Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells
Abstract
1. Introduction
2. Results
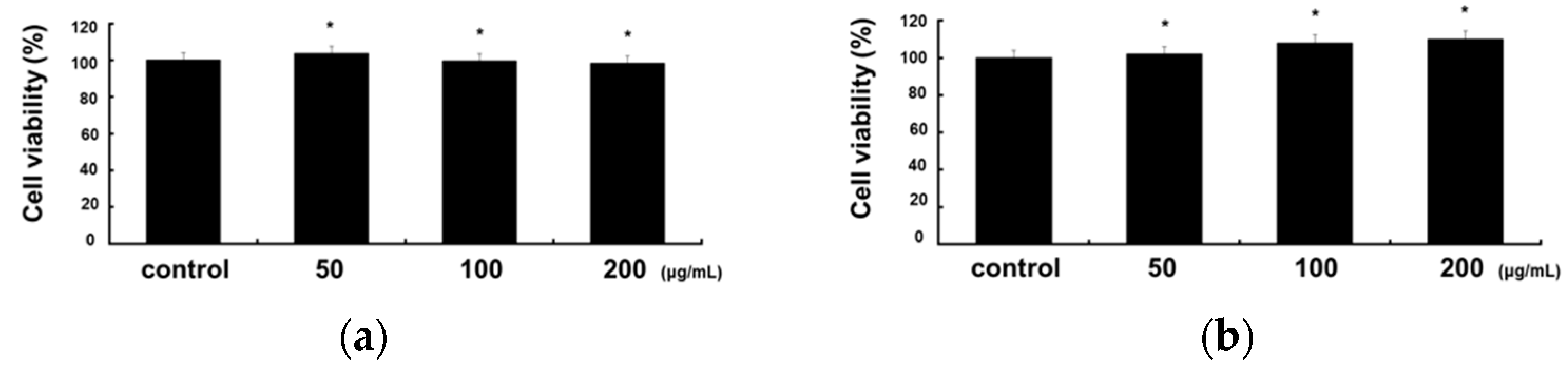
2.1. Cytotoxicity of RCE in RAW 264.7 and HaCaT Cells
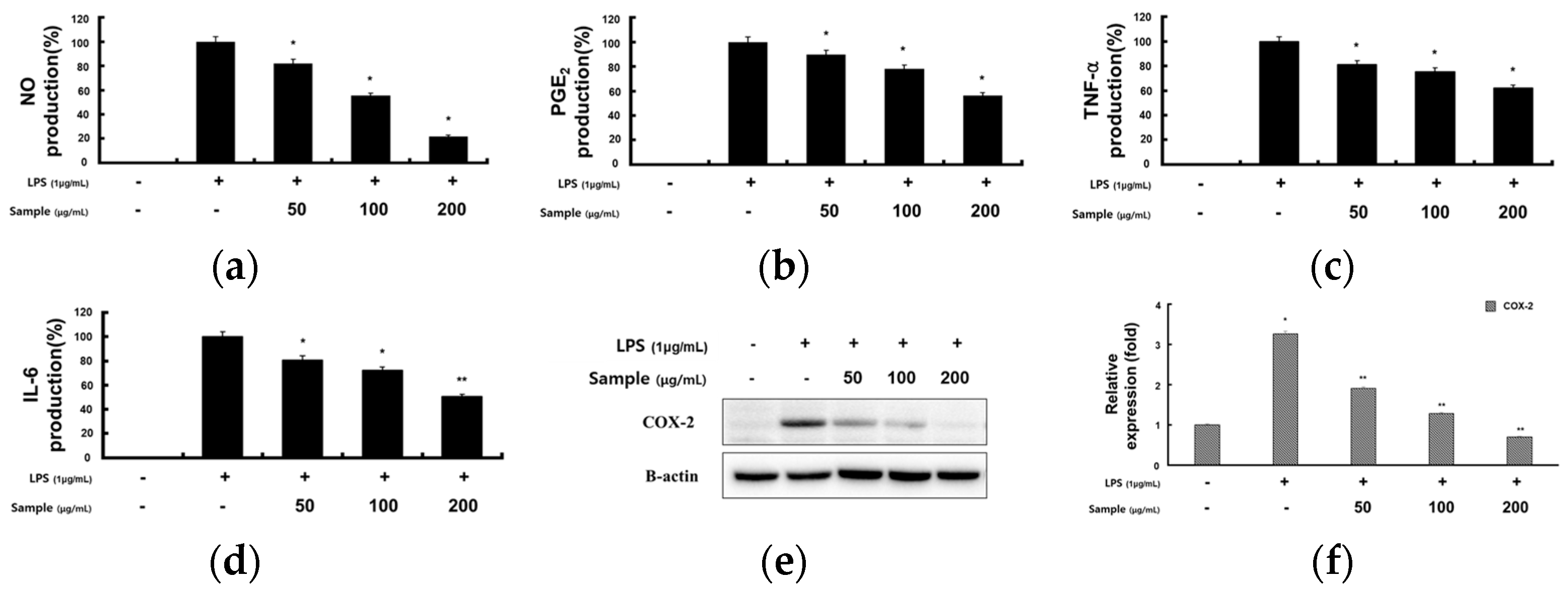
2.2. Inhibitory Effects of RCE on LPS-Induced Inflammatory Mediator and Proinflammatory Cytokines in RAW 264.7 Cells
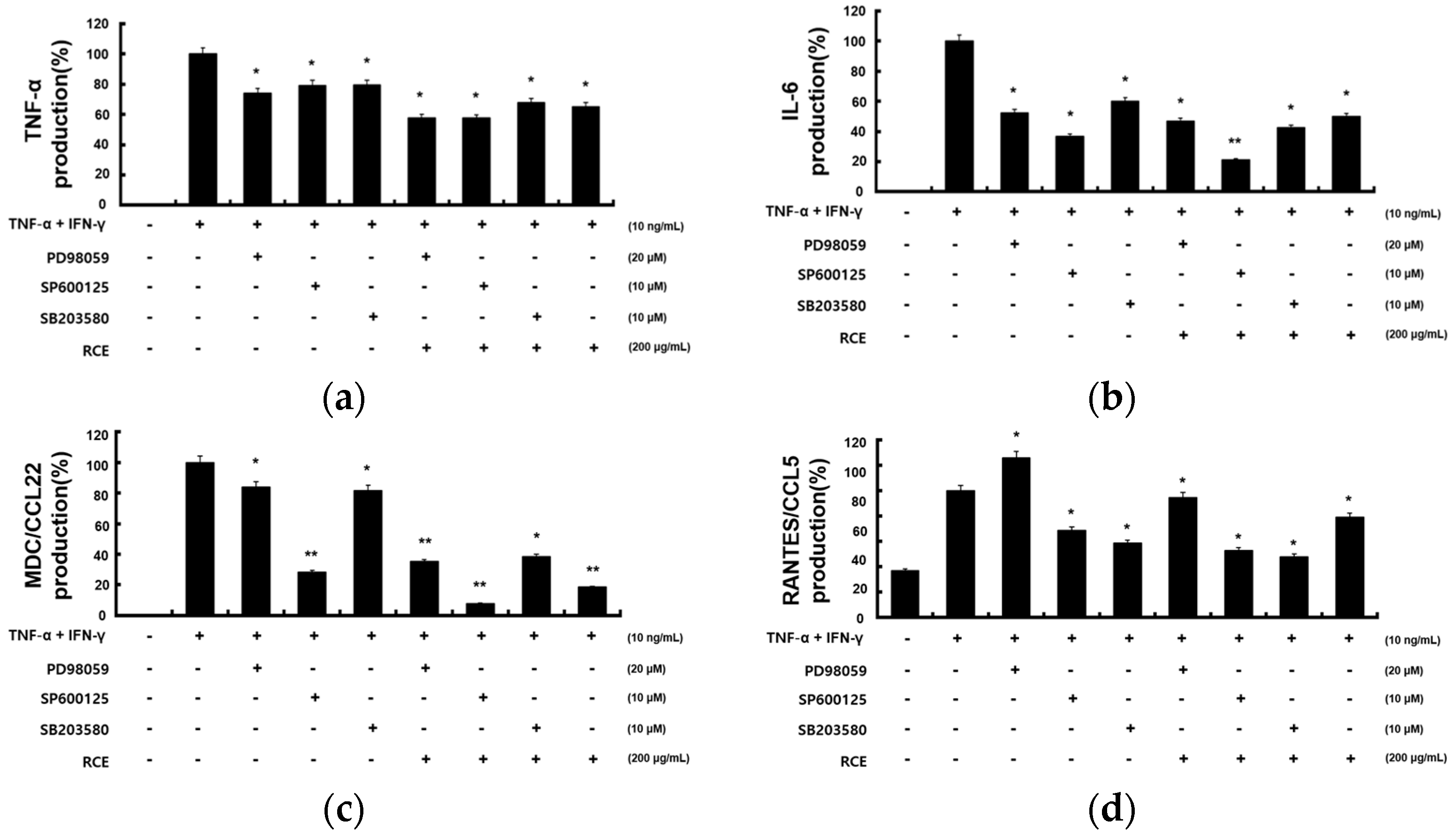
2.3. Inhibitory Effects of RCE on TNF-α/IFN-γ-Induced Proinflammatory Cytokines and Chemokines in HaCaT Cells
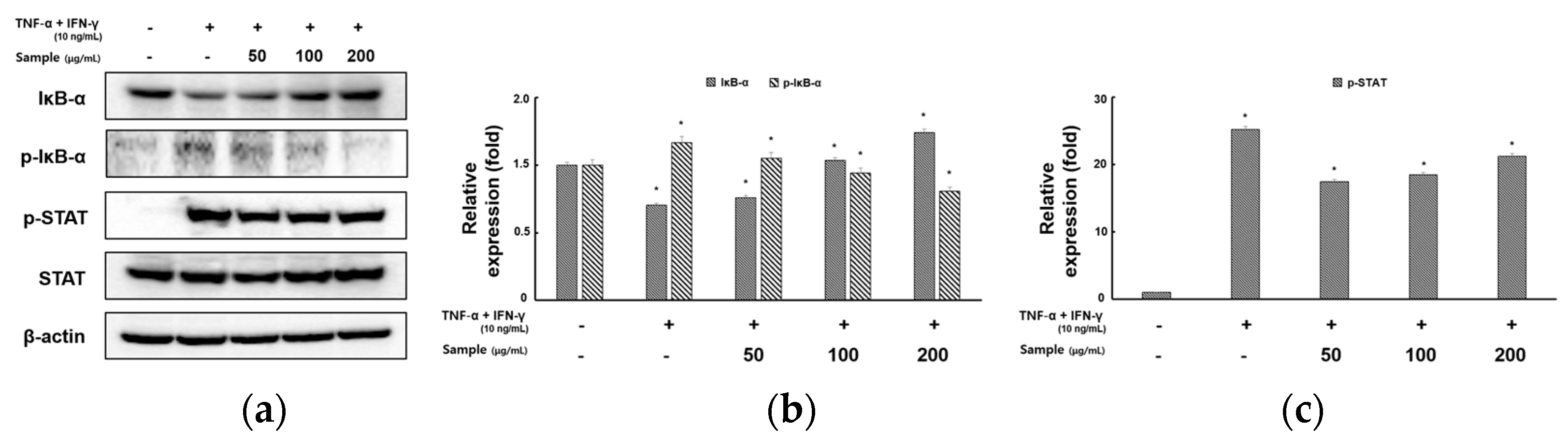
2.4. Inhibitory Effects of RCE on the Expression of NF-κB and STAT1 Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Cells
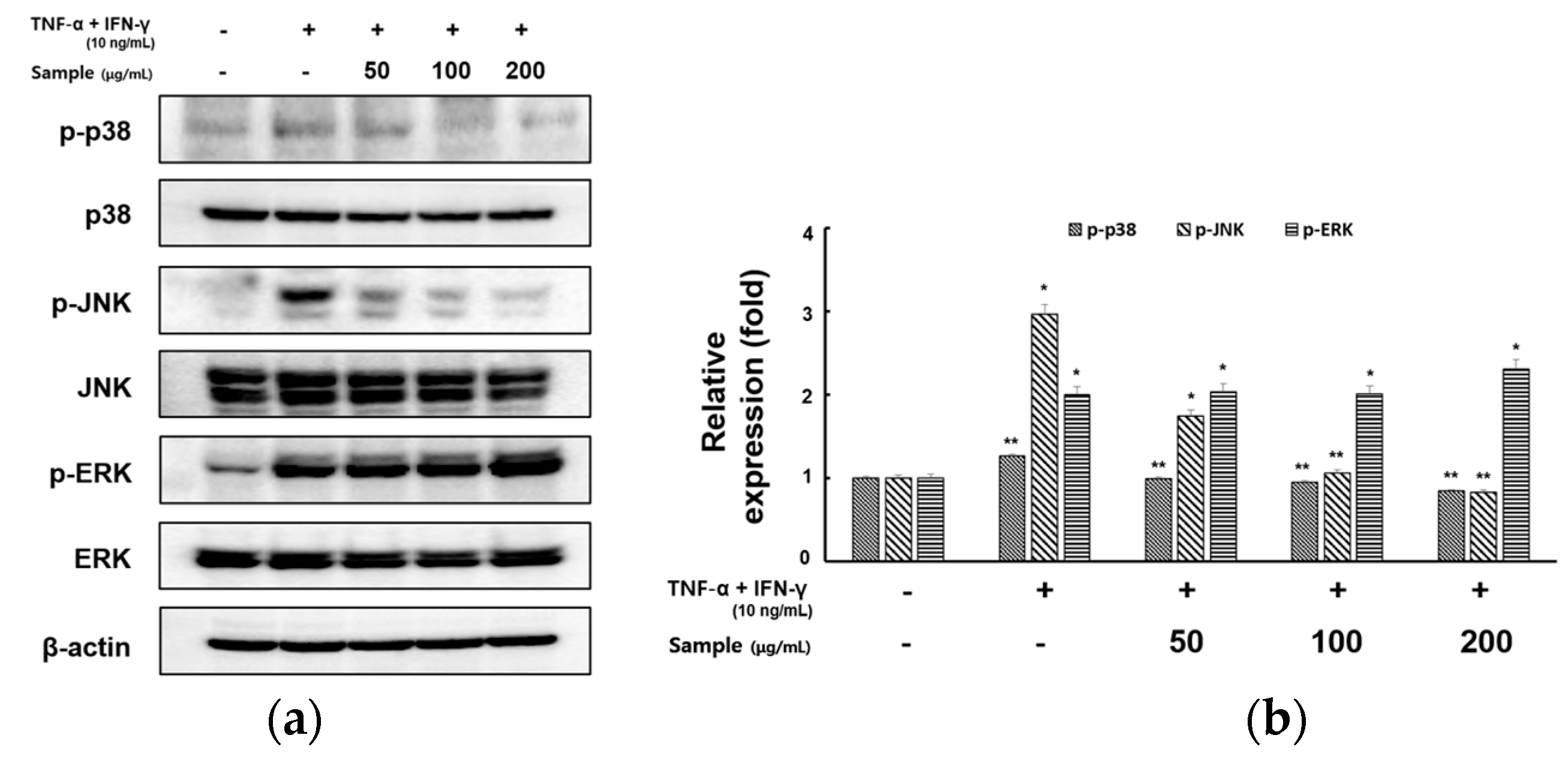
2.5. Inhibitory Effects of RCE on the Expression of MAPK Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Cells
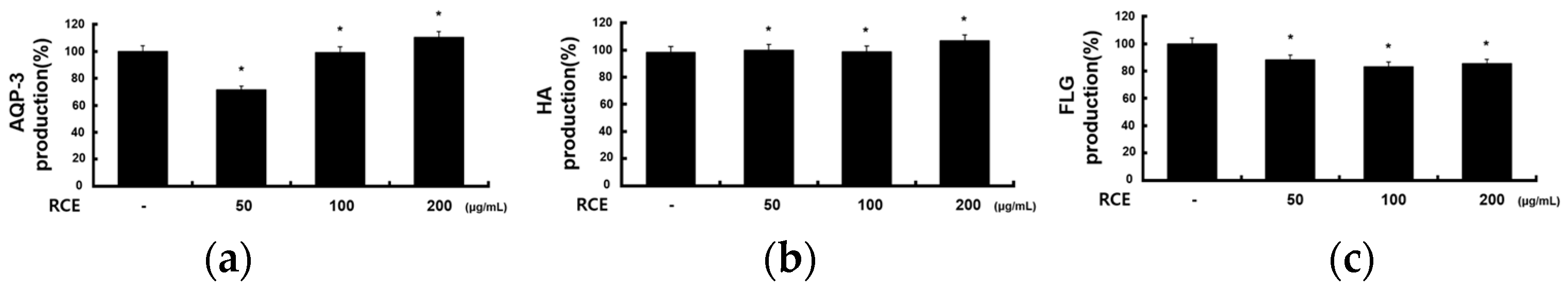
2.6. Effects of RCE on Other Molecules in HaCaT Cells
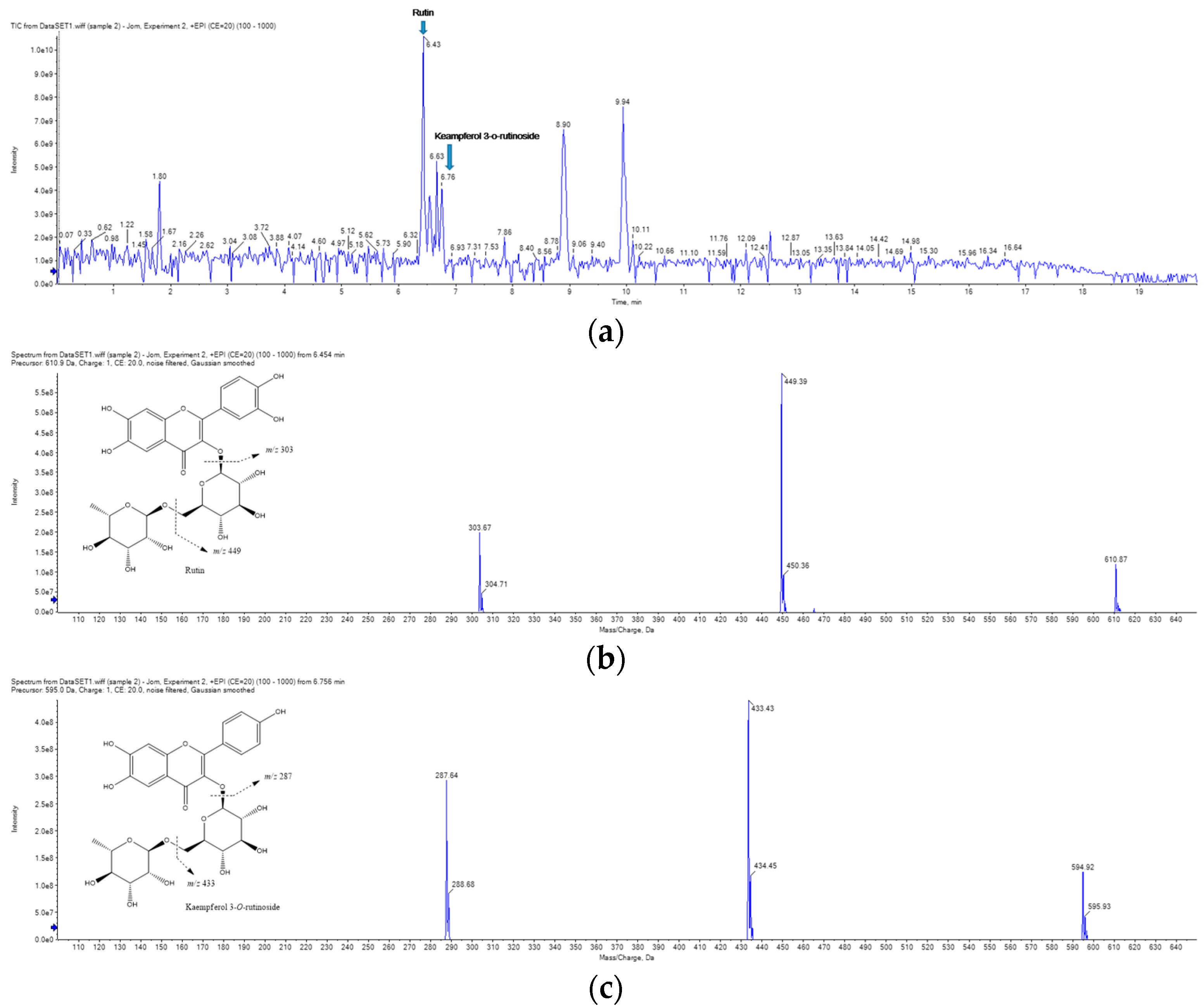
2.7. UPLC-MS/MS Analysis of RCE
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Nitric Oxide (NO) Determination
3.6. Enzyme-Linked Immunosorbent Assay (ELISA)
3.7. Western Blot Analysis
3.8. UPLC-MS/MS Analysis
