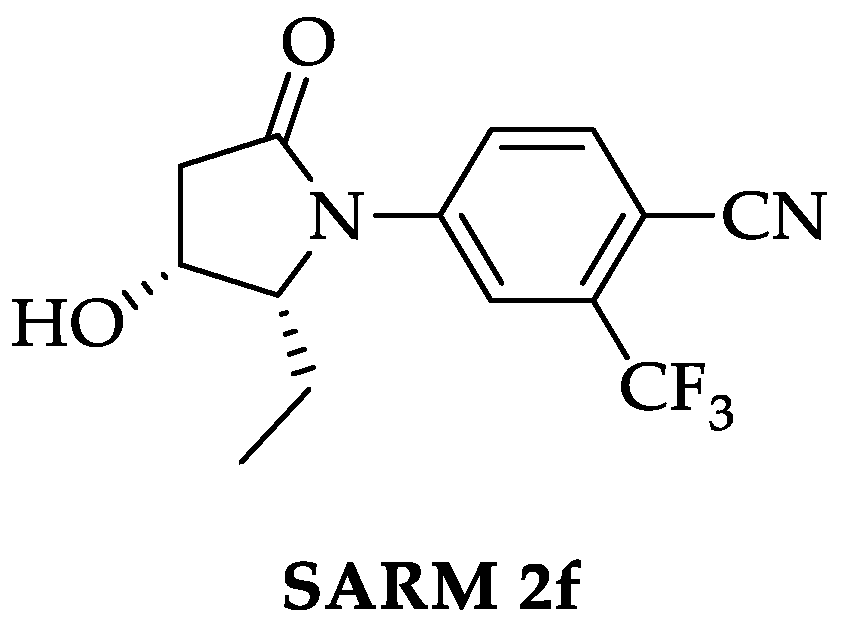
Identification and Synthesis of Selected In Vitro Generated Metabolites of the Novel Selective Androgen Receptor Modulator (SARM) 2f
Abstract
1. Introduction
2. Results and Discussion
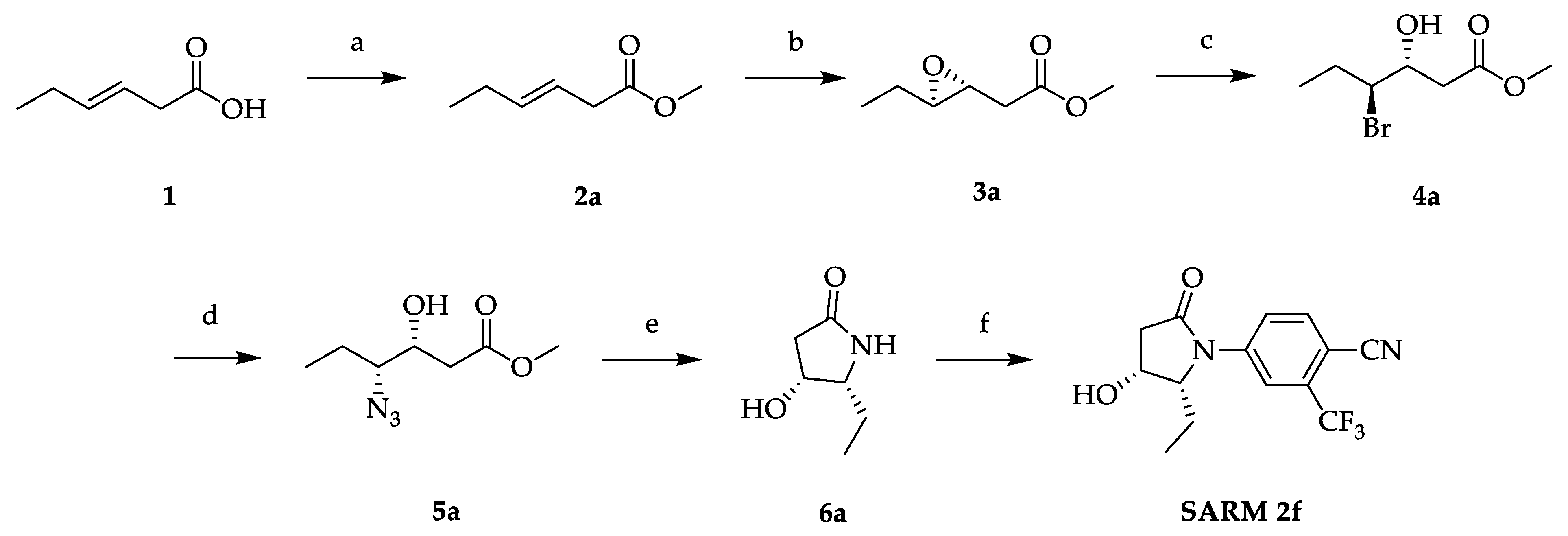
2.1. Synthesis of SARM 2f
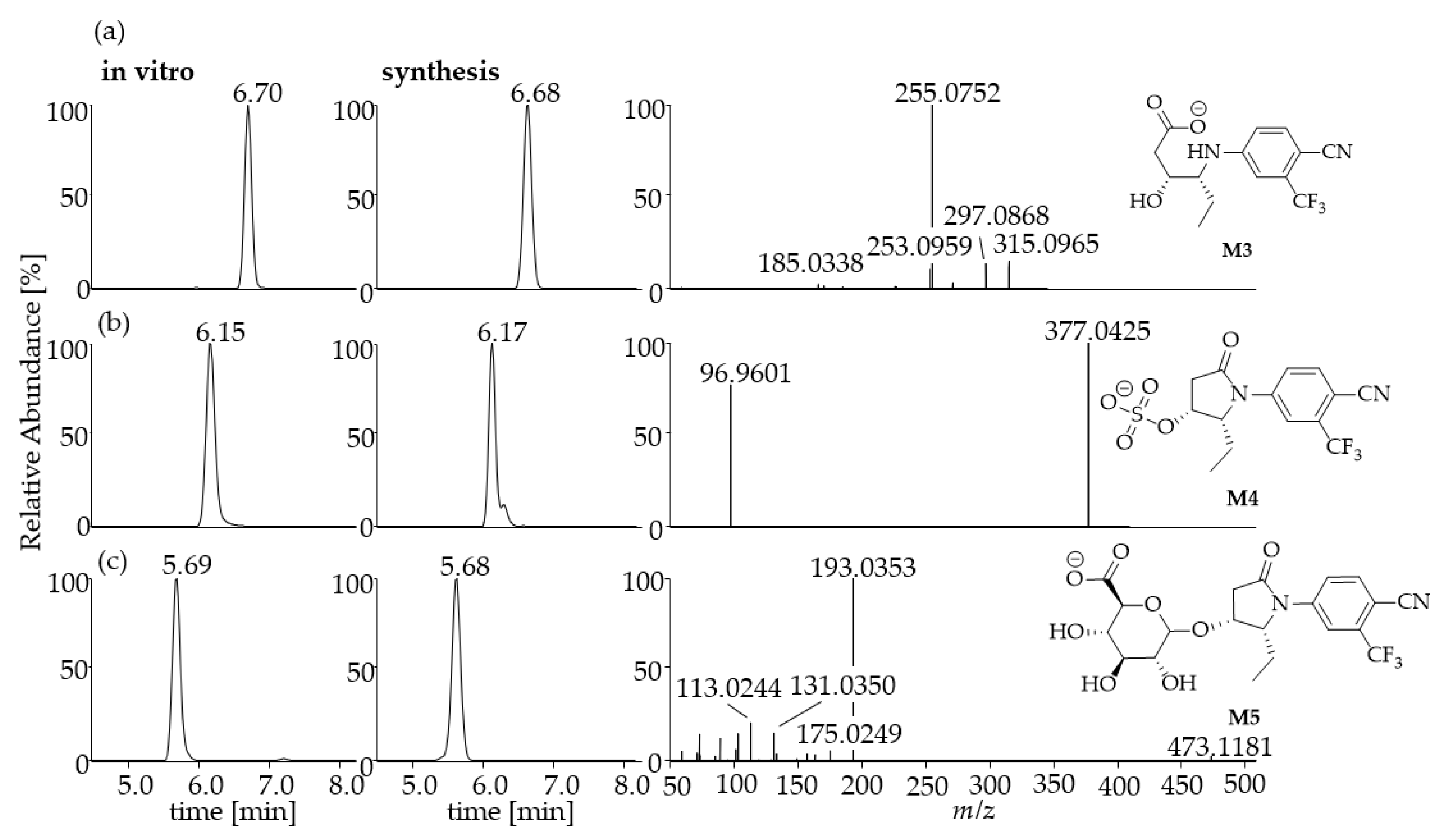
2.2. In Vitro Generated Metabolites
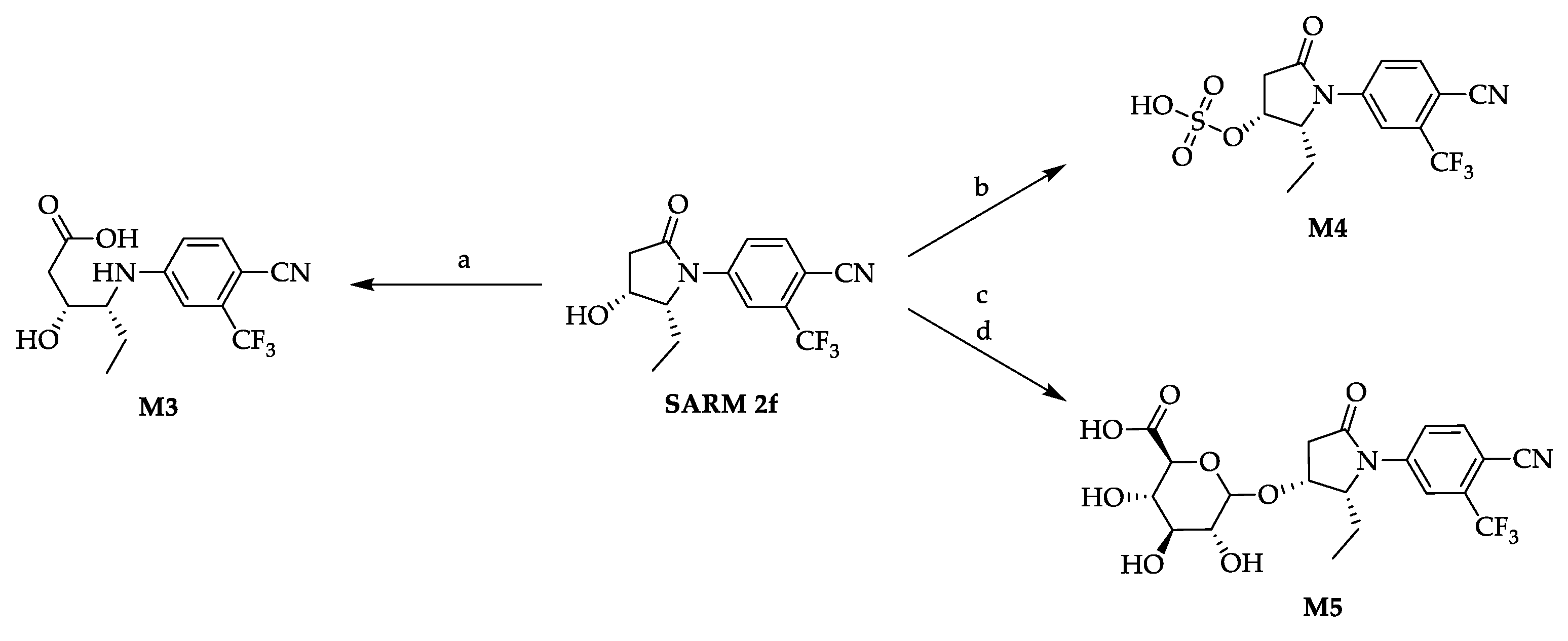
2.3. Synthesis of Selected In Vitro Generated Metabolites
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. NMR Spectroscopy
3.3. Synthesis
3.4. In Vitro Metabolic Assay
3.5. LC-HRMS
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gao, W.; Dalton, J.T. Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discov. Today 2007, 12, 241–248. [Google Scholar] [CrossRef]
