Modulating Crystallization and Defect Passivation by Butyrolactone Molecule for Perovskite Solar Cells
Abstract
1. Introduction
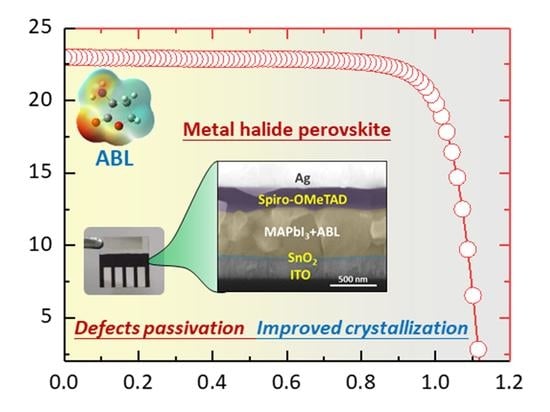
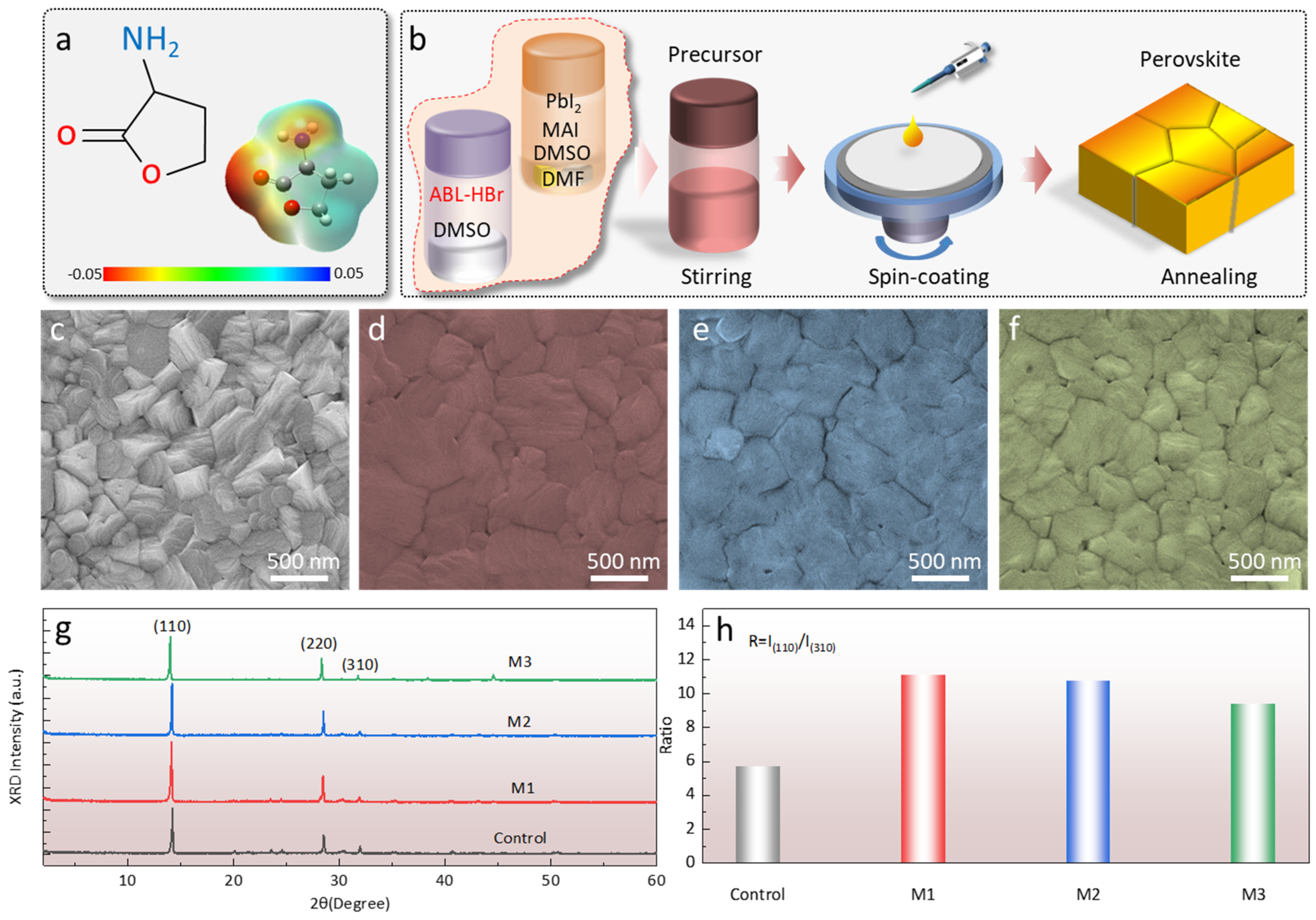
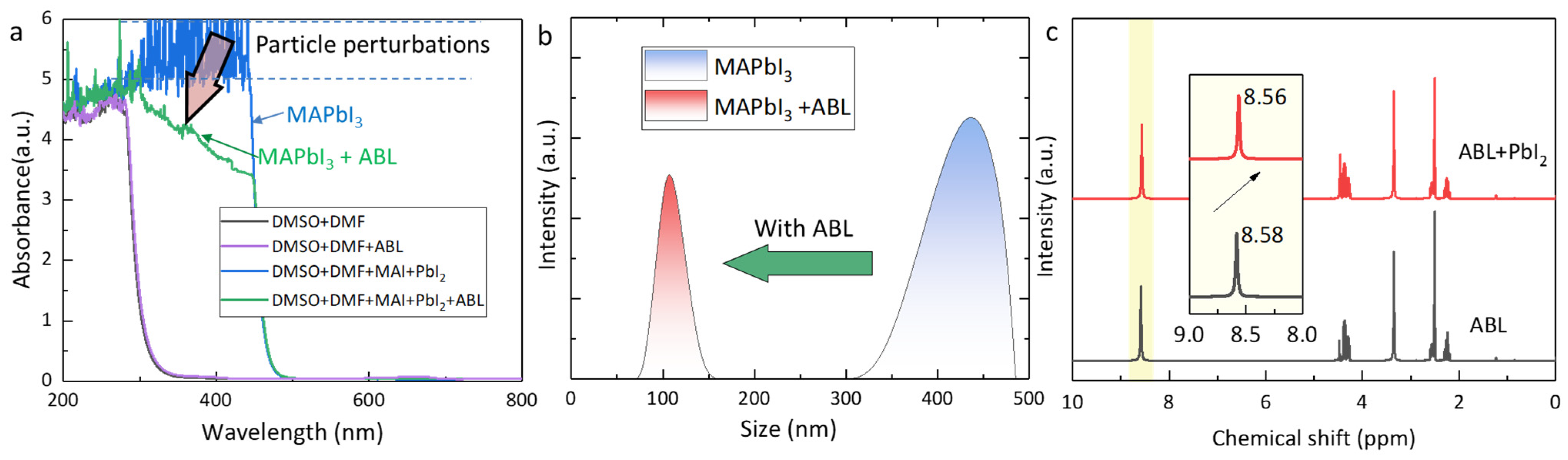
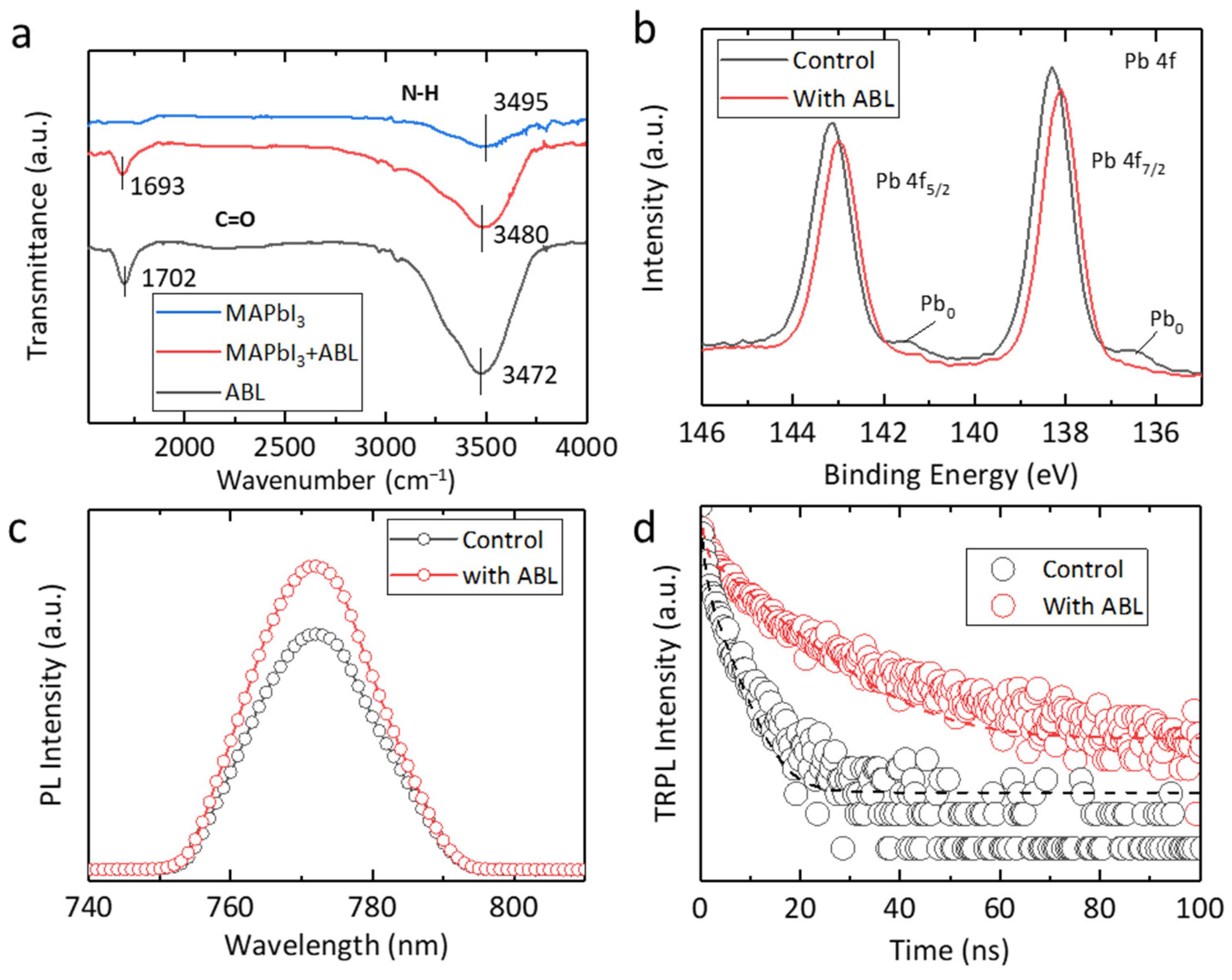
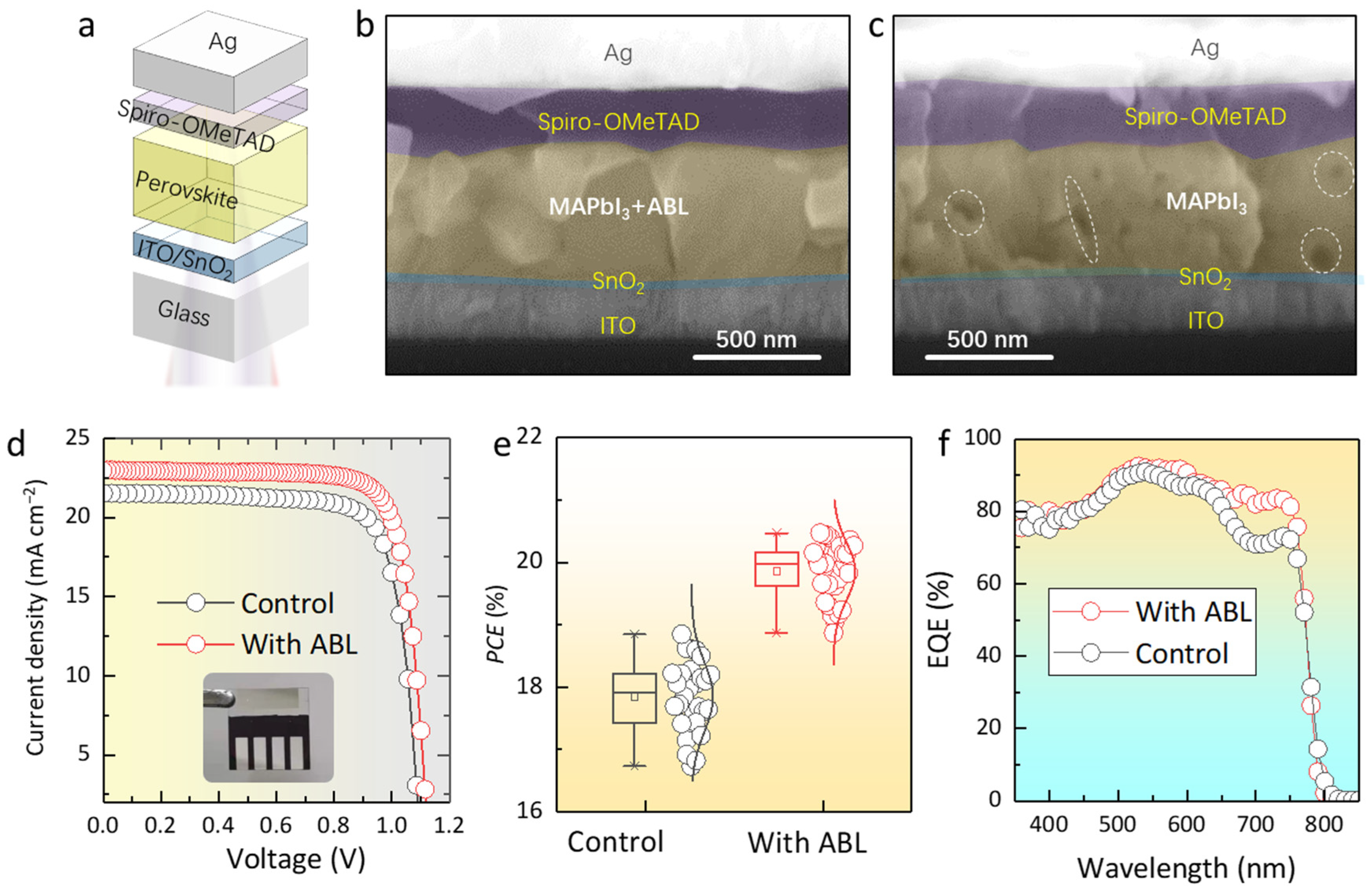
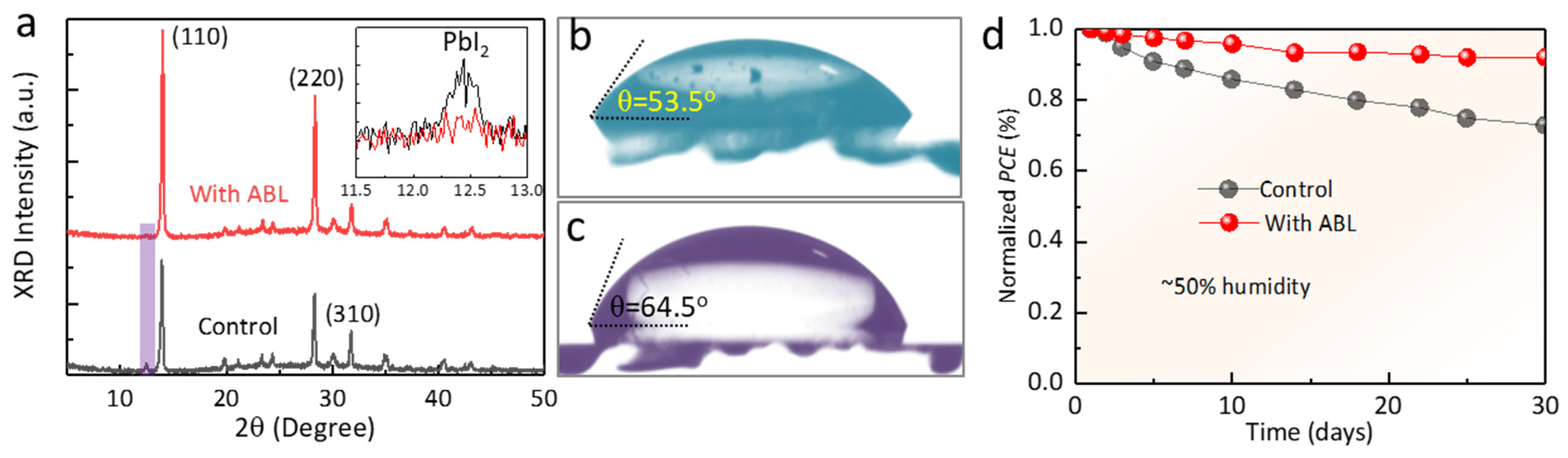
