Abstract
Smartphone-assisted fluorescence and colorimetric methods for the on-site detection of Hg2+ and Cl− were established based on the oxidase-like activity of the Au–Hg alloy on the surface of Au/Cu/Ti3C2 NSs. The Au nanoparticles (NPs) were constructed via in-situ growth on the surface of Cu/Ti3C2 NSs and characterized by different characterization techniques. After the addition of Hg2+, the formation of Hg–Au alloys could promote the oxidization of o-phenylenediamine (OPD) to generate a new fluorescence emission peak of 2,3-diaminopenazine (ADP) at 570 nm. Therefore, a turn-on fluorescence method for the detection of Hg2+ was established. As the addition of Cl− can influence the fluorescence of ADP, the fluorescence intensity was constantly quenched to achieve the continuous quantitative detection of Cl−. Therefore, a turn-off fluorescence method for the detection of Cl− was established. This method had good linear ranges for the detection of Hg2+ and Cl− in 8.0–200.0 nM and 5.0–350.0 µM, with a detection limit of 0.8 nM and 27 nM, respectively. Depending on the color change with the detection of Hg2+ and Cl−, a convenient on-site colorimetric method for an analysis of Hg2+ and Cl− was achieved by using digital images combined with smartphones (color recognizers). The digital picture sensor could analyze RGB values in concentrations of Hg2+ or Cl− via a smartphone app. In summary, the proposed Au/Cu/Ti3C2 NSs-based method provided a novel and more comprehensive application for environmental monitoring.
1. Introduction
Many anions and cations often have a significant influence on physiological functions and the environmental system. For example, Cl− is a common anion with essential physiological functions which takes part in many important biological processes, such as the regulation of cell volume, membrane potential, and intracellular pH. However, excess Cl− can cause severe dehydration and even plant death, as well as health issues such as high blood pressure and various heart diseases [1,2,3]. Hg2+ as global environmental pollutants were found in the air, soil, and water. Inorganic mercury (Hg2+) can transform into neurotoxin methylmercury (MeHg) through bacterial conversion within water bodies, which can damage the human brain, lungs, and central nervous system via the food chain [4,5,6,7]. Therefore, the detection of trace Cl− and Hg2+ is particularly urgent and necessary for disease diagnosis, environmental monitoring, and food safety evaluation [8,9,10,11,12,13]. Various conventional methods have been developed for the detection of Hg2+ and Cl−, including inductively coupled plasma mass spectrometry (ICP-MS), energy dispersive X-ray spectroscopy (EDX), and ion chromatography [14,15,16,17,18,19]. Despite the significant accuracy and precision of the above methods, they suffer from limitations including expensive equipment, tedious sample preparation, and on-site detection. Therefore, it is necessary to look for facile, portable, and cost-effective strategies for the detection of Cl− and Hg2+.
The colorimetric method has attracted the most attention due to its simple readout and obvious color change which can be observed by the naked eye [20,21]. There is a great demand to achieve a high-sensitivity colorimetric method which can greatly expand its applications field [22]. Recently, the nanozyme-based colorimetric method was used for the detection of ion at the nmol/L level, which could be equivalent to the analytical results from expensive equipment. Zhou et al. developed the colorimetric detection of Hg2+ by Au nanoparticles formed with the H2O2 reduction of HAuCl4 [23]. Logan et al. demonstrated amalgamated Au-nanoalloys with enhanced catalytical activity for the colorimetric detection of Hg2+ in seawater samples [24]. Wang et al. developed a single-nanozyme colorimetric array based on target-induced differential surface passivation for the quantification and discrimination of Cl−, Br−, and I− [25]. However, Cl− and Hg2+ usually coexist in the practical clinical or environmental matrix, with only a few works reported on colorimetric methods for the continuous monitoring of Cl− and Hg2+. Yan et al. proposed an efficient strategy for the visual detection and removal of toxic Cl− and Hg2+ based on the β-Cyclodextrin and graphene oxide co-strengthened AgRu bimetal mesoporous nanozyme [26]. Unfortunately, this method accelerated the oxidation of colorless 3,3′,5,5′-tetramethylbenzidine (TMB) with H2O2 in blue oxTMB. The instability of H2O2 could interfere with the accuracy of the corresponding sensing method. Therefore, it is still a challenge to explore the colorimetric methods for the continuous monitoring of Cl− and Hg2+ based on oxidase-like nanozymes which catalyzed the substrate without H2O2.
As a new two-dimensional material, Ti3C2 nanosheets (NSs) as the most promising MXene material derived from the MAX phase have intriguing metallic conductivity, high stability, a large surface area and ease of functionalization, as well as biocompatibility, which has wide applications in the fields of biosensing, catalysis, energy, and nanomedicine [27,28,29,30,31,32]. MXene cannot be directly synthesized from M and X elements for their thermodynamic metastability. The employment of the HF etching strategy is generally used for the fabrication of MXene, although HF acid is severely corrosive and toxic. Therefore, it is very important to develop a fluorine-free method for MXene preparation for its applications. Recently, Ti3C2 NSs attracted wide attention for its enzyme-like activity. Chen et al. prepared nitrogen-sulfur-doped Ti3C2 NSs with peroxidase-like activity and an electrochemical ability for the quantitative detection of uric acid [33]. Wu et al. synthesized histidine-modified Ti3C2 NSs, which can simulate the catalytic performance of peroxidase for a colorimetric paper-based sensor of glucose [34]. But until now, reports about the Ti3C2 NSs-based nanozyme with oxidase-like activity as well as nanozyme-based sensing are limited.
