Weight Loss Supplements
Abstract
1. Introduction
2. Search Methodology
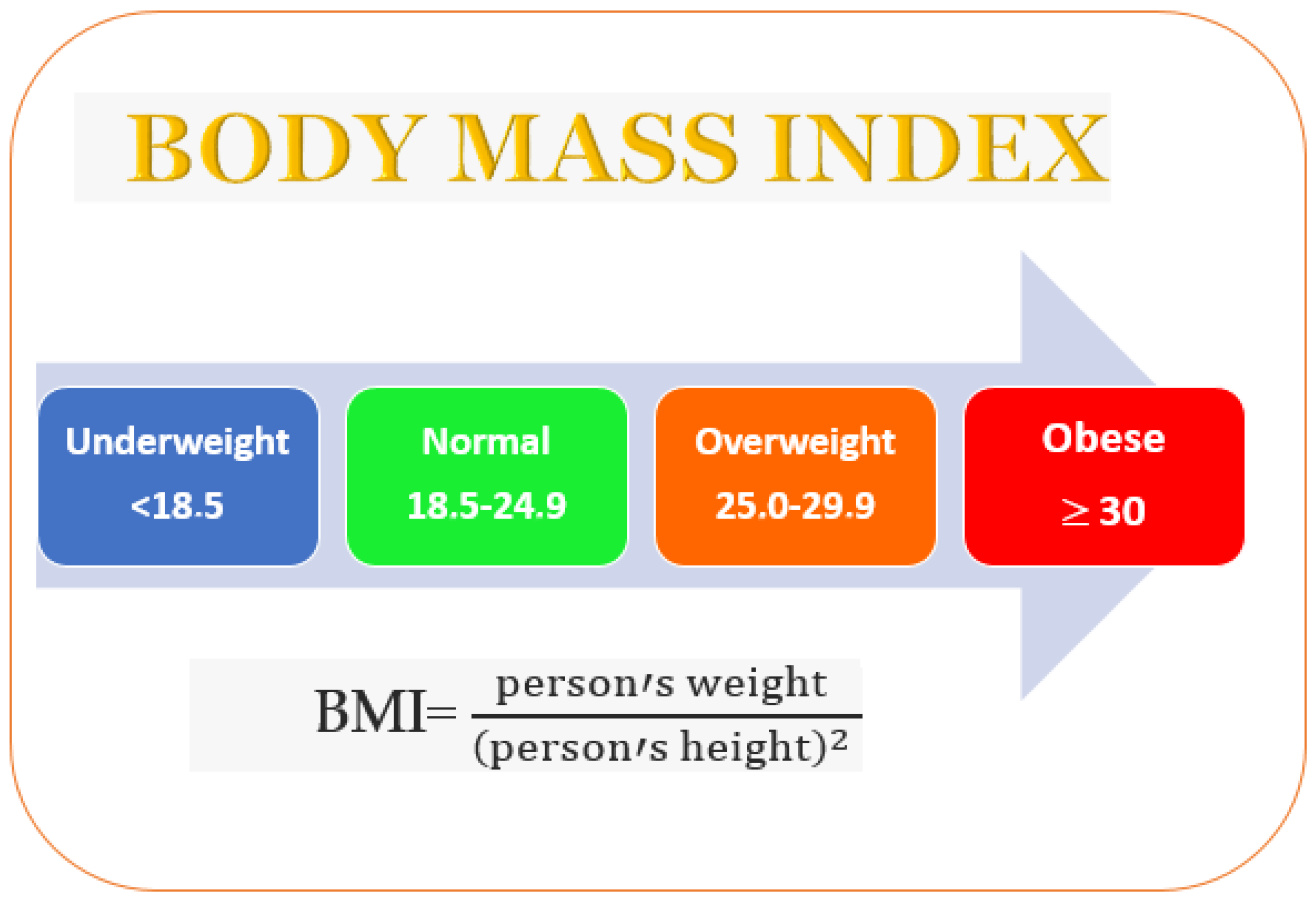
3. Obesity
4. Supplement Regulation
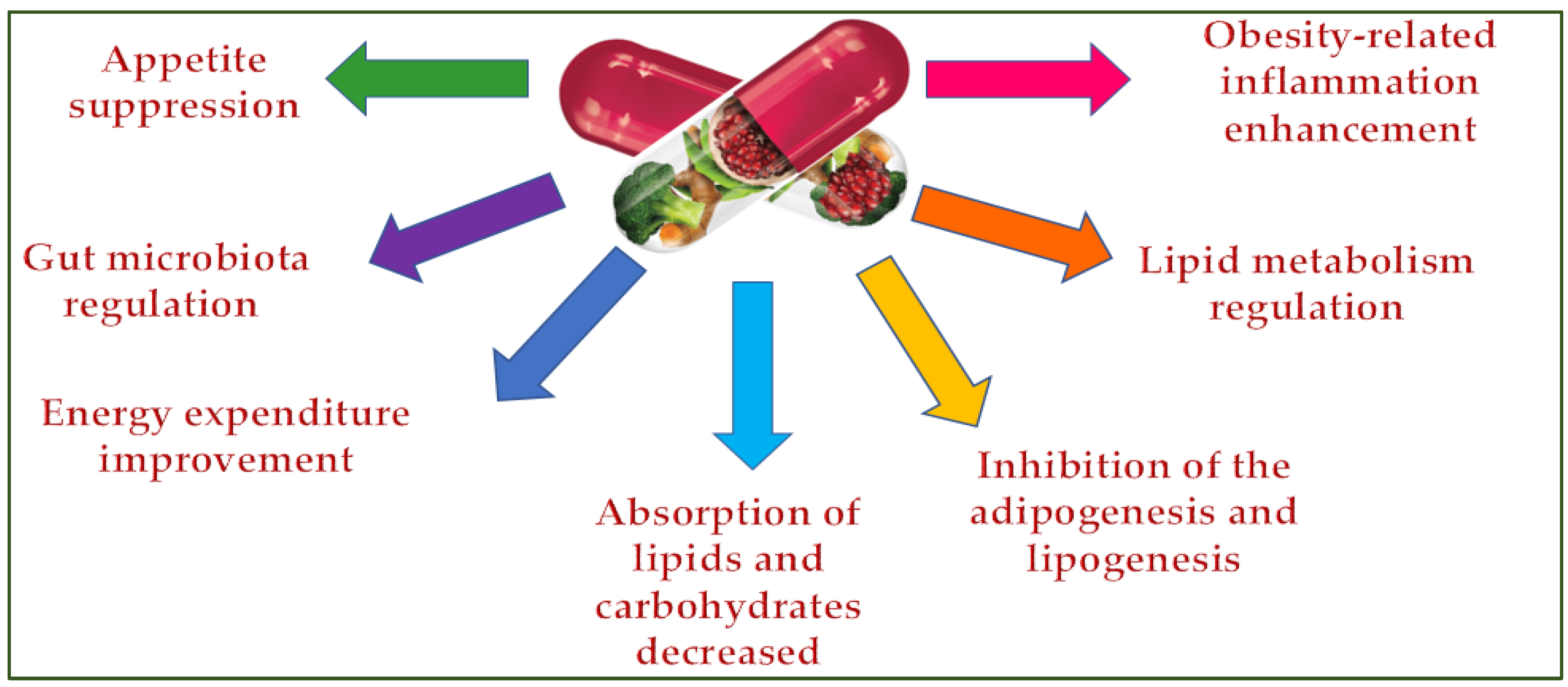
5. Weight Management Supplements
5.1. Plants Extract in Supplements for Weight Control Management
5.2. Dietary Supplements Able to Decrease the Appetite
5.3. Dietary Supplements Able to Interact with the Central Nervous System
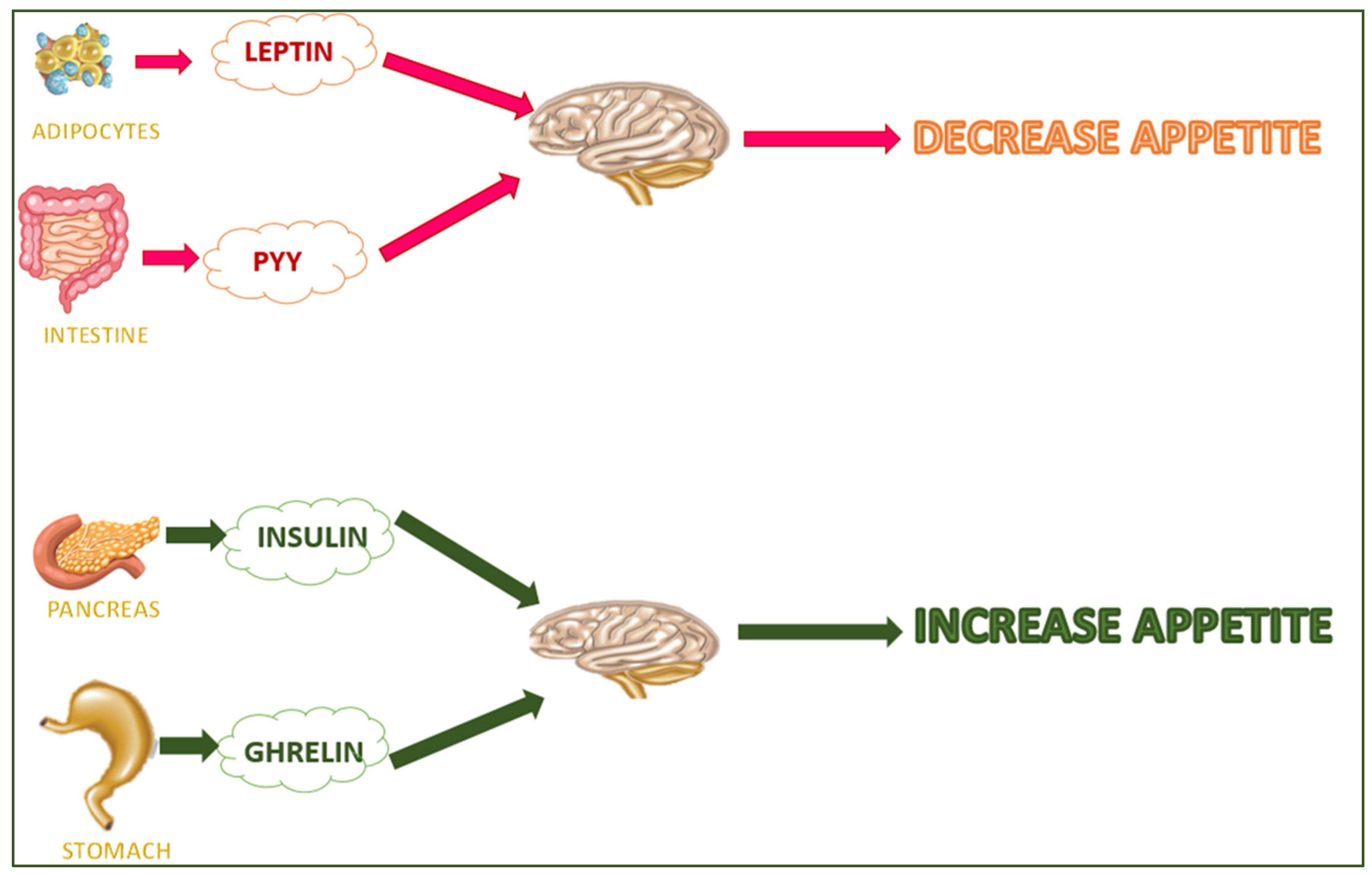
5.4. Dietary Supplements That Interact with the Hormones in the Digestive System and Adipose Tissue
5.5. Prebiotics in Weight Control Supplements
5.6. Probiotics in Weight Control Supplements
5.7. Symbiotics in Weight Control Supplements
5.8. Postbiotics in Weight Control Supplements
| Patent No | Title | Patent’s Country | Patent’s Year | Reference |
|---|---|---|---|---|
| Examples of dietary supplements in which plants or their metabolites are used as appetite suppressors | ||||
| JP2023041885A | Bioregulator-containing wheat flour and/or rice flour masterbatch for processed food and method for producing the same | Japan | 2023 | [151] |
| CN116058499A | Mediterranean diet fruit and vegetable fat-reducing meal replacement powder and preparation method and application thereof | China | 2023 | [152] |
| WO2010054469A1 | Appetite-suppressing weight management composition | Worldwide applications | 2008 | [153] |
| KR102041036B1 | Production Method of Crocetin and Health Supplement for Appetite Suppression Comprising Crocetin as an Active Ingredient | Republic of Korea | 2018 | [154] |
| WO2014020344A1 | Compounds and their effects on appetite control and insulin sensitivity | Worldwide applications | 2012 | [155] |
| CA2778381 | Dietary supplements and methods of use | United States | 2006 | [156] |
| US20060024388A1 | Plant-derived or derivable material with appetite-suppressing activity | United States | 2002 | [157] |
| US5945107A | Compositions and methods for weight reduction | United States | 1998 | [80] |
| Examples of dietary supplements in which plants or their metabolites are used as hormones and/or neurotransmitters activators | ||||
| KR20220026635A | Composition for preventing or treating obesity and/or metabolic syndrome comprising narcissoside | Republic of Korea | 2020 | [158] |
| KR102511950B1 | Dietary supplements for weight loss of pill type | Republic of Korea | 2020 | [159] |
| KR102461437B1 | Pharmaceutical composition for preventing or treating obesity with garcinia cambogia extract and health functional food with the same | Republic of Korea | 2022 | [160] |
| KR102511262B1 | A process for the preparation of five-grain bread comprising cheonggukjang and five-grain bread comprising cheonggukjang prepared therefrom | Republic of Korea | 2022 | [161] |
| US6759063B2 | Methods and compositions for reducing sympathomimetic-induced side effects | United States | 2002 | [162] |
| KR102438276B1 | Anti-inflammatory and antiobesity composition comprising Sargassum horneri extract and method for preparing the same | Republic of Korea | 2022 | [163] |
| Examples of dietary supplements in which plants or their metabolites interact with the hormones in the digestive system and adipose tissue | ||||
