Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review
Abstract
1. Introduction
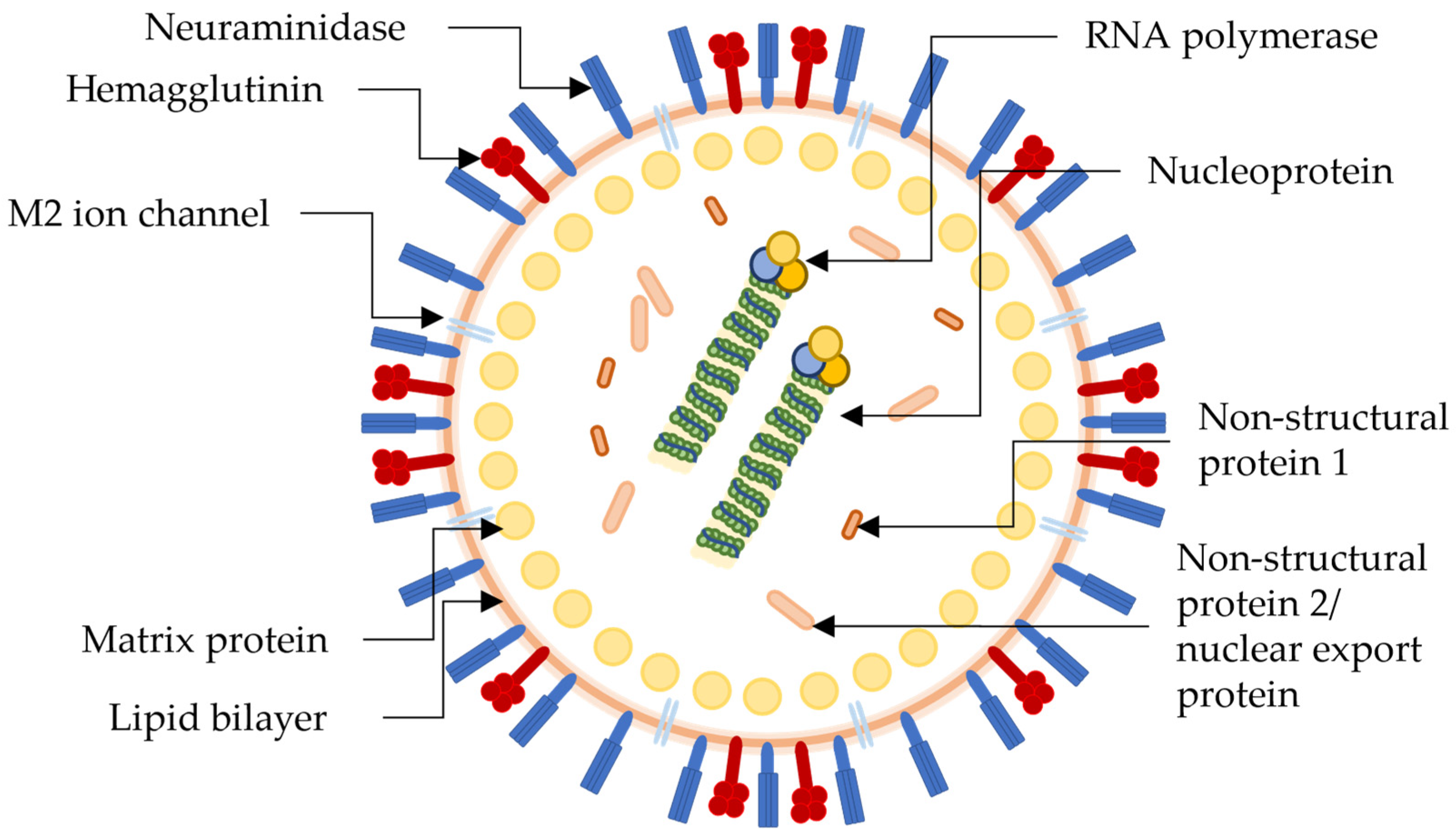
2. H1N1
3. Hepatitis
4. HIV
5. Discussion
6. Methodology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109208. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K. Occurrence and Content of Hydroxycinnamic and Hydroxybenzoic Acid Compounds in Foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Aliotta, G.E.; Johannesen, A.S.; Voetmann-Jensen, D.; Laustsen, F.H.; Andersen, L.A.; Rezai, A.; Fredsgaard, M.; Lo Vecchio, S.; Arendt-Nielsen, L.; et al. Effects of Salicornia-Based Skin Cream Application on Healthy Humans’ Experimental Model of Pain and Itching. Pharmaceuticals 2022, 15, 150. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin Bioactive Effects: Moving from Preclinical Evidence to Potential Clinical Applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, H.C.; Zhou, S.J.; Li, Y.; Zheng, T.T.; Zhou, C.Z.; Wan, X.H. Hyperoside: A Review on Its Sources, Biological Activities, and Molecular Mechanisms. Phyther. Res. 2022, 36, 2779–2802. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Phenolic Compounds in Natural and Roasted Nuts and Their Skins: A Brief Review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Li, W.; Hydamaka, A.W.; Lowry, L.; Beta, T. Comparison of Antioxidant Capacity and Phenolic Compounds of Berries, Chokecherry and Seabuckthorn. Cent. Eur. J. Biol. 2009, 4, 499–506. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial Properties of Phenolic Compounds from Berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cho, J.-Y.; Moon, J.-H.; Choi, G.-C.; Lee, K.-D.; Ham, K.-S.; Kim, S.-J. Change of Phenylpropanoic Acid and Flavonol Contents at Different Growth Stage of Glasswort (Salicornia herbacea L.). Food Sci. Biotechnol. 2014, 23, 685–691. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Rocha, R.M.; Trentin, R.; Fredsgaard, M.; Chaturvedi, T.; Custódio, L.; Thomsen, M.H. Bioactive Extracts from Salicornia ramosissima J. Woods Biorefinery as a Source of Ingredients for High-Value Industries. Plants 2023, 12, 1251. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In Vitro and in Silico Perspectives on Biological and Phytochemical Profile of Three Halophyte Species—A Source of Innovative Phytopharmaceuticals from Nature. Phytomedicine 2018, 38, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Beji-Serairi, R.; Selmi, S.; Saidani-Tounsi, M.; Abdelly, C. Salicornia fruticosa L. and Portulaca oleracea L. Antioxidants as Affected by Domestic Cooking Processes. Int. J. Gastron. Food Sci. 2022, 27, 100462. [Google Scholar] [CrossRef]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Ryu, Y.K.; Hong, J.M.; Cho, E.A.; Shin, H.S.; et al. Desalted Salicornia europaea Extract Attenuated Vascular Neointima Formation by Inhibiting the MAPK Pathway-Mediated Migration and Proliferation in Vascular Smooth Muscle Cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. A Comparative Metabolomics Analysis of the Halophyte Suaeda Salsa and Salicornia europaea. Environ. Geochem. Health 2021, 43, 1109–1122. [Google Scholar] [CrossRef]
