Abstract
As one of the key components of solid-contact ion-selective electrodes (SC-ISEs), the SC layer plays a crucial role in electrode performance. Carbon materials, known for their efficient ion–electron signal conversion, chemical stability, and low cost, are considered ideal materials for solid-state transducing layers. In this review, the application of different types of carbon materials in SC-ISEs (from 2007 to 2023) has been comprehensively summarized and discussed. Representative carbon-based materials for the fabrication of SC-ISEs have been systematically outlined, and the influence of the structural characteristics of carbon materials on achieving excellent performance has been emphasized. Finally, the persistent challenges and potential opportunities are also highlighted and discussed, aiming to inspire the design and fabrication of next-generation SC-ISEs with multifunctional composite carbon materials in the future.
1. Introduction
The ion-selective electrode (ISE) is an electrochemical sensor that converts the activity of a target ion into a measurable electromotive force (EMF), enabling the determination of ion concentration. The first study of ISEs originated with Cremer’s discovery in the early 20th century, wherein variations in potential across a glass membrane reflected differences in H+ activity [1]. This finding sparked extensive and profound investigations into ISEs. The glass electrode, introduced in the 1930s, marked a significant milestone and triggered widespread exploration. During the 1960s and 1970s, remarkable advancements were achieved, including the development of the silver membrane ISE [2], the selective response of zinc oxide to combustible gases [3], and the advent of modern carrier-based ISEs. These liquid-contact ISEs (LC-ISEs) typically comprise an ion-selective membrane [4], an internal solution [5], a reference electrode [6], and an inert cavity [7] (Figure 1a). In the 1990s, Pretsch et al. [8] proposed the theory of steady-state ion flux in liquid ISEs, propelling a significant leap forward in understanding ISE mechanisms.
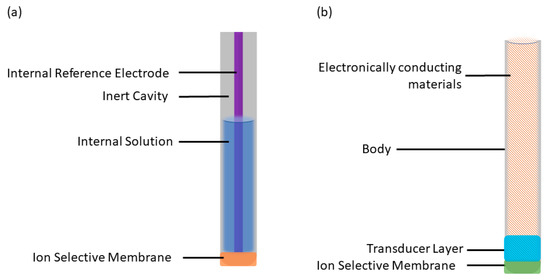
Figure 1.
Structure images of: (a) LC-ISEs; (b) SC-ISEs.
Today, liquid-contact ISEs are relatively mature and extensively employed. However, traditional LC-ISEs have their inherent limitations: (1) The evaporation and permeation of internal filling solution [9] and variations in sample temperature and pressure can affect the electrode response. (2) Osmotic pressure resulting from differences in ion strength between the sample and internal filling solution can cause the ingress of pure water into the internal filling solution, leading to significant volume changes or stratification of the ISM (internal solid membrane). This requires careful use and proper maintenance of ISEs, resulting in higher costs [10]. (3) There is a steady-state ion flux between the internal filling solution and the test solution. (4) It is challenging to reduce the volume of the internal filling solution to the milliliter level, making it difficult to miniaturize the electrode. To address these limitations, transitioning from the liquid interface to solid-state or solid-contact ISEs has emerged as a major research trend. SC-ISEs offer numerous advantages, including ease of storage, simplified maintenance, absence of external pressure requirements, low detection limits, reduced temperature dependence, and the potential for microfabrication [11]. Consequently, SC-ISEs have become a crucial research direction in the field of ISEs.
During the early development of SC-ISEs, the primary focus was on eliminating the need for filling solutions. In 1970, Hirata and Date et al. produced an all-solid-state Cu2+ ISE by coating platinum wire with CuS silica gel [12]. Subsequently, James et al. [13] conducted further research and coated a polymer-sensitive film containing a Ca2+ carrier onto a platinum wire, creating the first ion carrier-coated wire electrode. This resulted in the formation of a Ca2+ ISE, which is considered the first ion carrier-based SC-ISE. However, the coated electrode exhibited drawbacks such as poor stability and significant potential drift [13]. These issues arose from the absence of a stable ion–electron conversion between the sensitive film and the conductive substrate, as well as the small contact area of the wire-coated electrode, leading to noise and potential drift [14]. To overcome these challenges, it became necessary to introduce a solid contact material to facilitate the ion–electron conversion between the ion-selective membrane (ISM) and the conductive substrate. In 1985, Nikolskii and Materova [15] identified three key conditions for the solid contact (SC) layer to achieve stable and reliable SC-ISE responses: A reversible ion-to-electron conduction transition, an ideal non-polarized high exchange current density interface, and the absence of side reactions. Various SC materials have since been developed, including hydrogels [16,17], redox polymers [18,19,20], and self-assembled monolayers [21,22,23]. Hydrogel-based SC-ISEs, in particular, have gained significant attention. However, hydrogel-based electrolyte contacts do not completely eliminate the need for a filling solution but rather increase the sensor’s storage capacity under dry conditions. Moreover, the volume changes of hydrogels due to water adsorption/desorption are influenced by the ion concentration within the hydrogels.
In recent years, the field of SC-ISEs has witnessed significant advancements, driven by the need for improved stability, enhanced sensitivity, and the elimination of filling solutions. Using carbon materials as solid contacts in SC-ISEs is a promising area of research. Carbon materials, including carbon nanotubes (CNT) [24,25,26], graphene [27], carbon black (CB) [28,29], porous carbon, and other carbon materials [30,31,32] renowned for their unique properties, have emerged as highly suitable candidates for facilitating ion–electron conversion and addressing the limitations associated with traditional solid-contact materials. The incorporation of carbon-based solid contacts offers numerous benefits, including improved stability, reduced potential drift, hydrophobicity, and compatibility with various ion-selective membranes [33]. However, there is no systematic summary or analysis of the features of one specific carbon material that has been used for SC-ISEs. This paper has provided a comprehensive overview of the developments in the field of different carbon material-based SC-ISEs. The main objective of this research is to address the limitations associated with traditional solid-contact materials in SC-ISEs and explore the specific characteristics of the four main kinds of carbon materials (CNT, graphene, CB, porous carbon, and carbon-based composites) that have been researched for SCs. It is expected to offer more references for the selection of carbon materials as potential solid contacts to enhance stability, sensitivity, and wearability.
2. Carbon Materials for SC-ISEs
Carbon materials, known for their versatility and exceptional properties, have gained significant attention in various scientific and technological fields. Besides, carbon nanomaterials demonstrate structural diversity and often exist in the form of allotropes, including diamond, graphene, amorphous carbon, fullerene (C60), and carbon nanotubes (CNTs) (Figure 2) [34]. Moreover, they can also be classified based on spatial dimensions, such as zero-dimensional nanoparticles, one-dimensional carbon nanotubes, and two-dimensional layered materials such as graphene [35,36]. Over the past decade, carbon materials have gained attention as composite material carriers due to the following advantages: (1) Excellent acid and alkali resistance; (2) structural stability at high temperatures; (3) high chemical and mechanical reliability; (4) adjustable pore and surface properties; (5) controllable active sites; (6) good electrical and thermal conductivity; (7) versatile structures and morphologies for ease of manipulation; and (8) low production costs [37,38]. These materials exhibit unique properties in terms of size, surface area, strength, and electrical properties. They also possess chemical diversity, ease of manipulation, biocompatibility, and stability as carrier materials. Therefore, they have become attractive research subjects in various fields such as catalysis, energy storage, adsorption, and sensing.
