Research on the Mechanism of Low-Temperature Oxidation of Asphaltene
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
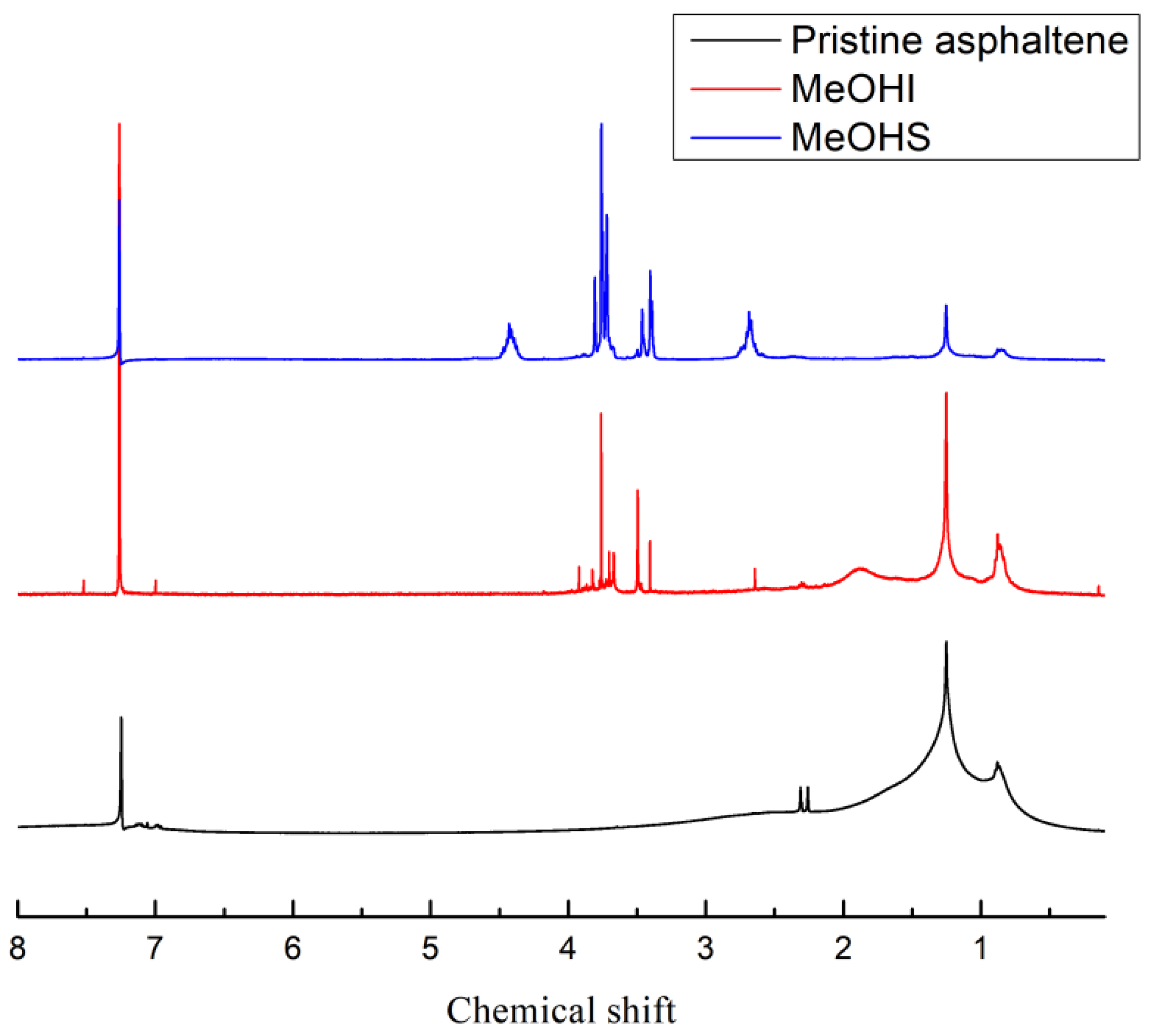
2.2. Nuclear Magnetic Spectrum Analysis
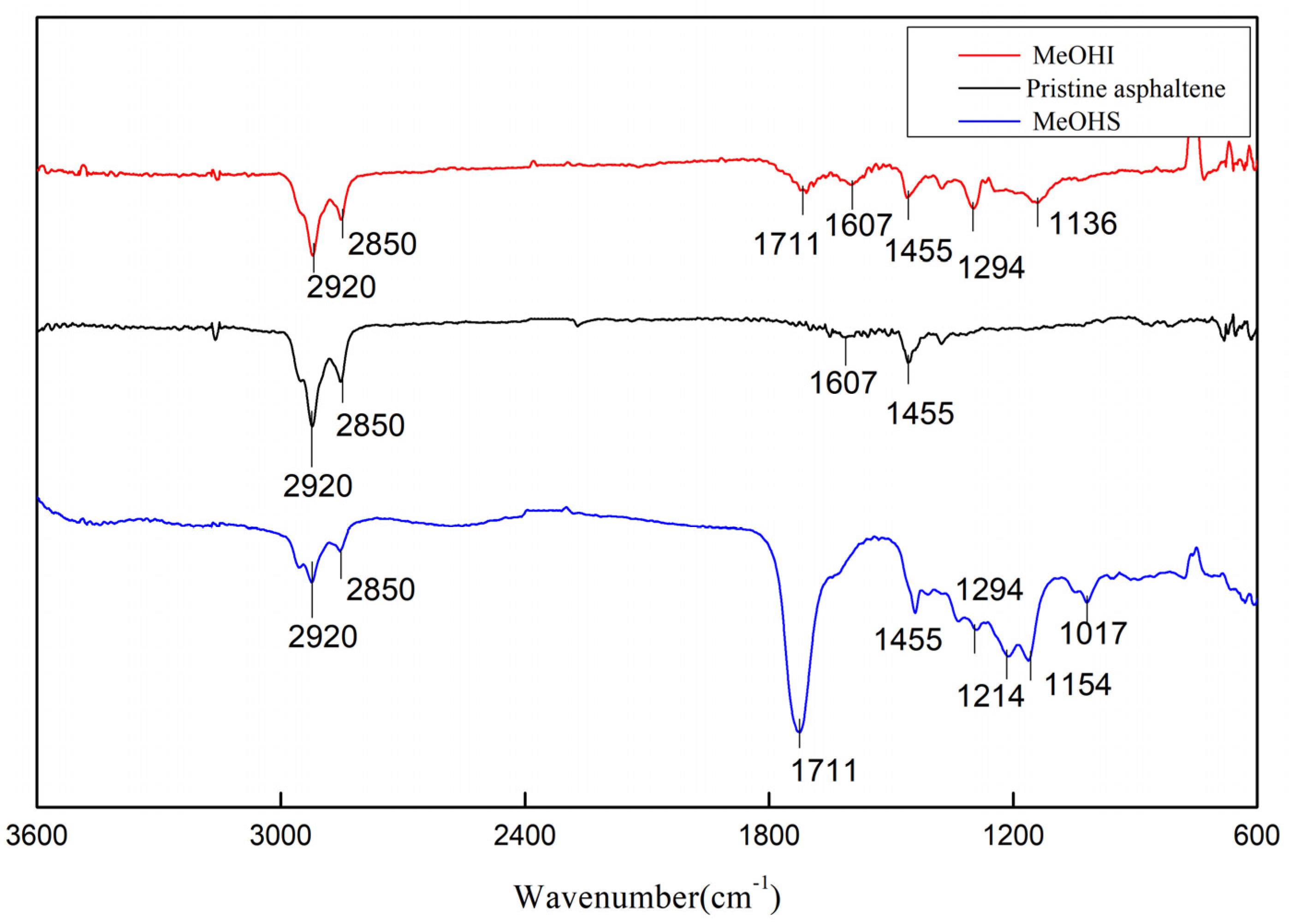
2.3. Infrared Spectroscopic Analysis
2.4. GC/MS Analysis of MeOHS
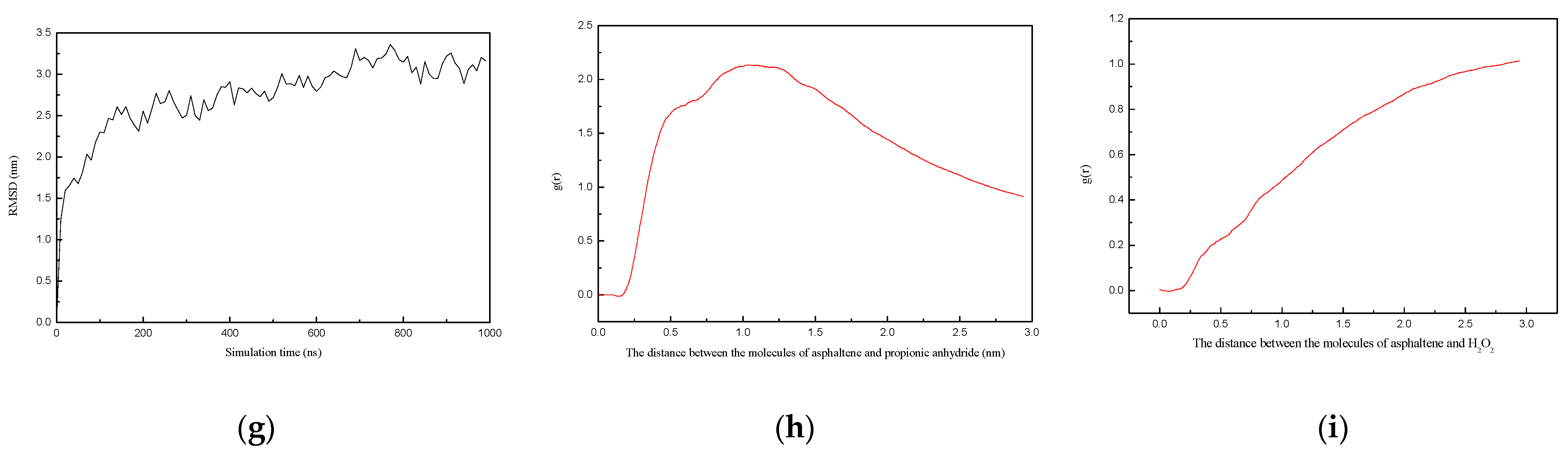
2.5. Molecular Dynamics Simulation Analysis
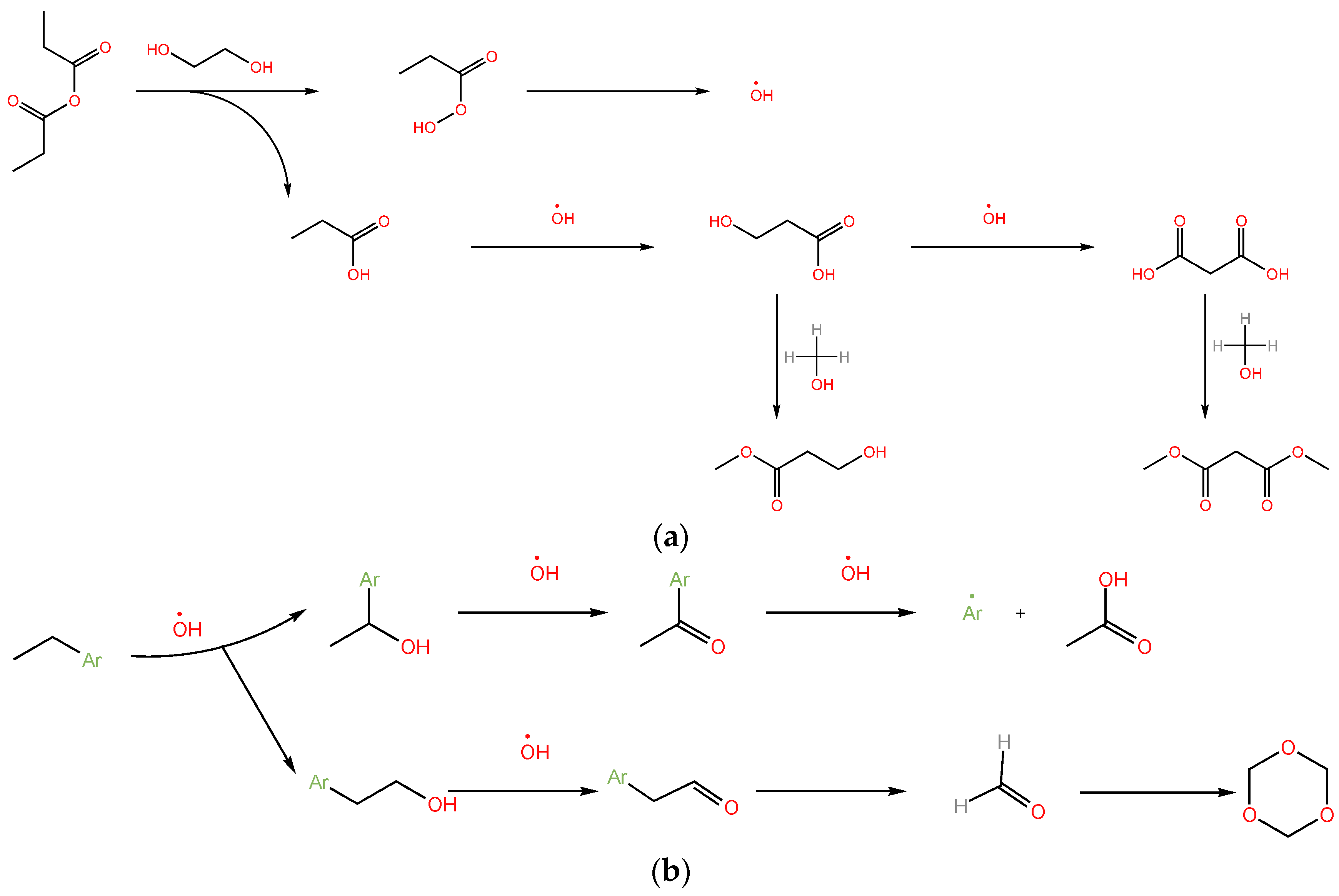
2.6. Oxidative Reaction Mechanism Analysis
3. Materials and Methods
3.1. Experimental Materials and Reagents
3.2. Asphaltene Oxidation Experimental Methods
3.3. Analysis Methods
3.4. Molecular Dynamics Simulation Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, S.; Tang, X.; Wang, J.; Zhang, B.; Sun, W.; Höök, M. Environmental impacts from conventional and shale gas and oil development in China considering regional differences and well depth. Resour. Conserv. Recycl. 2021, 167, 105368. [Google Scholar] [CrossRef]
- Li, X.; Chi, P.; Guo, X.; Sun, Q. Effects of asphaltene concentration and asphaltene agglomeration on viscosity. Fuel 2019, 255, 115825. [Google Scholar] [CrossRef]
- Lu, D.; Wang, W.; Chang, J.; Wang, X.; Wang, Y.; Song, H. Fabrication of Fe Nanoparticles into N-doped Mesoporous Carbon Nanotube Derived from Rice-Like Fe/N-MOF and its ORR Catalytic Performance for MFC. China Pet. Process. Petrochem. Technol. 2021, 23, 98–108. [Google Scholar]
- Nguyen, M.T.; Nguyen, D.L.T.; Xia, C.; Nguyen, T.B.; Shokouhimehr, M.; Sana, S.S.; Grace, A.N.; Aghbashlo, M.; Tabatabaei, M.; Sonne, C.; et al. Recent advances in asphaltene transformation in heavy oil hydroprocessing: Progress, challenges, and future perspectives. Fuel Process. Technol. 2021, 213, 106681. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Li, Y.; Song, H. Catalytic asphaltene upgrading under methane environment: Solvent effect and its interaction with oil components. Fuel 2021, 291, 120157. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Hou, J.; Zhou, C. A mechanism study on the viscosity evolution of heavy oil upon peroxide oxidation and pyrolysis. Fuel 2018, 214, 123–126. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.; Zhang, S.; Wang, F.; Yan, Y. Molecular structure models of asphaltene in crude and upgraded bio-oil. Chem. Eng. Technol. 2014, 37, 1198–1204. [Google Scholar] [CrossRef]
- Pomerantz, A.E.; Hammond, M.R.; Morrow, A.L.; Mullins, O.C.; Zare, R.N. Two-step laser mass spectrometry of asphaltenes. J. Am. Chem. Soc. 2008, 130, 7216–7217. [Google Scholar] [CrossRef]
- Korneev, D.S.; Pevneva, G.S.; Voronetskaya, N.G. Effects of the composition and molecular structure of heavy oil asphaltenes on their reactivity in thermal decomposition processes. Pet. Chem. 2021, 61, 152–161. [Google Scholar] [CrossRef]
- Fakher, S.; Ahdaya, M.; Elturki, M.; Imqam, A. Critical review of asphaltene properties and factors impacting its stability in crude oil. J. Pet. Explor. Prod. Technol. 2020, 10, 1183–1200. [Google Scholar] [CrossRef]
- Alshareef, A.H. Asphaltenes: Definition, properties, and reactions of model compounds. Energy Fuels 2020, 34, 16–30. [Google Scholar] [CrossRef]
- Strausz, O.; Mojelsky, T.; Lown, E. The molecular structure of asphaltene: An unfolding story. Fuel 1992, 77, 1355–1363. [Google Scholar] [CrossRef]
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef] [PubMed]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; Mcfarlane, R.; Goual, L.; et al. Advances in asphaltene science and the Yen-Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Mckenna, A.M.; Chacón-Patiño, M.L.; Weisbrod, C.R.; Blakney, G.T.; Rodgers, R.P. Molecular-level characterization of asphaltenes isolated from distillation cuts. Energy Fuels 2019, 33, 2018–2029. [Google Scholar] [CrossRef]
