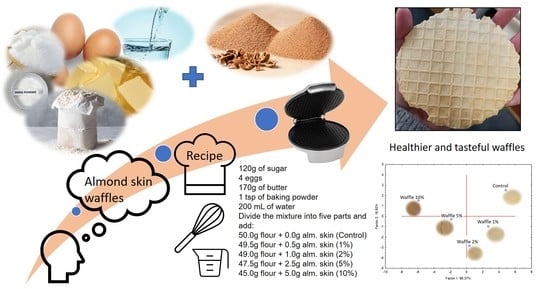
Chemical and Sensory Properties of Waffles Supplemented with Almond Skins
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Bioactive Compound Content and Antioxidant Activity
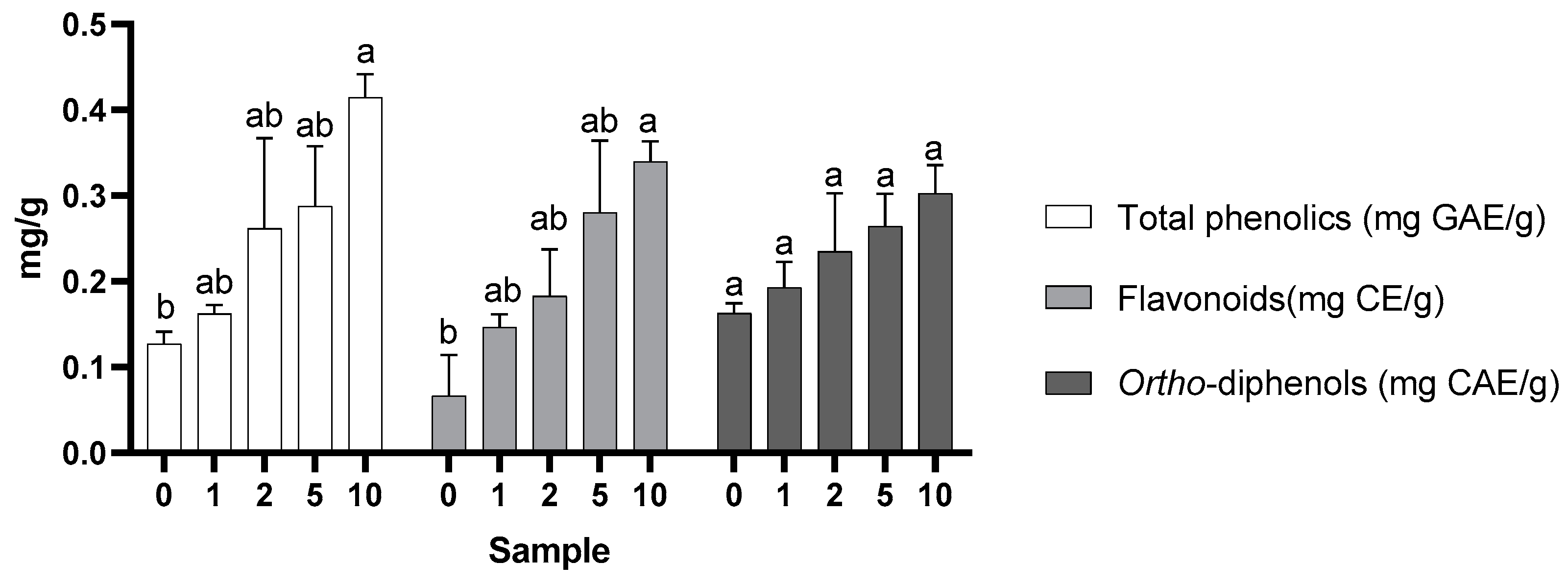
2.1.1. Total Phenol, Flavonoids, and Ortho-Diphenols Content Analysis
2.1.2. Analysis of Antioxidant Capacity by the DPPH, ABTS, and FRAP Methods
2.2. Quantification of Starch and Soluble Sugars
2.3. Texture Evaluation
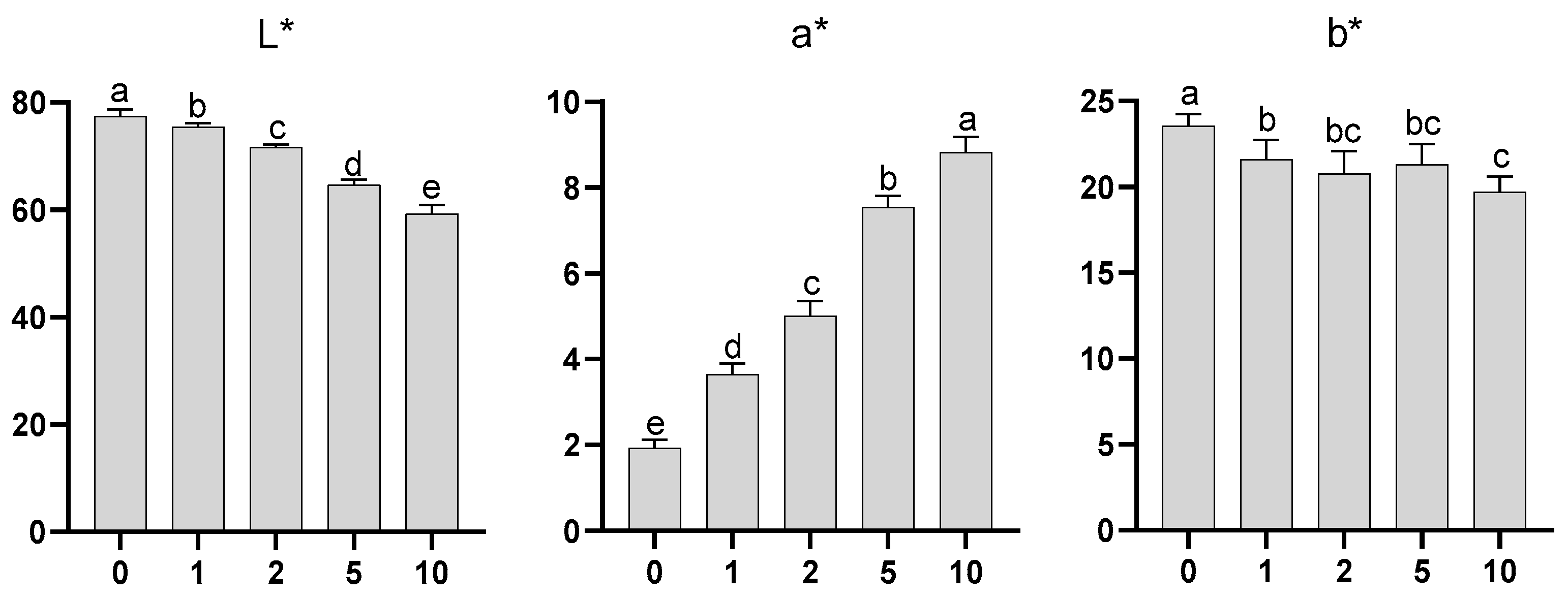
2.4. Instrumental Colour Evaluation
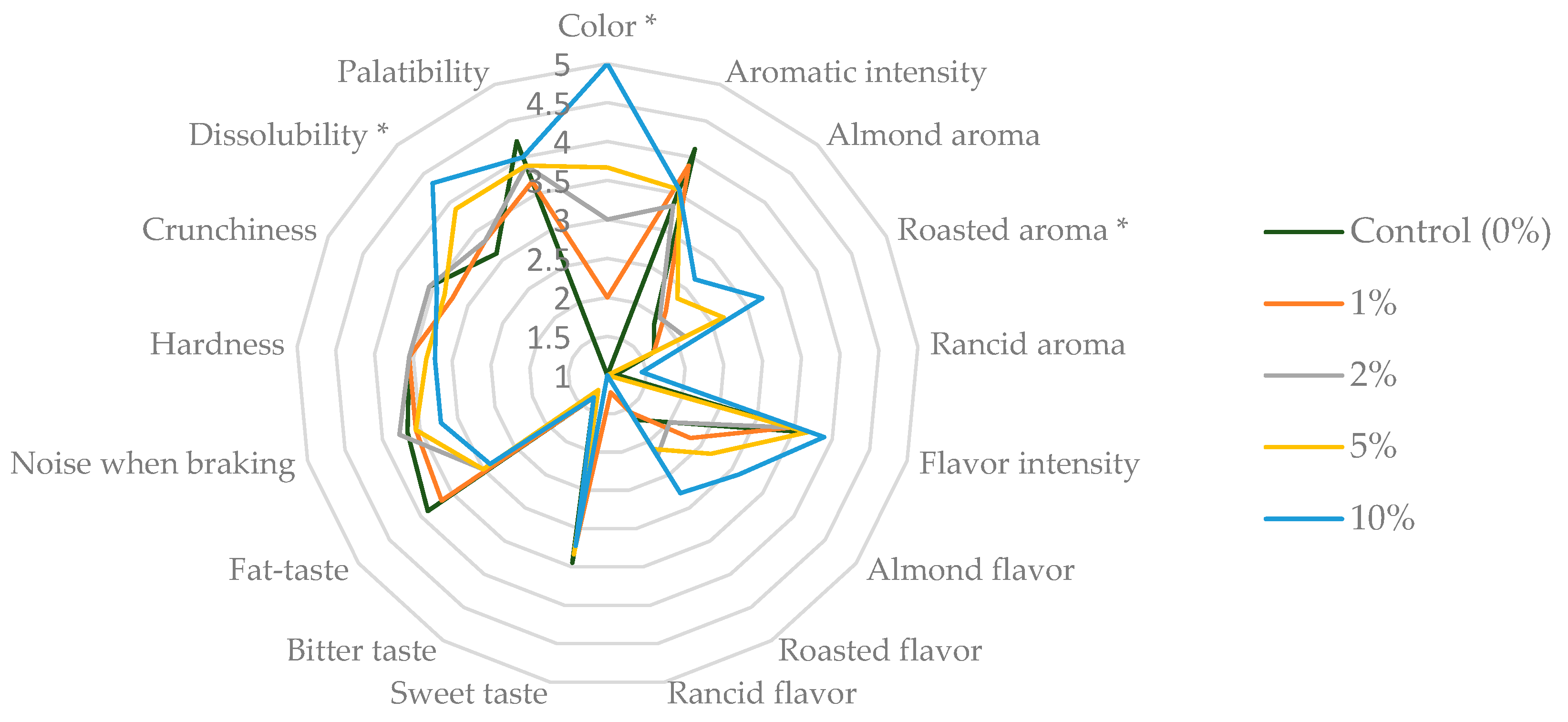
2.5. Waffle Sensory Profile
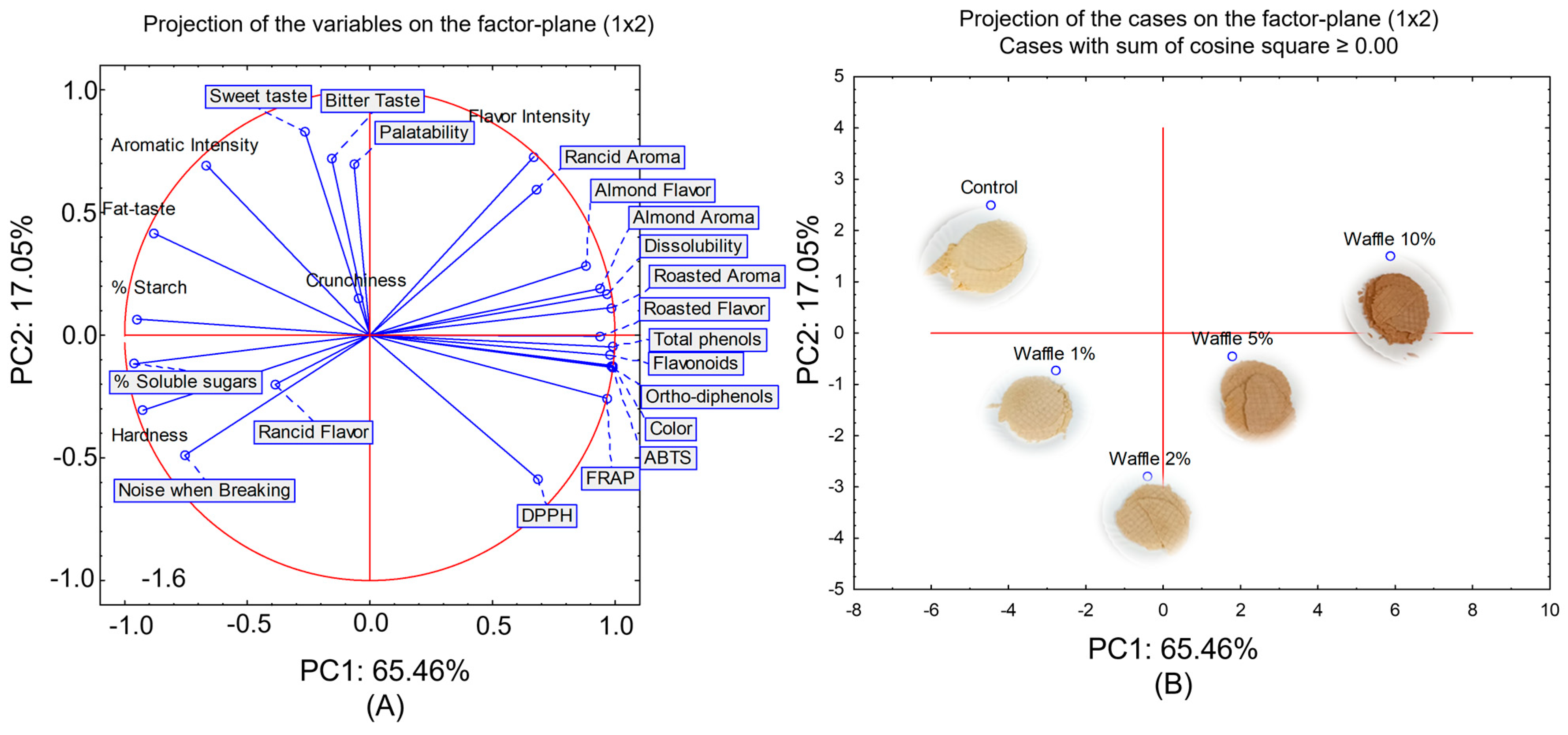
2.6. Chemical and Sensory Parameters Correlation by PCA Analysis
3. Materials and Methods
3.1. Almond Skin Extraction
3.2. Waffle Preparation
3.3. Preparation of Extracts for Bioactive Compounds and Antioxidant Assays
3.4. Bioactive Compounds: Total Phenolic Content, Total Flavonoid Content, and Ortho-Diphenols
3.5. Antioxidant Activities (AA)
3.6. Extraction and Quantification of Soluble Sugars
3.7. Extraction and Quantification of Starch
3.8. Texture Evaluation
3.9. Colour Determination
3.10. Sensory Evaluation of Waffles by Quantitative Descriptive Analysis (QDA)
3.11. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Esfahlan, J.; Jamei, R. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Prgomet, I.; Goncalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, 106–115. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Boukid, F.; Angelis, D.; Summo, C. Use of almond skins to improve nutritional and functional properties of biscuits: An example of upcycling. Foods 2020, 9, 1705. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Malayil, S.; Surendran, N.; Kate, K.; Satyavolu, J. Impact of acid hydrolysis on composition, morphology and xylose recovery from almond biomass (skin and shell). Bioresour. Technol. Rep. 2022, 19, 101150. [Google Scholar] [CrossRef]