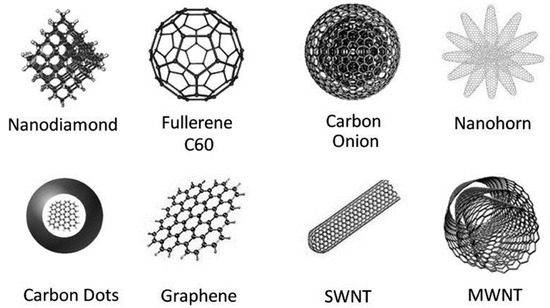
Figure 2.
Members of the carbon nanomaterial family [34].
Carbon-based SCs bring several benefits to ISEs, including enhanced stability, sensitivity, selectivity, and hydrophobicity. The incorporation of carbon materials in SC-ISEs facilitates efficient ion–electron conversion, enhances electrode–substrate interactions, and provides a stable and reliable sensing interface. Moreover, carbon materials enable miniaturization, flexibility, and compatibility with various substrates, expanding the possibilities for the development of wearable and portable SC-ISEs. The comparison of performance across typical carbon-based SC-ISEs is shown in Table 1.

Table 1.
Comparison of performance across typical carbon-based SC-ISEs.
2.1. Carbon Nanotubes (CNTs)
CNTs are cylindrical structures composed of rolled graphene sheets, offering exceptional mechanical strength, high electrical conductivity, and a large surface area. These properties make CNTs an excellent choice for SC-ISEs. The use of CNTs as solid contacts improves ion–electron transfer, reduces potential drift, and enhances the overall stability and sensitivity of the electrodes [46,47,48]. Rius et al. [26,49,50] first used a single-walled carbon nanotube (SWCNT) as a solid-state transfer layer to stabilize electrode potential because of its large capacitance property and then used CNT instead of an ion-selective membrane in the following research. It is used in the detection of biological macromolecules, proteins, etc. Then, to prove the flexibility and wearability, Huang et al. [51] successfully added a biological antifouling film to SWCNT SCs (Figure 3a). This ISE can be used for long-term, continuous monitoring of water quality. This research confirmed the role of SWCNT in reducing water layer formation and reinforcing the ISM and electrode link.
Another kind of material used as SCs is multi-walled CNT (MWCNT). In 2017, a remarkable advancement was presented. Roy et al. [39] produced a wearable sensor constructed using multi-walled CNT (MWCT) electrode arrays for sweat sensing. SC-ISEs with a specific sensitivity to Na+ ions were fabricated by drop coating plasticized poly (vinyl chloride) (PVC) doped with ionophore and ion exchanger onto CNT electrodes (Figure 3b). The ion-selective membrane (ISM) effectively filled the intratubular spaces within the highly porous CNT film, resulting in a stronger attachment compared to flat Au, Pt, or carbon electrodes. Remarkably, a sensitivity of 56 ± 3 mV/decade to Na+ ions was achieved. This study successfully demonstrates the immense potential of CNT-based devices integrated onto flexible supports as an enticing platform for future wearable technology devices. Then Wang et al. [52] proposed a smooth elastic fiber (SE-fiber) coated with CNT film. The effective combination of the wrinkle conductive electrode with the “island” region-designed ion-selective electrode and reference electrode could lead to a highly stable sensing performance, ignoring the stretching/releasing of the sensor from 0 to 200% strain. However, upon releasing the prestretched SE fiber, the CNT film could form a conformal wrinkle structure along with the substrate. In contrast, the sensing materials, including the Ag/AgCl ink film and ion-selective membrane, gradually detached from the CNT layer of the fiber. In order to fabricate a more stable electrochemical sensor on a highly stretchable elastic fiber based on wrinkle structures, carboxyl-functionalized multi-walled carbon nanotube (MWCNT) SCs were also used to make the first wearable, textile-based solid-state contact fluoride sensor (Figure 3c) [53].
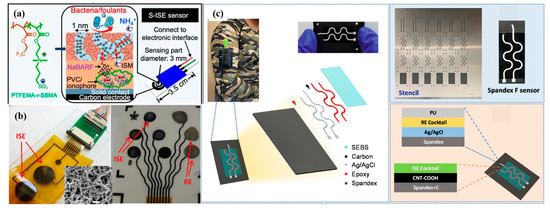
Figure 3.
(a) Chemical structure of self-assembled channel-type zwitterionic copolymer poly (trifluoromethyl methacrylate-random-sulfobetaine methacrylate) PTFEMA-r-SBMA (left); self-assembly of PTFEMA-r-SBMA from the network of hydrophilic nanochannels ∼1 nm in diameter for anti-biofouling and leaching prevention properties (middle) and diagram of S-ISE sensors (right) [51]. (b) CNT-based flexible ISEs, which contained ISE and reference electrode [39]. Fabrication flow of CNT electrodes. And ISEs on polyimide (Kapton) substrates. (inset) SEM image of the surface of the CNT electrodes. (c) Flexible and stretchable textile-based solid-contact potentiometric fluoride sensor [53].
2.2. Graphene
Graphene is a two-dimensional sheet of carbon atoms arranged in a hexagonal lattice, exhibiting extraordinary electrical, mechanical, and thermal properties. Its high electrical conductivity and large surface area make graphene an ideal material for SC-ISEs. Graphene-based SC-ISEs offer enhanced sensitivity, selectivity, and stability. The carbon atom in graphene is bonded to three other carbon atoms; however, the valency of carbon is four; hence, one of the electrons is free to move around in the sheet, leading to an exceptionally high carrier concentration in graphene [54]. The synthesis method used to prepare graphene is crucial since the defects in graphene, such as corrugations (wrinkles, ripples, or crumples), dislocations, adatoms, and Stone–Wales defects, determine its electrical behavior.
In 2011, graphene was first used as a solid ion-to-electron transducer in ISEs [55]. This novel electrode displayed a Nernstian response of 58.4 mV/decade and an impressively low detection limit of 10−6.2 M. Importantly, the potentiometric water layer test confirmed the absence of water film formation between the solid-contact layer and the polymeric membrane (Figure 4a). Furthermore, the developed electrode exhibited a fast response, was insensitive to oxygen and light, and had excellent potential stability, which makes it very promising for routine analysis and application (Figure 4a). The 3D graphene sponge (3D GS) was proven to be used as both an electrode substrate and a solid contact for the construction of Cu2+-ISE [56]. This electrode shows a stable potential response with a LOD of 2.5 × 10−9 M (Figure 4b). Then, the laser-induced graphene (LIG) technique [57], three-dimensional (3D) self-assembled porous graphene aerogel (PGA) [58], and cold atmospheric plasma surface modification [59] were also successfully electrodeposited on the electrode substrate surface. What needs to be mentioned is that ultra-thin and defect-free graphene ink was applied in the preparation of the screen-printed sensor, which significantly enhances the mechanical flexibility of ISEs [60]. On the other hand, Sudiptad et al. used ultraviolet-ozone irradiation to induce laser-induced graphene (LIG) electrodes and developed a disposable device for detecting biosignals such as Na+ ions in sweat [40]. The resulting device exhibited a sensitivity of 60.2 ± 0.9 mV/decade towards Na+. The stability characteristics and significantly expanded preparation methods for graphene-based SCs make it theoretically possible to produce wearable SC-ISEs on a large scale and finally achieve commercial application (Figure 4c).