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Thomsen, M.H. Optimizing Methods to Characterize Caffeic, Ferulic, and Chlorogenic Acids in Salicornia sinus-persica and Salicornia bigelovii Extracts by Tandem Mass Spectrometry (LC-MS/MS). BioResources 2021, 16, 5508–5523. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-O-Glucoside and Quercetin 3-O-Glucoside Isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Rebelo, J.; Silva, H.; Pinto, D.C.G.A. Gall Midge Baldratia salicorniae Kieffer (Diptera: Cecidomyiidae) Infestation on Salicornia europaea L. Induces the Production of Specialized Metabolites with Biotechnological Potential. Phytochemistry 2022, 200, 113207. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Park, S.-H. Isolation and Identification of Antioxidant Flavonoids from Salicornia herbacea L. Appl. Biol. Chem. 2004, 47, 120–123. [Google Scholar]
- Kong, C.S.; Lee, J.I.; Kim, Y.A.; Kim, J.A.; Bak, S.S.; Hong, J.W.; Park, H.Y.; Yea, S.S.; Seo, Y. Evaluation on Anti-Adipogenic Activity of Flavonoid Glucopyranosides from Salicornia herbacea. Process Biochem. 2012, 47, 1073–1078. [Google Scholar] [CrossRef]
- Ekanayake, S.; Egodawatta, C.; Attanayake, R.N.; Perera, D. From Salt Pan to Saucepan: Salicornia, a Halophytic Vegetable with an Array of Potential Health Benefits. Food Front. 2023, 4, 641–676. [Google Scholar] [CrossRef]
- Panth, N.; Park, S.-H.; Kim, H.; Kim, D.-H.; Oak, M.-H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Rhee, M.H.; Park, H.-J.; Cho, J.Y. Salicornia herbacea: Botanical, Chemical and Pharmacological Review of Halophyte Marsh Plant. J. Med. Plants Res. 2009, 3, 548–555. [Google Scholar]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima j. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Patz, S.; Ruppel, S. Salicornia europaea L. as an Underutilized Saline-Tolerant Plant Inhabited by Endophytic Diazotrophs. J. Adv. Res. 2019, 19, 49–56. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, M.; Cao, C.; Ju, Y.; Deng, Y.; Ye, T.; Xia, Z.; Chen, M. Effect and Mechanism of Salicornia bigelovii Torr. Plant Salt on Blood Pressure in SD Rats. Food Funct. 2015, 6, 920–926. [Google Scholar] [CrossRef]
- Cho, H.D.; Lee, J.H.; Jeong, J.H.; Kim, J.Y.; Yee, S.T.; Park, S.K.; Lee, M.K.; Seo, K.-i. Production of Novel Vinegar Having Antioxidant and Anti-Fatigue Activities from Salicornia herbacea L. J. Sci. Food Agric. 2016, 96, 1085–1092. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the Halophytic Extremophile as a Food and a Pharmaceutical Candidate. 3 Biotech 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Cole, G.M.; Lim, G.P.; Yang, F.; Teter, B.; Begum, A.; Ma, Q.; Harris-White, M.E.; Frautschy, S.A. Prevention of Alzheimer’s Disease: Omega-3 Fatty Acid and Phenolic Anti-Oxidant Interventions. Neurobiol. Aging 2005, 26, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible Role of Diet in Cancer: Systematic Review and Multiple Meta-Analyses of Dietary Patterns, Lifestyle Factors, and Cancer Risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLOS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef]
- Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and Long-Term Effects of Ferulic Acid on Blood Pressure in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2002, 15, 351–357. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, Toxicology, and Metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, S.K.; Choi, J.G.; Chung, S.H. Salicornia herbacea Prevents High Fat Diet-Induced Hyperglycemia and Hyperlipidemia in ICR Mice. Arch. Pharm. Res. 2006, 29, 256–264. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&sort=FDA_s_Letter&order=DESC&showAll=true&type=basic&search= (accessed on 23 February 2023).
