Solid-Phase Synthesis of 2-Benzothiazolyl and 2-(Aminophenyl)benzothiazolyl Amino Acids and Peptides
Abstract
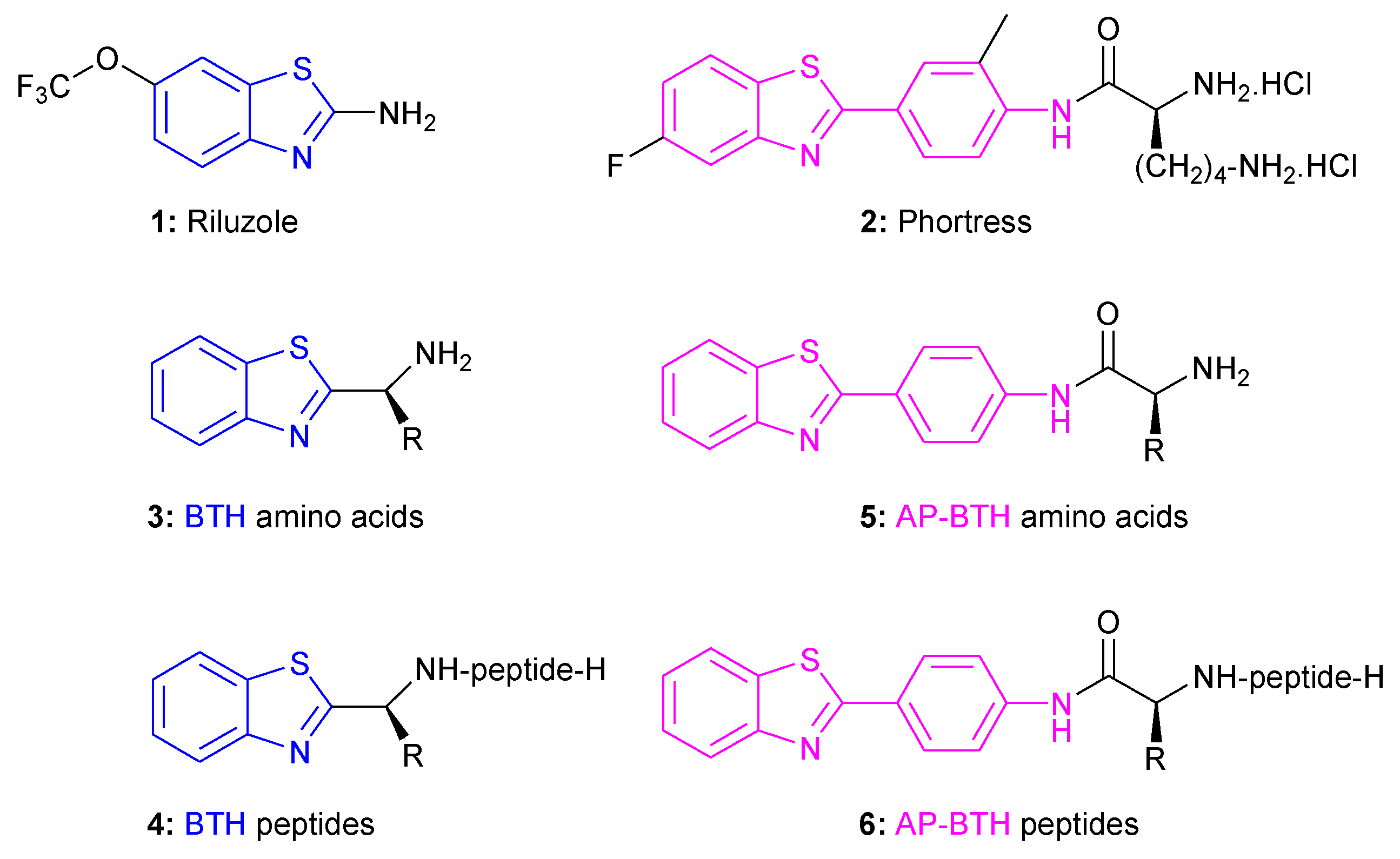
1. Introduction
2. Results and Discussion
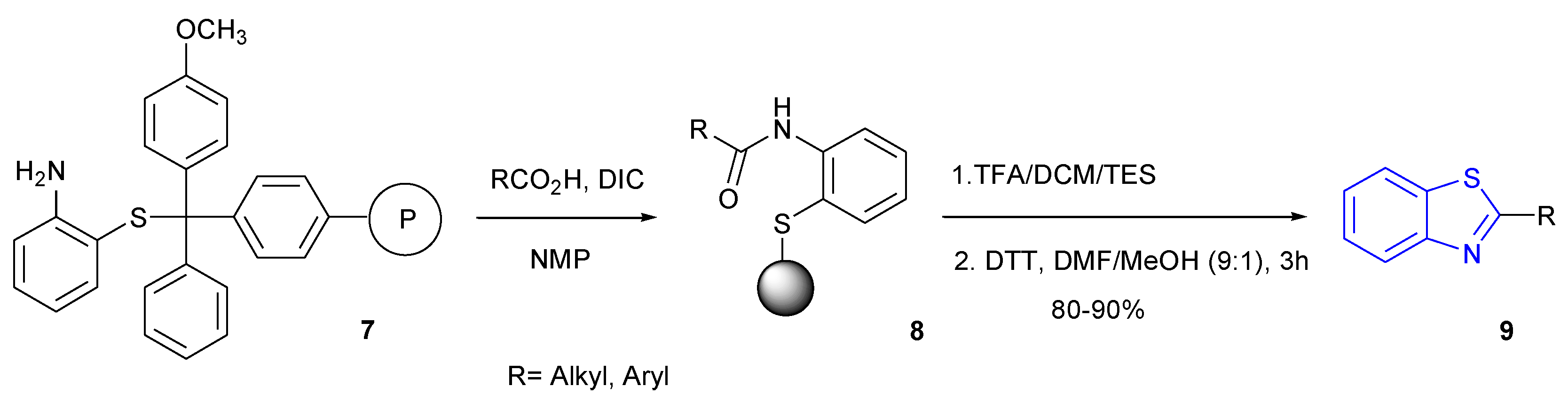
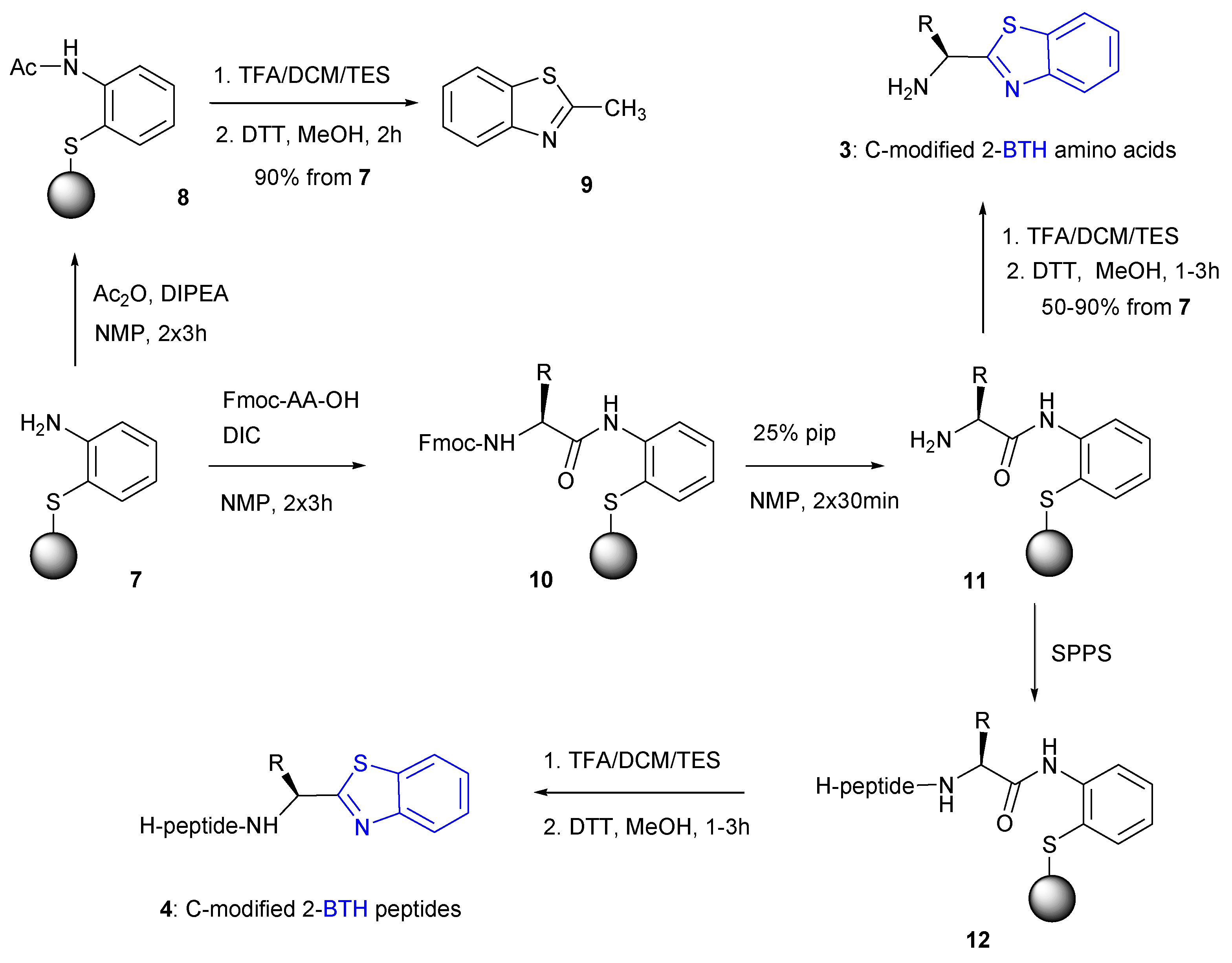
2.1. Synthesis of BTH-Amino Acids 3 and Peptides 4
2.1.1. General Method Analysis
2.1.2. Racemization during the First Fmoc-Amino Acid Coupling with Resin 7
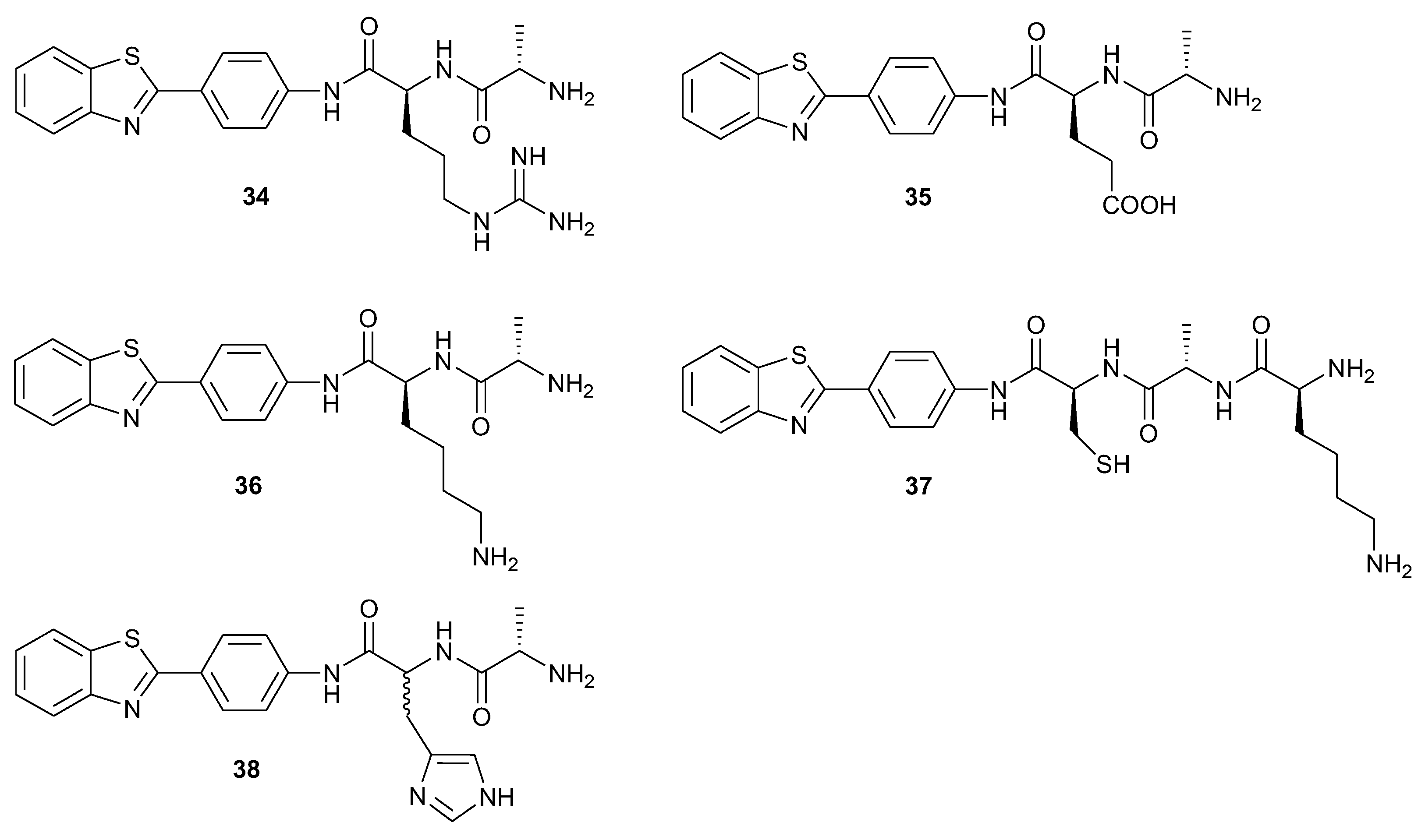
2.1.3. Applicability in the Solid-Phase Synthesis of BTH-Amino Acid Libraries and BTH-Peptides