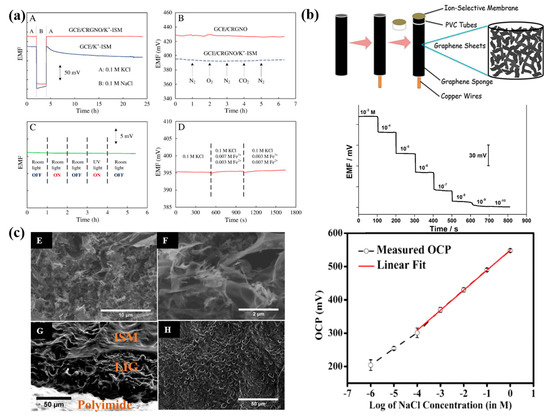
Figure 4.
(a) Stability test for the GCE/K+-ISM and GCE/CRGNO/K+-ISM in different environments [55]. (A): Water layer test for the GCE/K+-ISM and GCE/CRGNO/K+-ISM, the measurements were switched between 0.1 M KCl and 0.1 M NaCl. (B): Sensitivity to O2 and CO2 in 0.1 M KCl solution for the GCE/CRGNO (solid line) and GCE/CRGNO/K+-ISM (dashed line). (C): Effect of light on the potential stability of the GCE/CRGNO/K+-ISM in 0.1 M KCl solution. (D): Redox interference test for the GCE/CRGNO/K+-ISM, the solution was switched to the mixed solution containing 0.1 M KCl, 0.007 M FeCl3 and 0.003 M FeCl2 at 500 s, then replaced by the mixed solution containing 0.1 M KCl, 0.003 M FeCl3 and 0.007 M FeCl2 at 1000 s. (b) A freestanding all-solid-state polymeric membrane Cu2+-selective electrode based on three-dimensional graphene sponge was fabricated, and time-dependent responses of the GS/Cu2+-ISE to Cu2+ in the activity range of 7.9 × 10−4–1.0 × 10−10 M [56]. (c) Potentiometric ISEs based on UV-ozone-irradiated laser-induced graphene electrode [40]. (E,F): SEM images Ozone treated LIG. (G): Cross-sectional SEM image of ISE prepared by two-step drop-coating process showing the different layers. (H): SEM image showing the porous morphology of the top surface of an ISE.
2.3. Carbon Black (CB)
CB, which consists of fine particles of amorphous carbon, is widely used in several industrial processes. This nanomaterial features a set of remarkable properties, including high surface area, high thermal and electrical conductivity, and very low cost [61]. Several studies have explored the applicability of CB in electrochemical fields. Carbon black-based SC-ISEs have also been employed for the detection of various analytes due to their improved sensitivity and stability [62]. The porous nature of carbon black allows for increased ion diffusion and enhanced electrochemical performance. Previous studies show that CB has the ability to intercalate ions on graphite layers [63], which affects electrical conduction between particles by tunneling [64] and the surface reaction. These characteristics also make CB a valuable material for SC-ISEs.
In 2012, Paczosa-Bator successfully introduced carbon black into SC-ISE for the first time [65]. CB was functional as an intermediate layer between an ionophore-doped solvent polymeric membrane and an electrical conductor and a polymeric membrane component, respectively. As shown in Figure 5a, the developed electrodes show a very stable response over time. Even after a long time of conditioning in 0.01 M KCl (6–7 weeks), the electrodes still showed a linear response in the same range of potassium activity. Notably, in 2022, all-solid-state K+-selective sensors based on CB-modified thermoplastic electrodes were fabricated [66]. The result in Figure 5b shows that modification of CB can reduce noise and the formation of a water layer between graphene solid contact and polymeric membrane due to the small particle size of graphene and the CB hydrophobicity, which contributes a lot to the stability of SC-ISEs.
All these studies prove that CBs in SC-ISE can improve the long-term stability of the devices. However, compared to CNT and graphene, CB SC-ISEs have a much higher low detection limit (around 10−4~1 M [28]). And compared with SCs, CBs are more suitable for electrode modification. Due to the high capacitance, CB can efficiently reduce the resistance and mediate the charge transfer at the ISM and glassy carbon electrode interface [67]. Ozer et al. achieved a good sensitivity response to K+ and a LOD of 1 × 10−5 M after modifying stencil-printed carbon electrodes (SPCE) [68] and microfluidic paper-based thermoplastic electrodes (TPE) [69] with CB, respectively (Figure 5c,d).
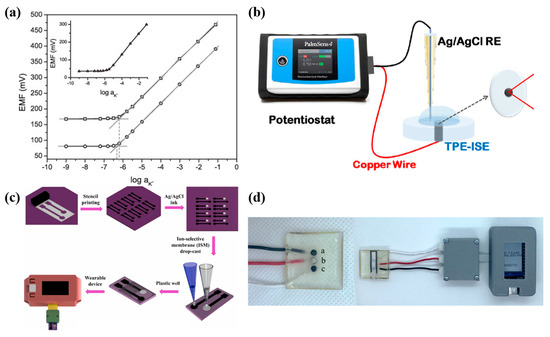
Figure 5.
(a) EMF dependence on K+ activities for GCD/CB/K+-ISM (○), GCD/(CB, K+-ISM) (□), and inset: GCD/K+-ISM (△) electrodes conditioned in 0.01 M KCl solution [65]. (b) CB-modified K+ sensitive thermoplastic electrode [66]. (c) Fabrication process of K+-selective SPCEs and integration into the wearable device (WISETIC) [68]. (d) Image of the TPE array, where a is the Na+-ISE, b is the reference electrode, and c is the K+-ISE, and the TPE array is coupled to a paper-based device (PAD) [69].
2.4. Porous Carbon
Porous carbon materials have high electrical conductivity and a large specific surface area [70], and they also have an adjustable pore size, which improves the selectivity of the ISE in the detection process [71]. Among all, three-dimensionally ordered microporous carbon (3DOMC) draws researchers’ attention. It consists of a glassy carbon skeleton with interconnected macropores that can be infiltrated with the ISM to form a continuous structure in which electrons are conducted through the carbon framework while ions move through the infused ISM. Its large interfacial contact area and high capacitance mean that it has a larger contact area and higher EDL capacitance when used as SCs, which is more conducive to electron transfer and potential stability.
Lai et al. [41] first employed 3DOMC as a solid-state transduction layer for K+-selective electrodes, as shown in Figure 6a. The SC-ISEs fabricated based on 3DOMC exhibited a long-term potential drift of only 11.7 mV/h, which remained unchanged over an extended period. The electrode demonstrated excellent resistance to interference from oxygen. Additionally, the team investigated the influence of 3DOMC structure and surface chemistry on the performance of ion-selective electrodes. It was shown that oxidized 3DOMC exhibited a typical water layer phenomenon that led to the potential stability of the deterioration of SC-ISEs [72]. In addition, Niu’s team also developed porous carbon submicrons (PC-SMSs) as SCs for K+ SC-ISE, as shown in Figure 6b. PC-SMSs have high hydrophobicity and a large EDL capacitance. The potential drift of PC-SMS-based ISEs is only 7 mV in two months [31].
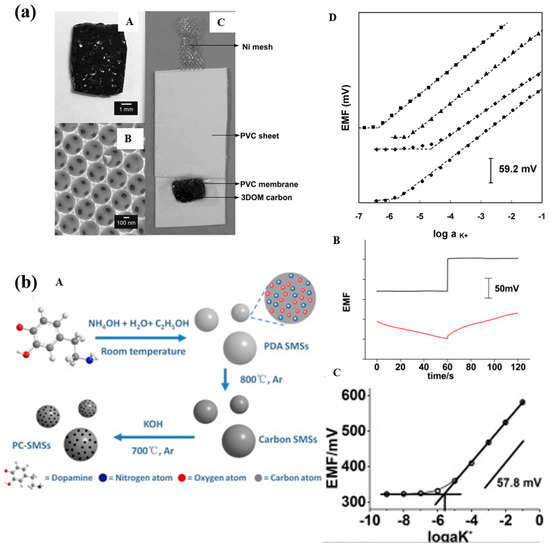
Figure 6.