| WO2010053949A1 | Phytochemical compositions and methods for activating amp-kinase | Worldwide applications | 2009 | [164] |
| WO2017064530A1 | Agavaceae extract comprising steroidal saponins to treat or prevent metabolic-disorder-related pathologies | Worldwide applications | 2015 | [165] |
| Examples of dietary supplements in which prebiotics are used for weight control | ||||
| JP2023075270A | Prebiotics for treating disorders associated with disturbed composition or function of the gut microbiome | Japan | 2023 | [166] |
| CN113750172A | Weight-reducing composition and application thereof in preparation of weight-reducing product | China | 2021 | [167] |
| CN115466687A | Composition for reducing body fat content and body weight and application thereof | China | 2021 | [168] |
| Examples of dietary supplements in which probiotics are used for weight control | ||||
| CN116004472A | Clostridium butyricum for relieving obesity and application thereof | China | 2023 | [169] |
| CN114480228A | Probiotics for relieving metabolic syndrome, metabolite formula, and application thereof | China | 2022 | [170] |
| CN115300605A | Probiotic powder for resisting obesity and losing weight and application thereof | China | 2022 | [171] |
| Examples of dietary supplements in which symbiotics are used for weight control | ||||
| CN114376235A | Weight-reducing probiotics and prebiotics composition beneficial for controlling in vivo fat and preparation method thereof | China | 2022 | [172] |
| WO2023070512A1 | Composition of prebiotics and probiotics and use thereof | Worldwide applications | 2021 | [173] |
6. Discussion
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organisation. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 November 2022).
- Avila, C.; Holloway, A.C.; Campbell, M.K. Biological, environmental, and social influences on childhood obesity. Pediatr. Res. 2016, 79, 205–211. [Google Scholar]
- Pietrabissa, G. Group Motivation-Focused Interventions for Patients with Obesity and Binge Eating Disorder. Front. Psychol. 2018, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Prajapati, P.K. Diet and lifestyle guidelines for diabetes: Evidence based ayurvedic perspective. Romanian J. Diabetes Nutr. Metab. Dis. 2014, 21, 335–346. [Google Scholar] [CrossRef]
- World Food and Agriculture 2022 Statistical Yearbook. 2022. Available online: https://www.fao.org/3/cc2211en/cc2211en.pdf (accessed on 18 June 2023).
- Hahn, M.K.; Morrison, K.M.; Restivo, M.; Anglin, R.; Taylor, V.H. An overview of links between obesity and mental health. Curr. Obes. Rep. 2015, 4, 303–310. [Google Scholar]
- Rajan, T.M.; Menon, V. Psychiatric disorders and obesity: A review of association studies. J. Postgrad. Med. 2017, 63, 182–190. [Google Scholar]
- Lung, T.; Jan, S.; Tan, E.J.; Killedar, A.; Hayes, A. Impact of overweight, obesity and severe obesity on life expectancy of Australian adults. Int. J. Obes. 2019, 43, 782. [Google Scholar] [CrossRef]
- Bluher, M.; Aras, M.; Aronne, L.J.; Batterham, R.L.; Giorgino, F.; Ji, L.; Pietilainen, K.H.; Schnell, O.; Tonchevska, E.; Wilding, J.P.H. New insights into the treatment of obesity. Diabetes Obes. Metab. 2023, 25, 2058–2072. [Google Scholar] [CrossRef]
