Application of Chromatographic and Spectroscopic-Based Methods for Analysis of Omega-3 (ω-3 FAs) and Omega-6 (ω-6 FAs) Fatty Acids in Marine Natural Products
Abstract
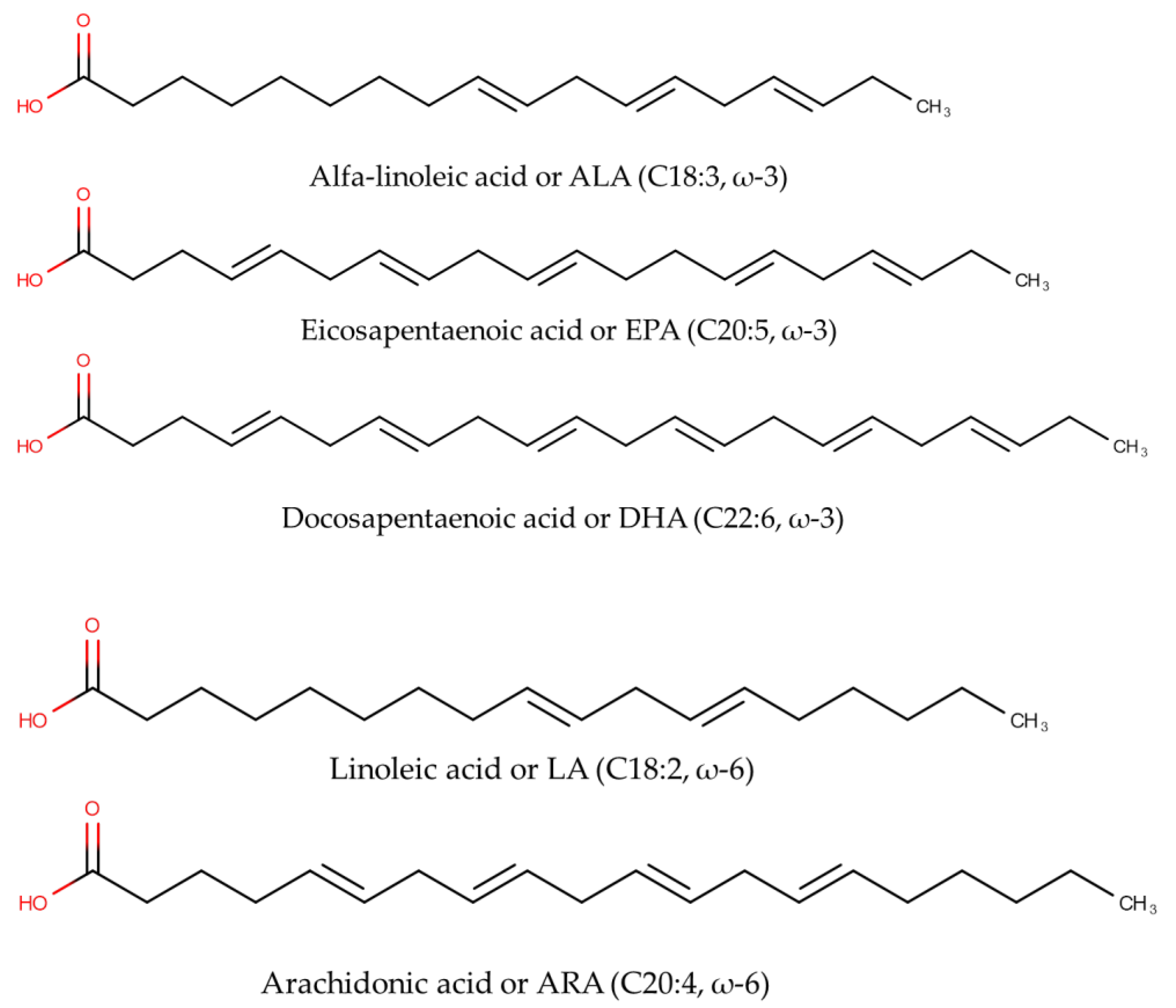
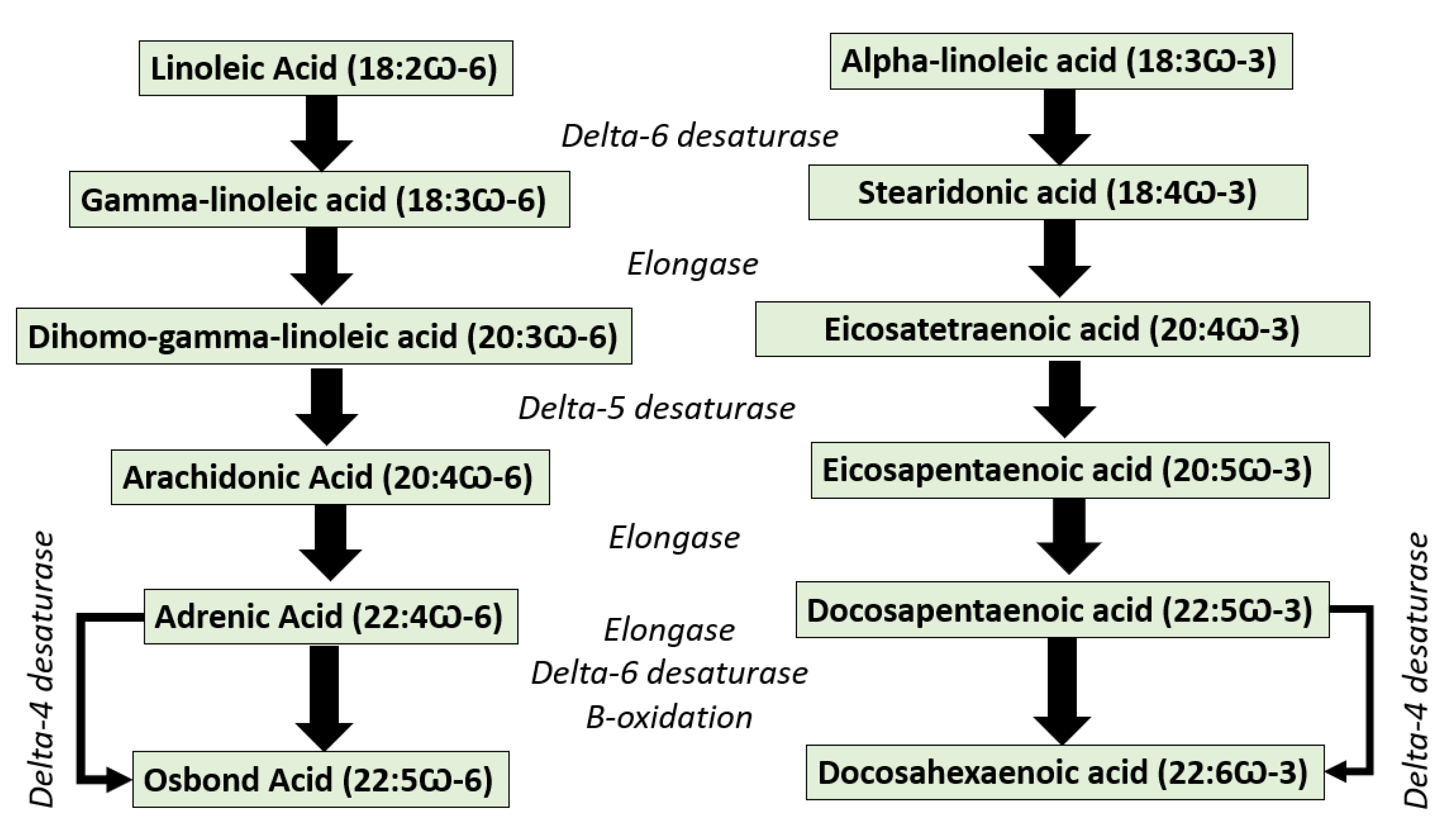
1. Introduction
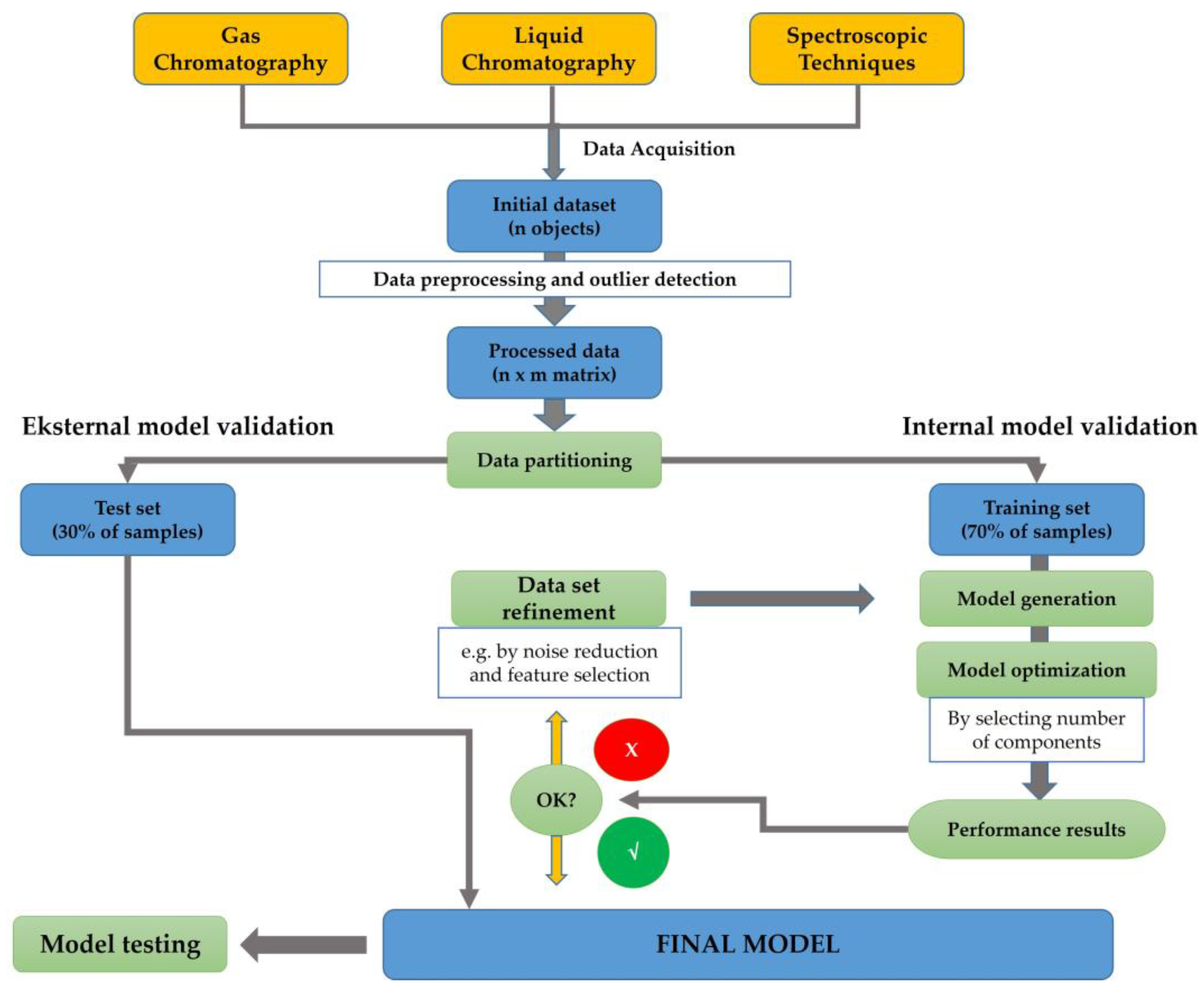
2. Chemometrics
3. Official Methods of Analysis of ω-3 FAs and Related Compounds
4. Chromatographic-Based Methods for Analysis of ω-3 FAs and ω-6 FAs
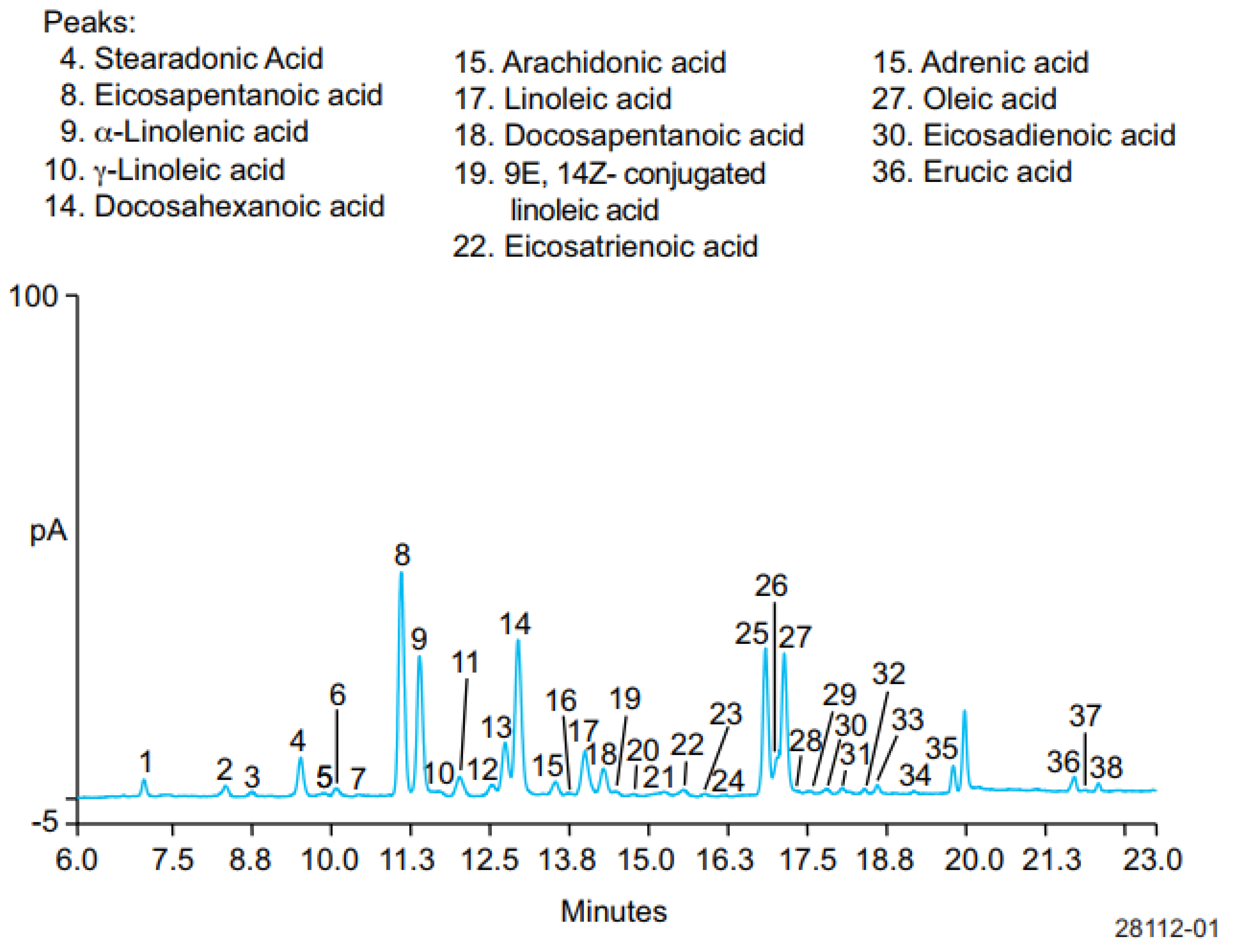
4.1. Gas Chromatography for Analysis of ω-3 FAs and ω-6 FAs
4.2. Liquid Chromatography for Analysis of ω-3 and ω-6 FAs
- Liquid chromatography (LC) is a chromatographic separation technique based on the difference in the distribution of species between two non-miscible phases in which the mobile phase is a liquid that eluted through a stationary phase contained in a column. The term LC is synonymous with high-pressure liquid chromatography (HPLC) [35,59]. Particle diameter of the stationary phase for classical HPLC is about 2–10 µm; if the particle diameter is less than 2 µm, the term becomes ultra-high-performance liquid chromatography (UHPLC) [60]. A liquid chromatograph comprises (1) a pumping system, (2) an injector, (3) a chromatographic column (a column temperature controller may be used), and (4) one or more detectors and data acquisition systems [59].
