Comparative Analysis of Volatile Flavor Compounds in Seven Mustard Pastes via HS-SPME–GC–MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Emulsion Type of MP
2.2. Comparison of Sensory Evaluation
2.3. Identification of Volatiles in MPs
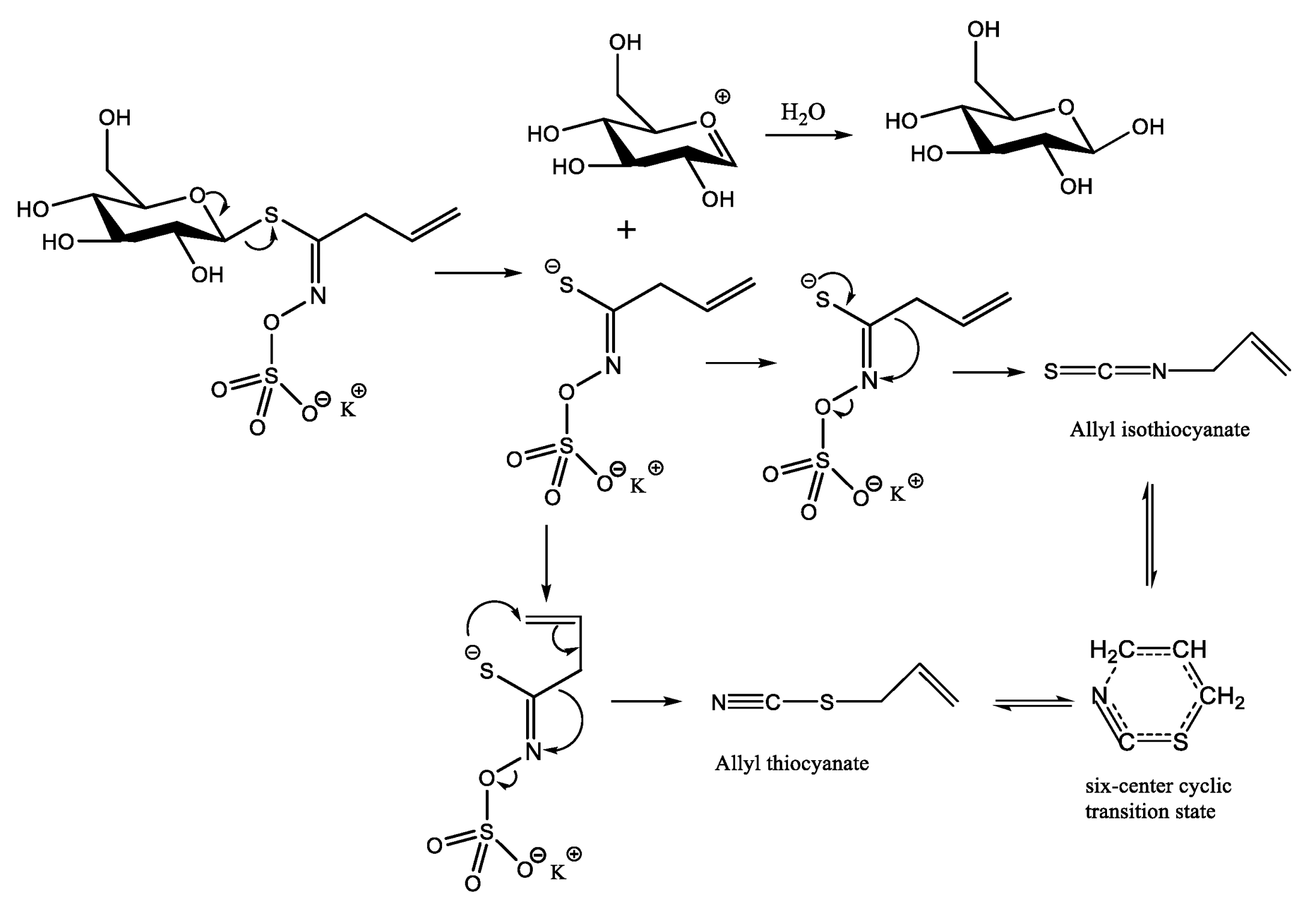
2.4. Degradation Products of AITC and Method for Stabilizing AITC
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Determination of MP Emulsion Type
3.4. Sensory Evaluation of MP
3.5. Extraction of Volatiles in MP
3.6. GC–MS Analysis
3.7. Qualitative Analyses
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nielsen, P.V.; Rios, R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Jideani, V.A.; Vogt, K. Antimicrobial packaging for extending the shelf life of bread—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Saladino, F.; Manyes, L.; Luciano, F.B.; Mañes, J.; Fernandez-Franzon, M.; Meca, G. Bioactive compounds from mustard flours for the control of patulin production in wheat tortillas. LWT-Food Sci. Technol. 2016, 66, 101–107. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Cai, J.; Liu, B.; Su, Q. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatogr. A 2001, 930, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Torrijos, R.; Righetti, L.; Cirlini, M.; Calani, L.; Mañes, J.; Meca, G.; Dall’Asta, C. Phytochemical profiling of volatile and bioactive compounds in yellow mustard (Sinapis alba) and oriental mustard (Brassica juncea) seed flour and bran. LWT-Food Sci. Technol. 2023, 173, 114221. [Google Scholar] [CrossRef]
- Zhao, D.; Tang, J.; Ding, X. Analysis of volatile components during potherb mustard (Brassica juncea, Coss.) pickle fermentation using SPME–GC–MS. LWT-Food Sci. Technol. 2007, 40, 439–447. [Google Scholar] [CrossRef]
- Paunovic, D.S.; Solevic-Knudsen, T.; Krivokapic, M.; Zlatković, B.P.; Antic, M. Sinalbin degradation products in mild yellow mustard paste. Hem. Ind. 2012, 66, 29–32. [Google Scholar] [CrossRef]
