Modulatory Effect of Rosmarinic Acid on H2O2-Induced Adaptive Glycolytic Response in Dermal Fibroblasts
Abstract
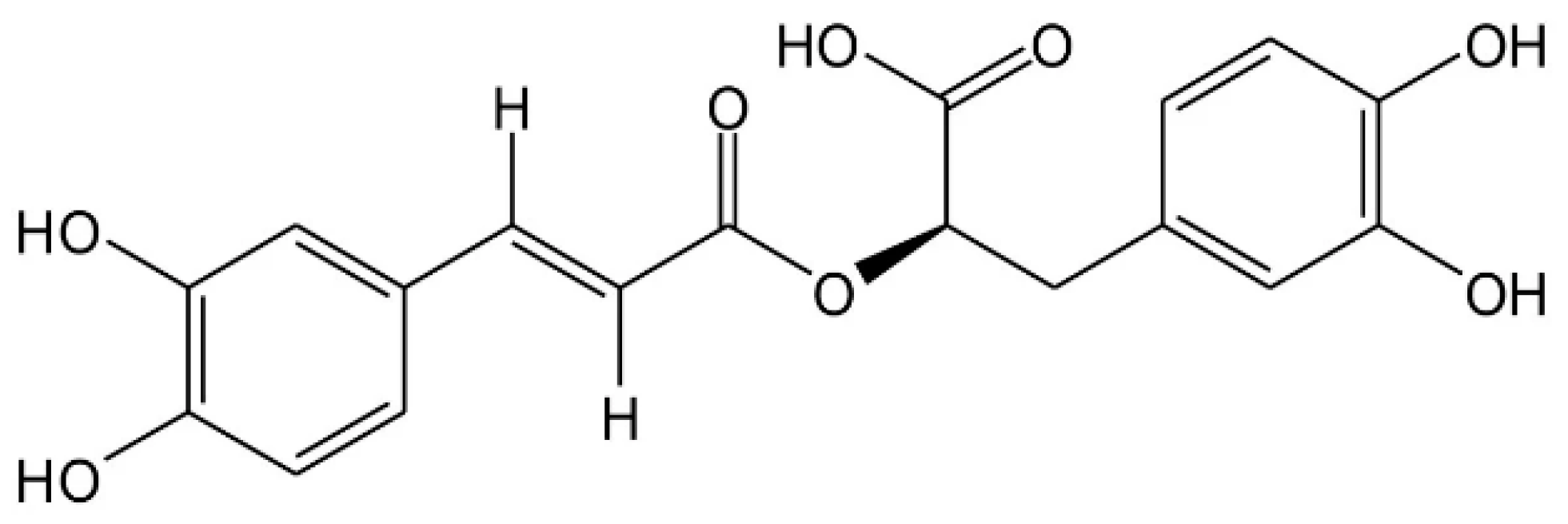
1. Introduction
2. Results
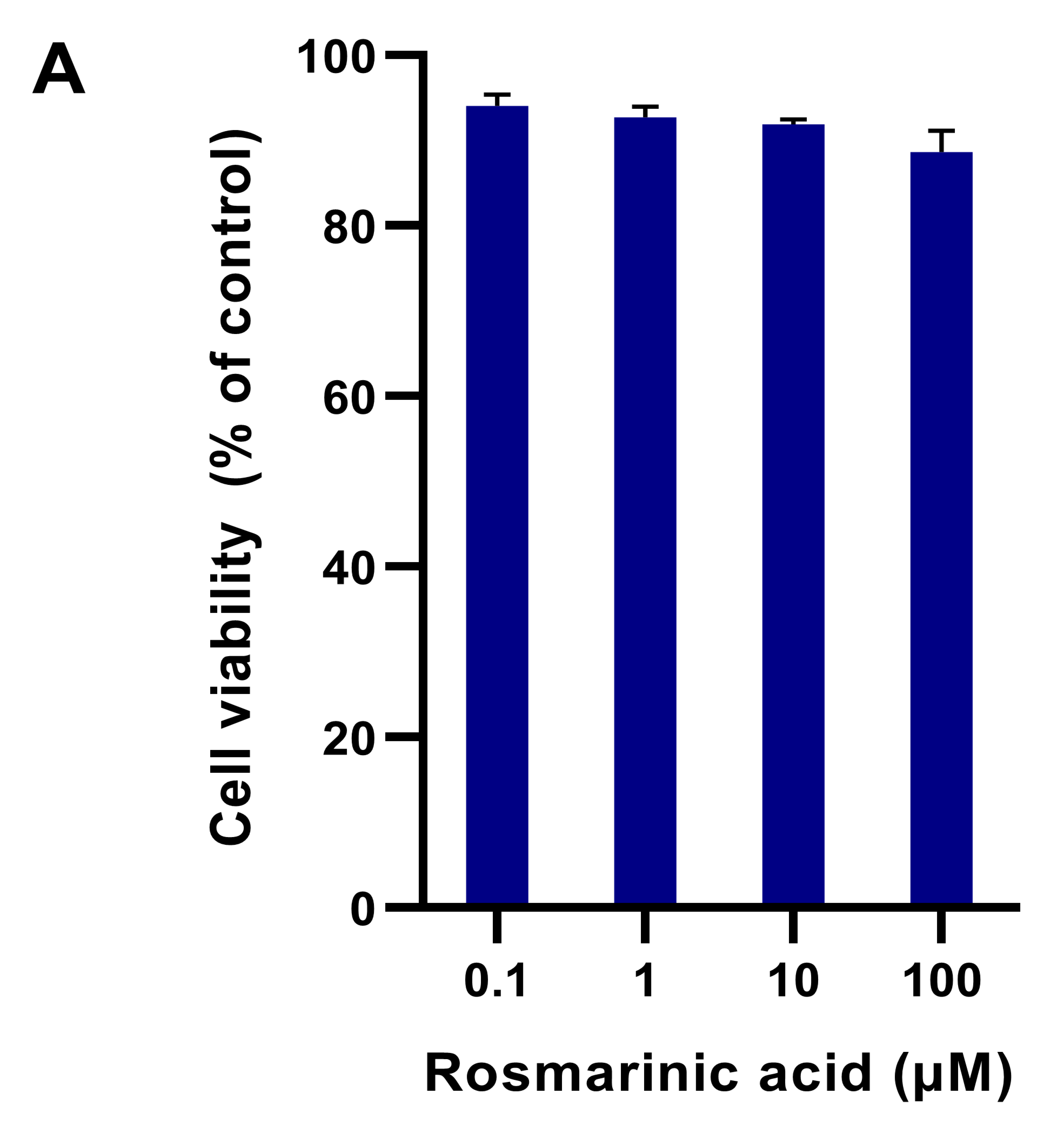
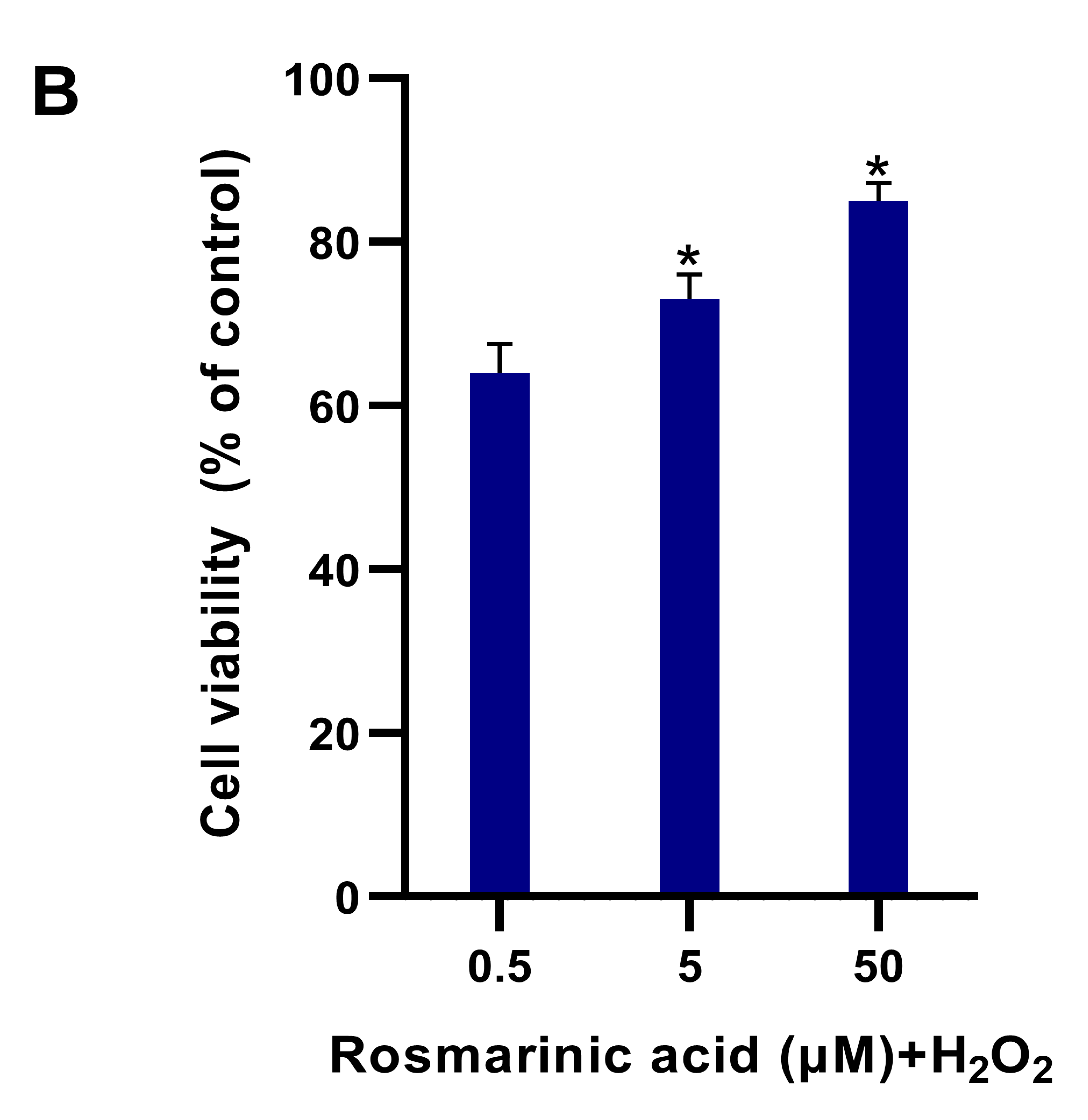
2.1. Fibroblast Viability in Different Treatments
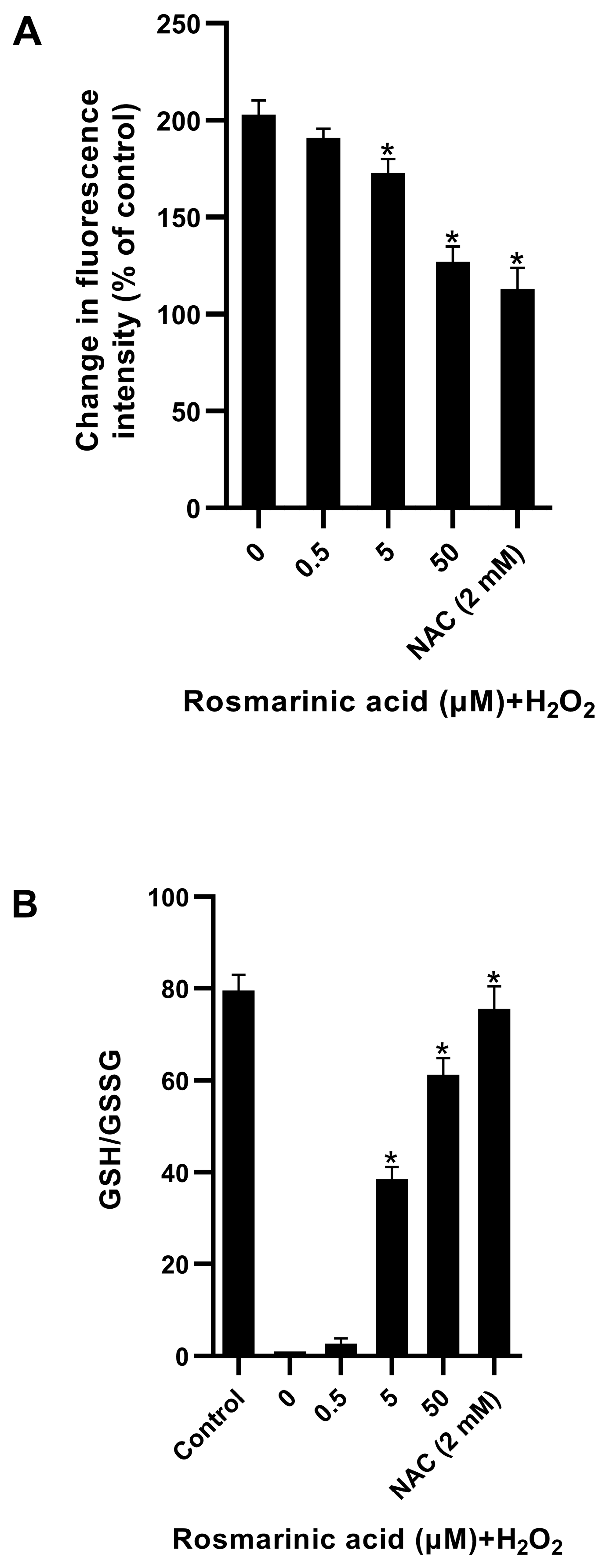
2.2. Effect of Rosmarinic Acid on H2O2-Induced ROS Formation in Fibroblasts
2.3. Modulatory Effect of Rosmarinic Acid on Glycolytic Adaptive Response Induced by H2O2
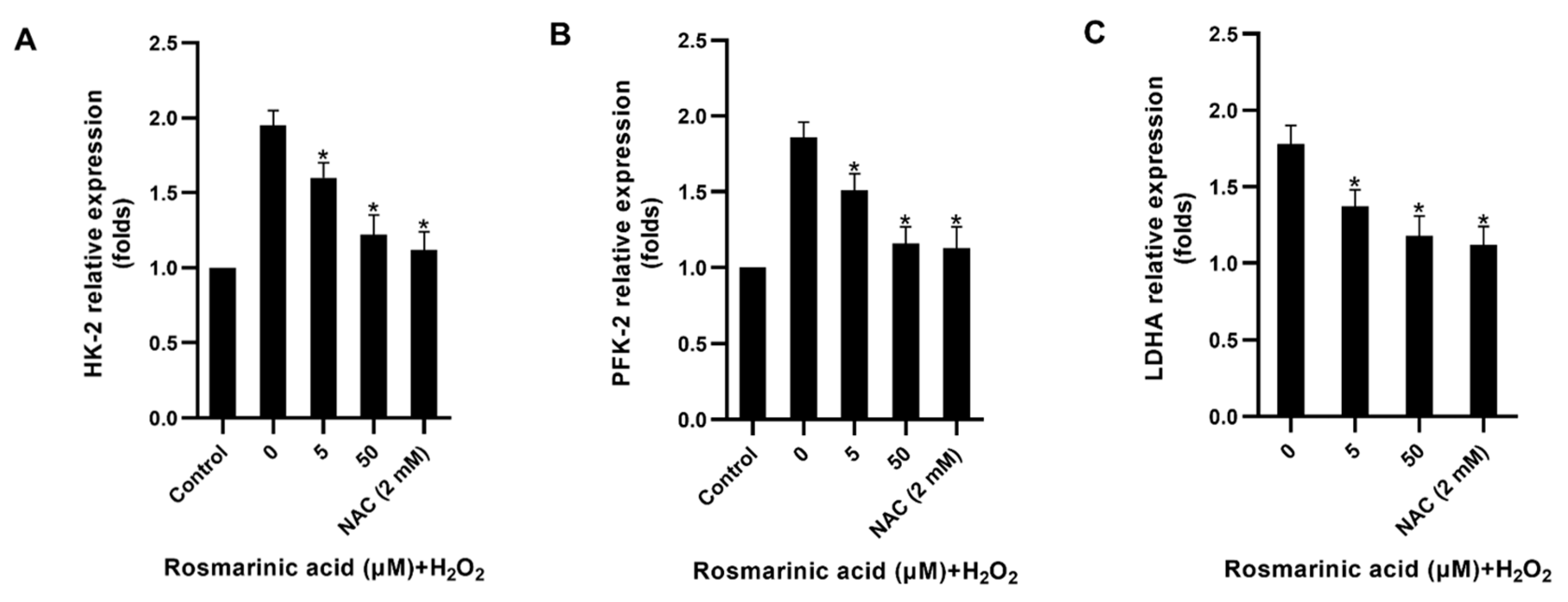
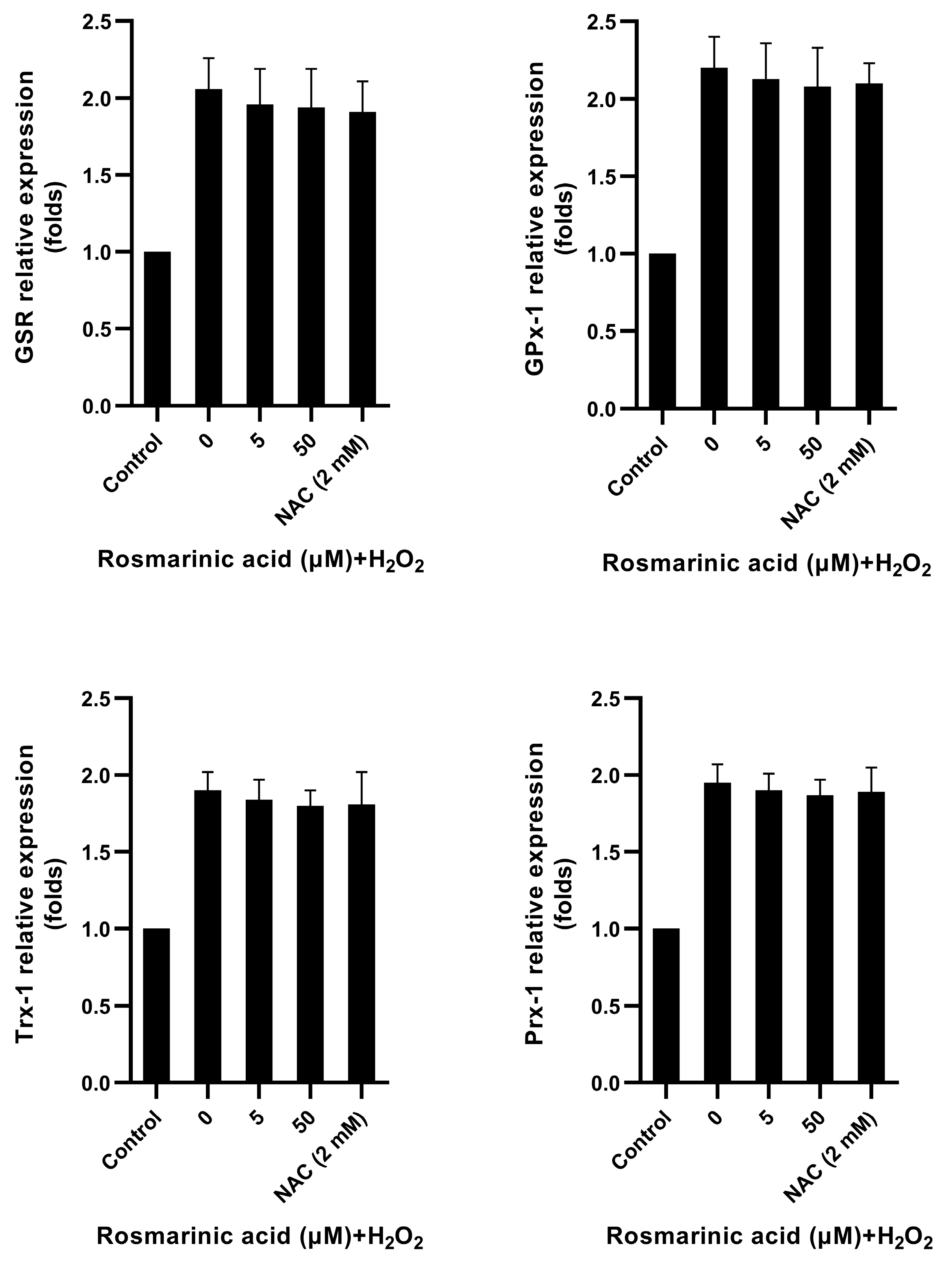
2.4. Effect of Rosmarinic Acid on Glycolytic Enzyme and Antioxidant Genes Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Treatments
4.3. Cytotoxicity Test
4.4. Reactive Oxygen Species (ROS) Measurement
4.5. Adenosine Triphosphate (ATP) Measurement
4.6. Determination of Glucose, Lactate, and NADPH/NADP+
4.7. GSH/GSSG Quantification
4.8. Ribonucleic Acid (RNA) Isolation and Quantitative Real-Time Polymerase Chain Reaction
4.9. Protein Quantification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Csekes, E.; Rackova, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Park, W.H. H2O2 inhibits the growth of human