5. Molecular Spectroscopic Methods for Analysis of ω-3 FAs and ω-6 FAs
6. Materials and Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| APCI-MS | Atmospheric pressure chemical ionization-mass spectrometry |
| ATR | Attenuated total reflectance |
| 13C-NMR | Proton nuclear magnetic resonance |
| CVD | Cardiovascular diseases |
| DHA | Docosahexaenoic acid |
| ECD | Electrochemical detector |
| EFSA | European Food Safety Authority |
| EPA | Eicosapentaenoic acid |
| FAO | Food and Agriculture Organization |
| FAs | Fatty acids |
| FAMEs | Fatty acids methyl |
| FTIR | Fourier Transform Infrared |
| GC-FID | Gas chromatography-flame ionization detector |
| GC-MS | Gas chromatography-mass spectrometry |
| 1H-NMR | Proton nuclear magnetic resonance |
| HP-88 | High polarity-88 |
| ICH | International Council for Harmonization |
| HPLC | High-performance liquid chromatography |
| LC–MS/MS | Liquid chromatography tandem mass spectrometry |
| LDA | Linear discriminant analysis |
| MHz | Megahertz |
| PCA | Principal component analysis |
| PCR | Principal component regression |
| PE-FFAP | Nitroterephthalic acid-modified polyethylene glycol, PEG bonded |
| PEG | Polyethylene glycol |
| PLSR | Partial least square regression |
| PUFA | Polyunsaturated fatty acid |
| R2 | Coefficient of determination |
| RMSEC | Root mean square error of calibration |
| RMSEP | Root mean square error of prediction |
| RRt | Relative retention time |
| Rt | Retention time |
| SIMCA | Soft independent modeling class analogy |
| SST | Standard solution test |
| TAGs | Triacylglycerols |
| TGs | Triglycerides |
| UPLC | Ultra-performance liquid chromatography |
| UV | Ultraviolet |
| WHO | World Health Organization |
| ω-3 | Omega-3 |
| ω-6 | Omega-6 |
References
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. OCL Ol. Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Maruba, P.; Jamaran, K.; Basuki, W.; Jansen, S. Determination and identification of omega 3 and 6 fatty acids position in nile tilapia oil. IOP Conf. Ser. Earth Environ. Sci. 2018, 205, 012045. [Google Scholar] [CrossRef]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef]
- Khalid, W.; Gill, P.; Arshad, M.S.; Ali, A.; Ranjha, M.M.A.N.; Mukhtar, S.; Afzal, F.; Maqbool, Z. Functional behavior of DHA and EPA in the formation of babies brain at different stages of age, and protect from different brain-related diseases. Int. J. Food Prop. 2022, 25, 1021–1044. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Mokos, Z.B. Omega-3 versus Omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 2005, 581–597. [Google Scholar] [CrossRef]
- Nishiyama, M.F.; de Souza, A.H.P.; Gohara, A.K.; dos Santos, H.M.C.; de Oliveira, C.A.L.; Ribeiro, R.P.; de Souza, N.E.; Gomes, S.T.M.; Matsushita, M. Chemometrics applied to the incorporation of omega-3 in tilapia fillet feed flaxseed flour. Food Sci. Technol. 2014, 34, 449–455. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hibbeln, J.R.; Domenichiello, A.F.; Ramsden, C.E.; Salem, N.M.; Chen, C.T.; Jin, H.; Courville, A.B.; Majchrzak-Hong, S.F.; Rapoport, S.I.; et al. Quantitation of Human Whole-Body Synthesis-Secretion Rates of Docosahexaenoic Acid and Eicosapentaenoate Acid from Circulating Unesterified α-Linolenic Acid at Steady State. Lipids 2018, 53, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The essentiality of arachidonic acid in infant developments. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and distribution of omega-3 fatty acids in South Pacific fish and shellfish species. Foods 2020, 9, 233. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Brereton, R.G. Pattern recognition in chemometrics. Chemom. Intell. Lab. Syst. 2015, 149, 90–96. [Google Scholar] [CrossRef]
- Brereton, R.G.; Jansen, J.; Lopes, J.; Marini, F.; Pomerantsev, A.; Rodionova, O.; Roger, J.M.; Walczak, B.; Tauler, R. Chemometrics in analytical chemistry—Part II: Modeling, validation, and applications. Anal. Bioanal. Chem. 2018, 410, 6691–6704. [Google Scholar] [CrossRef] [PubMed]
- Riswanto, F.D.O.; Windarsih, A.; Lukitaningsih, E.; Rafi, M.; Fadzilah, N.A.; Rohman, A. Metabolite Fingerprinting Based on 1H-NMR Spectroscopy and Liquid Chromatography for the Authentication of Herbal Products. Molecules 2022, 27, 952–980. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.; Charles, A.L. Application of multivariate statistical techniques to assess the phenolic compounds and the in vitro antioxidant activity of commercial grape cultivars. J. Chemom. 2018, 32, e3073. [Google Scholar] [CrossRef]
