Catalyzing Benzoxazine Polymerization with Titanium-Containing POSS to Reduce the Curing Temperature and Improve Thermal Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Catalytic Activity of Ti-Ph-POSS
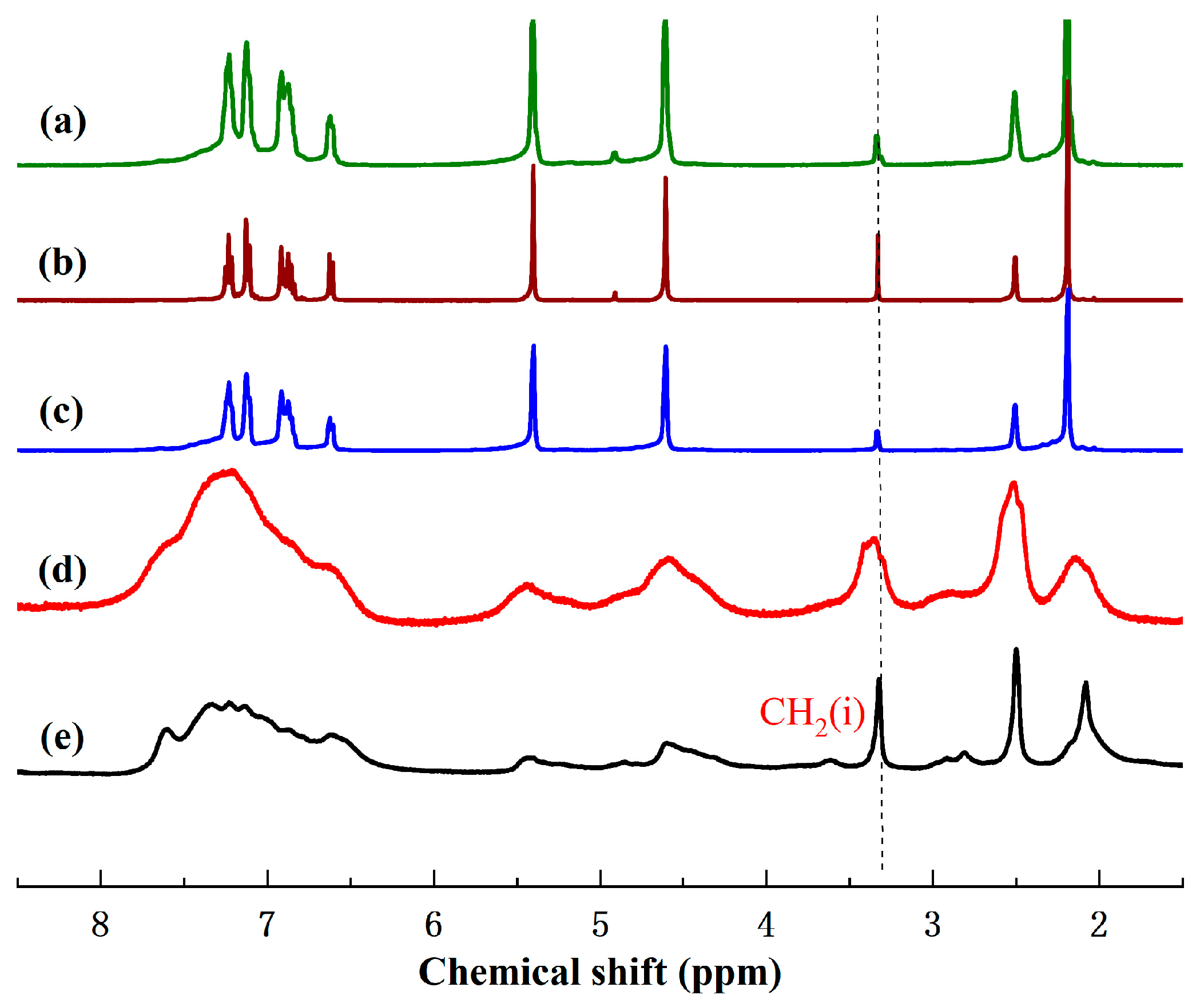
2.1.1. 1H-NMR Analysis
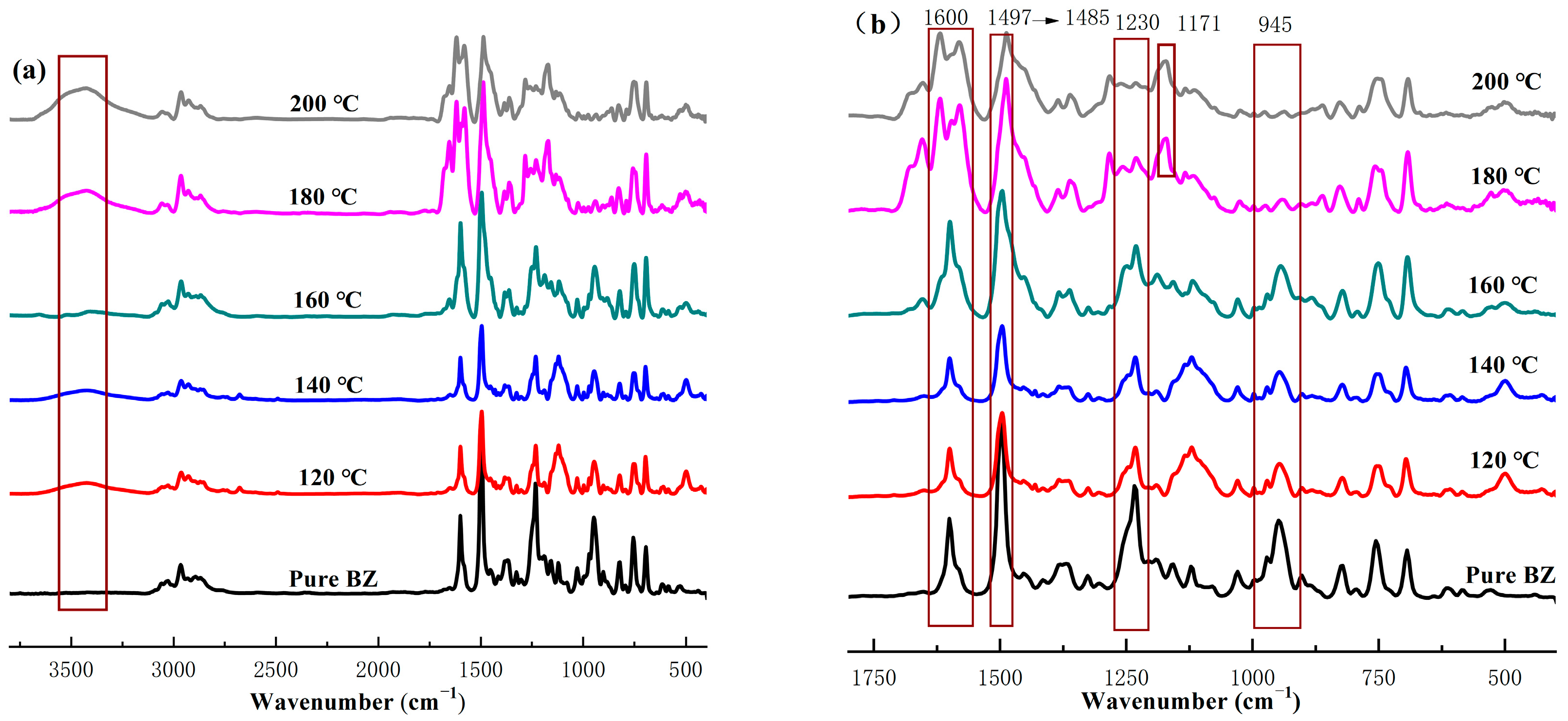
2.1.2. FTIR Analysis
2.2. Curing Behavior and Curing Kinetics of Ti-Ph-POSS/BZ
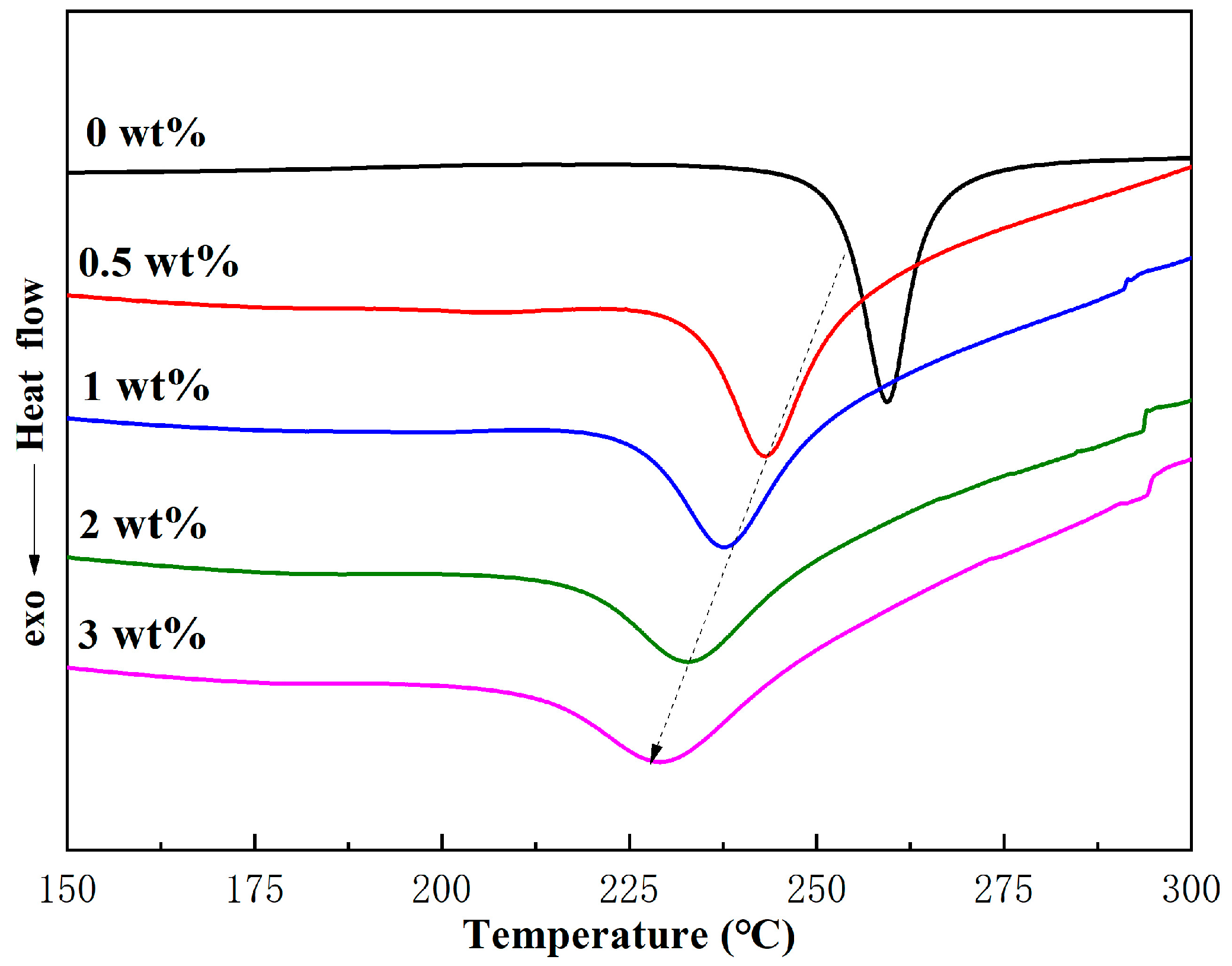
2.2.1. Curing Behavior of Ti-Ph-POSS/BZ
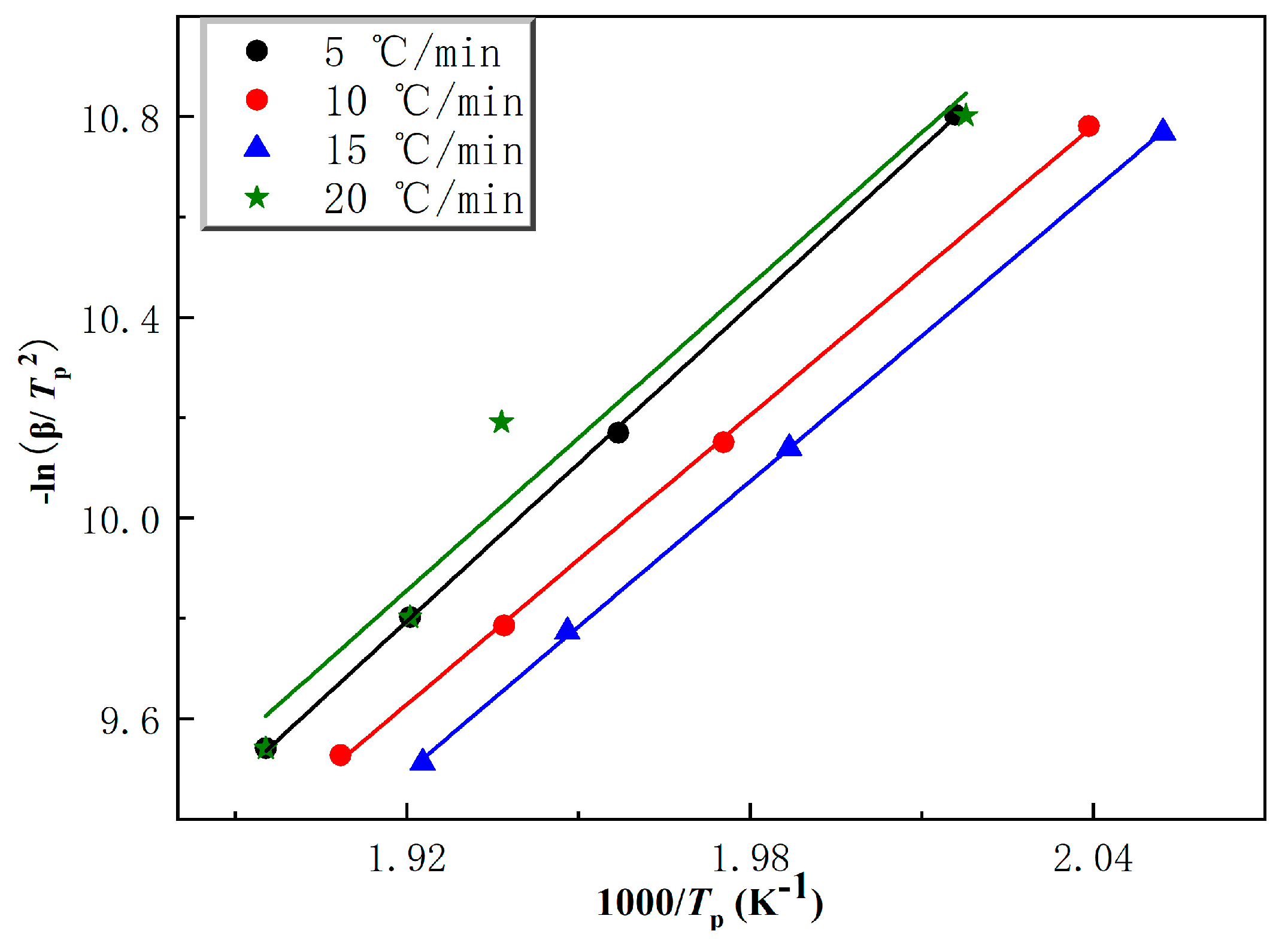
2.2.2. Curing Kinetics of Ti-Ph-POSS/BZ Composite Systems
2.3. Thermal Properties of Ti-Ph-POSS/PBZ
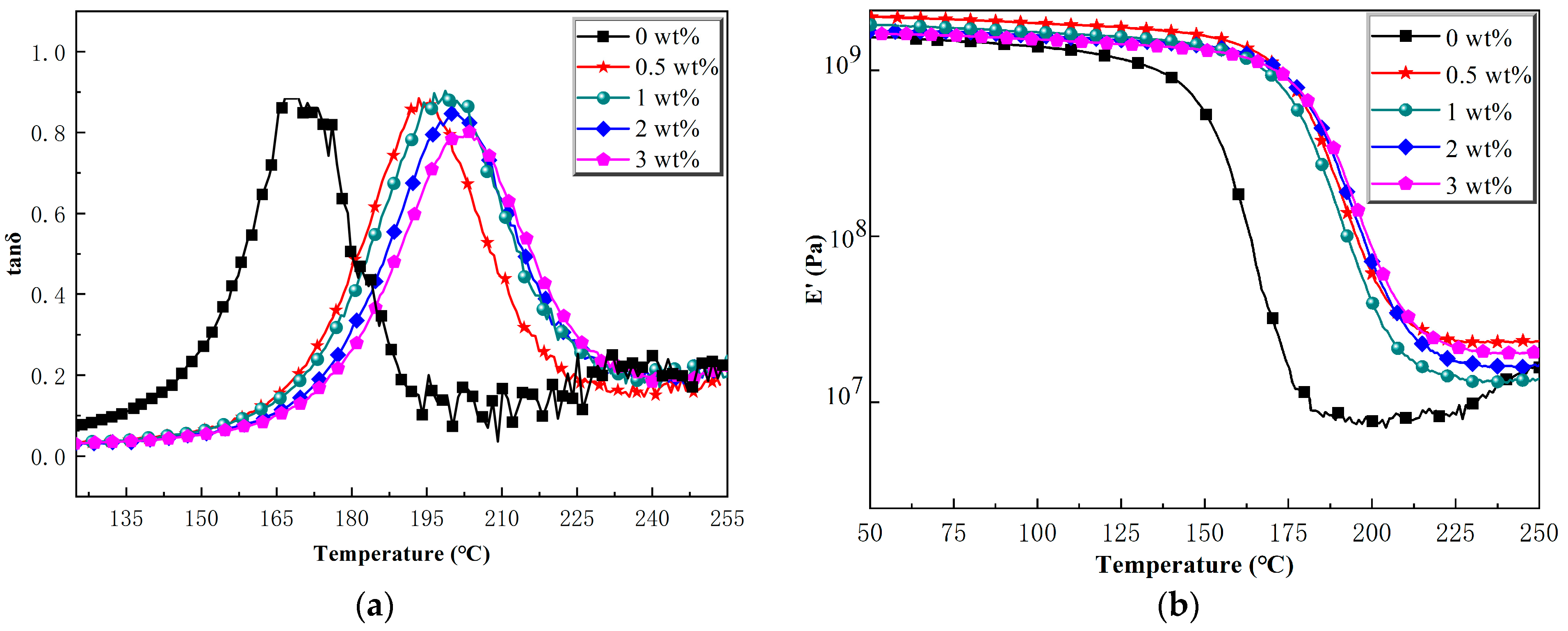
2.3.1. Dynamic Mechanical Thermal Analysis
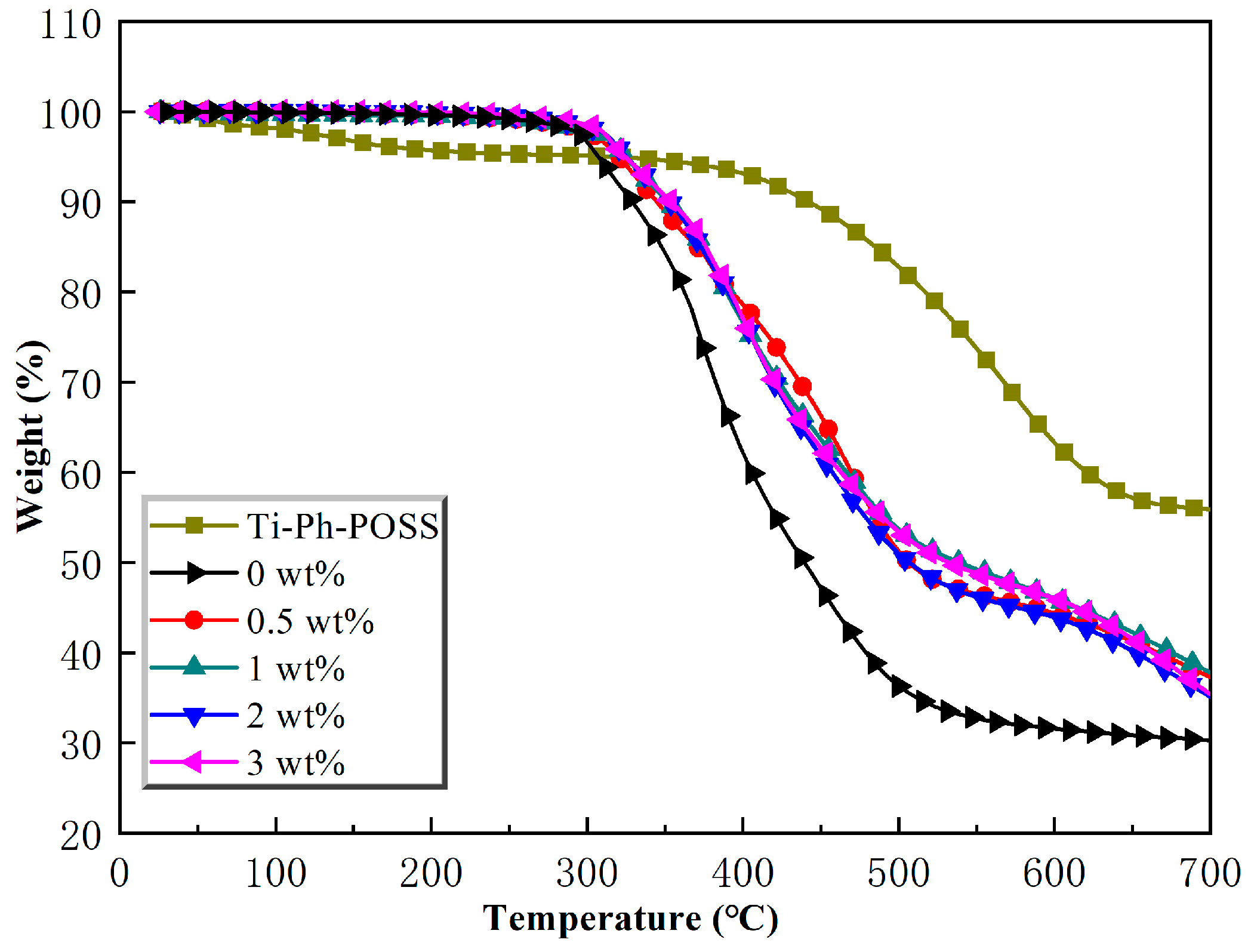
2.3.2. Thermogravimetric Analysis
2.4. Morphology of Ti-Ph-POSS/PBZ
3. Materials and Methods
3.1. Materials
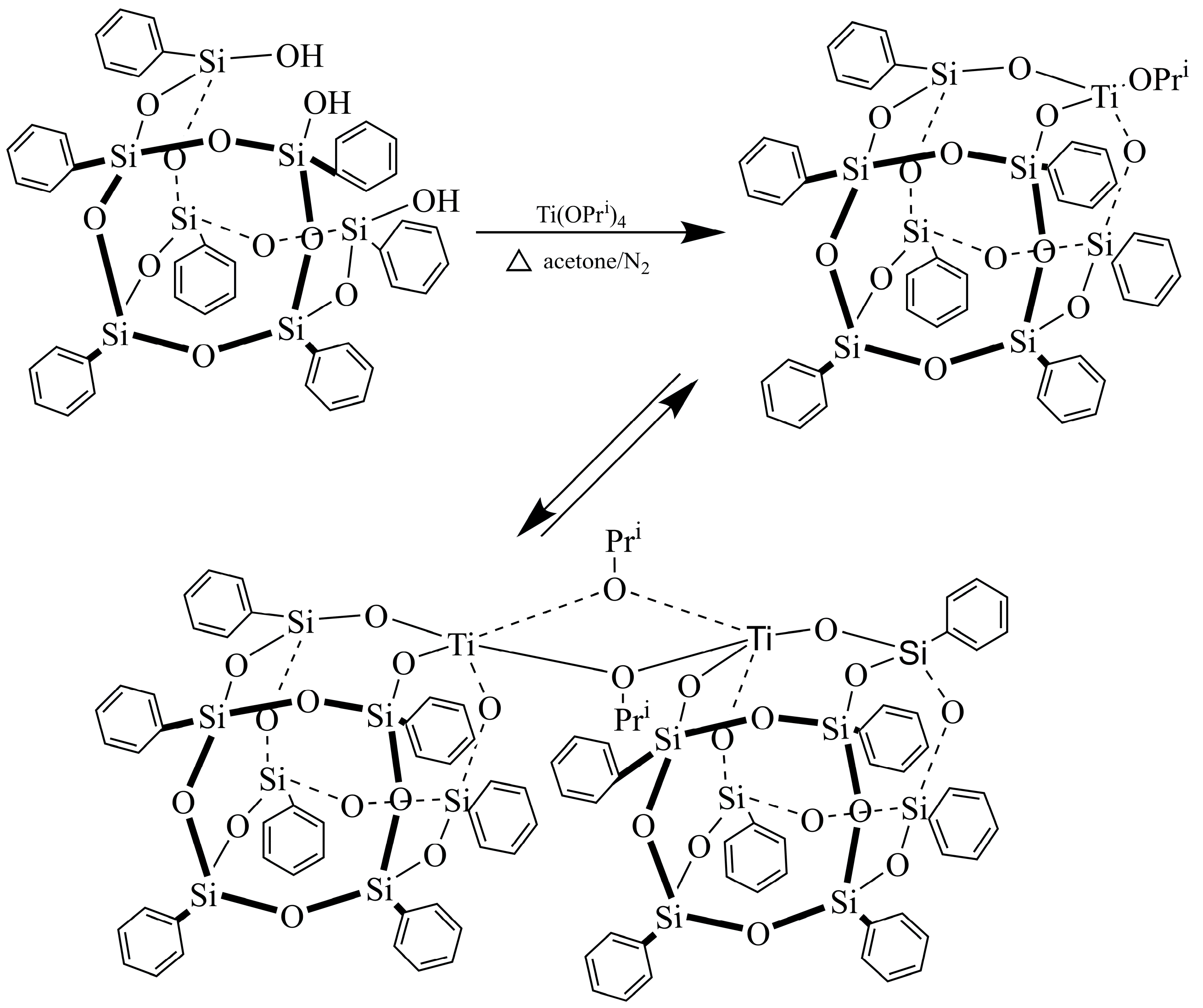
3.2. Syntheses of Trisilanolphenyl-POSS Titanium (Ti-Ph-POSS)
3.3. Preparation of Ti-Ph-POSS/PBZ Composites
3.3.1. Ti-Ph-POSS/PBZ Composites
3.3.2. Ti-Ph-POSS/pC-a Composites
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, R.; Wang, L.; Yu, D. Polybenzoxazine-POSS Nanocomposites. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 517–540. [Google Scholar]
- Hanssen, R.W.; Van Santen, R.A.; Abbenhuis, H.C. The dynamic status quo of polyhedral silsesquioxane coordination chemistry. Eur. J. Inorg. Chem. 2004, 2004, 675–683. [Google Scholar] [CrossRef]
- Monticelli, O.; Zunino, E.; Carniato, F.; Boccaleri, E.; Marchese, L.; Chincarini, A. Novel polymer nanocomposites based on polystyrene and Ti-functionalized polyhedral silsesquioxanes. Polym. Adv. Technol. 2010, 21, 848–853. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chen, Z.-Y.; Ba, Y.; Wang, B.; Chen, G.; Fang, X.; Kuo, S.-W. Double-decker-shaped polyhedral silsesquioxanes reinforced epoxy/bismaleimide hybrids featuring high thermal stability. Polymers 2022, 14, 2380. [Google Scholar] [CrossRef]
