Solubility and Solvation Properties of Pharmaceutically Active Ionic Liquid Benzocainium Ibuprofenate in Natural Deep Eutectic Solvent Menthol–Lauric Acid
Abstract
1. Introduction
2. Results and Discussion
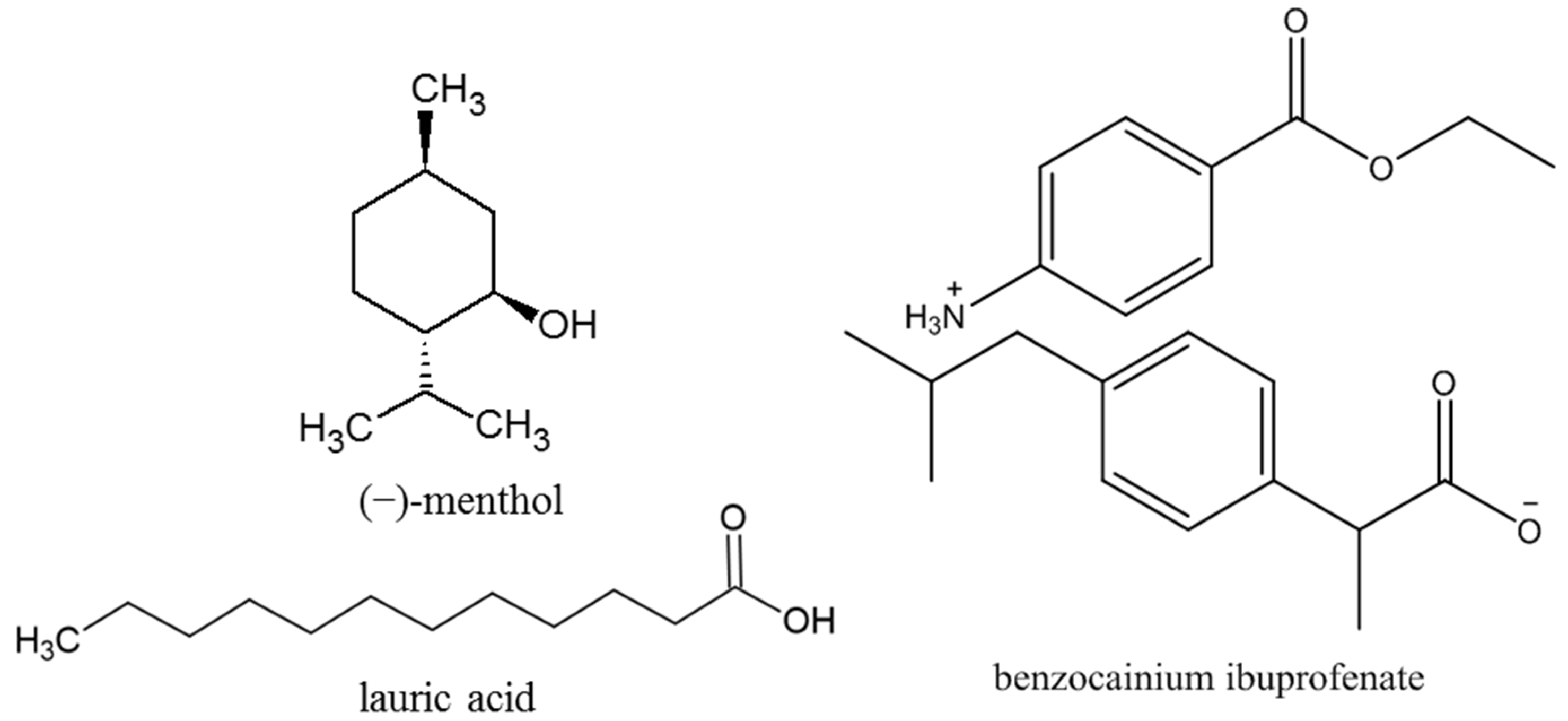
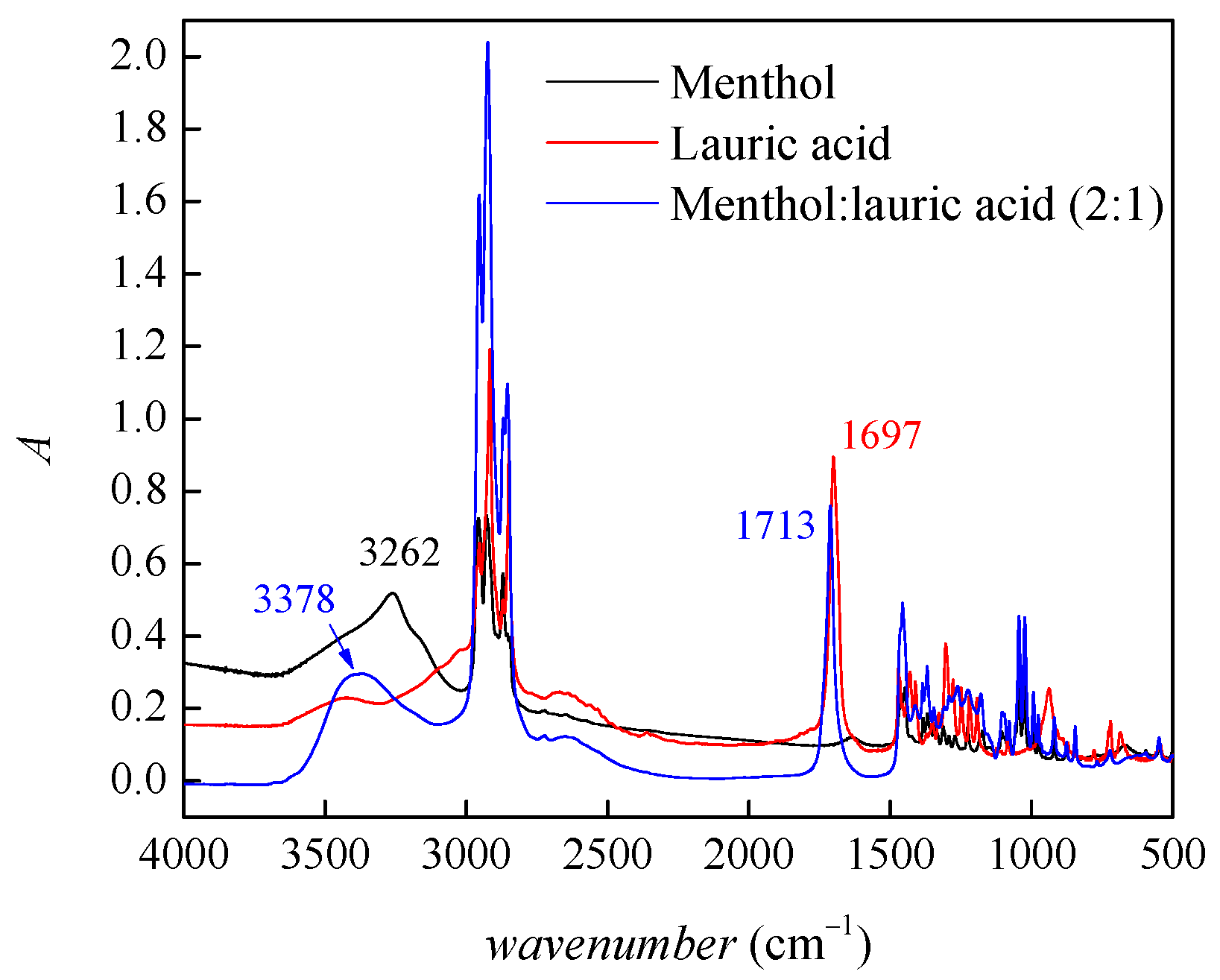
2.1. Characterization of Prepared Solvent
2.2. Solubility Determination
2.3. Volumetric and Viscosity Studies
3. Materials and Methods
3.1. Solvent Preparation
3.2. Spectroscopy Analysis
3.3. Solubility Determination
3.4. Density and Viscosity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 60, 264–300. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Row, K.H. Development of Deep Eutectic Solvents for Sustainable Chemistry. J. Mol. Liq. 2022, 362, 119654. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Mjalli, F.S.; AlNashef, I.M.; Hashim, M.A.; Hussain, M.A.; Bahadori, L.; Low, C.T.J. Prospects of Applying Ionic Liquids and Deep Eutectic Solvents for Renewable Energy Storage by Means of Redox Flow Batteries. Renew. Sustain. Energ. Rev. 2014, 30, 254–270. [Google Scholar] [CrossRef]
- Ali, S.A.; Mulk, W.U.; Ullah, Z.; Khan, H.; Zahid, A.; Shah, M.U.H.; Shah, S.N. Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review. Energies 2022, 15, 9098. [Google Scholar] [CrossRef]
- Azmi, S.; Koudahi, M.F.; Frackowiak, E. Reline Deep Eutectic Solvent as a Green Electrolyte for Electrochemical Energy Storage Applications. Energy Environ. Sci. 2022, 15, 1156–1171. [Google Scholar] [CrossRef]
