A Comprehensive Review on Polyphenols of White Wine: Impact on Wine Quality and Potential Health Benefits
Abstract
1. Introduction
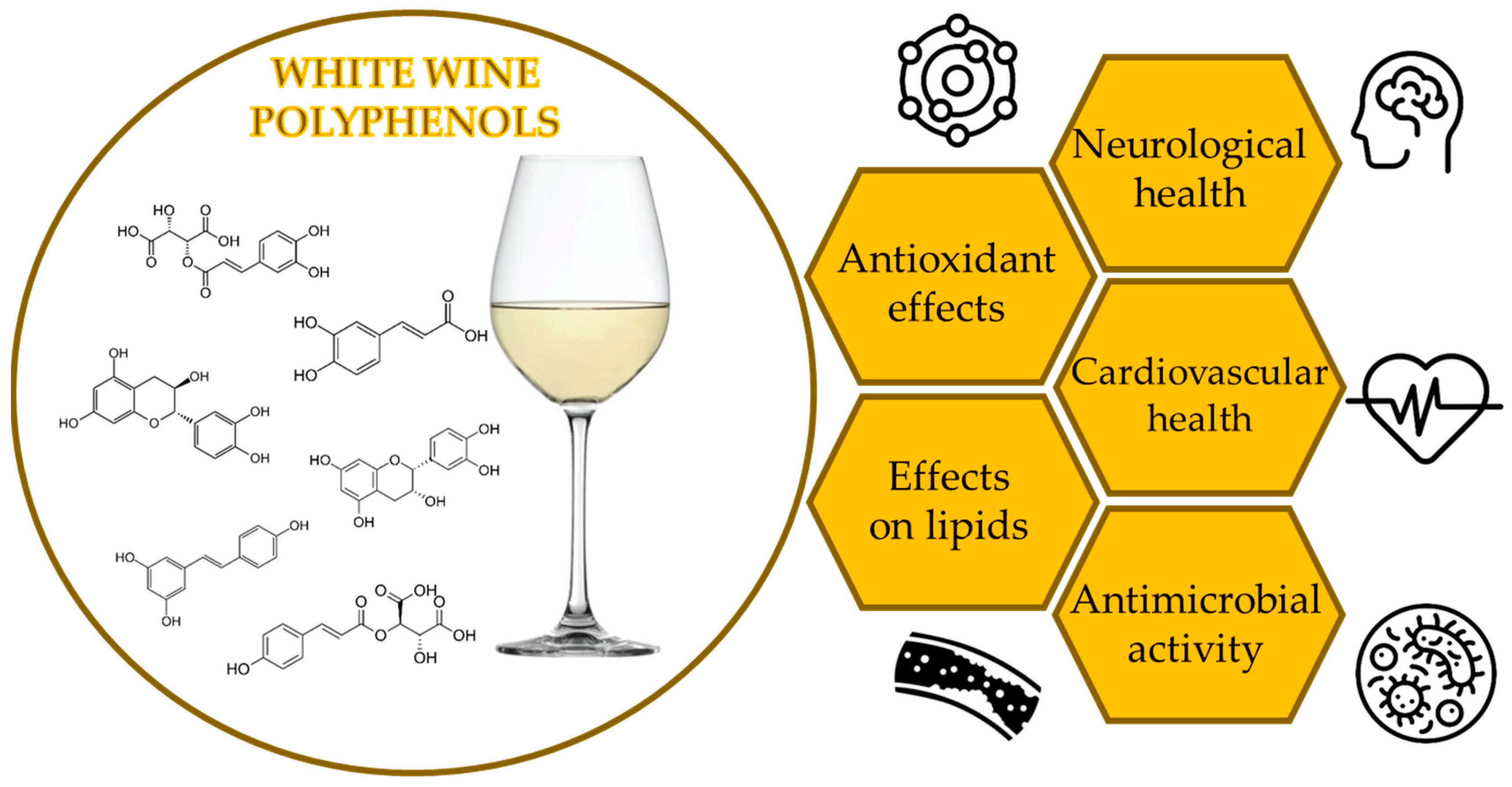
2. White Wine Polyphenols
2.1. Total Polyphenols of White Wines
2.2. Phenolic Acids
2.3. Flavonoids
2.4. Tannins
2.5. Stilbenes
2.6. Polyphenols of Macerated White Wines
3. Polyphenols and Quality of White Wines
3.1. Impact on the Mouthfeel
3.2. Impact on Flavor
3.3. Impact on Color
4. Potential Health Benefits of White Wine Polyphenols
4.1. Antioxidant Potential
4.2. Lipid Effects
4.3. Cardiovascular Health
4.4. Neuroprotection
4.5. Antimicrobial Activity
4.6. Anti-Inflammatory Properties
4.7. Negative Health Effects
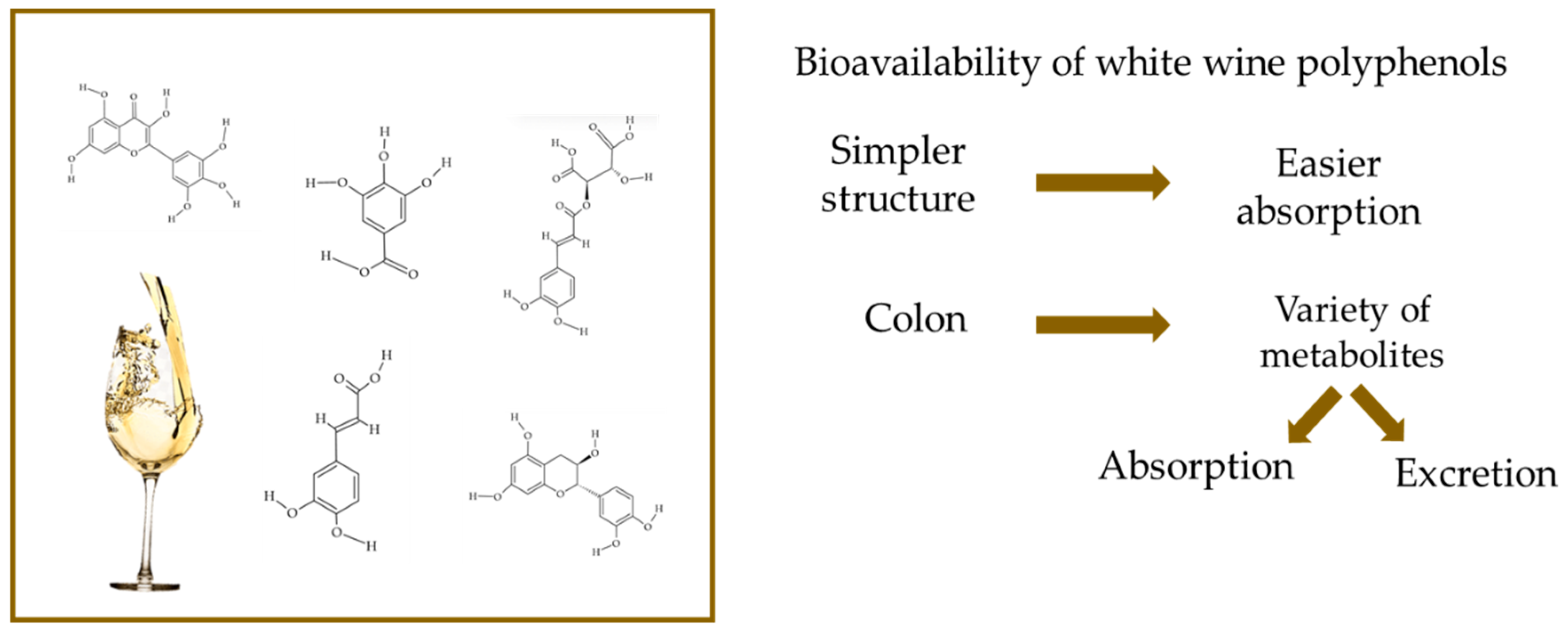
5. Issues Regarding Bioavailability of White Wine Polyphenols
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Ružić, I.; Škerget, M.; Knez, Ž.; Runje, M. Phenolic content and antioxidant potential of macerated white wines. Eur. Food Res. Technol. 2011, 233, 465–472. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Guilford, J.M.; Pezzuto, J.M. Wine and Health: A Review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef]
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Santi, R.L.; Liprandi, A.S.; Baranchuk, A. What’s in wine? A clinician’s perspective. Trends Cardiovasc. Med. 2018, 29, 97–106. [Google Scholar] [CrossRef]
- Thulile, N.; van Jaarsveld, F.; Caleb, O.J. French and Mediterranean-style diets: Contradictions, misconceptions and scientific facts-A review. Food Res. Int. 2018, 116, 840–858. [Google Scholar]
- Fuhrman, B.; Lavy, A.; Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995, 61, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; German, J.B.; Kinsella, J.E.; Parks, E.; Kanner, J. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993, 20, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, H.; Abdallah, A.M.; Prinyawiwatkul, W.; Xu, Z. Red and white wines inhibit cholesterol oxidation induced by free radicals. J. Agric. Food Chem. 2011, 59, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Ilak Peršurić, A.S.; Jeromel, A.; Radeka, S. Comparison of different maceration and non-maceration treatments for enhancement of phenolic composition, colour intensity, and taste attributes of Malvazija istarska (Vitis vinifera L.) white wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Gambacorta, G.; Paradiso, V.M. Influence of prolonged maceration on phenolic compounds, volatile profile and sensory properties of wines from Minutolo and Verdeca, two Apulian white grape varieties. LWT Food Sci. Technol. 2024, 192, 115698. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.; Maslov, L.; Jakobović, S.; Palčić, I.; Jeromel, A. Comparative Study of Aromatic and Polyphenolic Profiles of Croatian White Wines Produced by Cold Maceration. Czech J. Food Sci. 2018, 36, 459–469. [Google Scholar] [CrossRef]
- Buican, B.-C.; Colibaba, L.C.; Luchian, C.E.; Kallithraka, S.; Cotea, V.V. “Orange” wine—The resurgence of an ancient winemaking technique: A review. Agriculture 2023, 13, 1750. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Clarke, S.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. White wine phenolics: Current methods of analysis. J. Sci. Food Agric. 2022, 3, 7–25. [Google Scholar] [CrossRef]
- Arribas, A.S.; Martínez-Fernández, M.; Chicharro, M. The role of electroanalytical techniques in analysis of polyphenols in wine. TrAC Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- De Villiers, A.; Alberts, P.; Tredoux, A.G.J.; Nieuwoudt, H.H. Analytical techniques for wine analysis: An African perspective; A Review. Anal. Chim. Acta 2012, 730, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Pinelo, M.; Manzocco, L.; Nunez, M.J.; Nicoli, M.C. Interaction among phenols in food fortification: Negative synergism on antioxidant capacity. J. Agric. Food Chem. 2004, 52, 1177–1180. [Google Scholar] [CrossRef]
- Hang, D.T.N.; Hoa, N.T.; Bich, H.N.; Mechler, A.; Vo, Q.V. The hydroperoxyl radical scavenging activity of natural hydroxybenzoic acids in oil and aqueous environments: Insights into the mechanism and kinetics. Phytochemistry 2022, 201, 113281. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Lombardi-Boccia, G.; Souto, E.B.; Cecchini, F.; Santini, A. Wine Polyphenols and Health: Quantitative Research Literature Analysis. Appl. Sci. 2021, 11, 4762. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Cheynier, V.; Schneider, R.; Salmon, J.-M.; Fulcrand, H. Chemistry of wine. Compr. Nat. Prod. II 2010, 3, 1119–1172. [Google Scholar]
- Fang, F.; Li, J.-M.; Zhang, P.; Tang, K.; Wang, W.; Pan, Q.-H.; Huang, W.-D. Effects of grape variety, harvest date, fermentation vessel and wine ageing on flavonoid concentration in red wines. Food Res. Int. 2008, 41, 53–60. [Google Scholar] [CrossRef]
- Balík, J.; Kyseláková, M.; Tříska, J.; Vrchotová, N.; Veverka, J.; Híc, P.; Totušek, J.; Lefnerova, D. The changes of selected phenolic substances in wine technology. Czech J. Food Sci. 2008, 26, S3–S12. [Google Scholar] [CrossRef]
- Kennedy, J.A. Grape and wine phenolics: Observations and recent findings. Cienc. Investig. Agrar. 2008, 35, 107–120. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Soyollkham, B.; Valášek, P.; Fišera, M.; Fic, V.; Kubáň, V.; Hoza, I. Total polyphenolic compounds contents (TPC), total antioxidant activities (TAA) and HPLC determination of individual polyphenolic compounds in selected Moravian and Austrian wines. Cent. Eur. J. Chem. 2011, 9, 677–687. [Google Scholar] [CrossRef]
- Danilewicz, J.C. The Folin-Ciocalteu, FRAP, and DPPH Assays for Measuring Polyphenols Concentration in White Wine. Am. J. Enol. Vitic. 2015, 66, 463–471. [Google Scholar] [CrossRef]
- Ghatak, A.A.; Chaturvedi, P.A.; Desai, N.S. Indian Grape Wines: A Potential Source of Phenols, Polyphenols, and Antioxidants. Int. J. Food Prop. 2013, 17, 818–828. [Google Scholar] [CrossRef]