- Bhasin, S.; Jasuja, R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Büttner, A.; Thieme, D. Side effects of anabolic androgenic steroids: Pathological findings and structure-activity relationships. Handb. Exp. Pharmacol. 2010, 195, 459–484. [Google Scholar]
- Nieminen, M.S.; Rämö, M.P.; Viitasalo, M.; Heikkilä, P.; Karjalainen, J.; Mäntysaari, M.; Heikkila, J. Serious cardiovascular side effects of large doses of anabolic steroids in weight lifters. Eur. Heart J. 1996, 17, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Turner, L.; Walters, W.A.; Handelsman, D.J. The effects of chronic high dose androgen or estrogen treatment on the human prostate [corrected]. J. Clin. Endocrinol. Metab. 1996, 81, 4290–4295. [Google Scholar] [CrossRef]
- Dalton, J.T. The long and winding road for selective androgen receptor modulators. Br. J. Clin. Pharmacol. 2017, 83, 2131–2133. [Google Scholar] [CrossRef]
- Hoffmann, D.B.; Komrakova, M.; Pflug, S.; von Oertzen, M.; Saul, D.; Weiser, L.; Walde, T.A.; Wassmann, M.; Schilling, A.F.; Lehmann, W.; et al. Evaluation of ostarine as a selective androgen receptor modulator in a rat model of postmenopausal osteoporosis. J. Bone Miner. Metab. 2019, 37, 243–255. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Sullivan, R.D.; You, D.; Zafar, N.; He Yang, C.; Thiyagarajan, T.; Johnson, D.L.; Barrett, M.L.; Koehler, N.J.; Star, M.; et al. Androgen receptor agonists increase lean mass, improve cardiopulmonary functions and extend survival in preclinical models of Duchenne muscular dystrophy. Hum. Mol. Genet. 2017, 26, 2526–2540. [Google Scholar] [CrossRef]
- Srinath, R.; Dobs, A. Enobosarm (GTx-024, S-22): A potential treatment for cachexia. Future Oncol. 2014, 10, 187–194. [Google Scholar] [CrossRef]
- World Anti Doping Agency prohibited List. Available online: https://www.wada-ama.org/en/news/wada-publishes-2023-prohibited-list (accessed on 14 March 2023).