- Devaraju, S.; Krishnadevi, K.; Naveena, E.; Alagar, M. Design and development of polybenzoxazine-POSS hybrid materials from renewable starting materials for low k and low surface free energy applications. Mater. Res. Express 2019, 6, 104007. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Lin, Y.-C.; Kuo, S.-W. Highly thermally stable, transparent, and flexible polybenzoxazine nanocomposites by combination of double-decker-shaped polyhedral silsesquioxanes and polydimethylsiloxane. Macromolecules 2017, 50, 5739–5747. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Fu, Q.; Zhang, Q.; Xu, R. Synthesis of a novel bifunctional epoxy double-decker silsesquioxane: Improvement of the thermal stability and dielectric properties of polybenzoxazine. Polymers 2022, 14, 5154. [Google Scholar] [CrossRef]
- Niu, Z.; Tian, D.; Yan, L.; Ma, X.; Zhang, C.; Hou, X. Synthesis of 3,13-diglycidyloxypropyloctaphenyl double-decker polyhedral oligomeric silsesquioxane and the thermal reaction properties with thermosetting phenol-formaldehyde resin. J. Appl. Polym. Sci. 2020, 137, 49376. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Zhang, W.; He, J.; Yang, R.; Huang, J.; Zhang, X.; Zhang, W. Thermal protection materials based on epoxy-containing and phenyl-containing polyhedral oligomeric silsesquioxane modified phenolic resin and phenolic resin/carbon fiber composites. Polym. Adv. Technol. 2023, 34, 2198–2212. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Hreczycho, G. Highly efficient catalytic route for the synthesis of functionalized silsesquioxanes. Inorg. Chem. 2017, 56, 9337–9342. [Google Scholar] [CrossRef]
- Carniato, F.; Boccaleri, E.; Marchese, L. A versatile route to bifunctionalized silsesquioxane (POSS): Synthesis and characterisation of Ti-containing aminopropylisobutyl-POSS. Dalton Trans. 2008, 36–39. [Google Scholar] [CrossRef]
