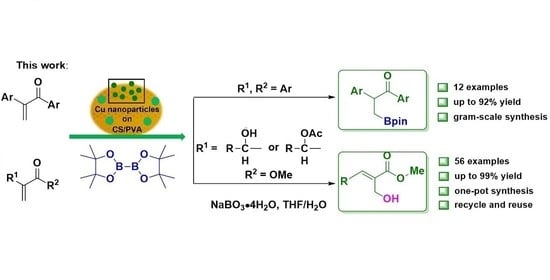
Application of Chitosan/Poly(vinyl alcohol) Stabilized Copper Film Materials for the Borylation of α, β-Unsaturated Ketones, Morita-Baylis-Hillman Alcohols and Esters in Aqueous Phase †
Abstract
1. Introduction
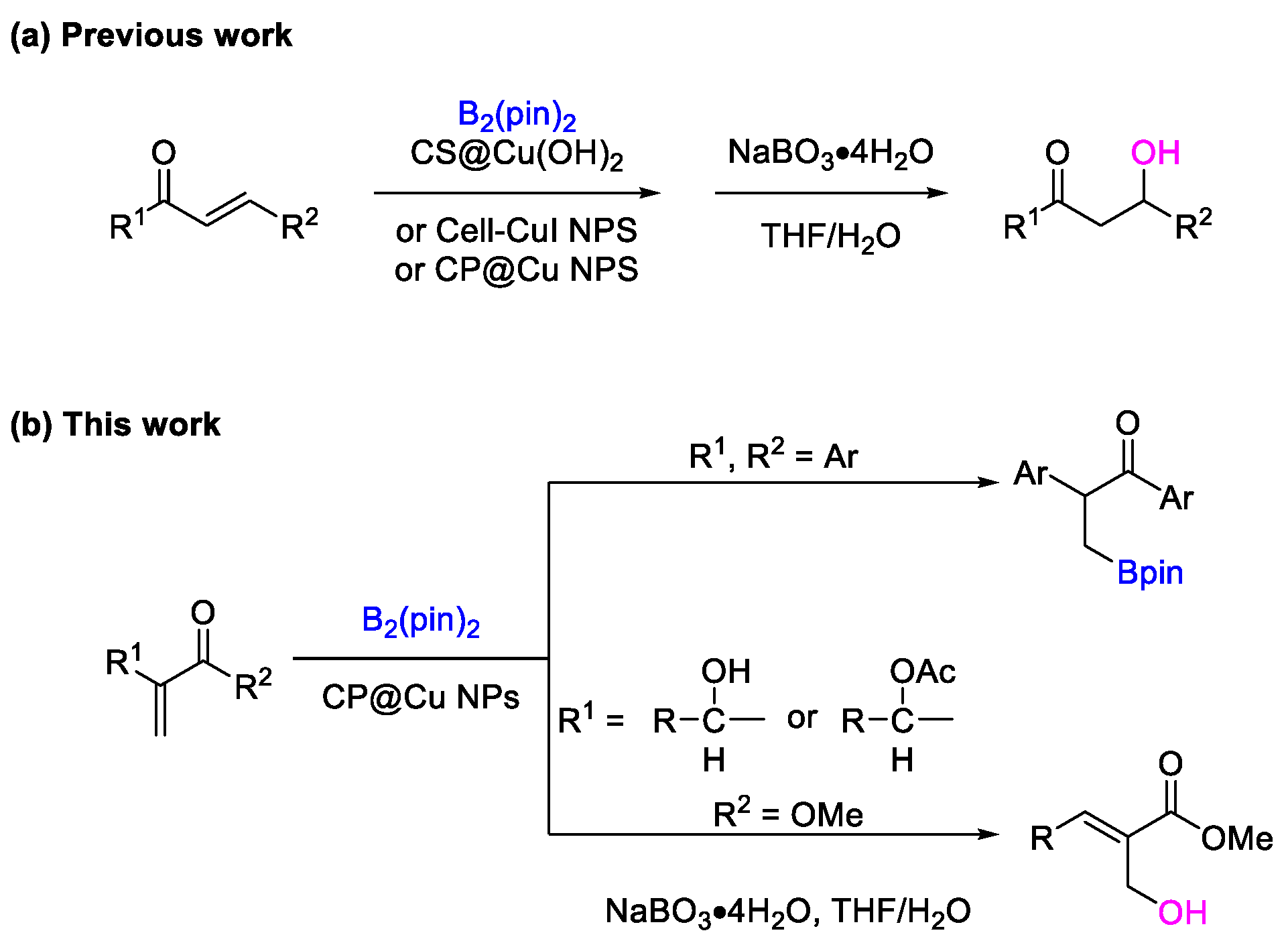
2. Results and Discussion
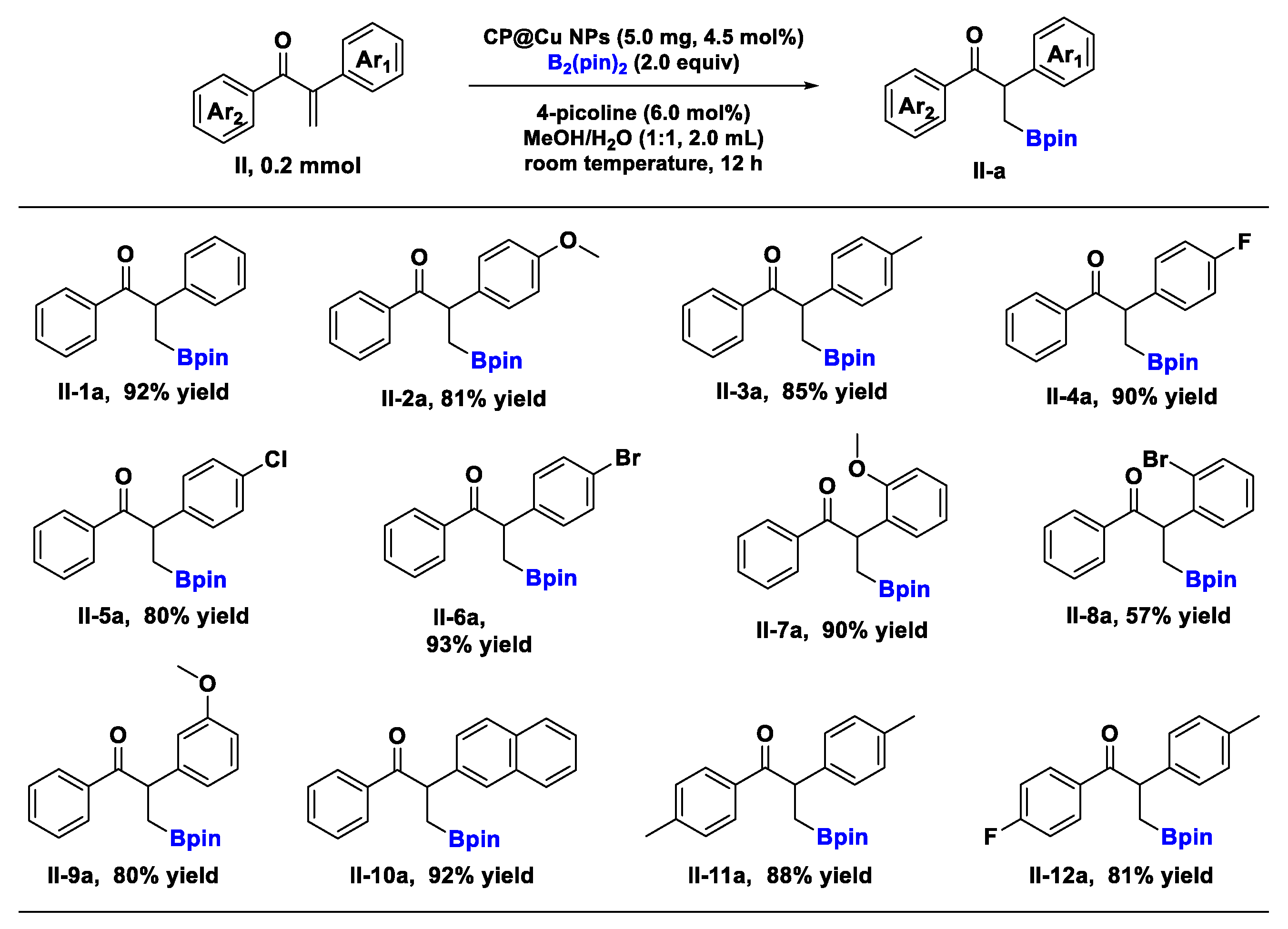
2.1. Catalysis of CP@Cu NPs in the Borylation Reaction of α, β-Unsaturated Ketones
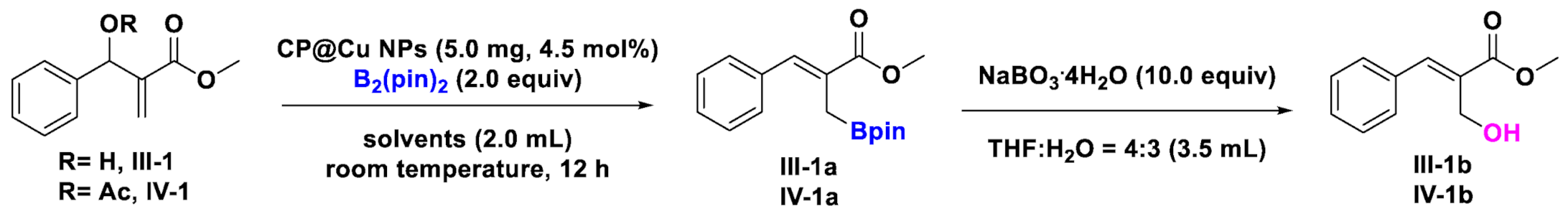
2.2. Catalysis of CP@Cu NPs in the Borylation Reaction of MBH Alcohols and Esters
2.3. Recycling Experiments of CP@Cu NPs in Borylation Reactions
3. Materials and Methods
3.1. Materials
3.2. Synthesis of α, β-Unsaturated Ketones II
3.3. Synthesis of MBH Alcohols III
3.4. Synthesis of MBH Esters IV
3.5. Analytical Methods
3.6. Copper-Catalyzed Borylation Reactions
3.6.1. Borylation Reactions of α, β-Unsaturated Ketones
3.6.2. Borylation Reactions of MBH Alcohols and Esters
3.7. General Procedure for ICP Test of CP@Cu NPs
3.8. General Procedure for the Sample Preparation for ICP Analysis to Determine Metal Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roccaro, A.M.; Vacca, A.; Ribatti, D. Bortezomib in the treatment of cancer. Recent Pat. Anti-Cancer Drug Discov. 2006, 1, 397. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.S.; Zhu, Y.; Xie, H.; Hosmane, N.S.; Zhang, Y. Organoboron compounds: Effective antibacterial and antiparasitic agents. Molecules 2021, 26, 3309. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, M.; Costa, G.P.; Barcellos, A.M.; Perin, G.; Lenardão, E.J.; Alves, D. Transition-metal-free C−S, C−Se, and C−Te bond formation from organoboron compounds. Chem. Rec. 2021, 21, 2855. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Das, B.C.; Das, S. Rhodium-catalyzed 1,4-addition reactions of diboron reagents to electron deficient olefins. Tetrahedron Lett. 2002, 43, 2323. [Google Scholar] [CrossRef]