3.9. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kader, H.A.; Azeem, M.; Jwayed, S.A.; Al-Shehhi, A.; Tabassum, A.; Ayoub, M.A.; Hetta, H.F.; Waheed, Y.; Iratni, R.; Al-Dhaheri, A.; et al. Current insights into immunology and novel therapeutics of atopic dermatitis. Cells 2021, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The Epithelial Immune Microenvironment (EIME) in Atopic Dermatitis and Psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate Immune Cells in Atopic Dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ Synergy Amplifies Senescence-Associated Inflammation and SARS-CoV-2 Receptor Expression via Hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, C.; Wang, F.; Dong, J.; Zhang, D.; Jiang, J.; Feng, Y.; Wu, B.; Xie, T.; Cheng, L. Qingxue Jiedu Formulation Ameliorated DNFB-Induced Atopic Dermatitis by Inhibiting STAT3/MAPK/NF-κB Signaling Pathways. J. Ethnopharmacol. 2021, 270, 113773. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Thyssen, J.P.; Bissonnette, R.; Bieber, T.; Kabashima, K.; Hijnen, D.J.; Guttman-Yassky, E. Biomarkers in atopic dermatitis—A review on behalf of the International Eczema Council. J. Allergy Clin. Immunol. 2021, 147, 1174–1190.e1. [Google Scholar] [CrossRef]
- Catherine, J.; Roufosse, F. What does elevated TARC/CCL17 expression tell us about eosinophilic disorders? Semin. Immunopathol. 2021, 43, 439–458. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Wang, R.; Moon, S.K.; Kim, W.J.; Dhandapani, S.; Kim, H.; Kim, Y.J. Biologically Synthesized Rosa rugosa-Based Gold Nanoparticles Suppress Skin Inflammatory Responses via MAPK and NF-κB Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Keratinocytes. ACS Omega 2022, 7, 35951–35960. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, G.H.; Jin, S.W.; Kim, J.Y.; Hwang, Y.P.; Han, E.H.; Kim, Y.H.; Jeong, H.G. Impressic Acid Ameliorates Atopic Dermatitis-Like Skin Lesions by Inhibiting ERK1/2-Mediated Phosphorylation of NF-κB and stat1. Int. J. Mol. Sci. 2021, 22, 2334. [Google Scholar] [CrossRef]
- Segarra, S.; Naiken, T.; Garnier, J.; Hamon, V.; Coussay, N.; Bernard, F.X. Enhanced In Vitro Expression of Filaggrin and Antimicrobial Peptides Following Application of Glycosaminoglycans and a Sphingomyelin-Rich Lipid Extract. Vet. Sci. 2022, 9, 323. [Google Scholar] [CrossRef]
- Tanei, R. Atopic Dermatitis in Older Adults: A Review of Treatment Options. Drugs Aging 2020, 37, 149–160. [Google Scholar] [CrossRef]
- Fleming, P.; Yang, Y.B.; Lynde, C.; O’Neill, B.; Lee, K.O. Diagnosis and Management of Atopic Dermatitis for Primary Care Providers. J. Am. Board Fam. Med. 2020, 33, 626–635. [Google Scholar] [CrossRef]
- Kim, M.J.; Hwang, B.S.; Oh, Y.T. Anti-inflammatory and Anti-atopic Effects of Rorippa cantoniensis Extracts of poster. In Proceedings of the International Meeting of the Microbiological Society of Korea, Jeju-si, Republic