- Miricescu, D.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, D.A.; Popa, C.C.; Enyedi, M.; Nedelea, A.S.; Nica, A.E.; et al. Impact of adipose tissue in chronic kidney disease development (Review). Exp. Ther. Med. 2021, 21, 539. [Google Scholar] [CrossRef]
- McNabney, S.M. Obesity, Body Image Dissatisfaction, and Sexual Dysfunction: A Narrative Review. Sexes 2022, 3, 20–39. [Google Scholar] [CrossRef]
- Hebebrand, J.; Albayrak, O.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef]
- Meule, A.; Heckel, D.; Jurowich, C.F.; Vogele, C.; Kubler, A. Correlates of food addiction in obese individuals seeking bariatric surgery. Clin. Obes. 2014, 4, 228–236. [Google Scholar] [CrossRef]
- Fazzino, T.L.; Courville, A.B.; Guo, J.; Hall, K.D. Ad libitum meal energy intake is positively influenced by energy density, eating rate and hyper-palatable food across four dietary patterns. Nat. Food 2023, 4, 144–147. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Shang, A.; Gan, R.-Y.; Xu, X.-Y.; Mao, Q.-Q.; Zhang, P.-Z.; Li, H.-B. Effects and mechanisms of edible and medicinal plants on obesity: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2061–2077. [Google Scholar] [CrossRef]
- Khanna, D.; Welch, B.S.; Rehman, A. Pathophysiology of Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; et al. Lipid reducing activity and toxicity profiles of a library of polyphenol derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef]
- Kim, K.S.; Jang, M.J.; Fang, S.; Yoon, S.G.; Kim, I.Y.; Seong, J.K.; Hahm, D.H. Antiobesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. J. Amino Acids 2019, 51, 245–254. [Google Scholar] [CrossRef]
- Fitzgerald, M.P.; Hennigan, K.; Gorman, C.S.O.; Mccarron, L. Obesity, diet and lifestyle in 9-year-old children with parentally reported chronic diseases: Findings from the growing up in Ireland longitudinal child cohort study. Ir. J. Med. Sci. 2018, 188, 29–34. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Anoruo, M.D.; Sharma, S. Biochemistry, Lipoprotein Lipase; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Zhang, D.; Wei, Y.; Huang, Q.; Chen, Y.; Zeng, K.; Yang, W.; Chen, J.; Chen, J. Important Hormones Regulating Lipid Metabolism. Molecules 2022, 27, 7052. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Rood, J.; Janderova, L.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; Smith, S.R. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J. Clin. Endocrinol. Metab. 2004, 89, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Campbel, L.V. Insulin resistance and obesity. Clin. Dermatol. 2004, 22, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Singh, P.; Rai, S.N. Factors affecting obesity and its treatment. Obes. Med. 2019, 16, 100140. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Scherer, T.; Sakamoto, K.; Buettner, C. Brain Insulin Signalling in Metabolic Homeostasis and Disease. Nat. Rev. Endocrinol. 2021, 17, 468–483. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A Review on Obesity Management through Natural Compounds and a Green Nanomedicine-Based Approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef]