2.2. Synthesis of AP-BTH-Amino Acids 5 and Peptides 6
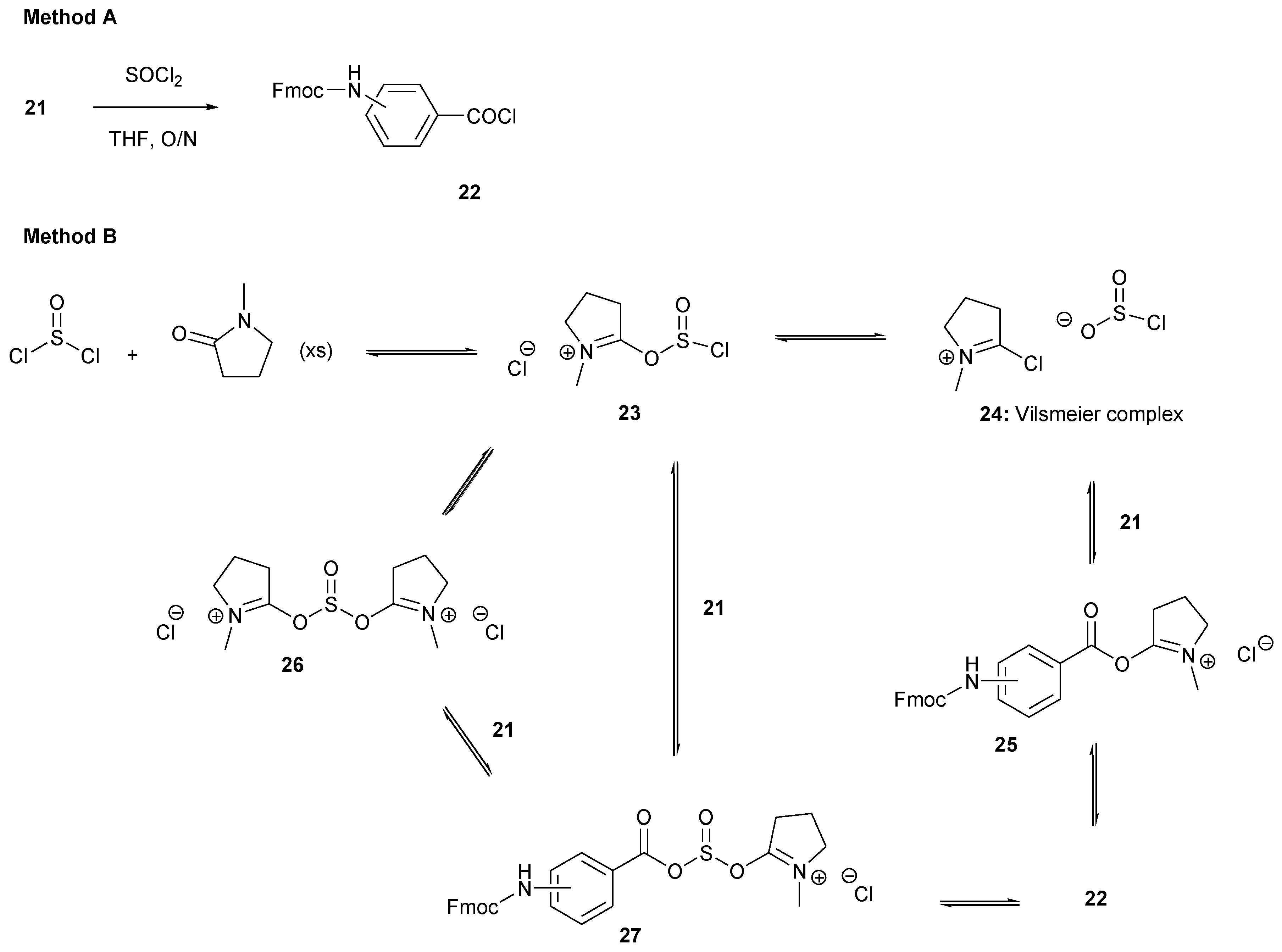
2.2.1. General Method—Coupling of Aminobenzoic Acids to Resin 7
2.2.2. Racemization during the First Fmoc-Amino Acid Coupling to Resin 30
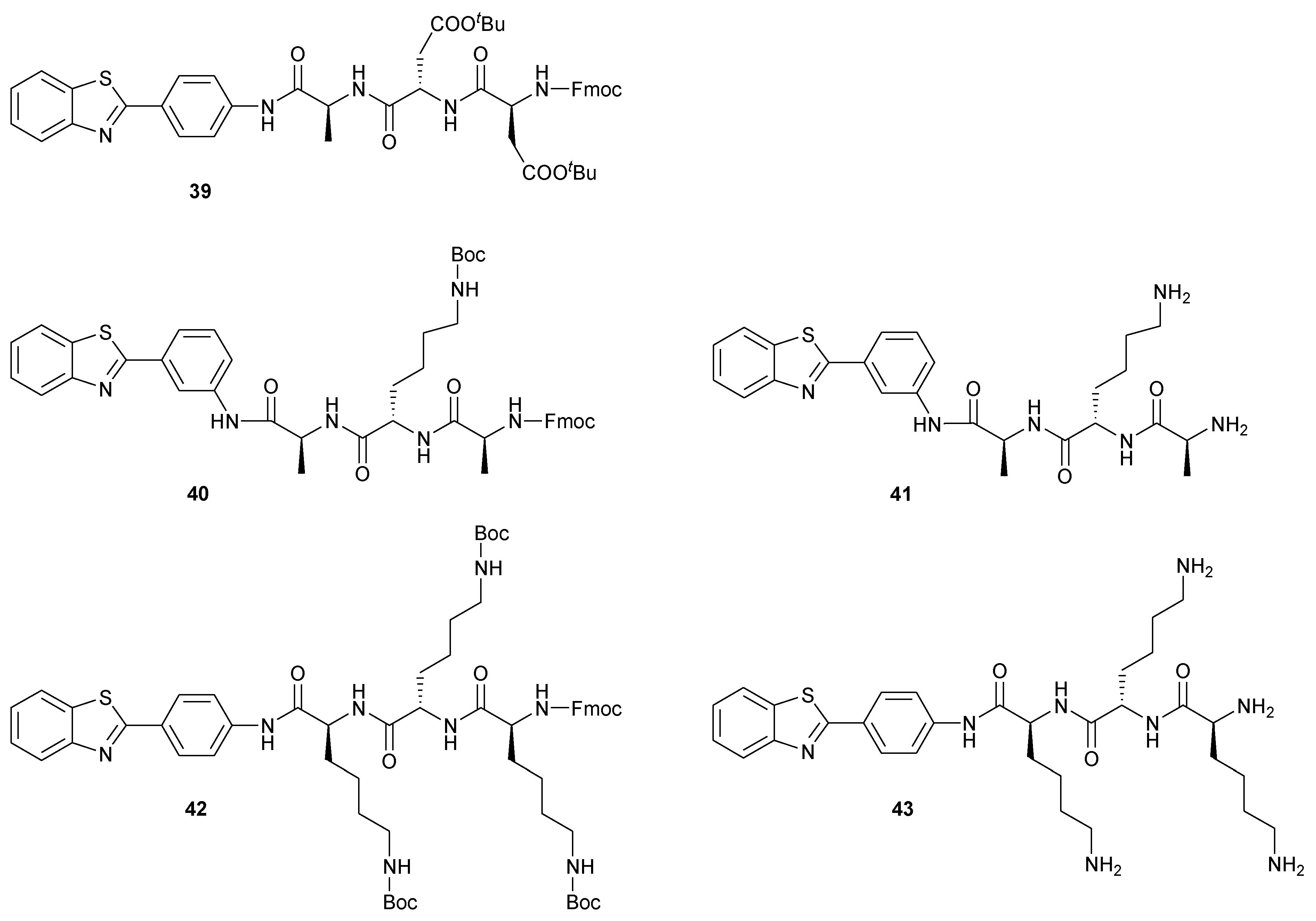
2.2.3. Applicability in the Solid-Phase Synthesis of AP-BTH Peptides
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthetic Procedures
3.3.1. Synthesis of Fmoc-4-aminobenzoic Acid 21a and Fmoc-3-aminobenzoic Acid 21b
3.3.2. Synthesis of 2-Aminobenzethiol-4-methoxytrityl Resin (7)