of Korea, 30 October–1 November 2022. [Google Scholar]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the Maintenance of Homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Vu, Y.H.; Furue, M.; Tsuji, G. The Role of Interleukin-24 in Atopic Dermatitis. Explor. Immunol. 2021, 1, 4–15. [Google Scholar] [CrossRef]
- Klonowska, J.; Gleń, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Yang, J.H.; Do, H.J.; Lee, E.; Yim, N.H.; Cho, W.K.; Park, K.I.; Ma, J.Y. Jageum-Jung Improves 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and Suppresses Pro-inflammatory Chemokine Production by Inhibiting TNF-α/IFN-γ-Induced STAT-1 and NFκB Signaling in HaCaT Cells. J. Ethnopharmacol. 2018, 221, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.M.; Hung, Y.L.; Wang, S.J.; Tsai, Y.J.; Wu, N.L.; Liang, C.W.; Chang, D.C.; Hung, C.F. Anti-allergic and Anti-Inflammatory Effects of Neferine on rbl-2h3 Cells. Int. J. Mol. Sci. 2021, 22, 10994. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Hu, Z.; Jia, C.; Yang, M.; Li, D.; Xu, A.; Jiang, J.; Chen, Z.; Li, Y.; Li, S.; et al. Deciphering the mechanisms of Yinlan Tiaozhi capsule in treating hyperlipidemia by combining network pharmacology, molecular docking and experimental verifcation. Sci. Rep. 2023, 13, 6424. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, L.; Landvan’t, B.; Sprikkelman, A.B.; Garssen, J. Role of Microbial Modulation in Management of Atopic Dermatitis in Children. Nutrients 2017, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Two, A.M.; Chun, K.A.; Narala, S.; Geha, R.S.; Hata, T.R.; Gallo, R.L. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J. Investig. Dermatol. 2016, 136, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.A. Overview of Atopic Dermatitis. Am. J. Manag. Care 2017, 23, 115–123. [Google Scholar]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-Like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Asahina, R.; Maeda, S. A Review of the Roles of Keratinocyte-Derived Cytokines and Chemokines in the Pathogenesis of Atopic Dermatitis in Humans and Dogs. Vet. Dermatol. 2017, 28, 15–25. [Google Scholar]
- Lee, H.; Lee, D.H.; Oh, J.H.; Chung, J.H. Skullcapflavone ii Suppresses tnf-α/ifn-γ-induced Tarc, mdc, and Ctss Production in Hacat Cells. Int. J. Mol. Sci. 2021, 22, 6428. [Google Scholar] [CrossRef]
- Townsley, H.; Crane, J.; Siebers, R. Effect of Haemolysis on the Determination of CCL17/Thymus and Activation-Regulated Chemokine (TARC) and CCL22/Macrophage-Derived Chemokine (MDC). Clin. Chem. Lab. Med. 2018, 56, 92–93. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, Y.H.; Koh, D.; Lim, Y.; Shin, S.Y. Chrysin inhibits nf-κb-dependent ccl5 transcription by targeting iκb kinase in the atopic dermatitis-like inflammatory microenvironment. Int. J. Mol. Sci. 2020, 21, 7348. [Google Scholar] [CrossRef]