(a)—(A): Photograph of monolithic 3DOM carbon. (B): SEM image of 3DOM carbon. (C): Photograph of 3DOM carbon-contacted ISE. (D): Potentiometric K+ response curves of SC-ISEs with different electrode assemblies in KCl solutions: (◼) Ni/3DOM carbon/PVC, (▲) Ni/HOPG/PVC, (◆) Ni/3DOM carbon, (●) Ni/PVC. For clarity, response curves have been shifted vertically relative to one another [41]. (b)—(A): Schematic illustration of the synthesis of PC-SMSs. (B): Chronopotentiometric results. Upper part: PC-SMSs/K+-ISE; applied current: 10 nA for 60 s and −10 nA for 60 s; bottom part: K+-CWE; applied current: 1 nA for 60 s and −1 nA for 60 s; solution: 0.1 M KCl. (C): Calibration curve for the PC-SMSs/K+-ISE in the varied concentration of KCl solution [31].
2.5. Other Carbon Materials
Apart from the four main carbon materials, a wide variety of carbon materials are available. This makes it possible for the researchers to choose the most appropriate SC material to satisfy the specific requirements and desirable performances. For example, zero-dimensional hollow spherical fullerenes with polygons [42,73], single-walled carbon nanohorns (SWCNHS) [43], NiO-doped porous biomass carbon materials [44], ZnCl2-KOH-activated kelp charcoal (ZKAKC) [45], graphdiyne oxide [74], and other functionalized carbon composites [75,76,77,78] were also used as SC-ISEs of SCs and achieved good results (Figure 7).
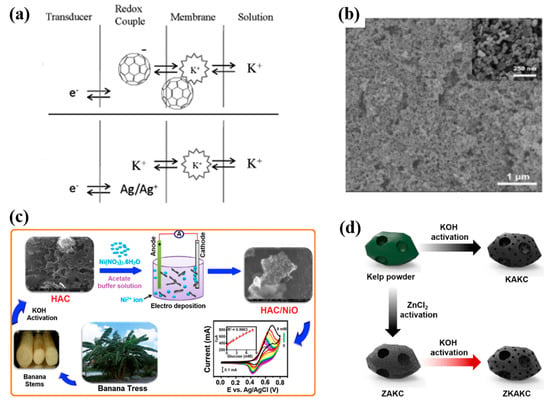
Figure 7.
(a) Schematic diagram of the charge-transfer process in a C60 SC-ISEs (upper) and conventional ISEs (down) [42]. (b) SEM image of GCE/SWCNHs [43]. (c) Illustrated synthesis of HAC/NiO nanocomposite from banana stems, their application as glucose sensor and CV curves [44]. (d) Graphical illustration of preparation of ZKAKC [45].
At present, another trend of SCs is to combine carbon materials with various materials in order to take advantage of each other. Due to the hydrophobic properties of the 3D graphene oxide–CNT composite, Hua et al. successfully achieved higher reversibility of ISEs (Figure 8a). Under a strain of 40%, the sensor still showed a sensitivity of 42.7 ± 3.1 mV/log (NH4+) [79]. Another study also proved that the ionic liquid trihexyltetradecylphosphonium chloride (THTDPCl) and various types of multi-walled carbon nanotubes (MWCNTs-THTDPCl) nanocomposite effectively act as an ion exchanger and ionic membrane component by decreasing membrane resistance. Such a device does not require additional lipophilic salt [80].
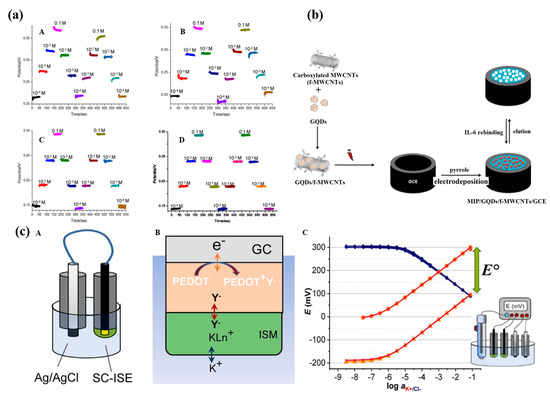
Figure 8.
The reversibility of the potentiometric NH4+ detection using sensors (a)—(A,B):without and (C,D): with 3D graphene–CNT modified electrodes, respectively [79]. (b) Schematic preparation of molecularly imprinting polymers (MIP)/GQDs/f-MWCNTs/GCE [81]. (c) Schematic representation of the instrument-free method to adjust the standard potential E0 of solid-contact ion-selective electrodes. (c)—(A): The SC-ISE is short-circuited with an Ag/AgCl QRE in a solution of constant concentration of the chloride salt of the primary ion. (B): The potential difference between the short-circuited electrodes causes oxidation/reduction or charging/discharging of the solid contact (depending on the type of solid-contact material) which (C): Alters the standard potential of the short-circuited SC-ISEs measured at open circuit potential, while the potential of the Ag/AgCl remains constant due to its large capacitance [82].
In addition, the carbon material’s surface can also be functionalized with various functional groups (such as oxides, amides, thiols, or different groups) to change the properties of nanomaterials. This strategy can satisfy the special properties of ISEs, such as small quantities of samples, free of calibration, and long-term stability, making it possible to work in specific conditions. Nermin et al. used graphene quantum dots (GQD)/carboxylated CNT (f-CNT) as SCs and found that GQDs/f-MWCNTs can not only prevent the stacking of GQDs but also have a larger surface area (Figure 8b). Their hydrophilic groups also contribute to the reduction of sample quantities [81]. In another work, a larger capacitance quasi-reference electrode (QRE) was used to calibrate SC-ISEs (Figure 8c). Therefore, upon short-circuiting, the potential of the QRE remains practically constant, while the potential of the SC-ISE is shifted until the potential difference between the SC-ISE and QRE approaches zero [82].
3. Relationship between Carbon Materials and Performance of the SC-ISEs
According to the International Union of Pure and Applied Chemistry (IUPAC) on the definition of ISE-related terms, evaluation of performance indicators, and identification methods, the performance evaluation of ISEs includes sensitivity, selectivity, detection limit, responsible time, and service life. Among these, the sensitivity of ISEs is described by the Nernst equation. However, the diffusion potential of ions between samples depends mainly on the ISM. So, it will not be discussed in detail in this work.
- Selectivity: Selectivity is one of the most important characteristics of the sensor because it determines whether the target ion can be reliably measured. In the actual measurement, ISEs will also respond to some interference ions, so that the response slope of the electrode deviates from the theoretical value and the sensitivity decreases. Therefore, it is necessary to introduce a selectivity coefficient to evaluate the anti-interference ability of electrodes against interfering ions. The smaller the selectivity coefficient , the better the ISE selectivity and the better the anti-jamming capability [83].
