Ni(II)-Salophen—Comprehensive Analysis on Electrochemical and Spectral Characterization and Biological Studies
Abstract
1. Introduction
2. Results
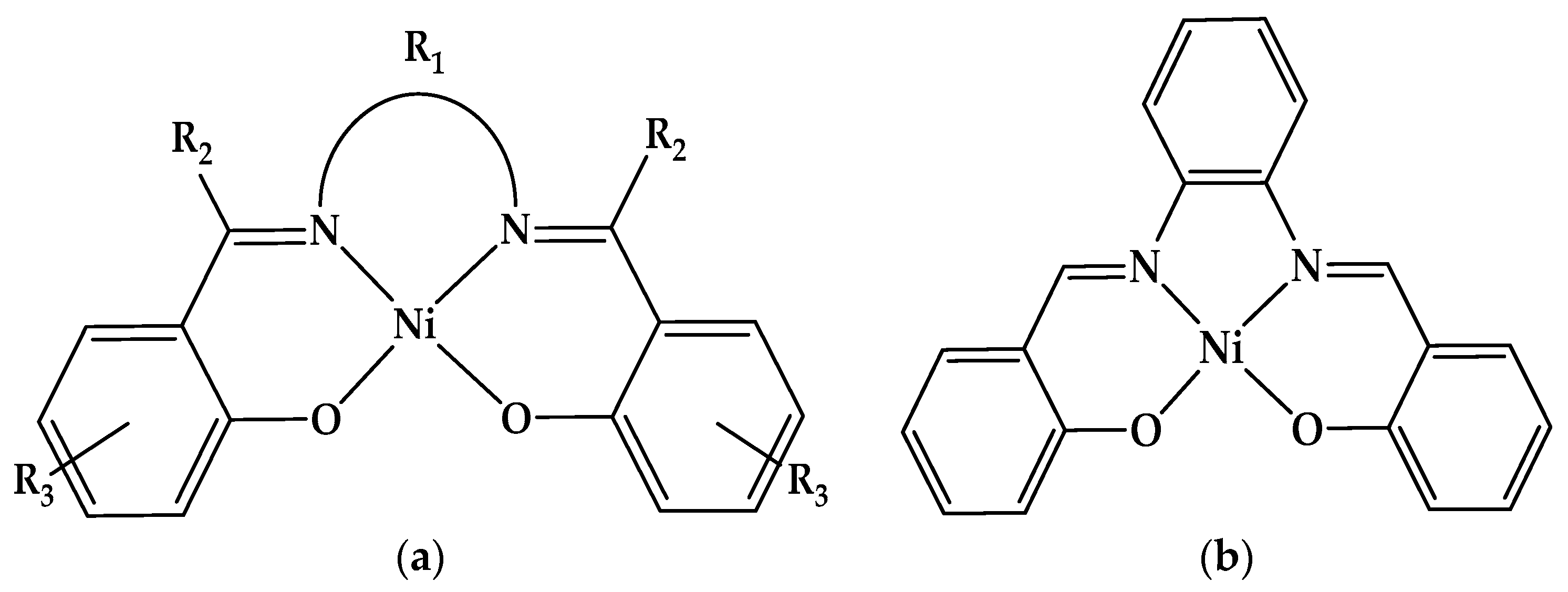
2.1. Electrochemical Studies of Salophen Ligand L and its Ni(II) Complex
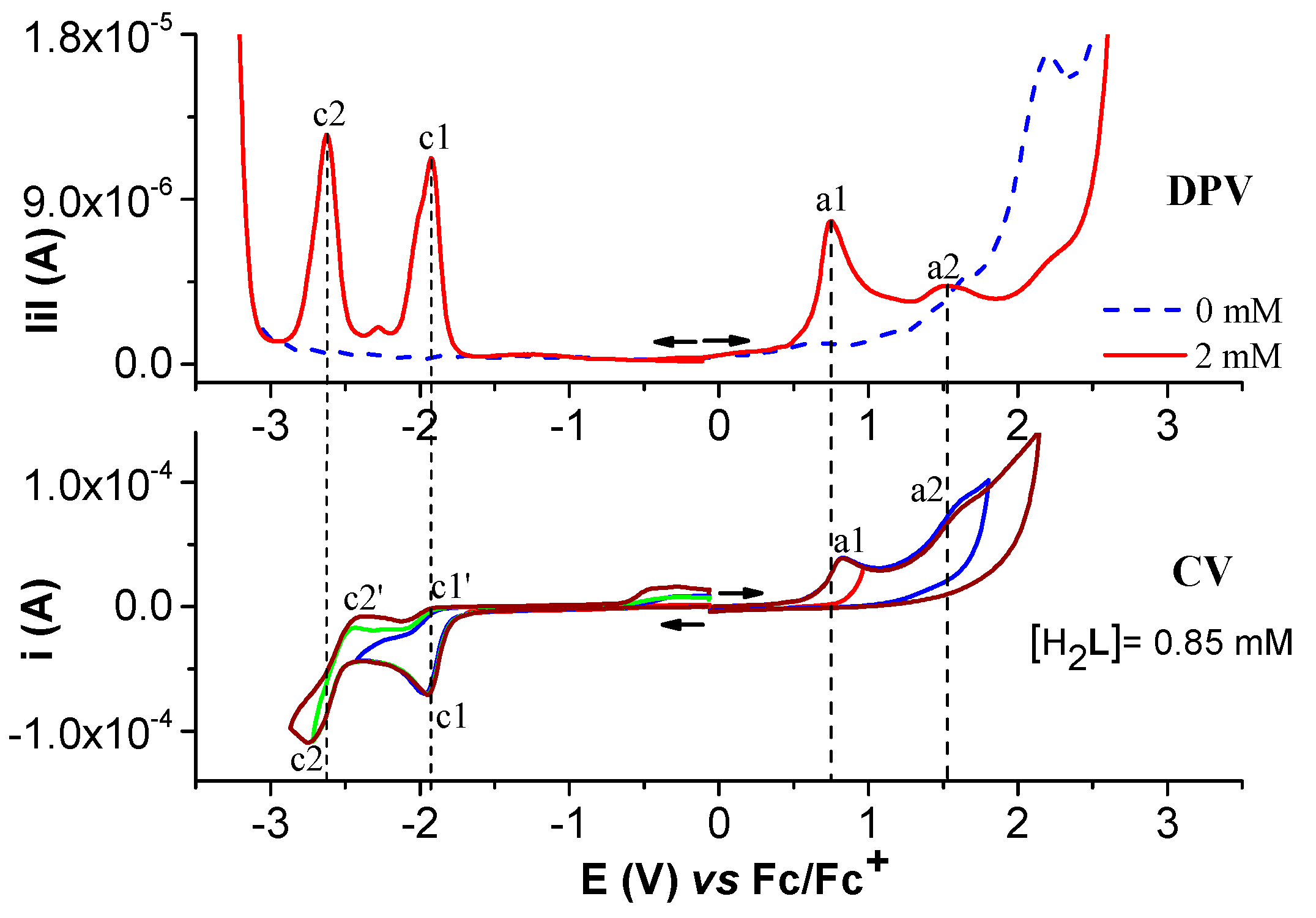
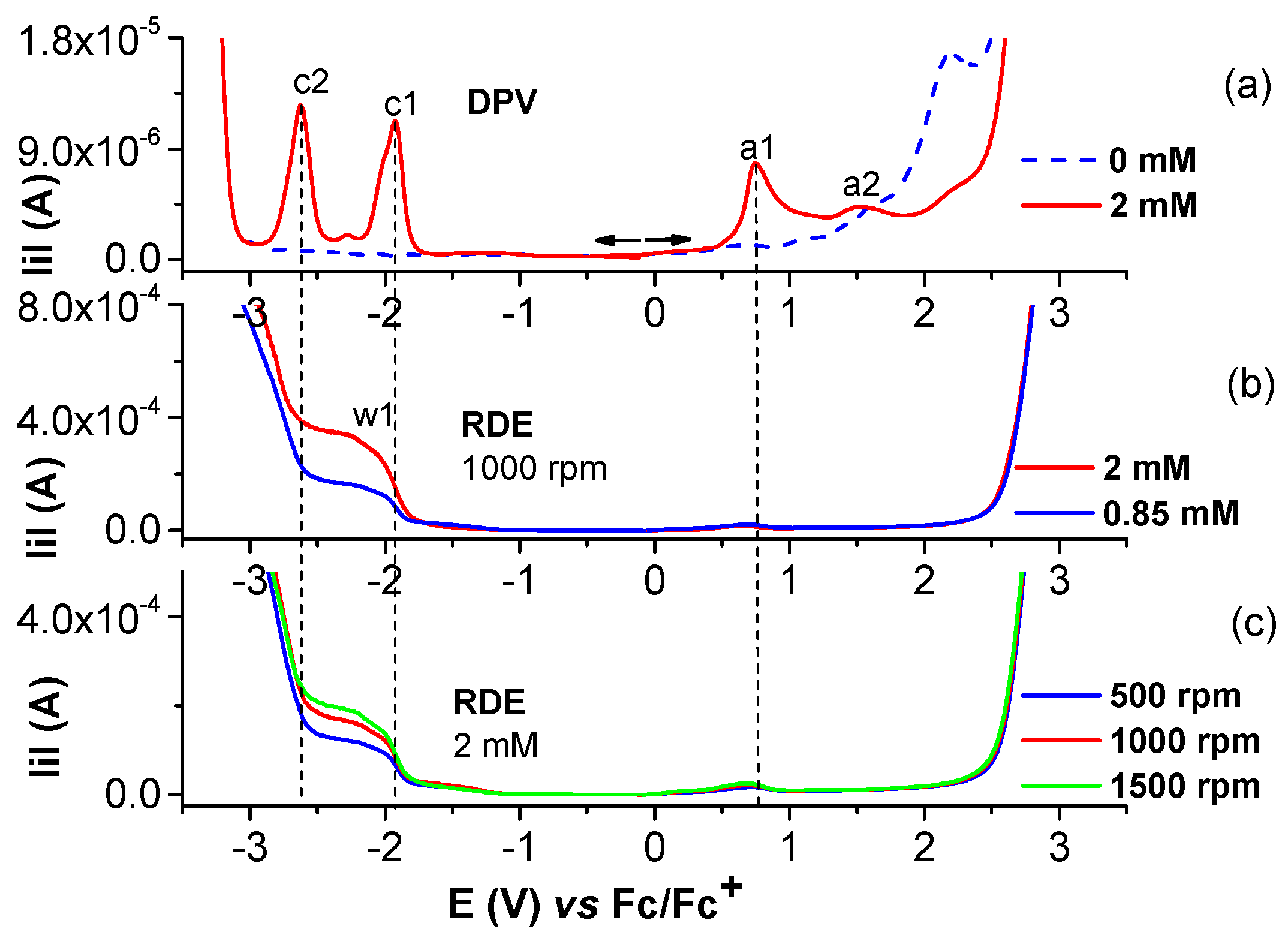
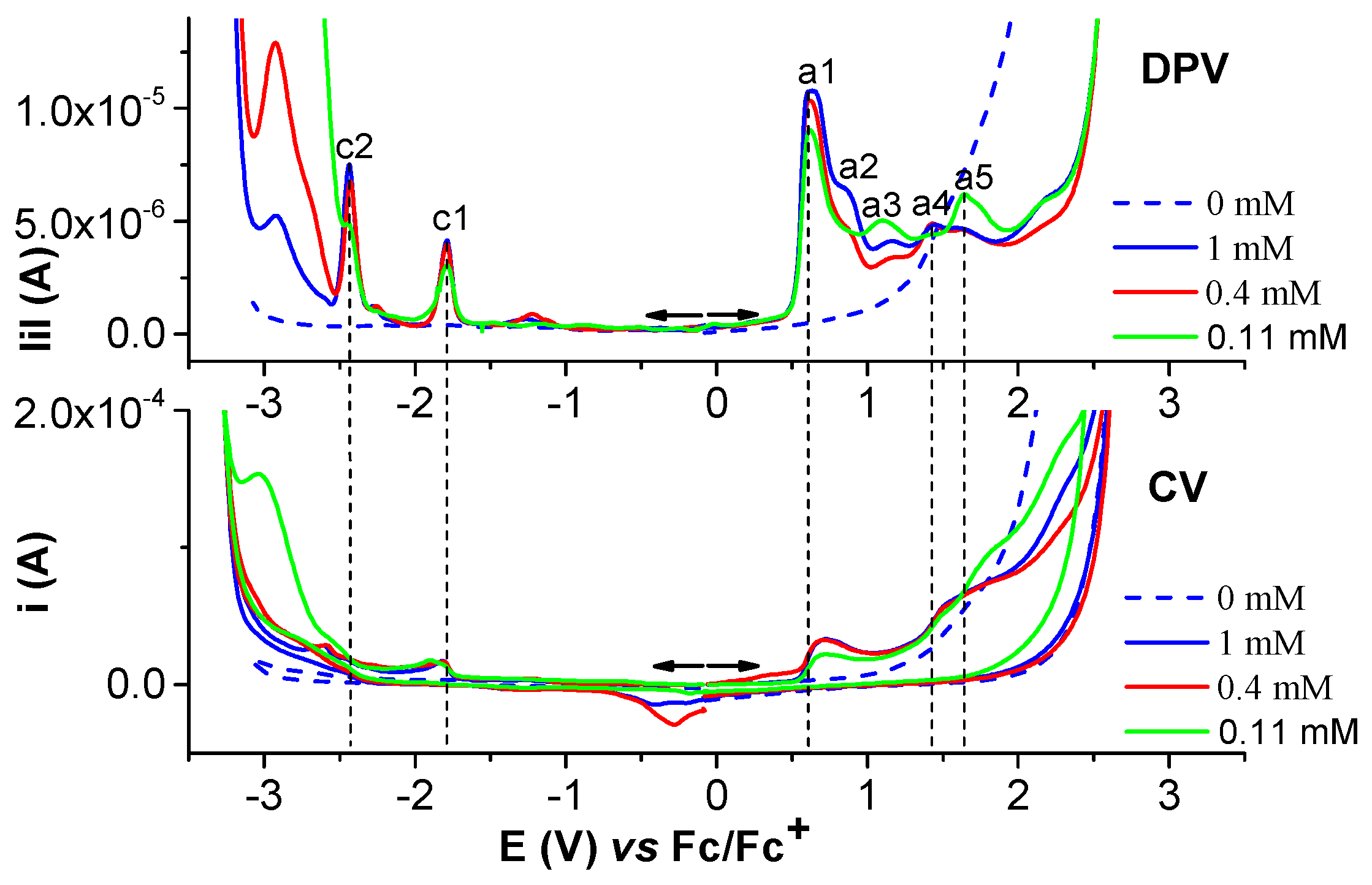
2.1.1. Electrochemical Study of Salophen Ligand L
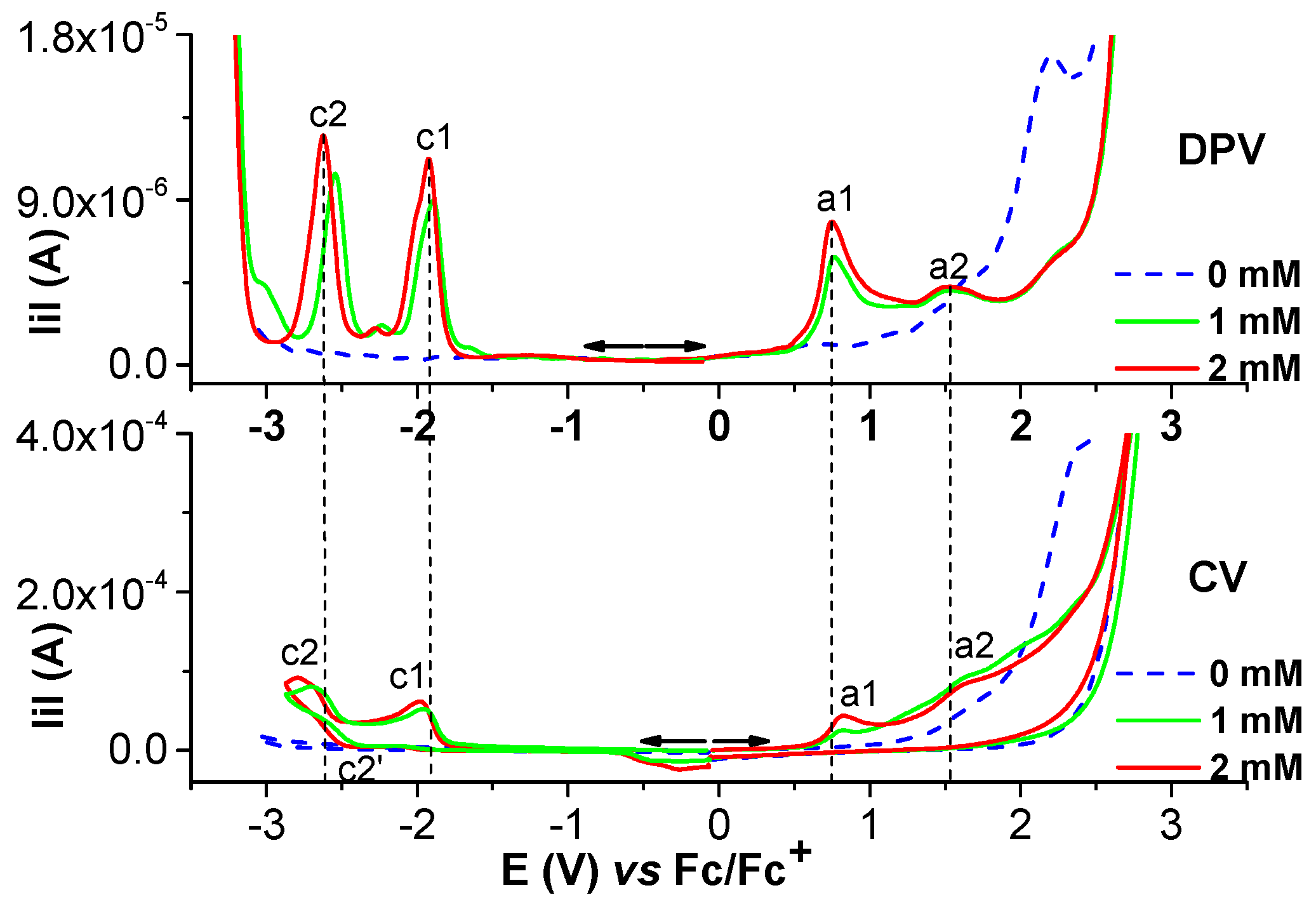
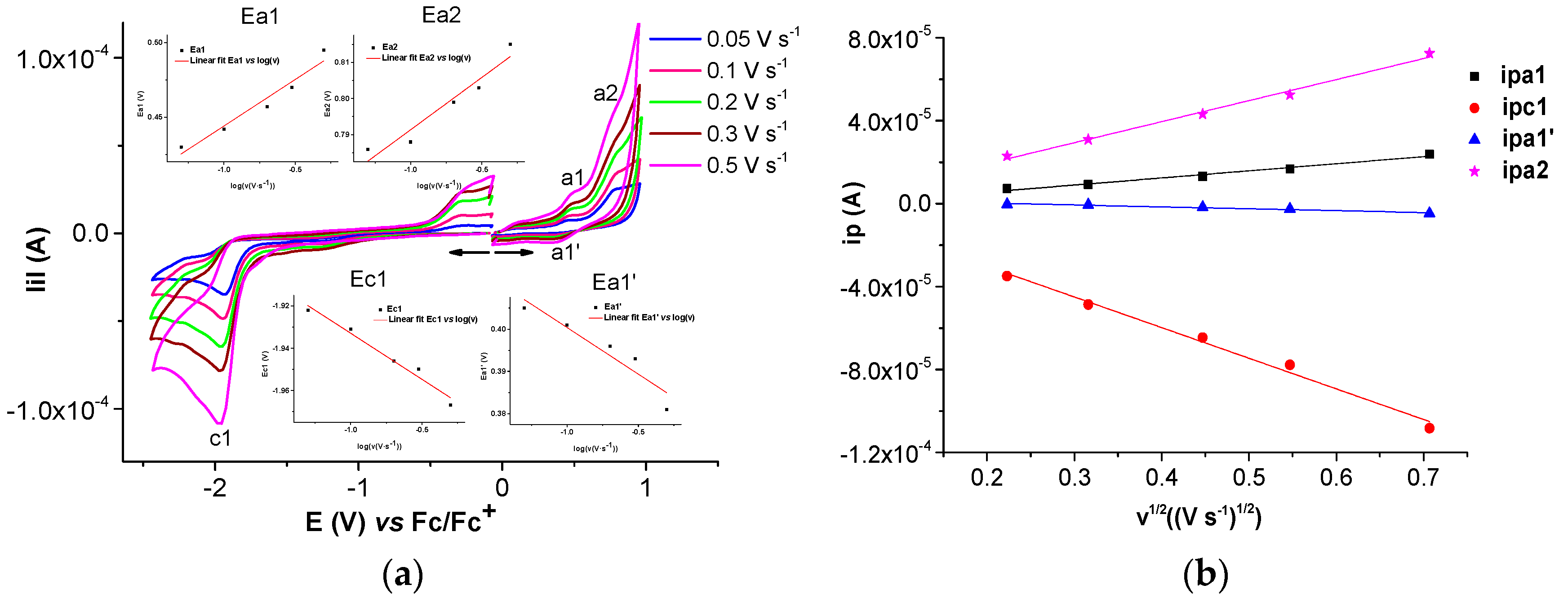
2.1.2. Electrochemical Study of Ni(II)L Complex
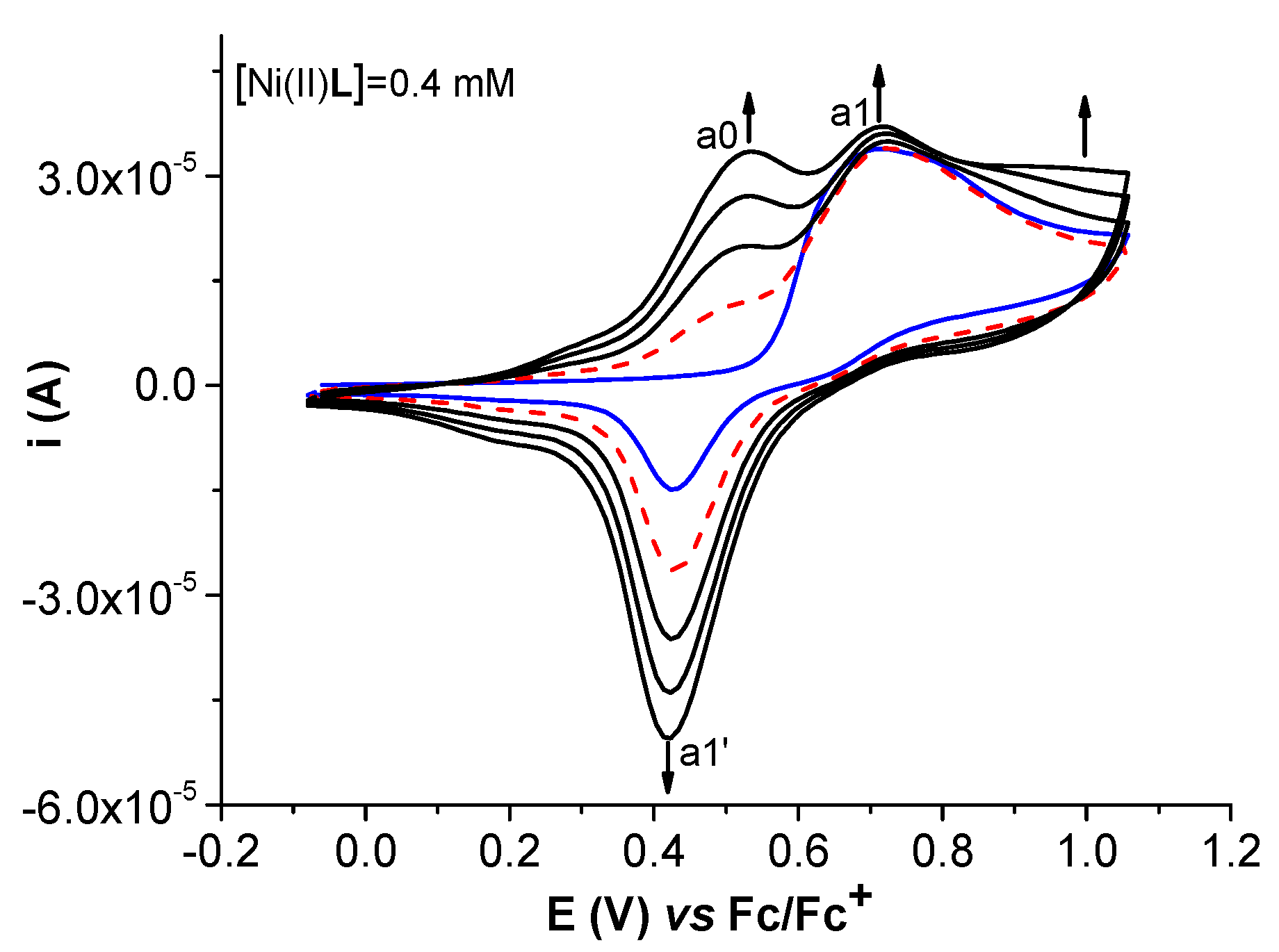
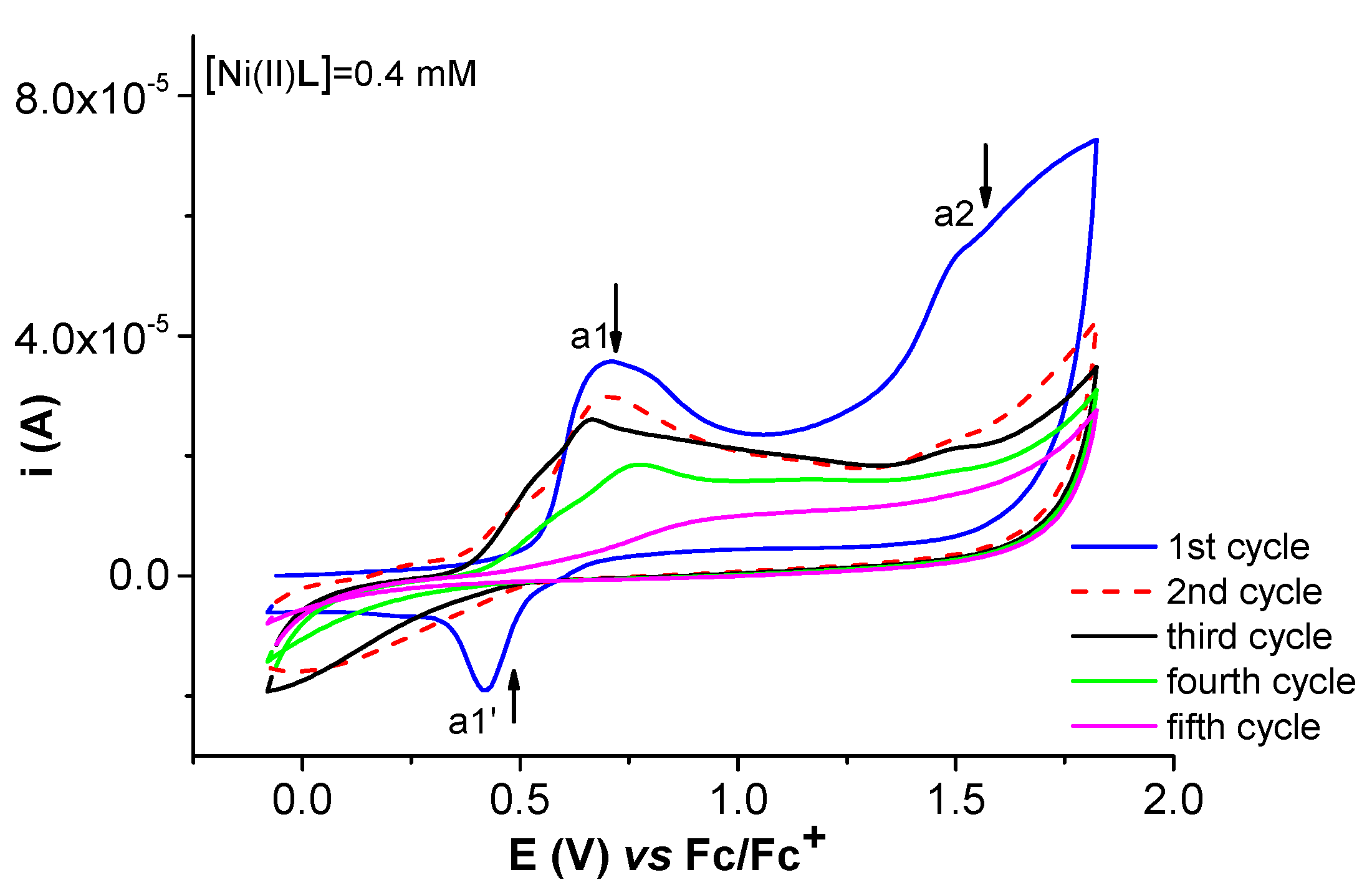
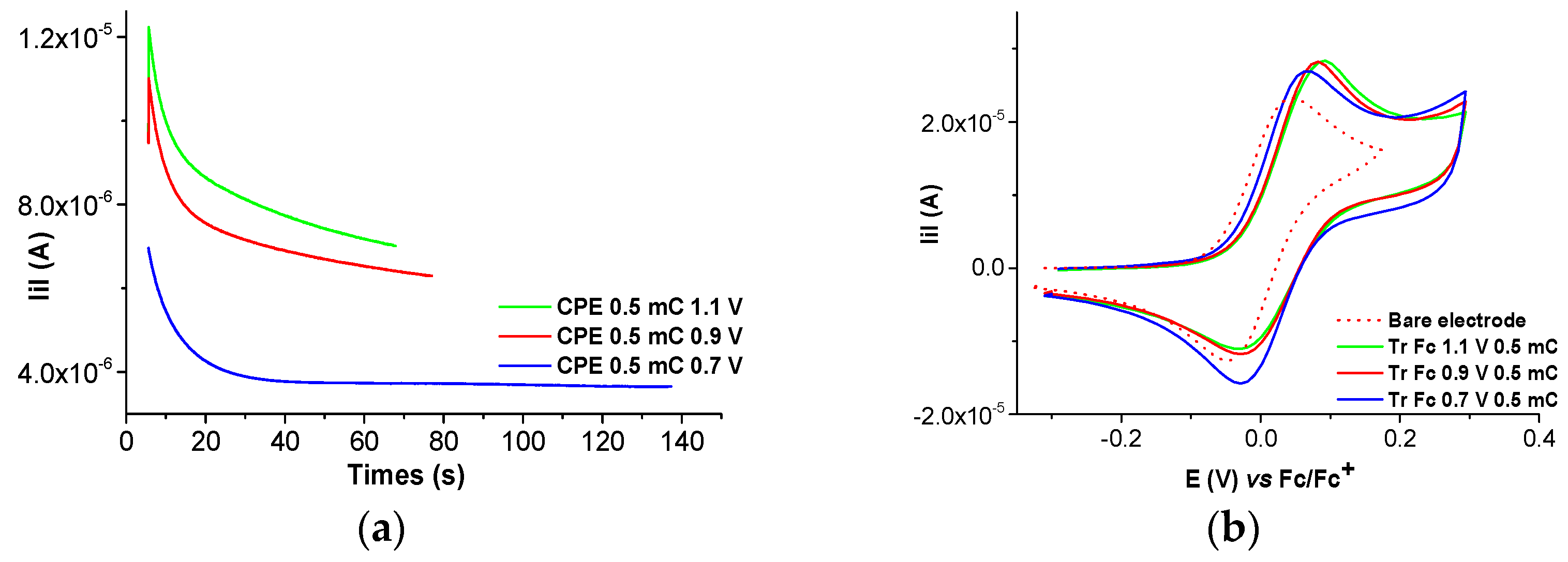
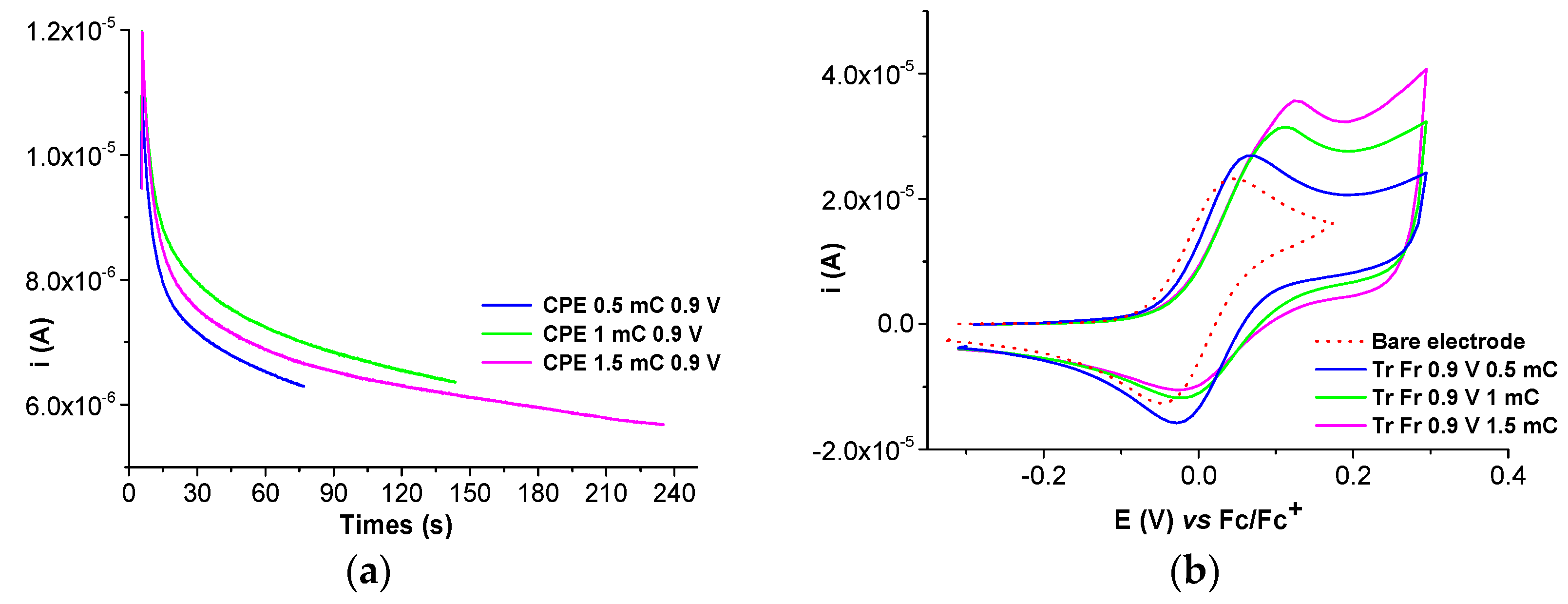
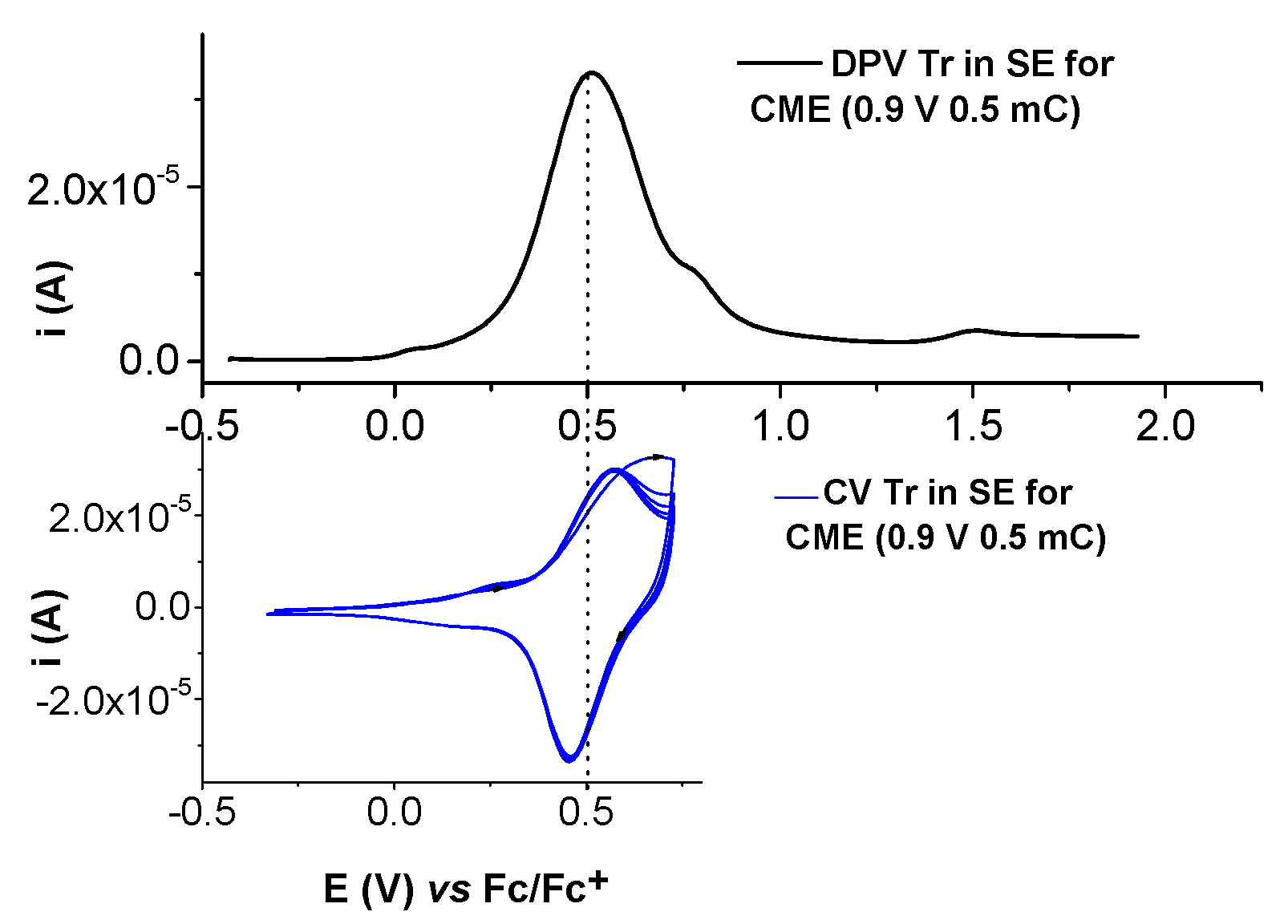
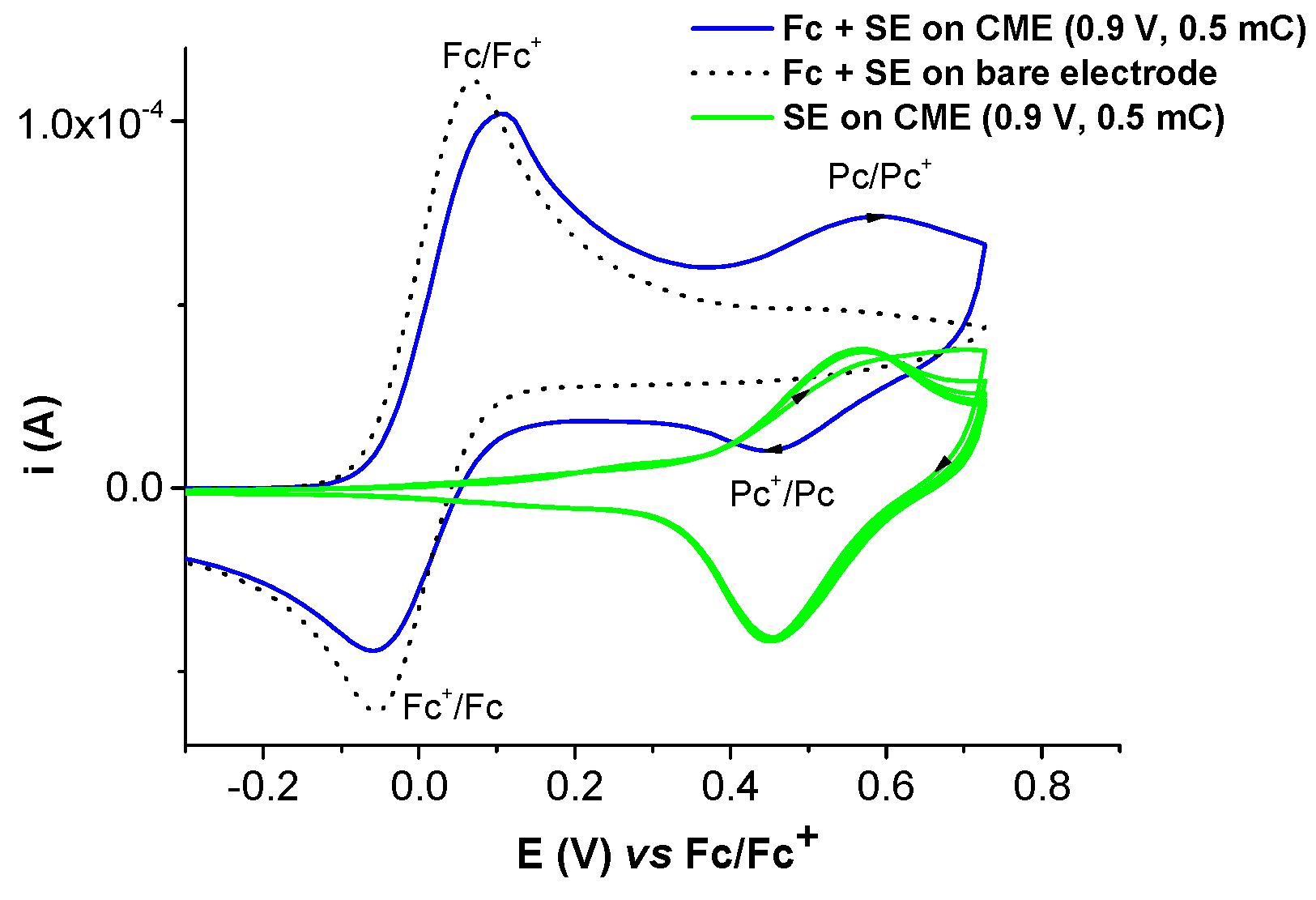
2.2. Film Formation from Ni(II)L Complexes and Their Electrochemical Characterization
2.3. Conductivity Studies
2.4. UV-Vis Studies
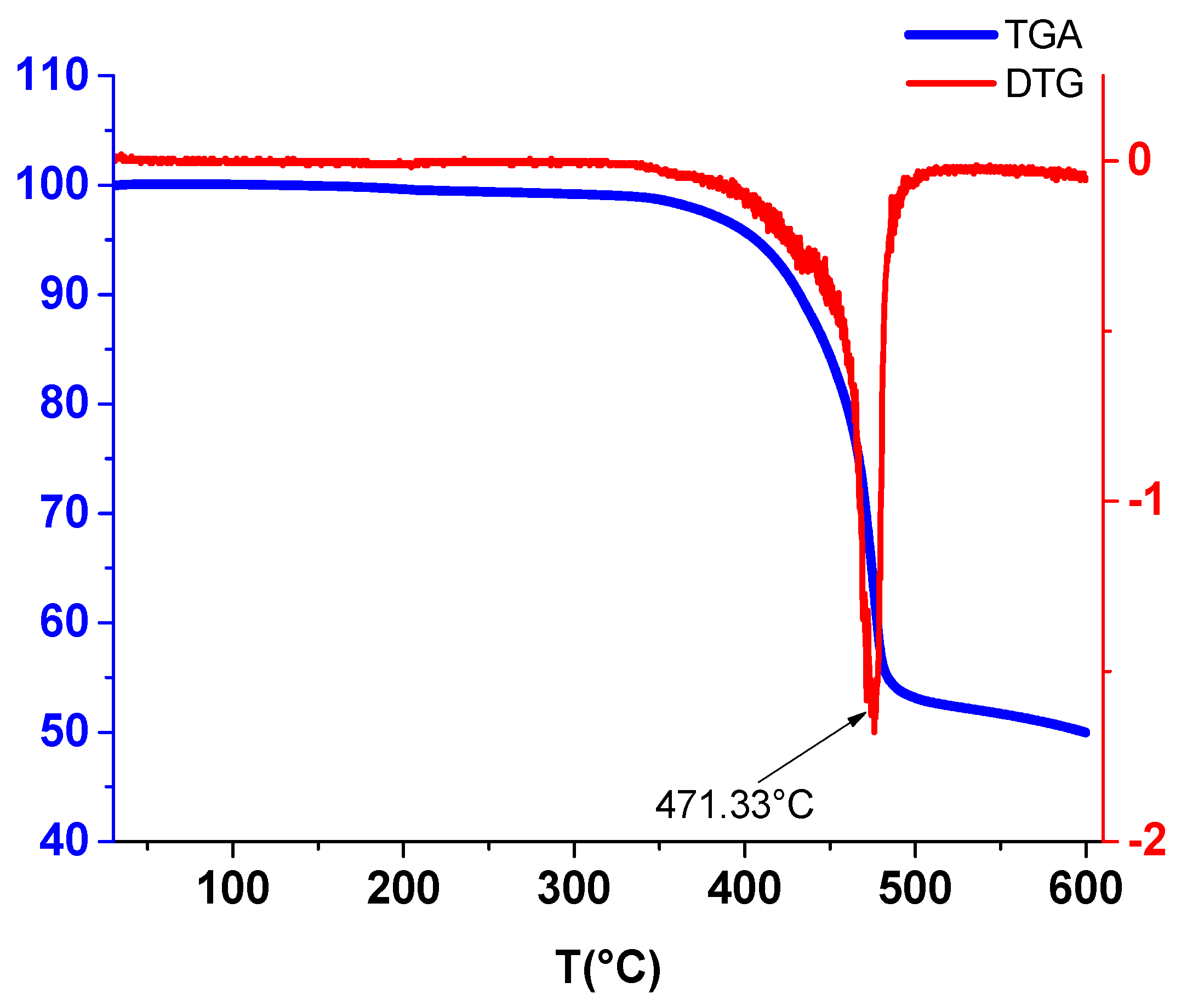
2.5. Thermogravimetric Studies
2.6. Antimicrobial Activity Studies
2.7. Antioxidant Activity Studies
3. Discussion
4. Materials and Methods
4.1. Reactants
4.2. Apparata
4.3. Procedures
4.3.1. Synthesis of Schiff Base N,N’-Bis-(2-hydroxybenzylidene)-benzene-1,2-diamine (H2L)
4.3.2. Synthesis of Ni(II)L Complex
4.3.3. Procedure for Voltammetric Investigations
4.3.4. Procedure for Film Formation
4.3.5. Procedure for Conductometric, UV-Vis, Thermogravimetric, Antimicrobial Activity, and Antioxidant Activity Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, J.; Xu, L.; Wong, W.-Y. Energy materials based on metal Schiff base complexes. Coord. Chem. Rev. 2018, 355, 180–198. [Google Scholar] [CrossRef]
- Trujillo, A.; Fuentealba, M.; Carrillo, D.; Manzur, C.; Ledoux-Rak, I.; Hamon, J.-R.; Saillard, J.-Y. Synthesis, Spectral, Structural, Second-Order Nonlinear Optical Properties and Theoretical Studies on New Organometallic Donor−Acceptor Substituted Nickel(II) and Copper(II) Unsymmetrical Schiff-Base Complexes. Inorg. Chem. 2010, 49, 2750–2764. [Google Scholar] [CrossRef]
- Vereschagin, A.A.; Sizov, V.V.; Vlasov, P.S.; Alekseeva, E.V.; Konev, A.S.; Levin, O.V. Water-stable [Ni(salen)]-type electrode material based on phenylazosubstituted salicylic aldehyde imine ligand. New J. Chem. 2017, 41, 13918–13928. [Google Scholar] [CrossRef]
- Wesley Jeevadason, A.; Kalidasa Murugavel, K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Sarwar, A.; Bin Shamsuddin, M.; Lingtang, H. Synthesis, Characterization and Luminescence Studies of Metal-Diimine Complexes. Mod. Chem. Appl. 2018, 6, 262. [Google Scholar] [CrossRef]
- Mendes, R.A.; Germino, J.C.; Fazolo, B.R.; Thaines, E.H.N.S.; Ferraro, F.; Santana, A.M.; Ramos, R.J.; de Souza, G.L.C.; Freitas, R.G.; Vazquez, P.A.M.; et al. Electronic and magnetic properties of the [Ni(salophen)]: An experimental and DFT study. J. Adv. Res. 2018, 9, 27–33. [Google Scholar] [CrossRef]