3.3.3. Solid-Phase Synthesis—General Protocols
3.3.3.1. Coupling of the First Fmoc-Amino Acid with Resins 7 and 30
3.3.3.2. Coupling of 21a/b with 2-Aminobenzethiol-4-methoxytrityl Resin (7)
3.3.3.3. Coupling of Fmoc-Amino Acids with HOBt/DIC—Peptide Assembly
3.3.3.4. Fmoc Removal during Solid-Phase Peptide Assembly
3.3.4. General Procedures for the Acidic Cleavage and Subsequent Cyclization to BTH-and AP-BTH-Amino Acids and Peptides
3.3.4.1. Cleavage and Cyclization into Side-Chain tBu-Protected/N-Terminus Fmoc-Protected BTH and AP-BTH Amino Acids and Peptides (9, 13, 14, 15, 16, 16a, 17, 18, 19, 20, 39, 40, 42)
- (a)
- The oily products (fully protected derivatives) were dissolved in MeOH and DTT (0.1–0.2 eq) was added, and the mixture was stirred for 1–3 h at rt to allow cyclization into BTH and AP-BTH amino acid/peptide derivatives (completeness of cyclization was monitored using HPLC analysis). Then, MeOH was removed and the oily product that was formed was washed with either DEE or a mixture of DEE/hexane (Hex) (or Hex), and the product was dried in vacuo.