- Diep Nguyen, T.M. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Xu, W.; Tian, M.; Zhou, Y. The relationship between insulin resistance, adiponectin and C-reactive protein and vascular endothelial injury in diabetic patients with coronary heart disease. Exp. Ther. Med. 2018, 16, 2022–2026. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Gar, C.; Thorand, B.; Herder, C.; Sujana, C.; Heier, M.; Meisinger, C.; Peters, A.; Koenig, W.; Rathmann, W.; Roden, M.; et al. Association of circulating MR-proADM with all-cause and cardiovascular mortality in the general population: Results from the KORA F4 cohort study. PLoS ONE 2022, 17, e0262330. [Google Scholar] [CrossRef]
- Abizaid, A. Stress and obesity: The ghrelin connection. J. Neuroendocrinol. 2019, 31, e12693. [Google Scholar] [CrossRef]
- Zhou, Y.; Murugan, D.D.; Khan, H.; Huang, Y.; Cheang, W.S. Roles and Therapeutic Implications of Endoplasmic Reticulum Stress and Oxidative Stress in Cardiovascular Diseases. Antioxidants 2021, 10, 1167. [Google Scholar] [CrossRef]
- Dietary Supplement Market. Available online: https://www.researchandmarkets.com/reports/4479727/dietary-supplements-market-size-share-and-trends (accessed on 21 March 2022).
- Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements (accessed on 3 June 2023).
- Food Supplements. Available online: https://www.food.gov.uk/business-guidance/food-supplements (accessed on 4 July 2023).
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. OJ L 183, 12.7.2002, pp. 51–57. Consolidated Version 26/07/2017. Available online: http://data.europa.eu/eli/dir/2002/46/oj (accessed on 11 November 2019).
- Shehzad, A.; Rabail, R.; Munir, S.; Jan, H.; Fernández-Lázaro, D.; Aadil, R.M. Impact of Oats on Appetite Hormones and Body Weight Management: A Review. Curr. Nutr. Rep. 2023, 12, 66–82. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Hwang, K.A.; Hwang, Y.J.; Im, P.R.; Hwang, H.J.; Song, J.; Kim, Y.J. Platycodon grandiflorum Extract Reduces High-Fat Diet-Induced Obesity Through Regulation of Adipogenesis and Lipogenesis Pathways in Mice. J. Med. Food 2019, 22, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger prevents obesity through regulation of energy metabolism and activation of browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, D.; Zhu, Y.; Cai, S.; Liang, X.; Tang, Y.; Jin, S.; Ding, C. Swertiamarin supplementation prevents obesity-related chronic inflammation and insulin resistance in mice fed a high-fat diet. Adipocyte 2021, 10, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.A.; Granner, M.L.; Conway, J.M.; Ainsworth, B.E.; Dobre, M. Availability of Weight-Loss Supplements: Results of an Audit of Retail Outlets in a Southeastern City. J. Am. Diet. Assoc. 2006, 106, 2045–2051. [Google Scholar] [CrossRef]
- Younus, H.; Anwar, S. Prevention of Non-Enzymatic Glycosylation (Glycation): Implication in the Treatment of Diabetic Complication. Int. J. Health Sci. 2016, 10, 261–277. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.-A.; Macit, M.S.; Nascimento, I.J.B.D.; Guimaraes, N.S. The effect of green tea supplementation on obesity: A systematic review and dose–response metaanalysis of randomized controlled trials. Phytother. Res. 2020, 34, 2459–2470. [Google Scholar] [CrossRef]
- Liver Tox: Clinical and Research Information on Drug-Induced Injury. Green Tea. National Institute of Diabetes and Digestive and Kidney Diseases. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 7 December 2020).