pulmonary fibroblast cells by inducing cell death, GSH depletion and G1 phase arrest. Mol. Med. Rep. 2013, 7, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Molavian, H.R.; Kohandel, M.; Sivaloganathan, S. High Concentrations of H2O2 Make Aerobic Glycolysis Energetically More Favorable for Cellular Respiration. Front. Physiol. 2016, 7, 362. [Google Scholar] [CrossRef][Green Version]
- Charoensin, S.; Eroglu, E.; Opelt, M.; Bischof, H.; Madreiter-Sokolowski, C.T.; Kirsch, A.; Depaoli, M.R.; Frank, S.; Schrammel, A.; Mayer, B.; et al. Intact mitochondrial Ca2+ uniport is essential for agonist-induced activation of endothelial nitric oxide synthase (eNOS). Free Radic. Biol. Med. 2017, 102, 248–259. [Google Scholar] [CrossRef]
- Wu, S.B.; Wei, Y.H. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: Implication of the cell survival in mitochondrial diseases. Biochim. Biophys. Acta 2012, 1822, 233–247. [Google Scholar] [CrossRef]
- Hariton, F.; Xue, M.; Rabbani, N.; Fowler, M.; Thornalley, P.J. Sulforaphane Delays Fibroblast Senescence by Curbing Cellular Glucose Uptake, Increased Glycolysis, and Oxidative Damage. Oxid. Med. Cell. Longev. 2018, 2018, 5642148. [Google Scholar] [CrossRef]