- Irnawati, I.; Riswanto, F.D.O.; Riyanto, S.; Martono, S.; Rohman, A. The use of software packages of R factoextra and FactoMineR and their application in principal component analysis for authentication of oils. Indones. J. Chemom. Pharm. Anal. 2021, 1, 1–10. [Google Scholar]
- Zhang, X.Y.; Hu, W.; Teng, J.; Peng, H.H.; Gan, J.H.; Wang, X.C.; Sun, S.Q.; Xu, C.H.; Liu, Y. Rapid recognition of marine fish surimi by one-step discriminant analysis based on near-infrared diffuse reflectance spectroscopy. Int. J. Food Prop. 2017, 20, 2932–2943. [Google Scholar] [CrossRef]
- Alamprese, C.; Casiraghi, E. Application of FT-NIR and FT-IR spectroscopy to fish fillet authentication. LWT 2015, 63, 720–725. [Google Scholar] [CrossRef]
- Nieto-Ortega, S.; Olabarrieta, I.; Saitua, E.; Arana, G.; Foti, G.; Melado-Herreros, Á. Improvement of Oil Valorization Extracted from Fish By-Products Using a Handheld near Infrared Spectrometer Coupled with Chemometrics. Foods 2022, 11, 1092. [Google Scholar] [CrossRef]
- Jahani, R.; Yazdanpanah, H.; van Ruth, S.M.; Kobarfard, F.; Alewijn, M.; Mahboubi, A.; Faizi, M.; Aliabadi, M.H.S.; Salamzadeh, J. Novel application of near-infrared spectroscopy and chemometrics approach for detection of lime juice adulteration. Iran. J. Pharm. Res. 2020, 19, 34–44. [Google Scholar] [CrossRef]
- Devos, O.; Downey, G.; Duponchel, L. Simultaneous data pre-processing and SVM classification model selection based on a parallel genetic algorithm applied to spectroscopic data of olive oils. Food Chem. 2014, 148, 124–130. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F.; Ruckebusch, C.; Vitale, R. Chemometric Strategies for Spectroscopy-Based Food Authentication. Appl. Sci. 2020, 10, 6544. [Google Scholar] [CrossRef]
- Karunathilaka, S.R.; Choi, S.H.; Mossoba, M.M.; Yakes, B.J.; Brückner, L.; Ellsworth, Z.; Srigley, C.T. Rapid classification and quantification of marine oil omega-3 supplements using ATR-FTIR, FT-NIR and chemometrics. J. Food Compos. Anal. 2019, 77, 9–19. [Google Scholar] [CrossRef]
- USP 46—NF 41; Monograph, Dietary Supplement: Omega-3 Free Fatty Acids. Available online: https://doi.org/10.31003/USPNF_M10475_03_01 (accessed on 28 May 2023). [CrossRef]
- USP 46—NF 41; Monograph, Dietary Supplement: Omega-3 Acid Triglycerides. Available online: https://doi.org/10.31003/USPNF_M2611_05_01 (accessed on 29 May 2023). [CrossRef]
- USP 46—NF 41; Monograph, Dietary Supplement: Omega-3-Acid Ethyl Esters. Available online: https://doi.org/10.31003/USPNF_M2608_06_01 (accessed on 29 May 2023). [CrossRef]
- Alaerts, G.; Van Erps, J.; Pieters, S.; Dumarey, M.; van Nederkassel, A.M.; Goodarzi, M.; Smeyers-Verbeke, J.; Vander Heyden, Y. Similarity analyses of chromatographic fingerprints as tools for identification and quality control of green tea. J. Chromatogr. B 2012, 910, 61–70. [Google Scholar] [CrossRef]
- USP 46—NF 41; General chapter <401> Fats and Fixed Oils. Available online: https://doi.org/10.31003/USPNF_M99160_04_01 (accessed on 28 May 2023). [CrossRef]
- USP 46—NF 41; Monograph, Dietary Supplement: Fish Oil Containing Omega-3 Acids. Available online: https://doi.org/10.31003/USPNF_M58636_06_01 (accessed on 29 May 2023). [CrossRef]
- USP-NF, USP44-NF39; <1226> Verification of Compendial Procedures. Available online: https://online.uspnf.com/uspnf/document/1_GUID-18A6E56B-8EA7-4B37-AB7D-82352B73A309_3_en-US (accessed on 20 April 2022).
- USP-NF, USP44-NF39; <1225> Validation of Compendial Procedures. Available online: https://online.uspnf.com/uspnf/document/1_GUID-E2C6F9E8-EA71-4B72-A7BA-76ABD5E72964_4_en-US (accessed on 26 March 2022).
- Viswanathan, S.; Verma, P.R.P.; Ganesan, M.; Manivannan, J. A novel liquid chromatography/tandem mass spectrometry (LC–MS/MS) based bioanalytical method for quantification of ethyl esters of Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) and its application in pharmacokinetic study. J. Pharm. Biomed. Anal. 2017, 141, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.X.; Araujo, P.; Du, Z.Y.; Nguyen, T.T.; Frøyland, L.; Grung, B. Elucidation of triacylglycerols in cod liver oil by liquid chromatography electrospray tandem ion-trap mass spectrometry. Talanta 2010, 82, 1261–1270. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11.1. 2.2.28. Gas Chromatography. Available online: https://www.google.com.ua/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiXxar5touAAxVNwjgGHY4gD8QQFnoECAsQAQ&url=https%3A%2F%2Ffile.wuxuwang.com%2Fyaopinbz%2FEP9%2FEP9.0_01__37.pdf&usg=AOvVaw0CKj2kZCcgPpt6R9bBYbQG&opi=89978449 (accessed on 15 May 2023).
- USP 46—NF 41; General Chapter <621> Chromatography. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/harmonization-november-2021-m99380.pdf (accessed on 20 May 2023).