- Nie, L.; Toufouki, S.; Yao, S.; Guo, D. Rethinking the Applications of Ionic Liquids and Deep Eutectic Solvents in Innovative Nano-Sorbents. Front. Chem. 2021, 9, 653238. [Google Scholar] [CrossRef]
- Sakhno, T.V.; Barashkov, N.N.; Irgibaeva, I.S.; Mendigaliyeva, S.; Bostan, D.S. Ionic Liquids and Deep Eutectic Solvents and Their Use for Dissolving Animal Hair. Adv. Chem. Eng. Sci. 2019, 10, 40–51. [Google Scholar] [CrossRef]
- Santos, M.M.; Branco, L.C. Ionic Liquids and Deep Eutectic Solvents for Application in Pharmaceutics. Pharmaceutics 2020, 12, 909. [Google Scholar] [CrossRef]
- Hayyan, M. Versatile Applications of Deep Eutectic Solvents in Drug Discovery and Drug Delivery Systems: Perspectives and Opportunities. Asian J. Pharm. Sci. 2023, 18, 100780. [Google Scholar] [CrossRef] [PubMed]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: A position paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 2nd ed.; Chapman & Hall: New York, NY, USA, 1988. [Google Scholar]
- Karpinski, P.H. Polymorphism of active pharmaceutical ingredients. Chem. Eng. Technol. 2006, 29, 233–238. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodriguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Panić, J.; Papović, S.; Šarac, B.; Cerc Korošec, R.; Rapaić, M.; Gadžurić, S.; Bešter-Rogač, M.; Vraneš, M. Active pharmaceutical ingredient ionic liquid in natural deep eutectic solvents—Influence of solvent composition. J. Mol. Liq. 2023, 384, 122282. [Google Scholar] [CrossRef]