- (b)
- The oily products (fully protected derivatives) were dissolved in MeOH (or NMP/MeOH 3:1) and DTT (0.1–0.2 eq) was added, to allow cyclization to BTH and AP-BTH amino acid/peptide derivatives for 1–3 h at rt (completeness of cyclization was monitored using HPLC analysis). Then, MeOH was concentrated and the resulting solution was extracted with water and EtOAc. The two phases were separated, and the aqueous phase was washed once more with EtOAc. The combined organic phases were washed twice with water and then dried with magnesium sulfate (MgSO4). The filtrates were condensed and the oily product that was formed was washed with either DEE or a mixture of DEE/Hex (or Hex), and the product was dried in vacuo.
3.3.4.2. Cleavage/Cyclization into Side-Chain tBu-Deprotected/Fmoc-Deprotected BTH and AP-BTH Amino Acids and Peptides (3a, 3b, 3c, 3d, 3e, 3f, 3g, 34, 35, 36, 37, 38, 41, 43)
- (a)
- The oily products (fully deprotected derivatives) were dissolved in MeOH and DTT (0.1–0.2 eq) was added, and the mixture was stirred for 1–3 h at rt to allow cyclization into BTH and AP-BTH amino acid/peptide derivatives (completeness of cyclization was monitored by HPLC analysis). Then, MeOH was removed and the oily product that was formed was washed with either DEE or a mixture of DEE/Hex (or Hex), and the product was dried in vacuo.
- (b)
- The oily products (fully deprotected derivatives) were dissolved in NMP/MeOH (2:1; 3:1) and DTT (0.1–0.2 eq) was added to allow cyclization into BTH and AP-BTH amino acid/peptide derivatives for 1–3 h at rt (completeness of cyclization was monitored using HPLC analysis). MeOH was then concentrated with a flash of nitrogen and the resulting solution was extracted with water and EtOAc. The two phases were separated, and the aqueous phase was washed twice with EtOAc. TLC analysis showed that all BTH and AP-BTH amino acids/peptides tested were collected in the aqueous phase, which was finally lyophilized to afford BTH/AP-BTH amino acid/peptides.
3.3.5. Synthesis of BTH-Amino Acid Library (9, 13, 14, 15, 16, 16a, 17)
3.3.6. Scale-Up Protocols
Synthesis of BTH-Peptides 18, 18a and 18b
Synthesis of 2-(4-Aminophenyl)benzothiazole (4-AP-BTH) (31a) and 2-(3-Aminophenyl)benzothiazole (3-AP-BTH) (31b)
Synthesis of AP-BTH Peptide 43
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zheng, X.J.; Li, C.S.; Cui, M.Y.; Song, Z.W.; Bai, X.Q.; Liang, C.W.; Zhang, T.Y. Synthesis, biological evaluation of benzothiazole derivatives bearing a 1,3,4-oxadiazole moiety as potential anti-oxidant and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2020, 30, 127237. [Google Scholar] [CrossRef] [PubMed]
- Ghonim, A.E.; Ligresti, A.; Rabbito, A.; Mahmoud, A.M.; Di Marzo, V.; Osman, N.; Abadi, A.H. Structure-activity relationships of thiazole and benzothiazole derivatives as selective cannabinoid CB2 agonists with in vivo anti-inflammatory properties. J. Med. Chem. 2019, 180, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Srivastava, V.K.; Saxena, K.K.; Goel, S.L. Synthesis of New Thiazolylthiazolidinylbenzothiazoles and Thiazolylazetidinylbenzothiazoles as Potential Insecticidal, Antifungal, and Antibacterial Agents. Arch. Pharm. 2006, 339, 466–472. [Google Scholar] [CrossRef]
- Asiri, Y.I.; Alsayari, A.; Muhsinah, A.B.; Mabkhot, Y.N.; Hassan, M.Z. Benzothiazoles as potential antiviral agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Zagaliotis, P.; Theodoroula, N.F.; Mystridis, G.A.; Vizirianakis, I.S.; Walsh, T.J.; Geronikaki, A. Thiazole/Thiadiazole/Benzothiazole Based Thiazolidin-4-One Derivatives as Potential Inhibitors of Main Protease of SARS-CoV-2. Molecules 2022, 27, 2180. [Google Scholar] [CrossRef]
- Kumar, K.R.; Karthik, K.N.S.; Begum, P.R.; Rao, C.M.M.P. Synthesis, characterization and biological evaluation of benzothiazole derivatives as potential antimicrobial and analgesic agents. Asian J. Pharm. Sci. 2017, 7, 115–119. [Google Scholar] [CrossRef]
- Mistry, B.M.; Patel, R.V.; Keum, Y.S.; Kim, D.H. Chrysin–benzothiazole conjugates as antioxidant and anticancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 5561–5565. [Google Scholar] [CrossRef]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free radical scavengers: An overview on heterocyclic advances and medicinal prospects. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef]