- Wendlinger, C.; Hammann, S.; Vetter, W. Various concentrations of erucic acid in mustard oil and mustard. Food Chem. 2014, 153, 393–397. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Niu, L.Y.; Liao, X.J.; Hu, X.S. Determination of volatile flavor compounds in mustard oil, wasabi, and Chongcai leaf mustard by SPME/GC/MS. China Condiment 2004, 29, 42–45. [Google Scholar]
- Ray, A.; Mohanty, S.; Jena, S.; Sahoo, A.; Acharya, L.; Panda, P.C.; Sial, P.; Duraisamy, P.; Nayak, S. Drying methods affects physicochemical characteristics, essential oil yield and volatile composition of turmeric (Curcuma longa L.). J. Appl. Res. Med. Aromat. Plants 2022, 26, 100357. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Heising, J.; Dekker, M. Multiresponse kinetic modelling of the formation, release, and degradation of allyl isothiocyanate from ground mustard seeds to improve active packaging. J. Food Eng. 2021, 292, 110370. [Google Scholar] [CrossRef]
- Gemert, L.J. Odour thresholds. In Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; pp. 207–359. [Google Scholar]
- Lin, X.H.; Li, R.; Jiang, Z.T. Study on the chemical composition in the volatile oil of Armoracia lapathifolia Gilib. Food Sci. 2001, 22, 373–375. [Google Scholar]
- Liu, Y.; Rossi, M.; Liang, X.; Zhang, H.; Zou, L.; Ong, C.N. An integrated metabolomics study of glucosinolate metabolism in different brassicaceae genera. Metabolites 2020, 10, 313. [Google Scholar] [CrossRef]
- Pechacek, R.; Velisek, J.; Hrabcova, H. Decomposition products of allyl isothiocyanate in aqueous solutions. J. Agric. Food Chem. 1997, 45, 4584–4588. [Google Scholar] [CrossRef]
- Li, Y.M.; Huang, J.; Zhang, Y.; Liu, Y.P. Extraction and analysis of volatile compounds in mustard oil by SPME-GC-MS. China Food Addit. 2016, 23, 188–193. [Google Scholar]
- Bell, L.; Kitsopanou, E.; Oloyede, O.O.; Lignou, S. Important odorants of four brassicaceae species, and discrepancies between glucosinolate profiles and observed hydrolysis products. Foods 2021, 10, 1055. [Google Scholar] [CrossRef]
- GB 29980-2013; Chinese Food Safety Standard. Food Additive-Allyl isothiocyanate. The National Health and Family Planning Commission: Beijing, China, 2013.