- Lajoie, L.; Fabiano-Tixier, A.S.; Chemat, F. Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals 2022, 15, 1507. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S.L. Drug Solubility Enhancement through the Preparation of Multicomponent Organic Materials: Eutectics of Lovastatin with Carboxylic Acids. Pharmaceutics 2019, 11, 112. [Google Scholar] [CrossRef]
- Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. MedChemComm 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Taylor, R., Jr.; LeQuang, J.A.; Raffa, R.B. The role and mechanism of action of menthol in topical analgesic products. J. Clin. Pharm. Ther. 2018, 43, 313–319. [Google Scholar] [CrossRef]
- Ranke, J.; Othman, A.; Fan, P.; Müller, A. Explaining Ionic Liquid Water Solubility in Terms of Cation and Anion Hydrophobicity. Int. J. Mol. Sci. 2009, 10, 1271–1289. [Google Scholar] [CrossRef]
- Masson, D.O. XXVIII. Solute molecular volumes in relation to solvation and ionization. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1929, 8, 218–235. [Google Scholar] [CrossRef]
- Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 1929, 51, 2950–2964. [Google Scholar] [CrossRef]


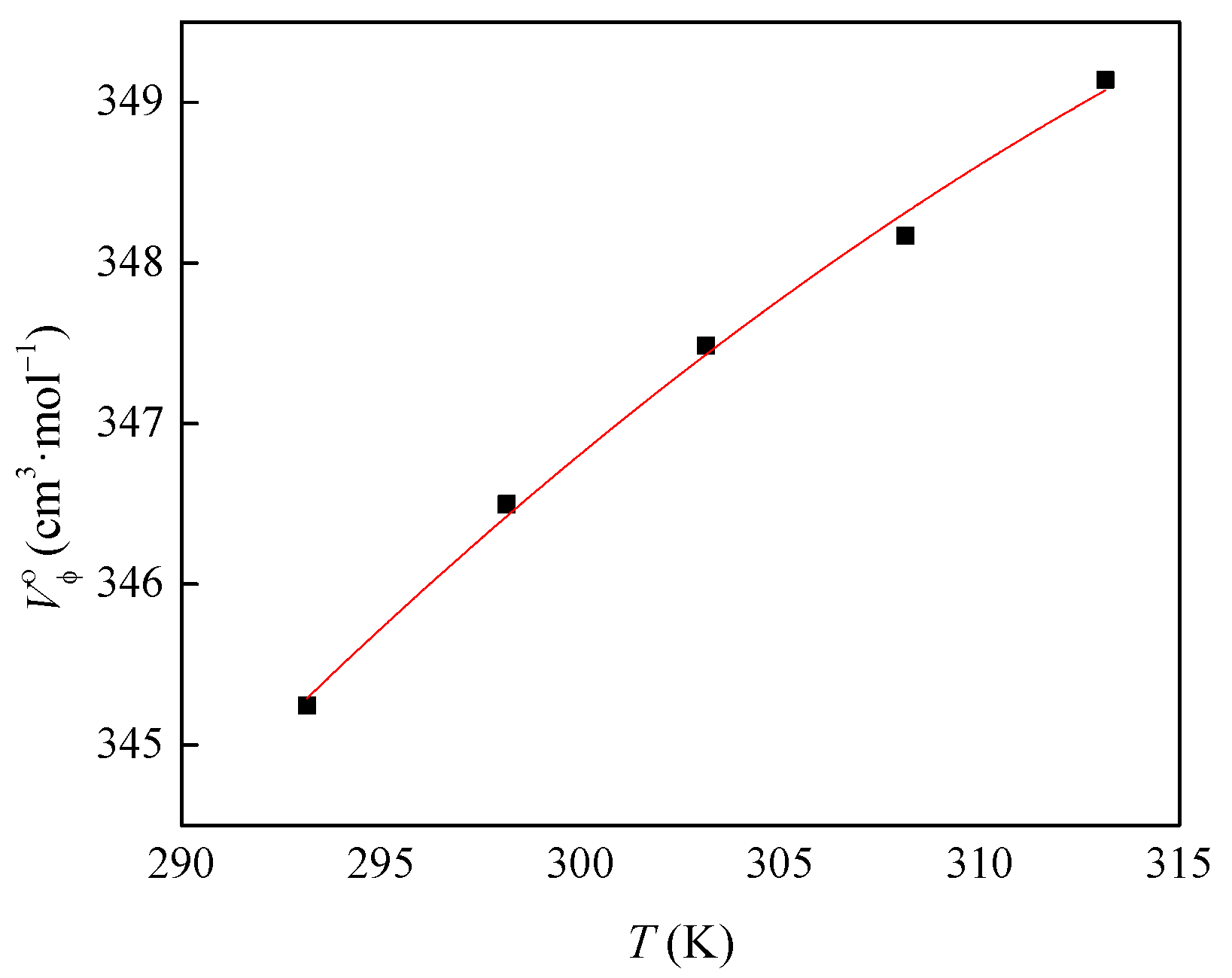


| T (K) | 293.15 | 298.15 | 303.15 | 308.15 | 313.15 |
| Vϕo (cm3·mol−1) | 345.24 | 346.50 | 347.49 | 348.17 | 349.14 |