- Recamales, Á.F.; Sayago, A.; González-Miret, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and color of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- De Quirós, R.B.; Lage-Yusty, M.A.; López-Hernández, J. HPLC-analysis of polyphenolic compounds in Spanish white wines and determination of their antioxidant activity by radical scavenging assay. Food Res. Int. 2009, 42, 1018–1022. [Google Scholar] [CrossRef]
- Bubola, M.; Rusjan, D.; Lukić, I. Crop level vs. leaf removal: Effects on Istrian Malvasia wine aroma and phenolic acids composition. Food Chem. 2020, 312, 126046. [Google Scholar] [CrossRef]
- Banc, R.; Socaciu, C.; Miere, D.; Filip, L.; Cozma, A.; Stanciu, O.; Loghin, F. Benefits of Wine Polyphenols on Human Health: A Review. Food Sci. Technol. Bull. 2014, 71, 79–87. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Vašková, R.; Snopek, L.; Mlček, J. Evolution of the Polyphenolic Content of Moravian White Grape Variety Winess of Different Vintages During Storage. S. Afr. J. Enol. Vitic. 2023, 44, 9–24. [Google Scholar]
- Milat, A.M.; Boban, M.; Teissedre, P.-L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Radeka, S.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganć, K.; Horvat, I.; Lukić, I.; Orbanić, F.; Zaninović Jurjević, T.; Dvornik, Š. Bioactive compounds and antioxidant activity of red and white wines produced from autochthonous Croatian varieties: Effect of moderate consumption on human health. Foods 2022, 11, 1804. [Google Scholar] [CrossRef]
- Pour Nikfardjam, M.; Köhler, H.J.; Schmitt, A.; Patz, C.D.; Dietrich, H. Polyphenolic composition of German white wines and its use for the identification of cultivar. Mitteilungen Klosterneubg. 2007, 57, 146–152. [Google Scholar]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef]
- Mitić, M.N.; Obradović, M.V.; Grahovac, Z.B.; Pavlović, A.N. Antioxidant Capacities and Phenolic Levels of Different Varieties of Serbian White Wines. Molecules 2010, 15, 2016–2027. [Google Scholar] [CrossRef]
- Lukić, I.; Jedrejčić, N.; Kovačević Ganić, K.; Staver, M.; Peršurić, Đ. Composition of white wine after prolonged maceration and maturation. Food Technol. Biotechnol. 2015, 53, 407–418. [Google Scholar]
- Paixão, N.; Pereira, V.; Marques, J.C.; Câmara, J.S. Quantification of polyphenols with potential antioxidant properties in wines using reverse phase HPLC. J. Sep. Sci. 2008, 31, 2189–2198. [Google Scholar] [CrossRef]
- Rastija, V.; Srečnik, G.; Medić-Šarić, M. Polyphenolic composition of Croatian wines with different geographical origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of the Winemaking Practices and Aging on Phenolic Content of Smederevka and Chardonnay Wines. Food Bioproc. Technol. 2011, 4, 1512–1518. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic composition and antioxidant activity of red, rosé and white wines originating from Romanian grape cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 716–734. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Flavan-3-ols and Condensed Tannin. In Understanding Wine Chemistry; Water John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; p. 117. [Google Scholar]
- Cíchová, M.; Petříček, J.; Fiala, J. Influence of tannin addition on the content and composition of polyphenolic compounds in wines. Czech J. Food Sci. 2009, 26, 33–38. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The mouthfeel of white wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Cvejic, J.M.; Djekic, S.V.; Petrovic, A.V.; Atanackovic, M.T.; Jovic, S.M.; Brceski, I.D.; Gojkovic-Bukarica, L.C. Determination of trans- and cis-Resveratrol in Serbian Commercial Wines. J. Chromatogr. Sci. 2010, 48, 229–234. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Busconi, M.; Flamini, R.; De Rosso, M. Wine Resveratrol: From the Ground Up. Nutrients 2016, 8, 222. [Google Scholar] [CrossRef]
- Fabjanowicz, M.; Płotka-Wasylka, J.; Namieśnik, J. Detection, identification and determination of resveratrol in wine. Problems and challenges. TrAC Trends Anal. Chem. 2018, 103, 21–33. [Google Scholar] [CrossRef]
- Bertelli, A.A. Wine, research and cardiovascular disease: Instructions for use. Atherosclerosis 2007, 195, 242–247. [Google Scholar] [CrossRef]
- Gresele, P.; Cerletti, C.; Guglielmini, G.; Pignatelli, P.; de Gaetano, G.; Violi, F. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 2011, 22, 201–211. [Google Scholar] [CrossRef]
- Hernanz, D.; Recamales, Á.F.; González-Miret, M.L.; Gómez-Míguez, M.J.; Vicario, I.M.; Heredia, F.J. Phenolic composition of white wines with a prefermentative maceration at experimental and industrial scale. J. Food Eng. 2007, 80, 327–335. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; González-Miret, M.L.; Hernanz, D.; Fernández, M.Á.; Vicario, I.M.; Heredia, F.J. Effects of prefermentative skin contact conditions on colour and phenolic content of white wines. J. Food Eng. 2007, 78, 238–245. [Google Scholar] [CrossRef]
- Arenas, I.; Ribeiro, M.; Filipe-Ribeiro, L.; Vilamarim, R.; Costa, E.; Siopa, J.; Cosme, F.; Nunes, F.M. Effect of Pre-Fermentative Maceration and Fining Agents on Protein Stability, Macromolecular, and Phenolic Composition of Albarino White Wines: Comparative Efficiency of Chitosan, k-Carrageenan and Bentonite as Heat Stabilisers. Foods 2021, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Kennedy, J.A. Wine and grape tannin interactions with salivary proteins and their impact on astringency: A review of current research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef]
- Pyrzynska, K. Chemical speciation and fractionation of metals in wine. Chem. Speciat. Bioavailab. 2007, 19, 1–8. [Google Scholar] [CrossRef]
- Fontana, A.R.; Bottini, R. High-throughput method based on quick, easy, cheap, effective, rugged and safe followed by liquid chromatography-multi-wavelength detection for the quantification of multiclass polyphenols in wines. J. Chromatogr. A 2014, 1342, 44–53. [Google Scholar] [CrossRef]
- Palade, L.; Popa, M.E. Polyphenol Fingerprinting Approaches in Wine Traceability and Authenticity: Assessment and Implications of Red Wines. Beverages 2018, 4, 75. [Google Scholar] [CrossRef]