- GB 2760-2014; Chinese Food Additive Use Standard. The National Health and Family Planning Commission: Beijing, China, 2014.
- Li, P.; Zhao, Y.M.; Wang, C.; Zhu, H.P. Antibacterial activity and main action pathway of benzyl isothiocyanate extracted from papaya seeds. J. Food Sci. 2021, 86, 169–176. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ye, W.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Wu, J.C.; Pan, M.H.; Ho, C.T. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef]
- Li, Y.; Teng, Z.; Chen, P.; Song, Y.; Luo, Y.; Wang, Q. Enhancement of aqueous stability of allyl isothiocyanate using nanoemulsions prepared by an emulsion inversion point method. J. Colloid Interface Sci. 2015, 438, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.Z.; Ding, R.X.; Ding, K.; Han, T.; Chen, X.N. Preparation of allyl isothiocyanate microencapsulation and its application in pork preservation. J. Food Process. Preserv. 2020, 44, e14709. [Google Scholar] [CrossRef]
- Chhajed, S.; Misra, B.B.; Tello, N.; Chen, S. Chemodiversity of the glucosinolate-myrosinase system at the single cell type resolution. Front. Plant Sci. 2019, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhang, Y.; Huang, J.; Liu, Y.P. The synthesis and odor characteristics of eight isothiocyanate flavor compounds. China Food Addit. 2016, 23, 119–123. [Google Scholar]

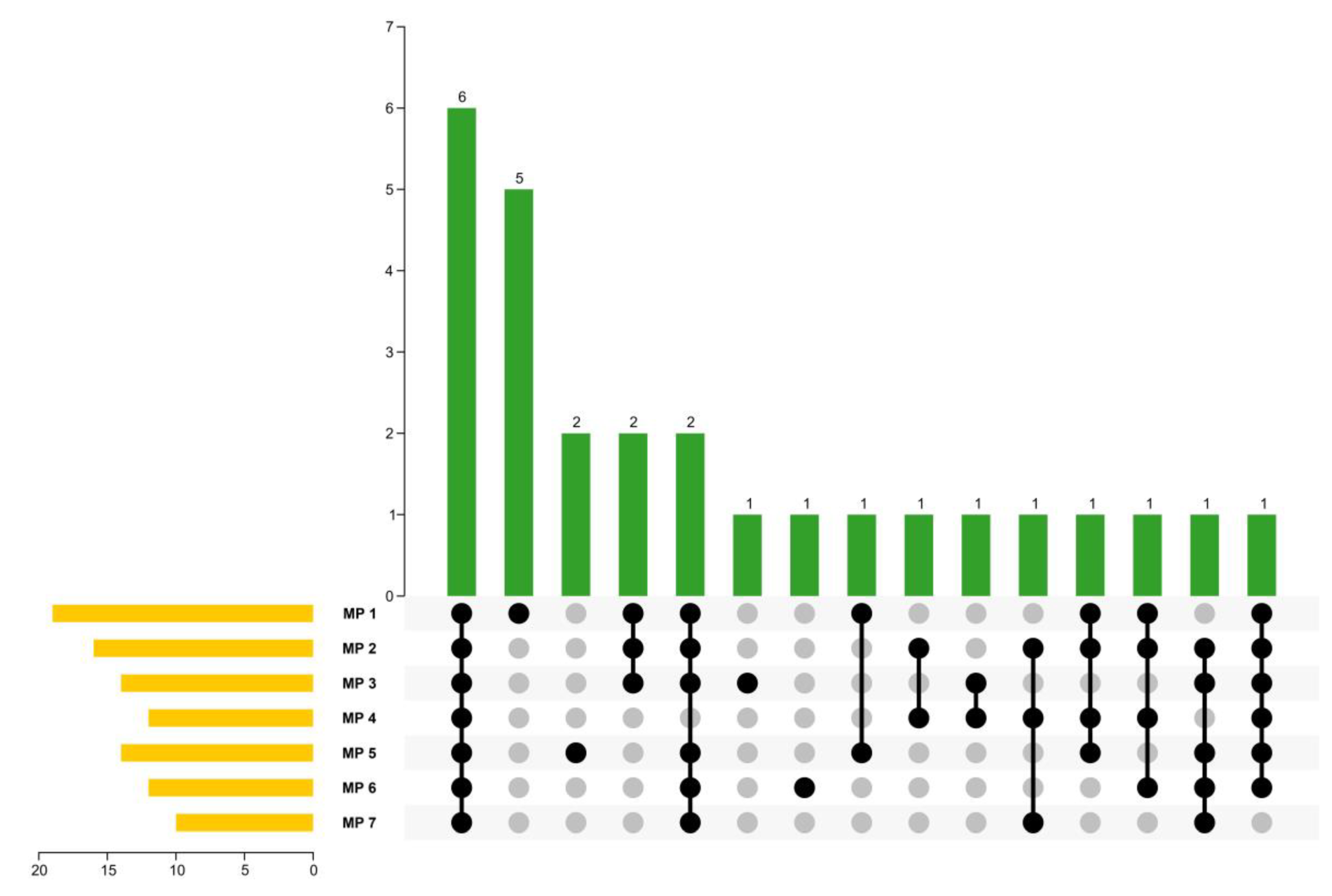
| Compounds | Concentration (μg/g) | Matching Degree | RI/RI *c | Qualitative Method e | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MP 1 | MP 2 | MP 3 | MP 4 | MP 5 | MP 6 | MP 7 | Match | R.Match | |||
| Esters (9) | |||||||||||
| Allyl isocyanate | + a | + | + | − b | + | + | + | 909 | 917 | 972/N d | MS |
| sec-Butylisothiocyanate- | + | + | − | + | + | − | − | 868 | 895 | 1289/1287 | MS, RI, S |
| Allyl isothiocyanate | + | + | + | + | + | + | + | 960 | 960 | 1363/1361 | MS, RI, S |