- Gawai, A.A.; Das, S.; Nemade, M. Synthesis, Preliminary Pharmacological and Acute Toxicity Studies of a New Series of 7-(2-(Benzo[d]thiazol-2-ylamino)ethoxy)-4-methyl-2H-chromen-2-one Derivatives with Atypical Antipsychotic Activity. Indian J. Pharm. Sci. 2019, 81, 241–248. [Google Scholar] [CrossRef]
- Murtuja, S.; Shaquiquzzaman, M.; Amir, M. Design, Synthesis, and screening of hybrid benzothiazolyl-oxadiazoles as anticonvulsant agents. Lett. Drug Des. Discov. 2018, 15, 398–405. [Google Scholar] [CrossRef]
- Bhutani, R.; Pathak, D.; Kapoor, G.; Husain, A.; Iqbal, M.A. Novel hybrids of benzothiazole-1,3,4-oxadiazole-4-thiazolidinone: Synthesis, in silico ADME study, molecular docking and in vivo anti-diabetic assessment. Bioorg. Chem. 2019, 83, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Pathak, A.; Rohilla, A.; Haider, M.R.; Ahmad, K.; Yar, M.S. Synthetic strategy and SAR studies of C-glucoside heteroaryls as SGLT2 inhibitor: A review. J. Med. Chem. 2019, 184, 111773. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Naqvi, S.A.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Pathak, N.; Rathi, E.; Kumar, N.; Kini, S.G.; Rao, C.M. A Review on Anticancer Potentials of Benzothiazole Derivatives. Mini-Rev. Med. Chem. 2020, 20, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Blyufer, A.; Lhamo, S.; Tam, C.; Tariq, I.; Thavornwatanayong, T.; Mahajan, S.S. Riluzole: A neuroprotective drug with potential as a novel anti-cancer agent (Review). Int. J. Oncol. 2021, 59, 95. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.A.; Jackson, C.E.; Heiman-Patterson, T.D.; Bettica, P.; Brooks, B.R.; Pioro, E.P. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.D.; Westwell, A.D. The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. Curr. Med. Chem. 2004, 11, 1009–1021. [Google Scholar] [CrossRef]
- Shi, D.F.; Bradshaw, T.D.; Wrigley, S.; McCall, C.J.; Lelieveld, P.; Fichtner, I.; Stevens, M.F. Antitumor benzothiazoles. 3. Synthesis of 2-(4-aminophenyl)benzothiazoles and evaluation of their activities against breast cancer cell lines in vitro and in vivo. J. Med. Chem. 1996, 39, 3375–3384. [Google Scholar] [CrossRef]
- Dubey, R.; Shrivastava, P.K.; Basniwal, P.K.; Bhattacharya, S.; Moorthy, N.S.H.N. 2-(4-aminophenyl) benzothiazole: A potent and selective pharmacophore with novel mechanistic action towards various tumour cell lines. Mini-Rev. Med. Chem. 2006, 6, 633–637. [Google Scholar] [CrossRef]
- Mylari, B.L.; Larson, E.R.; Beyer, T.A.; Zembrowski, W.J.; Aldinger, C.E.; Dee, M.F.; Siegel, T.W.; Singleton, D.H. Novel, potent aldose reductase inhibitors: 3,4-dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl] methyl]-1-phthalazineacetic acid (zopolrestat) and congeners. J. Med. Chem. 1991, 34, 108–122. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, H.; Zhang, L.; Zhao, Y.; Chen, S.; Chen, Y.; Peng, X.; Li, Q.; Yuan, M.; Hu, X. Zopolrestat as a human glyoxalase I inhibitor and its structural basis. Chem. Med. Chem. 2013, 8, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Abutaleb, N.S.; Elhassanny, A.E.M.; Nocentini, A.; Hewitt, C.S.; Elkashif, A.; Cooper, B.R.; Supuran, C.T.; Seleem, M.N.; Flaherty, D.P. Repurposing FDA-approved sulphonamide carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae. J. Enzym. Inhib. Med. Chem. 2022, 37, 51–61. [Google Scholar] [CrossRef]
- Sumit; Kumar, A.; Mishra, A.K. Advancement in Pharmacological Activities of Benzothiazole and its Derivatives: An Up to Date Review. Mini-Rev. Med. Chem. 2021, 21, 314–355. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Shrivastava, N.; Pathak, A.; Dewangan, R.P.; Yahya, S.; Yar, M.S. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Results Chem. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F.G. Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Mavroidi, B.; Marazioti, A.; Kannavou, M.; Sagnou, M.; Pelecanou, M.; Antimisiaris, S.G. Liposomes Decorated with 2-(4′-Aminophenyl)benzothiazole Effectively Inhibit Aβ1-42 Fibril Formation and Exhibit in Vitro Brain-Targeting Potential. Biomacromolecules 2020, 21, 4685–4698. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Xia, X.; He, W.Z.; Tang, Z.J.; Lv, Y.L.; Li, X.; Zhang, D.Y. Recent developments in benzothiazole-based iridium(Ⅲ) complexes for application in OLEDs as electrophosphorescent emitters. Org. Electron. 2019, 66, 126–135. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Kuznetsov, M.L.; Cherkasov, A.V.; Kryukov, D.M.; Bokach, N.A.; Kukushkin, V.Y. Metal-involved C⋯dz2-PtII tetrel bonding as a principal component of the stacking interaction between arenes and the platinum(ii) square-plane. Inorg. Chem. Front. 2023, 10, 3916–3928. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Kuznetsov, M.L.; Semenov, N.A.; Bokach, N.A.; Kukushkin, V.Y. A new look at the chalcogen bond: π-hole-based chalcogen (Se, Te) bonding which does not include a σ-hole interaction. Inorg. Chem. Front. 2023, 10, 3065–3081. [Google Scholar] [CrossRef]