- Yuwono, M.; Indrayanto, G. GC system instrumentation. In Dekker Encyclopedia of Chromatography; Cazes, J., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 682–686. ISBN 0824721535. [Google Scholar]
- Scott, R.P.W. Gas Chromatography–Mass Spectrometry Systems. In Encyclopedia of Chromatography; Marcel Dekker Inc.: New York, NY, USA, 2022; pp. 1–7. [Google Scholar]
- Harvey, D.J. Mass spectrometric detectors for gas chromatography. Gas Chromatogr. 2021, 399–424. [Google Scholar] [CrossRef]
- Yuwono, M.; Indrayanto, G. On-Column Injection for GC. In Encyclopedia of Chromatography, Update Supplement; Cazes, J., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 1135–1139. [Google Scholar]
- Swinley, J.; Coning, P. de A Practical Guide to Gas Analysis by Gas Chromatography; Elsevier Inc.: Amsterdam, The Netherlands; Oxford, UK; Cambridge, UK, 2019. [Google Scholar]
- Yuwono, M.; Indrayanto, G. Gas Chromatography. In Ewing’s Analytical Instrumentation Handbook; Cazes, J., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2005; pp. 727–757. [Google Scholar]
- Noviana, E.; Indrayanto, G.; Rohman, A. Advances in Fingerprint Analysis for Standardization and Quality Control of Herbal Medicines. Front. Pharmacol. 2022, 13, 853023. [Google Scholar] [CrossRef]
- Indrayanto, G. The importance of method validation in herbal drug research. J. Pharm. Biomed. Anal. 2022, 214, 114735. [Google Scholar] [CrossRef]
- Indrayanto, G. Validation of Chromatographic Methods of Analysis: Application for Drugs That Derived From Herbs. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2018; Volume 43, pp. 359–392. ISBN 9780128151259. [Google Scholar]
- Tang, B.; Row, K.H. Development of gas chromatography analysis of fatty acids in marine organisms. J. Chromatogr. Sci. 2013, 51, 599–607. [Google Scholar] [CrossRef]
- Petrović, M.; Kezić, N.; Bolanča, V. Optimization of the GC method for routine analysis of the fatty acid profile in several food samples. Food Chem. 2010, 122, 285–291. [Google Scholar] [CrossRef]
- Juárez, M.; Juárez, A.; Aldai, N.; Avilés, C.; Polvillo, O. Validation of a gas-liquid chromatographic method for analysing samples rich in long chain n-3 polyunsaturated fatty acids: Application to seafood. J. Food Compos. Anal. 2010, 23, 665–670. [Google Scholar] [CrossRef]
- Alinafiah, S.M.; Azlan, A.; Ismail, A.; Rashid, N.K.M.A. Method development and validation for omega-3 fatty acids (Dha and epa) in fish using gas chromatography with flame ionization detection (gc-fid). Molecules 2021, 26, 6592. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Li, S.M.; Fan, J.Y.; Fan, L.L.; Zhang, Z.F.; Luo, P.; Zhang, X.J.; Wang, J.G.; Zhu, L.; Zhao, Z.Z.; et al. Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC-MS. Lipids Health Dis. 2014, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, M.; Ren, J.; Djuric, Z.; Fisher, G.J.; Wang, X.; Li, Y. Gas chromatography-mass spectrometry analysis of effects of dietary fish oil on total fatty acid composition in mouse skin. Sci. Rep. 2017, 7, srep42641. [Google Scholar] [CrossRef]
- Arfan, T.; Harmita; Maggadani, B.P. Analysis of alpha-linolenic acid and docosahexaenoic acid in mackerel fish oil (Rastrelliger Kanagurta) using gas chromatography. Int. J. Appl. Pharm. 2018, 10, 28–34. [Google Scholar] [CrossRef]
- Brotas, M.S.C.; Carvalho, G.A.; Pereira, P.A.P. Determination, through Derivatization and GC-MS Analysis, of Omega-3 and Omega-6 Fatty Acids in Fish Oil Capsules Sold in Salvador, Bahia. J. Braz. Chem. Soc. 2020, 31, 447–455. [Google Scholar] [CrossRef]
- Lorensia, A.; Budiono, R.; Suryadinata, R.V.; Tiarasari, N. Quantitative determination of EPA and DHA in fish oil capsules for cardiovascular disease therapy in Indonesia by GC-MS. J. Public health Res. 2021, 10, jphr-2021. [Google Scholar] [CrossRef]
- Kleiner, A.C.; Cladis, D.P.; Santerre, C.R. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J. Sci. Food Agric. 2015, 95, 1260–1267. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Villarreal-Rubio, M.B.; Valenzuela, R.; Valenzuela, A. Comparison of fatty acid profiles of dried and raw by-products from cultured and wild fishes. Eur. J. Lipid Sci. Technol. 2017, 119, 1953–1967. [Google Scholar] [CrossRef]
- Archer, L.; Mc Gee, D.; Paskuliakova, A.; McCoy, G.R.; Smyth, T.; Gillespie, E.; Touzet, N. Fatty acid profiling of new Irish microalgal isolates producing the high-value metabolites EPA and DHA. Algal. Res. 2019, 44, 101671. [Google Scholar] [CrossRef]
- Bayir, A.; Haliloǧlu, H.I.; Sirkecioǧlu, A.N.; Aras, N.M. Fatty acid composition in some selected marine fish species living in Turkish waters. J. Sci. Food Agric. 2006, 86, 163–168. [Google Scholar] [CrossRef]
- Okada, T.; Morrissey, M.T. Production of n − 3 polyunsaturated fatty acid concentrate from sardine oil by lipase-catalyzed hydrolysis. Food Chem. 2007, 103, 1411–1419. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11.1, 2.2.29. Liquid Chromatography. Available online: https://www.google.com.ua/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi3m8S-touAAxWw-jgGHdZKAr8QFnoECBQQAQ&url=https%3A%2F%2Ffile.wuxuwang.com%2Fyaopinbz%2FEP9%2FEP9.0_01__38.pdf&usg=AOvVaw0swpPGkWRzIbNCaU6wOL_q&opi=89978449 (accessed on 15 May 2023).
- Merck KGaA. A Practical Guide to High Performance Liquid Chromatography. 2021. Available online: https://app.go.sigmaaldrich.com/e/er?utm_campaign=AP_AA_MRK_2023028066_48188_Pharma%20Chem_MAY23_Em%202&utm_medium=email&utm_source=Eloqua&s=832461399&lid=32798&elqTrackId=b0b25b4bdea04c1fbc49b43ac2cd2be7&elq=f5d245faaca04384bb2503404f50d98b&elqaid=44691&elqat=1 (accessed on 27 May 2023).
- The Ministry of Health, Labour, and Welfare. The Japanese Pharmacopoeia, 18th ed.; The Ministry of Health, Labour and Welfare Ministerial Notification: Tokyo, Japan, 2021.