- Gould, K.A.; Wiehe, I.A. Natural hydrogen donors in petroleum resids. Energy Fuels 2007, 21, 1199–1204. [Google Scholar] [CrossRef]
- Mullins, O.C. The asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef]
- George, G.N.; Gorbaty, M.L. Sulfur K-edge X-ray absorption spectroscopy of petroleum asphaltenes and model compounds. J. Am. Chem. Soc. 1989, 197, 3182–3186. [Google Scholar] [CrossRef]
- Mitrakirtley, S.; Mullins, O.C.; Vanelp, J.; George, S.J.; Cramer, S.P. Determination of the nitrogen chemical structures in petroleum asphaltenes using XANES spectroscopy. J. Am. Chem. Soc. 1993, 115, 252–258. [Google Scholar] [CrossRef]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Li, N.; Yan, B.; Xiao, X. Kinetic and reaction pathway of upgrading asphaltene in supercritical water. Chem. Eng. Sci. 2015, 134, 230–237. [Google Scholar] [CrossRef]
- Ashtari, M.; Carbognani Ortega, L.; Lopez-Linares, F.; Eldood, A.; Pereira-Almao, P. New pathways for asphaltenes upgrading using the Oxy-Cracking process. Energy Fuels 2016, 30, 4596–4608. [Google Scholar] [CrossRef]
- Dehghani, F.; Ayatollahi, S.; Bahadorikhalili, S.; Esmaeilpour, M. Synthesis and characterization of mixed-metal oxide nanoparticles (CeNiO3, CeZrO4, CeCaO3) and application in adsorption and catalytic oxidation-decomposition of asphaltenes with different chemical structures. Pet. Chem. 2020, 60, 731–743. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Olmos, C.M.; Chen, X.; Cortés, F.B.; Franco, C.A. Effect of multifunctional nanocatalysts on n-C7 asphaltene adsorption and subsequent oxidation under high-pressure conditions. Energy Fuels 2020, 34, 6261–6278. [Google Scholar] [CrossRef]
- Sadegh Mazloom, M.; Hemmati-Sarapardeh, A.; Husein, M.M.; Shokrollahzadeh Behbahani, H.; Zendehboudi, S. Application of nanoparticles for asphaltenes adsorption and oxidation: A critical review of challenges and recent progress. Fuel 2020, 279, 117763. [Google Scholar] [CrossRef]