- Detection range: Each ISE has an upper and lower detection limit, and the range between the upper and lower detection limits is the detection range of the electrode. With the decrease in the activity of the target ions, the interfering ions can enter the ISM, which results in a deviation of the response of the electrode from the theoretical value of the Nernst slope. And the lowest concentration of this solution is regarded as the lower limit of detection. When the target ion activity is too high, the ISM will produce a co-extraction effect with the solution to be measured, which also causes the response of the electrode to deviate from the theoretical value of the Nernst slope. It is defined as the upper limit of detection [84].
- Response time: In continuous monitoring, rapid response is an important parameter to obtain the dynamic change of target ion activity in real time. The response time is considered to be the time required for the sensor to reach 90% of its equilibrium potential [85,86]. The main factors affecting the electrode response time are the diffusion-controlled equilibrium of target ions at the membrane-water interface and the efficiency of ion–electron conversion, which depend on the ISM and SC, respectively.
- Lifetime: The service life of electrodes refers to the time that the electrode can be used normally while keeping its performance indexes unchanged. The service life of electrodes is mainly affected by the ISM, such as the aging of the membrane matrix, the loss of ionic carriers and additives, and the damage of the external environment to membrane components [87].
ISEs incorporate SCs to enable reversible ion–electron conversion and stabilize the SC|ISM interface potential. This maximizes the performance of SC-ISEs, aiming to create highly stable, maintenance-free, calibration-free, and interchangeable potential ion sensors. The exceptional performance of SC materials is crucial for ensuring stable and reproducible potential. Among these capabilities, a stable potential response is indicative of minimal potential drift, while poorer potential stability typically suggests defects in the SC material. Additionally, the reproducibility of potential among individual electrodes determines calibration frequency, while the reproducibility of electrode standard potential (E0) between electrodes determines their direct interchangeability. Therefore, when compared to LC-ISEs, the performance assessment of SC-ISEs includes evaluating potential stability, reproducibility, the presence of a water layer, and interference resistance [65].
- Stability: Ideally, SC materials should possess a non-polarizing interface with a high exchange current density. However, in practical measurements, the input current of the measuring amplifier inevitably causes charging and discharging, resulting in varying degrees of electrode polarization [88]. In addition to electrode polarization, mechanical failures of the electrode and hydrolysis of the ISM can also lead to potential drift. For instance, when the electrode is immersed in an aqueous solution for an extended period, the adhesion between the ISM and the SC gradually decreases, resulting in potential fluctuations [89]. Extensive theoretical and practical evidence demonstrates that a sufficiently large oxidation–reduction or electric double-layer (EDL) capacitance serves as a guarantee for electrode potential stability [90]. In the EDL model, the sum of the three interface potentials (ISM|(SC)|GC) is the total potential of SC-ISEs. However, due to the conductivity of SCs, the potential diversity of SC|GS can be ignored. And the potential diversity between SCs and ISMs cannot be accurately calculated because there is no electron exchange between SCs and ISMs. But according to the definition of potential (E = Q/C, E represents potential diversity, Q represents the amount of charge, and C represents capacity), it is easy to realize that a larger capacity of SCs would result in a smaller potential diversity of SC|ISM. Most carbon materials used for SCs belong to the capacitance-based transduction mechanism. It means carbon materials with a large surface area and abundant pores, such as graphene, SWCNT, and porous carbons, are advantageous for increasing capacitance and enhancing the stability of ISEs. At the same time, a hydrophobic surface can also help reduce drift currents.
- Reproducibility: The pre-calibration of potential is an essential step for SC-ISEs before testing, as it directly affects the accuracy and reproducibility of the measurement results. While SC-ISEs with low potential drift can meet the requirements of practical testing through periodic calibration, complex or frequent calibrations can significantly increase time and cost [87]. Achieving high reproducibility for the E0 remains a challenge. The Bühlmann group has pointed out that E0 is determined by the overall structure of SC-ISEs, including each bulk phase and interface [91]. It is very important to reduce the surface redox functional groups to maintain the stability of the interface potential. When it comes to carbon materials, The introduction of colloidal imprinted mesoporous carbon (CIMC) can effectively improve the reproducibility of the E0 [92]. Besides, redox buffer has already been introduced to design and fabricate calibration-free ion sensors [93], but their lifetime is not long because of the gradual loss of the redox buffer from the ISMs with usage time. In summary, research on E0 is still in its early stages, and there is room for improvement in terms of potential reproducibility. The development of calibration-free SC-ISEs remains a hot topic for future research.
- Water layer testing: The water layer effect at the SC|ISM interface is one of the major and persistent challenges in SC-ISEs (solid contact ion-selective electrodes). The continuous water layer formed between SCs and ISMs acts as a reservoir for transmembrane ions and neutral particles, but instead of providing a reversible interfacial potential, it irreversibly disrupts the long-term potential stability of SC-ISEs. As the water layer continues to diffuse at the interface, the adhesion between ISMs and SCs is further compromised, eventually leading to the separation of ISMs from the substrate [94]. This is also a reason that causes a change in the value of E0 [95]. Currently, enhancing the hydrophobicity of SC materials is considered the most effective approach to addressing the water layer effect [96]. Most carbon materials possess a high surface area and strong adsorption capabilities due to their porous nature. Therefore, methods to enhance the hydrophobicity of carbon materials include reducing the presence of oxygen-containing hydrophilic functional groups on the surface and defects.
- Interference testing: In addition to the water layer effect, various external interferences such as light, CO2, O2, and redox couples can also disrupt the ion–electron transduction process and lead to potential changes. This necessitates that SC functional materials possess sufficient capacitance and high chemical stability. Actually, there are four electrons in the outermost layer of carbon. These four electrons occupy the s and p sublayers of the second layer. There are four total orbitals in these two layers. According to the Hund rule, each electron occupies one orbital and chooses the same direction; the electrons are low in energy and relatively stable, which is why the carbon chemical property is so stable. In this regard, most carbon materials are relatively hard to react with disturbing effects and meet the requirements of SCs.
4. Conclusions and Outlook
Through the development and research process of ISEs, in order to address the issues of calibration, large volume, leakage, and short lifespan associated with LC-ISEs, researchers introduced the concept of SCs. This was performed with the aim of developing ISEs that exhibit strong stability, can adapt to complex testing environments, and facilitate miniaturization. However, in order to achieve stable and reliable responses in SC-ISEs, SCs need to meet certain criteria, including reversible ion-to-electron conduction, an ideal non-polarizing high exchange current density interface, and no side reactions. Various materials, including noble metals, conductive polymers, and carbon materials, have been studied as SCs. Among these materials, carbon materials have gained wide applications due to their abundance, good conductivity, and low cost. However, some current challenges and future directions for carbon materials in SC-ISEs are as follows:
- Different types of carbon-based SCs have their own advantages. CNT-SCs exhibit higher capacitance. Graphene SCs have good resistance to interference and are applicable to various preparation methods. CB-SCs demonstrate better repeatability and long-term stability but have a narrower measurement range. Porous carbon SCs exhibit stable calibration potential and hydrophobicity.
- Limited selectivity: The carbon-based solid transducing layer may exhibit lower selectivity towards certain ions, leading to cross-interference or inaccuracies in ISE measurements. This can restrict the accuracy and precision of ion analysis. Developing carbon-based solid transducing layers with improved selectivity towards specific ions is an important research direction. To enhance ion recognition and selectivity, the design and modification of carbon materials, such as introducing functional groups or surface modifications, are effective ways.