- Charoensin, S.; Weera, W. Preventive Effect of Nuciferine on H2O2-Induced Fibroblast Senescence and Pro-Inflammatory Cytokine Gene Expression. Molecules 2022, 27, 8148. [Google Scholar] [CrossRef]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef]
- Suttajit, M.; Thangaleela, S.; Sivamaruthi, B.S.; Charoensin, S. Dietary restriction, vegetarian diet, and aging intervention. In Plant Bioactives as Natural Panacea against Age-Induced Diseases: Nutraceuticals and Functional Lead Compounds for Drug Development; Elsevier: Amsterdam, The Netherlands, 2022; pp. 307–327. [Google Scholar]
- Kwon, D.Y.; Li, X.; Kim, J.K.; Park, S.U. Molecular cloning and characterization of rosmarinic acid biosynthetic genes and rosmarinic acid accumulation in Ocimum basilicum L. Saudi J. Biol. Sci. 2019, 26, 469–472. [Google Scholar] [CrossRef]
- Lamponi, S.; Baratto, M.C.; Miraldi, E.; Baini, G.; Biagi, M. Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis. Plants 2021, 10, 97. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef]
- Moazzami Farida, S.H.; Karamian, R.; Albrectsen, B.R. Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage. Physiol. Plant. 2020, 170, 415–432. [Google Scholar] [CrossRef]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive Effects of Rosmarinic Acid Rich Fraction from Perilla on Oxidative Stress, Inflammation and Metastasis Ability in A549 Cells Exposed to PM via C-Jun, p-65-NF-κB and Akt Signaling Pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Jin, Y.; Lin, A.; Wang, P.; Wang, Y.; Zhu, H. Rosmarinic acid relieves cisplatin-induced ovary toxicity in female mice via suppression of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22839. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, Y.; Yan, H.Z.; Xia, Z.R.; Wen, W.; Liu, D.Y.; Wan, L.H. Rosmarinic acid ameliorates acetaminophen-induced acute liver injury in mice via RACK1/TNF-α mediated antioxidant effect. Pharm. Biol. 2021, 59, 1286–1293. [Google Scholar] [CrossRef]
- Kangwan, N.; Pintha, K.; Lekawanvijit, S.; Suttajit, M. Rosmarinic Acid Enriched Fraction from Perilla frutescens Leaves Strongly Protects Indomethacin-Induced Gastric Ulcer in Rats. BioMed Res. Int. 2019, 2019, 9514703. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; An, I.S.; Ahn, K.J.; Han, H.J. Protective effects of rosmarinic acid against hydrogen peroxide-induced cellular senescence and the inflammatory response in normal human dermal fibroblasts. Mol. Med. Rep. 2017, 16, 9763–9769. [Google Scholar] [CrossRef]
