Electronic Properties and CO2-Selective Adsorption of (NiB)n (n = 1~10) Clusters: A Density Functional Theory Study
Abstract
1. Introduction
2. Results
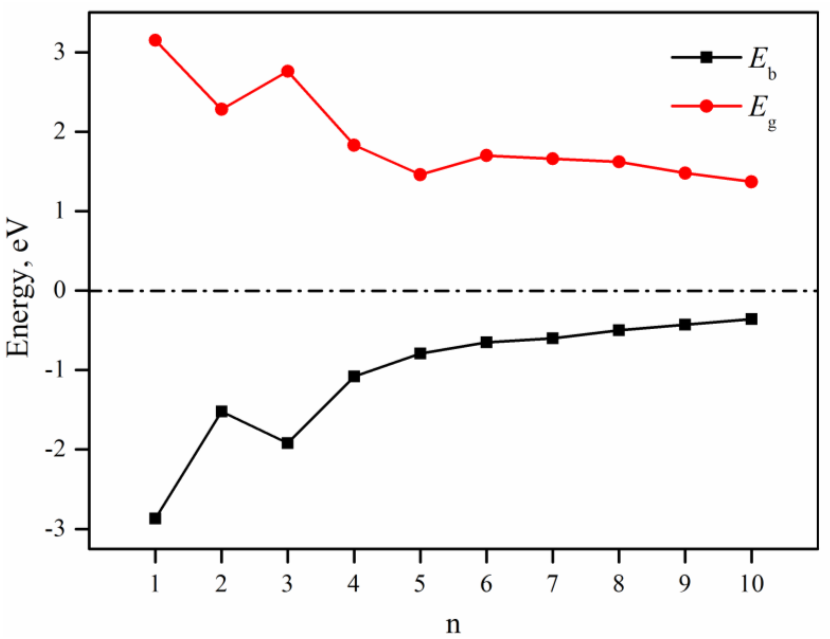
2.1. Geometrical Structures of Clusters
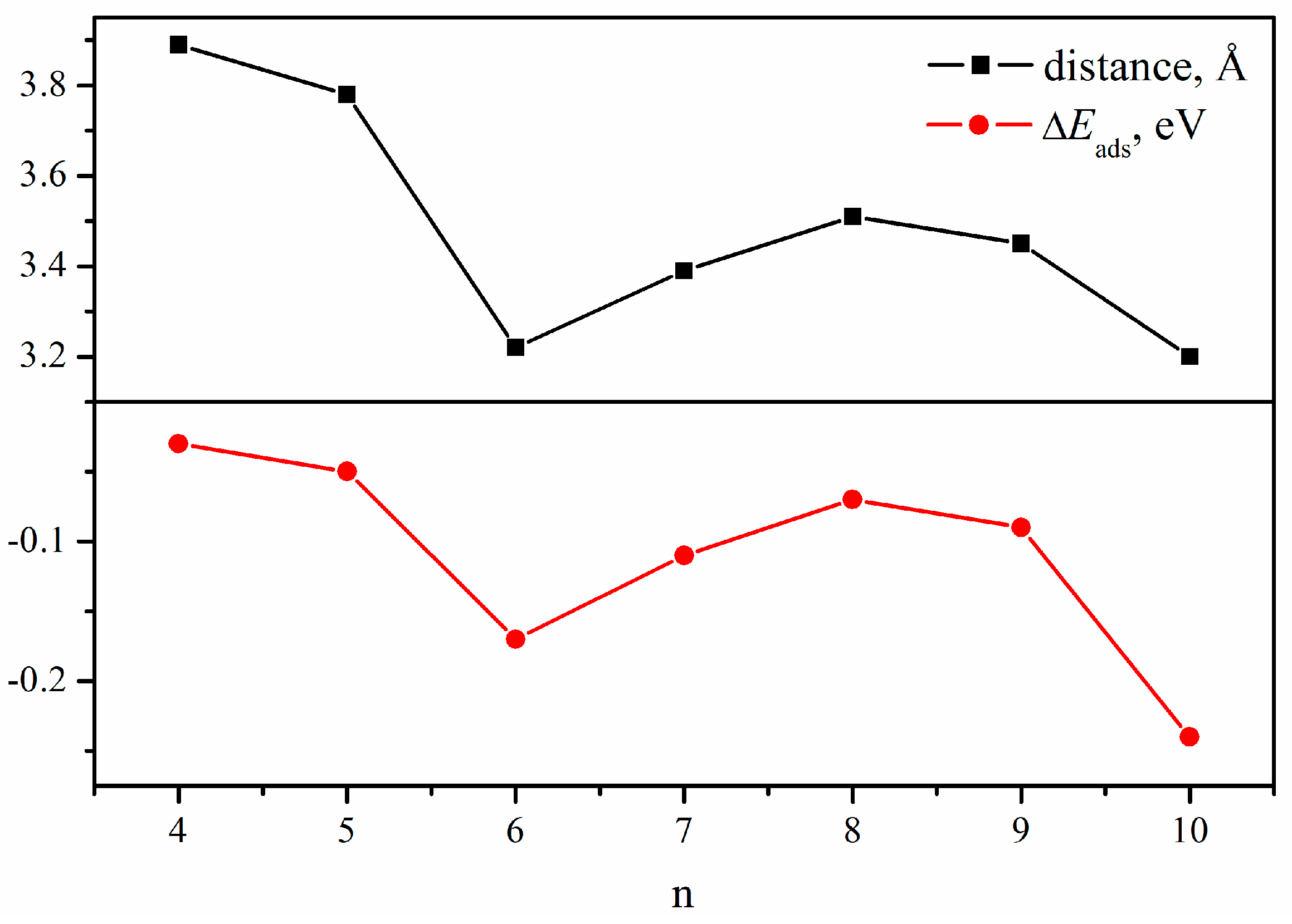
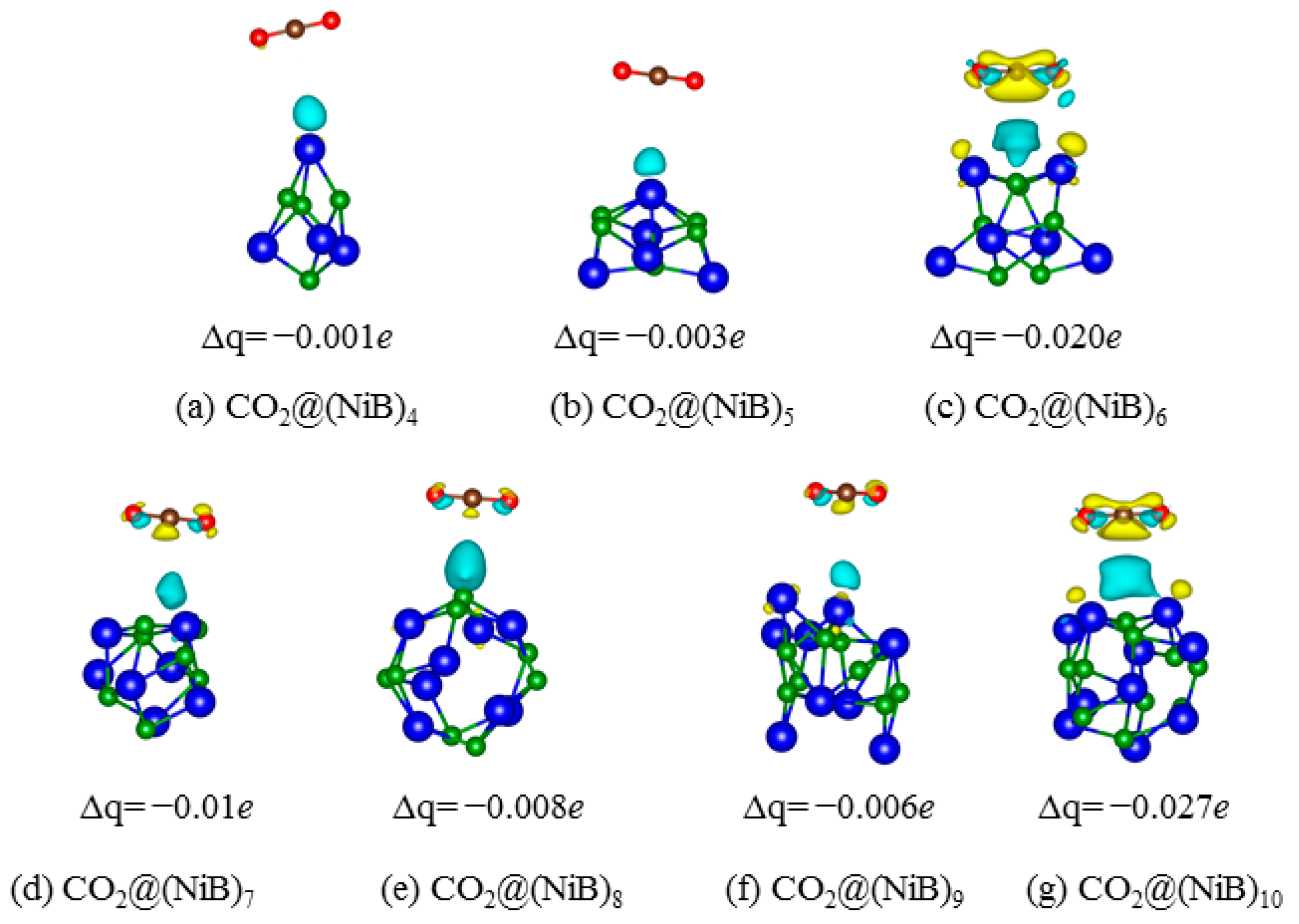
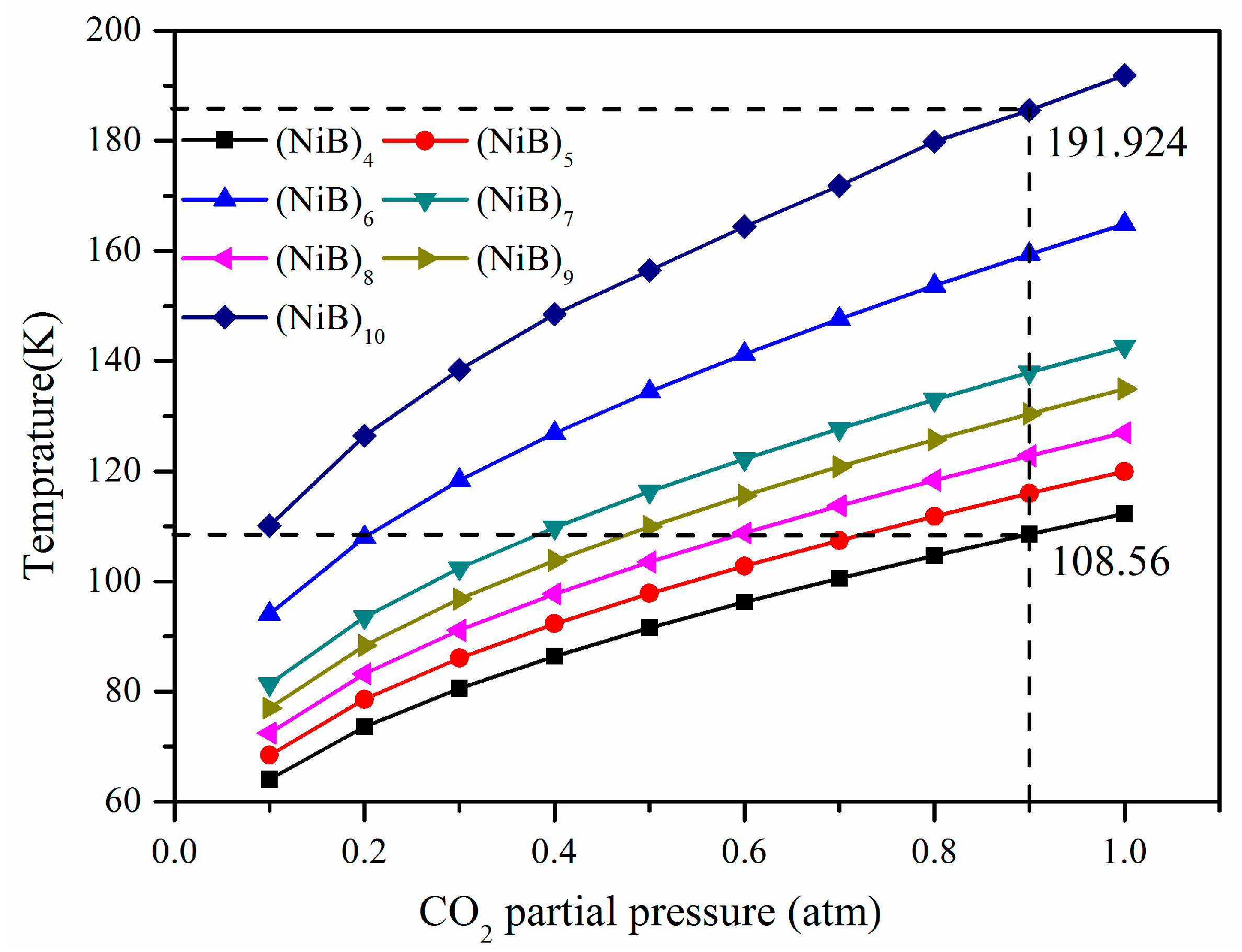
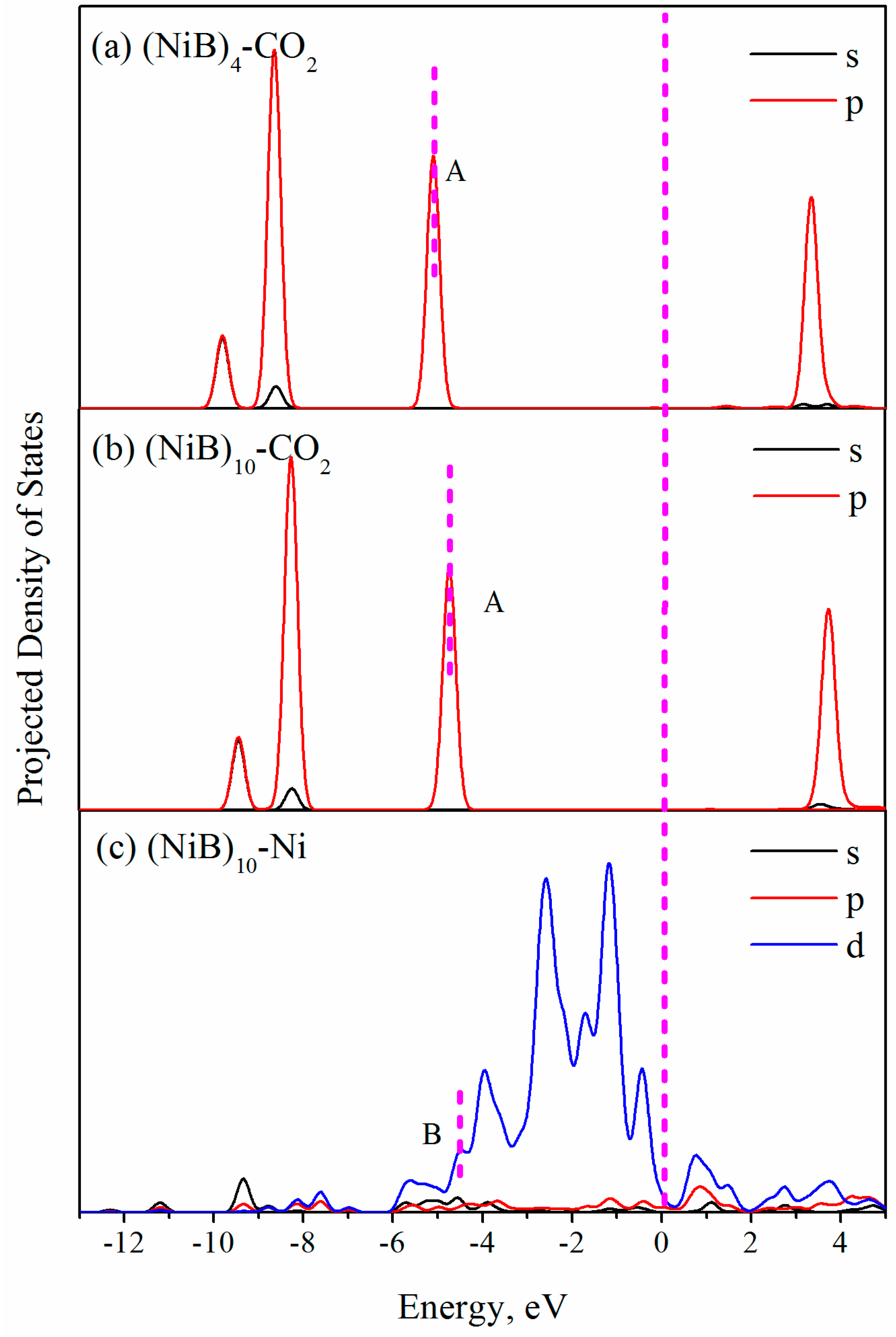
2.2. CO2 Adsorption
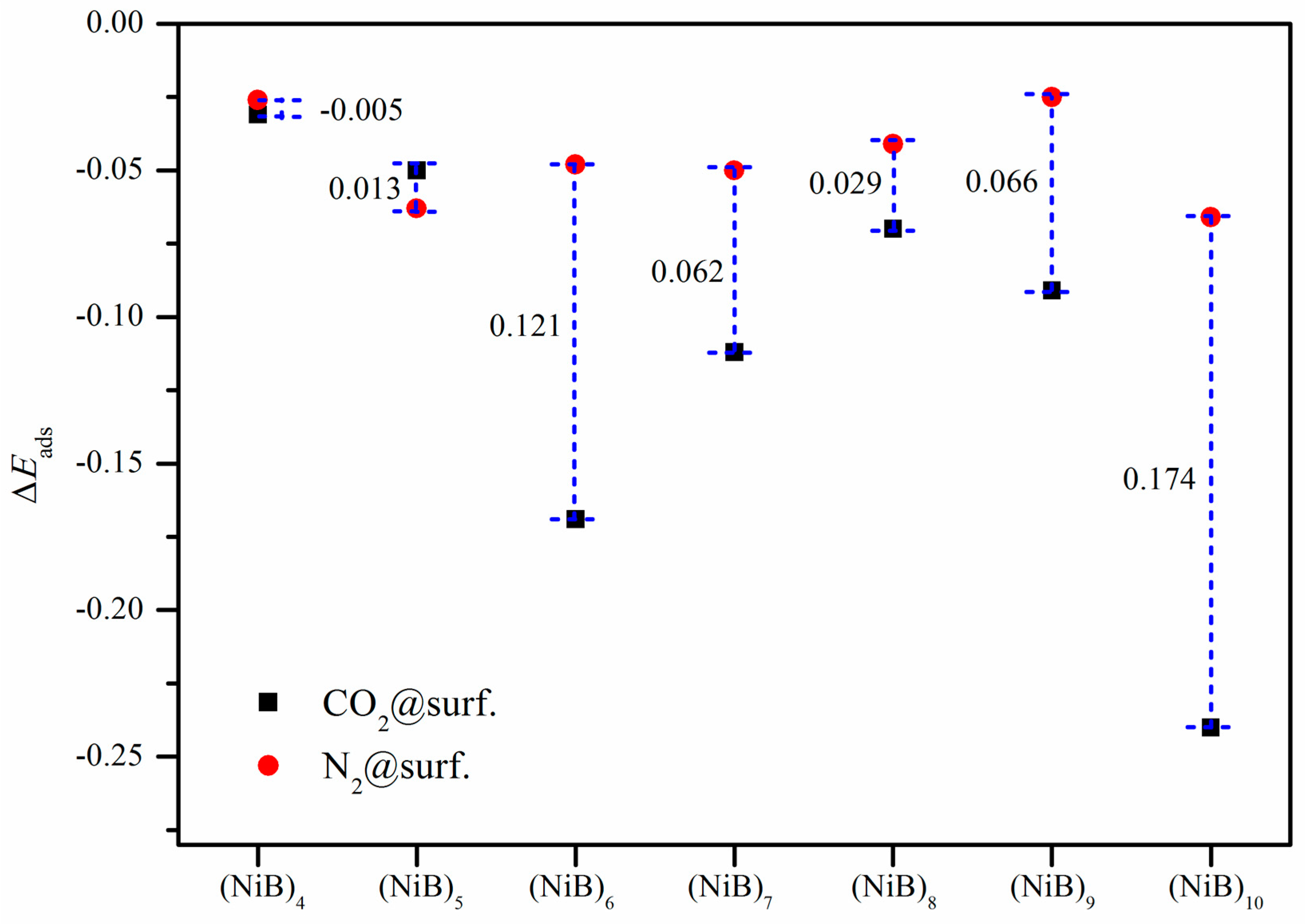
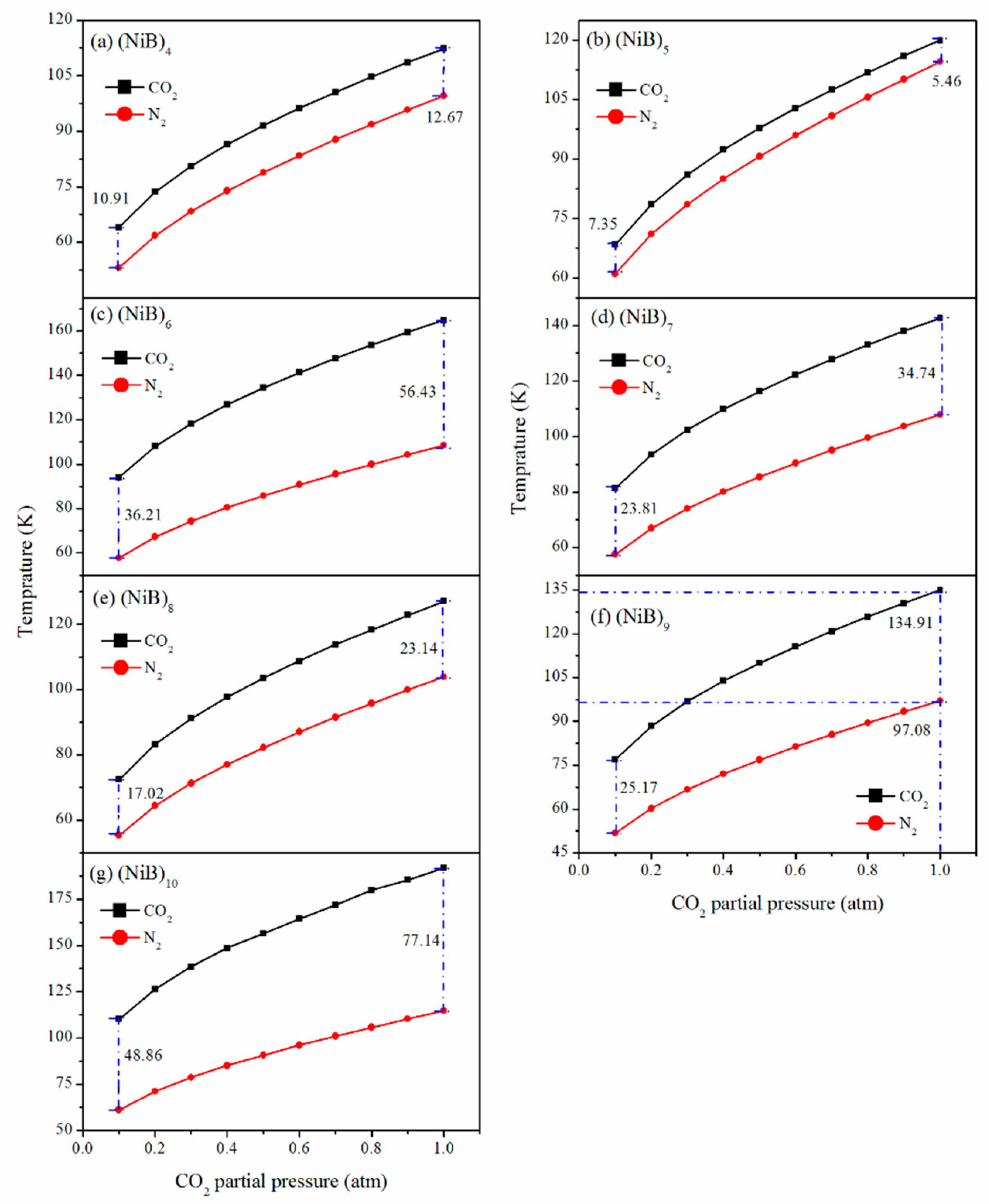
2.3. Adsorption Selectivity over N2
3. Discussion
4. Calculation Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mitchell, J.F. The “greenhouse” effect and climate change. Rev. Geophys. 1989, 27, 115–139. [Google Scholar] [CrossRef]
- Duyar, M.S.; Treviño, M.A.A.; Farrauto, R.J. Dual function materials for CO2 capture and conversion using renewable H2. Appl. Catal. B Environ. 2015, 168, 370–376. [Google Scholar] [CrossRef]
- Pramod, C.V.; Upendar, K.; Mohan, V.; Sarma, D.S.; Rao, K. Hydrotalcite-SBA-15 composite material for efficient carbondioxide capture. J. CO2 Util. 2015, 12, 109–115. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical reduction of CO2 at copper nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, Y.; Zeng, G.; Toma, F.M.; Goddard, W.A.; Bell, A.T. Effects of surface roughness on the electrochemical reduction of CO2 over Cu. ACS Energy Lett. 2020, 5, 1206–1214. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Nejadebrahimi, B. Theoretical insights into hydrogenation of CO2 to formic acid over a single CO atom incorporated nitrogen-doped graphene: A DFT study. Appl. Surf. Sci. 2019, 475, 363–371. [Google Scholar] [CrossRef]
- Xu, Z.; McNamara, N.D.; Neumann, G.T.; Schneider, W.F.; Hicks, J.C. Catalytic hydrogenation of CO2 to formic acid with silica-tethered iridium catalysts. ChemCatChem 2013, 5, 1769–1771. [Google Scholar] [CrossRef]
- Prajapati, A.; Sartape, R.; Galante, M.T.; Xie, J.; Leung, S.L.; Bessa, I.; Andrade, M.H.S.; Somich, R.T.; Reboucas, M.V.; Hutras, G.T.; et al. Fully-integrated electrochemical system that captures CO2 from flue gas to produce value-added chemicals at ambient conditions. Energy Environ. Sci. 2022, 15, 5105–5117. [Google Scholar] [CrossRef]