- Nworie, F. Bis(Salicylidene)Ethylenediamine(Salen) and Bis(Salicylidene)Ethylenediamine-Metal Complexes: From Structure to Biological Activity. J. Anal. Pharm. Res. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal–Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Lin, J.; Ma, B.; Chen, M.; Ding, Y. Water oxidation catalytic ability of polypyridine complex containing a μ-OH, μ-O2 dicobalt(III) core. Chin. J. Catal. 2018, 39, 463–471. [Google Scholar] [CrossRef]
- Azevedo, F.; Freire, C.; de Castro, B. Reductive electrochemical study of Ni(II) complexes with N2O2 Schiff base complexes and spectroscopic characterisation of the reduced species. Reactivity towards CO. Polyhedron 2002, 21, 1695–1705. [Google Scholar] [CrossRef]
- Aburas, N.; Lolic, A.; Stevanovic, N.; Tripkovic, T.; Nikolic-Mandic, S.; Baosic, R. Electrochemical behavior and antioxidant activity of tetradentate Schiff bases and their copper(II) complexes. J. Iran. Chem. Soc. 2012, 9, 859–864. [Google Scholar] [CrossRef]
- Djebbar-Sid, S.; Benali-Baitich, O.; Deloume, J.P. Synthesis, characterization, electrochemical behaviour and catalytic activity of manganese(II) complexes with linear and tripodal tetradentate ligands derived from Schiff bases. Transit. Met. Chem. 1998, 23, 443–447. [Google Scholar] [CrossRef]
- Chepurnaya, I.; Karushev, M.; Alekseeva, E.; Lukyanov, D.; Levin, O. Redox-conducting polymers based on metal-salen complexes for energy storage applications. Pure Appl. Chem. 2020, 92, 1239–1258. [Google Scholar] [CrossRef]
- Root, D.K.; Smith, W.H. Electrochemical behavior of selected imine derivatives, reductive carboxylation, α-amino acid synthesis. J. Electrochem. Soc. 1982, 129, 1231. [Google Scholar] [CrossRef]
- Kuder, J.E.; Gibson, H.W.; Wychick, D. Linear free energy relations. III. Electrochemical characterization of salicylaldehyde anils. J. Org. Chem. 1975, 40, 875–879. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A.; Vianello, E. A study of the electrochemical reduction mechanism of Ni(salophen) in DMF. Electrochim. Acta 1992, 37, 113–118. [Google Scholar] [CrossRef]
- Fry, A.J.; Reed, R.G. Electrochemical reduction of imines in dimethylformamide. J. Am. Chem. Soc. 1969, 91, 6448–6451. [Google Scholar] [CrossRef]
- Becker, J.Y.; Kerr, J.B.; Pletcher, D.; Rosas, R. The electrochemistry of square planar macrocyclic nickel complexes and the reaction of Ni(I) with alkyl bromides: Nickel tetraamine complexes. J. Electroanal. Chem. 1981, 117, 87–99. [Google Scholar] [CrossRef]
- Bakac, A.; Espenson, J.H. Kinetics and mechanism of the alkylnickel formation in one-electron reductions of alkyl halides and hydroperoxides by a macrocyclic nickel(I) complex. J. Am. Chem. Soc. 1986, 108, 713–719. [Google Scholar] [CrossRef]