- World Anti Doping Agency Testing Figures Report. Available online: https://www.wada-ama.org/en/resources/anti-doping-stats/anti-doping-testing-figures-report (accessed on 14 March 2023).
- Thevis, M.; Schänzer, W. Detection of SARMs in doping control analysis. Mol. Cell. Endocrinol. 2018, 464, 34–45. [Google Scholar] [CrossRef]
- Aikawa, K.; Asano, M.; Ono, K.; Habuka, N.; Yano, J.; Wilson, K.; Fujita, H.; Kandori, H.; Hara, T.; Morimoto, M.; et al. Synthesis and biological evaluation of novel selective androgen receptor modulators (SARMs) Part III: Discovery of 4-(5-oxopyrrolidine-1-yl)benzonitrile derivative 2f as a clinical candidate. Bioorg. Med. Chem. 2017, 25, 3330–3349. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Amano, Y.; Oka, M.; Harada, A.; Fujita, H.; Hikichi, Y.; Tozawa, R.; Yamaoka, M.; Hara, T. Amelioration of sexual behavior and motor activity deficits in a castrated rodent model with a selective androgen receptor modulator SARM-2f. PLoS ONE 2017, 12, e0189480. [Google Scholar] [CrossRef]
- Nyquist, M.D.; Ang, L.S.; Corella, A.; Coleman, I.M.; Meers, M.P.; Christiani, A.J.; Pierce, C.; Janssens, D.H.; Meade, H.E.; Bose, A.; et al. Selective androgen receptor modulators activate the canonical prostate cancer androgen receptor program and repress cancer growth. J. Clin. Investig. 2021, 131, e146777. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Yamaoka, M.; Hara, T. A selective androgen receptor modulator SARM-2f activates androgen receptor, increases lean body mass, and suppresses blood lipid levels in cynomolgus monkeys. Pharmacol. Res. Perspect. 2020, 8, e00563. [Google Scholar] [CrossRef] [PubMed]