- Murakami, A. Dose-Dependent Functionality and Toxicity of Green Tea Polyphenols in Experimental Rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible Controversy over Dietary Polyphenols: Benefits vs Risks. Chem. Res. Toxicol. 2007, 20, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; El Arbi, K.; Aydi, S.S.; Hzami, A.; Tlahig, S.; Najar, R.; Aydi, S.; Debouba, M. Biochemical Composition and Biological Activities of Salicornia europaea L. from Southern Tunisia. J. Food Meas. Charact. 2022, 16, 4833–4846. [Google Scholar] [CrossRef]
- De Souza, M.M.; Mendes, C.R.; Doncato, K.B.; Badiale-Furlong, E.; Costa, C.S. Growth, Phenolics, Photosynthetic Pigments, and Antioxidant Response of Two New Genotypes of Sea Asparagus (Salicornia neei Lag.) to Salinity under Greenhouse and Field Conditions. Agriculture 2018, 8, 115. [Google Scholar] [CrossRef]
- Chaturvedi, T.; Hulkko, L.S.S.; Fredsgaard, M.; Thomsen, M.H. Extraction, Isolation, and Purification of Value-Added Chemicals from Lignocellulosic Biomass. Processes 2022, 10, 1752. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An Overview of the Emerging Trends of the Salicornia L. Genus as a Sustainable Crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Qin, G.; Lei, J.; Li, S.; Jiang, Y.; Qiao, L.; Ren, M.; Gao, Q.; Song, C.; Fu, S.; Zhou, J.; et al. Efficient, Green Extraction of Two Biflavonoids from Selaginella Uncinata with Deep Eutectic Solvents. Microchem. J. 2022, 183, 108085. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, J.P. Ultrasonic-Assisted Extraction and Enrichment of the Flavonoids from Salicornia europaea Leaves Using Macroporous Resins and Response Surface Methodology. Chem. Pap. 2023, 77, 2769–2781. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, Concentration and Purification of Phenolic Compounds by Adsorption: A Review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Lampejo, T. Influenza and Antiviral Resistance: An Overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef]
- Kim, Y.; Narayanan, S.; Chang, K.O. Inhibition of Influenza Virus Replication by Plant-Derived Isoquercetin. Antivir. Res. 2010, 88, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Spreeuwenberg, P.; Kroneman, M.; Paget, J. Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic. Am. J. Epidemiol. 2018, 187, 2561–2567. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 25 January 2023).
- Soriano, V.; Barreiro, P.; Cachay, E.; Kottilil, S.; Fernandez-Montero, J.V.; de Mendoza, C. Advances in Hepatitis B Therapeutics. Ther. Adv. Infect. Dis. 2020, 7, 204993612096502. [Google Scholar] [CrossRef]
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 9 February 2023).