- Carniato, F.; Boccaleri, E.; Marchese, L.; Fina, A.; Tabuani, D.; Camino, G. Synthesis and characterisation of metal isobutylsilsesquioxanes and their role as inorganic–organic nanoadditives for enhancing polymer thermal stability. Eur. J. Inorg. Chem. 2007, 2007, 585–591. [Google Scholar] [CrossRef]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Ouyang, J.; Haotian, S.; Liang, Y.; Commisso, A.; Li, D.; Xu, R.; Yu, D. Recent Progress in Metal-containing Silsesquioxanes: Preparation and Application. Curr. Org. Chem. 2017, 21, 2829–2848. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Pan, Y.-T.; Yang, R. Facile synthesis of transition metal containing polyhedral oligomeric silsesquioxane complexes with mesoporous structures and their applications in reducing fire hazards, enhancing mechanical and dielectric properties of epoxy composites. J. Hazard. Mater. 2021, 401, 123439. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, X.; Jiang, Y.; Qiao, L.; Zhang, W.; Pan, Y.-T.; Yang, R.; Li, J.; Li, Y. Controllable dimensions and regular geometric architectures from self-assembly of lithium-containing polyhedral oligomeric silsesquioxane: Build for enhancing the fire safety of epoxy resin. Compos. Part B Eng. 2022, 229, 109483. [Google Scholar] [CrossRef]
- Zeng, B.; Hu, R.; Zhou, R.; Shen, H.; Liu, X.; Chen, G.; Xu, Y.; Yuan, C.; Luo, W.; Dai, L. Co-flame retarding effect of ethanolamine modified titanium-containing polyhedral oligomeric silsesquioxanes in epoxy resin. Appl. Organomet. Chem. 2020, 34, e5266. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, L.; Zeng, B.; Yi, X.; Huang, C.; Du, K.; Liu, X.; Xu, Y.; Yuan, C.; Dai, L. Diblock Copolymers Containing Titanium-Hybridized Polyhedral Oligomeric Silsesquioxane Used as a Macromolecular Flame Retardant for Epoxy Resin. Polymers 2022, 14, 1708. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Zhang, W.; Yang, R.; Li, J. Fabrication of eco-friendly and multifunctional sodium-containing polyhedral oligomeric silsesquioxane and its flame retardancy on epoxy resin. Compos. Part B Eng. 2020, 191, 107961. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.; Tabuani, D.; Camino, G. Metal functionalized POSS as fire retardants in polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Chmielewska, D.; Sterzyński, T.; Dudziec, B. Epoxy compositions cured with aluminosilsesquioxanes: Thermomechanical properties. J. Appl. Polym. Sci. 2014, 131, 40672. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene metal functionalised POSS nanocomposites: A study by thermogravimetric analysis. Polym. Degrad. Stab. 2006, 91, 1064–1070. [Google Scholar] [CrossRef]
- Hoyos, M.; Fina, A.; Carniato, F.; Prato, M.; Monticelli, O. Novel hybrid systems based on poly (propylene-g-maleic anhydride) and Ti-POSS by direct reactive blending. Polym. Degrad. Stab. 2011, 96, 1793–1798. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, B.; Chen, J.; Wu, T.; Li, Y.; Liu, Y.; Dai, L. An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Carniato, F.; Fina, A.; Tabuani, D.; Boccaleri, E. Polypropylene containing Ti-and Al-polyhedral oligomeric silsesquioxanes: Crystallization process and thermal properties. Nanotechnology 2008, 19, 475701. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Mechanistic studies on ring-opening polymerization of benzoxazines: A mechanistically based catalyst design. Macromolecules 2011, 44, 4616–4622. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, M.; Wang, L.; Lu, X.; Xin, Z. Preparation of bio-based polybenzoxazine/pyrogallol/polyhedral oligomeric silsesquioxane nanocomposites: Low dielectric constant and low curing temperature. Macromol. Mater. Eng. 2022, 307, 2100747. [Google Scholar] [CrossRef]
- Lochab, B.; Monisha, M.; Amarnath, N.; Sharma, P.; Mukherjee, S.; Ishida, H. Review on the Accelerated and low-temperature polymerization of benzoxazine resins: Addition polymerizable sustainable polymers. Polymers 2021, 13, 1260. [Google Scholar] [CrossRef]