- Prasetyo, N.; Wicaksono, H.R. Effect of Pt cluster size on CO2 adsorption and activation on (110) and (100) γ-alumina surfaces: Insights from DFT using a periodic boundary approach. J. Mol. Model. 2022, 28, 137. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, N.; Wang, D.; Wang, R.; Peng, W.; Zhang, J.; Liu, J.; Zhang, J. CO2 Capture and Separation by Mono-Vacancy Doped Graphene in Electric Field: A DFT study. ChemistrySelect 2023, 8, 13. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous organic polymers for CO2 storage and conversion reactions. ChemCatChem 2019, 1, 244–257. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Roque-Malherbe, R.; Uwakweh, O.N.; Lozano, C.; Polanco, R.; Hernandez-Maldonado, A.; Fierro, P.; Lugo, F.; Primera-Pedrozo, J.N. Structural effects and interactions of carbon dioxide molecules adsorbed on Ni, Zn, and Cd nitroprussides. J. Phys. Chem. 2011, 115, 15555–15569. [Google Scholar] [CrossRef]
- Li, J.; Croiset, E.; Ricardez-Sandoval, L. Effect of carbon on the Ni catalyzed methane cracking reaction: A DFT study. Appl. Surf. Sci. 2014, 311, 435–442. [Google Scholar] [CrossRef]
