Construction of N-Ferrocene Substituted Benzodihydrooxazoles via a Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization Studies
2.2. Substrate Scope Studies
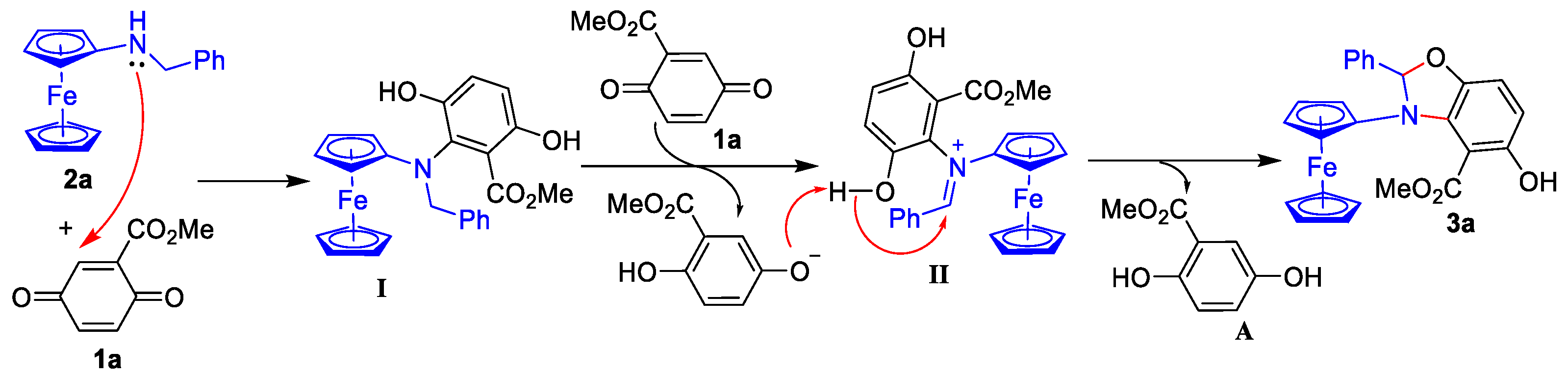
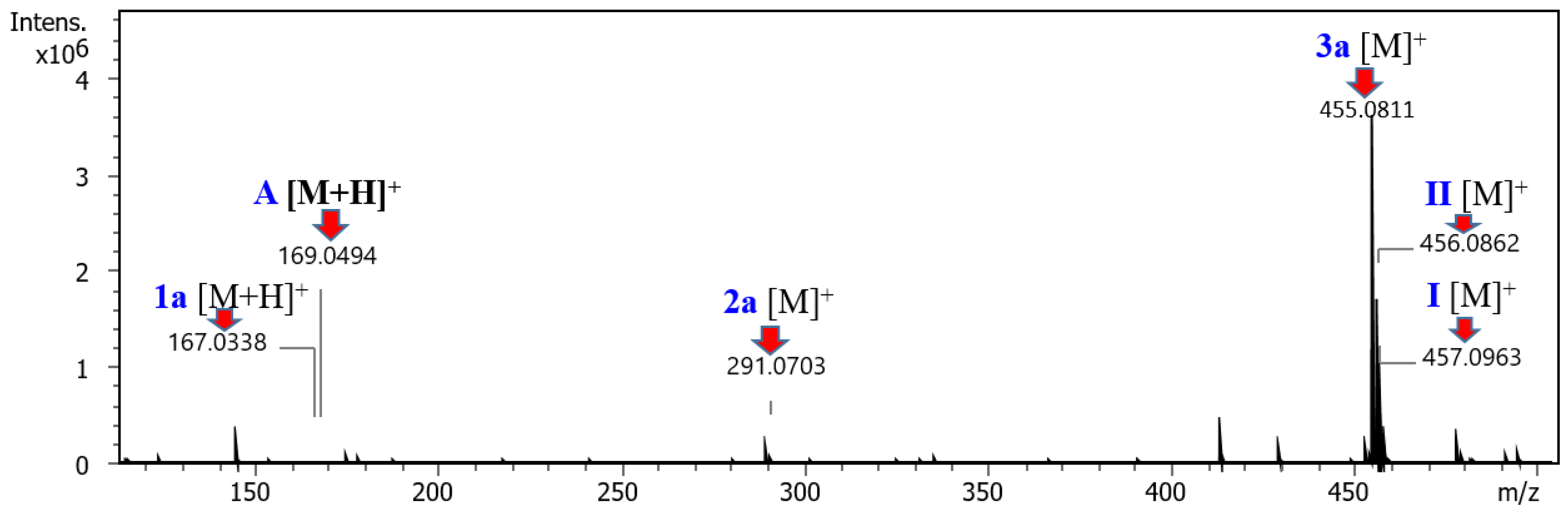
2.3. Proposed Mechanism for the Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction
3. Materials and Methods
3.1. General Information
3.2. Method for Crystal Growth of 3o
3.3. General Experimental Procedure for the Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction for the Synthesis of Products 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roy, S.; Panja, S.; Sahoo, S.R.; Chatterjeea, S.; Maiti, D. Enroute Sustainability: Metal free C–H Bond Functionalization. Chem. Soc. Rev. 2023, 52, 2391–2479. [Google Scholar] [CrossRef]
- Gao, G.-Y.; Ruppel, J.V.; Fields, K.B.; Xu, X.; Chen, Y.; Zhang, X.P. Synthesis of Diporphyrins via Palladium-Catalyzed C−O Bond Formation: Effective Access to Chiral Diporphyrins. J. Org. Chem. 2008, 73, 4855–4858. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.; Jeong, Y.; Kim, Y.R.; Lee, E.; Choi, W.; Lee, P.H. Palladium-Catalyzed C(sp2 and sp3)–H Activation/C–O Bond Formation: Synthesis of Benzoxaphosphole 1- and 2-Oxides. Org. Lett. 2013, 15, 5210–5213. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; White, M.C. A Sulfoxide-Promoted, Catalytic Method for the Regioselective Synthesis of Allylic Acetates from Monosubstituted Olefins via C−H Oxidation. J. Am. Chem. Soc. 2004, 126, 1346–1347. [Google Scholar] [CrossRef]
- Ma, R.; Young, J.; Promontorio, R.; Dannheim, F.M.; Pattillo, C.C.; White, M.C. Synthesis of anti-1,3 Amino Alcohol Motifs via Pd(II)/SOX Catalysis with the Capacity for Stereodivergence. J. Am. Chem. Soc. 2019, 141, 9468–9473. [Google Scholar] [CrossRef]
- Blieck, R.; Taillefer, M.; Monnier, F. Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds. Chem. Rev. 2020, 120, 13545–13598. [Google Scholar] [CrossRef]
- Newton, C.G.; Wang, S.-G.; Oliveira, C.C.; Cramer, N. Catalytic Enantioselective Transformations Involving C–H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [Google Scholar] [CrossRef]
- Hu, F.; Sun, Z.; Pan, M.; Wang, L.; Xu, L.; Liu, X.-L.; Li, S.-S. Divergent Synthesis of Nitrogen Heterocycles via H2O-Mediated Hydride Transfer Reactions. Green Chem. 2023, 25, 5134–5141. [Google Scholar] [CrossRef]
- Hu, F.; Li, X.; Ding, Z.; Wang, L.; Ge, C.; Xu, L.; Li, S.-S. Divergent Synthesis of [3,4]-Fused 3-Alkenyl-Oxindoles via Propargyl Alcohol-Triggered C(sp3)–H Functionalization. ACS Catal. 2022, 12, 943–952. [Google Scholar] [CrossRef]