Herein, we fabricated a smartphone-enabled fluorescence and colorimetric platform for the on-site detection of Hg2+ and Cl− based on the oxidase-like activity of Au/Cu/Ti3C2 NSs (Figure 1). The Au nanoparticles (NPs) were in-situ loaded onto the surface of Cu/Ti3C2 NSs to form Au/Cu/Ti3C2 NSs. The Au/Cu/Ti3C2 NSs were characterized and their enzyme-like activities were studied. After the addition of Hg2+ with different concentrations, the formation of Hg–Au alloys could promote the oxidization of o-phenylenediamine (OPD) to generate a new fluorescence emission peak at 570 nm. Therefore, a turn-on fluorescence method for the detection of Hg2+ could be established. Given the interaction between Hg2+ and Cl− and the inhibition of the formation of Hg–Au alloys, the fluorescence intensity was constantly quenched to achieve the quantitative detection of Cl−. Depending on the color change with the detection of Hg2+ and Cl−, a convenient colorimetric method for Hg2+ and Cl− could be developed by using digital images combined with smartphones (color recognizers) to analyze RGB values in concentrations of Hg2+ or Cl−. The digital picture sensor based on a smartphone tested Cl− and Hg2+ in water samples and held great promise for environmental monitoring.
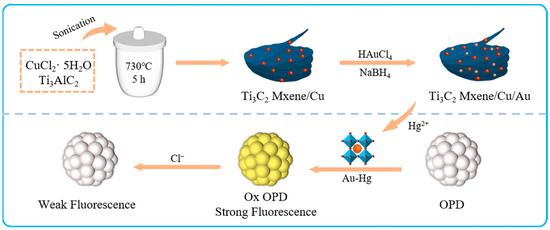
Figure 1.
Schematic illustration of synthesis of Au/Cu/Ti3C2 NSs and the recognition of Hg2+ and Cl−.
2. Results and Discussion
2.1. Characterization of Au/Cu/Ti3C2 NSs
Cu/Ti3C2 NSs was prepared by the one-step Lewis acidic etching route. Ti3AlC2 and CuCl2 were mixed evenly in a mortar and grinded for 10 min. The pulverized mixture was transferred to a porcelain crucible and roasted at 730 °C for 1 h. After cooling and quenching, FeCl3 solution and deionized water were added to wash the powder. The power was collected and dried in vacuum at 70 °C to obtain the products. The synthesized multilayer Cu/Ti3C2 NSs and Au/Cu/Ti3C2 NSs were characterized by SEM, TEM, XPS, and XRD. Figure 2a showed the XRD patterns of Ti3AlC2, Cu/Ti3C2 NSs, and Au/Cu/Ti3C2 NSs. Compared with the pristine Ti3AlC2, most diffraction peaks disappeared in the final Cu/Ti3C2 NSs. There were several wide low diffraction peaks in the 2θ ranging from 5° to 80°, which indicated that Ti3AlC2 had been successfully exfoliated into layered Cu/Ti3C2 NSs. The corresponding diffraction peak of Ti3C2 (002) shifted from 9.63° to 7.98°, demonstrating that the interlayer spacing had been expanded. The diffraction peaks at 2θ of 43.30°, 50.46°, and 74.09° corresponded to Cu, while those at 2θ of 38.1°, 44.5°, 65.1°, and 77.6° corresponded to Au, which demonstrated the successful loading of Au on the surface of Cu/Ti3C2 NSs [35,36]. Figure 2b shows the SEM image of multilayer Cu/Ti3C2 NSs, revealing that Cu/Ti3C2 NSs material had a good layered microstructure, which was consistent with the XRD data analysis and similar to what was previously reported for MXenes and obtained through etching methods with the HF- or F-containing electrolyte. Figure 2c,d shows EDS diagrams of multilayer Cu/Ti3C2 NSs. The element distribution map of Cu/Ti3C2 NSs showed that the nanosheets contained C, O, Cu, Ti, and a small amount of Al, which demonstrated that the majority of Al was etched by this method.
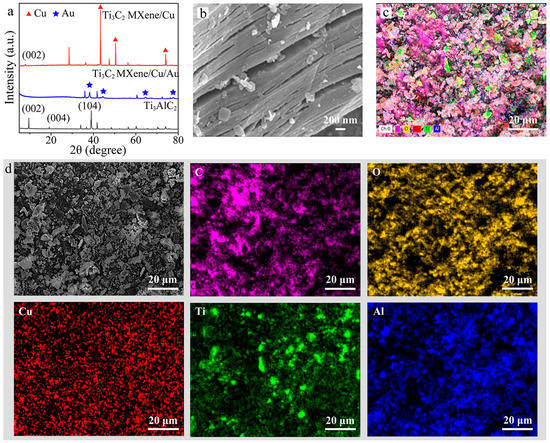
Figure 2.
(a) XRD patterns of Ti3AlC2, Cu/Ti3C2 NSs, and Au/Cu/Ti3C2 NSs, (b) SEM patterns of Cu/Ti3C2 NSs, (c) EDS element analysis of multilayer Cu/Ti3C2 NSs, and (d) the element distribution map.