- Ezeonyeka, N.L.; Hemmati-Sarapardeh, A.; Husein, M.M. Asphaltenes adsorption onto metal oxide nanoparticles: A critical evaluation of measurement techniques. Energy Fuels 2018, 32, 2213–2223. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Mayo, F.R. Application of sodium hypochlorite oxidations to the structure of coal. Fuel 1975, 54, 273–275. [Google Scholar] [CrossRef]
- Mayo, F.R.; Kirshen, N.A. Oxidations of coal by aqueous sodium hypochlorite. Fuel 1979, 58, 698–704. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Yan, H.; Liu, F.; Li, P.; Zong, Z. Sequential oxidation of Jincheng No. 15 anthracite with aqueous sodium hypochlorite. Fuel Process. Technol. 2014, 125, 182–189. [Google Scholar] [CrossRef]
- Strausz, O.P.; Mojelsky, T.W.; Lown, E.M.; Kowalewski, I.; Behar, F. Structural features of Boscan and Duri asphaltenes. Energy Fuels 1999, 13, 228–247. [Google Scholar] [CrossRef]
- Cheshkova, T.V.; Kovalenko, E.Y.; Sagachenko, T.A.; Min, R.S.; Golushkova, E.B. Composition of petroleum asphaltenes derived from ruthenium-catalyzed oxidation. Mendeleev Commun. 2022, 32, 139–141. [Google Scholar] [CrossRef]
- Djerassi, C.; Engle, R.R. Oxidations with ruthenium tetroxide. J. Am. Chem. Soc. 1953, 75, 3838–3840. [Google Scholar] [CrossRef]
- Miura, K.; Mae, K.; Okutsu, H.; Mizutani, N. New oxidative degradation method for producing fatty acids in high yields and high selectivity from low-rank coals. Energy Fuels 1996, 10, 1196–1201. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Liu, J.; Yan, H.; Wei, Z.; Li, Y.; Li, P.; Liu, F.; Zong, Z. Oxidation of Shenmu char powder with aqueous hydrogen peroxide–acetic anhydride. Fuel Process. Technol. 2015, 136, 56–63. [Google Scholar] [CrossRef]