- Troisi, L.; Granito, C.; Ronzini, L.; Rosato, F.; Videtta, V. An Economic and Efficient Tetrahydrofuranylation of Alcohols, Imines and Alkynes. Tetrahedron Lett. 2010, 51, 5980. [Google Scholar] [CrossRef]
- Fan, R.; Sun, Y.; Ye, Y. Iodine(III)-Mediated Tandem Acetoxylation−Cyclization of o-Acyl Phenols for the Facile Construction of α-Acetoxy Benzofuranones. Org. Lett. 2009, 11, 5174–5177. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, X.; Lin, G.; Negrerie, D.Z.; Du, Y. Chiral Aryliodine-Mediated Enantioselective Organocatalytic Spirocyclization: Synthesis of Spirofurooxindoles via Cascade Oxidative C–O and C–C Bond Formation. Org. Lett. 2016, 18, 5580–5583. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, X.; Zhang, B.; Cong, Y.; Wan, X.; Bao, M.; Zhao, X.; Li, B.; Negrerie, D.Z.; Du, Y. Synthesis of Spirofurooxindoles via Phenyliodine(III) Bis(trifluoroacetate) (PIFA)-Mediated Cascade Oxidative C−O and C−C Bond Formation. Adv. Synth. Catal. 2018, 360, 1634–1638. [Google Scholar] [CrossRef]
- Xing, Q.; Liang, H.; Bao, M.; Li, X.; Zhang, J.; Bi, T.; Zhang, Y.; Xu, J.; Du, Y.; Zhao, K. Metal-free Synthesis of Spiro-2,2′-benzo[b]furan-3,3′-ones via PhI(OAc)2-Mediated Cascade Spirocyclization. Adv. Synth. Catal. 2019, 361, 4669–4673. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, R.; Negrerie, D.Z.; Du, Y.; Zhao, K. Hypervalent Iodine-Mediated Oxygenation of N,N-Diaryl Tertiary Amines: Intramolecular Functionalization of sp3 C–H Bonds Adjacent to Nitrogen. J. Org. Chem. 2014, 79, 10581–10587. [Google Scholar] [CrossRef]
- Couto, I.; Tellitu, I.; Domıínguez, E. An Intramolecular PIFA-Mediated Metal-Free Allylic Oxycarbonylation Reaction and Its Application to the Preparation of Furopyrimidinones. J. Org. Chem. 2010, 75, 7954–7957. [Google Scholar] [CrossRef]
- Wang, X.; Donaire, J.G.; Martin, R. Mild ArI-Catalyzed C(sp2)–H or C(sp3)–H Functionalization/C-O Formation: An Intriguing Catalyst-Controlled Selectivity Switch. Angew. Chem. Int. Ed. 2014, 53, 11084–11087. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Santra, S.; Hajra, A.; Zyryanov, G.V.; Majee, A. Visible-Light-Induced Regioselective C(sp3)-H Acyloxylation of Aryl-2H-azirines with (Diacetoxy)iodobenzene. J. Org. Chem. 2019, 84, 11735–11740. [Google Scholar] [CrossRef]
- Shibutani, S.; Kodo, T.; Takeda, M.; Nagao, K.; Tokunaga, N.; Sasaki, Y.; Ohmiya, H. Organophotoredox-Catalyzed Decarboxylative C(sp3)–O Bond Formation. J. Am. Chem. Soc. 2020, 142, 1211–1216. [Google Scholar] [CrossRef]