The TEM images of Cu/Ti3C2 NSs and Au/Cu/Ti3C2 NSs clearly demonstrated that Cu nanoparticles and Au nanoparticles were uniformly distributed on the surface of Ti3C2 NSs (Figure 3a,b). The HRTEM image in the upper right corner of Figure 3b showed a lattice spacing of 0.29 nm, which corresponds to the (111) crystal plane of Au. The elemental composition and chemical bond of Au/Cu/Ti3C2 NSs were characterized by XPS spectra (Figure 3c). Au/Cu/Ti3C2 NSs contained five characteristic peaks according to the full spectrum analysis of XPS: Cu 2p (977.6 eV), O 1s (530.9 eV), Ti 2p (458.9 eV), C 1s (284.5 eV), and Au 4f (85.5 eV). It proved that Au/Cu/Ti3C2 NSs was successfully synthesized. The chemical bonds of Au/Cu/Ti3C2 NSs were analyzed by high-resolution XPS spectra, including Cu 2p, Ti 2p, C 1s, and Au 4f. As shown in Figure 3d–g, a deconvolution peak corresponding to C-C existed in the high-resolution XPS spectrum of C 1s. The high-resolution XPS spectrum of Ti 2p contained two deconvolution peaks corresponding to Ti-C and Ti-O. However, there were two deconvolution peaks in the high-resolution XPS spectra of Au 4f, indicating that Au mainly existed in the form of 0 valence and 1 valence.
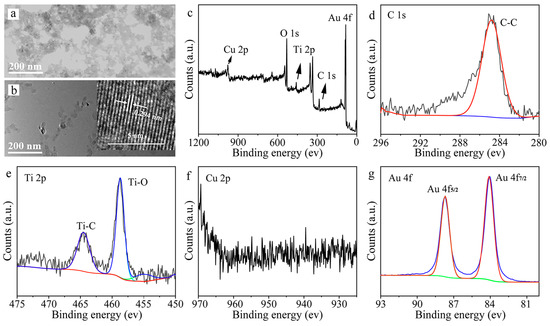
Figure 3.
(a) TEM image of Cu/Ti3C2 NSs, (b) TEM image of Au/Cu/Ti3C2 NSs, the inset of (b) was the HRTEM of Au/Cu/Ti3C2 NSs, (c) survey XPS spectra of Au/Cu/Ti3C2 NSs and spectra of Ti3C2 MXene/Cu/Au NSs, (d) C 1s, (e) Ti 2p, (f) Cu 2p, and (g) Au 4f.
2.2. Oxidase-like Activity of Au/Cu/Ti3C2 NSs
The feasibility of Au/Cu/Ti3C2 NSs + Hg2+ to catalysis substrate OPD was investigated by absorption spectra (Figure 4a) and fluorescence spectra (Figure 4b). In the absorption spectra, a new absorption peak at 420 nm appeared after the oxidation of OPD to 2,3-diaminopenazine (DAP) with the catalysis of Au/Cu/Ti3C2 NSs + Hg2+. In the fluorescence spectra, only when Au/Cu/Ti3C2 NSs and Hg2+ co-exist, OPD could be rapidly oxidized and a new fluorescence emission peak of DAP was generated at 570 nm. The oxidization process of OPD by dissolved oxygen was slow. The addition of Hg2+ to the solution resulted in the formation of Hg–Au alloys on the surface of Au/Cu/Ti3C2 NSs, which could accelerate the oxidization of OPD to generate DAP with both new absorption and a fluorescence signal [37]. Therefore, we intended to develop an absorption and fluorescent method for the sensing of Hg2+ via this phenomenon. To achieve the sensitive detection of Hg2+, several enzymatic factors that may influence the enzyme-substrate interactions were studied. The reaction time dependence of the Hg2+-induced fluorescence of DAP increasing was investigated. The fluorescence intensity increased when the reaction time was from 0 to 9 min and then kept stable with the reaction time from 9 to 12 min (Figure 4c,d). Therefore, 9 min was chosen as the optimal reaction time. The pH dependence of the Hg2+-induced fluorescence of DAP increasing was investigated in the pH range of 1.0 to 10.0. The fluorescence intensity increased abruptly from pH 1.0 to 6.0, and then decreased from pH 6.0 to 10.0 (Figure 4e). Therefore, a pH of 6.0 was chosen as the optimal pH.
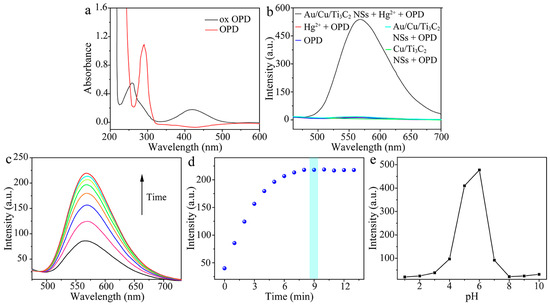
Figure 4.
(a) UV-vis spectra of OPD and DAP, (b) the feasibility of catalytic system for the detection of Hg2+ using florescence spectra (Au/Cu/Ti3C2 NSs + Hg2+ + OPD, Hg2+ + OPD, Au/Cu/Ti3C2 NSs + OPD, OPD, Cu/Ti3C2 NSs + OPD), (c) fluorescence spectra of DAP with time change, (d) scatter plot of fluorescence intensity of DAP and incubation time (n = 3), and (e) the influence of pH on fluorescence of the DAP (n = 3).
