. 2016, 199, 220–227. [Google Scholar] [CrossRef]
- Mok, W.S.; Antal, M.J.; Varhegyi, G. Productive and parasitic pathways in dilute acid-catalyzed hydrolysis of cellulose. Ind. Eng. Chem. Res. 1992, 31, 94–100. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wu, R.Q.; Zhou, Y.H.; Lin, Q.L.; Fang, C.Q. A novel surface-oxidized rigid carbon foam with hierarchical macro-nanoporous structure for efficient removal of malachite green and lead ion. J. Mater. Sci. Technol. 2022, 103, 15–28. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
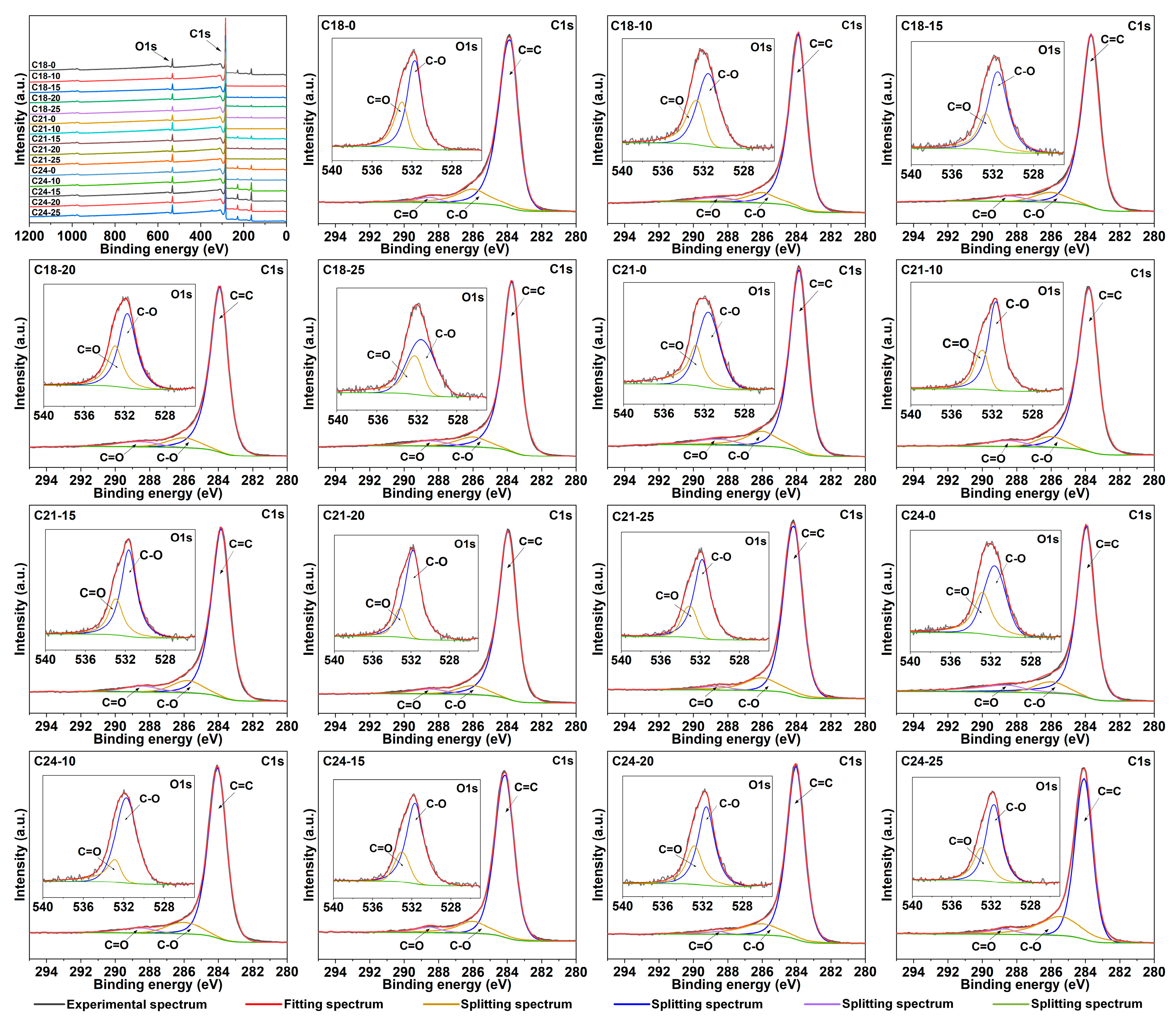

| Samples | C | O (C=O) | O (C-O) |
|---|---|---|---|
| C18-0 | 94.15 | 1.94 | 3.91 |
| C18-10 | 97.27 | 0.97 | 1.76 |
| C18-15 | 96.91 | 0.95 | 2.14 |
| C18-20 | 97.02 | 0.95 | 2.03 |
| C18-25 | 98.00 | 0.33 | 1.67 |
| C21-0 | 97.27 | 0.76 | 1.97 |
| C21-10 | 95.94 | 1.26 | 2.80 |
| C21-15 | 96.19 | 1.15 | 2.66 |
| C21-20 | 96.56 | 0.78 | 2.66 |
| C21-25 | 95.41 | 1.20 | 3.39 |
| C24-0 | 97.31 | 1.13 | 1.56 |
| C24-10 | 96.60 | 1.00 | 2.40 |
| C24-15 | 95.39 | 1.16 | 3.45 |
| C24-20 | 96.52 | 1.28 | 2.20 |
| C24-25 | 94.92 | 1.58 | 3.50 |
| Samples | D (002) | D (10) |
|---|---|---|
| C18-0 | 29 | 36 |
| C18-10 | 28 | 39 |
| C18-15 | 33 | 38 |
| C18-20 | 24 | 35 |
| C18-25 | 25 | 26 |
| C21-0 | 16 | 37 |
| C21-10 | 21 | 33 |
| C21-15 | 24 | 28 |
| C21-20 | 19 | 26 |
| C21-25 | 18 | 21 |
| C24-0 | 20 | 39 |
| C24-10 | 21 | 33 |
| C24-15 | 17 | 34 |
| C24-20 | 18 | 27 |