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC-MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J. Agric. Food Chem. 2020, 68, 3868–3880. [Google Scholar] [CrossRef] [PubMed]
- Ucar, Y.; Ozogul, F.; Durmus, M.; Ozogul, Y.; Kosker, A.R.; Boga, E.K.; Ayas, D. Purification of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fish oil using HPLC method and investigation of their antibacterial effects on some pathogenic bacteria. Turkish J. Marit. Mar. Sci. 2019, 5, 35–43. [Google Scholar]
- Oh, C.E.; Kim, G.J.; Park, S.J.; Choi, S.; Park, M.J.; Lee, O.M.; Seo, J.W.; Son, H.J. Purification of high purity docosahexaenoic acid from Schizochytrium sp. SH103 using preparative-scale HPLC. Appl. Biol. Chem. 2020, 63, 56. [Google Scholar] [CrossRef]
- Indelicato, S.; Di Stefano, V.; Avellone, G.; Piazzese, D.; Vazzana, M.; Mauro, M.; Arizza, V.; Bongiorno, D. HPLC/HRMS and GC/MS for Triacylglycerols Characterization of Tuna Fish Oils Obtained from Green Extraction. Foods 2023, 12, 1193. [Google Scholar] [CrossRef]
- Xue, J.; Ge, L.; Wang, H.; Liang, J.; Wang, Q.; Lu, W.; Cui, Y.; Xie, H.; Jian, S.; Jin, D.; et al. Comprehensive Screening for EPA/DHA-Structured Phospholipids in Aquatic Products by a Specific Precursor Ion Scanning-Based HILIC-MS/MS Method. J. Agric. Food Chem. 2023, 71, 7937–7946. [Google Scholar] [CrossRef]
- Cartens, M.; Molina Grima, E.; Robles Medina, A.; Giménez Giménez, A.; Ibánez Gonzalez, J. Eicosapentaenoic acid (20:5n-3) from the Marine Microalga Phaeodactylum tricornutum. J. Am. Oil Chem. Soc. 1996, 73, 1025–1031. [Google Scholar] [CrossRef]
- Dillon, J.T.; Aponte, J.C.; Tarozo, R.; Huang, Y. Purification of omega-3 polyunsaturated fatty acids from fish oil using silver-thiolate chromatographic material and high performance liquid chromatography. J. Chromatogr. A 2013, 1312, 18–25. [Google Scholar] [CrossRef]
- Yin, M.; Chen, M.; Matsuoka, R.; Song, X.; Xi, Y.; Zhang, L.; Wang, X. UHPLC-Q-Exactive Orbitrap MS/MS based untargeted lipidomics reveals fatty acids and lipids profiles in different parts of capelin (Mallotus villosus). J. Food Compos. Anal. 2023, 116, 105096. [Google Scholar] [CrossRef]
- Suh, J.H.; Niu, Y.S.; Hung, W.L.; Ho, C.T.; Wang, Y. Lipidomic analysis for carbonyl species derived from fish oil using liquid chromatography–tandem mass spectrometry. Talanta 2017, 168, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Cao, J.; Zeng, S.; Ge, J.; Li, P.; Li, C. Comprehensive evaluation of lipidomics profiles in golden threadfin bream (Nemipterus virgatus) and its by-products using UHPLC-Q-exactive Orbitrap-MS. LWT 2022, 165, 113690. [Google Scholar] [CrossRef]
- He, C.; Sun, Z.; Qu, X.; Cao, J.; Shen, X.; Li, C. A comprehensive study of lipid profiles of round scad (Decapterus maruadsi) based on lipidomic with UPLC-Q-Exactive Orbitrap-MS. Food Res. Int. 2020, 133, 109138. [Google Scholar] [CrossRef] [PubMed]
- Acworth, I.; Plante, M.; Crafts, C.; Bailey, B. Quantitation of Underivatized Omega-3 and Omega-6 Fatty Acids in Foods by HPLC and Charged Aerosol Detection. Planta Med. 2011, 77, PJ1. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Monedeiro, F.; Monedeiro-Milanowski, M.; Nowak, Z.; Krakowska-Sieprawska, A.; Pomastowski, P.; Gadzała-Kopciuch, R.; Buszewski, B. Isolation of omega-3 polyunsaturated fatty acids (eicosapentaenoic acid—EPA and docosahexaenoic acid—DHA) from diatom biomass using different extraction methods. Algal Res. 2022, 62, 102615. [Google Scholar] [CrossRef]
- Serafim, V.; Tiugan, D.A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and validation of a LC–MS/MS-based assay for quantification of free and total omega 3 and 6 fatty acids from human plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A. The application of molecular spectroscopy in combination with chemometrics for halal authentication analysis: A review. Int. J. Mol. Sci. 2020, 21, 5155. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Heraud, P.; Zhang, W.; Kralovec, J.A.; McNaughton, D.; Barrow, C.J. Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem. 2012, 135, 603–609. [Google Scholar] [CrossRef]
- Amorim, T.L.; de la Fuente, M.A.; de Oliveira, M.A.L.; Gómez-Cortés, P. ATR-FTIR and Raman Spectroscopies Associated with Chemometrics for Lipid Form Evaluation of Fish Oil Supplements: A Comparative Study. ACS Food Sci. Technol. 2021, 1, 318–325. [Google Scholar] [CrossRef]
- Prado, E.; Eklouh-Molinier, C.; Enez, F.; Causeur, D.; Blay, C.; Dupont-Nivet, M.; Labbé, L.; Petit, V.; Moreac, A.; Taupier, G.; et al. Prediction of fatty acids composition in the rainbow trout Oncorhynchus mykiss by using Raman micro-spectroscopy. Anal. Chim. Acta 2022, 1191, 339212. [Google Scholar] [CrossRef]
- Bekhit, M.Y.; Grung, B.; Mjøs, S.A. Determination of omega-3 fatty acids in fish oil supplements using vibrational spectroscopy and chemometric methods. Appl. Spectrosc. 2014, 68, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Killeen, D.P.; Marshall, S.N.; Burgess, E.J.; Gordon, K.C.; Perry, N.B. Raman Spectroscopy of Fish Oil Capsules: Polyunsaturated Fatty Acid Quantitation Plus Detection of Ethyl Esters and Oxidation. J. Agric. Food Chem. 2017, 65, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
- Killeen, D.P.; Card, A.; Gordon, K.C.; Perry, N.B. First Use of Handheld Raman Spectroscopy to Analyze Omega-3 Fatty Acids in Intact Fish Oil Capsules. Appl. Spectrosc. 2020, 74, 365–371. [Google Scholar] [CrossRef]
- Lv, J.; Wang, C.; Zhang, X.; Zhihua, L.; Yu, M. 1H-NMR quantification of DHA and EPA in fish oil. J. Ocean Univ. China. 2020, 19, 1193–1197. [Google Scholar] [CrossRef]
- Moros, J.; Garrigues, S.; Guardia, M. de la Vibrational spectroscopy provides a green tool for multi-component analysis. TrAC—Trends Anal. Chem. 2010, 29, 578–591. [Google Scholar] [CrossRef]
| ω-3 and ω-6 FAs (Samples) | Sample Preparation | GC Condition | Results | Ref. |
|---|---|---|---|---|
| ALA and DHA in mackerel fish oil | The fish was subjected to boiling, and the separated oils were extracted using ethanol-chloroform. Fish oils were subjected to derivatization to provide FAMEs with BF3. | Capillary column with DB-5 stationary phase (30 m × 0.32 mm, 0.25 μm film thickness), carrier gas: helium; splitness; oven was programmed at temperature 160–200 °C with flow rate 1.0, detector FID and the injector temperatures were set at 250 °C. | APA and DHA were well separated with good efficiency as indicated with low high equivalent theoretical plate. The recovery obtained was in the range of 100.36 ± 0.31 (APA) to 100.58 ± 0.32. The levels of APA and DHA found were 0.181–0.214% (APA) and 0.010–0.321%. | [51] |
| EPA and DHA in fish oil capsules | The fish oil was taken from fish oil capsules then used for derivatization of the polyunsaturated fatty acids into FAMEs. | Capillary column of nitroterephthalic acid-modified polyethylene glycol, PEG bonded (30 m × 0.32 mm ID × 0.25 µm), carrier gas: helium, oven temperature was programmed at temperature of 100–240 °C with total running time of 38 min using flow rate of 0.8 mL/min; injector temperature was 240 °C in split mode (20:1); the detector temperature (ion trap mass spectrometer) was 240 °C. | The combined concentrations of EPA and DHA ranged from 160.6–360.4 mg/g, with an average result of 197.3 ± 50.7 mg/g. The limit of detection and limit of quantification were 0.16–0.18 mg/g and 0.46–0.63 mg/g, respectively, with recoveries above 76%. | [52] |