- Abu, A.H.; Goldberg, I.; Srebnik, M. Addition reactions of bis(pinacolato)diborane(4) to carbonyl enones and synthesis of (pinacolato)2BCH2B and (pinacolato)2BCH2CH2B by insertion and coupling. Organometallics 2001, 20, 3962. [Google Scholar] [CrossRef]
- Bonet, A.; Gulyas, H.; Koshevoy, I.O.; Estevan, F.; Sanau, M.; Úbeda, M.A.; Fernandez, E. Tandem β-boration/arylation of α, β-unsaturated carbonyl compounds by using a single palladium complex to catalyse both steps. Chem.–A Eur. J. 2010, 16, 6382. [Google Scholar] [CrossRef]
- Lawson, Y.; Norman, N.; Rice, C.; Marder, T. Platinum catalysed 1,4-diboration of α, β-unsaturated ketones. Chem. Commun. 1997, 2051. [Google Scholar] [CrossRef]
- Kajiwara, T.; Terabayashi, T.; Yamashita, M.; Nozaki, K. Syntheses, structures, and reactivities of borylcopper and-zinc compounds: 1, 4-silaboration of an α, β-unsaturated ketone to form a γ-siloxyallylborane. Angew. Chem. 2008, 120, 6708. [Google Scholar] [CrossRef]
- Hirano, K.; Yorimitsu, H.; Oshima, K. Nickel-catalyzed β-boration of α, β-unsaturated esters and amides with bis(pinacolato)diboron. Org. Lett. 2007, 9, 5031. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Yamanaka, H.; Tateiwa, J.; Hosomi, A. Boration of an α, β-enone using a diboron promoted by a copper(I)–phosphine mixture catalyst. Tetrahedron Lett. 2000, 41, 6821. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722. [Google Scholar] [CrossRef] [PubMed]
- Kitanosono, T.; Xu, P.; Kobayashi, S. Heterogeneous versus homogeneous copper(II) catalysis in enantioselective conjugate-addition reactions of boron in water. Chem.–Asian J. 2014, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Hvolbæk, B.; Abild-Pedersen, F.; Chorkendorff, I.; Christensen, C.H. The nature of the active site in heterogeneous metal catalysis. Chem. Soc. Rev. 2008, 37, 2163. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358. [Google Scholar] [CrossRef]
- Rezayat, M.; Blundell, R.K.; Camp, J.E.; Walsh, D.A.; Thielemans, W. Green one-step synthesis of catalytically active palladium nanoparticles supported on cellulose nanocrystals. ACS Sustain. Chem. Eng. 2014, 2, 1241. [Google Scholar] [CrossRef]