- Loizzo, R.; Tundis, R.; Leporini, M.; D’Urso, G.; Gagliano-Candela, T.; Sottile, F. Almond (Prunus dulcis cv. Casteltermini) skin confectionery by-products: A new opportunity for the development of a functional blackberry (Rubus ulmifolius Schott) Jam. Antioxidants 2021, 10, 1218. [Google Scholar] [CrossRef]
- Arena, A.; Bisignano, C.; Stassi, G.; Filocamo, A.; Mandalari, G. Almond skin inhibits HSV-2 replication in peripheral blood mononuclear cells by modulating the cytokine network. Molecules 2015, 20, 8816–8822. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Rich, T.; Bisignano, C.; Saija, A.; Dugo, P.; Cross, L.; Waldron, W.; et al. Characterization of polyphenols, lipids, and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Reyes-De-Corcuera, J.; Cavalieri, P.; Powers, R.; Reyes, I.; Corcuera, D. Chapter 5—Blanching. In Encyclopedia of Agricultural, Food, and Biological Engineering; Heldman, D.R., Moraru, C.I., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–6. [Google Scholar]
- Mandalari, G.; Tomaino, A.; Rich, T.; lo Curto, R.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, L.; Waldron, W.; et al. Polyphenol and nutrient release from the skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Arena, A.; Bisignano, C.; Stassi, G.; Mandalari, G.; Wickham, S.; Bisignano, G. Immunomodulatory and antiviral activity of almond skins. Immunol. Lett. 2010, 132, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, M.; Wani, A.; Bhat, A. Pasting, rheology, antioxidant and texture profile of gluten-free cookies with added seed gum hydrocolloids. Food Sci. Technol. Int. 2021, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lusk, L. Consumer beliefs about healthy foods and diets. PLoS ONE 2019, 14, e0223098. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Mahloko, M.; Silungwe, H.; Mashau, E.; Kgatla, E. Bioactive compounds, antioxidant activity and physical characteristics of wheat-prickly pear and banana biscuits. Heliyon 2019, 5, e02479. [Google Scholar] [CrossRef]
- Olagunju, A.; Omoba, O.; Enujiugha, V.; Aluko, R. Development of value-added nutritious crackers with high antidiabetic properties from blends of Acha (Digitaria exilis) and blanched Pigeon pea (Cajanus cajan). Food Sci. Nutr. 2018, 6, 1791–1802. [Google Scholar] [CrossRef]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef]
- Dhillon, J.; Newman, J.; Fiehn, O.; Ortiz, R. Almond consumption for 8 weeks altered host and microbial metabolism in comparison to a control snack in young adults. J. Am. Nutr. Assoc. 2023, 42, 242–254. [Google Scholar] [CrossRef]
- Szymanowska, U.; Karaś, M.; Złotek, U.; Jakubczyk, A. Effect of fortification with raspberry juice on the antioxidant and potentially anti-inflammatory activity of waffles subjected to in vitro digestion. Foods 2021, 10, 791. [Google Scholar] [CrossRef]
- Szymanowska, U.; Karaś, M.; Bochnak-Niedźwiecka, J. Antioxidant and anti-inflammatory potential and consumer acceptance of waffles enriched with Freeze-Dried Raspberry Pomace. Appl. Sci. 2021, 11, 6807. [Google Scholar] [CrossRef]
- Urganci, U.; Fatma, K. Quality characteristics of biscuits fortified with pomegranate peel. Akad. Gıda 2021, 19, 10–20. [Google Scholar] [CrossRef]