- Wharton, S.; Bonder, R.; Jeffery, A.; Christensen, R.A.G. The safety and effectiveness of commonly-marketed natural supplements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016. Crit. Rev. Food Sci. Nutr. 2019, 60, 1614–1630. [Google Scholar] [CrossRef]
- Končić, M.Z. Getting More Than You Paid For: Unauthorized “Natural” Substances in Herbal Food Supplements on EU Market. Planta Med. 2018, 84, 394–406. [Google Scholar]
- Flis, P.; Mehrholz, D.; Nowicki, R.; Barańska-Rybak, W. Slim figure for high price. Urticaria due to weight loss products and performance enhancers—A review of three cases. Med. Ogólna Nauk. Zdrowiu 2015, 21, 369–371. [Google Scholar] [CrossRef]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The Use of Garcinia Extract (Hydroxycitric Acid) as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Obes. 2010, 2011, 509038. [Google Scholar]
- Huang, H.; Liao, D.; Zou, Y.; Chi, H. The effects of chitosan supplementation on body weight and body composition: A systematic review and metaanalysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 60, 1815–1825. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Milajerdi, A.; Varkaneh, H.K.; Gorjipour, M.M.; Esmaillzadeh, A. The effects of curcumin supplementation on body weight, body mass index and waist circumference: A systematic review and dose-response metaanalysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 171–180. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Bodzin, A.S.; Reino, D.C.; Wang, H.L.; Busuttil, R.W. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J. Gastroenter. 2016, 22, 10071–10076. [Google Scholar] [CrossRef]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Marconi, E.; Risaliti, F.; Bertoni, M.; Ippolito, F.M.; Maggini, V.; Gallo, E.; Firenzuoli, F.; et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: Case series and literature review. Intern. Emerg. Med. 2018, 13, 857–872. [Google Scholar] [CrossRef]
- Inayat, F.; Majeed, C.N.; Ali, N.S.; Hayat, M.; Vasim, I. The risky side of weight-loss dietary supplements: Disrupting arrhythmias causing sudden cardiac arrest. BMJ Case Rep. 2018, 11, e227531. [Google Scholar] [CrossRef]
- Escamilla-Ocañas, C.E.; Cantú-Martinez, L.; Martínez, H.R.; Cámara-Lemarroy, C.R. Acute toxic leukoencephalopathy associated with a non-prescription weight loss supplement: A report of two cases. Neurol. Sci. 2017, 38, 2199–2201. [Google Scholar] [CrossRef]
- Maharlouei, N.; Tabrizi, R.; Lankarani, K.B.; Rezaianzadeh, A.; Akbari, M.; Kolahdooz, F.; Rahimi, M.; Keneshlou, F.; Asemi, Z. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and metaanalysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1753–1766. [Google Scholar] [CrossRef]
- Gorji, Z.; Varkaneh, H.K.; Talaei, S.; Nazary-Vannani, A.; Clark, C.C.; Fatahi, S.; Rahmani, J.; Salamat, S.; Zhang, Y. The effect of green-coffee extract supplementation on obesity: A systematic review and dose-response metaanalysis of randomized controlled trials. Phytomedicine 2019, 63, 153018. [Google Scholar] [CrossRef]
- Whiting, S.; Derbyshire, E.J.; Tiwari, B. Could capsaicinoids help to support weight management? A systematic review and metaanalysis of energy intake data. Appetite 2014, 73, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Nachvak, S.M.; Rouhani, M.H. Effects of Spirulina supplementation on obesity: A systematic review and metaanalysis of randomized clinical trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the French Agency for Food, Environmental and Occupational Health & Safety. Available online: https://www.anses.fr/en/system/files/NUT2014SA0096EN.pdf (accessed on 18 June 2023).
- Luis, A.; Domingues, F.; Pereira, L. Metabolic changes after licorice consumption: A systematic review with metaanalysis and trial sequential analysis of clinical trials. Phytomedicine 2018, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Serna, A.; Marhuenda, J.; Arcusa, R.; Pérez-Piñeiro, S.; Sánchez-Macarro, M.; Victoria-Montesinos, D.; Cánovas, F.; Jones, J.; Caturla, N.; López-Román, J. Effectiveness of a Polyphenolic Extract (Lippia Citriodora and Hibiscus Sabdariffa) on Appetite Regulation in Overweight and Obese Grade I Population: A 8 weeks Randomized, Double-Blind, Cross-Over, Placebo-Controlled Trial. Randomized Control. Trial 2020, 61, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.; Tan, O.; Molina, J. Systematic Review and Metaanalysis of a Proprietary Alpha-Amylase Inhibitor from White Bean (Phaseolus vulgaris L.) on Weight and Fat Loss in Humans. Foods 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and Drugs for Weight Loss and Health in Obesity—An Update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef]
- Appetite Suppressant. 2004. Available online: https://patents.google.com/patent/US20050214362A1/en (accessed on 18 June 2023).
- Thompson, M.S.; Yan, T.H.; Saari, N.; Sarbini, S.R. A review: Resistant starch, a promising prebiotic for obesity and weight management. Food Biosci. 2022, 50, 101965. [Google Scholar] [CrossRef]
- Si, Y.; Sha, X.-S.; Shi, L.-L.; Wei, H.-Y.; Jin, Y.-X.; Ma, G.-X.; Zhang, J. Review on Pregnane Glycosides and Their Biological Activities. Phytochem. Lett. 2022, 47, 1–17. [Google Scholar] [CrossRef]
- Compositions and Methods for Weight Reduction. 1998. Available online: https://patents.google.com/patent/US5945107 (accessed on 18 June 2023).
- Satiating Dietetic Product. 2001. Available online: https://patents.google.com/patent/WO2002094038A1/en (accessed on 18 June 2023).