- Pinnavaia, T.J. Intercalated clay catalysts. Science 1983, 220, 365. [Google Scholar] [CrossRef]
- Kumar, D.D.; Jyoti, B.B.; Pollov, S.P. Recent advances in metal nanoparticles stabilization into nanopores of montmorillonite and their catalytic applications for fine chemicals synthesis. Catal. Rev. 2015, 57, 257. [Google Scholar] [CrossRef]
- Bhat, A.H.; Rangreez, T.A.; Chisti, H. Wastewater treatment and biomedical applications of montmorillonite based nanocomposites: A review. Curr. Anal. Chem. 2022, 18, 269. [Google Scholar] [CrossRef]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated carbon from lignocellulosic biomass as catalyst: A review of the applications in fast pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Ocón, P.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Highly efficient application of activated carbon as catalyst for wet peroxide oxidation. Appl. Catal. B Environ. 2013, 140, 663. [Google Scholar] [CrossRef]
- Matatov-Meytal, U.; Sheintuch, M. Activated carbon cloth-supported Pd–Cu catalyst: Application for continuous water denitrification. Catal. Today 2005, 102, 121. [Google Scholar] [CrossRef]
- Lee, M.; Chen, B.; Den, W. Chitosan as a natural polymer for heterogeneous catalysts support: A short review on its applications. Appl. Sci. 2015, 5, 1272. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sahu, P.K.; Gupta, S.K.; Agarwal, D.D. Chitosan: An efficient, reusable, and biodegradable catalyst for green synthesis of heterocycles. Ind. Eng. Chem. Res. 2014, 53, 2085. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344. [Google Scholar] [CrossRef]
- El, K.A. Chitosan as a sustainable organocatalyst: A concise overview. ChemSusChem 2015, 8, 217. [Google Scholar]
- Mironenko, A.Y.; Sergeev, A.; Nazirov, A.; Modin, E.; Voznesenskiy, S.; Bratskaya, S.Y. H2S optical waveguide gas sensors based on chitosan/Au and chitosan/Ag nanocomposites. Sens. Actuators B Chem. 2016, 225, 348. [Google Scholar] [CrossRef]