- Salem, R.; El-Sahy, M.; Sulieman, M.; Gouda, R. Use of tomato pomace, mango seeds kernel and pomegranate peels powders for the production of functional biscuits. J. Agric. Res. 2020, 47, 1011–1023. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Ribeiro, C.; Gonçalves, B. Morphological, mechanical and antioxidant properties of Portuguese almond cultivars. J. Food Sci. Technol. 2017, 55, 467–478. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of different processing treatments on almond (Prunus dulcis) bioactive compounds, antioxidant activities, fatty acids, and sensorial characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Ogunjobi, K.; Ogunwolu, O. Physicochemical and sensory properties of cassava flour biscuits supplemented with cashew apple powder. J. Food Technol. 2010, 8, 24–29. [Google Scholar] [CrossRef]
- Hooda, S.; Jood, S. Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour. Food Chem. 2005, 90, 427–435. [Google Scholar] [CrossRef]
- Ng, H.; Robert, D.; Ahmad, W.; Ishak, W. Incorporation of dietary fiber-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit. Food Chem. 2017, 227, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Sudha, L.; Vetrimani, R.; Leelavathi, K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007, 100, 1365–1370. [Google Scholar] [CrossRef]
- Ajila, M.; Leelavathi, S.; Rao, P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Aires, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Phenolic and fatty acid profiles, α-tocopherol and sucrose contents, and antioxidant capacities of understudied Portuguese almond cultivars. J. Food Biochem. 2019, 43, e12887. [Google Scholar] [CrossRef]