- Sutkowska, J.; Hupert, N.; Gawron, K.; Strawa, J.W.; Tomczyk, M.; Forlino, A.; Galicka, A. The Stimulating Effect of Rosmarinic Acid and Extracts from Rosemary and Lemon Balm on Collagen Type I Biosynthesis in Osteogenesis Imperfecta Type I Skin Fibroblasts. Pharmaceutics 2021, 13, 938. [Google Scholar] [CrossRef]
- Matwiejczuk, N.; Galicka, A.; Zareba, I.; Brzoska, M.M. The Protective Effect of Rosmarinic Acid Against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients 2020, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S. Regulation of oxidative phosphorylation in the mammalian cell. Am. J. Physiol. 1990, 258, C377–C389. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardini, C.; Trombetti, F.; Schena, E.; Zannoni, A.; Forni, M.; Nesci, S. Cellular Metabolism and Bioenergetic Function in Human Fibroblasts and Preadipocytes of Type 2 Familial Partial Lipodystrophy. Int. J. Mol. Sci. 2022, 23, 8659. [Google Scholar] [CrossRef]
- Hurbain, J.; Thommen, Q.; Anquez, F.; Pfeuty, B. Quantitative modeling of pentose phosphate pathway response to oxidative stress reveals a cooperative regulatory strategy. iScience 2022, 25, 104681. [Google Scholar] [CrossRef]