- Emmanuel, B.; Wilson, E.M.; O’Brien, T.R.; Kottilil, S.; Lau, G. Shortening the Duration of Therapy for Chronic Hepatitis C Infection. Lancet Gastroenterol. Hepatol. 2017, 2, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Shearer, D.; Gamkrelidze, I.; Nguyen, M.H.; Chen, D.S.; Van Damme, P.; Abbas, Z.; Abdulla, M.; Abou Rached, A.; Adda, D.; Aho, I.; et al. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ashfaq, U.A.; Ijaz, B.; Riazuddin, S. Anti-Hepatitis C Virus Activity and Synergistic Effect of Nymphaea Alba Extracts and Bioactive Constituents in Liver Infected Cells. Microb. Pathog. 2018, 121, 198–209. [Google Scholar] [CrossRef]
- Erken, R.; Loukachov, V.; van Dort, K.; van den Hurk, A.; Takkenberg, R.B.; de Niet, A.; Jansen, L.; Willemse, S.; Reesink, H.; Kootstra, N. Quantified Integrated Hepatitis B Virus Is Related to Viral Activity in Patients with Chronic Hepatitis B. Hepatology 2022, 76, 196–206. [Google Scholar] [CrossRef]
- Ghany, M.G.; Doo, E.C. Antiviral Resistance and Hepatitis B Therapy. Hepatology 2009, 49, S174–S184. [Google Scholar] [CrossRef]
- Marino, A.; Cosentino, F.; Ceccarelli, M.; Moscatt, V.; Pampaloni, A.; Scuderi, D.; D’andrea, F.; Venanzi Rullo, E.; Nunnari, G.; Benanti, F.; et al. Entecavir Resistance in a Patient with Treatment-Naïve Hbv: A Case Report. Mol. Clin. Oncol. 2021, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Wang, H.-D.; Lee, S.M.Y.; Wang, Y.T.; Du, G.H. Structure-Activity Relationship of Flavonoids as Influenza Virus Neuraminidase Inhibitors and Their in Vitro Anti-Viral Activities. Bioorganic Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Al-Dosari, M.S.; Arbab, A.H.; Al-Rehaily, A.J.; Abdelwahid, M.A.S. Bioassay-Guided Isolation of Anti-Hepatitis B Virus Flavonoid Myricetin-3-O-Rhamnoside along with Quercetin from Guiera Senegalensis Leaves. Saudi Pharm. J. 2020, 28, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of Hepatitis C Virus by the Flavonoid Quercetin Is Mediated by Inhibition of NS3 Protease Activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Arumugaswami, V.; Raychaudhuri, S.; Yeh, G.K.; Maloney, E.M.; Wang, J.; Dasgupta, A.; French, S.W. Divergent Antiviral Effects of Bioflavonoids on the Hepatitis C Virus Life Cycle. Virology 2012, 433, 346–355. [Google Scholar] [CrossRef]
- Ono, K.; Nakane, H.; Fukushima, M.; Chermann, J.-C.; Barre-Sinoussi, F.B. Differential Inhibitory Effects of Various Flavonoids on the Activities of Reverse Transcriptase and Cellular DNA and RNA Polymerases. Eur. J. Biochem. 1990, 190, 469–476. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200. [Google Scholar] [CrossRef]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.d.S.M.B.; Martinez-Gutierrez, M.; de Sousa, D.P. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2021, 11, 11. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral Activities of Flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Nagarajan, S. Polyphenolic Compounds-A Promising Leads for Antiviral Therapy Flavonoids from Plants View Project. Pharmacophore 2013, 12, 119–130. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A Review on Advanced Microencapsulation Technology to Enhance Bioavailability of Phenolic Compounds: Based on Its Activity in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-Covalent Dietary Fiber-Polyphenol Interactions and Their Influence on Polyphenol Bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Tomas, M.; Rocchetti, G.; Ghisoni, S.; Giuberti, G.; Capanoglu, E.; Lucini, L. Effect of Different Soluble Dietary Fibres on the Phenolic Profile of Blackberry Puree Subjected to in Vitro Gastrointestinal Digestion and Large Intestine Fermentation. Food Res. Int. 2020, 130, 108954. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Liu, L.; Du, Y.; Wang, L.; Gao, M.; Wu, L.; Yang, C. Simultaneous Determination and Pharmacokinetic Study of Four Phenol Compounds in Rat Plasma by Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry after Oral Administration of Echinacea purpurea Extract. J. Sep. Sci. 2016, 39, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.P.; Motilva, M.J. Distribution of Olive Oil Phenolic Compounds in Rat Tissues after Administration of a Phenolic Extract from Olive Cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Sun, Q.; Zhang, C.; Yang, S.Y.; Li, L.; Jia, Z. Progress of Small Molecular Inhibitors in the Development of Anti-Influenza Virus Agents. Theranostics 2017, 7, 826–845. [Google Scholar] [CrossRef]