- Murugadoss, A.; Chattopadhyay, A. A ‘green’ chitosan–silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology 2007, 19, 015603. [Google Scholar] [CrossRef]
- Baig, R.N.; Nadagouda, M.N.; Varma, R.S. Ruthenium on chitosan: A recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem. 2014, 16, 2122. [Google Scholar] [CrossRef]
- Rafiee, F. Recent advances in the application of chitosan and chitosan derivatives as bio supported catalyst in the cross coupling reactions. Curr. Org. Chem. 2019, 23, 390. [Google Scholar] [CrossRef]
- Pei, X.; Zheng, X.; Liu, X.; Lei, A.; Zhang, L.; Yin, X. Facile fabrication of highly dispersed Pd catalyst on nanoporous chitosan and its application in environmental catalysis. Carbohydr. Polym. 2022, 286, 119313. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, X.; Shao, L.; Qi, C.; Zhang, X. Highly porous chitosan microspheres supported palladium catalyst for coupling reactions in organic and aqueous solutions. J. Organomet. Chem. 2012, 704, 29. [Google Scholar] [CrossRef]
- Xie, H.; Yue, H.; Zhang, W.; Hu, W.; Zhou, X.; Prinsen, P.; Luque, R. A chitosan modified Pt/SiO2 catalyst for the synthesis of 3-poly (ethylene glycol) propyl ether-heptamethyltrisiloxane applied as agricultural synergistic agent. Catal. Commun. 2018, 104, 118. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Yu, W.; Zhang, P. A highly active and easily recoverable chitosan@copper catalyst for the C–S coupling and its application in the synthesis of zolimidine. Green Chem. 2014, 16, 3007. [Google Scholar] [CrossRef]
- Mounir, C.; Ahlafi, H.; Aazza, M.; Moussout, H.; Mounir, S. Kinetics and langmuir–hinshelwood mechanism for the catalytic reduction of para-nitrophenol over Cu catalysts supported on chitin and chitosan biopolymers. React. Kinet. Mech. Catal. 2021, 134, 285. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, M.; Zhu, Z.; Chen, Y.; He, H.; Qin, T. Chitosan modified Cu2O nanoparticles with high catalytic activity for p-nitrophenol reduction. Appl. Surf. Sci. 2019, 480, 601. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Qin, C.; Zhu, L. A green and recyclable chitosan supported catalyst for the borylation of α, β-unsaturated acceptors in water. Catal. Commun. 2016, 86, 23. [Google Scholar]