- Song, Y.; He, Y.; Hu, L.; Cheng, Q.; Chen, Z.; Liu, R.; Zhu, S.; Zhua, H. Panchromatic luminescent D–π–A benzothiazoles with different π-bridging modulation: Design, synthesis and application in WLED devices. Mater. Chem. Front. 2023, 7, 2860–2870. [Google Scholar] [CrossRef]
- Kiritsis, C.; Mavroidi, B.; Shegani, A.; Palamaris, L.; Loudos, G.; Sagnou, M.; Pirmettis, I.; Papadopoulos, M.; Pelecanou, M. 2-(4′-Aminophenyl)benzothiazole Labeled with 99mTc-Cyclopentadienyl for Imaging β-Amyloid Plaques. ACS Med. Chem. Lett. 2017, 8, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Morfin, J.-F.; Lacerda, S.; Geraldes, C.F.G.C.; Tóth, É. Metal complexes for the visualisation of amyloid peptides. Sens. Diagn. 2022, 1, 627–647. [Google Scholar] [CrossRef]
- Sagnou, M.; Mavroidi, B.; Shegani, A.; Paravatou-Petsotas, M.; Raptopoulou, C.; Psycharis, V.; Pirmettis, I.; Papadopoulos, M.S.; Pelecanou, M. Remarkable Brain Penetration of Cyclopentadienyl M(CO)3+ (M = 99mTc, Re) Derivatives of Benzothiazole and Benzimidazole Paves the Way for Their Application as Diagnostic, with Single-Photon-Emission Computed Tomography (SPECT), and Therapeutic Agents for Alzheimer’s Disease. J. Med. Chem. 2019, 62, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cui, M. Recent progress in the development of metal complexes as β-amyloid imaging probes in the brain. MedChemComm 2017, 8, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Uzuegbunam, B.C.; Librizzi, D.; Yousefi, B.H. PET Radiopharmaceuticals for Alzheimer’s Disease and Parkinson’s Disease Diagnosis, the Current and Future Landscape. Molecules 2020, 25, 977. [Google Scholar] [CrossRef]
- Shainyan, B.A.; Zhilitskaya, L.V.; Yarosh, N.O. Synthetic Approaches to Biologically Active C-2-Substituted Benzothiazoles. Molecules 2022, 27, 2598. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Merrifield, B. Concept and early development of solid-phase peptide synthesis. Methods Enzym. 1997, 289, 3–13. [Google Scholar] [CrossRef]
- Prajapati, N.P.; Vekariya, R.H.; Borad, M.A.; Patel, H.D. Recent advances in the synthesis of 2-substituted benzothiazoles: A review. RSC Adv. 2014, 4, 60176–60208. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Z.B. A Review on Domino Condensation/Cyclization Reactions for the Synthesis of 2-Substituted 1,3-Benzothiazole Derivatives. Eur. J. Org. Chem. 2020, 4, 408–419. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Zuo, X.; Feng, X.; Gao, Y. Recent Advances in Synthesis of Benzothiazole Compounds Related to Green Chemistry. Molecules 2020, 25, 1675. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F.; Lloyd-Williams, P.; Giralt, E. Convergent solid-phase peptide synthesis. Methods Enzym. 1997, 289, 313–336. [Google Scholar] [CrossRef]
- Barlos, K.; Gatos, D. Convergent peptide synthesis. In Fmoc Solid Phase Peptide Synthesis—A Practical Approach; Chan, W.C., White, P.D., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 215–228. [Google Scholar] [CrossRef]
- Mourtas, S.; Gatos, D.; Barlos, K. Solid phase synthesis of benzothiazolyl compounds. Tetrahedron Lett. 2001, 42, 2201–2204. [Google Scholar] [CrossRef]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Conda-Sheridan, M.; Krishnaiah, M. Protecting Groups in Peptide Synthesis. In Methods in Molecular Biology; Hussein, W., Skwarczynski, M., Toth, I., Eds.; Humana: New York, NY, USA, 2020; Volume 2103, pp. 111–128. [Google Scholar] [CrossRef]
- Spears, R.J.; McMahona, C.; Chudasama, V. Cysteine protecting groups: Applications in peptide and protein science. Chem. Soc. Rev. 2021, 50, 11098–11155. [Google Scholar] [CrossRef]
- Caprino, L.A. 1-Hydroxy-7-Azabenzotriazole. An Efficient Peptide Coupling Additive. J. Am. Chem. Soc. 1993, 115, 4397–4398. [Google Scholar] [CrossRef]