- Wu, J.; Gao, Y.; Zhang, W.; Tan, Y.; Tang, A.; Men, Y.; Tang, B. Deep oxidation desulfurization with a new imidazole-type acidic ionic liquid polymer. RSC Adv. 2014, 4, 58800–58804. [Google Scholar] [CrossRef]
- Fedorov, R.A.; Akopyan, A.V.; Anisimov, A.V.; Karakhanov, E.A. Peroxide Oxidative Desulfurization of Crude Petroleum in the presence of fatty acids. Int. J. Biol. Chem. 2018, 11, 173–178. [Google Scholar] [CrossRef]
- Li, B.; Song, H.; Han, F.; Wei, L. Photocatalytic oxidative desulfurization and denitrogenation for fuels in ambient air over Ti3C2/g-C3N4 composites under visible light irradiation. Appl. Catal. B Environ. 2020, 269, 118845. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, L.; Zhao, B.; Li, Y.; Lin, Z. Structural characteristics of humic acids derived from Chinese weathered coal under different oxidizing conditions. PLoS ONE 2019, 14, e217469. [Google Scholar] [CrossRef]
- Lu, T.; Lu, M. Remarkable effect of PEG-1000-based dicationic ionic liquid for n-hydroxyphthalimide-catalyzed aerobic selective oxidation of alkylaromatics. Croat. Chem. Acta 2012, 85, 277–282. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Blanquart, G.; Pitsch, H. A consistent chemical mechanism for oxidation of substituted aromatic species. Combust. Flame 2010, 157, 1879–1898. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Liu, Z.; Pu, W.; Huang, B.; Zhu, S.; Li, Y.; Ao, X.; Xiang, Z.; Lu, Y.; Wei, B. New insights on low temperature oxidation characteristics and possibility of auto-ignition in light oil reservoir. Geoenergy Sci. Eng. 2023, 223, 211583. [Google Scholar] [CrossRef]
- Cao, Y. Study on Molecular Structure Characteristics of Fractions and Catalytic Aquathermolysis of Shengli Heavy Oil. Ph.D. Thesis, China University of Petroleum (East China), Qingdao, China, 2017. [Google Scholar]
- Ok, S.; Mal, T.K. NMR spectroscopy analysis of asphaltenes. Energy Fuels 2019, 33, 10391–10414. [Google Scholar] [CrossRef]