- Zhou, L.; Han, B.; Zhang, Y.; Li, B.; Wang, L.; Wang, J.; Wang, X.; Zhu, L. Cellulosic CuI nanoparticles as a heterogeneous, recyclable catalyst for the borylation of α, β-unsaturated acceptors in aqueous media. Catal. Lett. 2021, 151, 3220. [Google Scholar] [CrossRef]
- Wen, W.; Han, B.; Yan, F.; Ding, L.; Li, B.; Wang, L.; Zhu, L. Borylation of α, β-unsaturated acceptors by chitosan composite film supported copper nanoparticles. Nanomaterials 2018, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.W.; John, T.G.H.; David, O.H.; Richard, J.R. Tropic acid biosynthesis: The incorporation of (RS)-phenyl[2-18O,2-2H]lactate into littorine and hyoscyamine in datura stramonium. Chem. Commun. 1998, 1045. [Google Scholar]
- Ushimaru, R.; Ruszczycky, M.W.; Liu, H.-W. Changes in regioselectivity of H atom abstraction during the hydroxylation and cyclization reactions catalyzed by hyoscyamine 6β-Hydroxylase. J. Am. Chem. Soc. 2019, 141, 1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ren, B.-H.; Liu, X.-F.; Wang, L.-L.; Zhang, M.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Direct and selective electrocarboxylation of styrene oxides with CO2 for accessing β-hydroxy acids. Angew. Chem. Int. Ed. 2022, 61, e202207660. [Google Scholar]
- Guillaume, D.; Nicklas, S.; Kálmán, J.S.; Varinder, K.A. Direct synthesis of functionalized allylic boronic esters from allylic alcohols and inexpensive reagents and catalysts. Synthesis 2008, 14, 2293. [Google Scholar]
- Veeraraghavan, R.; Debarshi, P.; Debanjan, B.; Amit, S.; Venkat, R.R. Novel functionalized trisubstituted allylboronates via hosomi−miyaura borylation of functionalized allyl acetates. Org. Lett. 2004, 6, 481. [Google Scholar]
- Soumya, M.; Srinivas, R.G.; Robert, S.C. Total synthesis of the eupomatilones. J. Org. Chem. 2007, 72, 8724. [Google Scholar]