- Al-Sayyar, A.; Hammad, M.M.; Williams, M.R.; Al-Onaizi, M.; Abubaker, J.; Alzaid, F. Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance. Metabolites 2023, 13, 384. [Google Scholar] [CrossRef]
- Goit, R.K.; Taylor, A.W.; Lo, A.C.Y. The central melanocortin system as a treatment target for obesity and diabetes: A brief overview. Eur. J. Pharmacol. 2022, 924, 174956. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Yoon, H.-Y.; Yee, J.; Gwak, H.-S. Effects of Ephedrine-Containing Products on Weight Loss and Lipid Profiles: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Pharmaceuticals 2021, 14, 1198. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Kim, J.H.; Hahm, D.H.; Yang, D.C.; Kim, J.H.; Lee, H.J.; Shim, I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J. Pharmacol. Sci. 2005, 97, 124–131. [Google Scholar] [CrossRef]
- Anilkumar, A.T.; Manoharan, S.; Balasubramanian, S.; Perumal, E. Garcinia gummi-gutta: Phytochemicals and pharmacological pplications. BioFactors 2023, 49, 584–599. [Google Scholar] [CrossRef]
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on antiobesity effect of garcinia origin (−)-HCA. Evid. Based Complement. Altern. Med. 2013, 2013, 751658. [Google Scholar] [CrossRef]
- The Regulation Of Appetite, Body Weight and Athletic Function with Materials Derived from Citrus Varieties. 1996. Available online: https://patents.google.com/patent/CA2248854C/en (accessed on 18 June 2023).
- Murakami, S.; Hirazawa, C.; Ohya, T.; Yoshikawa, R.; Mizutani, T.; Ma, N.; Moriyama, M.; Ito, T.; Matsuzaki, C. The Edible Brown Seaweed Sargassum horneri (Turner) C. Agardh Ameliorates High-Fat Diet-Induced Obesity, Diabetes, and Hepatic Steatosis in Mice. Nutrients 2021, 13, 551. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Laurà, R.; Abbate, F.; Levanti, M.; Maugeri, A.; Germanà, A.; Navarra, M. Effects of a Flavonoid-Rich Extract from Citrus sinensis Juice on a Diet-Induced Obese Zebrafish. Int. J. Mol. Sci. 2019, 20, 5116. [Google Scholar] [CrossRef]
- Kola, B. Role of AMP-Activated Protein Kinase in the Control of Appetite. J. Neuroendocr. 2008, 20, 942–951. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with antiobesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zdunczyk, Z. Physiological effect of low digestible oligosaccharides in diets for animals and humans. Pol. J. Food Nutr. Sci. 2004, 13, 115–130. [Google Scholar]
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Howlett, J.F.; Betteridge, V.A.; Champ, M.; Craig, S.A.S.; Meheust, A.; Jones, J.M. The definition of dietary fiber—Discussions at the Ninth Vahouny Fiber Symposium: Building scientific agreement. Food Nutr. Res. 2010, 54, 5750. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging 2020, 12, 5539–5550. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Gaudier, E.; Rival, M.; Buisine, M.-P.; Robineau, I.; Hoebler, C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol. Res. 2009, 58, 111–119. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: A systematic review and metaanalysis. Genes 2018, 9, 167. [Google Scholar] [CrossRef]
- Guazzelli Marques, C.; de Piano Ganen, A.; Zaccaro de Barros, A.; Thomatieli Dos Santos, R.V.; Dos Santos Quaresma, M.V.L. Weight Loss Probiotic Supplementation Effect in Overweight and Obesity Subjects: A Review. Clin. Nutr. 2020, 39, 694–704. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H.; Keramati, M.; Jamilian, P.; Jamilian, P.; Mohagheghi, A.; Farnam, A. Probiotics as an effective therapeutic approach in alleviating depression symptoms: An umbrella metaanalysis. Crit. Rev. Food Sci. Nutr. 2022; Online ahead of print. [Google Scholar]
- Barathikannan, K.; Chelliah, R.; Rubab, M.; Daliri, E.B.-M.; Elahi, F.; Kim, D.-H.; Agastian, P.; Oh, S.-Y.; Oh, D.H. Gut Microbiome Modulation Based on Probiotic Application for Antiobesity: A Review on Efficacy and Validation. Microorganisms 2019, 7, 456. [Google Scholar] [CrossRef]
- Savcheniuk, O.; Kobyliak, N.; Kondro, M.; Virchenko, O.; Falalyeyeva, T.; Beregova, T. Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of non-alcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement. Altern. Med. 2014, 14, 247. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Hijová, E. Synbiotic Supplements in the Prevention of Obesity and Obesity-Related Diseases. Metabolites 2022, 12, 313. [Google Scholar] [CrossRef]
- Mischke, M.; Arora, T.; Tims, S.; Engels, E.; Sommer, N.; van Limpt, K.; Baars, A.; Oozeer, R.; Oosting, A.; Bäckhed, F.; et al. Specific synbiotics in early life protect against diet-induced obesity in adult mice. Diabetes Obes. Metab. 2018, 20, 1408–1418. [Google Scholar] [CrossRef]
- Hoffmann, D.E.; Fraser, C.M.; Palumbo, F.; Ravel, J.; Rowthorn, V.; Schwartz, J. Probiotics: Achieving a better regulatory fit. Food Drug Law J. 2014, 69, 237–272. [Google Scholar] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Tomar, S.K.; Anand, S.; Sharma, P.; Sangwan, V.; Mandal, S. Role of probiotics, prebiotics, synbiotics and postbiotics in inhibition of pathogens. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; pp. 717–732. [Google Scholar]
- Zhang, J.; Du, G.-C.; Zhang, Y.; Liao, X.-Y.; Wang, M.; Li, Y.; Chen, J. Glutathione protects Lactobacillus sanfranciscensis against freeze-thawing, freeze-drying, and cold treatment. Appl. Environ. Microbiol. 2010, 76, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Liu, S.-Q. Fortifying foods with synbiotic and postbiotic preparations of the probiotic yeast, Saccharomyces boulardii. Curr. Opin. Food Sci. 2022, 43, 216–224. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut Microbiota Interacts with Markers of Adipose Tissue Browning, Insulin Action and Plasma Acetate in Morbid Obesity. Mol. Nutr. Food Res. 2017, 62, 1700721. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Jung, R.; Shetty, P.; James, W.; Barrand, M.; Callingham, B. Reduced thermogenesis in obesity. Nature 1979, 279, 322–323. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Hanatani, S.; Motoshima, H.; Takaki, Y.; Kawasaki, S.; Igata, M.; Matsumura, T.; Kondo, T.; Senokuchi, T.; Ishii, N.; Kawashima, J. Acetate alters expression of genes involved in beige adipogenesis in 3T3-L1 cells and obese KK-Ay mice. J. Clin. Biochem. Nutr. 2016, 59, 16–23. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Unser, A.M.; Tian, Y.; Xie, Y. Opportunities and challenges in three-dimensional brown adipogenesis of stem cells. Biotechnol. Adv. 2015, 33, 962–979. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: Current evidence, controversies, and perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef]