- Temiz-Arpacı, Ö.; Özdemir, A.; Yalcin, I.; Yildiz, I.; Aki-Sener, E.; Altanlar, N. Synthesis and Antimicrobial Activity of Some 5-[2-(Morpholin-4-yl)acetamido] and/or 5-[2-(4-Substituted piperazin-1-yl)acetamido]-2-(p-substituted phenyl)benzoxazoles. Arch. Pharm. Chem. Life Sci. 2005, 338, 105–111. [Google Scholar] [CrossRef]
- Shang, Y.; He, X.; Hu, J.; Wu, J.; Zhang, M.; Yu, S.; Zhang, Q. Copper-Catalyzed Efficient Multicomponent Reaction: Synthesis of Benzoxazoline-Amidine Derivatives. Adv. Synth. Catal. 2009, 351, 2709–2713. [Google Scholar] [CrossRef]
- Fu, Y.; Li, G.-Y.; Ye, F.; Zhang, S.-S.; Gao, S. Synthesis and Biological Activity of Some Novel N-dichloroacetyl-2,3-dihydrobenzoxazole Derivatives. Heterocycl. Commun. 2011, 17, 57–60. [Google Scholar] [CrossRef]
- Mader, M.M.; Shih, C.; Considine, E.; Dios, A.D.; Grossman, C.S.; Hipskind, P.A.; Lin, H.-S.; Lobb, K.L.; Lopez, B.; Lopez, J.E.; et al. Acyl Sulfonamide Anti-proliferatives. Part 2: Activity of Heterocyclic Sulfonamide Derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, P.; Yang, Z. Synthesis of 2-Aryl Benzoxazoles from Benzoxazoles and α-Ketoic Acids by Photoredox Catalysis. Chin. J. Org. Chem. 2022, 42, 1770–1777. [Google Scholar] [CrossRef]
- Li, F.; Xiao, J.; Wu, X.; Wang, X.; Deng, J.; Tang, Z. Metal-Free Formation of 2-Substitued Benzoxazoles with Amides and Esters. Chin. J. Org. Chem. 2022, 42, 1778–1785. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Mathew, T.; Panja, C.; Vaghoo, H.; Venkataraman, K.; Olah, G.A. Efficient One-Pot Synthesis of Fluorinated Benzimidazolines, Benzothiazolines, Benzoxazolines, and Dihydrobenzoxazinones Using Gallium(III) Triflate as a Catalyst. Org. Lett. 2007, 9, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Lu, X.; Xu, J.; Chen, J.; Deng, H.; Shao, M.; Jin, Y.; Zhang, H.; Cao, W. Facile Synthesis of 2-perfluoroalkylated Benzoxazolines and Benzothiazolines. J. Fluorine Chem. 2013, 151, 20–25. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Vaghoo, H.; Panja, C.; Molnar, A.; Mathew, T.; Olah, G.A. Nafion®-H Catalyzed Synthesis of Fluorinated Benzimidazolines, Benzothiazolines, Benzoxazolines and Dihydrobenzoxazinones. Synthesis 2008, 2008, 897–902. [Google Scholar] [CrossRef]
- Jin, G.; Werncke, C.G.; Escudie, Y.; Sabo-Etienne, S.; Bontemps, S. Iron-Catalyzed Reduction of CO2 into Methylene: Formation of C–N, C–O, and C–C Bonds. J. Am. Chem. Soc. 2015, 137, 9563–9566. [Google Scholar] [CrossRef]