- Bisol, T.B.; Bortoluzzi, A.J.; Sá, M.M. Nucleophilic Ring-Opening of Epoxide and Aziridine Acetates for the Stereodivergent Synthesis of β-Hydroxy and β-Amino γ-Lactams. J. Org. Chem. 2011, 76, 948–962. [Google Scholar] [CrossRef]
- Azzena, F.; Crotti, P.; Favero, L.; Pineschi, M. Regiochemical control of the ring opening of 1,2-Epoxides by means of chelating processes. 11. Ring opening reactions of aliphatic mono- and difunctionalized cis and trans 2,3- and 3,4-Epoxy Esters. Tetrahedron 1995, 51, 13409–13422. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, M.M.; Huffman, M.A.; McNamara, J.M. Pd-Catalyzed N-Arylation of Heteroarylamines. Org. Lett. 2002, 4, 3481–3484. [Google Scholar] [CrossRef]
- Thevis, M.; Lagojda, A.; Kuehne, D.; Thomas, A.; Dib, J.; Hansson, A.; Hedeland, M.; Bondesson, U.; Wigger, T.; Karst, U.; et al. Characterization of a non-approved selective androgen receptor modulator drug candidate sold via the Internet and identification of in vitro generated phase-I metabolites for human sports drug testing. Rapid Commun. Mass Spectrom. 2015, 29, 991–999. [Google Scholar] [CrossRef]
- Pitsinos, E.N.; Angelis, Y.S.; Petrou, M. Structure revision and chemical synthesis of ligandrol’s main bishydroxylated long-term metabolic marker. Org. Biomol. Chem. 2022, 20, 9112–9116. [Google Scholar] [CrossRef]
