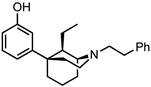
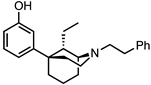
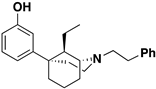
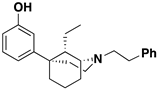
Abstract
The 5-(3-hydroxy)phenylmorphan structural class of compounds are unlike the classical morphinans, 4,5-epoxymorphinans, and 6,7-benzomorphans, in that they have an equatorially oriented aromatic ring rather than the axial orientation of that ring found in the classical opioids. This modified and simplified opioid-like structure has been shown to retain antinociceptive activity, depending on its stereochemistry and substituents, and some of them have been found to be much more potent than morphine. A simple C9-hydroxy-5-(3-hydroxy)phenylmorphan enantiomer was found to be about 500 times more potent than morphine in vivo. We have previously examined C9-alkenyl and hydroxyalkyl substituents in the N-phenethyl-5-(3-hydroxy)phenylmorphan class of compounds. Comparable C9-alkyl (methyl through butyl) substituents, with their sets of diastereomers, have not been explored. All these compounds have now been synthesized to determine the effect chain-length and stereochemistry at the C9 position in the molecule might have on their interaction with opioid receptors. We now report the synthesis and in vitro activity of 16 compounds, the C9-methyl, ethyl, propyl, and butyl diastereomers, using the inhibition of forskolin-induced cAMP accumulation assay. Several potent (sub-nanomolar and nanomolar) MOR compounds were found to be selective agonists with varying efficacy. Of greatest interest, a selective MOR antagonist was discovered; it did not display any DOR or KOR agonist activity in vitro, was three times more potent than naltrexone, and was found to antagonize the EC90 of fentanyl at MOR to a greater extent than naltrexone.
1. Introduction
The Center for Disease Control and Prevention (CDC) reported that more than 932,000 people have died since 1999 from a drug overdose. In 2020, opioids were involved in 68,630 overdose deaths (URL (accessed on 7 June 2023) https://www.cdc.gov/drugoverdose/deaths/index.html). According to the United States Department of Justice, the direct and indirect monetary cost of illicit drug use in the U.S. totaled around $193 billion in 2007. The cost in lives destroyed by the illicit use of drugs is immeasurable and, unfortunately, with increasingly potent analgesics reaching the market, those totals will only increase in future years. For these reasons, researchers have sought new types of analgesics as well as new and more selective and potent antagonists. Although no theory has succeeded in predicting the properties of a molecule that could act as a selective potent opioid antagonist, several theories have been developed that relate the properties of potential analgesics to their undesired side effects [1,2,3,4,5,6]. Some new types of analgesics (e.g., PZM21, SR17018, tramadol, tianeptine, oliceridine, Figure 1) have structures that appear to be quite different from the opioids used clinically (e.g., morphine, oxymorphone, and oxycodone (Figure 1)). Of these new compounds, only oliceridine has been approved for human use as an analgesic. It was shown to have somewhat fewer side effects than those associated with the opioids (the major opioid side effects are respiratory depression, gastrointestinal effects, tolerance, dependence, and OUD (opioid use disorder)).
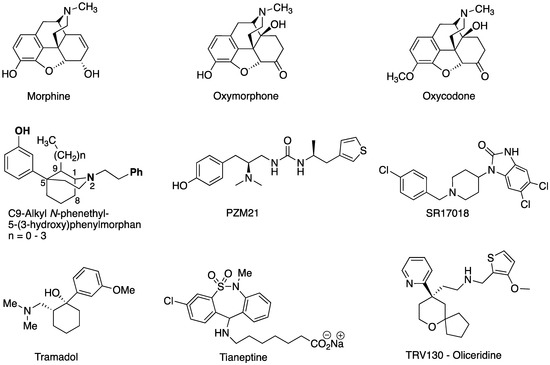
Figure 1.
Molecular structure of potential analgesics.
The 5-(3-hydroxy)phenylmorphan molecule (Figure 1) was designed [7] as a simplified form of morphine, but that simplification introduced major changes. Morphine, and all the classical 4,5-epoxymorphinan opioids, have a rigid aromatic phenyl ring axially oriented towards the piperidine ring, unlike the 5-phenylmorphans where the phenyl ring is detached from morphine’s B-ring and equatorially oriented towards the piperidine ring. The phenyl ring in the 5-phenylmorphans can rotate around a C-C bond, unlike the rigid phenyl ring in morphine. We have employed an N-phenethyl substituent, rather than N-methyl substituent, since it has been found to increase the potency of the molecule in the chiral C9-alkyl series; although in a racemic 5-phenylmorphan, it was initially found to decrease antinociceptive activity in mice [8].
Many other types of N-substituents have been noted in the literature [9,10]. We have used the N-phenethyl substituent for all of our C9-diastereomers, and we maintain that, initially, as a constant for comparison of their activity in vitro. In earlier work with the 5-phenylmorphans, we determined the effect of the enantiomers of a C9-hydroxyl group and a C9-methyl group in a few chiral N-phenethyl-substituted 5-phenylmorphans [11]. The 1R,5R,9S-OH compound and the 1R,5S,9R-methyl compound were found to have sub-nanomolar MOR affinity in a receptor binding assay. A later study with a 1S,5R,9R-propyl group at C9 in the phenylmorphans was also intriguing [12]. In an inhibition of forskolin-induced cAMP accumulation assay (cAMP assay), it acted as a partial agonist (EC50 (potency) = 1.42 nM; %Emax (efficacy) = 45%). Further testing in vivo has indicated that it was unlike morphine in its locomotor effects [13]. The C9-propyl compound appeared to have some antinociceptive activity, depending on the animal species used and the pain stimulus [13]. With our previous discovery that a chiral C9-methyl [11] and the C9-propyl [12] compound had interesting activity, we thought we should design compounds that would explore the complete 3-dimensional space around the C9 area of the 5-phenylmorphan, while maintaining the N-phenethyl substituent as a constant. A C9-substituent introduced a third chiral atom in the molecule. However, with one chiral atom (at C5) fixed, we would only need four diastereomers for every C9-substituent. To examine C9-alkyl substituents from methyl through butyl, we synthesized a total of 16 compounds. Three of these (two C9-methyl compounds and the C9-propyl) had previously been prepared [11,12], and they were reexamined in vitro for comparison with the novel compounds. We have, in this same manner, examined C9-alkenyl compounds [14] and hydroxyalkyl compounds and found interesting MOR partial agonists and potent MOR antagonists among them. The agonists and antagonists were distinguishable using molecular modeling [14]. The antagonists were found to interact with the inactive (4DKL) MOR crystal structures and agonists were found to interact with the active (6DDF) MOR crystal structures [14]. The determination that several C9-alkenyl compounds were of interest [15] gave us hope that we would find interesting alkyl compounds.
2. Results and Discussion
2.1. Synthesis of Diastereomeric C9-Alkyl-5-phenylmorphans
The synthesis of the C9 ethyl though butyl series (n = 1–3, Figure 1) of phenylmorphans was achieved through the route shown in Scheme 1. The racemic N-methyl compound 1 (Scheme 1) was synthesized from commercially available compounds following literature procedures [7,16]. Using the procedure of Hiebel et al. [11], optical resolution to separate the enantiomers of ±1 was achieved using tartaric acid, and the enantiomers were purified to >99% ee (as determined using 1H NMR). The tartrate salt of (1S,5S)-9-ketophenylmorpohan (1S, 5S-1) is shown in Scheme 1. For each enantiomer, the N-methyl substituent was replaced with an N-phenethyl moiety. Treatment of N-methyl compound 1 was with cyanogen bromide and subsequent hydrolysis with 3 N HCl yielded secondary amine 2 in high yield and purity. Further purification was unnecessary before alkylation. Treatment of secondary amine 2 with phenethyl bromide gave the alkylation product 3. Formation of alkylated product 3 gave a >60% yield over two steps. This procedure was scalable, and reactions were run on 1 g to 26 g scale without loss of yield.
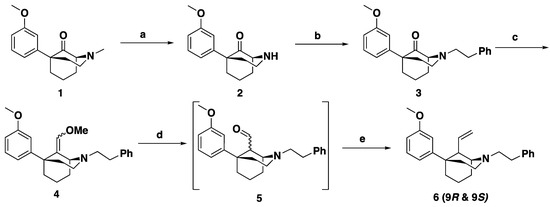
Scheme 1.