- Parlov Vuković, J.; Novak, P.; Jednačak, T. NMR spectroscopy as a tool for studying asphaltene composition. Croat. Chem. Acta 2019, 92, 323–329. [Google Scholar] [CrossRef]
- Fergoug, T.; Bouhadda, Y. Determination of Hassi Messaoud asphaltene aromatic structure from 1H & 13C NMR analysis. Fuel 2014, 115, 521–526. [Google Scholar]
- Zojaji, I.; Esfandiarian, A.; Taheri-Shakib, J. Toward molecular characterization of asphaltene from different origins under different conditions by means of FT-IR spectroscopy. Adv. Colloid Interface Sci. 2021, 289, 102314. [Google Scholar] [CrossRef]
- Zuo, P.; Qu, S.; Shen, W. Asphaltenes: Separations, structural analysis and applications. J. Energy Chem. 2019, 34, 186–207. [Google Scholar] [CrossRef]
- Zhao, J.; Nanjo, T.; de Lucca, E.C.; White, M.C. Chemoselective methylene oxidation in aromatic molecules. Nat. Chem. 2019, 11, 213–221. [Google Scholar] [CrossRef]
- Shen, H.S.; Oehlschlaeger, M.A. The autoignition of C8H10 aromatics at moderate temperatures and elevated pressures. Combust. Flame 2009, 156, 1053–1062. [Google Scholar] [CrossRef]
- Grützner, T.; Hasse, H.; Lang, N.; Siegert, M.; Ströfer, E. Development of a new industrial process for trioxane production. Chem. Eng. Sci. 2007, 62, 5613–5620. [Google Scholar] [CrossRef]
- Johannsen, J.; Baek, G.; Fieg, G.; Waluga, T. An innovative approach for fatty acid reduction to fatty aldehydes. Green Chem. Lett. Rev. 2021, 14, 455–461. [Google Scholar] [CrossRef]
- Yang, H.; Yang, H.; Yan, X. Low-Temperature oxidation of heavy oil asphaltene with and without catalyst. Molecules 2022, 27, 7075. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Dodda, L.S.; de Vaca, I.C.; Tirado-Rives, J.; Jorgensen, W.L. 1.14*CM1A-LBCC: Localized Bond-Charge Corrected CM1A Charges for Condensed-Phase Simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef]