- Surface adsorption and contamination: Carbon materials have a high adsorption capacity, making them prone to the adsorption of impurity ions or organic substances. This can result in surface adsorption and contamination and affect the selectivity and stability of ISE. In terms of surface design, surface modification of SCs by adding redox buffers, hydrophobic layers, and other methods is used to achieve stable E0 and drift potential. Investigating surface engineering strategies is essential to reducing adsorption and fouling on the carbon-based solid transducing layers. Besides, surface modifications, coatings, and nanostructured surfaces are all efficient in minimizing interference from impurities and enhancing the stability and performance of SCs.
- Structural degradation and deterioration: Prolonged usage or cycling processes may lead to structural changes or degradation of the carbon-based SCs and cause performance deterioration or irreversible damage. It would also limit the long-term stability and reliability of ISEs. Exploring the integration of carbon-based SCs with other advanced technologies, such as nanomaterials, nanoelectronics, or microfluidics, can unlock new possibilities for enhanced and multifunctional ISEs. Such a way includes exploring composite materials, hybrid structures, and novel device architectures that leverage the unique properties of carbon materials.
Author Contributions
Writing—original draft, P.W. and H.L.; investigation, H.L., S.Z. and L.C.; funding acquisition, H.L., L.C., S.Y. and J.W.; writing—review and editing, L.C., S.Y. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Shenzhen Science and Technology Program (Grant Nos. KQTD20200820113045083, ZDSYS20190902093220279 and JCYJ20220818102403007), National Natural Science Foundation of China (52201257) and Shenzhen Research Fund for Returned Scholars (DD11409017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Lina Chen, Suzhu Yu, and Jun Wei for their contributions to editing and proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Cremer, M. Über die Ursache der elektromotorischen Eigenschaften der Gewebe, zugleich ein Beitrag zur Lehre von den polyphasischen Elektrolytketten. Z. Biol. 1906, 47, 562–608. [Google Scholar]
- Pungor, E.; Hollos-Rokosinyi, E. Uber die Anwendung von Membranelektroden bei der Untersuchung von Ionenkonzentrationen. Acta Chim. Acad. Sci. Hung. 1961, 27, 63–68. [Google Scholar]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Mirsky, V.M. Combinatorial and high-throughput development of sensing materials: The first 10 years. Chem. Rev. 2008, 108, 770–813. [Google Scholar]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of electrochemical hydrogen sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar]
- Michalska, A. All-Solid-State Ion Selective and All-Solid-State Reference Electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- Durst, R.A. Ion-Selective Electrodes-The Early Years. Electroanalysis 2012, 24, 15–22. [Google Scholar] [CrossRef]
- Sokalski, T.; Ceresa, A.; Zwickl, T.; Pretsch, E. Large improvement of the lower detection limit of ion-selective polymer membrane electrodes. J. Am. Chem. Soc. 1997, 119, 11347–11348. [Google Scholar] [CrossRef]
- Kalinichev, A.V.; Solovyeva, E.V.; Ivanova, A.R.; Khripoun, G.A.; Mikhelson, K.N. Non-constancy of the bulk resistance of ionophore-based Cd2+-selective electrode: A correlation with the water uptake by the electrode membrane. Electrochim. Acta 2020, 334, 135541. [Google Scholar] [CrossRef]
- Hoekstra, R.; Blondeau, P.; Andrade, F.J. Distributed electrochemical sensors: Recent advances and barriers to market adoption. Anal. Bioanal. Chem. 2018, 410, 4077–4089. [Google Scholar] [CrossRef]
- Bobacka, J.; Lindfors, T.; McCarrick, M.; Ivaska, A.; Lewenstam, A. Single-piece all-solid-state ion-selective electrode. Anal. Chem. 1995, 67, 3819–3823. [Google Scholar] [CrossRef]
- Hirata, H.; Dato, K. Copper (I) sulphide-impregnated silicone rubber membranes as selective electrodes for copper (II) ions. Talanta 1970, 17, 883–887. [Google Scholar] [CrossRef]
- James, H.J.; Carmack, G.; Freiser, H. Coated wire ion-selective electrodes. Anal. Chem. 1972, 44, 856–857. [Google Scholar] [CrossRef]
- Hulanicki, A.; Trojanowicz, M. Calcium-selective electrodes with PVC membranes and solid internal contacts. Anal. Chim. Acta 1976, 87, 411–417. [Google Scholar] [CrossRef]
- Vasconcellos, E.; Evenson, K. New far infrared laser lines obtained by optically pumping13CD3OD. Int. J. Infrared Millim. Waves 1985, 6, 1157–1167. [Google Scholar] [CrossRef]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Johnson, T.A.; Ash, R.B.; Nagle, H.T.; Neuman, M.R.; Buck, R.P. In vivo and in vitro testing of microelectronically fabricated planar sensors designed for applications in cardiology. Fresenius’ J. Anal. Chem. 1993, 346, 584–588. [Google Scholar] [CrossRef]
- McElhoney, K.; O’Neil, G.D.; Chaniotakis, N.A.; Kounaves, S.P. Stability and Lifetime of Potassium Solid-Contact Ion Selective Electrodes for Continuous and Autonomous Measurements. Electroanal. 2012, 24, 2071–2078. [Google Scholar] [CrossRef]
- Hauser, P.C.; Chiang, D.W.; Wright, G.A. A potassium-ion selective electrode with valinomycin based poly (vinyl chloride) membrane and a poly (vinyl ferrocene) solid contact. Anal. Chim. Acta 1995, 302, 241–248. [Google Scholar] [CrossRef]
- Heng, L.Y.; Hall, E.A. Producing “self-plasticizing” ion-selective membranes. Anal. Chem. 2000, 72, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hupa, E.; Vanamo, U.; Bobacka, J. Novel ion-to-electron transduction principle for solid-contact ISEs. Electroanalysis 2015, 27, 591–594. [Google Scholar] [CrossRef]
- Fibbioli, M.; Bandyopadhyay, K.; Liu, S.-G.; Echegoyen, L.; Enger, O.; Diederich, F.; Bühlmann, P.; Pretsch, E. Redox-active self-assembled monolayers as novel solid contacts for ion-selective electrodes. Chem. Commun. 2000, 5, 339–340. [Google Scholar] [CrossRef]
- Fibbioli, M.; Bandyopadhyay, K.; Liu, S.-G.; Echegoyen, L.; Enger, O.; Diederich, F.; Gingery, D.; Bühlmann, P.; Persson, H.; Suter, U.W. Redox-active self-assembled monolayers for solid-contact polymeric membrane ion-selective electrodes. Chem. Mater. 2002, 14, 1721–1729. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Mousavi, Z.; Teter, A.; Lewenstam, A.; Maj-Zurawska, M.; Ivaska, A.; Bobacka, J. Comparison of Multi-walled Carbon Nanotubes and Poly (3-octylthiophene) as Ion-to-Electron Transducers in All-Solid-State Potassium Ion-Selective Electrodes. Electroanalysis 2011, 23, 1352–1358. [Google Scholar] [CrossRef]
- Guinovart, T.; Parrilla, M.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst 2013, 138, 5208–5215. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Rius, F.X. Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Zhou, M.; Gan, S.; Zhang, Q.; Han, D.; Niu, L. All-solid-state potassium-selective electrode using graphene as the solid contact. Analyst 2012, 137, 618–623. [Google Scholar] [CrossRef]