- Stiles, M. Nickel Complexes as Soluble Catalysts for Reductive Dehalogenation of Aromatic Halides. J. Org. Chem. 1994, 59, 5381–5385. [Google Scholar] [CrossRef]
- Dahm, C.E.; Peters, D.G.; Simonet, J. Electrochemical and spectroscopic characterization of anodically formed nickel salen polymer films on glassy carbon, platinum, and optically transparent tin oxide electrodes in acetonitrile containing tetramethylammonium tetrafluoroborate. J. Electroanal. Chem. 1996, 410, 163–171. [Google Scholar] [CrossRef]
- Audebert, P.; Hapiot, P.; Capdevielle, P.; Maumy, M. Electrochemical polymerization of several salen-type complexes. Kinetic studies in the microsecond time range. J. Electroanal. Chem. 1992, 338, 269–278. [Google Scholar] [CrossRef]
- Gonciarz, A.; Zuber, M.; Zwoździak, J. Spectrochemical Properties and Solvatochromism of Tetradentate Schiff Base Complex with Nickel: Calculations and Experiments. ChemistryOpen 2018, 7, 677–687. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A.; Vianello, E. Electrochemical carboxylation of arylmethyl chlorides catalysed by [Co(salen)][H2salen =N,N′-bis(salicylidene)ethane-1,2-diamine]. J. Chem. Soc. Dalton Trans. 1996, 8, 1613–1618. [Google Scholar] [CrossRef]
- Soroceanu, A.; Bargan, A. Advanced and Biomedical Applications of Schiff-Base Ligands and Their Metal Complexes: A Review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Akila, E.; Usharani, M.; Vimala, S.; Rajavel, R. Synthesis, spectroscopic, characterization and biological evaluation studies of mixed-ligand Schiff base with metal(II) complexes derived from O-phenylenediamine. Chem. Sci. Rev. Lett. 2012, 1, 181–194. [Google Scholar]
- Kuate, M.; Mainsah, E.N.; Ngounoue Kamga, F.A.; Paboudam, A.G.; Mariam, C.A.; Peucheu, C.N.; Ignas, T.K.; Ndifon, P.T. Cobalt (II), Nickel (II) and Copper (II) complexes of Tetradentate Schiff bases ligands derived from 4-Nitro-O-phenylenediamine: Synthesis, Characterization, cyclic voltammetry and biological studies. Egypt. J. Chem. 2022, 65, 477–495. [Google Scholar] [CrossRef]
- Borthakur, R.; Kumar, A.; De, A.K.; Lal, R.A. Synthesis, characterization and electrochemical properties of copper(II) complexes derived from succinoyldihydrazine Schiff base ligands. Arab. J. Chem. 2019, 12, 2192–2205. [Google Scholar] [CrossRef]
- Gosden, C.; Healy, K.P.; Pletcher, D. Reaction of electrogenerated square-planar nickel(I) complexes with alkyl halides. J. Chem. Soc. Dalton Trans. 1978, 8, 972–976. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A.; Vianello, E. Electrochemical reduction of Schiff base ligands H2salen and H2salophen. Electrochim. Acta 1997, 42, 2065–2071. [Google Scholar] [CrossRef]