- Gould, T.D.; Izar, A.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Stabilizing Ni Catalysts by Molecular Layer Deposition for Harsh, Dry Reforming Conditions. ACS Catal. 2014, 4, 2714–2717. [Google Scholar] [CrossRef]
- Chai, K.H.; Leong, L.K.; Wong, D.S.H.; Tsai, D.H.; Sethupathi, S. Effect of CO2 adsorbents on the Ni-based dual-function materials for CO2 capturing and in situ methanation. J. Chin. Chem. Soc. 2020, 67, 998–1008. [Google Scholar] [CrossRef]
- Fouskas, A.; Kollia, M.; Kambolis, A.; Papadopoulou, C.; Matralis, H. Boron-modified Ni/Al2O3 catalysts for reduced carbon deposition during dry reforming of methane. Appl. Catal. A-Gen. 2014, 474, 125–134. [Google Scholar] [CrossRef]
- Shin, J.H.; Kan, M.; Oh, J.W.; Yu, H.J.; Lin, L.C.; Kim, J.H.; Kang, D.; Lee, J.S. Solubility selectivity-enhanced SIFSIX-3-Ni-containing mixed matrix membranes for improved CO2/CH4 separation efficiency. J. Membr. Sci. 2021, 633, 119390. [Google Scholar] [CrossRef]