General route for the synthesis of the 9R and 9S-alkenyl intermediate (6). Reagents and conditions: (a) 1. CNBr, K2CO3, MeCN, reflux 4 h, 2. 3 N aq HCl, MeOH, reflux 16 h; (b) Ph(CH2)2Br, K2CO3, MeCN, reflux 16 h; (c) LiHMDS (methoxymethyl)triphenylphosphonium chloride, THF, 0 °C; (d) 6 N aq HCl; (e) Wittig conditions.
Functionalization of the ketone at the C9 position via the formation of enol ether 4 (Scheme 1) was achieved by a Wittig reaction of 3 with methoxymethyl triphenylphosphonium chloride and LiHMDS. Installation of the C9 stereocenter was accomplished by the hydrolysis of enol ether 4 with aqueous HCl. The concentration of HCl, reaction time, and quenching conditions affect the C9 R:S ratio, as noted previously [14]. Aldehyde 5 was used immediately upon formation without purification because it was unstable to silica gel column chromatography and not stable at room temperature for longer than a few hours. From the common intermediate 5, Wittig reactions using varying phosphonium salts (Scheme 2) were performed to obtain alkenes at C9 [14]. The Wittig reactions resulted in a mixture of epimers at the C9 position, which were not separable by silica gel column chromatography. The mixture of epimers was subject to O-demethylation conditions using BBr3 to form phenols 9–14 in 60–80% yields (Scheme 2). The phenol compounds 9–14 could be separated into their C9S and C9R diastereomers by column chromatography.
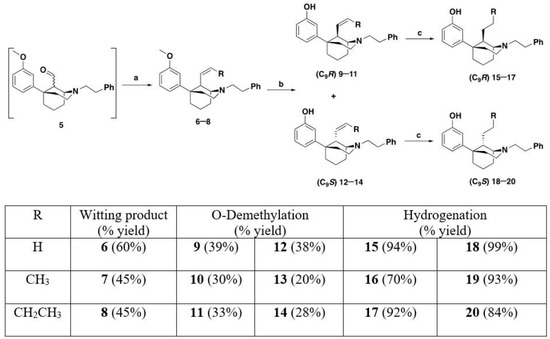
Scheme 2.
Formation and separation of C9R (15–17) and C9S (18–20) epimers. Reagents and conditions: (a) Wittig conditions; (b) BBr3, CH2Cl2, 0 °C–rt 4 h; (c) Pd/C, H2, HCl, 16 h.
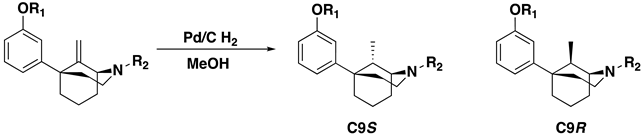
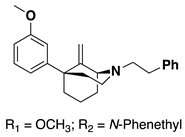
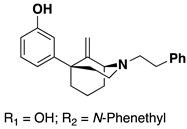
Alkenes 9–14 were hydrogenated in the presence of Pd/C (0.1 equiv) to give the desired alkyl compounds 15–20 (Scheme 2) in the 1S,5R series. The use of HCl salts of 9–14, or the treatment of the free base with one equivalent of HCl, for the hydrogenation reactions was necessary for high yielding reductions. When HCl was not employed, reaction yields dropped significantly. Alkyl compounds 15–20 were purified by column chromatography and isolated as oils. All the free base oils were treated with HCl in isopropanol to form the HCl salts as white solids, except for the C9 butyl compound 20, which was isolated as the tartrate salt.
The enantiomers (21–26) of compounds 15–20 were synthesized from the tartrate salt of (1R,5S)-5-(3-methoxyphenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-9-one (1R,5S–21, Scheme 3) by the same synthetic steps as in Scheme 1.
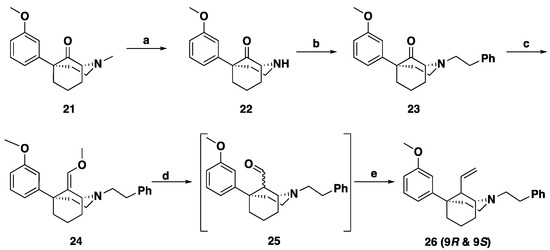
Scheme 3.
Synthesis of 1R,5S enantiomers (26, 9R & 9S). Reagents and conditions: (a) 1. CNBr, K2CO3, MeCN, reflux h, 2. 3 N aq HCl, MeOH, reflux 16 h; (b) Ph(CH2)2Br, K2CO3, MeCN, relux 16 h; (c) LiHMDS (methoxymethyl)triphenylphosphonium chloride, THF, 0 °C; (d) 6 N aq HCl; (e) Wittig conditions.
Using the same procedures described in Scheme 2, the Wittig reactions with aldehyde 25 (Scheme 4) yielded compounds 26–28 as a mixture of epimers at the C9 position that were not easily separable. O-Demethylation of 26–28 with BBr3 gave phenols 29–34, respectively. The phenolic compounds were separable by silica gel column chromatography and provided C9R and C9S compounds. Hydrogenation using Pd/C gave the final products 35–40.
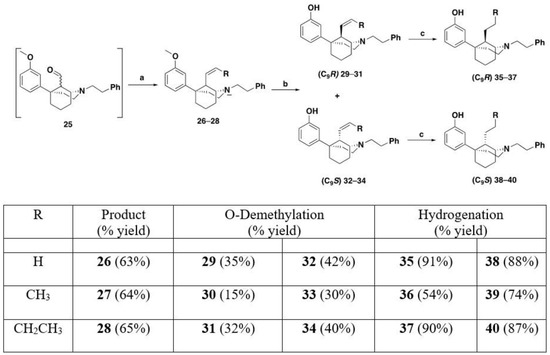
Scheme 4.
Synthesis of C9R (35–37) and C9S (38–40) epimers. Reagents and conditions: (a) Wittig conditions; (b) BBr3, CH2Cl2, 0 °C–rt 4 h; (c) H2, Pd/C, MeOH, 16 h.
The C9S-methyl compound (43) in the 1S,5R-series were synthesized (Scheme 5) from compound 3 using the literature procedure [11]. The alkylation product 3 olefination using Tebbe’s reagent gave alkene 41 in a 71% yield (Scheme 5). Reduction of 41 gave stereo-selectively the C9S-methyl 42 with only a small impurity of the C9R-methyl diastereomer. The final O-demethylated product 43 [11] was achieved by treating 42 with BBr3.
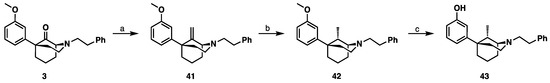
Scheme 5.
Resynthesis of 9S-methyl-1S,5R-5-phenylmorphan (43) [11]. Reagents and conditions: (a) Tebbe’s reagent, THF, 3 h; (b) H2, Pd/C, MeOH, quant; (c) BBr3, CH2Cl2, 4 h.
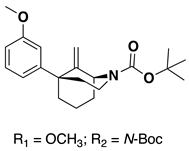
The C9R-methyl diastereomer (48, Scheme 6) had not been previously made as the literature method [11] selectively formed only the C9S-methyl epimer 43. To obtain the C9R-epimer, N-methyl phenylmorphan 1 (Scheme 6) was subjected to N-demethylation conditions followed by a Boc protection resulting in 44. Using a Tebbe’s olefination reaction to form the C9 methylene compound 45, followed by a hydrogenation reaction, resulted in a mixture of the desired C9R-stereochemistry in 46 and the C9S-epimer. Isolation and subsequent deprotection of the C9R isomer and the introduction of the phenethyl moiety resulted in compound 47. Finally, O-demethylation using standard conditions yielded the desired C9S-methyl compound 48.
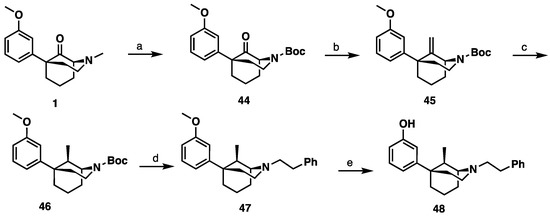
Scheme 6.