- Sibous, L.; Bentouhami, E.; Khan, M.A. Synthesis, Characterization, and Electrochemical Behaviour of Cobalt(II) and Nickel(II) Complexes with N2O2 Chelating Ligand 4,4′-(Biphenyl-4,4′-diyldinitrilo)dipentan-2-one. J. Inorg. Chem. 2013, 2013, 104979. [Google Scholar] [CrossRef]
- Zanello, P.; Cinquantini, A. Electrochemical behaviour of a series of N,N′-1,2-Phenylenebis(salicylideneimin)ato transition metal complexes. Transit. Met. Chem. 1985, 10, 370–374. [Google Scholar] [CrossRef]
- Aligholivand, M.; Shaghaghi, Z.; Bikas, R.; Kozakiewicz, A. Electrocatalytic water oxidation by a Ni(II) salophen-type complex. RSC Adv. 2019, 9, 40424–40436. [Google Scholar] [CrossRef]
- Santos, I.C.; Vilas-Boas, M.; Piedade, M.F.M.; Freire, C.; Duarte, M.T.; Castro, B.D. Electrochemical and X-ray studies of nickel(II) Schiff base complexes derived from salicylaldehyde. Polyhedron 2000, 19, 655–664. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Bagihalli, G.B.; Patil, S.A.; Badami, P.S. Synthesis, characterization, electrochemical and in-vitro antimicrobial studies of Co(II), Ni(II), and Cu(II) complexes with Schiff bases of formylcoumarin derivatives. J. Coord. Chem. 2009, 62, 3060–3072. [Google Scholar] [CrossRef]
- Shaju, K.; Joby, T.; Vinod, P.; Nimmy, K. Spectral and Cyclic Voltammetric Studies on Cu(II)-Schiff Base Complex Derived from Anthracene-9(10 H)-one. IOSR J. Appl. Chem. 2014, 7, 64–68. [Google Scholar]
- Feizi, H.; Shiri, F.; Bagheri, R.; Singh, J.P.; Chae, K.H.; Song, Z.; Najafpour, M.M. The application of a nickel(II) Schiff base complex in water oxidation: The importance of nanosized materials. Catal. Sci. Technol. 2018, 8, 3954–3968. [Google Scholar] [CrossRef]
- Tomczyk, D.; Bukowski, W.; Bester, K. Redox processes in the solution of Ni(II) complex with salen type ligand and in the polymer films. Electrochim. Acta 2018, 267, 181–194. [Google Scholar] [CrossRef]
- Peverari, C.R.; David-Parra, D.N.; Barsan, M.M.; Teixeira, M.F.S. Mechanistic study of the formation of multiblock π-conjugated metallopolymer. Polyhedron 2016, 117, 415–421. [Google Scholar] [CrossRef]
- Tomczyk, D.; Nowak, L.; Bukowski, W.; Bester, K.; Urbaniak, P.; Andrijewski, G.; Olejniczak, B. Reductive and oxidative electrochemical study and spectroscopic properties of nickel(II) complexes with N2O2 Schiff bases derived from (±)-trans-N,N′-bis(salicylidene)-1,2-cyclohexanediamine. Electrochim. Acta 2014, 121, 64–77. [Google Scholar] [CrossRef]
- Tomczyk, D.; Bukowski, W.; Bester, K.; Urbaniak, P.; Seliger, P.; Andrijewski, G.; Skrzypek, S. The mechanism of electropolymerization of nickel(II) salen type complexes. New J. Chem. 2017, 41, 2112–2123. [Google Scholar] [CrossRef]
- Aminabhavi, T.; Biradar, N.; Divakar, M. Bimetallic Complexes of Nickel (II)-Tetradentate Schiff Bases with Tin (IV), Selenium (IV) & Tellurium (IV) Chlorides. Indian J. Chem. 1986, 25, 283–284. [Google Scholar]