2.3. Detection of Hg2+
The feasibility of this method for the detection of Hg2+ was verified. Au/Cu/Ti3C2 NSs reacted with different concentrations of Hg2+ for 9 min to obtain the oxidase-like Au–Hg alloy, which catalyzed the oxidation of OPD to produce DAP. As shown in Figure 5a, when the concentration of Hg2+ gradually increased in the range from 0 to 200 nM, the fluorescence emission at 570 nm from the catalytic product DAP also increased continuously. Therefore, we established the fluorescence method for the detection of Hg2+. The linear relationship between the fluorescence intensity of DAP and the Hg2+ concentration was I = 1.434C + 159.575 (C is the concentration of Hg2+, nM). The linearly range for the detection of Hg2+ was 8.0 to 200.0 nM, with the detection limit of 0.8 nM. Since the new absorption peak of DAP at 420 nm also enhanced with the increased concentration of Hg2+, the correlation between the absorbance and concentration of Hg2+ was analyzed, as shown in Figure 5c. Therefore, we also established the colorimetric method for the detection of Hg2+. Within the concentration range of 24.0 to 200.0 nM, the linear equation of absorbance and Hg2+ concentration was A = 1.82 × 10−4C + 0.0334 (C is the concentration of Hg2+, nM), and the detection limit was 2.4 nM.
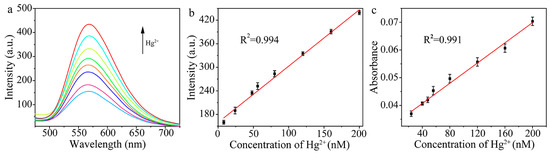
Figure 5.
(a) Fluorescence spectra of the system with different concentrations of Hg2+, (b) linear relationship between fluorescence intensity and Hg2+ concentration (n = 3), and (c) linear relationship between absorbance and Hg2+ concentration (n = 3).
2.4. Detection of Cl−
To study whether the Cl− can be detected via the influence on the oxidase-like activity of Hg–Au alloys on the surface of Au/Cu/Ti3C2 NSs, a series of Cl− with concentrations in the range from 0 to 350 μM was added to the system Au/Cu/Ti3C2 NSs + Hg2+ (Figure 6). Figure 6a showed that as the concentration of Cl− increased, the fluorescence intensity of DAP decreased gradually. As shown in Figure 6b, there were two linear relationships between the fluorescence intensity of DAP and Cl− concentration. Therefore, we established the fluorescence method for the detection of Cl−. When Cl− concentration increased from 10.0 μM to 150.0 μM, the linear equation of fluorescence intensity and Cl− concentration was I = −1.076C + 749.776 (C is the concentration of Cl−, μM). When the concentration of Cl− increased gradually from 150.0 μM to 350.0 μM, the linear equation of fluorescence intensity and Cl− concentration was I = −0.546C + 676.657 (C is the concentration of Cl−, μM). The detection limit was 27.0 nM. In addition, the relationship between the absorbance and the added Cl− concentration was also explored, as shown in Figure 6c. Therefore, we also established the colorimetric method for the detection of Cl−. In the range of 0.0 to 350.0 μM, the absorbance of the system had a good response to Cl− concentration. The linear equation was A = −1.24 × 10−4C + 0.109 (C is the concentration of Cl−, μM), and the detection limit was 1.0 μM.
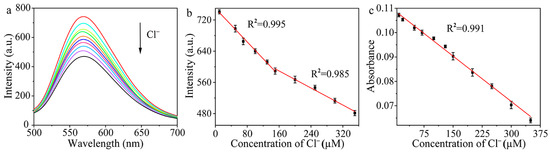
Figure 6.
(a) Fluorescence spectra of the system with different concentrations of Cl−, (b) linear relationship between fluorescence intensity and Cl− concentration (n = 3), and (c) linear relationship between absorbance and Cl− concentration (n = 3).
2.5. POCT for Hg2+ and Cl−
In order to simplify the detection procedure and realize instrument-free detection, portable test strips were prepared for the detection of Hg2+ and Cl−. The smartphone-assist platform was established and consisted of test strips containing Au/Cu/Ti3C2 NSs and a smartphone with an app as the signal reader and analyzer for the test strips. As the concentration of Hg2+ increased, the fluorescence color of the test strips changed from purple to pink (Figure 7a). Figure 7b showed the fluorescence color picture of the test strips with different concentrations of Cl−, and with the continuous addition of Cl− with different concentrations, the fluorescence color of the test strips changed from yellow to light green. The app software (color recognizer) in the smartphone acquired the color parameters (R, G, B value) of the photos. The RGB ratio (R/B) had a linear relationship with the concentration of Hg2+ over the range of 8.0 to 200.0 nM (Figure 7c). The linear equation of R/B and Hg2+ concentration was R/B = 0.0048C + 0.79 (C is the concentration of Hg2+, nM). The detection limit was 0.8 nM. There are two linear relationships between R/B and Cl− concentration, as shown in Figure 7d. Within the concentration range of 30.0 to 150.0 μM and 150.0 to 350.0 μM, the linear equations were R/B = −0.0016C + 1.309 and R/B = −7.396 × 10−4C + 1.191 (C is the concentration of Cl−, μM), and the detection limit was 3.0 μM. Compared with the fluorescence and colorimetric methods, the digital image method based on Au/Cu/Ti3C2 NSs was expected to be applied to the real-time and rapid detection of Hg2+ and Cl− concentrations in the field.