Synthesis of 9R-methyl-1S,5R-5-phenylmorphan (48). Reagents and conditions: (a) 1. BrCN, K2CO3, CH3CN, reflux, 2 h; 2. 3 M HCl, MeOH, reflux, 16 h; 3. Boc2O, Et3N, DMAP, MeOH, 1 h; (b) Tebbe’s reagent, THF, 16 h; (c) H2, Pd/C, MeOH; (d) 1. TFA, CH2Cl2, 1.5 h; 2. Phenethyl bromide, K2CO3, CH3CN, 16 h. (e) BBr3, CH2Cl2, 4 h.
The selectivity of hydrogenation could be shifted based on the substituent on the oxygen and nitrogen (Table 1). With a phenethyl group on nitrogen and a m-methoxy substituted aromatic ring, the C9S epimer is formed exclusively. After O-demethylation the same hydrogenation conditions result in a 5:1 ratio still favoring the C9S epimer. Alternatively, when a Boc group is exchanged for the phenethyl group on the nitrogen and a meta-methoxoyphenyl moiety is present the selectivity shifts to favor the C9R epimer in a 3:1 ratio. This observation was exploited to obtain all four diastereomers of the C9-methyl phenylmorphan (e.g., Scheme 5 for the C9R-methyl in the 1S,5R series using the starting material in reaction #3 in Table 1; the C9S epimer was obtained using the starting material in reaction 1 in Table 1).

Table 1.
Selectivity of hydrogenation reactions based on R1 and R2 groups.
Using the same procedures described in Scheme 5 and Scheme 6, the two C9-methyl diastereomers were obtained for the 1R,5S series (Scheme 7 and Scheme 8). The C9S and C9R-methyl compounds were differentiated based on the previously determined X-ray diffraction analyses of 51 [11].
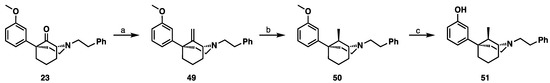
Scheme 7.
Synthesis of 9R-methyl-1R,5S-5-phenylmorphan (51). Reagents and conditions: (a) Tebbe’s reagent, THF, 3 h; (b) H2, Pd/C, MeOH, quantitative; (c) BBr3, CH2Cl2, 4 h.
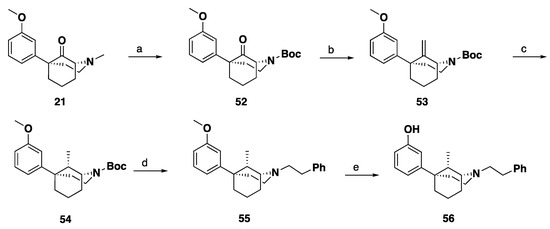
Scheme 8.
Synthesis of 9S-methyl-1R,5S-5-phenylmorphan (56). Reagents and conditions: (a) 1. Cyanogen bromide, K2CO3, CH3CN, HCl, 16 h; 2. Et3N, 4-dimethylaminopyridine, di-tert-butyl decarbonate, MeOH, 1 h; (b) Tebbe’s reagent, THF, 16 h; (c) H2, Pd/C, MeOH; (d) 1. TFA, CH2Cl2, 1.5 h; 2. Phenethyl bromide, K2CO3, CH3CN, 16 h. (e) BBr3, CH2Cl2, 4 h.
2.2. Forskolin-Induced cAMP Accumulation Assay for In Vitro Determination of the Potency and Efficacy of the Diastereomers
Compounds were examined for their functional activity using the inhibition of forskolin-induced cAMP accumulation assay (Table 2). Four diastereomers for each of the C9-alkyl compounds, C9-methyl, ethyl, propyl, and butyl were evaluated. All except three of these (compounds 43 [11], 51 [11] and 16 [12]) in Table 2 are novel, and the three formerly known compounds have been included and re-evaluated so that the data on the complete series could be compared.

Table 2.
Opioid Receptor Activity Measured in the Forskolin-induced cAMP Accumulation Assay a.
The C9-methyl series were of most interest in that one of them, 48, was a selective potent MOR antagonist (IC50 = 3.9 nM) with a high %Imax = 153%. It did not have DOR or KOR agonist activity, unlike naltrexone, which acts as a potent partial kappa agonist (KOR EC50 = 0.6 nM). The C9-methyl antagonist 48 was also found to antagonize the EC90 of fentanyl at MOR to a greater extent than naltrexone (%Imax = 153% vs 104% for naltrexone).
The enantiomer of 48, compound 56, was found to be a potent sub-nanomolar partial agonist (EC50 = 0.8 nM, %Emax = 39%), and the diastereomer of 56, compound 51, was a potent full agonist (EC50 = 1.89 nM, %Emax = 95.7%). As agonists, compounds 56 and 51 were found to be highly selective at MOR (EC50 > 10,000 nM for DOR and KOR). Whereas the reference MOR agonist morphine was less potent (EC50 = 5.8 nM) than the three compounds 48, 56, and 51, and was a partial agonist at DOR (EC50 = 525 nM, Emax = 75%) and KOR (EC50 = 346 nM, Emax = 86%). The remaining, previously described diastereomer 43 was the least potent partial C9-methyl agonist (EC50 = 16.1 nM, %Emax = 78%). These data exemplified our findings with diastereomers at C9 in the 5-phenylmorphan series and could have very different properties in vitro, and their activity was not predictable in silico. We previously determined that agonists and antagonist C9-substituted phenylmorphans could be distinguished using molecular modeling [14], but that partial and full agonists could not be differentiated.
The C9-ethyl diastereomers were all potent agonists: one set of these isomers, 15 and 38 were potent partial agonists ((EC50 = 1.4 nM and 4.9 nM), with weak efficacy (%Emax = 48% and 31%, respectively), and the other set of enantiomers, 18 and 35, were potent full agonists (EC50 = 3.4 nM and 1.0 nM, %Emax = 97% and 96%), respectively. Compound 35 was also found to be a low efficacy DOR partial agonist and to have weak antagonist activity at KOR. With the C9-propyl diastereomers, only one compound was a potent full agonist, 36. It had nanomolar potency (EC50 = 3.2 nM, %Emax = 94%). The other three C9-propyl compounds were partial agonists, compound 16 with very high potency and low efficacy (EC50 = 1.4 nM, %Emax = 46%), and 19 and 39 with lower potency (EC50 = 36 nM and 7.3 nM, respectively). The latter compound, 39, had very little efficacy (%Emax = 24%). The C9-butyl compounds 20, 37, and 40, were relatively inactive (EC50 = 53 (37) to 880 nM (20)), and only 17 had some potency (EC50 = 7.2 nM), as a partial agonist, but it had little efficacy (%Emax = 32%).
3. Materials and Methods
3.1. General Information
Melting points were determined on a Mettler Toledo MP70 and are uncorrected. Proton and carbon nuclear magnetic resonance (1H and 13C NMR) spectra (Figures S1–S12 in Supplementary Material) were recorded on a Varian Gemini-400 spectrometer in CDCl3 (unless otherwise noted) with the values given in ppm (TMS as internal standard) and J (Hz) assignments of 1H resonance coupling. The analyses were performed on the free base, unless otherwise noted. Mass spectra (HRMS) were recorded on a Waters (Mitford, MA, USA) Xevo-G X5 QTof. The optical rotation data were obtained on a PerkinElmer polarimeter model 341. Thin layer chromatography (TLC) analyses were carried out on Analtech silica gel GHLF 0.25 mm plates using various gradients of CHCl3/MeOH containing 1% NH4OH or gradients of EtOAc/n-hexane. Visualization was accomplished under UV light or by staining in an iodine chamber. Flash column chromatography was performed with Fluka silica gel 60 (mesh 220–400). Flash column chromatography was performed using RediSep Rf normal phase silica gel cartridges. Robertson Microlit Laboratories, Ledgewood, N.J., performed elemental analyses, and the results were within ±0.4% of the theoretical values. Experimental procedures for intermediates, and their characterization, can be found in a previous publication [14]. Compounds 16, 43, and 51 in Table 2 were discussed in previous publications [11,12].