- Veeraraghavan, R.; Debarshi, P.; Hari, N.G.N.; Matthew, W.; Sadie, S.; Michele, T.Y.; Huangbing, W.; Max, S. Tailored a-methylene-c-butyrolactones and their effects on growth suppression in pancreatic carcinoma cells. Bioorg. Med. Chem. Lett. 2010, 20, 6620. [Google Scholar]
- Veeraraghavan, R.; Hari, N.G.N.; Pravin, G. Syntheses of β- and γ-fluorophenyl cis- and trans-α-methylene-γ-butyrolactones. Tetrahedron Lett. 2014, 55, 5722. [Google Scholar]
- Henry, J.H.; Nader, S.A.; Rusha, P.; Mohamed, N.S.; Veeraraghavan, R. β,γ-Diaryl α-methylene-γ-butyrolactones as potent antibacterials against methicillin-resistant Staphylococcus aureus. Bioorg. Chem. 2020, 104, 104183. [Google Scholar]
- Daniel, P.D.; Chong-Lei, J.; Peng, L.; Kay, M.B. Thiol reactivity of n-aryl α-methylene-γ-lactams: Influence of the guaianolide structure. J. Org. Chem. 2022, 87, 11204. [Google Scholar]
- Xuan, Q.; Wei, Y.; Chen, J.; Song, Q. An expedient E-stereoselective synthesis of multi-substituted functionalized allylic boronates from Morita-Baylis-Hillman alcohols. Org. Chem. Front. 2017, 4, 1220. [Google Scholar] [CrossRef]
- George, W.K.; Venkataiah, B.; Dong, G. Pd-catalyzed cross-coupling of baylis-hillman acetate adducts with bis(pinacolato)diboron: An efficient route to functionalized allyl borates. J. Org. Chem. 2004, 69, 5807. [Google Scholar]
- George, W.K.; Bollu, V. The total synthesis of eupomatilones 2 and 5. Tetrahedron Lett. 2005, 46, 7325. [Google Scholar]
- Feng, J.; Lei, X.; Bao, R.; Li, Y.; Xiao, C.; Hu, L.; Tang, Y. Enantioselective and collective total syntheses of xanthanolides. Angew. Chem. Int. Ed. 2017, 56, 16323. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, Q.; Zhao, Y.; Ding, C. Copper-promoted oxidative intramolecular C–H amination of hydrazones to synthesize 1H-indazoles and 1H-pyrazoles using a cleavable directing group. Eur. J. Org. Chem. 2019, 33, 5801. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, S.; Ke, C.; Liu, X.; Feng, X. Chiral lewis acid-catalyzed enantioselective cyclopropanation and C–H insertion reactions of vinyl ketones with α-diazoesters. Chem. Commun. 2018, 54, 9837. [Google Scholar] [CrossRef]