| Allyl thiocyanate | + | + | + | + | + | + | + | 878 | 910 | 1447/1440 | MS, RI |
| 3-Butenyl isothiocyanate | + | + | − | + | − | + | − | 789 | 803 | 1451/1453 | MS, RI |
| Diglycolmonoethylether acetate | + | + | + | − | − | − | − | 886 | 901 | 1684/N | MS |
| 3-(Methylthio)propyl isothiocyanate | − | + | − | + | − | − | − | 901 | 912 | 1938/1979 | MS, S |
| Benzyl isothiocyanate | + | + | + | + | + | + | − | 854 | 883 | 2097/2107 | MS, RI, S |
| Phenylethyl isothiocyanate | + | + | + | + | + | + | + | 948 | 948 | 2227/2234 | MS, RI, S |
| Sulfur-containing compounds (3) | |||||||||||
| Allyl mercaptan | + | + | + | − | − | − | − | 942 | 946 | 886/887 | MS, RI, S |
| Diallyl sulfide | − | + | + | − | + | + | + | 925 | 925 | 1148/1148 | MS, RI, S |
| Diallyl disulphide | − | − | − | − | + | − | − | 910 | 932 | 1477/1475 | MS, RI, S |
| Nitriles (2) | |||||||||||
| 3-Butenenitrile | + | + | + | − | + | + | + | 977 | 977 | 1176/1186 | MS, RI |
| Benzenepropanenitrile | + | + | + | + | + | + | + | 936 | 936 | 2035/2041 | MS, RI |
| Ketones (3) | |||||||||||
| α-Turmerone | + | − | − | − | − | − | − | 825 | 838 | 2183/2245 | MS |
| β-Turmerone | + | − | − | − | − | − | − | 850 | 867 | 2250/N | MS |
| ar-Turmerone | + | − | − | − | − | − | − | 845 | 880 | 2262/N | MS |
| Alkenes (3) | |||||||||||
| α-Zingiberene | + | − | − | − | + | − | − | 861 | 892 | 1718/1715 | MS, RI |
| (−)-β-Sesquiphellandrene | + | − | − | − | − | − | − | 905 | 919 | 1765/1765 | MS, RI |
| Curcumene | + | − | − | − | − | − | − | 913 | 965 | 1769/1768 | MS, RI |
| Others (7) | |||||||||||
| Carbon disulfide | + | + | + | + | + | + | + | 930 | 960 | 730/733 | MS, RI, S |
| Allyl chloride | + | + | + | + | + | + | + | 866 | 893 | 806/814 | MS, RI, S |
| Isothiazole | − | − | + | + | − | − | − | 899 | 908 | 1214/N | MS |
| N-Allylacetamide | − | − | − | − | + | − | − | 831 | 852 | 1761/N | MS |
| Hexanoic acid | − | + | − | + | − | − | + | 949 | 952 | 1850/1850 | MS, RI, S |