3.2. Synthesis
General procedure for hydrogenation of C9-alkene to C9-alkane. The HCl salt of the alkene was dissolved in MeOH (10 mL) and was transferred to a Parr shaker degassed with argon. The solution was treated with palladium on carbon (Escat 103), and the Parr shaker was pressurized to 20 psi with H2 and was shaken at 23 °C for 16 h. Upon completion, the reaction mixture was filtered through Celite to remove the palladium and was washed several times with methanol. The filtrate was washed with saturated NaHCO3, dried, and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography 0–60% EtOAc: Hexanes.
3-((1S,5R,9R)-9-Ethyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (15). The HCl salt of alkene 9 (145 mg, 1 equiv, 378 µmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (4.02 mg, 0.1 equiv, 37.8 µmol) to give a white foam (125 mg, 95% yield). The HCl salt of 15 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from ethanol to give a white solid: mp 247–250 °C. 1H NMR (400 MHz; CD3OD): δ 7.39–7.33 (m, 4H), 7.32–7.26 (m, 1H), 7.18–7.15 (m, 1H), 6.80 (t, J = 5.6 Hz, 1H), 6.76 (s, 1H), 6.65 (dd, J = 8.0, 2.0 Hz, 1H), 3.93–3.92 (m, 1H), 3.73–3.51 (m, 3H), 3.44–3.35 (m, 1H), 3.25–3.17 (m, 1H), 3.06–2.98 (m, 1H), 2.51–2.39 (m, 2H), 2.31–2.21 (m, 2H), 2.11–1.99 (m, 3H), 1.90–1.82 (m, 2H), 1.48–1.40 (m, 1H), 1.29–1.23 (m, 1H), 1.19 (d, J = 7.0 Hz), and 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz; CD3OD): δ 158.8, 150.2, 137.5, 130.6, 130.0, 129.9, 128.4, 117.3, 114.2, 113.3, 56.5, 55.6, 51.7, 46.7, 42.0, 39.0, 31.5, 28.9, 25.1, 22.1, 19.2, and 12.0; HRMS-ESI (m/z): [M + H+] calcd for: C24H32NO 350.2484; found: 350.2487; Anal. Calcd. For C24H32ClNO·0.65 H2O: C, 72.49%; H, 8.44%; and N, 3.52%. Found C, 72.54%; H, 8.64%; N, 3.58%; 18.6° (c 0.4, CHCl3).
3-((1S,5R,9R)-9-Butyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (17). The HCl salt of alkene 11 (273 mg,1 equiv, 663 µmol) was was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (4.02 mg, 0.1 equiv, 37.8 µmol) to afford 17 as a white foam (230 mg, 92% yield). The HCl salt of 17 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from ethanol to give a white solid: mp 257–260 °C. 1H NMR (400 MHz; CD3OD): δ 7.39–7.34 (m, 4H), 7.32–7.27 (m, 1H), 7.19–7.15 (m, 1H), 6.81 (d, J = 8.0 Hz, 1H), 6.76 (s, 1H), 6.66 (dd, J = 8.0, 1.9 Hz, 1H), 3.88–3.86 (m, 1H), 3.73–3.65 (m, 1H), 3.57 (ddd, J = 18.9, 12.7, 6.4 Hz, 2H), 3.45–3.38 (m, 1H), 3.20 (dddd, J = 11.4, 6.1, 6.0, 5.4 Hz, 1H), 3.05–2.98 (m, 1H), 2.48–2.34 (m, 3H), 2.26–2.21 (m, 1H), 2.07–1.99 (m, 3H), 1.89–1.81 (m, 2H), 1.46–1.10 (m, 6H), 0.85 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz; CD3OD): δ 158.8, 150.2, 137.5, 130.6, 130.0, 129.9, 128.4, 117.3, 114.2, 113.4, 56.4, 56.4, 51.7, 44.9, 41.8, 38.9, 31.5, 30.7, 28.9, 26.1, 25.1, 23.5, 22.1, and 14.3. HRMS-ESI (m/z): [M + H+] calcd for: C26H36NO 378.2797; found 378.2796; C26H36ClNO·0.15 H2O calc. C: 74.94%; H: 8.78%; N: 3.36%; found C: 74.87%; H: 8.75%; N: 3.31%; and 23.4° (c 0.46, CHCl3).
3-((1S,5R,9S)-9-Ethyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (18). The HCl salt of alkene 12 (276 mg,1 equiv, 719 µmol) was dissolved in MeOH (15 mL) and was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (7.65 mg, 0.1 equiv, 71.9 µmol) to afford 18 as a white foam (249 mg, 99% yield). The HCl salt of 18 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from ethanol to give a white solid: mp 249–253 °C. 1H NMR (400 MHz; CD3OD): δ 7.39–7.34 (m, 4H), 7.32–7.26 (m, 1H), 7.17 (t, J = 8.0 Hz, 1H), 6.87 (d, J = 7.8 Hz, 1H), 6.85–6.81 (m, 1H), 6.66 (dd, J = 8.0, 2.2 Hz, 1H), 3.81 (s, 1H), 3.71–3.58 (m, 2H), 3.58–3.46 (m, 2H), 3.19–3.11 (m, 2H), 2.40–2.32 (m, 2H), 2.18–1.89 (m, 7H), 1.40–1.31 (m, 1H), 1.22–1.14 (m, 1H), and 0.86 (t, J = 7.5 Hz, 3H). 13C NMR (100 MHz; CD3OD): δ 158.8, 149.9, 137.8, 130.6, 130.02, 129.89, 128.3, 117.6, 114.3, 113.7, 59.1, 57.2, 51.1, 46.5, 39.6, 38.6, 31.9, 28.8, 20.8, 20.5, 17.3, and 12.0; HRMS-ESI (m/z): [M + H+] calcd for: C24H32NO 350.2484; found: 350.2487; Anal. Calcd. For C24H32ClNO·0.2 H2O: C, 73.99%; H, 8.38%; N, 3.60%. Found C, 73.61%; H, 7.98%; N, 3.51%; −22.2° (c 1.20, CHCl3).
3-((1S,5R,9S)-2-Phenethyl-9-propyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (19). The HCl salt of alkene 13 (425 mg,1 equiv, 1.07 mmol) was dissolved in MeOH (15 mL) and was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (11.4 mg, 0.1 equiv, 107 µmol) to afford 19 as a white foam (360 mg, 93% yield). The HCl salt of 19 was formed in iPrOH (1 mL) with 37% HCl (0.1 mL) and recrystallized from hot ethanol (4 mL) to give a white solid: mp 240–242 °C; 1H NMR (400 MHz; CD3OD): δ 7.37–7.33 (m, 4H), 7.28 (dt, J = 8.6, 4.3 Hz, 1H), 7.17 (t, J = 7.9 Hz, 1H), 6.89–6.84 (m, 2H), 6.66 (dd, J = 8.0, 2.0 Hz, 1H), 3.76 (s, 1H), 3.65–3.58 (m, 2H), 3.54–3.49 (m, 2H), 3.20–3.13 (m, 2H), 2.55 (d, J = 10.2 Hz, 1H), 2.42–2.35 (m, 1H), 2.18–1.91 (m, 8H), 1.40–1.30 (m, 2H), 1.20–1.00 (m, 2H), 0.83 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz; CD3OD): δ 158.7, 149.9, 137.8, 130.5, 129.99, 129.91, 128.3, 117.7, 114.3, 113.8, 59.4, 57.2, 51.1, 44.1, 39.5, 38.5, 31.8, 29.8, 28.8, 21.2, 20.9, 17.3, 14.3. HRMS-ESI (m/z): [M + H+] calcd for: C25H34NO 364.2640; found 364.2635; Anal. Calcd. For C25H34ClNO·0.75 H2O·0.05 C2H6O: C: 72.5%; H: 8.68%; N: 3.37%; found C: 72.54%; H: 8.63%; N: 3.39%; −31.0° (c 0.59, CHCl3).