- Merkytė, V.; Longo, E.; Windisch, G.; and Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Gerbi, V.; Motta, G.; Río Segade, S. Mouthfeel subqualities in wines: A current insight on sensory descriptors and physical–chemical markers. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3328–3365. [Google Scholar] [CrossRef]
- Gawel, R.; Van Sluyter, S.C.; Smith, P.A.; Waters, E.J. Effect of pH and Alcohol on Perception of Phenolic Character in White Wine. Am. J. Enol. Vitic. 2013, 64, 425–429. [Google Scholar] [CrossRef]
- Gawel, R.; Schulkin, A.; Day, M.; Barker, A.; Smith, P.A. Interactions between phenolics, alcohol and acidity in determining mouthfeel and bitterness of white wine. Wine Vitic. J. 2016, 31, 30–34. [Google Scholar]
- Gawel, R.; Schulkin, A.; Smith, P.A.; Waters, E.J. Taste and textural characters of mixtures of caftaric acid and Grape Reaction Product in model wine. Aust. J. Grape Wine Res. 2014, 20, 25–30. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Castro-Vázquez, L.; Hermosín-Gutiérrez, I.; and Pérez-Coello, M.S. Combined effects of prefermentative skin maceration and oxygen addition of must on color-related phenolics, volatile composition, and sensory characteristics of Airén wine. J. Agric. Food Chem. 2011, 59, 12171–12182. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Lund, C.M.; Nicolau, L.; Gardner, R.C.; KIilmartin, P.A. Effect of polyphenols on the perception of key aroma compounds from Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2009, 15, 18–26. [Google Scholar] [CrossRef]
- Dufour, C.; Bayonove, C.L. Interactions between wine polyphenols and aroma substances. An insight at the molecular level. J. Agric. Food Chem. 1999, 47, 678–684. [Google Scholar] [CrossRef]
- Jung, D.M.; de Ropp, J.S.; Ebeler, S.E. Study of interactions between food phenolics and aromatic flavors using one- and two-dimensional 1 H NMR spectroscopy. J. Agric. Food Chem. 2000, 48, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Hatzidimitriou, E.; Paraskevopoulou, A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides, and artificial saliva. Food Res. Int. 2011, 44, 1561–1570. [Google Scholar] [CrossRef]
- Petruzziello, M.; Asproudi, A.; Guaita, M.; Borsa, D.; Motta, S.; Panero, L.; Bosso, A. Influence of the matrix composition on the volatility and sensory perception of 4-ethylphenol and 4-ethylguaiacol in model wine solutions. Food Chem. 2014, 149, 197–202. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. 2006, 62, 191–202. [Google Scholar] [CrossRef]
- Salacha, M.I.; Kallithraka, S.; Tzourou, I. Browning of white wines: Correlation with antioxidant characteristics, total polyphenolic composition and flavanol content. Int. J. Food Sci. Technol. 2008, 43, 1073–1077. [Google Scholar] [CrossRef]
- Sioumis, N.; Kallithraka, S.; Tsoutsouras, E.; Makris, D.P.; Kefalas, P. Browning development in white wines: Dependence on compositional parameters and impact on antioxidant characteristics. Eur. Food Res. Technol. 2004, 220, 326–330. [Google Scholar] [CrossRef]
- Mlček, J.; Jurikova, T.; Bednaříková, R.; Snopek, L.; Ercisli, S.; Tureček, O. The Influence of Sulfur Dioxide Concentration on Antioxidant Activity, Total Polyphenols, Flavonoid Content and Individual Polyphenolic Compounds in White Wines during Storage. Agriculture 2023, 13, 1439. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Villaño, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Meral, R. Antioxidant effects of wine polyphenols. Trakia J. Sci. 2008, 6, 57–62. [Google Scholar]
- Tekos, F.; Makri, S.; Skaperda ZVPatouna, A.; Terizi, K.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; Demetrios, K. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Alonso, Á.M.; Domínguez, C.; Guillén, D.A.; Barroso, C.G. Determination of Antioxidant Power of Red and White Wines by a New Electrochemical Method and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A.C. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Psarra, E.; Makris, D.P.; Kallithraka, S.; Kefalas, P. Evaluation of the antiradical and reducing properties of selected Greek white wines: Correlation with polyphenolic composition. J. Sci. Food Agric. 2002, 82, 1014–1020. [Google Scholar] [CrossRef]
- Makris, D.P.; Psarra, E.; Kallithraka, S.; Kefalas, P. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res. Int. 2003, 36, 805–814. [Google Scholar] [CrossRef]
- Pignatelli, P.; Ghiselli, A.; Buchetti, B.; Carnevale, R.; Natella, F.; Germanò, G.; Fimognari, F.; Di Santo, S.; Lenti, L.; Violi, F. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atherosclerosis 2006, 188, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Landrault, N.; Poucheret, P.; Azay, J.; Krosniak, M.; Gasc, F.; Jenin, C.; Cros, G.; Teissedre, P.L. Effect of a polyphenols-enriched chardonnay white wine in diabetic rats. J. Agric. Food Chem. 2003, 51, 311–318. [Google Scholar] [CrossRef]
- Flechtner-Mors, M.; Biesalski, H.K.; Jenkinson, C.P.; Adler, G.; Ditschuneit, H.H. Effects of moderate consumption of white wine on weight loss in overweight and obese subjects. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1420–1426. [Google Scholar] [CrossRef]
- Rajdl, D.; Racek, J.; Trefil, L.; Siala, K. Effect of white wine consumption on oxidative stress markers and homocysteine levels. Physiol Res. 2007, 56, 203–212. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Garovic-Kocic, V.; Diamandis, E.P.; Pace-Asciak, C.R. Wine: Does the colour count? Clin. Chim. Acta 1996, 246, 183–193. [Google Scholar] [CrossRef]
- Sharpe, P.C.; McGrath, L.T.; McClean, E.; Young, I.S.; Archbold, G.P. Effect of red wine consumption on lipoprotein (a) and other risk factors for atherosclerosis. QJM Int. J. Med. 1995, 88, 101–108. [Google Scholar]
- Milat, A.; Mudnić, I.; Grković, I.; Ključević, N.; Grga, M.; Jerčić, I.; Jurić, D.; Ivanković, D.; Benzon, B.; Boban, M. Effects of White Wine Consumption on Weight in Rats: Do Polyphenols Matter? Oxid. Med. Cell Longev. 2017, 2017, 8315803. [Google Scholar] [CrossRef]