- Panda, N.; Yadav, S.A. Palladium-catalyzed Oxamidation of Alkenes: A New Approach to Benzoxazolidines. Tetrahedron 2018, 74, 1497–1504. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G. Haloamination of An Aminoallenylether and Subsequent Palladium-catalyzed Cross Coupling Reactions to Afford Dihydrobenzoxazole Derivatives Containing Conjugated Substituents. Tetrahedron Lett. 2017, 58, 4436–4439. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.B.; Jang, S.S.; Youn, S.W. Pd-Catalyzed Regioselective Intramolecular Allylic C–H Amination of 1,1-Disubstituted Alkenyl Amines. J. Org. Chem. 2022, 87, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, D.B.; Youn, S.W. Simple Tandem Olefin Isomerization/Intramolecular Hydroamination of Alkenyl Amines with Various Allylic Tethers. J. Org. Chem. 2022, 87, 11919–11924. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, S.; Beccalli, E.M.; Bernasconi, A.; Borelli, T.; Broggini, G.; Mazza, A. Palladium-Catalysed Carbo- and Hydroamination of Allenyl Ethers and Aminoallenes: Available Entry to Nitrogen-Containing Benzo-Fused Rings. Eur. J. Org. Chem. 2016, 2016, 4534–4544. [Google Scholar] [CrossRef]
- Ward, A.F.; Wolfe, J.P. Stereoselective Synthesis of Substituted 1,3-Oxazolidines via Pd-Catalyzed Carboamination Reactions of O-Vinyl-1,2-Amino Alcohols. Org. Lett. 2011, 13, 4728–4731. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beveridge, R.E.; Black, D.A.; Arndtsen, B.A. Copper-Catalyzed Multicomponent Coupling of Organoindium Reagents with Nitrogen-Containing Aromatic Heterocycles. Eur. J. Org. Chem. 2010, 2010, 3650–3656. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessieres, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Mu, J.; Xie, X.; Xiong, S.; Zhang, Y.; Wang, Y.; Zhao, Q.; Zhu, H.; Huang, W.; He, G. Discovery of Spirooxindole-Ferrocene Hybrids As Novel MDM2 Inhibitors. Chin. Chem. Lett. 2021, 32, 1897–1901. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Li, W.; Yan, J.; Dansette, P.M.; Othman, M.; McGlinchey, M.J.; Jaouen, G. Diversity-oriented Synthesis and Bioactivity Evaluation of N-substituted Ferrocifen Compounds As Novel Antiproliferative Agents Against TNBC Cancer Cells. Eur. J. Med. Chem. 2022, 234, 114202. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, Z.; Ye, J.; Zhang, C.; Zhang, X.; Zhao, Y. Multicore Ferrocene Derivative as a Highly Soluble Cathode Material for Nonaqueous Redox Flow Batteries. ACS. Appl. Energy Mater. 2021, 4, 855–861. [Google Scholar] [CrossRef]