| C24-25 | 16 | 28 |
| Components | Cellulose | Hemicellulose | Lignin | Ash-Inorganic Salt | Other |
|---|---|---|---|---|---|
| Content | 65.247 | 6.706 | 0.181 | 0.676 | 27.385 |
| Samples | Temperature (°C) | H2SO4 (mL) |
|---|---|---|
| C18-0 | 180 | 0 |
| C18-10 | 180 | 10 |
| C18-15 | 180 | 15 |
| C18-20 | 180 | 20 |
| C18-25 | 180 | 25 |
| C21-0 | 210 | 0 |
| C21-10 | 210 | 10 |
| C21-15 | 210 | 15 |
| C21-20 | 210 | 20 |
| C21-25 | 210 | 25 |
| C24-0 | 240 | 0 |
| C24-10 | 240 | 10 |
| C24-15 | 240 | 15 |
| C24-20 | 240 | 20 |
| C24-25 | 240 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Su, J.; Fang, C.; Cheng, Y.; Li, Y.; Yan, Y.; Lei, W. Synthesis and Properties of Carbon Microspheres from Waste Office Paper. Molecules 2023, 28, 5756. https://doi.org/10.3390/molecules28155756
Yang M, Su J, Fang C, Cheng Y, Li Y, Yan Y, Lei W. Synthesis and Properties of Carbon Microspheres from Waste Office Paper. Molecules. 2023; 28(15):5756. https://doi.org/10.3390/molecules28155756
Chicago/Turabian StyleYang, Mannan, Jian Su, Changqing Fang, Youliang Cheng, Yangyang Li, Yubo Yan, and Wanqing Lei. 2023. "Synthesis and Properties of Carbon Microspheres from Waste Office Paper" Molecules 28, no. 15: 5756. https://doi.org/10.3390/molecules28155756
APA StyleYang, M., Su, J., Fang, C., Cheng, Y., Li, Y., Yan, Y., & Lei, W. (2023). Synthesis and Properties of Carbon Microspheres from Waste Office Paper. Molecules, 28(15), 5756. https://doi.org/10.3390/molecules28155756