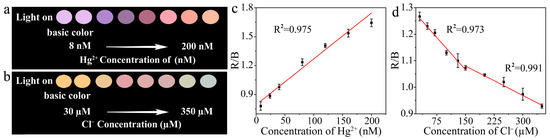
Figure 7.
(a) Pictures taken under the irradiation of 365 nm UV lamp when different concentrations of Hg2+ were added, (b) pictures taken under the irradiation of 365 nm UV lamp when different concentrations of Cl− were added, (c) linear relationship between R/B value and Hg2+ concentration (n = 3), and (d) linear relationship between R/B value and Cl− concentration (n = 3).
2.6. Selectivity for Hg2+ and Cl−
Selectivity is an important factor to evaluate the practicality of the method. In order to explore the practicability of this system for the detection of Hg2+, different anions and cations, including Na+, Ca2+, Mg2+, K+, Zn2+, Cu2+, CO32−, F−, Ac−, SO42−, and OH− were added to the Au/Cu/Ti3C2 NSs + OPD system. As shown in Figure 8a, the Au/Cu/Ti3C2 NSs + OPD system had an obvious response to Hg2+, while the fluorescence intensity of the system did not change significantly when detecting other ions.
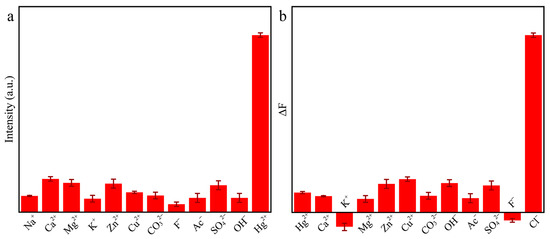
Figure 8.
The selectivity of the catalytic system to (a) Hg2+ and (b) Cl− (n = 3).
In addition, the reliability of the Ti3C2 MXene/Cu/Au + Hg2+ + OPD system for the detection of Cl− concentration was also discussed. Different anions and cations were added to the Ti3C2 MXene/Cu/Au + Hg2+ + OPD system, and the fluorescence intensity of the detection system was recorded by a fluorescence spectrometer. As shown in Figure 8b, the Ti3C2 MXene/Cu/Au + Hg2+ + OPD system had good specificity for the analysis of Cl−, while there was no obvious change in fluorescence intensity when detecting other ions. Therefore, it can be concluded that the detection system has good selectivity for Hg2+ and Cl−. Meanwhile, the influence of Cl− concentration on the detection of mercury ions was studied (Figure S1). When the concentration of Cl− ranged from 0 to 500 μM, there was no obvious effect on the detection of mercury ions.
2.7. Real Sample Detection
In order to verify the applicability of this method in detecting Hg2+ concentration in actual samples, water from Shahu Lake, East Lake, and Yangtze River was selected as actual samples, and the content of Hg2+ was evaluated using test strips. The specific results are shown in Table 1. The recoveries of Hg2+ concentration was 93.3 to 109.7%, and RSD ranged from 1.4% to 5.5%. The real sample detection for chloride ions was also investigated. As shown in Table S1, the recoveries of Cl− concentration vary from 93.3 to 109.7% with the RSDs of recoveries less than 5.5%. The results demonstrate that the developed method is practical and reliable for the detection of Hg2 and Cl−.

Table 1.
Analytical results for detection of Hg2+ in real samples (n = 3).
3. Experimental
3.1. Materials
All reagents were of at least analytical grade and used as received without purification. Ti3AlC2 was acquired from Forsman Technology Co., Ltd. (Beijing, China). Hg(NO3)2, CuSO4·5H2O, and HAuCl4 were supplied by Macklin Biochemical Co., Ltd. (Shanghai, China). O-Phenylenediamine (OPD) was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). NaBH4, NaCl, Na2CO3, FeCl3, MgCl2·6H2O, ZnCl2, KCl, CaCl2, Na2SO4, and KNO3 were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All solutions were freshly prepared before use.
3.2. Instuments
The UV-vis absorption measurements were observed by a Lambda 35 UV analyzer (Perkin-Elmer, Waltham, MA, USA). The excitation and emission spectra were obtained on a LS55 spectrophotometer (Perkin-Elmer, Waltham, MA, USA). The transmission electron microscopy (TEM) was performed using a TecnaiG20 transmission electron microscope (FEI, Hillsboro, OR, USA) and LEPL-Model 2100 F instrument. High-resolution transmission electron microscopy (HRTEM) was measured by a JEM-2100 UHR (JEOL, Tokyo, Japan). X-ray photo electron spectroscopy (XPS) was performed with a VGEscalab 200 spectrometer using an aluminum anode (AlKα) operating at 510 W with a background pressure of 2 × 10−9 mbar (Escalab, Waltham, MA, USA).