- Lin, K.; Yang, X.; Ma, X.; Han, L.; Li, X.; Wang, W.; Zhan, H.; Ma, B. Efficient bimetal loaded (Rh-Ni)/αβ-MoxC catalyst for CO2 methanation. J. Chem. Sci. 2021, 133, 108. [Google Scholar] [CrossRef]
- Xu, J.; Saeys, M. Improving the coking resistance of Ni-based catalysts by promotion with subsurface boron. J. Catal. 2006, 242, 217–226. [Google Scholar] [CrossRef]
- Xu, J.; Saeys, M. First principles study of the coking resistance and the activity of a boron promoted Ni catalyst. Chem. Eng. Sci. 2007, 62, 5039–5041. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Tan, K.; Borana, A.; Saeys, M. Effect of boron on the stability of Ni catalysts during steam methane reforming. J. Catal. 2009, 261, 158–165. [Google Scholar] [CrossRef]
- Shakir, M.D.; Sengupta, S.; Sinhamahapatra, A.; Liu, S.; Vuthaluru, H. B-Ni/MgAl2O4 catalyzed dry reforming of methane: The role of boron to resist the formation of graphitic carbon. Fuel 2022, 320, 123950. [Google Scholar] [CrossRef]
- Shein, I.R.; Medvedeva, N.I.; Ivanovskii, A.L. Electronic and structural properties of cementite-type M3X (M = Fe, Co, Ni; X = C or B) by first principles calculations. Phys. B-Condens. Matter 2006, 371, 126–132. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Feng, Z.; Li, Z. Electronic Structure and Mechanical Properties of NiB: A Promising Interphase Material for Future UHTCf/UHTC Composites. J. Am. Ceram. Soc. 2016, 99, 2110–2119. [Google Scholar] [CrossRef]
- Jain, P.K. A DFT-Based Study of the Low-Energy Electronic Structures and Properties of Small Gold Clusters. Struct. Chem. 2005, 16, 421–426. [Google Scholar] [CrossRef]
- Juárez-Sánchez, J.O.; Galván, D.H.; Posada-Amarillas, A. Combined DFT and NBO approach to analyze reactivity and stability of (CuS)n (n = 1–12) clusters. Comput. Theor. Chem. 2017, 1103, 71–82. [Google Scholar] [CrossRef]
- Arab, A.; Ziari, F.; Fazli, M. Electronic structure and reactivity of (TiO2)(n) (n = 1-10) nano-clusters: Global and local hardness based DFT study. Comput. Mater. Sci. 2016, 117, 90–97. [Google Scholar] [CrossRef]
