Reactive Paper Spray Ionization Mass Spectrometry for Rapid Detection of Estrogens in Cosmetics
Abstract
1. Introduction
2. Results and Discussion
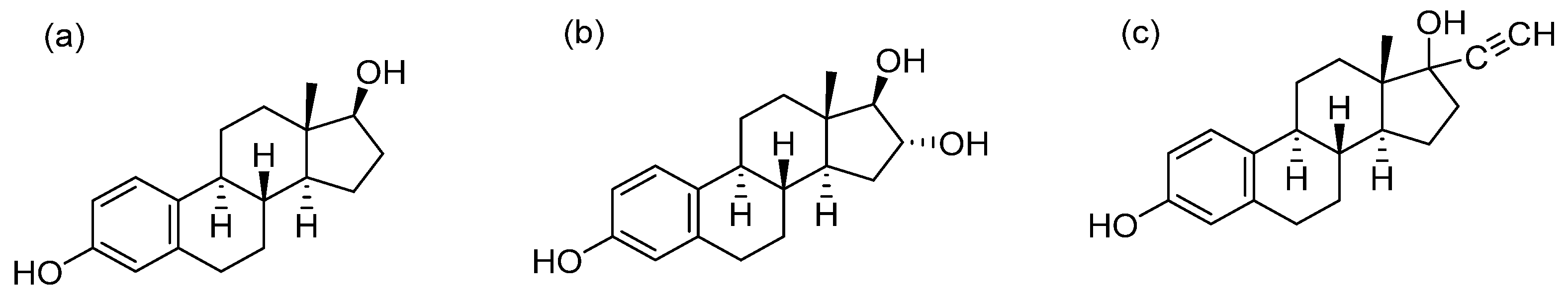
2.1. The Requirements of Online Derivatization
2.2. The Optimization of FluMP’s Concentration and Volume
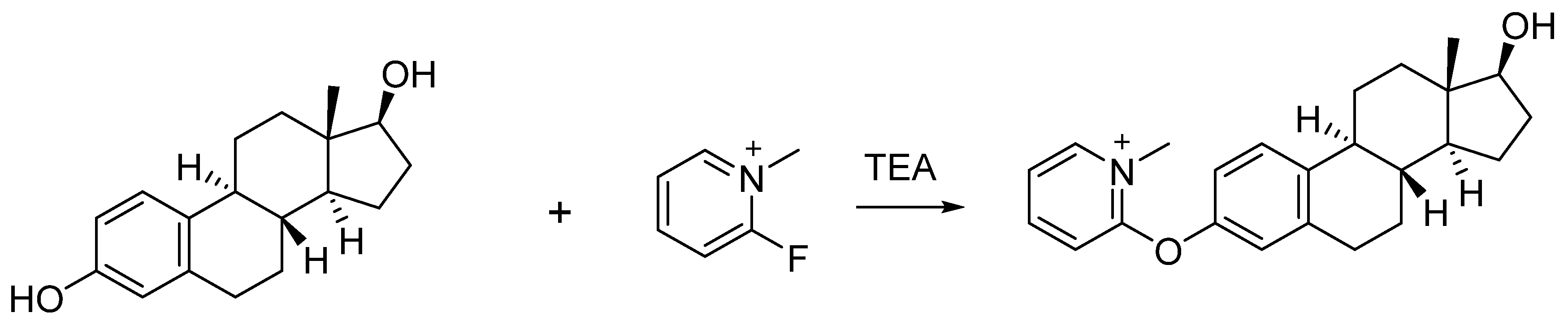
2.3. Examination of the Addition Position and Amount of Triethylamine
2.4. Optimize the Time of the Product Loaded on the Paper
2.5. Linearity, Lower Limits of Detection
2.6. Recovery
2.7. Detection Sample with Complex Matrix
2.8. Future Applications
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrument
3.3. Sample Preparation
3.4. Mass Spectrometry Parameters
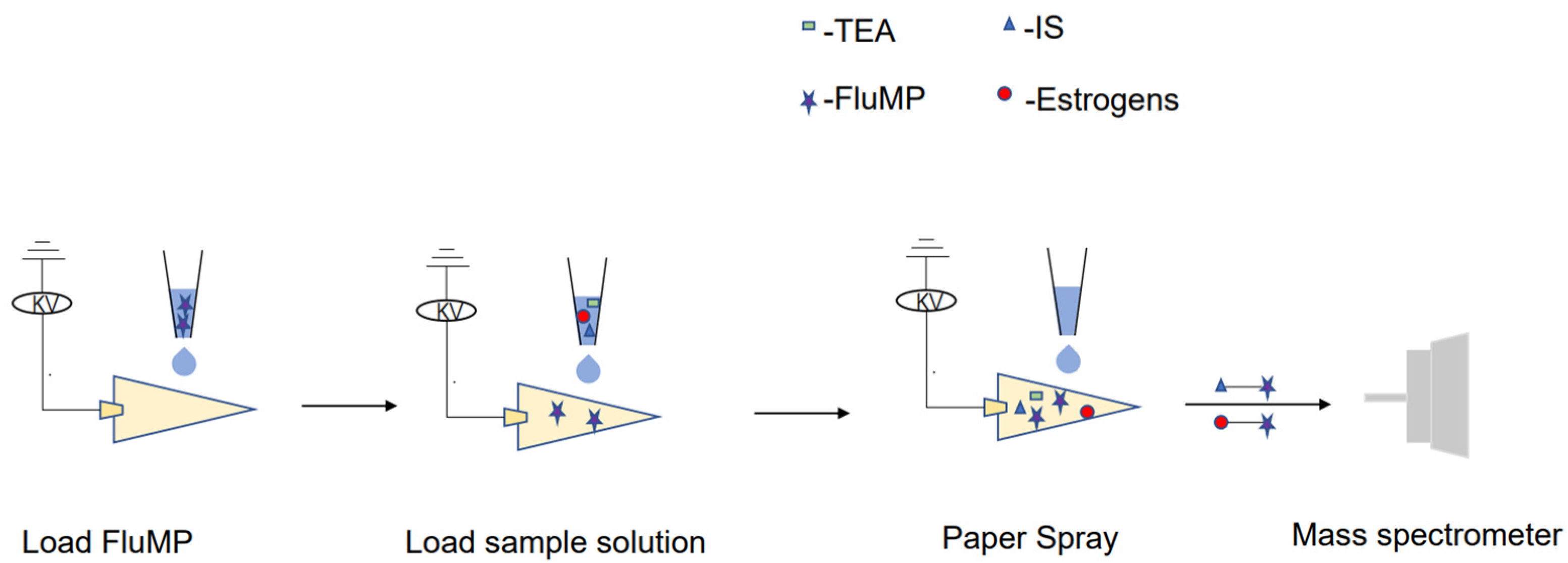
3.5. RPSI-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bahadoran, Z.; Mirmiran, P.; Azizi, F.; Ghasemi, A. A Brief History of Modern Endocrinology and Definitions of a True Hormone. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 1116–1121. [Google Scholar] [PubMed]
- Ing, N.H. Steroid Hormones Regulate Gene Expression Posttranscriptionally by Altering the Stabilities of Messenger RNAs. Biol. Reprod. 2005, 72, 1290–1296. [Google Scholar]
- Gupta, S.; McCarson, K.E.; Welch, K.M.A.; Berman, N.E.T. Mechanisms of Pain Modulation by Sex Hormones in Migraine. Headache 2011, 51, 905–922. [Google Scholar]