- Garg, B.S.; Nandan Kumar, D. Spectral studies of complexes of nickel(II) with tetradentate schiff bases having N2O2 donor groups. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Rehman, S.; Shah, A.; Jamal, Q. Activity on Leishmania tropica of Metal Complexes with NNOO Tetradentate Schiff Base Ligand: Kinetic and Thermodynamic Studies from TG-DTA Analysis. J. Chem. Soc. Pak. 2015, 37, 869–878. [Google Scholar]
- Baecker, D.; Sesli, Ö.; Knabl, L.; Huber, S.; Orth-Höller, D.; Gust, R. Investigating the antibacterial activity of salen/salophene metal complexes: Induction of ferroptosis as part of the mode of action. Eur. J. Med. Chem. 2021, 209, 112907. [Google Scholar] [CrossRef] [PubMed]
- Kargar, H.; Ashfaq, M.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Tahir, M.N. Unsymmetrical Ni(II) Schiff base complex: Synthesis, spectral characterization, crystal structure analysis, Hirshfeld surface investigation, theoretical studies, and antibacterial activity. J. Mol. Struct. 2022, 1265, 133381. [Google Scholar] [CrossRef]
- El-Ghamry, M.A.; Elzawawi, F.M.; Aziz, A.A.A.; Nassir, K.M.; Abu-El-Wafa, S.M. New Schiff base ligand and its novel Cr(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II) complexes: Spectral investigation, biological applications, and semiconducting properties. Sci. Rep. 2022, 12, 17942. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Arif, M.; Akhtar, M.A.; Supuran, C.T. Metal-Based Antibacterial and Antifungal Agents: Synthesis, Characterization, and In Vitro Biological Evaluation of Co(II), Cu(II), Ni(II), and Zn(II) Complexes with Amino Acid-Derived Compounds. Bioinorg. Chem. Appl. 2006, 2006, 83131. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Mohsin, A.; Maurya, R.R.; Singh, J.; Negi, P.S.; Rajor, H.K.; Bahadur, I. Schiff base complexes of Cu(II) and Ni(II) derived from N,N’-bis(salicylidene)-o-phenylenediamine as potential ionophores in the construction of PVC membrane iodide sensors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128369. [Google Scholar] [CrossRef]
- Tailor, S.B.; Manzotti, M.; Asghar, S.; Rowsell, B.J.S.; Luckham, S.L.J.; Sparkes, H.A.; Bedford, R.B. Revisiting Claims of the Iron, Cobalt-, Nickel-, and Copper-Catalyzed Suzuki Biaryl Cross-Coupling of Aryl Halides with Aryl Boronic Acids. Organometallics 2019, 38, 1770–1777. [Google Scholar] [CrossRef]
- Afsan, F.; Dalia, S.; Hossain, M.; Sarker, M.S.; Zahan, M.K.-E. Synthesis, Spectral and Thermal Characterization of Selected Metal Complexes Containing Schiff Base Ligands with Antimicrobial Activities. Asian J. Chem. Sci. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Asadi, M.; Aein Jamshid, K.; Hossein Kyanfar, A. Thermodynamic Studies of the Interaction of Nickel(II) Schiff Base Complexes with Diorganotin (IV) Dichlorides in Non-Aqueous Solvents. Synth. React. Inorg. Met. Org. Chem. 2007, 37, 77–83. [Google Scholar] [CrossRef]