- Ye, J.; Fan, Z.; Zhang, S.; Liu, X. Improved curing reactivity, thermal resistance and mechanical properties of furylamine-based benzoxazine using melamine as an amine source. Polym. Adv. Technol. 2023, 34, 1253–1264. [Google Scholar] [CrossRef]
- Wang, L.; Du, W.; Wu, Y.; Xu, R.; Yu, D. Synthesis and characterizations of a latent polyhedral oligomeric silsequioxane-containing catalyst and its application in polybenzoxazine resin. J. Appl. Polym. Sci. 2012, 126, 150–155. [Google Scholar] [CrossRef]
- Wang, G.; Yu, L.; Mei, Z.; Xin, J.; Pan, W.; Wang, Q. Curing mechanism and kinetics of benzoxazine co-catalyzed by transition metal salt and phenolic resin. Thermochim. Acta 2022, 710, 179182. [Google Scholar]
- Yan, H.; Hu, J.; Wang, H.; Zhan, Z.; Cheng, J.; Fang, Z. Effect of acetylacetone metal salts on curing mechanism and thermal stability of polybenzoxazine. High Perform. Polym. 2020, 32, 953–962. [Google Scholar] [CrossRef]
- Coban, Z.G.; Yagci, Y.; Kiskan, B. Catalyzing the Ring-Opening Polymerization of 1,3-Benzoxazines via Thioamide from Renewable Sources. ACS Appl. Polym. Mater. 2021, 3, 4203–4212. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, N.; Maurya, S.K.; Avecilla, F. Influence of coordination geometry on the ring opening of benzoxazine: Oxidovanadium (V), dioxidomolybdenum (VI) and dioxidotungsten (VI) complexes of benzoxazine based ligands and their bio inspired catalytic applications. Eur. J. Inorg. Chem. 2023, 26, e202200536. [Google Scholar] [CrossRef]
- Ai, T.; Zhang, Y.; Zhong, D.; Yang, Z.; Zong, J.; Niu, Y. Hybrids from silazane and allyl-containing benzoxazine for low-curing temperature and high-flame retardance. J. Appl. Polym. Sci. 2022, 139, 51801. [Google Scholar] [CrossRef]
- Deliballi, Z.; Kiskan, B.; Yagci, Y. Catalyzing benzoxazine polymerization with borohydrides to reduce the cure temperature and coloring. Eur. Polym. J. 2022, 181, 111679. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, G.; Lu, K.; Wang, J.; Ji, H.; Wang, Z.; Yang, Z.; Yang, Y.; Xiao, Y.; Ding, F. Polybenzoxazine aerogels created in Lewis acid catalysis for thermal insulation matrix in deep space exploration. Eur. Polym. J. 2022, 178, 111491. [Google Scholar] [CrossRef]