- Waller, C.C.; McLeod, M.D. A simple method for the small scale synthesis and solid-phase extraction purification of steroid sulfates. Steroids 2014, 92, 74–80. [Google Scholar] [CrossRef]
- Koenigs, W.; Knorr, E. Ueber einige Derivate des Traubenzuckers und der Galactose. Berichte Der Dtsch. Chem. Ges. 1901, 34, 957–981. [Google Scholar] [CrossRef]
- Herzig, J.; Nudelman, A.; Gottlieb, H.E.; Fischer, B. Studies in sugar chemistry. 2. A simple method for O-deacylation of polyacylated sugars. J. Org. Chem. 1986, 51, 727–730. [Google Scholar] [CrossRef]
- Kuuranne, T.; Leinonen, A.; Schänzer, W.; Kamber, M.; Kostiainen, R.; Thevis, M. Aryl-propionamide-derived selective androgen receptor modulators: Liquid chromatography-tandem mass spectrometry characterization of the in vitro synthesized metabolites for doping control purposes. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Peeters, L.; Vervliet, P.; Foubert, K.; Hermans, N.; Pieters, L.; Covaci, A. A comparative study on the in vitro biotransformation of medicagenic acid using human liver microsomes and S9 fractions. Chem. Biol. Interact. 2020, 328, 109192. [Google Scholar] [CrossRef]
- Van den Eede, N.; Maho, W.; Erratico, C.; Neels, H.; Covaci, A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 2013, 223, 9–15. [Google Scholar] [CrossRef] [PubMed]

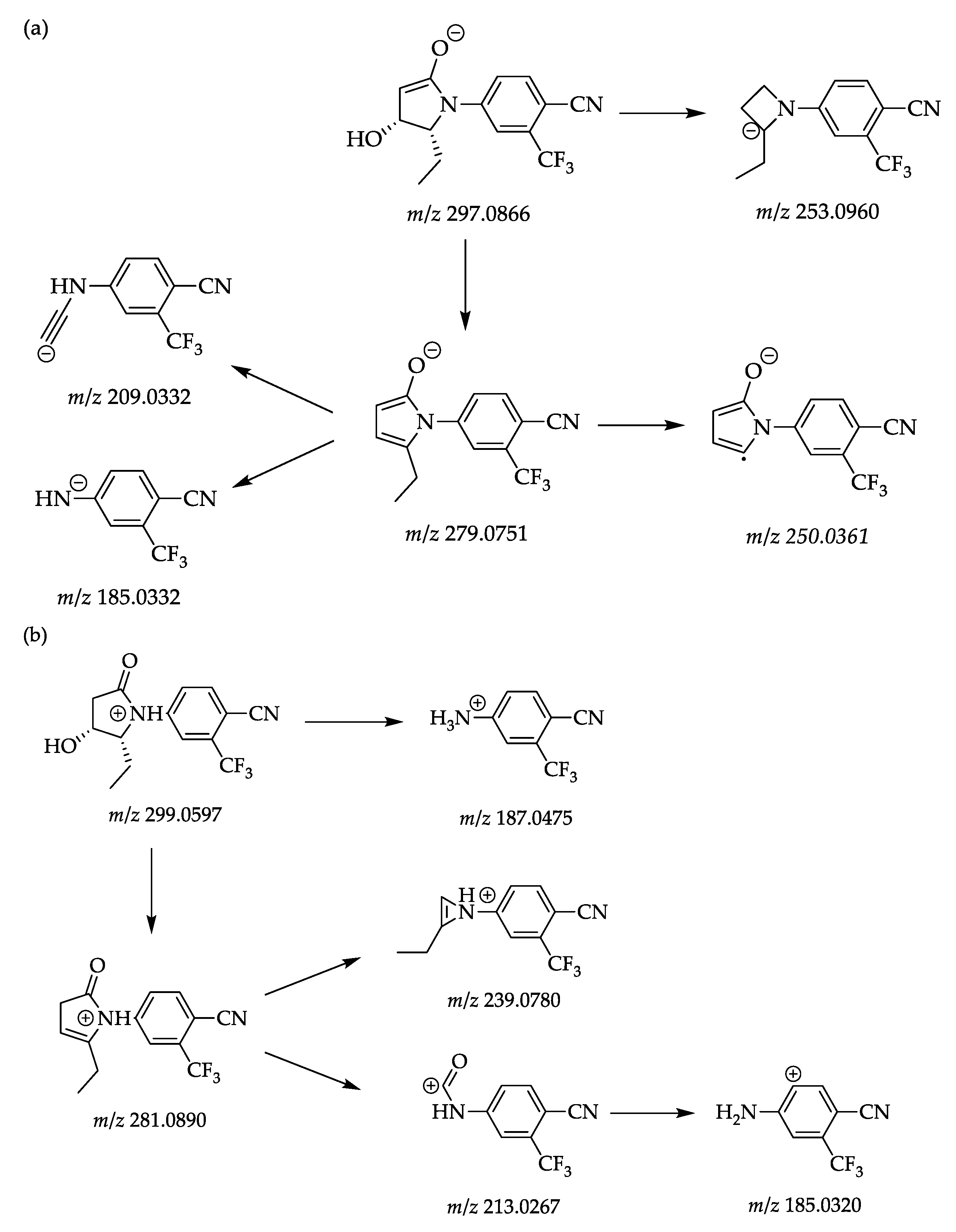

| Metabolic Reaction | Precursor Ion [M−H]− [m/z] | Elemental Composition | Retention Time [min] | Product Ions [m/z] | Elemental Composition | |