3.3. Preparation of Au/Cu/Ti3C2 NSs
Cu/Ti3C2 NSs was synthesized by a one-step Lewis acidic etching route. After being placed in a mortar, 0.5 g of Ti3AlC2 and 2.6 g of CuCl2·2H2O were mixed evenly and ground for 10 min. The pulverized mixture was transferred to a 50 mL porcelain crucible and roasted at 730 °C for 1 h. After cooling and quenching, 50.0 mL of 3.0 M FeCl3 solution was added to the powder and stirred continuously for 3 h. The precipitate was washed with deionized water, collected, and dried for 12 h in vacuum at 70 °C.
Then, 100.0 mg of Cu/Ti3C2 NSs was dispersed in 25.0 mL of deionized water and ultrasonicated for 5 h. The suspension with Cu/Ti3C2 NSs was gently collected with centrifugation at 5000 r for 8 min. Then the suspension was further centrifuged at 9500 r for 30 min and the collected precipitates were dispersed in 5 mL of deionized water for future use.
Subsequently, 500 µL of 0.5 mol/L HAuCl4 was added to the above solution, followed by 250 µL of 0.1 M NaBH4 quickly, and stirred for 1 h. Then, the precipitates of Au/Cu/Ti3C2 NSs were collected by centrifugation at 9500 r for 30 min and dispersed in 6 mL of deionized water for future use.
3.4. Fluorescence and Colorimetric Method for the Detection of Hg2+ and Cl−
The method for the detection of Hg2+ is described below. First, 60 µL of Au/Cu/Ti3C2 NSs and 30 µL of Hg2+ with different concentrations were successively added into 880 µL of deionized water. The Au–Hg alloys with oxidase activity were obtained after being incubated for 5 min. Finally, 30 µL of 0.1 M OPD was added into the mixture. The concentration of Hg2+ was monitored by fluorescence and colorimetric signals after incubation at 37 °C for 9 min.
The method for the detection of Cl− is described below. First, 60 µL of Au/Cu/Ti3C2 NSs, 50 µL of 8 µM Hg2+, and 20 µL of Cl− with different concentrations were successively added to 840 µL of deionized water. Then, 30 µL of 0.1 M OPD was added into the mixture after incubation for 5 min. The concentration of Cl− was monitored by fluorescence colorimetric signals after incubation at 37 °C for 9 min.
3.5. Preparation of Test Papers
Commercial fiber filter paper was used to make test paper with a diameter of 1 cm. The fiber filter paper was immersed in Au/Cu/Ti3C2 NSs solution for 10 min and then dried in an oven at 60 °C to obtain the test paper. Afterwards, 30 µL of 0.1 M OPD and 30 µL of Hg2+ with different concentrations were successively dropped on the test paper. Then, the colorimetric signals were recorded by a Lambda 35 UV analyzer. The colorimetric images were collected with a smartphone under 365 nm ultraviolet light. The test paper for Cl− was prepared with the same process with test paper for Hg2+.
3.6. Real Sample Analysis
The potential application of the proposed method was demonstrated by applying it to detecting Hg2+ and Cl− in water samples. All the water as the real sample was taken from a nearby lake and river in Wuhan (China). The water from East Lake, Shahu Lake, and Yangtze River was selected as the research objects. The samples were filtered by 0.22 μm microporous membrane and diluted to 20 times as the real samples to be tested.
4. Conclusions
In this work, an efficient fluorescence and colorimetric method with the favor of a smartphone for the on-site detection of Hg2+ and Cl− was fabricated based on Au/Cu/Ti3C2 NSs. Characterization techniques confirmed the sucessful synthesis of Au/Cu/Ti3C2 NSs. Because of the formation of the Au–Hg alloy with oxidase-like activity, the OPD was transferred into ADP with a fluorescence emission at 570 nm and an obvious color change. Therefore, a turn-on fluorescence and colorimetric method for the detection of Hg2+ was established. As the addition of Cl−, the fluorescence intensity was constantly quenched to achieve the continuous quantitative detection of Cl−. A series of color variations of the paper strip can be observed by the naked eye and digital images with the introduction of different concentrations of Hg2+ or Cl−. The smartphone with the app (color recognizers) could favor the digital images of Hg2+ or Cl−. The Au/Cu/Ti3C2 NSs-based platform displayed potent sensitivity, broad applicability, favorable selectivity, and significant simplicity. Our proposed Au/Cu/Ti3C2 NSs-based method is a promising method for environmental monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28145355/s1. Figure S1: The influence of Cl− concentration on the detection of mercury ions (n = 3, the concentration of Hg2+ is 40 nM); Table S1: Analytical results for the detection of Cl− in real samples (n = 3).