- Subirós-Funosas, R.; El-Faham, A.; Albericio, F. PyOxP and PyOxB: The Oxyma-based novel family of phosphonium salts. Org. Biomol. Chem. 2010, 8, 3665–3673. [Google Scholar] [CrossRef]
- Barlos, K.; Gatos, D.; Hatzi, O.; Koch, N.; Koutsogianni, S. Synthesis of the very acid-sensitive Fmoc-Cys(Mmt)-OH and its application in solid-phase peptide synthesis. Int. J. Pept. Protein Res. 1996, 47, 148–153. [Google Scholar] [CrossRef]
- Mourtas, S.; Katakalou, C.; Nicolettou, A.; Tzavara, C.; Gatos, D.; Barlos, K. Resin-bound aminothiols: Synthesis and application. Tetrahedron Lett. 2003, 44, 179–182. [Google Scholar] [CrossRef]
- De Tar, D.F.; Silverstein, R. The mechanisms of the reactions of acetic acid with dicyclohexylcarbodiimide. J. Am. Chem. Soc. 1966, 88, 1013–1019. [Google Scholar] [CrossRef]
- Bosshard, H.H.; Mory, R.; Schmid, M.; Zollinger, H. Eine Method zur Katalysierten Herstellung von Carbonsaureund Sulfosaure-Chloriden mit Thionylchloride. Helv. Chim. Acta 1959, 42, 1653–1658. [Google Scholar] [CrossRef]
- Kimura, Y.; Matsuura, D. Novel Synthetic Method for the Vilsmeier-Haack Reagent and Green Routes to Acid Chlorides, Alkyl Formates, and Alkyl Chlorides. Int. J. Org. Chem. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Kumagai, T.; Anki, T.; Ebi, T.; Konishi, A.; Matsumoto, K.; Kurata, H.; Kubo, T.; Katsumoto, K.; Kitamura, C.; Kawase, T. An effective synthesis of N,N-dimethylamides from carboxylic acids and a new route from N,N-dimethylamides to 1,2-diaryl-1,2-diketones. Tetrahedron 2010, 66, 8968–8973. [Google Scholar] [CrossRef]
- Leggio, A.; Belsito, E.L.; De Luca, G.; Di Gioia, M.L.; Leotta, V.; Romio, E.; Siciliano, C.; Liguori, A. One-pot synthesis of amides from carboxylic acids activated using thionyl chloride. RSC Adv. 2016, 6, 34468–34475. [Google Scholar] [CrossRef]
- Higashi, F.; Nishi, T. Direct polyamidation with thionyl chloride in N-methyl-pyrrolidone. J. Polym. Sci. 1986, 24, 701–706. [Google Scholar] [CrossRef]
- Tortoioli, S.; Marchal, D.; Kesselgruber, M.; Pabst, T.; Skranc, W.; Abele, S. Short Synthesis of a Proline Amide Orexin Receptor Antagonist on the Pilot Plant Scale. Org. Process Res. Dev. 2014, 18, 1759–1762. [Google Scholar] [CrossRef]
- Dozeman, G.J.; Fiore, P.J.; Puls, T.P.; Walker, J.C. Chemical Development of a Pilot Scale Process for the ACAT Inhibitor 2,6-Diisopropylphenyl [(2,4,6-Triisopropylphenyl)acetyl]sulfamate. Org. Process Res. Dev. 1997, 1, 137–148. [Google Scholar] [CrossRef]
- Bremmer, S.C.; McNeil, A.J.; Soellner, M.B. Enzyme-triggered gelation: Targeting proteases with internal cleavage sites. Chem. Commun. 2014, 50, 1691–1693. [Google Scholar] [CrossRef]
- Seyler, H.; Kilbinger, A.F.M. Tuning the solubility of hepta(p-benzamide)s via the monomer sequence. Tetrahedron Lett. 2013, 54, 753–756. [Google Scholar] [CrossRef]




| AA1 | Rac% 1 | Rac% 2 | Rac% 3 | Rac% 4 |
|---|---|---|---|---|
| Leu | 0.25 | 0.14 | ||
| Ser(tBu) | 0.25 | <0.10 | ||
| Arg(Pbf) | 0.41 | 0.32 | ||
| Glu(tBu) | 1.13 | 0.98 | ||
| Lys(Boc) | 1.16 | 0.58 | ||
| Cys(Trt) | 1.89 | 2.22 | 1.32 | |
| His(Trt) | 44.9 | 40.2 | 7.64 | 24.9 |
| AA1 | Rac% 1 | Rac% 2 |
|---|---|---|
| Arg(Pbf) | 0.14 | |
| Glu(tBu) | 0.23 | |
| Lys(Boc) | <0.10 | |
| Cys(Trt) 3 | 1.08 | |
| His(Trt) | 44.5 | 7.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourtas, S.; Athanasopoulos, V.; Gatos, D.; Barlos, K. Solid-Phase Synthesis of 2-Benzothiazolyl and 2-(Aminophenyl)benzothiazolyl Amino Acids and Peptides. Molecules 2023, 28, 5412. https://doi.org/10.3390/molecules28145412
Mourtas S, Athanasopoulos V, Gatos D, Barlos K. Solid-Phase Synthesis of 2-Benzothiazolyl and 2-(Aminophenyl)benzothiazolyl Amino Acids and Peptides. Molecules. 2023; 28(14):5412. https://doi.org/10.3390/molecules28145412
Chicago/Turabian StyleMourtas, Spyridon, Vasileios Athanasopoulos, Dimitrios Gatos, and Kleomenis Barlos. 2023. "Solid-Phase Synthesis of 2-Benzothiazolyl and 2-(Aminophenyl)benzothiazolyl Amino Acids and Peptides" Molecules 28, no. 14: 5412. https://doi.org/10.3390/molecules28145412
APA StyleMourtas, S., Athanasopoulos, V., Gatos, D., & Barlos, K. (2023). Solid-Phase Synthesis of 2-Benzothiazolyl and 2-(Aminophenyl)benzothiazolyl Amino Acids and Peptides. Molecules, 28(14), 5412. https://doi.org/10.3390/molecules28145412