- Cavallari, J.F.; Fullerton, M.D.; Duggan, B.M.; Foley, K.P.; Denou, E.; Smith, B.K.; Desjardins, E.M.; Henriksbo, B.D.; Kim, K.J.; Tuinema, B.R. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab. 2017, 25, 1063–1074.e1063. [Google Scholar] [CrossRef]
- Cavallari, J.F.; Barra, N.G.; Foley, K.P.; Lee, A.; Duggan, B.M.; Henriksbo, B.D.; Anhê, F.F.; Ashkar, A.A.; Schertzer, J.D. Postbiotics for NOD2 require nonhematopoietic RIPK2 to improve blood glucose and metabolic inflammation in mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E579–E585. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Duggan, B.M.; Singh, A.M.; Chan, D.Y.; Schertzer, J.D. Postbiotics engage IRF4 in adipocytes to promote sex-dependent changes in blood glucose during obesity. Physiol. Rep. 2022, 10, e15439. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Park, S.-J.; Sharma, A.; Lee, H.-J. Postbiotics against Obesity: Perception and Overview Based on Pre-Clinical and Clinical Studies. Int. J. Mol. Sci. 2023, 24, 6414. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, H.G.; Han, S.; Seo, K.-H.; Kim, H. Effect of surface layer proteins derived from paraprobiotic kefir lactic acid bacteria on inflammation and high-fat diet-induced obesity. J. Agric. Food Chem. 2021, 69, 15157–15164. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.Y.; Kang, S.-S.; Yun, C.-H.; Han, S.H. Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Mol. Immunol. 2015, 64, 183–189. [Google Scholar] [CrossRef]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y. Lipoteichoic acid is involved in the ability of the immunobiotic strain Lactobacillus plantarum CRL1506 to modulate the intestinal antiviral innate immunity triggered by TLR3 activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Álvarez, B.; Chenoll, E.; Ramon, D.; Tortajada, M.; Martorell, P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2022, 15, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Li, S.; Shen, P.; Park, Y. Caenorhabditis elegans as a model for obesity research. Curr. Res. Food Sci. 2021, 4, 692–697. [Google Scholar] [CrossRef]
- Garsin, D.A.; Villanueva, J.M.; Begun, J.; Kim, D.H.; Sifri, C.D.; Calderwood, S.B.; Ruvkun, G.; Ausubel, F.M. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 2003, 300, 1921. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Ryan, P.; Ross, R.; Fitzgerald, G.; Caplice, N.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Li, Y.; Zhou, L.; Ding, Q.; Xu, L. Isolated exopolysaccharides from Lactobacillus rhamnosus GG alleviated adipogenesis mediated by TLR2 in mice. Sci. Rep. 2016, 6, 36083. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Oh, N.; Park, J.; Kwon, M.; Seo, J.; Roh, S. Oral intake of Lactobacillus plantarum L-14 extract alleviates TLR2- and AMPK-mediated obesity-associated disorders in high-fat-diet-induced obese C57BL/6J mice. Cell Prolif. 2021, 54, e13039. [Google Scholar] [CrossRef]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic postbiotic from Lactobacillus paracasei manages metabolic syndrome in albino wistar rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef]
- Hossain, M.; Park, D.-S.; Rahman, M.; Ki, S.-J.; Lee, Y.R.; Imran, K.; Yoon, D.; Heo, J.; Lee, T.-J.; Kim, Y.-S. Bifidobacterium longum DS0956 and Lactobacillus rhamnosus DS0508 culture-supernatant ameliorate obesity by inducing thermogenesis in obese-mice. Benef. Microbes 2020, 11, 361–373. [Google Scholar]
- Imperial, I.C.; Ibana, J.A. Addressing the antibiotic resistance problem with probiotics: Reducing the risk of its double-edged sword effect. Front. Microbiol. 2016, 7, 1983. [Google Scholar] [CrossRef]
- Bioregulator-Containing Wheat Flour and/or Rice Flour Masterbatch for Processed Food and Method for Producing the Same. 2023. Available online: https://patents.google.com/patent/JP2023041885A/en?q=(Appetite+suppressing+supplement)&before=priority:20231231&after=priority:20230101&oq=Appetite+suppressing+supplement+2023 (accessed on 18 June 2023).
- Mediterranean Diet Fruit and Vegetable Fat-Reducing Meal Replacement Powder and Preparation Method and Application Thereof. 2023. Available online: https://patents.google.com/patent/CN116058499A/en?q=(weight+loss+supplement)&before=priority:20231231&after=priority:20230101&oq=weight+loss+supplement+2023&page=1 (accessed on 18 June 2023).
- Appetite Suppressing Weight Management Composition. 2008. Available online: https://patents.google.com/patent/WO2010054469A1 (accessed on 18 June 2023).
- Production Method of Crocetin and Health Supplement for Appetite Suppression Comprising Crocetin as an Active Ingredient. 2018. Available online: https://patents.google.com/patent/KR102041036B1/en?q=(Appetite+suppressing+supplement)&oq=Appetite+suppressing+supplement (accessed on 18 June 2023).
- Compounds and Their Effects on Appetite Control and Insulin Sensitivity. 2012. Available online: https://patents.google.com/patent/WO2014020344A1/tr (accessed on 18 June 2023).
- Dietary Supplement and Methods of Use. 2006. Available online: https://brevets-patents.ic.gc.ca/opic-cipo/cpd/eng/patent/2778381/summary.html (accessed on 18 June 2023).
- Plant Derived or Derivable Material with Appetite Suppressing Activity. 2002. Available online: https://patents.google.com/patent/US20060024388A1 (accessed on 19 September 2005).
- Composition for Preventing or Treating Obesity and/or Metabolic Syndrome Comprising Narcissoside. 2020. Available online: https://patents.google.com/patent/KR20220026635A/en?q=(%22neuropeptide+Y%22+%22food+supplement%22)&before=priority:20201231&after=priority:20200101&oq=%22neuropeptide+Y%22++%22food+supplement%22+2020 (accessed on 18 June 2023).