| EPA and DHA in fish oil capsules | The fish oil from fish oil capsules was pipetted, weighed, and subjected to derivatization to form FAMEs using BF3. | Capillary column of RTX-5SM (60 m × 0.25 mm, layer thickness 0.25 μm), carrier gas: helium with flow rate of 0.73 mL/min, oven temperature was programmed from 80–280 °C, injector temperature was 250 °C with split ratio 1:200, the temperature of the electron impact source was set at 200 °C | The EPA and DHA in fish oil capsules were determined in relative areas. All tested products had relative content of EPA and DHA in accordance with the respective labels. | [53] |
| EPA and DHA in fish oil nutritional capsules | The fish oil nutritional samples were subjected to derivatization to obtain FAMEs using BF3. | A DB5-MS capillary column (30 m × 0.32 mm ID × 0.25 µm) was used, carrier gas: helium (0.8 mL/min), oven temperature was programmed at 80–280 °C, injector temperature was 250 °C with a split ratio of 10:1, ion source temperature of EI was 200 °C. | The GC-MS method had good sensitivity, accuracy, and precision. The LoDs for EPA and DHA were 0.08 ng and 0.21 ng, respectively. The content of EPA was 39.52 to 509.16 mg/g, and the content of DHA was 35.14 to 645.70 mg/g. The obtained recovery was 100.50% and 103.83% for EPA and DHA, respectively, with RSD less than 1.05% for both EPA and DHA. | [49] |
| EPA and DHA in fish | Fish samples were divided into three groups: raw, baking, and steaming. The fat content was extracted using the Bligh and Dyer method. The obtained fats were then subjected to derivatization to provide FAMEs using BF3. | A capillary column with high polarity of GC HP-88 (60 m length, 0.25 mm ID, 0.2 µm DF) was used, carrier gas: helium with flow rate of 1 mL/min, the oven temperature was programmed from 40 °C to 230 °C, the temperature of the injector was 250 °C with a split ratio of 20:1, the FID temperature was 250 °C. | The method could separate EPA and DHA with good resolution. The method had a high accuracy shown by the recovery (>95%) and good precision (RSD ≤ 2%). | [48] |
| EPA and DHA in commercial omega-3 dietary supplements | Supplements of cod liver oils, algal oils, krill oils, and fish oils were subjected to derivatization to obtain FAMEs using BF3. | A capillary column of wax CP-52CB (CP 8843, 30 m × 0.32 mm I.D., DF-25 coating thickness 0.25 μm) was used. The oven temperature was programmed from 170–240 °C. Carrier gas was helium at a flow rate of 2.5 mL/min. The temperature of the injector was set at 250 °C, whereas FID was operated at 300 °C. | The contents of EPA and DHA for fish and krill-based supplements were 81.8–456.4 mg/g oil and 51.6–220.4 mg/g oil, respectively. For algal oil, the content of EPA was 7.7–151.1 mg/g and the content of DHA was 237.8–423.5 mg/g oil. | [54] |
| EPA and DHA in South Pacific fish and shellfish species | Samples were subjected to total lipids extraction applying the Folch method then used for the derivatization of the fatty acids to obtain FAMEs using BF3. | Analysis was performed using GC-FID using programmed temperature with helium as the carrier gas. | Red cusk eel contained EPA and DHA levels of 40.8 and 74.4 mg/100 g, respectively. Mackerel contained 414.7 and 956.0 mg/100 g of EPA and DHA, respectively. Sea squirt (shellfish species) contained EPA and DHA levels of 375.0 and 165.7 mg/100 g, respectively. In addition, EPA + DHA content in Chilean abalone was 63.6 mg/100 g. | [55] |
| EPA and DHA in Irish microalgal isolates (three marine strains: diatom cf. Stauroneis sp. LACW24, chrophyte cf. Phaeothamnion sp. LACW34, and haptophyte Diacronema sp. GMC30) | The total lipids of microalgal isolates were extracted using the Bligh and Dyer method. The lipids were further processed and used in derivatization to obtain FAMEs using BF3. | A capillary column of BPX70 120 m length and 0.22 mm internal diameter was used. The oven temperature was programmed from 50–240 °C. The carrier gas was helium operated at 2 mL/min. The sample was injected at a split ratio of 100:1 at an injector temperature of 250 °C. The detector temperature of the MS source was set at 230 °C, and the MS Quad was at 150 °C. | The average yields of EPA were 3.9, 11.9, and 1.3 mg EPA/g DW for GMC30, LACW24, and LACW34, respectively. The average yields of DHA were 3.0 and 2.0 mg DHA/g DW for GMC30 and LACW34, respectively. | [56] |
| EPA and DHA in marine fish species in Turkish waters | The total lipids from twelve marine fish species in Turkey were extracted, and the fatty acids content was further derivatized to provide FAMEs. | A fused silica capillary column (25 m × 0.2 mm ID) was used, and the oven temperature was set from 170–300 °C. The carrier gas was hydrogen. The injector temperature was 250 °C, whereas the detector temperature (FID) was 300 °C. | DHA ranged from 43.7 to 75.2%. The n-3/n-6 FA ratio ranged from 2.67 to 12.61. | [57] |
| EPA and DHA in sardine oil | The lipids of sardine oil were subjected to derivatization to obtain FAMEs. | Analysis was performed using a capillary column (EC-wax, 30 m × 0.25 mm i.d.) at oven temperature programmed from 50 °C to 220 °C. The split ratio of sample injection was 100:1 with an injector temperature of 250 °C. The detector (FID) temperature was set at 270 °C. | The unhydrolyzed oil contained EPA and DHA levels of 26.86% and 13.62%, respectively. The hydrolyzed oil contained 33.74% EPA and 29.94% DHA. | [58] |
| ω-3 and ω-6 FAs (Samples) | Sample Preparation | HPLC/LC-MS or LC-MS/MS Condition | Results | Ref. |
|---|---|---|---|---|
| EPA and DHA from fish oils (rainbow trout oil) | The fatty acids were extracted, then the EPA and DHA were further purified using HPLC. | HPLC method using mobile phase of ethanol HPLC grade and ultra-pure water. Elution of 5 µL injected sample was performed for 20 min and detected using UV detector at 254 nm. | The fractions of EPA and DHA could be separated from other fatty acids after purification and could be detected using the HPLC-UV system. The retention time was between 3.5 and 4.7 min. | [63] |
| DHA from marine microalgae (Schizochytrium sp. SH103) | The lipids were extracted from dried cells using chloroform: methanol (2:1 v/v) for 20 min. The chloroform layer was taken and concentrated using a rotary evaporator. After that, the extracted lipids were converted into fatty acids ethyl esters (FAEEs) using acid-catalyzed transesterification. | Semi-preparative HPLC with UV detector. Reverse-phase system (C18 column 250 mm, 4.6 mm, 2.0 µm). Two small columns were connected using a connector with dead volume of 20 µL in order to optimize the semi-preparative condition. Methanol at various concentrations was used as the mobile phase. | The optimum separation of DHA was obtained using the isocratic condition employing the mobile phase of methanol/water (96:4 v/v) with velocity of 0.5 mL/min. The purity of DHA was 98.5%. | [64] |