- Mine, Y.; Takahashi, T.; Okamoto, T. Protective effects of coenzyme Q10 on cell damage induced by hydrogen peroxides in cultured skin fibroblasts. J. Clin. Biochem. Nutr. 2021, 69, 247–255. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hong, Y.; Zhang, T.Y.; Chen, Y.X.; Wei, Z.J.; Gao, C.Y. Rosmarinic acid exerts anti-inflammatory effect and relieves oxidative stress via Nrf2 activation in carbon tetrachloride-induced liver damage. Food Nutr. Res. 2022, 66, 8359. [Google Scholar] [CrossRef]
- Cai, X.; Yang, F.; Zhu, L.; Xia, Y.; Wu, Q.; Xue, H.; Lu, Y. Rosmarinic Acid, the Main Effective Constituent of Orthosiphon stamineus, Inhibits Intestinal Epithelial Apoptosis Via Regulation of the Nrf2 Pathway in Mice. Molecules 2019, 24, 3027. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Julsrigival, J.; Ungsurungsie, M.; Samuhasaneetoo, S.; Chulasiri, P.; Kwankhao, P.; Pitiporn, S.; Ogata, F.; Kawasaki, N.; et al. Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves. Plants 2021, 10, 1648. [Google Scholar] [CrossRef]

| Target Genes | Forward Primers | Reverse Primers |
|---|---|---|
| HK-2 | GAGTTTGACCTGGATGTGGTTGC | CCTCCATGTAGCAGGCATTGCT |
| PFK-2 | TACCGACCTCTTGACCCAGACA | TAAATGGTGCGAGGCTGGACGT |