- Singleton, L.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.; Liu, H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Gutfinger, T. Polyphenols in olive oils. J. Am. Oil Chem. Soc. 1981, 58, 966–996. [Google Scholar] [CrossRef]
- Garcia, B.; Coelho, J.; Costa, M.; Pinto, J.; Paiva-Martins, F. A simple method for the determination of bioactive antioxidants in virgin olive oils. J. Sci. Food Agric. 2013, 93, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Sci. Food Agric. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, T. Kinetics and mechanisms of antioxidant activity using the DPPH. Free radical method. Food Sci. Technol. 1995, 30, 609–615. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Siddhraju, P.; Becker, K. Antioxidant properties of various solvents extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Irigoyen, J.; Einerich, W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Osaki, K.; Doi, M. On the concentration gradient of polymer solution in a rotating rheometer. J. Rheol. 1991, 35, 89–92. [Google Scholar] [CrossRef]
- Fragoso, P. Desenvolvimento de Bolachas com Incorporação de Diferentes Microalgas. Ph.D. Thesis, Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, Portugal, 2016. [Google Scholar]
- Civille, V.; Lapsley, K.; Huang, G.; Yada, S.; Seltsam, J. Development of an almond lexicon to assess the sensory properties of almond varieties. J. Sens. Stud. 2010, 25, 146–162. [Google Scholar] [CrossRef]
- Ormenese, C.; Abreu, N.; Coelho, D.; Silva, D. Perfil sensorial e teste de consumidor de biscoito recheado sabor chocolate. Bol. Cent. Pesqui. Process. Aliment. 2001, 19, 277–300. [Google Scholar] [CrossRef]





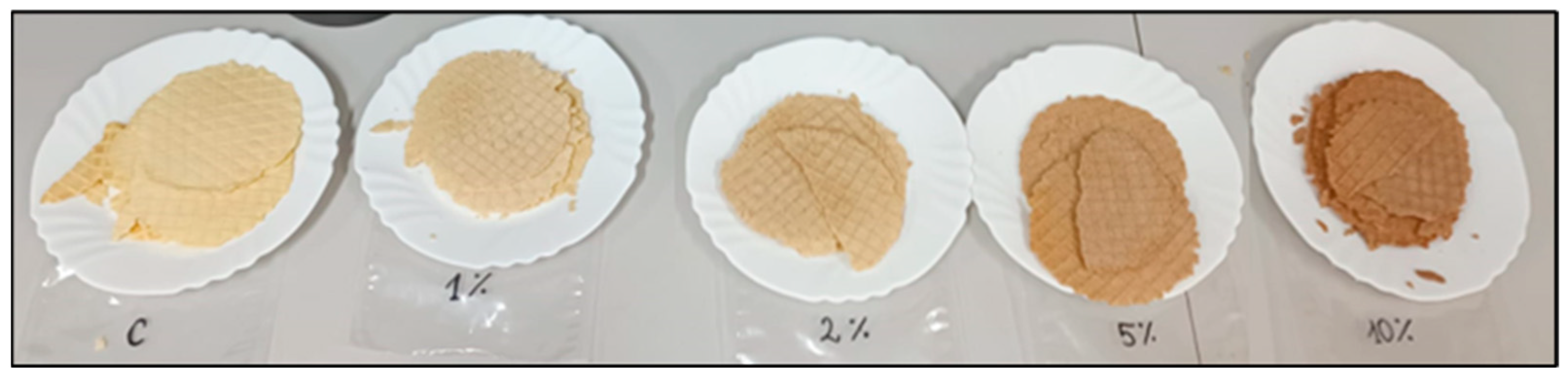

| Samples | Mean Weight ± Standard Deviation |
|---|---|
| Control | 10.25 ± 1.99 g |
| 1% | 8.34 ± 0.81 g |