- Boban, D.; Dželalija, A.M.; Gujinović, D.; Benzon, B.; Ključević, N.; Boban, Z.; Mudnić, I.; Grković, I. Differential Effects of White Wine and Ethanol Consumption on Survival of Rats after a Myocardial Infarction. Appl. Sci. 2023, 13, 1450. [Google Scholar] [CrossRef]
- Cui, J.; Tosaki, A.; Cordis, G.A.; Bertelli, A.A.E.; Bertelli, A.; Maulik, N.; Das, D.K. Cardioprotective Abilities of White Wine. Ann. N. Y. Acad. Sci. 2002, 957, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Lenti, L.; Pulcinelli, F.M.; Catasca, R.; Saccani, G.; Germano, G.; Marcoccia, A.; Silvestri, M.A.; Ghiselli, A.; Violi, F. Red and white wine differently affect collagen-induced platelet aggregation. Pathophysiol. Haemost. Thromb. 2002, 32, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Lavy, A.; Fuhrman, B.; Markel, A.; Dankner, G.; Ben-Amotz, A.; Presser, D.; Aviram, M. Effect of Dietary Supplementation of Red or White Wine on Human Blood Chemistry, Hematology and Coagulation: Favorable Effect of Red Wine on Plasma High-Density Lipoprotein. Ann. Nutr. Metab. 1994, 38, 287–294. [Google Scholar] [CrossRef]
- Auger, C.; Rouanet, J.; Vanderlinde, R.; Bornet, A.; Décordé, K.; Lequeux, N.; Cristol, J.; Teissedre, J. Polyphenols-Enriched Chardonnay White Wine and Sparkling Pinot Noir Red Wine Identically Prevent Early Atherosclerosis in Hamsters. J. Agric. Food Chem. 2005, 53, 9823–9829. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef]
- Ates, O.; Cayli, S.; Altinoz, E.; Gurses, I.; Yucel, N.; Sener, M.; Kocak, A.; Yologlu, S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol. Cell Biochem. 2007, 294, 137–144. [Google Scholar] [CrossRef]
- Tsai, S.K.; Hung, L.M.; Fu, Y.T.; Cheng, H.; Nien, M.W.; Liu, H.Y.; Zhang, F.B.Y.; Huang, S.S. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J. Vasc. Surg. 2007, 46, 346–353. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.G.; Fang, D.; Le, W.D. (−)- Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004, 78, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Aβ(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.; Chibane, M.; Monti, J.; Richard, T. Wine Polyphenols: Potential Agents in Neuroprotection. Oxid. Med. Cell Longev. 2012, 2012, 805762. [Google Scholar] [CrossRef]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Dacarro, C.; Gazzani, G. Antibacterial activity of red and white wine against oral streptococci. J. Agric. Food Chem. 2007, 55, 5038–5042. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Migliori, M.; Panichi, V.; Longoni, B.; Origlia, N.; Ferretti, A.; Cuttano, M.G.; Giovannini, L. Oxidative stress and inflammatory reaction modulation by white wine. Ann. N. Y. Acad. Sci. 2002, 957, 295–301. [Google Scholar] [CrossRef]
- Migliori, M.; Panichi, V.; de la Torre, R.; Fitó, M.; Covas, M.; Bertelli, A.; Muñoz-Aguayo, D.; Scatena, A.; Paoletti, S.; Ronco, C. Anti-Inflammatory Effect of White Wine in CKD Patients and Healthy Volunteers. Blood Purif. 2015, 39, 218–223. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Stockley, C.; Teissedre, P.-L.; Boban, M.; Di Lorenzo, C.; Restani, P. Bioavailability of wine-derived phenolic compounds in humans: A review. Food Funct. 2012, 3, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Yan, J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Forte, M.; Vrhovsek, U.; Mattivi, F.; Viola, R.; Scaccini, C. White wine phenolics are absorbed and extensively metabolized in humans. J. Agric. Food Chem. 2009, 57, 2711–2718. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, X.; Zhang, J.; Ju, Y.; Que, Z.; Liao, X.; Lao, F.; Fang, Y.; Ma, T. Letting wine polyphenols functional: Estimation of wine polyphenols bioaccessibility under different drinking amount and drinking patterns. Food Res. Int. 2020, 127, 108704. [Google Scholar] [CrossRef]
- Proca, A.C.; Horodincu, L.; Solcan, C.; Solcan, G. The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs. Agriculture 2024, 14, 1142. [Google Scholar] [CrossRef]
| Polyphenol | Concentration (mg/L) | Wine Type | Applied Method | Reference |
|---|---|---|---|---|
| Phenolic acids | ||||
| trans-caftaric acid | 33.14 ± 0.99 5.90 ± 0.07 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 11.36 ± 0.17 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 3.42 ± 0.16 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 11.54 ± 0.19 0.88 ± 0.01 | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 2.08 ± 0.03 3.49 ± 0.04 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 53.10 ± 0.80 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 33.11 ± 0.05 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 10.54 ± 0.05 5.71 ± 0.06 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 4.48 ± 0.03 3.83 ± 0.04 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 25.05 ± 0.25 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 43.60 ± 0.20 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 7.17 ± 0.20 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 21.07 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 45.92 ± 0.20 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 46.70 ± 0.04 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 33.2–85.7 | Commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] | |
| 2.97 ± 0.33 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 3.46 ± 0.26 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 8.99 ± 2.02 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 16.96 ± 0.85 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0–21.1 | Traminer from Germany | HPLC-DAD | [45] | |
| 0–40.2 | Silvaner from Germany | HPLC-DAD | [45] | |
| 0.5–25.9 | Riesling from Germany | HPLC-DAD | [45] | |
| 15.50–43.47 | 12 commercially available white wines | HPLC-DAD | [46] | |
| 14.71 ± 1.1 | Chardonnay from Serbia | HPLC-DAD | [47] | |
| 43.53 ± 1.5 | Banatski Riesling from Serbia | HPLC-DAD | [47] | |