- Garg, S.; Kaur, N.; Goel, N. Conceptual DFT and TDDFT study on electronic structure and reactivity of pure and sulfur doped (CrO3)(n) (n = 1–10) clusters. J. Mol. Graph. Model. 2020, 99, 107617. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio totalenergy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Wei, S.; Ma, D.; Sun, Q. The effect of environment on the reaction of water on the Ceria(111) surface: A DFT plus U study. J. Phys. Chem. C 2010, 114, 14891–14899. [Google Scholar] [CrossRef]
- Reuter, K.; Scheffler, M. Composition, structure, and stability of RuO2(110) as a function of oxygen pressure. Phys. Rev. B—Condens. Matter Mater. Phys. 2002, 65, 035406. [Google Scholar] [CrossRef]
- Fronzi, M.; Piccinin, S.; Delley, B.; Traversa, E.; Stampfl, C. Water adsorption on the stoichiometric and reduced CeO2(111) surface: A first-principles investigation. R. Soc. Chem. 2009, 11, 9188–9199. [Google Scholar] [CrossRef]



| Cluster Form | VIE | VEA | μ |
|---|---|---|---|
| (NiB)1 | 5.22 | 2.84 | −4.03 |
| (NiB)2 | 5.58 | 2.43 | −4.01 |
| (NiB)3 | 4.84 | 3.56 | −4.20 |
| (NiB)4 | 3.85 | 2.56 | −3.21 |
| (NiB)5 | 3.40 | 2.49 | −2.94 |
| (NiB)6 | 3.21 | 2.41 | −2.81 |
| (NiB)7 | 3.37 | 2.67 | −3.02 |
| (NiB)8 | 4.52 | 1.77 | −3.18 |