- Tahara, H.; Uranaka, K.; Hirano, M.; Ikeda, T.; Sagara, T.; Murakami, H. Electrochromism of Ferrocene- and Viologen-Based Redox-Active Ionic Liquids Composite. ACS Appl. Mater. Inter. 2019, 11, 1–6. [Google Scholar] [CrossRef]
- Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Asymmetric Catalysis with Chiral Ferrocene Ligands. Acc. Chem. Res. 2003, 36, 659–667. [Google Scholar] [CrossRef]
- Cunningham, L.; Benson, A.; Guiry, P.J. Recent developments in the synthesis and applications of chiral ferrocene ligands and organocatalysts in asymmetric catalysis. Org. Biomol. Chem. 2020, 18, 9329–9370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Nie, Y.-H.; Liu, C.-X.; Zhang, W.-W.; Wu, Z.-J.; Gu, Q.; Zheng, C.; You, S.-L. Rhodium(III)-Catalyzed Enantioselective C–H Activation/Annulation of Ferrocenecarboxamides with Internal Alkynes. ACS Catal. 2022, 12, 3083–3093. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Shen, L.-W.; Yang, P.; You, Y.; Zhao, J.-Q.; Yuan, W.-C. Access to 4-Trifluoromethyl Quinolines via Cu-Catalyzed Annulation Reaction of Ketone Oxime Acetates with ortho-Trifluoroacetyl Anilines under Redox-Neutral Conditions. J. Org. Chem. 2022, 87, 5804–5816. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Xu, L.; Wang, L.; Li, S.-S. Catalytic Formal Benzylic C–H Bond Functionalization of 2,5-Dialkylfuran Derivatives with Ferrocenyl Alcohols as Alkylation Reagents. Org. Lett. 2019, 21, 627–631. [Google Scholar] [CrossRef]
- Yan, J.; Yue, K.; Fan, X.; Xu, X.; Wang, J.; Qin, M.; Zhang, Q.; Hou, X.; Li, X.; Wang, Y. Synthesis and Bioactivity Evaluation of Ferrocene-based Hydroxamic Acids As Selective Histone Deacetylase 6 Inhibitors. Eur. J. Med. Chem. 2023, 246, 115004. [Google Scholar] [CrossRef]
- Skoupilova, H.; Bartosik, M.; Sommerova, L.; Pinkas, J.; Vaculovic, T.; Kanicky, V.; Karban, J.; Hrstka, R. Ferrocenes As New Anticancer Drug Candidates: Determination of the Mechanism of Action. Eur. J. Pharmacol. 2020, 867, 172825. [Google Scholar] [CrossRef]
- Dai, L.; Xu, D.; Mao, Y.; Zhu, J.; Yang, M. Structures and Synthetic Strategies of Chiral Oxazolinyl Ferrocene Derivatives. Chin. J. Org. Chem. 2022, 42, 2364–2375. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Yin, D. Progress of Ferrocene-Based Metal Cation Recognition Receptor. Chin. J. Org. Chem. 2021, 41, 158–170. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, F. Advances in Catalytic Asymmetric Reactions Using 2-Indolylmethanols as Platform Molecules. Chin. J. Org. Chem. 2022, 42, 3351–3372. [Google Scholar] [CrossRef]