| 21.96 ± 1.2 | Graševina from Serbia | HPLC-DAD | [47] | |
| 7.56 ± 0.85 | Traminac from Serbia | HPLC-DAD | [47] | |
| cis-caftaric acid | 0.71 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] |
| 0.50 ± 0.00 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.35 ± 0.00 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.32 ± 0.00 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| trans-coutaric acid | 3.01 ± 0.04 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [44] |
| 3.45 ± 0.01 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 0.20 ± 0.07 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.36 ± 0.22 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.22 ± 0.26 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 3.04 ± 0.76 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0–7.5 | Traminer from Germany | HPLC-DAD | [45] | |
| 0–6.9 | Silvaner from Germany | HPLC-DAD | [45] | |
| 0.7–3.0 | Riesling from Germany | HPLC-DAD | [45] | |
| 2.66 ± 0.74 | Chardonnay from Serbia | HPLC-DAD | [47] | |
| 5.74 ± 0.76 | Banatski Riesling from Serbia | HPLC-DAD | [47] | |
| 3.71 ± 0.90 | Graševina from Serbia | HPLC-DAD | [47] | |
| 1.36 ± 0.53 | Traminac from Serbia | HPLC-DAD | [47] | |
| 1.21 ± 0.22 | Semillon from Serbia | HPLC-DAD | [47] | |
| para-coutaric acid | 8.02 ± 0.09 5.12 ± 0.05 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 6.07 ± 0.14 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 6.43 ± 0.08 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 3.36 ± 0.05 1.95 ± 0.01 | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 3.16 ± 0.03 2.58 ± 0.03 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 17.09 ± 0.02 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 4.25 ± 0.04 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 3.89 ± 0.06 2.65 ± 0.03 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 2.65 ± 0.03 1.84 ± 0.03 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| cis-coutaric acid | 1.53 ± 0.01 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] |
| 1.90 ± 0.03 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| fertaric acid | 1.53 ± 0.01 1.37 ± 0.02 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 1.59 ± 0.04 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 0.92 ± 0.01 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 0.81 ± 0.01 0.97 ± 0.03 | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.92 ± 0.03 0.98 ± 0.01 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.77 ± 0.00 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 1.90 ± 0.05 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 0.93 ± 0.03 0.76 ± 0.02 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.77 ± 0.01 0.79 ± 0.02 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.49 ± 0.07 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.59 ± 0.25 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.36 ± 0.12 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.24 ± 0.03 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2-S-glutathionylcaftaric acid | 1.51 ± 0.02 1.34 ± 0.01 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 5.30 ± 0.07 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 2.30 ± 0.06 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 2.96 ± 0.23 0.97 ± 0.01 | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 3.60 ± 0.02 2.46 ± 0.10 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 6.69 ± 0.07 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 1.81 ± 0.01 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 2.41 ± 0.04 1.55 ± 0.03 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.33 ± 0.01 1.81 ± 0.01 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.13 ± 0.15 | Chardonnay from Serbia | HPLC-DAD | [47] | |
| 2.39 ± 0.35 | Banatski Riesling from Serbia | HPLC-DAD | [47] | |
| 1.91 ± 0.48 | Traminac from Serbia | HPLC-DAD | [47] | |
| 4.14 ± 0.52 | Semillon from Serbia | HPLC-DAD | [47] | |
| caffeic acid | 3.14 ± 0.05 2.81 ± 0.03 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 1.69 ± 0.02 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 1.72 ± 0.03 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 1.42 ± 0.29 - | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.06 ± 0.03 2.36 ± 0.02 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 5.45 ± 0.05 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 3.20 ± 0.03 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 1.24 ± 0.06 0.78 ± 0.01 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.68 ± 0.01 - | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 5.3 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] | |
| 3.39 ± 0.08 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 7.34 ± 0.01 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 11.71 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.22 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 8.13 ± 0.04 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 10.61 ± 0.011 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 2.04 ± 0.88 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.10 ± 0.09 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.35 ± 0.02 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.78 ± 0.13 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.1–25.9 | Traminer from Germany | HPLC-DAD | [45] | |
| 0–13.5 | Silvaner from Germany | HPLC-DAD | [45] | |
| 0–3.9 | Riesling from Germany | HPLC-DAD | [45] | |
| 0.48–1.51 | 12 commercially available white wines | HPLC-DAD | [46] | |
| 17.76–114.99 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 7.65–16.16 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 1.57 ± 0.18 | Chardonnay from Serbia | HPLC-DAD | [47] | |
| 3.39 ± 0.65 | Banatski Riesling from Serbia | HPLC-DAD | [47] | |
| 2.27 ± 0.82 | Graševina from Serbia | HPLC-DAD | [47] | |
| 7.70 ± 0.98 | Traminac from Serbia | HPLC-DAD | [47] | |
| 3.88 ± 0.80 | Semillon from Serbia | HPLC-DAD | [47] | |