- Yuan, T.-F.; Le, J.; Cui, Y.; Peng, R.; Wang, S.-T.; Li, Y. An LC-MS/MS analysis for seven sex hormones in serum. J. Pharm. Biomed. Anal. 2019, 162, 34–40. [Google Scholar]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, sex hormones, and immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [PubMed]
- Siddiqui, A.N.; Siddiqui, N.; Khan, R.A.; Kalam, A.; Jabir, N.R.; Kamal, M.A.; Firoz, C.K.; Tabrez, S. Neuroprotective Role of Steroidal Sex Hormones: An Overview. CNS Neurosci. Ther. 2016, 22, 342–350. [Google Scholar]
- Vegeto, E.; Villa, A.; Torre, S.D.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [PubMed]
- Tu, D.-Z.; Wu, H.-L.; Li, Y.-N.; Zhang, J.; Li, Y.; Nie, C.-C.; Zhang, X.-H.; Yu, R.-Q. Measuring estriol and estrone simultaneously in liquid cosmetic samples using second-order calibration coupled with excitation–emission matrix fluorescence based on region selection. Anal. Methods 2012, 4, 222–229. [Google Scholar]
- Komori, S.; Ito, Y.; Nakamura, Y.; Aoki, M.; Takashi, T.; Kinuta, T.; Tanaka, H.; Koyama, K. A long-term user of cosmetic cream containing estrogen developed breast cancer and endometrial hyperplasia. Menopause 2008, 15, 1191–1192. [Google Scholar]
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products[S]; The European Parliament and of the Council: Brussels, Belgium, 2009.