- Cho, W.K.; Yang, H.J.; Ma, J.Y. Lotus (Nelumbo Nucifera Gaertn.) Leaf Water Extracts Suppress Influenza a Viral Infection via Inhibition of Neuraminidase and Hemagglutinin. J. Funct. Foods 2022, 91, 105019. [Google Scholar] [CrossRef]
- Cho, W.-K.; Lee, M.-M.; Ma, J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23, 13112. [Google Scholar] [CrossRef]
- Nile, S.H.; Kim, D.H.; Nile, A.; Park, G.S.; Gansukh, E.; Kai, G. Probing the Effect of Quercetin 3-Glucoside from Dianthus superbus L. against Influenza Virus Infection—In Vitro and in Silico Biochemical and Toxicological Screening. Food Chem. Toxicol. 2020, 135, 110985. [Google Scholar] [CrossRef]
- Hariono, M.; Abdullah, N.; Damodaran, K.V.; Kamarulzaman, E.E.; Mohamed, N.; Hassan, S.S.; Shamsuddin, S.; Wahab, H.A. Potential New H1N1 Neuraminidase Inhibitors from Ferulic Acid and Vanillin: Molecular Modelling, Synthesis and in Vitro Assay. Sci. Rep. 2016, 6, 38692. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hong, Q.; Wang, Y.; Liang, Q.; Tan, H.; Xiao, C.; Tang, X.; Shao, S.; Zhou, S.; Gao, Y. Ferulic Acid Induces Heme Oxygenase-1 via Activation of ERK and Nrf2. Drug Discov. Ther. 2011, 5, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stavchansky, S.; Kerwin, S.M.; Bowman, P.D. Structure-Activity Relationships in the Cytoprotective Effect of Caffeic Acid Phenethyl Ester (CAPE) and Fluorinated Derivatives: Effects on Heme Oxygenase-1 Induction and Antioxidant Activities. Eur. J. Pharmacol. 2010, 635, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shao, Y.; Qu, X.; Guo, J.; Yang, J.; Zhou, Z.; Wang, S. Sodium Ferulate Protects against Influenza Virus Infection by Activation of the TLR7/9-MyD88-IRF7 Signaling Pathway and Inhibition of the NF-ΚB Signaling Pathway. Biochem. Biophys. Res. Commun. 2019, 512, 793–798. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; Maria John, K.M.; Ayyanar, M.; Sekar, T.; Jin, K.J.; Kim, D.H. Anti-Influenza (H1N1) Potential of Leaf and Stem Bark Extracts of Selected Medicinal Plants of South India. Saudi J. Biol. Sci. 2015, 22, 532–538. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural Product-Derived Phytochemicals as Potential Agents against Coronaviruses: A Review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, H.S.; Park, H.; Kim, Y.C.; Yun, Y.G.; Park, S.; Shin, H.J.; Kim, K. In Vitro Inhibition of Coronavirus Replications by the Traditionally Used Medicinal Herbal Extracts, Cimicifuga Rhizoma, Meliae Cortex, Coptidis Rhizoma, and Phellodendron Cortex. J. Clin. Virol. 2008, 41, 122–128. [Google Scholar] [CrossRef]
- Spasova, M.; Philipov, S.; Nikolaeva-Glomb, L.; Galabov, A.S.; Milkova, T. Cinnamoyl- and Hydroxycinnamoyl Amides of Glaucine and Their Antioxidative and Antiviral Activities. Bioorganic Med. Chem. 2008, 16, 7457–7461. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Inhibition of the Main Protease of SARS-CoV-2 (Mpro) by Repurposing/Designing Drug-like Substances and Utilizing Nature’s Toolbox of Bioactive Compounds. Comput. Struct. Biotechnol. J. 2022, 20, 1306–1344. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid From Plant Biomass: A Phytochemical With Promising Antiviral Properties. Front. Nutr. 2022, 8, 1358. [Google Scholar] [CrossRef]
- Salman, S.; Shah, F.H.; Idrees, J.; Idrees, F.; Velagala, S.; Ali, J.; Khan, A.A. Virtual Screening of Immunomodulatory Medicinal Compounds as Promising Anti-SARS-CoV-2 Inhibitors. Future Virol. 2020, 15, 267–275. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Commandeur, J.N.M.; van Vugt-Lussenburg, B.; Vermeulen, N.P.E. Inhibition of Human Recombinant Cytochrome P450s by Curcumin and Curcumin Decomposition Products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, H.; Wang, T.; Liu, Y.; Wang, J.; Sun, B. Feruloylated Oligosaccharides Modulate the Gut Microbiota in Vitro via the Combined Actions of Oligosaccharides and Ferulic Acid. J. Funct. Foods 2019, 60, 103453. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. Pentadecyl Ferulate, a Potent Antioxidant and Antiproliferative Agent from the Halophyte Salicornia herbacea. Food Chem. 2013, 141, 2066–2074. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals Have Potential for Boosting the Type 1 Interferon Response to RNA Viruses Including Influenza and Coronavirus. Prog. Cardiovasc. Dis. 2020, 63, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral Activity of Chlorogenic Acid against Influenza A (H1N1/H3N2) Virus and Its Inhibition of Neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral Effect of Methylated Flavonol Isorhamnetin against Influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Kanazawa, R.; Morimoto, R.; Horio, Y.; Sumitani, H.; Isegawa, Y. Inhibition of Influenza Virus Replication by Apiaceae Plants, with Special Reference to Peucedanum Japonicum (Sacna) Constituents. J. Ethnopharmacol. 2022, 292, 115243. [Google Scholar] [CrossRef]
- Kai, H.; Obuchi, M.; Yoshida, H.; Watanabe, W.; Tsutsumi, S.; Park, Y.K.; Matsuno, K.; Yasukawa, K.; Kurokawa, M. In Vitro and in Vivo Anti-Influenza Virus Activities of Flavonoids and Related Compounds as Components of Brazilian Propolis (AF-08). J. Funct. Foods 2014, 8, 214–223. [Google Scholar] [CrossRef]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking Study of Flavonoid Derivatives as Potent Inhibitors of Influenza H1N1 Virus Neuraminidas. Biomed. Rep. 2019, 10, 33–38. [Google Scholar] [CrossRef]
- Kaihatsu, K. Potential Anti-Influenza Virus Agents Based on Coffee Ingredients and Natural Flavonols. Nat. Prod. Chem. Res. 2014, 2, 1000129. [Google Scholar] [CrossRef]
- Albeshri, A.; Baeshen, N.A.; Bouback, T.A.; Aljaddawi, A.A. Evaluation of Cytotoxicity and Antiviral Activity of Rhazya Stricta Decne Leaves Extract against Influenza A/PR/8/34 (H1N1). Saudi J. Biol. Sci. 2022, 29, 103375. [Google Scholar] [CrossRef]
- Motlhatlego, K.E.; Mehrbod, P.; Fotouhi, F.; Abdalla, M.A.; Eloff, J.N.; McGaw, L.J. Anti-Influenza A Virus Activity of Two Newtonia Species and the Isolated Compound Myricetin-3-o-Rhamnoside. BMC Complement. Med. Ther. 2021, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Harish, D.R.; Vetrivel, U.; Deshpande, S.H.; Khanal, P.; Hegde, H.V.; Roy, S.; Jalalpure, S.S. Pharmacoinformatics Analysis Reveals Flavonoids and Diterpenoids from Andrographis Paniculata and Thespesia Populnea to Target Hepatocellular Carcinoma Induced by Hepatitis B Virus. Appl. Sci. 2022, 12, 10691. [Google Scholar] [CrossRef]
- Shin, M.S.; Kang, E.H.; Lee, Y.I. A Flavonoid from Medicinal Plants Blocks Hepatitis B Virus-e Antigen Secretion in HBV-Infected Hepatocytes. Antivir. Res. 2005, 67, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M. A Survey on Herbal Management of Hepatocellular Carcinoma. World J. Hepatol. 2011, 3, 175. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ijaz, B.; Fatima, N.; Muhammad, S.A.; Riazuddin, S. Therapeutic Potential of Taraxacum Officinale against HCV NS5B Polymerase: In-Vitro and In Silico Study. Biomed. Pharmacother. 2016, 83, 881–891. [Google Scholar] [CrossRef]
- Lam, B.; Henry, L.; Younossi, Z. Sofosbuvir (Sovaldi) for the Treatment of Hepatitis C. Expert Rev. Clin. Pharmacol. 2014, 7, 555–566. [Google Scholar] [CrossRef]
- Watashi, K.; Urban, S.; Li, W.; Wakita, T. NTCP and Beyond: Opening the Door to Unveil Hepatitis B Virus Entry. Int. J. Mol. Sci. 2014, 15, 2892–2905. [Google Scholar] [CrossRef]
- Lv, H.; An, B.; Yu, Q.; Cao, Y.; Liu, Y.; Li, S. The Hepatoprotective Effect of Myricetin against Lipopolysaccharide and D-Galactosamine-Induced Fulminant Hepatitis. Int. J. Biol. Macromol. 2020, 155, 1092–1104. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Truant, R.; Antunovic, J.; Greenblatt, J.; Prives, C.; Cromlish, J.A. Direct Interaction of the Hepatitis B Virus HBx Protein with P53 Leads to Inhibition by HBx of P53 Response Element-Directed Transactivation. J. Virol. 1995, 69, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Gottlob, K.; Fulco, M.; Levrero, M.; Graessmann, A. The Hepatitis B Virus HBx Protein Inhibits Caspase 3 Activity. J. Biol. Chem. 1998, 273, 33347–33353. [Google Scholar] [CrossRef]
- Chung, T.; Lee, Y.; Kim, C. Hepatitis B Viral HBx Induces Matrix Metalloproteinase-9 Gene Expression through Activation of ERKs and PI-3K/AKT Pathways: Involvement of Invasive Potential. FASEB J. 2004, 18, 1123–1125. [Google Scholar] [CrossRef]