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, J.; Wang, Q.; Jin, Z.; Zhang, X.; Lei, J.; Bin, H.; Zhang, Z.-G.; Li, Y.; Liu, S.F. Polymer Doping for High-Efficiency Perovskite Solar Cells with Improved Moisture Stability. Adv. Energy Mater. 2018, 8, 1701757. [Google Scholar] [CrossRef]
- Reichert, S.; An, Q.; Woo, Y.W.; Walsh, A.; Vaynzof, Y.; Deibel, C. Probing the ionic defect landscape in halide perovskite solar cells. Nat. Commun. 2020, 11, 6098. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Q.; Yang, Z.; Rudd, P.N.; Shao, Y.; Dai, X.; Wei, H.; Zhao, J.; Fang, Y.; Wang, Q.; Liu, Y.; et al. Bilateral alkylamine for suppressing charge recombination and improving stability in blade-coated perovskite solar cells. Sci. Adv. 2019, 5, 8925. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, L.; Mu, X.; Chen, B.; Xiong, Q.; Wang, W.D.; Ding, J.; Gao, P.; Wu, Y.; Cao, J. Grain Boundary Engineering with Self-Assembled Porphyrin Supramolecules for Highly Efficient Large-Area Perovskite Photovoltaics. J. Am. Chem. Soc. 2021, 143, 18989–18996. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, J.; Duan, H.; Wang, H.; Fan, L.; Sun, Y.; Sui, Y.; Yang, J.; Wang, F.; Yang, L. Moisture-preventing MAPbI3 solar cells with high photovoltaic performance via multiple ligand engineering. Nano Res. 2021, 15, 1375–1382. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev220630.pdf (accessed on 20 March 2023).