3-((1S,5R,9S)-9-Butyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (20). The HCl salt of alkene 14 (324 mg,1 equiv, 786 µmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (8.37 mg, 0.1 equiv, 78.6 µmol) to afford 20 as a white foam (250 mg, 84% yield). The tartrate salt of 20 was formed in iPrOH with D-tartaric acid and recrystallized from hot ethanol to give a white solid: mp 193–196 °C. 1H NMR (400 MHz; CD3OD): δ 7.35–7.32 (m, 4H), 7.26 (ddd, J = 8.4, 5.7, 2.9 Hz, 1H), 7.15 (dd, J = 10.4, 6.0 Hz, 1H), 6.87 (d, J = 7.0 Hz, 2H), 6.66–6.64 (m, 1H), 4.47 (s, 2H), 3.75 (s, 1H), 3.63–3.58 (m, 2H), 3.48 (dd, J = 10.5, 6.2 Hz, 2H), 3.18–3.11 (m, 2H), 2.52–2.37 (m, 2H), 2.18–2.01 (m, 4H), 2.00–1.88 (m, 3H), 1.35–1.03 (m, 7H), 0.80 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz; CD3OD): δ 177.1, 158.7, 150.2, 138.1, 130.5, 130.0, 129.9, 128.2, 117.8, 114.3, 113.8, 74.3, 59.3, 57.3, 50.6, 43.8, 39.2, 38.5, 31.8, 30.3, 28.9, 27.2, 23.6, 20.8, 17.7, and 14.2; HRMS-ESI (m/z): [M + H+] calcd for: C26H36NO 378.2797; found 378.2799; Anal. Calcd. For C25H32ClNO ·0.1 H2O: C, 75.11%; H, 8.12%; N, 3.5%. Found C, 75.00%; H, 7.87%; N, 3.42%; C30H41NO7·0.2 H2O C: 67.83; H: 7.85; N: 2.64; found C: 67.62; H: 7.63; N: 2.68; and −28.4° (c 0.40, CHCl3).
3-((1R,5S,9R)-9-Ethyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (35). The HCl salt of alkene 29 (300 mg,1 equiv, 781 µmol) was dissolved in MeOH (15 mL) and was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (8.31 mg, 0.1 equiv, 78.1 µmol) to afford 36 and isolated as a white foam (250 mg, 91% yield). The HCl salt of 36 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from ethanol to give a white solid: mp 231–235°; 1H NMR (400 MHz; CD3OD): δ 7.35–7.31 (m, 4H), 7.26 (dq, J = 8.5, 4.3 Hz, 1H), 7.17–7.13 (m, 1H), 6.86–6.82 (m, 2H), 6.64 (dd, J = 8.0, 1.8 Hz, 1H), 3.78–3.76 (m, 1H), 3.65–3.57 (m, 2H), 3.53–3.46 (m, 2H), 3.19–3.08 (m, 2H), 2.42–2.31 (m, 2H), 2.17–1.85 (m, 7H), 1.37–1.27 (m, 1H), 1.20–1.10 (m, 1H), 0.84 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz; CD3OD): δ 158.8, 150.0, 137.8, 130.6, 129.99, 129.91, 128.3, 117.6, 114.3, 113.7, 59.0, 57.2, 51.1, 46.4, 39.5, 38.6, 31.8, 28.8, 20.8, 20.5, 17.3, and 12.0; HRMS-ESI (m/z): [M + H+] calcd for: C24H32NO 350.2484; found: 350.2484; Anal. Calcd. For C24H32ClNO: C, 74.68%; H, 8.36%; N, 3.63%. Found C, 74.49%; H, 7.98%; N, 3.59%; 20.4° (c 0.80, CHCl3).
3-((1R,5S,9R)-2-Phenethyl-9-propyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (36). The HCl salt of alkene 30 (250 mg,1 equiv, 628 µmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (6.68 mg, 0.1 equiv, 62.8 µmol) to afford 37 as a white foam (123 mg, 54% yield). The HCl salt of 37 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from hot ethanol to give a white solid: mp 238–242 °C; 1H NMR (400 MHz; CD3OD): δ 7.36–7.30 (m, 4H), 7.29–7.23 (m, 1H), 7.15 (td, J = 7.9, 4.0 Hz, 1H), 6.86–6.79 (m, 2H), 6.65–6.63 (m, 1H), 3.78–3.71 (m, 1H), 3.66–3.44 (m, 4H), 3.18–3.08 (m, 2H), 2.49–2.44 (m, 1H), 2.40–2.29 (m, 1H), 2.20–1.85 (m, 7H), 1.42–1.22 (m, 3H), 1.22–0.98 (m, 2H), 0.89–0.74 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz; CD3OD): δ 158.7, 149.9, 137.8, 130.5, 129.99, 129.91, 128.3, 117.7, 114.3, 113.8, 59.4, 57.2, 51.1, 44.1, 39.5, 38.5, 31.8, 29.8, 28.8, 21.2, 20.9, 17.3, and 14.3; HRMS-ESI (m/z): [M + H+] calcd for: C25H34NO 364.2640; found 364.2643; Anal. Calcd. For C25H34ClNO·0.35 H2O: C, 73.9%; H, 8.61%; N, 3.45%. Found C, 73.56%; H, 8.25%; N, 3.18%; 25.4° (c 0.7, CHCl3).
3-((1R,5S,9R)-9-Butyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (37). The HCl salt of alkene 31 (150 mg,1 equiv, 364 µmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (3.87 mg, 0.1 equiv, 36.4 µmol) to afford 37 as a white foam (125 mg, 90% yield). The tartrate salt of 37 was formed in iPrOH with L-tartaric acid and recrystallized from hot ethanol to give a white solid: mp 194–197 °C. 1H NMR (400 MHz; CD3OD): δ 7.36–7.31 (m, 4H), 7.26 (ddd, J = 8.9, 5.7, 2.9 Hz, 1H), 7.15 (t, J = 8.0 Hz, 1H), 6.87 (d, J = 7.2 Hz, 2H), 6.66–6.63 (m, 1H), 4.47 (s, 2H), 3.74 (s, 1H), 3.66–3.55 (m, 2H), 3.48 (t, J = 8.3 Hz, 2H), 3.18–3.10 (m, 2H), 2.53–2.38 (m, 2H), 2.17–2.02 (m, 4H), 1.99–1.86 (m, 3H), 1.34–1.05 (m, 6H), 0.80 (t, J = 6.9 Hz, 3H). 13C NMR (101 MHz; CD3OD): δ 177.1, 158.7, 150.2, 138.2, 130.5, 130.0, 129.9, 128.1, 117.8, 114.3, 113.9, 74.3, 59.3, 57.3, 50.6, 43.7, 39.2, 38.5, 31.8, 30.2, 28.9, 27.2, 23.6, 20.8, 17.7, 14.2. HRMS-ESI (m/z): [M + H+] calcd for: C26H36NO 378.2797; found 378.2800; C30H41NO7·0.1 H2O C: 68.06; H: 7.84; N: 2.65; found C: 67.73; H: 7.51; N: 2.59, 38.0° (c 0.58, CHCl3).
3-((1R,5S,9S)-9-Ethyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (38). The HCl salt of alkene 32 (180 mg,1 equiv, 469 µmol) was dissolved in MeOH (15 mL) and hydrogenated according to the general procedure using palladium on carbon (Escat 103) (4.99 mg, 0.1 equiv, 46.9 µmol) to afford 38 and isolated as a white foam (160 mg, 88% yield). The HCl salt of 38 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from ethanol to give a white solid: mp 249–253 °C; 1H NMR (400 MHz; CD3OD): δ 7.39–7.35 (m, 4H), 7.32–7.26 (m, 1H), 7.17 (t, J = 8.0 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 6.76 (t, J = 2.0 Hz, 1H), 6.65 (dd, J = 8.0, 2.2 Hz, 1H), 3.93 (s, 1H), 3.73–3.52 (m, 3H), 3.46–3.35 (m, 1H), 3.21 (td, J = 12.2, 5.5 Hz, 1H), 3.01 (td, J = 12.2, 4.9 Hz, 1H), 2.49–2.39 (m, 2H), 2.30–2.22 (m, 2H), 2.11–2.00 (m, 3H), 1.89–1.83 (m, 2H), 1.45–1.38 (m, 1H), 1.26 (ddd, J = 14.8, 7.5, 3.0 Hz, 1H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz; CD3OD): δ 158.8, 150.2, 137.5, 130.6, 130.0, 129.9, 128.4, 117.3, 114.2, 113.3, 56.5, 55.6, 51.7, 46.7, 42.0, 39.0, 31.5, 28.9, 25.1, 22.1, 19.2, 12.0; HRMS-ESI (m/z): [M + H+] calcd for: C24H32NO 350.2484; found: 350.2487; Anal. Calcd. For C24H32ClNO·0.1 H2O: C, 74.34%; H, 8.37%; N, 3.61%. Found C, 74.29%; H, 8.33%; N, 3.6%; −18.3° (c 0.6, CHCl3).