- Tu, M.-S.; Chen, K.-W.; Wu, P.; Zhang, Y.-C.; Liu, X.-Q.; Shi, F. Advances in Organocatalytic Asymmetric Reactions of Vinylindoles: Powerful Access to Enantioenriched Indole Derivatives. Org. Chem. Front. 2021, 8, 2643–2672. [Google Scholar] [CrossRef]
- Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Progresses in Organocatalytic Asymmetric Dearomatization Reactions of Indole Derivatives. Org. Chem. Front. 2020, 7, 3967–3998. [Google Scholar] [CrossRef]
- Zhang, M.; He, X.-W.; Xiong, Y.; Zuo, X.; Zhou, W.; Liu, X.-L. Asymmetric Construction of Six Vicinal Stereogenic Centers on Hexahydroxanthones via Organocatalytic One-pot Reactions. Chem. Commun. 2021, 57, 6764–6767. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, H.; Gu, L.; Zhang, W.; Zhao, J.; Wang, Q. Skeletal Remodeling of Chalcone-based Pyridinium Salts to Access Isoindoline Polycycles and Their Bridged Derivatives. Chem. Sci. 2021, 12, 15389–15398. [Google Scholar] [CrossRef]
- Lin, B.; Huang, J.-F.; Liu, X.-W.; Ma, X.-T.; Liu, X.-L.; Lu, Y.; Zhou, Y.; Guo, F.-M.; Feng, T.-T. Rapid, Microwave-accelerated Synthesis and Anti-osteoporosis Activities Evaluation of Morusin Scaffolds and Morusignin L Scaffolds. Bioorg. Med. Chem. Lett. 2017, 27, 2389–2396. [Google Scholar] [CrossRef]
- Miao, H.-J.; Wang, L.-L.; Han, H.-B.; Zhao, Y.-D.; Wang, Q.-L.; Bu, Z.-W. Regio- and Diastereoselective Dearomatizations of N-alkyl Activated Azaarenes: The Maximization of the Reactive Sites. Chem. Sci. 2020, 11, 1418–1424. [Google Scholar] [CrossRef]
- Wang, H.-J.; Zhou, Y.-Y.; Liu, X.-L.; Zhang, W.-H.; Chen, S.; Liu, X.-W.; Zhou, Y. Regioselective Synthesis and Evaluation of 2-amino 3-cyano Chromene-chrysin hybrids as Potential Anticancer Agents. Bioorg. Med. Chem. Lett. 2020, 30, 127087. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, A.-A.; Zhao, R.-J.; Li, F.; Meng, T.-J.; Ishida, N.; Murakami, M.; Zhao, W.-X. Asymmetric Synthesis of Planar Chiral Ferrocenes by Enantioselective Intramolecular C–H Arylation of N-(2-Haloaryl)ferrocenecarboxamides. Org. Lett. 2014, 16, 5336–5338. [Google Scholar] [CrossRef]
- Jia, L.; Liu, X.; Zhang, A.-A.; Wang, T.; Hua, Y.; Li, H.; Liu, L. Synthesis of planar chiral ferrocenes via a Pd(0)-catalyzed syn-carbopalladation/asymmetric C–H alkenylation process. Chem. Commun. 2020, 56, 1737–1740. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zuo, Z.; Zhang, A.-A.; Li, Z.; Meng, T.; Wu, W.; Hua, Y.; Mao, G. Synthesis of Planar Chiral Isoquinolinone-fused Ferrocenes Through Palladium-catalyzed C-H Functionalization Reaction. Chin. Chem. Lett. 2021, 32, 239–242. [Google Scholar] [CrossRef]