| 3-Methylphenol | − | − | + | − | − | − | − | 936 | 945 | 2087/2085 | MS, RI, S |
| Dehydroacetic acid | − | − | − | − | − | + | − | 920 | 921 | 2374/N | MS |
| Sample | Common Raw Materials | Flavor Material | Other Raw Materials |
|---|---|---|---|
| MP 1 | Water; edible oil; sorbitol; salt; tartrazine; brilliant blue (pigment) | Mustard oil | Lactose, refined cane sugar, citric acid, corn starch, xanthan gum, turmeric |
| MP 2 | Horseradish, edible spice | Lactose, citric acid, glyceryl monostearate, xanthan gum, turmeric | |
| MP 3 | Horseradish, edible spice | Lactose, citric acid, glyceryl monostearate, avicel, xanthan gum, turmeric | |
| MP 4 | Wasabi, fresh wasabi | Glucose, citric acid | |
| MP 5 | Horseradish, spice, flavoring essence | Lactose, ansemi, xanthan gum, sodium diacetate, turmeric | |
| MP 6 | Horseradish powder | Citric acid, edible starch | |
| MP 7 | Horseradish powder | No other ingredient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Wang, R.; Wu, Y.; Xin, R.; Guan, W.; Liu, Y. Comparative Analysis of Volatile Flavor Compounds in Seven Mustard Pastes via HS-SPME–GC–MS. Molecules 2023, 28, 5482. https://doi.org/10.3390/molecules28145482
Liang M, Wang R, Wu Y, Xin R, Guan W, Liu Y. Comparative Analysis of Volatile Flavor Compounds in Seven Mustard Pastes via HS-SPME–GC–MS. Molecules. 2023; 28(14):5482. https://doi.org/10.3390/molecules28145482
Chicago/Turabian StyleLiang, Miao, Rui Wang, Yajian Wu, Runhu Xin, Wei Guan, and Yuping Liu. 2023. "Comparative Analysis of Volatile Flavor Compounds in Seven Mustard Pastes via HS-SPME–GC–MS" Molecules 28, no. 14: 5482. https://doi.org/10.3390/molecules28145482
APA StyleLiang, M., Wang, R., Wu, Y., Xin, R., Guan, W., & Liu, Y. (2023). Comparative Analysis of Volatile Flavor Compounds in Seven Mustard Pastes via HS-SPME–GC–MS. Molecules, 28(14), 5482. https://doi.org/10.3390/molecules28145482