3-((1R,5S,9S)-2-Phenethyl-9-propyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (39). The HCl salt of alkene 33 (325 mg,1 equiv, 817 µmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (8.69 mg, 0.1 equiv, 81.7 µmo to afford 39 as a white foam (220 mg, 74% yield). The HCl salt of 39 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from hot ethanol to give a white solid: mp 253–256 °C. 1H-NMR (400 MHz; CD3OD): δ 7.37–7.33 (m, 4H), 7.29 (dq, J = 8.5, 4.2 Hz, 1H), 7.17 (dd, J = 10.4, 5.5 Hz, 1H), 6.82–6.80 (m, 1H), 6.76 (s, 1H), 6.66 (dd, J = 8.0, 1.8 Hz, 1H), 3.88 (s, 1H), 3.72–3.51 (m, 3H), 3.41 (td, J = 12.0, 5.0 Hz, 1H), 3.24–3.16 (m, 1H), 3.06–2.99 (m, 1H), 2.48–2.36 (m, 3H), 2.23 (dd, J = 14.1, 5.0 Hz, 1H), 2.12–1.99 (m, 3H), 1.89–1.81 (m, 2H), 1.51–1.38 (m, 2H), 1.24–1.05 (m, 2H), 0.86 (t, J = 7.0 Hz, 3H). 13C NMR (10 MHz; CD3OD): δ 158.8, 150.2, 137.5, 130.6, 130.02, 129.93, 128.4, 117.3, 114.2, 113.4, 56.4, 56.2, 51.7, 44.6, 41.9, 38.9, 31.5, 28.9, 28.5, 25.1, 22.1, 21.5, and 14.2. HRMS-ESI (m/z): [M + H+] calcd for: C25H34NO 364.2640; found 364.2637; Anal. Calcd. For C25H34ClNO·0.05 H2O; C: 74.9%; H: 8.57%; N: 3.49%; found: C: 74.97%, H: 8.73%; N: 3.49%; −27.7° (c 0.35, CHCl3).
3-((1R,5S,9S)-9-Butyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (40). The HCl salt of alkene 34 (150 mg,1 equiv, 364 µmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (3.87 mg, 0.1 equiv, 36.4 µmol) hydrogenated according to the general procedure using to afford 40 as a white foam (120 mg, 87% yield). The HCl salt of 40 was formed in iPrOH (0.5 mL) with 37% HCl (0.05 mL) and recrystallized from ethanol to give a white solid: mp 254–259 °C. 1H NMR (400 MHz; CD3OD): δ 7.37–7.26 (m, 5H), 7.15 (dt, J = 10.4, 6.5 Hz, 1H), 6.81 (d, J = 8.0 Hz, 1H), 6.76 (s, 1H), 6.71–6.62 (m, 1H), 3.88 (s, 1H), 3.70–3.49 (m, 4H), 3.46–3.37 (m, 1H), 3.20 (td, J = 12.1, 5.3 Hz, 1H), 3.02 (td, J = 12.1, 5.1 Hz, 1H), 2.48–2.33 (m, 3H), 2.22 (dt, J = 14.0, 6.6 Hz, 1H), 2.05 (t, J = 8.5 Hz, 3H), 1.87 (q, J = 11.2 Hz, 2H), 1.49–1.33 (m, 2H), 1.29–1.10 (m, 4H), 0.85 (t, J = 7.2 Hz, 3H). 13-C NMR (101 MHz; CD3OD): δ 158.7, 150.2, 130.6, 130.01, 129.93, 128.4, 117.4, 114.2, 113.4, 56.44, 56.28, 51.7, 44.9, 41.9, 38.9, 31.5, 30.7, 28.9, 26.1, 25.2, 23.5, 22.1, and 14.3. HRMS-ESI (m/z): [M + H+] calcd for: C26H36NO 378.2797; found 378.2800; C26H36ClNO ·0.35 C2H6O calc. C: 74.55%; H: 8.93%; N: 3.26%; found C: 74.65%, H: 9.06%; N: 3.37%; −17.9° (c 0.75, CHCl3).
tertert-Butyl (1S,5S)-5-(3-methoxyphenyl)-9-oxo-2-azabicyclo[3.3.1]nonane-2-carboxylate (44). To a vial containing 1 (2.5 g, 1 equiv, 9.6 mmol) in acetonitrile (15 mL), under argon, was added potassium carbonate (2.7 g, 2.0 equiv, 19 mmol) and cyanogen bromide (3.9 mL, 2.0 equiv, 19 mmol). The reaction mixture was stirred for 2 h at room temperature followed by 2 h at reflux. Upon completion, the reaction mixture was filtered through Celite, washed several times with CHCl3, and the filtrate was concentrated in vacuo. The resulting oil was taken up in MeOH (3.0 mL) and treated with 3 M HCl (29 mL). The reaction mixture was stirred at reflux for 16 h and subsequently quenched with 7 N NH4OH in MeOH, extracted with CHCl3 and concentrated in vacuo. To the crude solution was added dry dichloromethane (12 mL) at 0 °C, di-tert-butyl dicarbonate (2.9 g, 1.4 equiv, 13 mmol), triethylamine (1.9 mL, 1.4 equiv, 13 mmol), and 4-dimethylaminopyridine (0.12 g, 0.1 equiv, 0.96 mmol) dropwise. The solution was allowed to stir under argon for 30 min at 0 °C then warmed to room temperature. After 1 h, TLC showed consumption of starting material. Saturated ammonium chloride was added, and the mixture was extracted with CH2Cl2, washed with brine, and dried over sodium sulfate. The crude mixture was loaded onto silica and purified via flash chromatography eluting with 0–30% ethyl acetate in hexane.
tert-Butyl (1S,5S)-5-(3-methoxyphenyl)-9-methylene-2-azabicyclo[3.3.1]nonane-2-carboxylate (45). To a vial containing ketone 44 (1.85 g, 1 equiv, 5.36 mmol) in THF (25.0 mL) at 0 °C was added Tebbe’s Reagent (10.7 mL, 1 equiv, 5.36 mmol). The resulting mixture was stirred at 0 °C for 1 h and then slowly warmed up to room temperature for an additional 4 h. Upon completion by TLC, the reaction was cooled to 0 °C and 50 mL of Et2O was added. The reaction was quenched carefully with 1.8 N NaOH. A very vigorous gas evolution took place and a thick red/orange precipitate formed. Magnesium sulfate was added, and the mixture was allowed to stir an additional 5 min. The solids were filtered and washed with EtOAc. Flash column chromatography using 1–5% CMA in CHCl3 yielded 45 as an orange foam (0.81 g, 44% yield).
tert-Butyl (1S,5R,9R)-5-(3-methoxyphenyl)-9-methyl-2-azabicyclo[3.3.1]nonane-2-carboxylate (46). Methylene 45 (810 mg,1 equiv, 2.13 mmol) was hydrogenated according to the general procedure using palladium on carbon (Escat 103) (22.7 mg, 0.1 equiv, 213 µmol) to afford 46 as a white foam (700 mg, 95% yield). The reaction gave a mixture of epimers that were used without further purification.
(1S,5R,9R)-5-(3-Methoxyphenyl)-9-methyl-2-phenethyl-2-azabicyclo[3.3.1]nonane (47). To a solution of 46 (1 g, 1 equiv, 3 mmol) in dichloromethane (30 mL) at 0 °C was added trifluoroacetic acid (2 mL, 10 equiv, 0.03 mol), dropwise. After 15 min, the reaction was allowed to warm to room temperature and allowed to stir for 1 h. Upon completion as determined using TLC, the reaction mixture was cooled to 0 °C and quenched with NaHCO3 and extracted with dichloromethane. The crude oil was taken up in acetonitrile (32 mL), was treated with K2CO3, and the mixture was purged with argon. (2-Bromoethyl)benzene (0.8 g, 1.5 equiv, 4 mmol) was added, and the reaction was refluxed under argon for 16 h. Upon completion, the reaction mixture was concentrated in vacuo then extracted with CHCl3. The organic extracts were washed with water and brine, dried with sodium sulfate and concentrated in vacuo. Purification by flash column chromatography on silica using 0–100% EtOAc: Hexanes to isolate 47 as a yellow oil (830 mg, 80% yield). The resulting oil was a mixture of epimers and was used without further purification.