- Mazzaracchio, V.; Serani, A.; Fiore, L.; Moscone, D.; Arduini, F. All-solid state ion-selective carbon black-modified printed electrode for sodium detection in sweat. Electrochim. Acta 2021, 394, 139050. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupień, K. Potentiometric sensors with carbon black supporting platinum nanoparticles. Anal. Chem. 2013, 85, 10255–10261. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Cabaj, L.; Pięk, M.; Piech, R.; Kubiak, W.W. Carbon-Supported Platinum Nanoparticle Solid-State Ion Selective Electrodes for the Determination of Potassium. Anal. Lett. 2015, 48, 2773–2785. [Google Scholar] [CrossRef]
- Ye, J.; Li, F.; Gan, S.; Jiang, Y.; An, Q.; Zhang, Q.; Niu, L. Using sp2-C dominant porous carbon sub-micrometer spheres as solid transducers in ion-selective electrodes. Electrochem. Commun. 2015, 50, 60–63. [Google Scholar] [CrossRef]
- Jiang, T.; Yin, B.; Liu, X.; Yang, L.; Pang, H.; Song, J.; Wu, S. Porous carbon-based robust, durable, and flexible electrochemical device for K+ detection in sweat. Analyst 2022, 147, 1144–1151. [Google Scholar] [CrossRef]
- Miller, P.R.; Xiao, X.; Brener, I.; Burckel, D.B.; Narayan, R.; Polsky, R. Microneedle-based transdermal sensor for on-chip potentiometric determination of K+. Adv. Health Mater. 2014, 3, 876–881. [Google Scholar] [CrossRef]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Flavin, K.; Kopf, I.; Radics, G.; Hearnden, C.H.; McManus, G.J.; Moran, B.; Villalta-Cerdas, A.; Echegoyen, L.A.; Giordani, S. Functionalization of carbon nanoparticles modulates inflammatory cell recruitment and NLRP3 inflammasome activation. Small 2013, 9, 4194–4206. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Buriak, J.M. Diamond surfaces: Just big organic molecules? Angew. Chem. Int. Ed. 2001, 40, 532–534. [Google Scholar] [CrossRef]
- Noked, M.; Okashy, S.; Zimrin, T.; Aurbach, D. Composite carbon nanotube/carbon electrodes for electrical double-layer super capacitors. Angew. Chem. 2012, 124, 1600–1603. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon nanotube-based ion selective sensors for wearable applications. Acs Appl. Mater. Inter. 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Choudhury, S.; Roy, S.; Bhattacharya, G.; Fishlock, S.; Deshmukh, S.; Bhowmick, S.; McLaughlign, J.; Roy, S.S. Potentiometric ion-selective sensors based on UV-ozone irradiated laser-induced graphene electrode. Electrochim. Acta 2021, 387, 138341. [Google Scholar] [CrossRef]
- Lai, C.-Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef]
- Fouskaki, M.; Chaniotakis, N. Fullerene-based electrochemical buffer layer for ion-selective electrodes. Analyst 2008, 133, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yao, Y.; Cai, Y.; Ping, J. All-solid-state potentiometric sensor using single-walled carbon nanohorns as transducer. Sens. Actuators B Chem. 2019, 283, 284–289. [Google Scholar] [CrossRef]
- Veeramani, V.; Madhu, R.; Chen, S.-M.; Veerakumar, P.; Hung, C.-T.; Liu, S.-B. Heteroatom-enriched porous carbon/nickel oxide nanocomposites as enzyme-free highly sensitive sensors for detection of glucose. Sens. Actuators B Chem. 2015, 221, 1384–1390. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.M.; Jeon, Y.; Lee, J.; Oh, J.; Antink, W.H.; Kim, D.; Piao, Y. Novel two-step activation of biomass-derived carbon for highly sensitive electrochemical determination of acetaminophen. Sens. Actuators B Chem. 2018, 259, 50–58. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Soda, Y.; Gao, W.; Bosset, J.; Bakker, E. Emulsion Doping of Ionophores and Ion-Exchangers into Ion-Selective Electrode Membranes. Anal. Chem. 2020, 92, 14319–14324. [Google Scholar] [CrossRef]
- Appiah-Ntiamoah, R.; Gadisa, B.T.; Kim, H. An effective electrochemical sensing platform for fluoride ions based on fluorescein isothiocyanate-MWCNT composite. New J. Chem. 2018, 42, 11341–11350. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Bobacka, J.; Rius, F.X. Transduction mechanism of carbon nanotubes in solid-contact ion-selective electrodes. Anal. Chem. 2009, 81, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Sedeno, P.; Pingarrón, J.M.; Riu, J.; Rius, F.X. Electrochemical sensing based on carbon nanotubes. TrAC Trends Anal. Chem. 2010, 29, 939–953. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, X.; Wang, X.; Wang, T.; Lounder, S.J.; Ravindran, T.; Demitrack, Z.; McCutcheon, J.; Asatekin, A.; Li, B. Electrospraying Zwitterionic Copolymers as an Effective Biofouling Control for Accurate and Continuous Monitoring of Wastewater Dynamics in a Real-Time and Long-Term Manner. Environ. Sci. Technol. 2022, 56, 8176–8186. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, Y.; Yang, X.; Liu, L.; Li, L.; Lu, Q.; Li, T.; Zhang, T. Highly stretchable potentiometric ion sensor based on surface strain redistributed fiber for sweat monitoring. Talanta 2020, 214, 120869. [Google Scholar] [CrossRef]
- Goud, K.Y.; Sandhu, S.S.; Teymourian, H.; Yin, L.; Tostado, N.; Raushel, F.M.; Harvey, S.P.; Moores, L.C.; Wang, J. Textile-based wearable solid-contact flexible fluoride sensor: Toward biodetection of G-type nerve agents. Biosens. Bioelectron. 2021, 182, 113172. [Google Scholar] [CrossRef]
- Joshi, P.; Haque, A.; Gupta, S.; Narayan, R.J.; Narayan, J. Synthesis of multifunctional microdiamonds on stainless steel substrates by chemical vapor deposition. Carbon. 2021, 171, 739–749. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Li, J.; Qin, W. A freestanding all-solid-state polymeric membrane Cu2+-selective electrode based on three-dimensional graphene sponge. Anal. Chim. Acta 2019, 1068, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Sanborn, D.; Chen, B.; Garland, N.; Serhan, M.; Forzani, E.; Gomes, C.; Claussen, J.C. Ion-Selective Sensors Based on Laser-Induced Graphene for Evaluating Human Hydration Levels Using Urine Samples. Adv. Mater. Technol 2020, 5, 1901037. [Google Scholar] [CrossRef]
- Li, J.; Qin, W. An integrated all-solid-state screen-printed potentiometric sensor based on a three-dimensional self-assembled graphene aerogel. Microchem. J. 2020, 159, 105453. [Google Scholar] [CrossRef]
- Sedaghat, S.; Kasi, V.; Nejati, S.; Krishnakumar, A.; Rahimi, R. Improved performance of printed electrochemical sensors via cold atmospheric plasma surface modification. J. Mater. Chem. C 2022, 10, 10562–10573. [Google Scholar] [CrossRef]
- Kim, D.S.; Jeong, J.-M.; Park, H.J.; Kim, Y.K.; Lee, K.G.; Choi, B.G. Highly concentrated, conductive, defect-free graphene ink for screen-printed sensor application. Nano-Micro Lett. 2021, 13, 87. [Google Scholar] [CrossRef]