Author Contributions
Conceptualization, K.C., S.F., Q.R. and X.W.; methodology, K.C., Q.R. and X.W.; software, K.C., C.J. and F.G.; validation, K.C., S.F., F.G. and Y.H.; formal analysis, S.F., C.J. and F.G.; data curation, K.C. and Y.H.; writing—original draft preparation, K.C.; writing—review and editing, Q.R. and X.W.; supervision, Q.R. and X.W.; funding acquisition, K.C., Y.H., Q.R. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (21707030), Natural Science Foundation of Hebei, China (H2022209018), Open Project Funding of the State Key Laboratory of Biocatalysis and Enzyme Engineering (SKLBEE2020017), the Project of High Level Group for Research and Innovation of School of Public Health, North China University of Science and Technology, grant number KYTD202302, and the Hebei Province Graduate Student Innovation Ability Cultivation Funding Project (CXZZSS2023074).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Dimitrijevs, P.; Arsenyan, P. Cardiolipin in the spotlight: Quantitative analysis and fluorescence-based competitive binding assay. Sens. Actuators B-Chem. 2021, 346, 130537. [Google Scholar] [CrossRef]
- Boyd-Shiwarski, C.R.; Weaver, C.J.; Beacham, R.T.; Shiwarski, D.J.; Connolly, K.A.; Nkashama, L.J.; Mutchler, S.M.; Griffiths, S.E.; Knoell, S.A.; Sebastiani, R.S.; et al. Effects of extreme potassium stress on blood pressure and renal tubular sodium transport. Am. J. Physiol. Renal 2020, 318, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Addis, D.R.; Aggarwal, S.; Lazrak, A.; Jilling, T.; Matalon, S. Halogen-induced chemical injury to the mammalian cardiopulmonary systems. Physiology 2021, 36, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Madhesan, T.; Mitra, S.; Deivasigamani, P.; Nagarajan, S.; Brahmmananda Rao, C.V.S.; Mohan, A.M. Probe anchored porous organic polymer monolithic architectures as optical sensor for ultra-trace analysis of Hg2+ in water samples. Micropor. Mesopor. Mat. 2022, 333, 111724. [Google Scholar] [CrossRef]
- Wu, H.B.; Xie, R.Y.; Hao, Y.Q.; Pang, J.Y.; Gao, H.; Qu, F.Y.; Tian, M.M.; Guo, C.H.; Mao, B.D.; Chai, F. Portable smartphone-integrated AuAg nanoclusters electrospun membranes for multivariate fluorescent sensing of Hg2+, Cu2+and l-histidine in water and food samples. Food Chem. 2023, 418, 135961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Wang, D.; Zhang, D.; Zhang, T.T.; Yang, L.K.; Li, Z.P. In situ microfluidic sers chip for ultrasensitive Hg2+ sensing based on I−-functionalized silver aggregates. ACS Appl. Mater. Interfaces 2022, 14, 2211–2218. [Google Scholar] [CrossRef]
- Fang, Y.M.; Zhang, Y.; Cao, L.G.; Yang, J.Z.; Hu, M.H.; Pang, Z.L.; He, J.H. Portable Hg2+ Nanosensor with ppt level sensitivity using nanozyme as the recognition unit, enrichment carrier, and signal amplifier. ACS Appl. Mater. Interfaces 2020, 12, 11761–11768. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Guo, X.Y.; Liu, W.; Zhang, L.; Lv, C.C.; Xu, Y.L.; Jin, Y.; Li, B.X. Aggregation-induced chemiluminescence system for sensitive detection of mercury ions. Anal. Bioanal. Chem. 2021, 413, 625–633. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Xiu, F.R.; Yu, G.D.; Huang, L.L.; Li, B.X. Simple and rapid chemiluminescence aptasensor for Hg2+ in contaminated samples: A new signal amplification mechanism. Biosens. Bioelectron. 2017, 87, 439–446. [Google Scholar] [CrossRef]
- Wang, X.B.; Ma, X.Y.; Wen, J.H.; Geng, Z.R.; Wang, Z.L. A novel bimacrocyclic polyamine-based fluorescent probe for sensitive detection of Hg2+ and glutathione in human serum. Talanta 2020, 207, 120311. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M. Synthesis of metal nanoclusters and their application in Hg2+ ions detection: A review. J. Hazard. Mater. 2022, 424, 127565. [Google Scholar] [CrossRef]
- Yin, P.C.; Niu, Q.F.; Liu, J.Q.; Wei, T.; Hu, T.T.; Li, T.D.; Qin, X.Y.; Chen, J.B. A new AIEE-active carbazole based colorimetric/fluorimetric chemosensor for ultra-rapid and nano-level determination of Hg2+ and Al3+ in food/environmental samples and living cells. Sens. Actuators B-Chem. 2021, 331, 129418. [Google Scholar] [CrossRef]
- Ismail, S.; Yusof, N.A.; Abdullah, J.; Rahman, S.F.A. Development of electrochemical sensor based on silica/gold nanoparticles modified electrode for detection of arsenite. IEEE Sens. J. 2019, 20, 1558–1748. [Google Scholar] [CrossRef]
- Cetin, D.; Yavuz, O.; Alcay, Y.; Yildirim, M.S.; Kaplan, M.; Aribuga, H.; Ozdemir, E.; Ertugral, U.; Yilmaz, I. Development of a new near-infrared, spectrophotometric, and colorimetric probe based on phthalocyanine containing mercaptoquinoline unit for discriminative and highly sensitive detection of Ag+, Cu2+, and Hg2+ ions. Spectrochim. Acta A 2023, 297, 122725. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Pecyna-Utylska, P.; Kernert, J. Ion chromatography and related techniques in carboxylic acids analysis. Crit. Rev. Anal. Chem. 2020, 51, 549–564. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Jiang, S.J. Simultaneous speciation of arsenic and mercury in fish by high-performance liquid chromatography inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2021, 36, 938–945. [Google Scholar] [CrossRef]