3-((1S,5R,9R)-9-Methyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (48). In an oven-dried round-bottom flask, 47 (600 mg, 1 equiv, 2.9 mmol) was suspended in dichloromethane (30 mL) and the mixture was cooled to −78 °C. Tribromoborane (0.3 mL, 2 equiv, 5.8 mmol) was added dropwise and the reaction was stirred at −78 °C. The reaction mixture was allowed to warm to room temperature and stirred 2 h. Upon completion, the reaction mixture was cooled to 0 °C and quenched with 15 mL MeOH dropwise and stirred for 30 min. A total of 20 mL 1N HCl was added, and the reaction mixture was refluxed at 100 °C for 1 h. The reaction mixture was then cooled to 0 °C and made basic (pH > 10.5) with NH4OH and extracted with 9:1 CHCl3:MeOH. The combined organic layers were washed with water and brine, dried with sodium sulfate, and concentrated in vacuo. Purification by silica gel flash chromatography using 10–100% EtOAc Hexanes resulted in a tan foam (420 mg, 73%). The HCl salt of 48 was formed in iPrOH with 37% HCl (0.1 mL) and recrystallized from ethanol to give a white solid: mp 264–269 °C. 1H NMR (400 MHz; CD3OD): δ 7.39–7.34 (m, 4H), 7.29 (m, 1H), 7.17 (t, J = 7.9 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 6.77 (t, J = 1.9 Hz, 1H), 6.65 (dd, J = 8.0, 2.3 Hz, 1H), 3.77 (s, 1H), 3.71–3.59 (m, 2H), 3.52 (td, J = 12.2, 5.5 Hz, 1H), 3.40 (td, J = 12.1, 5.2 Hz, 1H), 3.16 (td, J = 12.2, 5.4 Hz, 1H), 3.03 (td, J = 12.2, 5.2 Hz, 1H), 2.66–2.60 (m, 1H), 2.54–2.38 (m, 2H), 2.24 (dd, J = 14.5, 5.0 Hz, 1H), 2.13–1.98 (m, 3H), 1.91–1.82 (m, 2H), 0.97 (d, J = 7.3 Hz, 3H); 13C NMR (100 MHz; CD3OD): δ 158.8, 150.1, 137.6, 130.6, 130.02, 129.91, 128.4, 117.3, 114.2, 113.3, 60.6, 56.6, 51.6, 41.9, 39.3, 38.4, 31.5, 28.1, 25.0, 22.2, and 13.7; HRMS-ESI (m/z): [M + H]+ calcd. for C23H29NO 336.2327, found 336.2321; Calcd for C23H30ClNO·0.05 H2O: C, 74.09%; H, 8.14%; N, 3.76%. Found C: 74.18% H: 8.07% N: 3.72%; 33.3° (c 1.4, CHCl3).
3-((1R,5S,9S)-9-Methyl-2-phenethyl-2-azabicyclo[3.3.1]nonan-5-yl)phenol (56). In an oven-dried round-bottom flask, 55 (400 mg, 1 equiv, 1.14 mmol) was suspended in dichloromethane (20 mL) and the mixture was cooled to −78 °C. Tribromoborane (0.2 mL, 2 equiv, 2.29 mmol) was added dropwise and the reaction was stirred at −78 °C. The reaction mixture was allowed to warm to room temperature and stirred 2 h. Upon completion, the reaction mixture was cooled to 0 °C and quenched with 15 mL MeOH dropwise and stirred for 30 min. A total of 20 mL 1N HCl was added, and the reaction mixture was refluxed at 100 °C for 1 h. The reaction mixture was then cooled to 0 °C and made basic (>10.5) with NH4OH and extracted with 9:1 CHCl3:MeOH. The combined organic layers were washed with water and brine, dried with sodium sulfate, and concentrated. Purification by silica gel flash chromatography 10–100% EtOAc Hexanes resulted in a tan foam (380 mg, 99%). The HCl salt was isolated as a white solid (380 mg, 99% yield): mp 268–272 °C. 1H NMR (400 MHz; CD3OD): δ 7.39–7.33 (m, 4H), 7.29 (ddd, J = 9.1, 5.9, 2.7 Hz, 1H), 7.17 (t, J = 8.0 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 6.76 (t, J = 2.0 Hz, 1H), 6.65 (dd, J = 8.0, 2.0 Hz, 1H), 3.77 (d, J = 0.6 Hz, 1H), 3.72–3.58 (m, 2H), 3.56–3.49 (m, 1H), 3.44–3.37 (m, 1H), 3.19–3.12 (m, 1H), 3.02 (ddd, J = 14.6, 9.9, 4.9 Hz, 1H), 2.66–2.61 (m, 1H), 2.54–2.39 (m, 2H), 2.27–2.22 (m, 1H), 2.13–1.98 (m, 3H), 1.91–1.82 (m, 2H), 0.96 (d, J = 7.3 Hz, 3H). 13C NMR (101 MHz; CD3OD): δ 158.8, 150.1, 137.6, 130.6, 130.03, 129.89, 128.4, 117.3, 114.2, 113.3, 60.7, 56.6, 51.6, 41.9, 39.3, 38.4, 31.5, 28.1, 25.0, 22.2, and 13.6; HRMS-ESI (m/z): [M + H]+ calcd. for C23H29NO 336.2327, found 336.2326; Calcd for C23H30ClNO: C, 74.27%; H, 8.13%; N, 3.77%. Found C: 74.39% H: 8.27% N: 3.82%; %; −34.8° (c 0.79, CHCl3).
3.3. In Vitro Assay
3.3.1. Cell Lines and Cell Culture
Cell lines and cell culture: cAMP HunterTM Chinese hamster ovary cells (CHO-K1) that express human μ-opioid receptor (OPRM1), human κ-opioid receptor (OPRMK1), and human δ-receptor (OPRMD1) were purchased from Eurofins DiscoverX (Fremont, CA, USA) and used for the forskolin-induced cAMP accumulation assays [16]. All cell lines were maintained in F-12 media with 10% fetal bovine serum (Life Technologies, Grand Island, NY, USA), 1% penicillin/streptomycin/l-glutamine (Life Technologies), and 800 µg/mL Geneticin (Mirus Bio, Madison, WI, USA). All cells were grown at 37 °C and 5% CO2 in a humidified incubator.
3.3.2. Forskolin-Induced cAMP Accumulation Assays
Briefly, cells were plated at 10,000 cells/well density in a 384-well tissue culture plate and incubated overnight at 37 °C in 5% CO2. Stock solutions of compound were made in 100% DMSO at a 5 mM concentration. A serial dilution of 10 concentrations was made using 100% DMSO, creating 100X working solutions of the compound for treatment. The 100X working solutions were then diluted to 5X working solutions using assay buffer consisting of Hank’s Buffered Salt Solution, HEPES, and forskolin. For the agonist assay, cells were incubated at 37 °C with compounds for 30 min at a 1X final concentration. For the antagonist assay, cells were incubated at 37 °C with compounds for 15 min before 30-min incubation at 37 °C with selected agonist at their EC50 or EC90 dose. Detection was made by using the HitHunter cAMP Assay for Small Molecules by DiscoverX according to manufacturer’s directions, and the BioTek Synergy H1 hybrid plate reader (BioTek, Winooski, VT, USA) and Gen5 Software version 2.01 were used to quantify luminescence (BioTek, Winooski, VT, USA) [16].
4. Conclusions
Four series of C9-alkyl 5-phenylmorphans have been synthesized. Eight of these diastereomers were more potent than morphine (C9-methyl compounds 51 and 56, C9-ethyl compounds 15, 18, 35, and 38, and the C9-propyl compounds 16, and 36). Among them, compounds 51, 18, 35, and 36 showed full or close to full MOR agonism. One C9-methyl compound, 48, was a potent antagonist, more potent, selective, and with higher antagonist efficacy than naltrexone. The MOR antagonist 48 did not interact at all with DOR or KOR as an agonist and it had very little DOR antagonist activity. It was about as potent as the reference kappa ligand, nor-BNI, as a KOR antagonist. It will be further evaluated in the future in vivo as a possible replacement for naloxone in treating respiratory depression caused by the more potent agonists that are currently being abused.