- Cunningham, D.; Fitzgerald, J.; Little, M. Transition metal Schiff-base complexes as ligands in tin chemistry. Part 1. A tin-119 Mössbauer spectroscopic investigation of the adducts SnX4·ML, SnMe2(NCS)2·ML, and SnRCl3·ML (X = halide; M = Cu or Ni; L = quadridentate Schiff-base ligand; R = phenyl or n-butyl). J. Chem. Soc. Dalton Trans. 1987, 9, 2261–2266. [Google Scholar] [CrossRef]
- Onozawa-Komatsuzaki, N.; Katoh, R.; Himeda, Y.; Sugihara, H.; Arakawa, H.; Kasuga, K. Synthesis and Photochemical Properties of Novel Ruthenium(II)-Nickel(II) and Ruthenium(II)-Copper(II) Dinuclear Complexes. Bull. Chem. Soc. Jpn. 2003, 76, 977–984. [Google Scholar] [CrossRef]
- Xavier, A.J.M.; Srividhya, N. Synthesis and Study of Schiff base Ligands. J. Appl. Chem. 2014, 7, 6–15. [Google Scholar] [CrossRef]
- Franceschi, F.; Solari, E.; Scopelliti, R.; Floriani, C. Metal-Mediated Transfer of Electrons between Two Different C−C Single Bonds That Function as Electron-Donor and Electron-Acceptor Units. Angew. Chem. Int. Ed. 2000, 39, 1685–1687. [Google Scholar] [CrossRef]
- Ourari, A.; Khelafi, M.; Aggoun, D.; Bouet, G.; Khan, M. Synthesis, characterization, and electrochemical study of tetradentate ruthenium-Schiff base complexes: Dioxygen activation with a cytochrome P450 model using 1-or 2-methylimidazole as axial bases. Adv. Phys. Org. Chem. 2011, 2011, 157484. [Google Scholar] [CrossRef]
- Freire, C.; de Castro, B. Spectroscopic characterisation of electrogenerated nickel(III) species. Complexes with N2O2 Schiff-base ligands derived from salicylaldehyde. J. Chem. Soc. Dalton Trans. 1998, 9, 1491–1498. [Google Scholar] [CrossRef]
- Rani, J.J.; Jayaseeli, A.M.I.; Rajagopal, S.; Seenithurai, S.; Chai, J.-D.; Raja, J.D.; Rajasekaran, R. Synthesis, characterization, antimicrobial, BSA binding, DFT calculation, molecular docking and cytotoxicity of Ni(II) complexes with Schiff base ligands. J. Mol. Liq. 2021, 328, 115457. [Google Scholar] [CrossRef]
- Ansari, R.M.; Kumar, L.M.; Bhat, B.R. Air-Stable Cobalt(II) and Nickel(II) Complexes with Schiff Base Ligand for Catalyzing Suzuki–Miyaura Cross-Coupling Reaction. Russ. J. Coord. Chem. 2018, 44, 1–8. [Google Scholar] [CrossRef]
- Abd-Elzaher, M.M. Synthesis and Spectroscopic Characterization of Some Tetradentate Schiff Bases and Their Nickel, Copper and Zinc Complexes. Sythesis React. Inorg. Matel-Org. Chem. 2000, 30, 1805–1816. [Google Scholar] [CrossRef]