- Wang, F.; Li, X.; Duan, H.; Wang, H.; Fan, L.; Sun, Y.; Sui, Y.; Yang, J.; Yang, L. Toward efficient, moisture-resistant and lead-leakproofness perovskite solar cells: Coordination-driven reconstructing homogeneous amorphous perovskitoid/crystalline perovskite photoabsorber. Chem. Eng. J. 2022, 428, 132528. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Du, J.; Duan, H.; Wang, H.; Gou, Y.; Yang, L.; Fan, L.; Yang, J.; Rosei, F. Coordinating light management and advance metal nitride interlayer enables MAPbI3 solar cells with >21.8% efficiency. Nano Energy 2022, 92, 106765. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.-S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef]
- Zuo, C.; Bolink, H.J.; Han, H.; Huang, J.; Cahen, D.; Ding, L. Advances in Perovskite Solar Cells. Adv. Sci. 2016, 3, 1500324. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Li, W.; Zhang, Y.; Liu, Z.; Wang, D.; Liu, S. Additive Engineering to Grow Micron-Sized Grains for Stable High Efficiency Perovskite Solar Cells. Adv. Sci. 2019, 6, 1901241. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Wang, Z.; Xia, Y.; Chen, Y.; Huang, W. Additive engineering for highly efficient organic–inorganic halide perovskite solar cells: Recent advances and perspectives. J. Mater. Chem. A 2017, 5, 12602–12652. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive Engineering for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902579. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Wang, H.; Gou, Y.; Yang, S.; Han, D.; Yang, L.; Fan, L.; Yang, J.; Rosei, F. Supramolecular bridging strategy enables high performance and stable organic–inorganic halide perovskite solar cells. Chem. Eng. J. 2022, 446, 137431. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Li, H.; Yan, Y.; Zhou, W.; Yu, D.; Zhao, Q. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016, 7, 10228. [Google Scholar] [CrossRef]
- Hua, W.; Niu, Q.; Zhang, L.; Chai, B.; Yang, J.; Zeng, W.; Xia, R.; Min, Y. Enhancing the Performance of Perovskite Solar Cells by Introducing 4-(Trifluoromethyl)-1H-imidazole Passivation Agents. Molecules 2023, 28, 4976. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, P.; Zhong, S.; Luo, X.; Wu, S.; Wang, Q.; Gao, J.; Lu, X.; Gao, X.; Shui, L.; et al. Dual Effects of Slow Recrystallization and Defects Passivation Achieve Efficient Tin-Based Perovskite Solar Cells with Good Stability Up to One Year. Adv. Funct. Mater. 2023, 33, 2212106. [Google Scholar] [CrossRef]
- Xu, P.; Xie, L.; Yang, S.; Han, B.; Liu, J.; Chen, J.; Liu, C.; Jia, R.; Yang, M.; Ge, Z. Manipulating Halide Perovskite Passivation by Controlling Amino Acid Derivative Isoelectric Point for Stable and Efficient Inverted Perovskite Solar Cells. Sol. RRL 2023, 7, 2200858. [Google Scholar] [CrossRef]
- He, D.; Li, R.; Liu, B.; Zhou, Q.; Yang, H.; Yu, X.; Gong, S.; Chen, X.; Xu, B.; Yang, S.; et al. Unraveling abnormal buried interface anion defect passivation mechanisms depending on cation-induced steric hindrance for efficient and stable perovskite solar cells. J. Energy Chem. 2023, 80, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Bao, Z.; Liu, H.; Lv, Y.; Guo, X.; Liu, X.; Chang, Y.; Li, B. Simultaneous defect passivation and energy level modulation by multifunctional phthalocyanine for efficient and stable perovskite solar cells. Chem. Eng. J. 2023, 459, 141573. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.R.; Park, B.; Hong, S.; Hwang, I.-W.; Kim, J.; Kwon, S.; Kim, G.; Kim, H.; Lee, K. Simultaneously Passivating Cation and Anion Defects in Metal Halide Perovskite Solar Cells Using a Zwitterionic Amino Acid Additive. Small 2021, 17, 2005608. [Google Scholar] [CrossRef]