5. Patents
(1) K. C. Rice. A. E. Jacobson, A. Sulima, and D. C. Chambers, “Selective Opioid Receptor Agonists and Antagonists”, US Patent Application Serial No. 63/393,035, filed 28 July 2022; (2) Rice, K. C.; Jacobson, A. E.; LI, F.; Gutman, E. S.; Bow, E. W. Biased potent opioid-like agonists as improved medications to treat chronic and acute pain andmethods of using the same. US Application Serial No.: 62/644,791 filed on 19 March 2018. US Patent 11,352,365 issued 6-7-2022. International Publication WO 2019/182950 A1 26 September 2019.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28145411/s1, Figures S1–S12: 1H and 13C-NMR spectra of novel compounds are shown.
Author Contributions
Conceptualization, A.E.J. and A.S.; methodology, D.L., D.R.C., A.S. and T.E.P.; validation, D.L., T.E.P. and A.S.; investigation, D.R.C. and D.L; resources, K.C.R. and T.E.P.; writing—original draft preparation, A.E.J. and D.R.C.; writing—review and editing, A.S., T.E.P., D.L., D.R.C., A.E.J. and K.C.R.; supervision, A.S., A.E.J. and T.E.P.; project administration, A.E.J., K.C.R. and T.E.P.; funding acquisition, K.C.R. and T.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was also supported in part by DA051377 (to T.E.P.) and the Kentucky Medical Services Foundation Endowed Chair in Pharmacy (T.E.P.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article or in the supplementary material.
Acknowledgments
The work of D.R.C., A.S., A.E.J. and K.C.R. was supported by the NIH Intramural Research Program (IRP) of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism. We thank John Lloyd (Mass Spectrometry Facility, NIDDK) for the mass spectral data.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Patents have been obtained for some of the compounds (see reference [15]).
Sample Availability
Not applicable.
Abbreviations
MOR: μ-opioid receptor; DOR, δ-opioid receptor; KOR, κ-opioid receptor; cAMP, cyclic adenosine monophosphate.
References
- Kenakin, T. Biased Receptor Signaling in Drug Discovery. Pharmacol. Rev. 2019, 71, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.L.; Bohn, L.M. Low Intrinsic Efficacy Alone Cannot Explain the Improved Side Effect Profiles of New Opioid Agonists. Biochemistry 2022, 61, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.M.; Clark, M.J.; Agius, M.P.; Traynor, J.R.; Mosberg, H.I. Synthesis and evaluation of 4-substituted piperidines and piperazines as balanced affinity μ opioid receptor (MOR) agonist/δ opioid receptor (DOR) antagonist ligands. Bioorg. Med. Chem. Lett. 2014, 24, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.M.; Keresztes, A.; Tashiro, J.K.; Daconta, L.V.; Hruby, V.J.; Streicher, J.M. Synthesis and Evaluation of a Novel Bivalent Selective Antagonist for the Mu-Delta Opioid Receptor Heterodimer that Reduces Morphine Withdrawal in Mice. J. Med. Chem. 2018, 61, 6075–6086. [Google Scholar] [CrossRef] [PubMed]
- Wade, P.R.; Palmer, J.M.; McKenney, S.; Kenigs, V.; Chevalier, K.; Moore, B.A.; Mabus, J.R.; Saunders, P.R.; Wallace, N.H.; Schneider, C.R.; et al. Modulation of gastrointestinal function by MuDelta, a mixed µ opioid receptor agonist/ µ opioid receptor antagonist. Br. J. Pharmacol. 2012, 167, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Conibear, A.; Henderson, G. Biased Agonism: Lessons from Studies of Opioid Receptor Agonists. Ann. Rev. Pharmacol. Toxicol. 2023, 63, 491–515. [Google Scholar] [CrossRef] [PubMed]
- May, E.L.; Murphy, J.G. Structures Related to Morphine. IV. m-Substituted Phenylcyclohexane Derivatives. J. Org. Chem. 1955, 20, 1197–1201. [Google Scholar] [CrossRef]
- May, E.L. Structures Related to Morphine. VI. N-Phenylethyl Derivatives of Some Phenyl- and Benz-morphans. J. Org. Chem. 1956, 21, 899–901. [Google Scholar] [CrossRef]
- Ong, H.; Oh-ishi, T.; May, E.L. Phenylmorphan Agonists-Antagonists. J. Med. Chem. 1974, 17, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Kim, I.J.; Lee, M.J.; Adah, S.A.; Raymond, T.J.; Bilsky, E.J.; Aceto, M.D.; May, E.L.; Harris, L.S.; Coop, A.; et al. Opioid Ligands With Mixed Properties from Substituted Enantiomeric N-Phenethyl-5-phenylmorphans. Synthesis of a μ-Agonist δ-Antagonist and δ-Inverse Agonists. Org. Biomol. Chem. 2007, 5, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Hiebel, A.C.; Lee, Y.S.; Bilsky, E.J.; Giuvelis, D.; Deschamps, J.R.; Parrish, D.A.; Aceto, M.D.; May, E.L.; Harris, E.M.; Coop, A.; et al. Probes for Narcotic Receptor Mediated Phenomena. 34. Synthesis and Structure-Activity Relationships of a Potent μ-Agonist δ-Antagonist and an Exceedingly Potent Antinociceptive in the Enantiomeric C9-Substituted 5-(3-Hydroxyphenyl)-N-phenylethylmorphan Series. J. Med. Chem. 2007, 50, 3765–3776. [Google Scholar] [PubMed]
- Gutman, E.S.; Bow, E.; Li, F.; Sulima, A.; Kaska, S.; Crowley, R.; Prisinzano, T.E.; Lee, Y.-S.; Hassan, S.A.; Imler, G.H.; et al. G-Protein biased opioid agonists: 3-hydroxy-N-phenethyl-5-phenylmorphans with three-carbon chain substituents at C9. RSC Med. Chem. 2020, 11, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.J.; Nassehi, N.; Bow, E.W.; Chambers, D.R.; Gutman, E.S.; Jacobson, A.E.; Lutz, J.A.; Marsha, S.A.; Kenner, C.; Rice, K.C.; et al. Role of Efficacy as a Determinant of Locomotor Activation by Mu Opioid Receptor (MOR) Ligands in Female and Male Mice. II. Effects of Novel MOR-Selective Phenylmorphans with High to Low MOR Efficacy. Pharmacol. Res. Perspect. 2023, 11, e01111. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.R.; Sulima, A.; Luo, D.; Prisinzano, T.E.; Goldberg, A.; Xie, B.; Shi, L.; Paronis, C.A.; Bergman, J.; Selley, D.; et al. A Journey Through Diastereomeric Space: The Design, Synthesis, in vitro and in vivo Pharmacological Activity, and Molecular Modeling of Novel Potent Diastereomeric MOR Agonists and Antagonists. Molecules 2022, 27, 6455. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.C.; Jacobson, A.E.; Li, F.; Gutman, E.S.; Bow, E.W. Biased Potent Opioid-like Agonists as Improved Medications to Treat Chronic and Acute Pain and Methods of Using the Same. US Application Serial No. 62/644,791, 19 March 2018; International Publication WO 2019/182950 A1, 26 September 2019. U.S. Patent 11,352,365 6 July 2022. [Google Scholar]
- Linders, J.T.M.; Flippen-Anderson, J.L.; George, C.F.; Rice, K.C. An expedient synthesis of 9-keto-2-methyl-5-(dimethoxyphenyl)morphans. Tetrahedron Lett. 1999, 40, 3905–3908. [Google Scholar] [CrossRef]
- Hedrick, S.L.; Luo, D.; Kaska, S.; Niloy, K.K.; Jackson, K.; Sarma, R.; Horn, J.; Baynard, C.; Leggas, M.; Eduardo, R.; et al. Design, synthesis, and preliminary evaluation of a potential synthetic opioid rescue agent. J. Biomed. Sci. 2021, 28, 62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).