- Dodda, L.S.; Cabeza De Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Ren, Q.; Wang, L.; Yan, X. Influence of oxygen-containing functional groups on asphaltene self-diffusion coefficient in asphaltene-xylene systems. China Pet. Process. Petrochem. Technol. 2022, 24, 118–125. [Google Scholar]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Ok, S.; Mahmoodinia, M.; Rajasekaran, N.; Sabti, M.A.; Lervik, A.; van Erp, T.S.; Cabriolu, R. Molecular structure and solubility determination of asphaltenes. Energy Fuels 2019, 33, 8259–8270. [Google Scholar] [CrossRef]


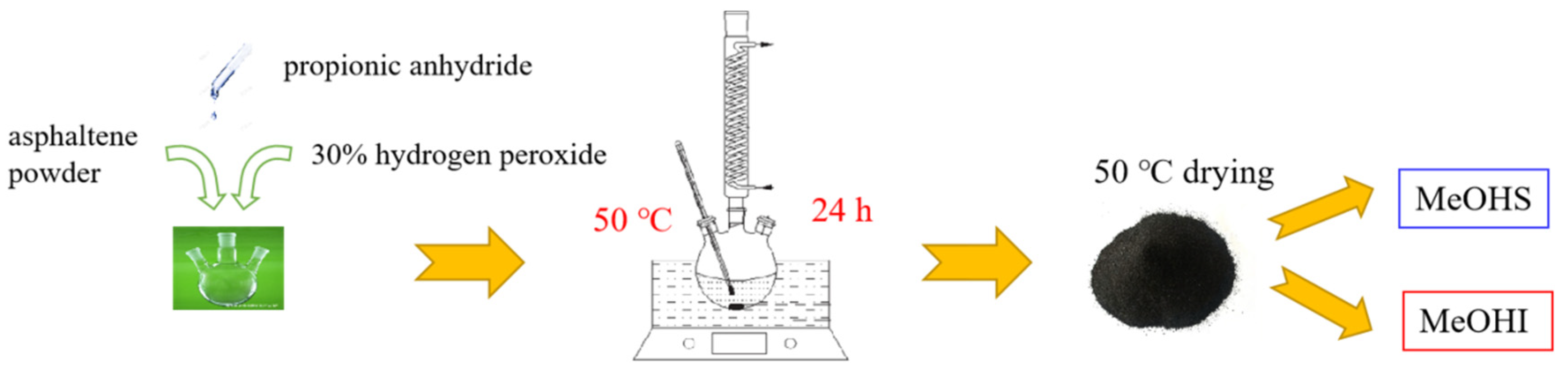
| Pristine Asphaltene | 30 wt.% Hydrogen Peroxide | Propionic Anhydride | MeOHS | MeOHI | |
|---|---|---|---|---|---|
| Dosage | 0.5520 g | 30 mL | 6.9 g | — | — |
| Production | — | — | — | 0.1974 g | 0.4982 g |
| Elemental Composition, wt.% | Atomic Ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | S | N | O | H/C | O/C | O/H | |
| before oxidation | ||||||||
| asphaltene, wt.% | 84.70 | 6.11 | 6.33 | 1.32 | 1.54 | 0.87 | 0.014 | 0.016 |
| after oxidation | ||||||||
| MeOHS, wt.% | 45.16 | 5.60 | 1.83 | 0.65 | 46.76 | 1.50 | 0.78 | 0.52 |
| MeOHI, wt.% | 71.13 | 4.70 | 5.12 | 1.05 | 18.00 | 0.79 | 0.19 | 0.24 |
| Pristine Asphaltene | MeOHS | MeOHI | |
|---|---|---|---|
| Relative value of integral area of NMR spectrum | |||
| Aromatic hydrogens (HA) | 0.16 | 0.19 | 0.22 |
| Hydrogens in α-position to aromatic ring (Hα) | 0.17 | 0.46 | 0.17 |
| CH2 and CH hydrogens other than in α-position to aromatic ring (Hβ) | 0.42 | 0.15 | 0.39 |
| Terminal (t-) CH3 hydrogens other than in α-position on aliphatic chain (Hγ) | 0.17 | 0.06 | 0.11 |
| C/H atomic ratio | 1.15 | 0.67 | 1.27 |
| Total hydrogens (HT) | 0.92 | 0.86 | 0.89 |
| Total carbons (CT) | 1.06 | 0.58 | 1.13 |
| Aromatic rate (fA) | 0.64 | 0.42 | 0.70 |
| Aromatic carbons (CA) | 0.68 | 0.24 | 0.80 |
| Peripheral hydrogen substitution rate of aromatic rings (σ) | 0.35 | 0.55 | 0.28 |