| EPA and DHA from tuna fish oils | The heads, skins, fishbones, and gullets of tuna fish were used for fish oil extraction. The homogenate was added with distilled water at a ratio of 1:1 then heated for 60 min at 50 °C. The oil phase was separated from the water layer and used for analysis. | LC-APCI/MS was used, equipped with a Hypersil column (50 mm, 2.1 mm, 1.8 µm), and the temperature was maintained at 25 °C. Analysis was performed in positive ionization with APCI parameters of corona probe current: 4 µA, corona voltage: 3.6 kV, and probe temperature: 450.0 °C. | EPA and DHA could be detected using HPLC/HRMS along with other TAG compounds. | [65] |
| EPA and DHA in several aquatic products | The lipids were extracted from each aquatic product using solvent extraction techniques. | HILIC-MS/MS method using specific precursor ion scanning. | The total identified PLEPA/DHA molecules were 80. The best resource for PLEPA/DHA was Antarctic krill (2574.69 µg/g), followed by mackerel (2330.11 µg/g), salmon (2109.91 µg/g), and Farrer’s scallop (1883.59 µg/g). Sea cucumber contained the highest contents of EPA-structured phospholipids, whereas sea bass contained the highest DHA-structured phospholipids. | [66] |
| EPA from marine microalgae of Phaeodactylum tricornutum | Three step preparation process: extraction of fatty acids (direct saponification of biomass), PUFA concentration, and EPA isolation. | A semi-preparative HPLC with reverse-phase system using a C18 column 25 cm × 10 mm i.d.. The mobile phase was methanol:water 80:20 v/v containing 1% acetic acid eluted in isocratic mode. | An amount of 65.7% EPA present in biomass was recovered in highly pure form. In addition, without the PUFA concentration step, 93.6% EPA could be isolated from pure fatty acid extracts. | [67] |
| EPA and DHA in fish oils | The fish oil was extracted using n-hexane and used for esterification in ethanol to obtain fatty acid ethyl esters. Subsequently, the purification was conducted to obtain concentrated EPA ethyl ester (EPA-EE) and DHA ethyl ester (DHA-EE). | An HPLC binary pump connected to APCI-MS was used. Analysis was performed using an AgTCM (150 mm × 3.0 mm i.d., 3 μm) column set at 25 °C. For APCI-MS parameters: vaporization temperature was 400 °C; nebulization pressure was 60 psig; drying gas flow and temperature were 5.0 L/min and 300 °C; corona current of 3.5 μA with capillary voltage of 3500 V. | The EPA-EE and DHA-EE could be separated from other fatty acid peaks, and the purification process could yield EA-EE and DHA-EE with purity above 95%. | [68] |
| EPA and DHA in capelin (Mallotus villosus) fish | Crude fat was extracted according to the AOAC method. The lipids were extracted using chloroform:methanol (2:1, −20 °C). | LC-HRMS Orbitrap using a C18 column (100 mm × 2.1 mm × 1.7 µm) set at a temperature of 55 °C. The mobile phase used was acetonitrile:water (60:40) (A) and isopropanol:acetonitrile (90:10) (B) both of them containing 10 mM ammonium formate. Elution was performed in gradient mode for 18 min. | The content of EPA was 7.79–16.91%, whereas the content of DHA was 7.65–19.83%. | [69] |
| ω-3 FAs in fish oils | Fish oils were subjected to oxidation then used for solid phase extraction (SPE) for both oxidized and non-oxidized forms. | LC-MS/MS using a reverse-phase system (C18 column, 100 mm, 5 mm, 1.8 µm) with water and acetonitrile containing 0.1% formic acid as mobile phases. Analytes were eluted using gradient mode with running time 35 min. The MS detector was a triple quadrupole MS operated at negative ionization mode. The temperature for the ion transfer tube was 325 °C with vaporizer temperature of 150 °C. | The LC-MS/MS method could distinguish carbonyl species from omega-3 and omega-6 fatty acids. The validated method could be applied to monitor the formation of carbonyl species in different fish oils caused by lipid peroxidation. | [70] |
| ω-3 FAs and ω-6 FAs in golden threadfin bream (Nemipterus virgatus) fish | Lipids were extracted using the Folch method and redissolved in methanol:isopropanol (1:1 v/v) then added with internal standard of phosphatidylethanolamine (15:0–18:1-d7-PE). | LC-Orbitrap HRMS using a reverse-phase system (C18 column 100 mm, 2.1 mm, 1.7 µm). The mobile phase was acetonitrile:water (60:40) and acetonitrile:propanol (10:90) both containing 10 mM ammonium formate. The MS detector was operated in both (+) and (−) ionization modes with capillary temperature 320 °C in ESI+ and 300 °C in ESI−. The resolution was 70,000 (full MS) and 17,500 (MS/MS). | High contents of phospholipids and saccharolipids were observed. The EPA, DHA, and ARA (arachidonic acid) PUFA were found to be dominant. The EPA content was 3.89–5.29%, the DHA content was 11.07–21.54%, and the ARA content was 2.36–3.64%. | [71] |
| EPA and DHA from Decapterus maruadsi fish | Fish samples were freeze dried, then the lipids were extracted using a modified Folch method. Isopropanol:methanol 1:1 v/v was used to resuspend the lipids. | The reverse-phase LC system using a C18 column (BEH 100 mm, 2.1 mm, 1.7 µm) was used. The mobile phase was acetonitrile:water (60:40) and acetonitrile:propanol (10:90) both containing ammonium formate (10 mM) and acetic acid (0.1%). The detection was performed using Orbitrap HRMS operated at positive and negative ionization modes with capillary temperature set at 320 °C and the scan range of 150–2000 m/z. | Higher proportions of EPA and DHA were found. The content of EPA was 148.84 ± 18.52 mg/100 g raw sample, whereas the content of DHA was 384.30 ± 17.67 mg/100 g raw sample. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohman, A.; Irnawati; Windarsih, A.; Riswanto, F.D.O.; Indrayanto, G.; Fadzillah, N.A.; Riyanto, S.; Bakar, N.K.A. Application of Chromatographic and Spectroscopic-Based Methods for Analysis of Omega-3 (ω-3 FAs) and Omega-6 (ω-6 FAs) Fatty Acids in Marine Natural Products. Molecules 2023, 28, 5524. https://doi.org/10.3390/molecules28145524
Rohman A, Irnawati, Windarsih A, Riswanto FDO, Indrayanto G, Fadzillah NA, Riyanto S, Bakar NKA. Application of Chromatographic and Spectroscopic-Based Methods for Analysis of Omega-3 (ω-3 FAs) and Omega-6 (ω-6 FAs) Fatty Acids in Marine Natural Products. Molecules. 2023; 28(14):5524. https://doi.org/10.3390/molecules28145524
Chicago/Turabian StyleRohman, Abdul, Irnawati, Anjar Windarsih, Florentinus Dika Octa Riswanto, Gunawan Indrayanto, Nurrulhidayah A. Fadzillah, Sugeng Riyanto, and Nor Kartini Abu Bakar. 2023. "Application of Chromatographic and Spectroscopic-Based Methods for Analysis of Omega-3 (ω-3 FAs) and Omega-6 (ω-6 FAs) Fatty Acids in Marine Natural Products" Molecules 28, no. 14: 5524. https://doi.org/10.3390/molecules28145524
APA StyleRohman, A., Irnawati, Windarsih, A., Riswanto, F. D. O., Indrayanto, G., Fadzillah, N. A., Riyanto, S., & Bakar, N. K. A. (2023). Application of Chromatographic and Spectroscopic-Based Methods for Analysis of Omega-3 (ω-3 FAs) and Omega-6 (ω-6 FAs) Fatty Acids in Marine Natural Products. Molecules, 28(14), 5524. https://doi.org/10.3390/molecules28145524