| (NiB)9 | 5.17 | 1.29 | −3.23 |
| (NiB)10 | 2.15 | 2.07 | −2.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Zhou, X.; Fu, C.; Nie, T.; Meng, Y. Electronic Properties and CO2-Selective Adsorption of (NiB)n (n = 1~10) Clusters: A Density Functional Theory Study. Molecules 2023, 28, 5386. https://doi.org/10.3390/molecules28145386
Hou M, Zhou X, Fu C, Nie T, Meng Y. Electronic Properties and CO2-Selective Adsorption of (NiB)n (n = 1~10) Clusters: A Density Functional Theory Study. Molecules. 2023; 28(14):5386. https://doi.org/10.3390/molecules28145386
Chicago/Turabian StyleHou, Meiling, Xing Zhou, Chao Fu, Tingting Nie, and Yu Meng. 2023. "Electronic Properties and CO2-Selective Adsorption of (NiB)n (n = 1~10) Clusters: A Density Functional Theory Study" Molecules 28, no. 14: 5386. https://doi.org/10.3390/molecules28145386
APA StyleHou, M., Zhou, X., Fu, C., Nie, T., & Meng, Y. (2023). Electronic Properties and CO2-Selective Adsorption of (NiB)n (n = 1~10) Clusters: A Density Functional Theory Study. Molecules, 28(14), 5386. https://doi.org/10.3390/molecules28145386