| 5.0 ± 0.5 | Pelješac from Dalmatia, Croatia | HPLC-DAD | [50] | |
| para-coumaric acid | 1.22 ± 0.00 0.68 ± 0.02 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 6.07 ± 0.14 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 1.37 ± 0.03 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 0.81 ± 0.01 0.97 ± 0.03 | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.79 ± 0.02 0.92 ± 0.03 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.32 ± 0.02 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 1.5 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] | |
| 1.51 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.79 ± 0.00 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 2.79 ± 0.00 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 3.91 ± 0.00 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.30 ± 0.04 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.12 ± 0.61 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.26 ± 0.03 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.55 ± 0.09 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 8.5–47.2 | Traminer from Germany | HPLC-DAD | [45] | |
| 0.8–33.3 | Silvaner from Germany | HPLC-DAD | [45] | |
| 0–3.7 | Riesling from Germany | HPLC-DAD | [45] | |
| 1.38–4.36 | 12 commercially available white wines | HPLC-DAD | [46] | |
| 3.52–33.83 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 5.19–12.40 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 0.62 ± 0.09 | Banatski Riesling from Serbia | HPLC-DAD | [47] | |
| 0.71 ± 0.08 | Graševina from Serbia | HPLC-DAD | [47] | |
| 2.02 ± 0.65 | Traminac from Serbia | HPLC-DAD | [47] | |
| 1.72 ± 0.06 | Semillon from Serbia | HPLC-DAD | [47] | |
| trans-ferulic acid | 0.78 ± 0.02 0.49 ± 0.02 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 1.75 ± 0.08 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| - 0.49 ± 0.02 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.04 ± 0.02 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 1.0 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] | |
| 3.30 ± 0.01 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 5.20 ± 0.02 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 0.95 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.54 ± 0.00 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| - | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.79 ± 0.01 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.88 ± 0.22 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.01 ± 0.46 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.34 ± 0.06 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.34 ± 0.12 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| cis-ferulic acid | 1.93 ± 0.01 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] |
| 2.81 ± 0.03 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| gallic acid | 5.65 ± 0.10 - | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 1.75 ± 0.08 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 3.24 ± 0.13 0.96 ± 0.04 | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 3.66 ± 0.07 3.06 ± 0.05 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 13.47 ± 0.06 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 19.38 ± 0.12 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 11.57 ± 0.12 8.30 ± 0.07 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 7.56 ± 0.10 9.77 ± 0.08 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 2.18 ± 0.01 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 20.69 ± 0.03 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 28.53 ± 0.01 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 3.08 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 4.64 ± 0.02 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 30.93 ± 0.03 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.45 ± 0.11 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.26 ± 0.11 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 2.57 ± 1.20 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.21 ± 0.24 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0–2.8 | Traminer from Germany | HPLC-DAD | [45] | |
| 0–14.8 | Silvaner from Germany | HPLC-DAD | [45] | |
| 5.10–9.89 | 12 commercially available white wines | HPLC-DAD | [46] | |
| 0–30.95 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 8.41–35.17 | 12 white wines from Madeira Island | RP HPLC-DAD | [49] | |
| 2.4 ± 0.2 | Traminac from Slavonia, Croatia | HPLC-DAD | [50] | |
| 8.4 ± 0.3 | Pelješac from Dalmatia, Croatia | HPLC-DAD | [50] | |
| 0.7 ± 0.2 | Malvazija from Istria, Croatia | HPLC-DAD | [50] | |
| protocatechuic acid | 6.4 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] |
| 0.64 ± 0.02 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.02 ± 0.17 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 4.56 ± 0.05 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.03 ± 0.04 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| vanillic acid | 4.7 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] |
| 0.70 ± 0.03 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.50 ± 0.27 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.46 ± 0.19 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.33 ± 0.11 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| syringic acid | 0.7 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] |
| 2.60 ± 0.01 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 20.83 ± 0.23 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 0.43 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.14 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.16 ± 0.00 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.28 ± 0.00 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.56 ± 0.09 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.85 ± 0.07 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.09 ± 0.02 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.20 ± 0.10 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| para-hydroxybenzoic acid | 0.67 ± 0.01 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] |