- Brown, R.; Nath, S.; Lora, A.; Samaha, G.; Elgamal, Z.; Kaiser, R.; Taggart, C.; Weldon, S.; Geraghty, P. Cathepsin S: Investigating an old player in lung disease pathogenesis, comorbidities, and potential therapeutics. Respir. Res. 2020, 21, 111. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.S. Dendritic Cell-Mediated th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, O.K.; Yuniato, P.; Marwoto, B.; Lee, J.; Oh, S.R.; Kim, J.H.; Ahn, K.S. Amelioration of an LPS-Induced Inflammatory Response Using a Methanolic Extract of Lagerstroemia Ovalifolia to Suppress the Activation of NF-κB in RAW264.7 Macrophages. Int. J. Mol. Med. 2016, 38, 482–490. [Google Scholar] [CrossRef]
- Safdar, M.; Ozaslan, M.; Junejo, Y.; Channa, I.S. Cytotoxic and Anticancer Activity of a Novel Synthesized Tet-AuNPs Simultaneously Activates p53 and Inhibits NF-kB Signaling in SKBR3 Cell Line. Toxicol. Environ. Health Sci. 2022, 14, 69–76. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Correa, D.; Navarrete-Meneses, P.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E.; Rodríguez-Martínez, S. NF-κB and Its Regulators During Pregnancy. Front. Immunol. 2021, 12, 679106. [Google Scholar] [CrossRef]