- Dietary Supplements for Weight Loss of Pill Type. 2020. Available online: https://patents.google.com/patent/KR102511950B1/en?q=(red+ginseng+obesity+%22food+supplement%22)&before=priority:20201231&after=priority:20200101&oq=red+ginseng++obesity+%22food+supplement%22+2020 (accessed on 18 June 2023).
- Pharmaceutical Composition for Preventing or Treating Obesity Having Garcinia Cambogia Extract and Health Functional Food Having the Same. 2022. Available online: https://patents.google.com/patent/KR102461437B1/en?q=(dietary+supplement+anti+obesity+amines)&before=priority:20221231&after=priority:20220101&oq=dietary+supplement+anti+obesity+++amines++2022 (accessed on 18 June 2023).
- A process for the Preparation of Five Grain Bread Comprising Cheonggukjang and the Five Grain Bread Comprising Cheonggukjang Prepared There from. 2022. Available online: https://patents.google.com/patent/KR102511262B1/en?q=(dietary+supplement+anti+obesity+amines)&before=priority:20221231&after=priority:20220101&oq=dietary+supplement+anti+obesity+++amines++2022&page=2 (accessed on 18 June 2023).
- Methods and Compositions for Reducing Sympathomimetic-Induced Side Effects. 2002. Available online: https://patents.google.com/patent/US6759063B2/en?q=(dietary+supplement+Citrus+aurantium+weightloss)&assignee=L.&oq=dietary+supplement+Citrus+aurantium+L.++weightloss&page=1 (accessed on 18 June 2023).
- Anti-Inflammatory and Antiobesity Composition Comprising Sargassum Horneri Extract and Method for Preparing the Same. 2022. Available online: https://patents.google.com/patent/KR102438276B1/en?q=(insulin+obesity+%22food+supplement%22)&before=priority:20221231&after=priority:20220101&oq=insulin++obesity+%22food+supplement%22+2022 (accessed on 18 June 2023).
- Phytochemical Compositions and Methods for Activating Amp-Kinase. 2009. Available online: https://patents.google.com/patent/WO2010053949A1/en?q=(dietary+supplement+Citrus+aurantium+weightloss)&assignee=L.&oq=dietary+supplement+Citrus+aurantium+L.++weightloss (accessed on 18 June 2023).
- Agavaceae extract Comprising Steroidal Saponins to Treat or Prevent Metabolic Disorder-Related Pathologies. 2016. Available online: https://patents.google.com/patent/WO2017064530A1/en?q=(carnitine+palmitoyl+transferase+1A+obese+%22food+supplement%22)&oq=carnitine+palmitoyl+transferase+1A+obese++%22food+supplement%22++++&page=2 (accessed on 18 June 2023).
- Prebiotics for Treating Disorders Associated with Disturbed Composition or Function of the Gut Microbiome. 2023. Available online: https://patents.google.com/patent/JP2023075270A/en?q=(prebiotic+weightloss)&before=priority:20231231&after=priority:20230101&oq=prebiotic+weightloss+2023 (accessed on 18 June 2023).
- Weight-Reducing Composition and Application Thereof in Preparation of Weight-Reducing Product. 2021. Available online: https://patents.google.com/patent/CN113750172A/en?q=(prebiotic+prebiotic+weightloss)&before=priority:20211231&after=priority:20210101&oq=prebiotic+prebiotic+weightloss+2021&page=10 (accessed on 18 June 2023).
- Composition for Reducing Body Fat Content and Body Weight and Application Thereof. 2021. Available online: https://patents.google.com/patent/CN115466687A/en?q=(prebiotic+prebiotic+weightloss)&before=priority:20211231&after=priority:20210101&oq=prebiotic+prebiotic+weightloss+2021&page=11 (accessed on 18 June 2023).
- Clostridium Butyricum for Relieving Obesity and Application Thereof. 2023. Available online: https://patents.google.com/patent/CN116004472A/en?q=(prebiotic+weightloss)&before=priority:20231231&after=priority:20230101&oq=prebiotic+weightloss+2023 (accessed on 18 June 2023).
- Probiotics for Relieving Metabolic Syndrome, Metabolite Formula and Application Thereof. 2022. Available online: https://patents.google.com/patent/CN114480228A/en?q=(probiotic+weightloss)&before=priority:20221231&after=priority:20220101&oq=probiotic+weightloss+2022 (accessed on 18 June 2023).
- Probiotic Powder for Resisting Obesity and Losing Weight and Application Thereof. 2022. Available online: https://patents.google.com/patent/CN115300605A/en?q=(probiotic+weightloss)&before=priority:20221231&after=priority:20220101&oq=probiotic+weightloss+2022&page=2 (accessed on 18 June 2023).
- Weight-Reducing Probiotics and Prebiotics Composition Beneficial to Controlling In Vivo Fat and Preparation Method Thereof. 2022. Available online: https://patents.google.com/patent/CN114376235A/en?q=(probiotic+weightloss)&before=priority:20221231&after=priority:20220101&oq=probiotic+weightloss+2022&page=2 (accessed on 18 June 2023).
- Composition of Prebiotics and Probiotics and Use Thereof. 2021. Available online: https://patents.google.com/patent/WO2023070512A1/en?q=(probiotic+prebiotic+weightloss)&before=priority:20211231&after=priority:20210101&oq=probiotic+prebiotic+weightloss+2021&page=1 (accessed on 18 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; Mancusi, A. Weight Loss Supplements. Molecules 2023, 28, 5357. https://doi.org/10.3390/molecules28145357
Dini I, Mancusi A. Weight Loss Supplements. Molecules. 2023; 28(14):5357. https://doi.org/10.3390/molecules28145357
Chicago/Turabian StyleDini, Irene, and Andrea Mancusi. 2023. "Weight Loss Supplements" Molecules 28, no. 14: 5357. https://doi.org/10.3390/molecules28145357
APA StyleDini, I., & Mancusi, A. (2023). Weight Loss Supplements. Molecules, 28(14), 5357. https://doi.org/10.3390/molecules28145357