|---|---|---|---|---|---|---|
| SARM 2f | 297.0866 | C14H12O2N2F3 | 6.58 | 279.0751 | C14H10ON2F3 | |
| 253.0960 | C13H12N2F3 | |||||
| 250.0361 | C12H5ON2F3 | |||||
| 209.0332 | C10H4N2F3 | |||||
| 185.0332 | C8H4N2F3 | |||||
| M1 | Oxidation | 295.0702 | C14H10O2N2F3 | 5.51 | 265.0597 | C13H8ON2F3 |
| 250.0359 | C12H5ON2F3 | |||||
| 186.0172 | C8H3ONF3 | |||||
| 185.0333 | C8H4N2F3 | |||||
| M2 a | Hydroxylation | 313.0794 | C14H12O3N2F3 | 5.94 | 185.0332 | C8H4N2F3 |
| 127.0402 | C6H7O3 | |||||
| M2 b | 6.17 | 185.0332 | C8H4N2F3 | |||
| 127.0402 | C6H7O3 | |||||
| 101.0608 | C5H9O2 | |||||
| M2 c | 6.74 | 185.0332 | C8H4N2F3 | |||
| 127.0402 | C6H7O3 | |||||
| 101.0608 | C5H9O2 | |||||
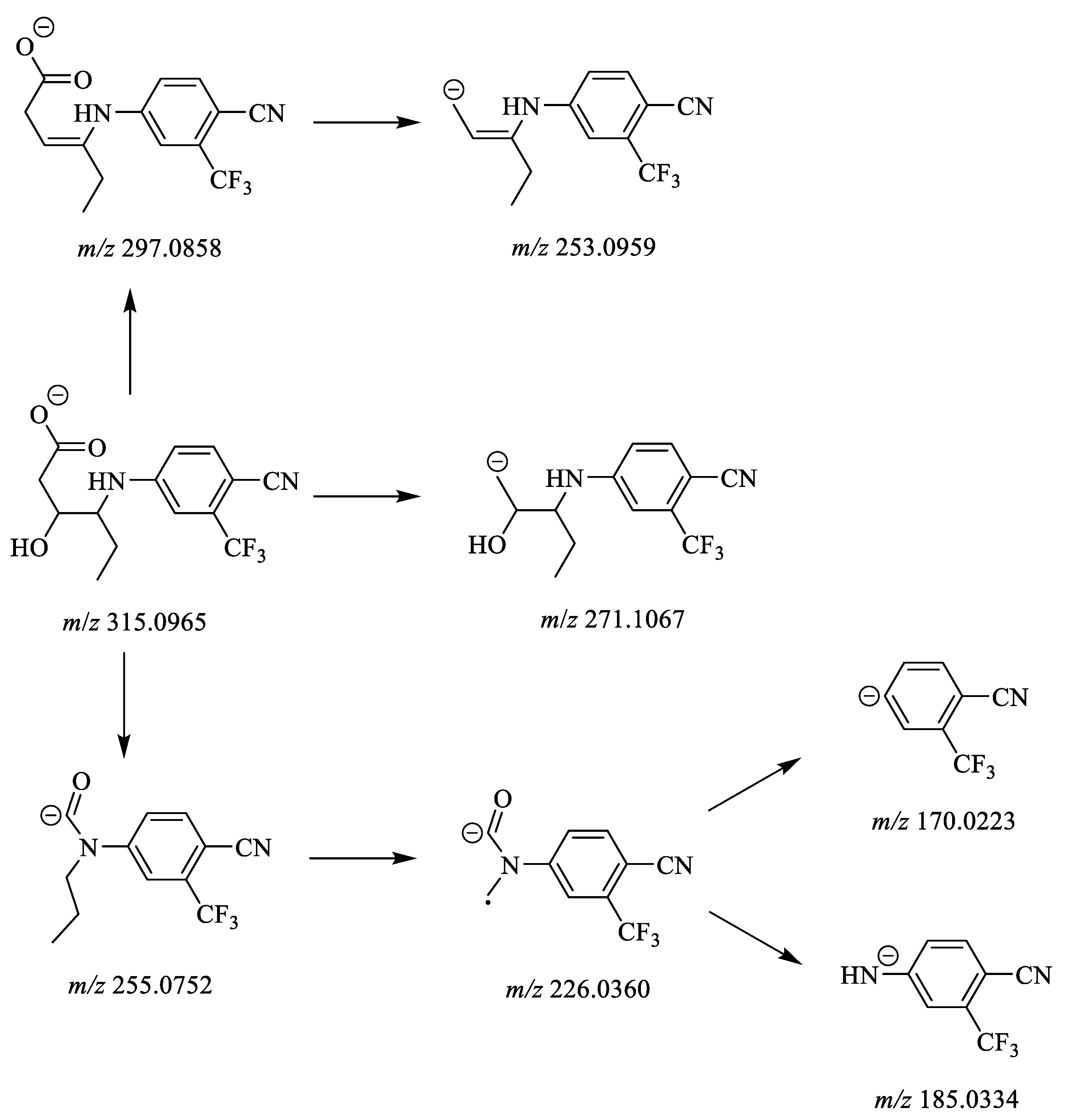
| M3 | Hydration | 315.0965 | C14H14O3N2F3 | 6.70 | 297.0858 | C14H12O2N2F3 |
| 271.1067 | C13H14ON2F3 | |||||
| 255.0752 | C12H10ON2F3 | |||||
| 253.0959 | C13H12N2F3 | |||||
| 226.0360 | C10H5ON2F3 | |||||
| 185.0334 | C8H4N2F3 | |||||
| 170.0223 | C8H3NF3 | |||||
| M4 | Sulfation | 377.0425 | C14H12O5N2F3S | 6.17 | 96.9601 | HSO4 |
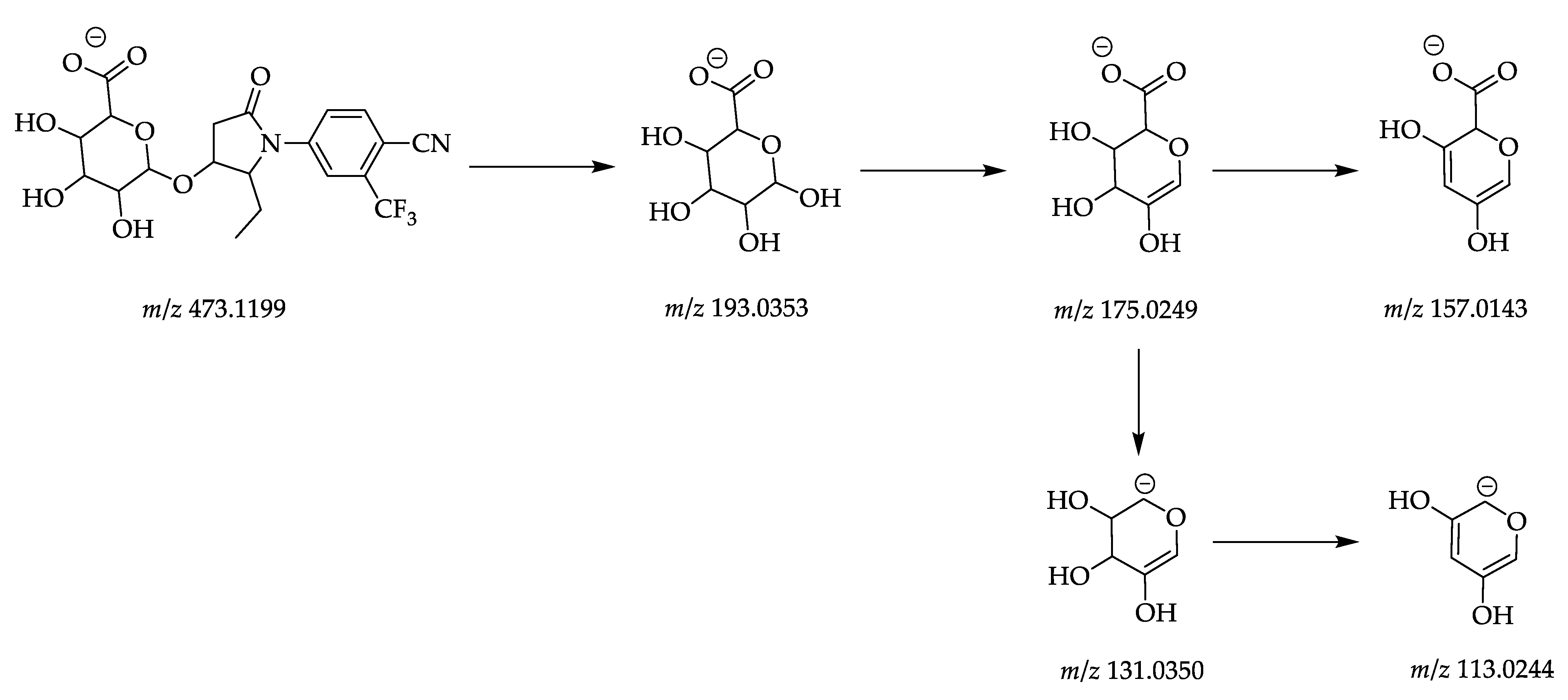
| M5 | Glucuronidation | 473.1181 | C20H20O8N2F3 | 5.70 | 193.0353 | C6H9O7 |
| 175.0249 | C6H7O6 | |||||
| 157.0143 | C6H5O5 | |||||
| 131.0350 | C5H7O4 | |||||
| 113.0244 | C5H5O3 | |||||
| M6 | Hydroxylation + Glucuronidation | 489.1143 | C20H20O9N2F3 | 4.93 | 471.1033 | C20H18O8N2F3 |
| 277.0597 | C14H8ON2F3 | |||||
| 193.0353 | C6H9O7 | |||||
| 175.0249 | C6H7O6 | |||||