- Xia, B.; Xu, J.; Xiang, Z.; Cen, Y.; Hu, Y.; Lin, X.; Wu, Q. Stereoselectivity-tailored, metal-free hydrolytic dynamic kinetic resolution of Morita-Baylis-Hillman acetates using an engineered lipase–organic base cocatalyst. ACS Catal. 2017, 7, 4542. [Google Scholar] [CrossRef]


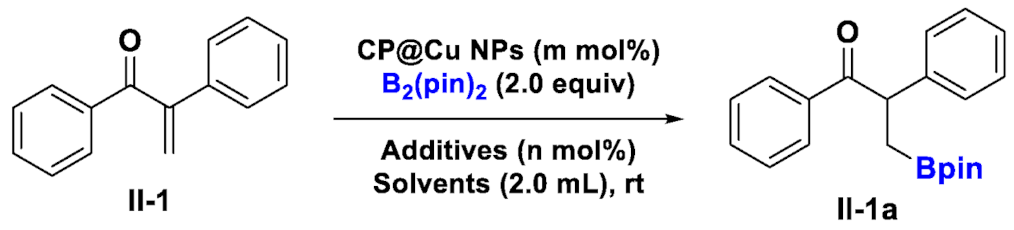 | |||||
| Entries | CP@Cu NPs | Solvents (2.0 mL) | Additives | Time (h) | NMR Yields (%) |
| 1 | (9.0 mol%) | THF | - | 12 | N.R. |
| 2 | (9.0 mol%) | Toluene | - | 12 | N.R. |
| 3 | (9.0 mol%) | Et2O | - | 12 | 8 |
| 4 | (9.0 mol%) | MeOH | - | 12 | 16 |
| 5 | (9.0 mol%) | Acetone | - | 12 | 8 |
| 6 | (9.0 mol%) | H2O | - | 12 | 19 |
| 7 | (9.0 mol%) | MeOH/H2O = 3:1 | - | 12 | 28 |
| 8 | (9.0 mol%) | MeOH/H2O = 3:1 | 2,2-bipyridine | 12 | 35 |
| 9 | (9.0 mol%) | MeOH/H2O = 3:1 | DMAP | 12 | 33 |
| 10 | (9.0 mol%) | MeOH/H2O = 3:1 | 2-cyanopyridine | 12 | 54 |
| 11 | (9.0 mol%) | MeOH/H2O = 3:1 | 2-chloropyridine | 12 | 57 |
| 12 | (9.0 mol%) | MeOH/H2O = 3:1 | 2-bromopyridine | 12 | 50 |
| 13 | (9.0 mol%) | MeOH/H2O = 3:1 | 2,6-dibromopyridine | 12 | 48 |
| 14 | (9.0 mol%) | MeOH/H2O = 3:1 | 4-picoline | 12 | 60 |
| 15 | (9.0 mol%) | MeOH/H2O = 2:1 | 4-picoline | 12 | 72 |
| 16 b | (9.0 mol%) | MeOH/H2O = 1:1 | 4-picoline | 12 | 92 |
| 17 | (9.0 mol%) | MeOH/H2O = 1:2 | 4-picoline | 12 | 89 |
| 18 | (9.0 mol%) | MeOH/H2O = 1:4 | 4-picoline | 12 | 80 |
| 19 | (9.0 mol%) | MeOH/H2O = 1:1 | 4-picoline | 4 | 79 |
| 20 | (9.0 mol%) | MeOH/H2O = 1:1 | 4-picoline | 8 | 87 |
| 21 b | (9.0 mol%) | MeOH/H2O = 1:1 | 4-picoline | 16 | 90 |
| 22 b,c | (9.0 mol%) | MeOH/H2O = 1:1 | 4-picoline | 12 | 90 |
| 23 b,d | (9.0 mol%) | MeOH/H2O = 1:1 | 4-picoline | 12 | 90 |
| 24 b | (4.5 mol%) | MeOH/H2O = 1:1 | 4-picoline | 12 | 92 |
| 25 b | (13.5 mol%) | MeOH/H2O = 1:1 | 4-picoline | 12 | 90 |
 | ||||
| Entries | Substrates | CP@Cu NPs | Solvents (2.0 mL) | NMR Yields (%) |