| Aromatic ring condensation degree parameters (HAU/CA) | 0.36 | 1.74 | 0.38 |
| Branching index of alkyl side chains (BI) | 0.40 | 0.40 | 0.28 |
| No. | Retention Time, min | Peak Area of Gas Chromatography, % | Corresponding Compounds | Molecular Structures |
|---|---|---|---|---|
| 1 | 5.708 | 21.54 | 1,3,5-trioxane | 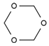 |
| 2 | 6.130 | 3.98 | 2-(methylsulfonylmethylsulfanyl) ethanol | 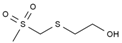 |
| 3 | 7.249 | 1.45 | methyl 2-hydroxyacetate | 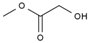 |
| 4 | 7.715 | 22.05 | acetic acid | 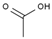 |
| 5 | 8.214 | 34.02 | dimethyl propanedioate |  |
| 6 | 8.616 | 10.03 | methyl 3-hydroxypropanoate | 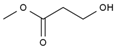 |
| 7 | 8.720 | 6.93 | pentan-2-yl acetate | 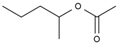 |
| Total | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Yang, H.; He, J.; Hu, F.; Cheng, F.; Liu, H.; Gong, C.; Wen, S. Research on the Mechanism of Low-Temperature Oxidation of Asphaltene. Molecules 2023, 28, 5362. https://doi.org/10.3390/molecules28145362
Zhao Z, Yang H, He J, Hu F, Cheng F, Liu H, Gong C, Wen S. Research on the Mechanism of Low-Temperature Oxidation of Asphaltene. Molecules. 2023; 28(14):5362. https://doi.org/10.3390/molecules28145362
Chicago/Turabian StyleZhao, Zhengchong, Haiyang Yang, Jingjing He, Fuqiang Hu, Fan Cheng, Hai Liu, Chunli Gong, and Sheng Wen. 2023. "Research on the Mechanism of Low-Temperature Oxidation of Asphaltene" Molecules 28, no. 14: 5362. https://doi.org/10.3390/molecules28145362
APA StyleZhao, Z., Yang, H., He, J., Hu, F., Cheng, F., Liu, H., Gong, C., & Wen, S. (2023). Research on the Mechanism of Low-Temperature Oxidation of Asphaltene. Molecules, 28(14), 5362. https://doi.org/10.3390/molecules28145362