- Hubinger, J.C. Determination of estriol, estradiol, estrone, and progesterone in cosmetic products. J. Cosmet. Sci. 2015, 66, 113–128. [Google Scholar] [PubMed]
- De Orsi, D.; Pellegrini, M.; Pichini, S.; Mattioli, D.; Marchei, E.; Gagliardi, L. High-performance liquid chromatography–diode array and electrospray-mass spectrometry analysis of non-allowed substances in cosmetic products for preventing hair loss and other hormone-dependent skin diseases. J. Pharm. Biomed. Anal. 2008, 48, 641–648. [Google Scholar] [PubMed]
- Takats, Z.; Wiseman, J.; Gologan, B.; Cooks, R. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar]
- Wang, H.; Liu, J.; Cooks, R.; Ouyang, Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew. Chem. 2010, 122, 889–892. [Google Scholar]
- Zhou, X.; Pei, J.; Huang, G. Reactive paper spray mass spectrometry for in situ identification of quinones. Rapid Commun. Mass. Spectrom. 2015, 29, 100–106. [Google Scholar]
- Mazzotti, F.; Di Donna, L.; Taverna, D.; Nardi, M.; Aiello, D.; Napoli, A.; Sindona, G. Evaluation of dialdehydic anti-inflammatory active principles in extra-virgin olive oil by reactive paper spray mass spectrometry. Int. J. Mass. Spectrom. 2013, 352, 87–91. [Google Scholar]
- Yan, X.; Augusti, R.; Li, X.; Cooks, R.G. Chemical Reactivity Assessment Using Reactive Paper Spray Ionization Mass Spectrometry: The Katritzky Reaction. ChemPlusChem 2013, 78, 1142–1148. [Google Scholar] [PubMed]
- Mueller, T.; Badu-Tawiah, A.; Cooks, R.G. Accelerated carbon–carbon bond-forming reactions in preparative electrospray. Angew. Chem. Int. Ed. 2012, 51, 11832–11835. [Google Scholar]
- Badu-Tawiah, A.K.; Campbell, D.I.; Cooks, R.G. Accelerated CN bond formation in dropcast thin films on ambient surfaces. J. Am. Soc. Mass. Spectrom. 2012, 23, 1461–1468. [Google Scholar]
- Espy, R.D.; Wleklinski, M.; Yan, X.; Cooks, R.G. Beyond the flask: Reactions on the fly in ambient mass spectrometry. Trends Anal. Chem. 2014, 57, 135–146. [Google Scholar]
- Quirke, J.M.E.; Hsu, Y.L.; Berkel, G.J.V. Selective detection of derivatized alcohols and phenols in essential oils by electrospray-tandem mass spectrometry. Essent. Oil Res. 2001, 13, 324–331. [Google Scholar]
- Quirke, J.M.E.; Berkel, G.J.V. Electrospray tandem mass spectrometric study of alkyl 1-methylpyridinium ether derivatives of alcohols. J. Mass. Spectrom. 2001, 36, 1294–1300. [Google Scholar] [PubMed]