| 1 | III-1 | 5.0 mg | MeOH | 31 |
| 2 | IV-1 | 5.0 mg | MeOH | 45 |
| 3 | III-1 | 5.0 mg | H2O | 33 |
| 4 | IV-1 | 5.0 mg | H2O | 20 |
| 5 | III-1 | 5.0 mg | MeOH/H2O =3:1 | 94 |
| 6 | IV-1 | 5.0 mg | MeOH/H2O =3:1 | 90 |
| 7 b | III-1 | 5.0 mg | MeOH/H2O =2:1 | 93 |
| 8 b | IV-1 | 5.0 mg | MeOH/H2O =2:1 | 92 |
| 9 | III-1 | 5.0 mg | MeOH/H2O =1:1 | 90 |
| 10 | IV-1 | 5.0 mg | MeOH/H2O =1:1 | 86 |
| 11 | III-1 | 5.0 mg | MeOH/H2O =1:2 | 89 |
| 12 | IV-1 | 5.0 mg | MeOH/H2O =1:2 | 83 |
| 13 | III-1 | 5.0 mg | MeOH/H2O =1:3 | 88 |
| 14 | IV-1 | 5.0 mg | MeOH/H2O =1:3 | 85 |
| 15 | III-1 | 10.0 mg | MeOH/H2O =2:1 | 93 |
| 16 | IV-1 | 10.0 mg | MeOH/H2O =2:1 | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wen, W.; Wen, W.; Guo, H.; Fu, C.; Zhang, Y.; Zhu, L. Application of Chitosan/Poly(vinyl alcohol) Stabilized Copper Film Materials for the Borylation of α, β-Unsaturated Ketones, Morita-Baylis-Hillman Alcohols and Esters in Aqueous Phase. Molecules 2023, 28, 5609. https://doi.org/10.3390/molecules28145609
Li B, Wen W, Wen W, Guo H, Fu C, Zhang Y, Zhu L. Application of Chitosan/Poly(vinyl alcohol) Stabilized Copper Film Materials for the Borylation of α, β-Unsaturated Ketones, Morita-Baylis-Hillman Alcohols and Esters in Aqueous Phase. Molecules. 2023; 28(14):5609. https://doi.org/10.3390/molecules28145609
Chicago/Turabian StyleLi, Bojie, Wu Wen, Wei Wen, Haifeng Guo, Chengpeng Fu, Yaoyao Zhang, and Lei Zhu. 2023. "Application of Chitosan/Poly(vinyl alcohol) Stabilized Copper Film Materials for the Borylation of α, β-Unsaturated Ketones, Morita-Baylis-Hillman Alcohols and Esters in Aqueous Phase" Molecules 28, no. 14: 5609. https://doi.org/10.3390/molecules28145609
APA StyleLi, B., Wen, W., Wen, W., Guo, H., Fu, C., Zhang, Y., & Zhu, L. (2023). Application of Chitosan/Poly(vinyl alcohol) Stabilized Copper Film Materials for the Borylation of α, β-Unsaturated Ketones, Morita-Baylis-Hillman Alcohols and Esters in Aqueous Phase. Molecules, 28(14), 5609. https://doi.org/10.3390/molecules28145609