| SV (cm3·dm3/2·mol−3/2) | 8.20 | 8.87 | 9.46 | 10.19 | 11.37 |
| Eφo (cm3·mol−1·K−1) | 0.2390 | 0.2142 | 0.1893 | 0.1645 | 0.1396 |
| A (dm3/2·mol−1/2) | −0.0913 | −0.0703 | −0.0690 | −0.0865 | −0.0951 |
| B (dm3∙mol−1) | 0.9727 | 0.8494 | 0.7960 | 0.7820 | 0.7619 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panić, J.; Rapaić, M.; Gadžurić, S.; Vraneš, M. Solubility and Solvation Properties of Pharmaceutically Active Ionic Liquid Benzocainium Ibuprofenate in Natural Deep Eutectic Solvent Menthol–Lauric Acid. Molecules 2023, 28, 5723. https://doi.org/10.3390/molecules28155723
Panić J, Rapaić M, Gadžurić S, Vraneš M. Solubility and Solvation Properties of Pharmaceutically Active Ionic Liquid Benzocainium Ibuprofenate in Natural Deep Eutectic Solvent Menthol–Lauric Acid. Molecules. 2023; 28(15):5723. https://doi.org/10.3390/molecules28155723
Chicago/Turabian StylePanić, Jovana, Maksim Rapaić, Slobodan Gadžurić, and Milan Vraneš. 2023. "Solubility and Solvation Properties of Pharmaceutically Active Ionic Liquid Benzocainium Ibuprofenate in Natural Deep Eutectic Solvent Menthol–Lauric Acid" Molecules 28, no. 15: 5723. https://doi.org/10.3390/molecules28155723
APA StylePanić, J., Rapaić, M., Gadžurić, S., & Vraneš, M. (2023). Solubility and Solvation Properties of Pharmaceutically Active Ionic Liquid Benzocainium Ibuprofenate in Natural Deep Eutectic Solvent Menthol–Lauric Acid. Molecules, 28(15), 5723. https://doi.org/10.3390/molecules28155723