- Song, D.; Yuan, S.; Zhang, C.; Luan, L.; Liu, T.; Zhang, Q. Rapid Detection of Estrogens in Cosmetics by Chemical Derivatization and Paper-Spray Ionization Mass-Spectrometry. Molecules 2023, 28, 1130. [Google Scholar] [PubMed]
- Beasley, E.; Francese, S.; Bassindale, T. Detection and Mapping of Cannabinoids in Single Hair Samples through Rapid Derivatization and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 10328–10334. [Google Scholar] [PubMed]
- Thieme, D.; Sachs, H.; Thevis, M. Formation of the N-methylpyridinium derivative to improve the detection of buprenorphine by liquid chromatography-mass spectrometry. J. Mass. Spectrom. 2008, 43, 974–979. [Google Scholar]
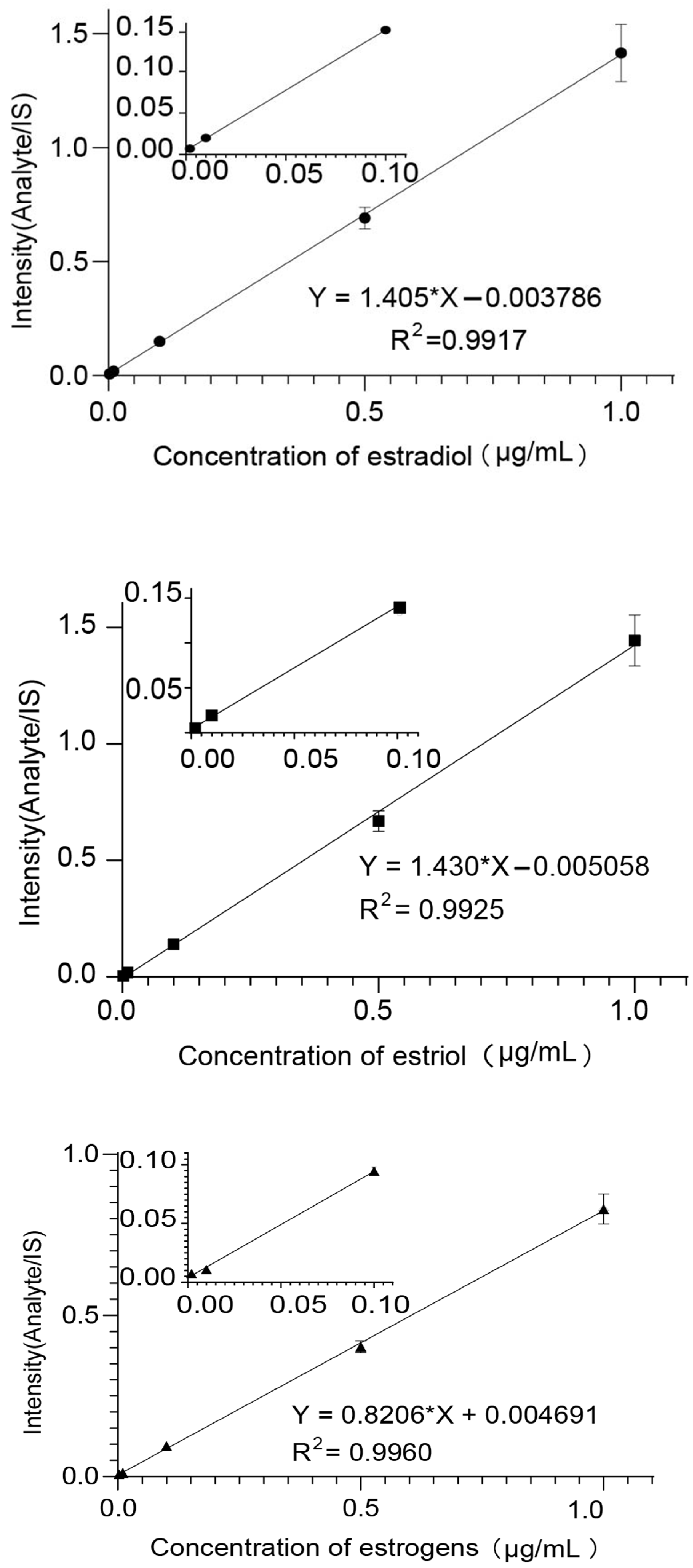
| Compound | Linearity Range | Linear Regression | LOD (μg/mL) | R2 |
|---|---|---|---|---|
| Estradiol | 0.002~1 μg/mL | Y = 1.405x − 0.003786 | 0.001 | 0.9917 |
| Estriol | 0.002~1 μg/mL | Y = 1.430x − 0.005058 | 0.001 | 0.9925 |
| Ethinyloestradiol | 0.002~1 μg/mL | Y = 0.8206x − 0.004691 | 0.001 | 0.9960 |
| Estradiol | Estriol | Ethinyloestradiol | ||||||
|---|---|---|---|---|---|---|---|---|
| Addition (μg/g, n = 3) | Detection (μg/g, n = 3) | RSD (%, n = 3) | Addition (μg/g, n = 3) | Detection (μg/g, n = 3) | RSD (%, n = 3) | Addition (μg/g, n = 3) | Detection (μg/g, n = 3) | RSD (%, n = 3) |
| 0.1 | 0.11 | 5.9 | 0.1 | 0.11 | 5.1 | 0.1 | 0.08 | 3.6 |
| 1 | 0.95 | 9.0 | 1 | 0.97 | 9.8 | 1 | 0.80 | 2.1 |
| 10 | 8.57 | 2.3 | 10 | 8.30 | 8.0 | 10 | 8.50 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Liu, J.; Liu, Y. Reactive Paper Spray Ionization Mass Spectrometry for Rapid Detection of Estrogens in Cosmetics. Molecules 2023, 28, 5675. https://doi.org/10.3390/molecules28155675
Song D, Liu J, Liu Y. Reactive Paper Spray Ionization Mass Spectrometry for Rapid Detection of Estrogens in Cosmetics. Molecules. 2023; 28(15):5675. https://doi.org/10.3390/molecules28155675
Chicago/Turabian StyleSong, Dongning, Jing Liu, and Yang Liu. 2023. "Reactive Paper Spray Ionization Mass Spectrometry for Rapid Detection of Estrogens in Cosmetics" Molecules 28, no. 15: 5675. https://doi.org/10.3390/molecules28155675
APA StyleSong, D., Liu, J., & Liu, Y. (2023). Reactive Paper Spray Ionization Mass Spectrometry for Rapid Detection of Estrogens in Cosmetics. Molecules, 28(15), 5675. https://doi.org/10.3390/molecules28155675