| 0.29 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 1.48 ± 0.05 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.23 ± 0.01 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.87 ± 0.11 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.55 ± 0.03 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 3.24 ± 0.38 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.88 ± 0.57 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| Ester | ||||
| caffeic acid ethyl ester | 1.31 ± 0.01 1.46 ± 0.02 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 0.85 ± 0.04 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 0.96 ± 0.01 | Sauvignon Blanc wine from Istria, Croatia | LC-MS | [2] | |
| 0.71 ± 0.12 - | 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.62 ± 0.00 0.88 ± 0.02 | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 2.09 ± 0.02 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 1.51 ± 0.01 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 0.66 ± 0.00 0.62 ± 0.01 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.64 ± 0.01 0.61 ± 0.01 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| Polyphenol | Concentration (mg/L) | Wine Type | Applied Method | Reference |
|---|---|---|---|---|
| (+)-catechin | 6.84 ± 0.56 - | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 7.30 ± 0.26 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 21.35 ± 0.07 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 11.46 ± 0.14 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 15.61 ± 0.28 9.36 ± 0.13 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 4.06 ± 0.25 13.81 ± 0.10 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 2.9 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] | |
| 5.18 ± 0.10 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q- TOF | [43] | |
| 64.85 ± 1.30 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q- TOF | [43] | |
| 2.96 ± 0.32 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 8.08 ± 0.30 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 5.06 ± 0.10 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 6.16 ± 0.79 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 7.2–15.6 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] | |
| 1.33 ± 0.14 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 2.03 ± 0.10 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.80 ± 0.44 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.36 ± 0.11 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0–26.3 | Silvaner from Germany | HPLC-DAD | [45] | |
| 25.29–42.05 | 4 commercial white wines from Romania | HPLC-DAD-ESI(+)MS | [52] | |
| 1.3 ± 0.2 | Traminac from Slavonia, Croatia | HPLC-DAD | [50] | |
| 4.1 ± 0.2 | Pelješac from Dalmatia, Croatia | HPLC-DAD | [50] | |
| (-)-epicatechin | 5.16 ± 0.19 3.41 ± 0.02 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 8.09 ± 0.23 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 4.05 ± 0.22 - | 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 7.9 | Malvazija wine from Istria, Croatia | HPLC-DAD | [48] | |
| 13.70 ± 0.06 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 12.01 ± 0.06 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 8.63 ± 0.15 3.13 ± 0.11 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 3.85 ± 0.19 7.09 ± 0.03 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 1.16 ± 0.02 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q- TOF | [43] | |
| 42.52 ± 0.09 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q- TOF | [43] | |
| 3.40 ± 0.26 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 3.61 ± 0.09 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.99 ± 0.01 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 2.64 ± 0.21 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 2.6–28.4 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] | |
| 0.30 ± 0.18 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.23 ± 0.02 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.35 ± 0.03 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.29 ± 0.05 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0–10.3 | Silvaner from Germany | HPLC-DAD | [45] | |
| 0–6.4 | Riesling from Germany | HPLC-DAD | [45] | |
| 0–89.36 | 6 white wines from the Canary Island | RP HPLC-DAD | [49] | |
| rutin | 4.4–33.6 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| myricetin | 1.6–4.5 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| quercetin | 1.4–10.3 | Commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| 0–30.53 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 0–20.18 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 4.1 ± 0.4 | Pelješac from Dalmatia, Croatia | HPLC-DAD | [50] | |
| 0.17 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 2.37 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.78 ± 0.04 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.21 ± 0.00 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| quercetin-3-glucoside | 0.28 ± 0.03 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] |
| 0.30 ± 0.06 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.25 ± 0.02 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.28 ± 0.04 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 5.26–6.37 | 4 commercial white wines from Romania | HPLC-DAD-ESI(+)MS | [52] | |
| taxifolin | 0.38 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] |
| 0.90 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.50 ± 0.00 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.70 ± 0.01 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| apigenin | 0.2–0.9 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| kaempferol | 0.6–7.4 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| 0–0.91 | 12 commercially available white wines | HPLC-DAD | [46] | |
| 0–4.24 | 4 commercial white wines from Romania | HPLC-DAD-ESI(+)MS | [52] | |