- Long, C.M.; Nascarella, M.A.; Valberg, P.A. Carbon black vs. black carbon and other airborne materials containing elemental carbon: Physical and chemical distinctions. Environ. Pollut. 2013, 181, 271–286. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.; Fatibello-Filho, O. Nanostructured carbon black for simultaneous sensing in biological fluids. Sens. Actuators B Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Reboul, J.-P.; Moussalli, G. About some DC conduction processes in carbon black filled polymers. Int. J. Polym. Mater. 1976, 5, 133–146. [Google Scholar] [CrossRef]
- Balberg, I.; Wagner, N.; Goldstein, Y.; Weisz, S. Tunneling and percolation behavior in granular metals. MRS Online Proc. Libr. (OPL) 1990, 195, 233. [Google Scholar] [CrossRef]
- Paczosa-Bator, B. All-solid-state selective electrodes using carbon black. Talanta 2012, 93, 424–427. [Google Scholar] [CrossRef]
- Ozer, T.; Henry, C.S. All-solid-state potassium-selective sensor based on carbon black modified thermoplastic electrode. Electrochim. Acta 2022, 404, 139762. [Google Scholar] [CrossRef]
- Pięk, M.; Paczosa-Bator, B.; Smajdor, J.; Piech, R. Molecular organic materials intermediate layers modified with carbon black in potentiometric sensors for chloride determination. Electrochim. Acta 2018, 283, 1753–1762. [Google Scholar] [CrossRef]
- Ozer, T.; Agir, I.; Henry, C.S. Low-cost Internet of Things (IOT)-enabled a wireless wearable device for detecting potassium ions at the point of care. Sens. Actuators B Chem. 2022, 365, 131961. [Google Scholar] [CrossRef]
- Ozer, T.; Henry, C.S. Microfluidic-based ion-selective thermoplastic electrode array for point-of-care detection of potassium and sodium ions. Microchim. Acta 2022, 189, 152. [Google Scholar] [CrossRef]
- Neal, J.N.; Wesolowski, D.J.; Henderson, D.; Wu, J. Ion distribution and selectivity of ionic liquids in microporous electrodes. J. Chem. Phys. 2017, 146, 174701. [Google Scholar] [CrossRef]
- Park, S.; Maier, C.S.; Koley, D. Anodic stripping voltammetry on a carbon-based ion-selective electrode. Electrochim. Acta 2021, 390, 138855. [Google Scholar] [CrossRef] [PubMed]
- Fierke, M.A.; Lai, C.-Z.; Buhlmann, P.; Stein, A. Effects of architecture and surface chemistry of three-dimensionally ordered macroporous carbon solid contacts on performance of ion-selective electrodes. Anal. Chem. 2010, 82, 680–688. [Google Scholar] [CrossRef]
- Li, J.; Yin, T.; Qin, W. An all-solid-state polymeric membrane Pb2+-selective electrode with bimodal pore C60 as solid contact. Anal. Chim. Acta 2015, 876, 49–54. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Y.; Hao, J.; Wei, H.; Zheng, W.; Mao, L. Graphdiyne oxide enhances the stability of solid contact-based ionselective electrodes for excellent in vivo analysis. Sci. China Chem. 2019, 62, 1414–1420. [Google Scholar] [CrossRef]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Poly(3,4-ethylenedioxythiophene) (PEDOT) doped with carbon nanotubes as ion-to-electron transducer in polymer membrane-based potassium ion-selective electrodes. J. Electroanal. Chem. 2009, 633, 246–252. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Roushani, M.; Shamsipur, M. Development of a highly selective voltammetric sensor for nanomolar detection of mercury ions using glassy carbon electrode modified with a novel ion imprinted polymeric nanobeads and multi-wall carbon nanotubes. J. Electroanal. Chem. 2013, 693, 16–22. [Google Scholar] [CrossRef]
- Sun, Q.; Li, W.; Su, B. Highly hydrophobic solid contact based on graphene-hybrid nanocomposites for all solid state potentiometric sensors with well-formulated phase boundary potentials. J. Electroanal. Chem. 2015, 740, 21–27. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Lindfors, T. Few-layer graphene and polyaniline composite as ion-to-electron transducer in silicone rubber solid-contact ion-selective electrodes. Sens. Actuators B Chem. 2016, 224, 624–631. [Google Scholar] [CrossRef]
- Hua, Y.; Guan, M.; Xia, L.; Chen, Y.; Mai, J.; Zhao, C.; Liao, C. Highly Stretchable and Robust Electrochemical Sensor Based on 3D Graphene Oxide–CNT Composite for Detecting Ammonium in Sweat. Biosensors 2023, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Wardak, C. Comparative study of nitrate all solid state ion-selective electrode based on multiwalled carbon nanotubes-ionic liquid nanocomposite. Sens. Actuators B Chem. 2021, 348, 130720. [Google Scholar] [CrossRef]
- Ozcan, N.; Karaman, C.; Atar, N.; Karaman, O.; Yola, M.L. A Novel Molecularly Imprinting Biosensor Including Graphene Quantum Dots/Multi-Walled Carbon Nanotubes Composite for Interleukin-6 Detection and Electrochemical Biosensor Validation. ECS J. Solid. State Sci. Technol. 2020, 9, 121010. [Google Scholar] [CrossRef]
- Han, T.; Mattinen, U.; Song, T.; Bobacka, J. Expanding the possibilities for an instrument-free method to reproducible resetting of the standard potential (E0) of solid-contact ion-selective electrodes. Sens. Actuators B Chem. 2023, 390, 134005. [Google Scholar] [CrossRef]
- Ross, J.W. Calcium-selective electrode with liquid ion exchanger. Science 1967, 156, 1378–1379. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Santos, L.; Neto, J.P.; Crespo, A.; Nunes, D.; Costa, N.; Fonseca, I.M.; Barquinha, P.; Pereira, L.; Silva, J.; Martins, R. WO3 nanoparticle-based conformable pH sensor. Acs Appl. Mater. Inter. 2014, 6, 12226–12234. [Google Scholar] [CrossRef]
- Bakker, E. Can calibration-free sensors be realized? ACS Sens. 2016, 1, 838–841. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-contact ion-selective electrodes: Response mechanisms, transducer materials and wearable sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- BruceáAsh, R.; TroyáNagle, H. Flexible (Kapton-based) microsensor arrays of high stability for cardiovascular applications. J. Chem. Soc. Faraday Trans. 1993, 89, 361–367. [Google Scholar]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Hu, J.; Zou, X.U.; Stein, A.; Buhlmann, P. Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.U.; Zhen, X.V.; Cheong, J.H.; Bühlmann, P. Calibration-Free Ionophore-Based Ion-Selective Electrodes With a Co(II)/Co(III) Redox Couple-Based Solid Contact. Anal. Chem. 2014, 86, 8687–8692. [Google Scholar] [CrossRef] [PubMed]
- Zdrachek, E.; Bakker, E. Electrochemically switchable polymeric membrane ion-selective electrodes. Anal. Chem. 2018, 90, 7591–7599. [Google Scholar] [CrossRef]
- Morf, W.E.; Badertscher, M.; Zwickl, T.; Reichmuth, P.; de Rooij, N.F.; Pretsch, E. Calculated effects of membrane transport on the long-term response behavior of polymeric membrane ion-selective electrodes. J. Phys. Chem. B 2000, 104, 8201–8209. [Google Scholar] [CrossRef]
- Zhou, M.; Gan, S.; Cai, B.; Li, F.; Ma, W.; Han, D.; Niu, L. Effective solid contact for ion-selective electrodes: Tetrakis (4-chlorophenyl) borate (TB−) anions doped nanocluster films. Anal. Chem. 2012, 84, 3480–3483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).