- Torresi, J.; Tran, B.M.; Christiansen, D.; Earnest-Silveira, L.; Schwab, R.H.M.; Vincan, E. HBV-Related Hepatocarcinogenesis: The Role of Signalling Pathways and Innovative Ex Vivo Research Models. BMC Cancer 2019, 19, 707. [Google Scholar] [CrossRef] [PubMed]
- Kang-Park, S.; Im, J.H.; Lee, J.H.; Lee, Y.I. PTEN Modulates Hepatitis B Virus-X Protein Induced Survival Signaling in Chang Liver Cells. Virus Res. 2006, 122, 53–60. [Google Scholar] [CrossRef]
- Lin, H.J.; Ku, K.L.; Lin, I.H.; Yeh, C.C. Naringenin Attenuates Hepatitis B Virus X Protein-Induced Hepatic Steatosis. BMC Complement. Altern. Med. 2017, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Zhou, W.; Cheng, G.; Wu, J.; Guo, S.; Jia, S.; Liu, Y.; Li, B.; Zhang, X.; et al. A Bioinformatics Investigation into Molecular Mechanism of Yinzhihuang Granules for Treating Hepatitis B by Network Pharmacology and Molecular Docking Verification. Sci. Rep. 2020, 10, 11448. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B.; Nascimbeni, M. Immunology of Hepatitis B Virus and Hepatitis C Virus Infection. Nat. Rev. Immunol. 2005, 5, 215–229. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wu, Y.-H.; Tseng, C.-K.; Lin, C.-K.; Chen, W.-C.; Hsu, Y.-C.; Lee, J.-C. Green Tea Phenolic Epicatechins Inhibit Hepatitis C Virus Replication via Cycloxygenase-2 and Attenuate Virus-Induced Inflammation. PLoS ONE 2013, 8, e54466. [Google Scholar] [CrossRef]
- Ma, J.Q.; Li, Z.; Xie, W.R.; Liu, C.M.; Liu, S.S. Quercetin Protects Mouse Liver against CCl4-Induced Inflammation by the TLR2/4 and MAPK/NF-ΚB Pathway. Int. Immunopharmacol. 2015, 28, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Berzsenyi, M.D.; Roberts, S.K.; Preiss, S.; Woollard, D.J.; Beard, M.R.; Skinner, N.A.; Bowden, D.S.; Visvanathan, K. Hepatic TLR2 & TLR4 Expression Correlates with Hepatic Inflammation and TNF-α in HCV & HCV/HIV Infection. J. Viral Hepat. 2011, 18, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Tyurina, D.A.; Ivanova, O.N.; Kochetkov, S.N.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress, a Trigger of Hepatitis C and B Virus-Induced Liver Carcinogenesis. Oncotarget 2017, 8, 3895–3932. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Valgimigli, L.; Trerè, D.; Gaiani, S.; Pedulli, G.F.; Gramantieri, L.; Bolondi, L. Oxidative Stress EPR Measurement in Human Liver by Radical-Probe Technique. Correlation with Etiology, Histology and Cell Proliferation. Free Radic. Res. 2002, 36, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Higgs, M.R.; Chouteau, P.; Lerat, H. “Liver Let Die”: Oxidative DNA Damage and Hepatotropic Viruses. J. Gen. Virol. 2014, 95, 991–1004. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, Y.; Niedernhofer, L.J.; Wang, Y. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Shen, J.; Wang, G.; Zuo, J. Caffeic Acid Inhibits HCV Replication via Induction of IFNα Antiviral Response through P62-Mediated Keap1/Nrf2 Signaling Pathway. Antivir. Res. 2018, 154, 166–173. [Google Scholar] [CrossRef]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-Hepatitis B Virus Activity of Chlorogenic Acid, Quinic Acid and Caffeic Acid in Vivo and in Vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum Basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Jong, C.P. Effects of Triterpenoids and Flavonoids Isolated from Alnus Firma on HIV-1 Viral Enzymes. Arch. Pharm. Res. 2007, 30, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, C.; Sanches, T.; Zhong, Y.; Liu, B.; Xiong, J.; Neamati, N.; Zhao, G. Design and Synthesis of Novel Nitrogen-Containing Polyhydroxylated Aromatics as HIV-1 Integrase Inhibitors from Caffeic Acid Phenethyl Ester. Bioorganic Med. Chem. Lett. 2009, 19, 4574–4578. [Google Scholar] [CrossRef] [PubMed]
- Tchertanov, L.; Mouscadet, J.F. Target Recognition by Catechols and β-Ketoenols: Potential Contribution of Hydrogen Bonding and Mn/Mg Chelation to HIV-1 Integrase Inhibition. J. Med. Chem. 2007, 50, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Cotelle, P. Anti-HIV Activities of Natural Antioxidant Caffeic Acid Derivatives: Toward an Antiviral Supplementation Diet. Curr. Med. Chem. 2005, 12, 1811–1818. [Google Scholar] [CrossRef]