- Zhang, A.-A.; Chen, C.; Gao, Y.; Mo, M.; Shen, R.-Z.; Zhang, Y.-H.; Ishida, N.; Murakami, M.; Liu, L. Planar Chiral 2-(trifluoromethyl)quinoline-fused Ferrocenes via Palladium(0)-catalyzed C-H Functionalization of Trifluoroacetimidoyl Chlorides. Green Synth. Catal. 2021, 2, 311–314. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, P.; Wu, D.; Qiu, Z.; Zhao, C.; Zhang, W.; Li, F.; Zhou, J.; Liu, L. Brønsted Acid-Catalyzed Reaction of N-arylnaphthalen-2-amines with Quinone Esters for the Construction of Carbazole and C–N Axially Chiral Carbazole Derivatives. J. Org. Chem. 2023, 88, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Wachter, V. Chemical Synthesis of Small Molecule Libraries around the P-Benzoquinone Scaffold. Ph.D. Thesis, Technical University of Braunschweig, Braunschweig, Germany, 2007. [Google Scholar]


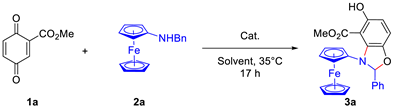 | |||
|---|---|---|---|
| Entry | Solvent | Cat. | Yield (%) [b] |
| 1 | DCM | DABCO | 67 |
| 2 | DCM | DMAP | 22 |
| 3 | DCM | (PhO)2PO2H | 85 |
| 4 | DCM | TsOH | 45 |
| 5 | DCM | PhCO2H | 75 |
| 6 | DCM | - | >99 |
| 7 | CH3CN | - | 50 |
| 8 | EtOAc | - | 67 |
| 9 | THF | - | 35 |
| 10 | toluene | - | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhao, P.; Liu, Q.; Liu, X.; Hu, J.; Wu, D.; Liu, L. Construction of N-Ferrocene Substituted Benzodihydrooxazoles via a Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction. Molecules 2023, 28, 5615. https://doi.org/10.3390/molecules28145615
Zhang M, Zhao P, Liu Q, Liu X, Hu J, Wu D, Liu L. Construction of N-Ferrocene Substituted Benzodihydrooxazoles via a Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction. Molecules. 2023; 28(14):5615. https://doi.org/10.3390/molecules28145615
Chicago/Turabian StyleZhang, Mingliang, Pin Zhao, Qilv Liu, Xinlei Liu, Jingya Hu, Dongqing Wu, and Lantao Liu. 2023. "Construction of N-Ferrocene Substituted Benzodihydrooxazoles via a Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction" Molecules 28, no. 14: 5615. https://doi.org/10.3390/molecules28145615
APA StyleZhang, M., Zhao, P., Liu, Q., Liu, X., Hu, J., Wu, D., & Liu, L. (2023). Construction of N-Ferrocene Substituted Benzodihydrooxazoles via a Catalyst-Free Aza-Michael Addition/C(sp3)-O Bond Formation Tandem Reaction. Molecules, 28(14), 5615. https://doi.org/10.3390/molecules28145615