- Yang, W.; Xie, Y.; Chen, J.; Huang, C.; Xu, Y.; Lin, Y. Metal Ion-Catalyzed Low-Temperature Curing of Urushiol-Based Polybenzoxazine. Front. Chem. 2022, 10, 879605. [Google Scholar] [CrossRef]
- Varga, V.; Hodík, T.; Lamač, M.; Horáček, M.; Zukal, A.; Žilková, N.; Parker, W.O., Jr.; Pinkas, J. Homogeneous and heterogeneous cyclopentadienyl-arene titanium catalysts for selective ethylene trimerization to 1-hexene. J. Organomet. Chem. 2015, 777, 57–66. [Google Scholar] [CrossRef]
- Aish, E.H. Synthesis and Catalytic Applications of Chemically Grafted SiH-Functionalized Tripodal Ti-POSS Complexes in Crosslinked Hyperbranched Poly (siloxysilane). Aust. J. Chem. 2015, 68, 1091–1101. [Google Scholar] [CrossRef]
- Carniato, F.; Bisio, C.; Sordelli, L.; Gavrilova, E.; Guidotti, M. Ti-POSS covalently immobilized onto mesoporous silica: A model for active sites in heterogeneous catalytic epoxidation. Inorg. Chim. Acta 2012, 380, 244–251. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like metallasilsesquioxanes in catalysis: A review. J. Mol. Catal. A Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Wada, K.; Sakugawa, S.; Inoue, M. Facile preparation of silica-supported Ti catalysts effective for the epoxidation of cyclooctene using Ti-bridged silsesquioxanes. Chem. Commun. 2012, 48, 7991–7993. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Tembe, G.L.; Parikh, P.A.; Mehta, G.N. Catalytic ethylene polymerization by the titanium-polyhedral oligomeric silsesquioxane-Et 3 Al 2 Cl 3 system. React. Kinet. Mech. Catal. 2011, 104, 369–375. [Google Scholar] [CrossRef]
- Monticelli, O.; Cavallo, D.; Bocchini, S.; Frache, A.; Carniato, F.; Tonelotto, A. A novel use of Ti-POSS as initiator of l-lactide ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4794–4799. [Google Scholar] [CrossRef]