| 2% | 7.73 ± 0.80 g |
| 5% | 8.78 ± 0.29 g |
| 10% | 7.16 ± 1.40 g |
| Descriptors | Factor 1 | Factor 2 |
|---|---|---|
| Colour | −1.53094 | 0.088359 |
| Aromatic Intensity | 0.21600 | −0.219561 |
| Almond Aroma | 0.28705 | −0.102452 |
| Roasted Aroma | −0.65894 | −0.082851 |
| Rancid Aroma | −0.12207 | −0.99538 |
| Flavour Intensity | −0.11662 | −0.119623 |
| Almond Flavour | −0.41306 | −0.214428 |
| Roasted Flavour | −0.45348 | 0.047448 |
| Rancid Flavour | 0.03684 | −0.013759 |
| Sweet taste | 0.04925 | −0.135587 |
| Bitter Taste | 0.01238 | −0.047488 |
| Fat Taste | 0.41027 | −0.189357 |
| Noise when Breaking | 0.15110 | 0.132059 |
| Hardness | 0.14321 | 0.058049 |
| Crunchiness | 0.01060 | 0.039401 |
| Dissolubility | −0.48132 | −0.132656 |
| Palatability | 0.02239 | −0.068781 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.; Marinho, B.; Szymanowska, U.; Karas, M.; Vilela, A. Chemical and Sensory Properties of Waffles Supplemented with Almond Skins. Molecules 2023, 28, 5674. https://doi.org/10.3390/molecules28155674
Oliveira I, Marinho B, Szymanowska U, Karas M, Vilela A. Chemical and Sensory Properties of Waffles Supplemented with Almond Skins. Molecules. 2023; 28(15):5674. https://doi.org/10.3390/molecules28155674
Chicago/Turabian StyleOliveira, Ivo, Beatriz Marinho, Urszula Szymanowska, Monika Karas, and Alice Vilela. 2023. "Chemical and Sensory Properties of Waffles Supplemented with Almond Skins" Molecules 28, no. 15: 5674. https://doi.org/10.3390/molecules28155674
APA StyleOliveira, I., Marinho, B., Szymanowska, U., Karas, M., & Vilela, A. (2023). Chemical and Sensory Properties of Waffles Supplemented with Almond Skins. Molecules, 28(15), 5674. https://doi.org/10.3390/molecules28155674