| 157.0143 | C6H5O5 | |||||
| 131.0350 | C5H7O4 | |||||
| 113.0244 | C5H5O3 | |||||
| M7 | Hydration + Glucuronidation | 491.1283 | C20H22O9N2F3 | 5.97 | 315.0965 | C14H14O3N2F3 |
| 255.0753 | C12H10ON2F3 | |||||
| 193.0353 | C6H9O7 | |||||
| 175.0249 | C6H7O6 | |||||
| 157.0143 | C6H5O5 | |||||
| 131.0350 | C5H7O4 | |||||
| 113.0244 | C5H5O3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, T.; Wen, H.-C.; Naumann, N.; Krug, O.; Thevis, M. Identification and Synthesis of Selected In Vitro Generated Metabolites of the Novel Selective Androgen Receptor Modulator (SARM) 2f. Molecules 2023, 28, 5541. https://doi.org/10.3390/molecules28145541
Möller T, Wen H-C, Naumann N, Krug O, Thevis M. Identification and Synthesis of Selected In Vitro Generated Metabolites of the Novel Selective Androgen Receptor Modulator (SARM) 2f. Molecules. 2023; 28(14):5541. https://doi.org/10.3390/molecules28145541
Chicago/Turabian StyleMöller, Tristan, Hui-Chung Wen, Nana Naumann, Oliver Krug, and Mario Thevis. 2023. "Identification and Synthesis of Selected In Vitro Generated Metabolites of the Novel Selective Androgen Receptor Modulator (SARM) 2f" Molecules 28, no. 14: 5541. https://doi.org/10.3390/molecules28145541
APA StyleMöller, T., Wen, H.-C., Naumann, N., Krug, O., & Thevis, M. (2023). Identification and Synthesis of Selected In Vitro Generated Metabolites of the Novel Selective Androgen Receptor Modulator (SARM) 2f. Molecules, 28(14), 5541. https://doi.org/10.3390/molecules28145541