- Tverdova, N.V.; Pelevina, E.D.; Giricheva, N.I.; Girichev, G.V.; Kuzmina, N.P.; Kotova, O.V. Molecular structures of 3d metal complexes with various Schiff bases studied by gas-phase electron diffraction and quantum-chemical calculations. J. Mol. Struct. 2012, 1012, 151–161. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I.; Ledoux, I.; Diaz-Garcia, M.A.; Marks, T.J. Synthesis, Characterization, Optical Spectroscopic, Electronic Structure, and Second-Order Nonlinear Optical (NLO) Properties of a Novel Class of Donor−Acceptor Bis(salicylaldiminato)nickel(II) Schiff Base NLO Chromophores. J. Am. Chem. Soc. 1997, 119, 9550–9557. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bajaj, H.C.; Halligudi, S.B.; Bhatt, K.N. Catalysis of alkene hydrogenation and oxidation by nickel-saloph complex; A novel bifunctional catalyst. J. Mol. Catal. 1993, 84, L1–L5. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, Characterization and Biological Studies of Metal(II) Complexes of (3E)-3-[(2-{(E)-[1-(2,4-Dihydroxyphenyl) ethylidene]amino}ethyl)imino]-1-phenylbutan-1-one Schiff Base. Molecules 2015, 20, 9788–9802. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.H.; Ismail, E.H.; Mohamed, G.G.; Zayed, E.M.; Badr, A. Synthesis and characterization of a novel schiff base metal complexes and their application in determination of iron in different types of natural water. Open J. Inorg. Chem. 2012, 2, 9. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragala, I.; Ledoux, I.; Marks, T.J. Role of Metal Electronic Properties in Tuning the Second-Order Nonlinear Optical Response of Coordination Complexes. A Combined Experimental and Theoretical Investigation of a Homologous Series of (N,N’-Disalicylidene-1,2-phenylenediaminato)M(II) (M = Co, Ni, Cu) Complexes. J. Am. Chem. Soc. 1995, 117, 9481–9485. [Google Scholar] [CrossRef]
- Yamada, M.; Ochi, S.; Suzuki, H.; Hisazumi, A.; Kuroda, S.; Shimao, I.; Araki, K. Catalytic activities of nickel (II) complexes of a salen analog, [Ni(babp)], in epoxidation of olefins. J. Mol. Catal. 1994, 87, 195–202. [Google Scholar] [CrossRef]
- Geary, W.-J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Gwrd. Them. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Khalaji, A. Preparation and Characterization of NiO Nanoparticles Via Solid-State Thermal Decomposition of Nickel(II) Schiff Base Complexes [Ni(salophen)] and [Ni(Me-salophen)]. J. Clust. Sci. 2012, 24, 209–215. [Google Scholar] [CrossRef]
- El-Sawaf, A.; Azzam, M.; Abdou, A.; Anouar, E.H. Synthesis, spectroscopic characterization, DFT and antibacterial studies of newly synthesized cobalt(II, III), nickel(II) and copper(II) complexes with Salicylaldehyde N(4)-antipyrinylthiosemicarbazone. Inorganica Chim. Acta 2018, 483, 116–128. [Google Scholar] [CrossRef]




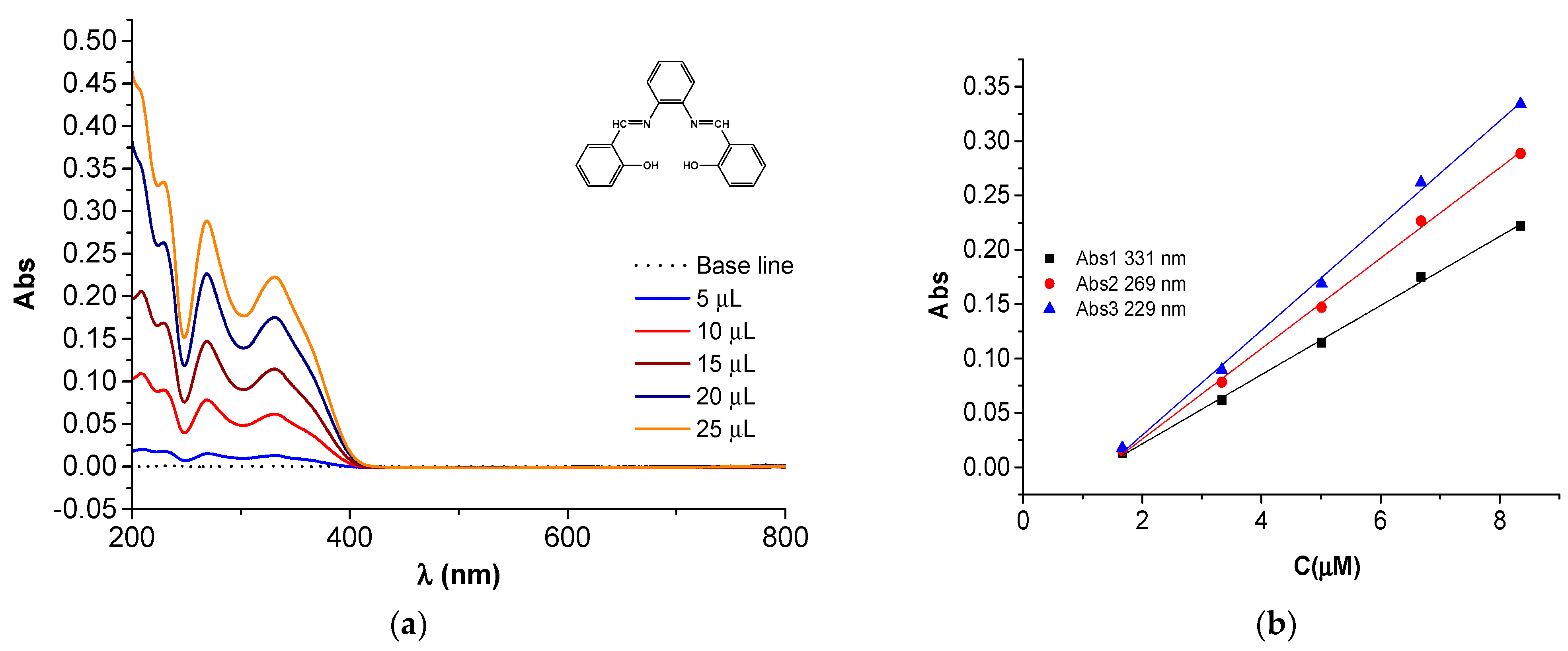
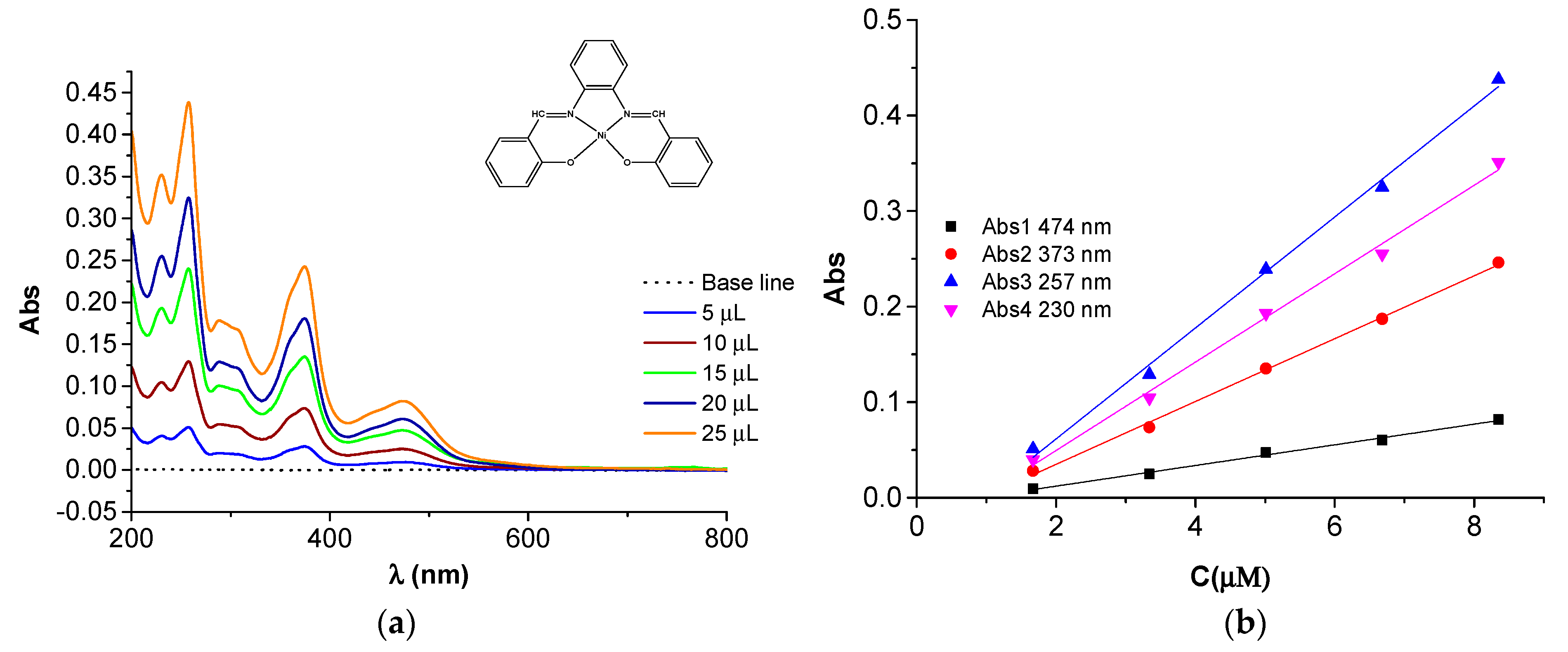

| Compound | Band Maxima (cm−1) | Wavelength (nm) | Assignment | Calibration Equation/Figure (C in µM) |
|---|---|---|---|---|
| H2L | 37,175 | 269 | n→π* | Abs2 = −0.0573 + 0.0416·C/Figure 17b |