- Kajal, S.; Jeong, J.; Seo, J.; Anand, R.; Kim, Y.; Bhaskararao, B.; Park, C.B.; Yeop, J.; Hagdfeldt, A.; Kim, J.Y.; et al. Coordination modulated passivation for stable organic-inorganic perovskite solar cells. Chem. Eng. J. 2023, 451, 138740. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Khrustalev, V.N.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Solution Processing of Methylammonium Lead Iodide Perovskite from γ-Butyrolactone: Crystallization Mediated by Solvation Equilibrium. Chem. Mater. 2018, 30, 5237. [Google Scholar] [CrossRef]
- Dai, Z.; Yadavalli, S.K.; Chen, M.; Abbaspourtamijani, A.; Qi, Y.; Padture, N.P. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 2021, 372, 618–622. [Google Scholar] [CrossRef]
- Cheng, Y.; So, F.; Tsang, S.-W. Progress in air-processed perovskite solar cells: From crystallization to photovoltaic performance. Mater. Horiz. 2019, 6, 1611–1624. [Google Scholar] [CrossRef]
- Liu, S.; Guan, Y.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. A Review on Additives for Halide Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902492. [Google Scholar] [CrossRef]
- Yan, K.; Long, M.; Zhang, T.; Wei, Z.; Chen, H.; Yang, S.; Xu, J. Hybrid Halide Perovskite Solar Cell Precursors: Colloidal Chemistry and Coordination Engineering behind Device Processing for High Efficiency. J. Am. Chem. Soc. 2015, 137, 4460–4468. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Zhou, P.; Li, S.; Ren, J.; He, H.; Wang, J.; Wang, N.; Guo, S. Efficient Bifacial Passivation with Cross-linked Thioctic Acid for High Performance Methylammonium Lead Iodide Perovskite Solar Cells. Adv. Mater. 2020, 32, 1905661. [Google Scholar] [CrossRef]
- Xu, T.; Zou, K.; Lv, S.; Tang, H.; Zhang, Y.; Chen, Y.; Chen, L.; Li, Z.; Huang, W. Efficient and Stable Carbon-Based Perovskite Solar Cells via Passivation by a Multifunctional Hydrophobic Molecule with Bidentate Anchors. Appl. Mater. Interfaces 2021, 13, 16485–16497. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Li, Y.; Chen, L. Interfacial Dipole in Organic and Perovskite Solar Cells. J. Am. Chem. Soc. 2020, 142, 18281–18292. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-Structure Perovskite Solar Cells with Efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Correa-Baena, J.-P.; Saracco, G.; Grätzel, M.; Hagfeldt, A.; Turri, S.; Gerbaldi, C. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science 2016, 354, 203–206. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Du, J.; Zhao, C.; Li, Y.; Wei, M.; Liu, H.; Yang, J.; Yang, L. Modulating Crystallization and Defect Passivation by Butyrolactone Molecule for Perovskite Solar Cells. Molecules 2023, 28, 5542. https://doi.org/10.3390/molecules28145542
Wang F, Du J, Zhao C, Li Y, Wei M, Liu H, Yang J, Yang L. Modulating Crystallization and Defect Passivation by Butyrolactone Molecule for Perovskite Solar Cells. Molecules. 2023; 28(14):5542. https://doi.org/10.3390/molecules28145542
Chicago/Turabian StyleWang, Fengyou, Jinyue Du, Chenyu Zhao, Yutao Li, Maobin Wei, Huilian Liu, Jinghai Yang, and Lili Yang. 2023. "Modulating Crystallization and Defect Passivation by Butyrolactone Molecule for Perovskite Solar Cells" Molecules 28, no. 14: 5542. https://doi.org/10.3390/molecules28145542
APA StyleWang, F., Du, J., Zhao, C., Li, Y., Wei, M., Liu, H., Yang, J., & Yang, L. (2023). Modulating Crystallization and Defect Passivation by Butyrolactone Molecule for Perovskite Solar Cells. Molecules, 28(14), 5542. https://doi.org/10.3390/molecules28145542