- Komagata, Y.; Iimura, T.; Shima, N.; Kudo, T. A Theoretical Study of the Insertion of Atoms and Ions into Titanosilsequioxane (Ti-POSS) in Comparison with POSS. Int. J. Polym. Sci. 2012, 2012, 391325. [Google Scholar] [CrossRef]
- Cozza, E.S.; Bruzzo, V.; Carniato, F.; Marsano, E.; Monticelli, O. On a novel catalytic system based on electrospun nanofibers and M-POSS. ACS Appl. Mater. Interfaces 2012, 4, 604–607. [Google Scholar] [CrossRef]
- Ullah, A.; Alongi, J.; Russo, S. Recent findings in (Ti) POSS-based polymer systems. Polym. Bull. 2011, 67, 1169–1183. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; Yu, D. Multiwalled carbon nanotube/polybenzoxazine nanocomposites: Preparation, characterization and properties. Polymer 2006, 47, 7711–7719. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Catalyst effects on the ring-opening polymerization of 1,3-benzoxazine and on the polymer structure. Polymer 2013, 54, 2873–2878. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Kuo, S.-W. Synthesis and characterization of polyhedral oligomeric silsesquioxane (POSS) with multifunctional benzoxazine groups through click chemistry. Polymer 2010, 51, 3948–3955. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Klunduk, M.C.; Martin, C.M.; Shephard, D.S.; Johnson, B.F.; Thomas, J.M. Modelling the active sites of heterogeneous titanium-centred epoxidation catalysts with soluble silsesquioxane analogues. Chem. Commun. 1997, 1847–1848. [Google Scholar] [CrossRef]

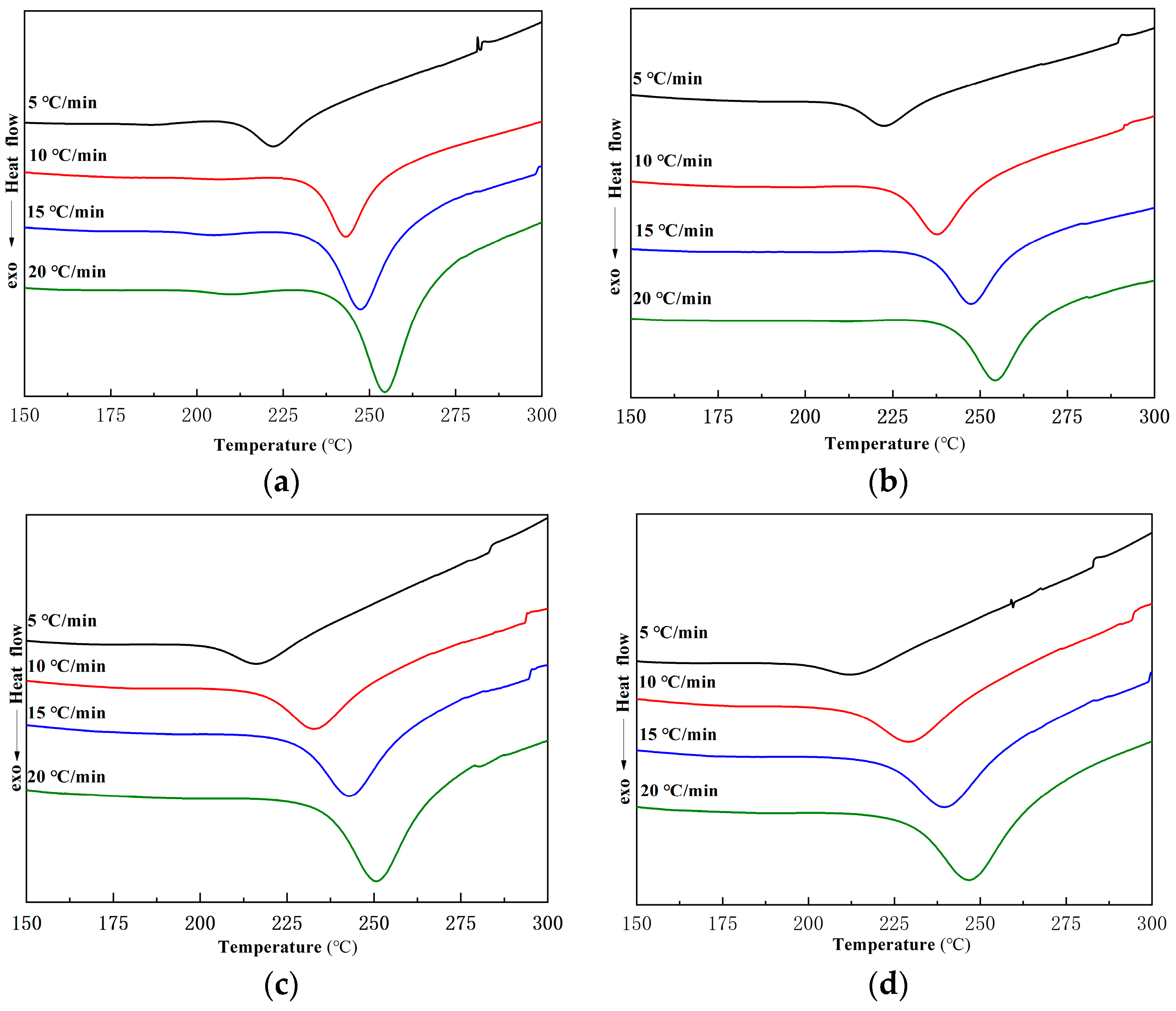


| Ti-Ph-POSS (wt%) | β (K/min) | ||||
|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | ||
| Ti (°C) | 0.5 | 208.04 | 232.23 | 233.70 | 240.88 |
| 1 | 209.54 | 223.7 | 233.96 | 240.88 | |
| 2 | 199.45 | 215.51 | 226.09 | 234.50 | |
| 3 | 191.33 | 207.43 | 219.20 | 226.85 | |
| TP (°C) | 0.5 | 222.46 | 243.23 | 247.52 | 254.45 |
| 1 | 222.93 | 237.82 | 247.51 | 254.44 | |