| 30,215 | 331 | π→π* | Abs1 = −0.0422 + 0.0318·C/Figure 17b | |
| Ni(II)L | 38,911 | 257 | π→π* | Abs3 = −0.0546 + 0.0581·C/Figure 18b |
| 26,738 | 373 | n→π* | Abs2 = −0.0308+ 0.0329·C/Figure 18b | |
| 21,097 | 474 | 1A1g→1B1g | Abs1 = −0.0095 + 0.0108·C/Figure 18b |
| Compound | Bacteria * | Fungi ** | |||||
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | Yeast | Mold | Fungus | |||
| B. cereus | S. aureus | S. typhi | E. coli | C. albicans | A. niger | A. carbonarius | |
| 40 mg/mL | |||||||
| Chloramphenicol | 16.00 ± 2.00 | 22. 00 ± 3.00 | 19.00 ± 1.00 | 18.00 ± 2.00 | / | / | / |
| Nystatine | / | / | / | / | 19.00 ± 2.00 | 23.00 ± 5.00 | 27.00 ± 3.00 |
| OPD *** | 7.00 ± 0.00 | 14.00 ± 4.00 | 22.00 ± 4.00 | 17.00 ± 1.00 | 9.00 ± 2.00 | 6.00 ± 0.00 | 17.00 ± 3.00 |
| SA **** | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| H2L | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| Ni(II)L | 6.00 ± 0.00 | 6.00 ± 0.00 | 11.00 ± 0.00 | 10.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| 30 mg/mL | |||||||
| H2L | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| Ni(II)L | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 18.00 ± 0.00 |
| 20 mg/mL | |||||||
| H2L | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| Ni(II)L | 6.00 ± 0.00 | 7.00 ± 0.00 | 15.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 11.00 ± 0.00 | 14.00 ± 9.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngounoue Kamga, F.A.; Hrubaru, M.-M.; Enache, O.; Diacu, E.; Draghici, C.; Tecuceanu, V.; Ungureanu, E.-M.; Nkemone, S.; Ndifon, P.T. Ni(II)-Salophen—Comprehensive Analysis on Electrochemical and Spectral Characterization and Biological Studies. Molecules 2023, 28, 5464. https://doi.org/10.3390/molecules28145464
Ngounoue Kamga FA, Hrubaru M-M, Enache O, Diacu E, Draghici C, Tecuceanu V, Ungureanu E-M, Nkemone S, Ndifon PT. Ni(II)-Salophen—Comprehensive Analysis on Electrochemical and Spectral Characterization and Biological Studies. Molecules. 2023; 28(14):5464. https://doi.org/10.3390/molecules28145464
Chicago/Turabian StyleNgounoue Kamga, Francis Aurelien, Madalina-Marina Hrubaru, Oana Enache, Elena Diacu, Constantin Draghici, Victorita Tecuceanu, Eleonora-Mihaela Ungureanu, Stephanie Nkemone, and Peter T. Ndifon. 2023. "Ni(II)-Salophen—Comprehensive Analysis on Electrochemical and Spectral Characterization and Biological Studies" Molecules 28, no. 14: 5464. https://doi.org/10.3390/molecules28145464
APA StyleNgounoue Kamga, F. A., Hrubaru, M.-M., Enache, O., Diacu, E., Draghici, C., Tecuceanu, V., Ungureanu, E.-M., Nkemone, S., & Ndifon, P. T. (2023). Ni(II)-Salophen—Comprehensive Analysis on Electrochemical and Spectral Characterization and Biological Studies. Molecules, 28(14), 5464. https://doi.org/10.3390/molecules28145464