| LDHA | GGATCTCCAACATGGCAGCCTT | AGACGGCTTTCTCCCTCTTGCT |
| GSR | TATGTGAGCCGCCTGAATGCCA | CACTGACCTCTATTGTGGGCTTG |
| GPx-1 | GTGCTCGGCTTCCCGTGCAAC | CTCGAAGAGCATGAAGTTGGGC |
| Trx-1 | GTAGTTGACTTCTCAGCCACGTG | CTGACAGTCATCCACATCTACTTC |
| Prx-1 | CTGCCAAGTGATTGGTGCTTCTG | AATGGTGCGCTTCGGGTCTGAT |
| β-actin | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoensin, S.; Dansakda, S. Modulatory Effect of Rosmarinic Acid on H2O2-Induced Adaptive Glycolytic Response in Dermal Fibroblasts. Molecules 2023, 28, 5599. https://doi.org/10.3390/molecules28145599
Charoensin S, Dansakda S. Modulatory Effect of Rosmarinic Acid on H2O2-Induced Adaptive Glycolytic Response in Dermal Fibroblasts. Molecules. 2023; 28(14):5599. https://doi.org/10.3390/molecules28145599
Chicago/Turabian StyleCharoensin, Suphachai, and Suwatsak Dansakda. 2023. "Modulatory Effect of Rosmarinic Acid on H2O2-Induced Adaptive Glycolytic Response in Dermal Fibroblasts" Molecules 28, no. 14: 5599. https://doi.org/10.3390/molecules28145599
APA StyleCharoensin, S., & Dansakda, S. (2023). Modulatory Effect of Rosmarinic Acid on H2O2-Induced Adaptive Glycolytic Response in Dermal Fibroblasts. Molecules, 28(14), 5599. https://doi.org/10.3390/molecules28145599