| 2 | 217.22 | 233.08 | 243.10 | 250.83 | |
| 3 | 214.131 | 230.161 | 240.172 | 268.012 | |
| Tf (°C) | 0.5 | 239.32 | 254.87 | 261.92 | 268.50 |
| 1 | 239.11 | 253.76 | 261.82 | 268.09 | |
| 2 | 235.43 | 253.38 | 262.16 | 267.90 | |
| 3 | 239.58 | 256.54 | 262.39 | 267.77 | |
| Ti-Ph-POSS (wt%) | Ea (kJ/mol) | n | A |
|---|---|---|---|
| 0.5 | 84.33 | 1.22 | 1.53 × 108 |
| 1 | 87.16 | 1.22 | 3.23 × 108 |
| 2 | 79.95 | 1.14 | 6.6 × 107 |
| 3 | 80.33 | 1.07 | 8.29 × 107 |
| The Content of Ti-Ph-POSS (wt%) | Tg (°C) | E′ (Pa) × 109 (100 °C) |
|---|---|---|
| 0 | 168.8 | 1.39 |
| 0.5 | 193.4 | 1.92 |
| 1 | 198.7 | 1.67 |
| 2 | 200.5 | 1.61 |
| 3 | 203.5 | 1.51 |
| The Content of Ti-Ph-POSS (wt%) | Td (°C) | Yc (%) |
|---|---|---|
| 0 | 322.6 | 30.3 |
| 0.5 | 344.8 | 37.3 |
| 1 | 352.1 | 37.8 |
| 2 | 352.9 | 35.1 |
| 3 | 353.7 | 35.4 |
| 100 | 442.0 | 55.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Fu, Q.; Dai, P.; Zhang, C.; Xu, R. Catalyzing Benzoxazine Polymerization with Titanium-Containing POSS to Reduce the Curing Temperature and Improve Thermal Stability. Molecules 2023, 28, 5450. https://doi.org/10.3390/molecules28145450
Sun X, Fu Q, Dai P, Zhang C, Xu R. Catalyzing Benzoxazine Polymerization with Titanium-Containing POSS to Reduce the Curing Temperature and Improve Thermal Stability. Molecules. 2023; 28(14):5450. https://doi.org/10.3390/molecules28145450
Chicago/Turabian StyleSun, Xiaoyi, Qixuan Fu, Pei Dai, Caili Zhang, and Riwei Xu. 2023. "Catalyzing Benzoxazine Polymerization with Titanium-Containing POSS to Reduce the Curing Temperature and Improve Thermal Stability" Molecules 28, no. 14: 5450. https://doi.org/10.3390/molecules28145450
APA StyleSun, X., Fu, Q., Dai, P., Zhang, C., & Xu, R. (2023). Catalyzing Benzoxazine Polymerization with Titanium-Containing POSS to Reduce the Curing Temperature and Improve Thermal Stability. Molecules, 28(14), 5450. https://doi.org/10.3390/molecules28145450