- Yu, C.H.; Suh, B.; Shin, I.; Kim, E.H.; Kim, D.; Shin, Y.J.; Chang, S.Y.; Baek, S.H.; Kim, H.; Bae, O.N. Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation. Int. J. Mol. Sci. 2019, 20, 2607. [Google Scholar] [CrossRef]
- Gil, T.Y.; Kang, Y.M.; Eom, Y.J.; Hong, C.H.; An, H.J. Anti-atopic Dermatitis Effect of Seaweed Fulvescens Extract via Inhibiting the STAT1 Pathway. Mediat. Inflamm. 2019, 2019, 3760934. [Google Scholar] [CrossRef]
- Ye, Q.; Luo, F.; Yan, T. Transcription Factor KLF4 Regulated STAT1 to Promote M1 Polarization of Macrophages in Rheumatoid Arthritis. Aging 2022, 14, 5669. [Google Scholar] [CrossRef]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The Function of MAPK Cascades in Response to Various Stresses in Horticultural Plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, W.H.; Jeong, J.; Park, M.; Ko, J.Y.; Kwon, O.W.; Lee, J.; Kim, Y.J. Pyropia yezoensis Extract Suppresses ifn-gamma and tnf-Alpha-Induced Proinflammatory Chemokine Production in Hacat Cells via the Down-Regulation of NF-κB. Nutrients 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK Signalling Pathway and Tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of Apigenin on rbl-2h3, raw264.7, and Hacat Cells: Anti-allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the Epidermis: More than Skin Deep. Am. J. Physiol. Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Li, B.; Ji, Y.; Yi, C.; Wang, X.; Liu, C.; Wang, C.; Lu, X.; Xu, X.; Wang, X. Rutin Inhibits Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation by Inducing Autophagy and Modulating PI3K/ATK Signaling. Molecules 2022, 27, 4201. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-κB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Kang, C.W.; Kim, G.D. Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Hwang, B.S.; Hwang, Y.; Jeong, Y.T.; Jeong, D.W.; Oh, Y.T. Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells. Molecules 2023, 28, 5463. https://doi.org/10.3390/molecules28145463
Kim M-J, Hwang BS, Hwang Y, Jeong YT, Jeong DW, Oh YT. Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells. Molecules. 2023; 28(14):5463. https://doi.org/10.3390/molecules28145463
Chicago/Turabian StyleKim, Min-Jin, Buyng Su Hwang, Yong Hwang, Yong Tae Jeong, Dae Won Jeong, and Young Taek Oh. 2023. "Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells" Molecules 28, no. 14: 5463. https://doi.org/10.3390/molecules28145463
APA StyleKim, M.-J., Hwang, B. S., Hwang, Y., Jeong, Y. T., Jeong, D. W., & Oh, Y. T. (2023). Anti-Inflammatory and Antiatopic Effects of Rorippa cantoniensis (Lour.) Ohwi in RAW 264.7 and HaCaT Cells. Molecules, 28(14), 5463. https://doi.org/10.3390/molecules28145463