| 0.4 ± 0.2 | Pelješac from Dalmatia, Croatia | HPLC-DAD | [50] |
| Polyphenol | Concentration (mg/L) | Wine Type | Applied Method | Reference |
|---|---|---|---|---|
| procyanidin B1 | 12–25.2 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| 1.19 ± 0.25 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 1.07 ± 0.03 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.34 ± 0.08 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.50 ± 0.08 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.73 ± 0.13 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 37.10 ± 0.38 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 0.56 ± 0.07 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.89 ± 0.19 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.98 ± 0.09 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.94 ± 0.27 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| procyanidin B2 | 5.1–30.4 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] |
| 0–6.7 | Silvaner from Germany | HPLC-DAD | [46] | |
| 1.17 ± 0.01 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 9.30 ± 0.02 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 0.38 ± 0.05 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.96 ± 0.08 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.53 ± 0.06 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.50 ± 0.08 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| procyanidin B3 | 4.48 ± 1.08 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] |
| 5.15 ± 0.84 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 2.26 ± 0.08 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.50 ± 0.08 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 2.88 ± 0.41 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 1.14 ± 0.01 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| 3.60 ± 0.01 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 4.68 ± 0.03 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 1.14 ± 0.07 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 1.58 ± 0.07 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| procyanidin B4 | 2.66 ± 0.10 | Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] |
| 22.93 ± 0.26 | macerated Graševina wine from Slavonia region, Croatia | UPLC with ESI-Q TOF | [43] | |
| procyanidin C1 | 0.36 ± 0.01 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] |
| 0.29 ± 0.01 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.35 ± 0.01 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.42 ± 0.01 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] |
| Polyphenol | Concentration (mg/L) | Wine Type | Applied Method | Reference |
|---|---|---|---|---|
| trans-resveratrol | 0.61 ± 0.02 0.46 ± 0.02 | 2 Malvazija wines from Istria, Croatia | LC-MS | [2] |
| 0.37 ± 0.01 | Pinot Gris wine from Istria, Croatia | LC-MS | [2] | |
| 1.95 ± 0.01 | macerated wine of bland of Malvazija, Sauvignon Blanc and Pinot Gris from Istria, Croatia | LC-MS | [2] | |
| 1.18 ± 0.02 | macerated wine of Malvazija from Istria, Croatia | LC-MS | [2] | |
| 0.79 ± 0.02 0.63 ± 0.00 | macerated 2 Ribolla Gialla wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.49 ± 0.01 0.63 ± 0.00 | macerated 2 Tocai Friulano wines from Friuli-Venezia Giulia, Italy | LC-MS | [2] | |
| 0.12 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.12 ± 0.00 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.12 ± 0.01 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.17 ± 0.00 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 1.1–4.1 | 8 commercial white wine from the Galicia region | LC-FID and LC-UV/Vis | [38] | |
| 0.19 ± 0.03 | Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.20 ± 0.06 | macerated Pošip from Dalmatia, Croatia | HPLC-DAD | [16] | |
| 0.40 ± 0.14 | Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0.44 ± 0.04 | macerated Škrlet from Moslavina, Croatia | HPLC-DAD | [16] | |
| 0–6.29 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 0–3.91 | 6 white wines from the Canary Islands | RP HPLC-DAD | [49] | |
| 0.67–1.46 | 4 commercial white wines from Romania | HPLC-DAD-ESI(+)MS | [49] | |
| 0.8 ± 0.2 | Pelješac from Dalmatia, Croatia | HPLC-DAD | [50] | |
| 0.1 ± 0.0 | Malvazija from Istria, Croatia | HPLC-DAD | [50] | |
| piceatannol | 0.09 ± 0.01 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] |
| 0.10 ± 0.02 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.08 ± 0.00 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.06 ± 0.00 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| cis-piceid | 0.18 ± 0.00 | Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] |
| 0.39 ± 0.03 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| 0.30 ± 0.02 | macerated Malvazija wine from Istria, Croatia | HPLC with DAD and FLD | [44] | |
| 0.40 ± 0.01 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] | |
| trans-piceid | 0.35 ± 0.08 | Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] |
| 0.85 ± 0.08 | macerated Pošip wine from Dalmatia, Croatia | HPLC with DAD and FLD | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. A Comprehensive Review on Polyphenols of White Wine: Impact on Wine Quality and Potential Health Benefits. Molecules 2024, 29, 5074. https://doi.org/10.3390/molecules29215074
Ćorković I, Pichler A, Šimunović J, Kopjar M. A Comprehensive Review on Polyphenols of White Wine: Impact on Wine Quality and Potential Health Benefits. Molecules. 2024; 29(21):5074. https://doi.org/10.3390/molecules29215074
Chicago/Turabian StyleĆorković, Ina, Anita Pichler, Josip Šimunović, and Mirela Kopjar. 2024. "A Comprehensive Review on Polyphenols of White Wine: Impact on Wine Quality and Potential Health Benefits" Molecules 29, no. 21: 5074. https://doi.org/10.3390/molecules29215074
APA StyleĆorković, I., Pichler, A., Šimunović, J., & Kopjar, M. (2024). A Comprehensive Review on Polyphenols of White Wine: Impact on Wine Quality and Potential Health Benefits. Molecules, 29(21), 5074. https://doi.org/10.3390/molecules29215074