- Xing, Y.Q.; Han, Y.; Pierce, D.T.; Zhao, X.J. Aggregation-based determination of mercury (II) using DNA-modified single gold nanoparticle, T-Hg (II)-T interaction, and single-particle ICP-MS. Microchim. Acta 2020, 187, 56. [Google Scholar] [CrossRef]
- Das, D.; Dutta, R.K. N-doped carbon dots synthesized from ethylene glycol and β-alanine for detection of Cr (VI) and 4-nitrophenol via photoluminescence quenching. ACS Appl. Nano Mater. 2021, 4, 3444–3454. [Google Scholar] [CrossRef]
- Wazuddin, D.A.; Mujawar, L.H.; Abduljabbar, T.N.; El-Shahawi, M.S. In-situ droplet assay on wax-modified paper for rapid and trace determination of Fe3+ in water. Microchem. J. 2021, 170, 106723. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.N.; Chen, Z.B. Highly sensitive colorimetric detection of glucose through glucose oxidase and Cu2+-catalyzed 3,3′,5,5′-tetramethylbenzidine oxidation. Spectrochim. Acta A 2019, 213, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Li, J.S.; Song, Y.L. Toward high sensitivity: Perspective on colorimetric photonic crystal sensors. Anal. Chem. 2022, 94, 9497–9507. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z.F. Colorimetric detection of Hg2+ by Au nanoparticles formed by H2O2 reduction of HAuCl4 using Au nanoclusters as the catalyst. Sens. Actuators B-Chem. 2017, 241, 1063–1068. [Google Scholar] [CrossRef]
- Logan, N.; McVey, C.; Elliott, C.; Cao, C. Amalgamated gold-nanoalloys with enhanced catalytic activity for the detection of mercury ions (Hg2+) in seawater samples. Nano Res. 2020, 13, 989–998. [Google Scholar] [CrossRef]
- Wang, L.J.; Xu, X.C.; Liu, P.; Wang, M.Z.; Niu, X.H.; Pan, J.M. A single-nanozyme colorimetric array based on target-induced differential surface passivation for quantification and discrimination of Cl−, Br−and I− ions. Anal. Chim. Acta 2021, 1160, 338451. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Xing, L.; Zhao, L.; Zhang, X.Y.; Zhang, Y.F.; Tang, Y.L.; Zhou, X.M.; Hu, L.; Zhu, N.L. β-Cyclodextrin and graphene oxide co-strengthened AgRu bimetal mesoporous nanozyme: An efficient strategy for visual detection and removal of toxic Hg2+ and Cl−. J. Environ. Chem. Eng. 2022, 10, 108242. [Google Scholar] [CrossRef]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef]
- Qin, R.; Shan, G.; Hu, M.; Huang, W. Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Mater. Today Phys. 2021, 21, 100527. [Google Scholar] [CrossRef]
- Li, Y.J.; Ding, L.; Guo, Y.C.; Liang, Z.Q.; Cui, H.Z.; Tian, J. Boosting the photocatalytic ability of g-C3N4 for hydrogen production by Ti3C2 MXene quantum dots. ACS Appl. Mater. Interfaces 2019, 11, 41400–41407. [Google Scholar] [CrossRef]
- Peng, X.Y.; Zhang, Y.L.; Lu, D.T.; Guo, Y.J.; Guo, S.J. Ultrathin Ti3C2 nanosheets based “off-on” fluorescent nanoprobe for rapid and sensitive detection of HPV infection. Sens. Actuators B-Chem. 2019, 286, 222–229. [Google Scholar] [CrossRef]
- Mai, Y.J.; Li, Y.G.; Li, S.L.; Zhang, L.Y.; Liu, C.S.; Jie, X.H. Self-lubricating Ti3C2 nanosheets/copper composite coatings. J. Alloys Compd. 2019, 770, 1–5. [Google Scholar] [CrossRef]
- Wu, S.S.; Su, Y.M.; Zhu, Y.; Zhang, Y.M.; Zhu, M.S. In-situ growing Bi/BiOCl microspheres on Ti3C2 nanosheets for upgrading visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 520, 146339. [Google Scholar] [CrossRef]
- Chen, D.; Shao, S.B.; Zhang, W.; Zhao, J.B.; Lian, M.L. Nitrogen and sulfur co-doping strategy to trigger the peroxidase-like and electrochemical activity of Ti3C2 nanosheets for sensitive uric acid detection. Anal. Chim. Acta 2022, 1197, 339520. [Google Scholar] [CrossRef]
- Wu, X.J.; Chen, T.M.; Chen, Y.; Yang, G.W. Modified Ti3C2 nanosheets as peroxidase mimetics for use in colorimetric detection and immunoassays. J. Mater Chem. B 2020, 8, 2650–2659. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Lu, X.T.; Xu, J.R.; Luo, H.J. Characterization and tribological properties of graphene/copper composites fabricated by electroless plating and powder metallurgy. Acta Metall. Sin.-Engl. 2020, 33, 903–912. [Google Scholar] [CrossRef]
- Poorshamohammad, C.; Liu, L.G.; Cheng, X.R.; Momtazi-Borojeni, A.A. Green synthesis of plant-stabilized Au nanoparticles for the treatment of gastric carcinoma. Arab. J. Chem. 2023, 16, 104386. [Google Scholar] [CrossRef]
- Yan, L.X.; Chen, Z.P.; Zhang, Z.Y.; Qu, C.L.; Chen, L.X.; Shen, D.Z. Fluorescent sensing of mercury (II) based on formation of catalytic gold nanoparticles. Analyst 2013, 138, 4280–4283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).