- Wyatt, R.; Moore, J.; Accola, M.; Desjardin, E.; Robinson, J.; Sodroski, J. Involvement of the V1/V2 Variable Loop Structure in the Exposure of Human Immunodeficiency Virus Type 1 Gp120 Epitopes Induced by Receptor Binding. J. Virol. 1995, 69, 5723–5733. [Google Scholar] [CrossRef]
- Delelis, O.; Malet, I.; Li, N.; Tchertanov, L.; Calvez, V.; Marcelin, A.-G.; Subra, F.; Deprez, E.; Mouscadet, J.-F. The G140S Mutation in HIV Integrases from Raltegravir-Resistant Patients Rescues Catalytic Defect Due to the Resistance Q148H Mutation. Nucleic Acids Res. 2009, 37, 1193–1201. [Google Scholar] [CrossRef]
- King, P.J.; Lee, D.J.; Reinke, R.A.; Victoria, J.G.; Beale, K.; Robinson, W.E. Human Immunodeficiency Virus Type-1 Integrase Containing a Glycine to Serine Mutation at Position 140 Is Attenuated for Catalysis and Resistant to Integrase Inhibitors. Virology 2003, 306, 147–161. [Google Scholar] [CrossRef]
- Singh, S.B.; Jayasuriya, H.; Dewey, R.; Polishook, J.D.; Dombrowski, A.W.; Zink, D.L.; Guan, Z.; Collado, J.; Platas, G.; Pelaez, F.; et al. Isolation, Structure, and HIV-1-Integrase Inhibitory Activity of Structurally Diverse Fungal Metabolites. J. Ind. Microbiol. Biotechnol. 2003, 30, 721–731. [Google Scholar] [CrossRef]
- Abraham, R.T.; Weiss, A. Jurkat T Cells and Development of the T-Cell Receptor Signalling Paradigm. Nat. Rev. Immunol. 2004, 4, 301–308. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Ji, H.; Deng, J.; Lu, P.; Jiang, Z.; Li, X.; Wang, Y.; Wang, C.; Zhao, J.; et al. Quercetin Synergistically Reactivates Human Immunodeficiency Virus Type 1 Latency by Activating Nuclear Factor-ΚB. Mol. Med. Rep. 2018, 17, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Ono1, K.; Nakane, H. Mechanisms of Inhibition of Various Cellular DNA and RNA Polymerases by Several Flavonoids. J. Biochem. 1990, 108, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Fesen, M.R.; Pommier, Y.; Leteurtre, F.; Hiroguchi, S.; Yung, J.; Kohn, K.W. Inhibition of HIV-1 Integrase by Flavones, Caffeic Acid Phenethyl Ester (CAPE) and Related Compounds. Biochem. Pharmacol. 1994, 48, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Pasetto, S.; Pardi, V.; Murata, R.M. Anti-HIV-1 Activity of Flavonoid Myricetin on HIV-1 Infection in a Dual-Chamber In Vitro Model. PLoS ONE 2014, 9, e115323. [Google Scholar] [CrossRef]
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV Activation in Latently Infected Cells by Flavonoid Compounds. AIDS Res. Hum. Retroviruses 2009, 12, 39–46. [Google Scholar] [CrossRef]
- Tamura, H.; Akioka, T.; Ueno, K.; Chujyo, T.; Okazaki, K.I.; King, P.J.; Robinson, W.E. Anti-Human Immunodeficiency Virus Activity of 3,4,5-Tricaffeoylquinic Acid in Cultured Cells of Lettuce Leaves. Mol. Nutr. Food Res. 2006, 50, 396–400. [Google Scholar] [CrossRef]
- Kreis, W.; Kaplan, M.H.; Freeman, J.; Sun, D.K.; Sarin, P.S. Inhibition of HIV Replication by Hyssop Officinalis Extracts. Antivir. Res. 1990, 14, 323–337. [Google Scholar] [CrossRef]
- Sonar, V.P.; Corona, A.; Distinto, S.; Maccioni, E.; Meleddu, R.; Fois, B.; Floris, C.; Malpure, N.V.; Alcaro, S.; Tramontano, E.; et al. Natural Product-Inspired Esters and Amides of Ferulic and Caffeic Acid as Dual Inhibitors of HIV-1 Reverse Transcriptase. Eur. J. Med. Chem. 2017, 130, 248–260. [Google Scholar] [CrossRef]
- Sanna, C.; Rigano, D.; Corona, A.; Piano, D.; Formisano, C.; Farci, D.; Franzini, G.; Ballero, M.; Chianese, G.; Tramontano, E.; et al. Dual HIV-1 Reverse Transcriptase and Integrase Inhibitors from Limonium Morisianum Arrigoni, an Endemic Species of Sardinia (Italy). Nat. Prod. Res. 2019, 33, 1798–1803. [Google Scholar] [CrossRef]
- Tamayose, C.I.; Torres, P.B.; Roque, N.; Ferreira, M.J.P. HIV-1 Reverse Transcriptase Inhibitory Activity of Flavones and Chlorogenic Acid Derivatives from Moquiniastrum floribundum (Asteraceae). S. Afr. J. Bot. 2019, 123, 142–146. [Google Scholar] [CrossRef]
- Wu, T.S.; Tsang, Z.J.; Wu, P.L.; Lin, F.W.; Li, C.Y.; Teng, C.M.; Lee, K.H. New Constituents and Antiplatelet Aggregation and Anti-HIV Principles of Artemisia Capillaris. Bioorganic Med. Chem. 2001, 9, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.C.F.; Salatino, A.; da Motta, L.B.; Negri, G.; Salatino, M.L.F. Chemical Characterization, Antioxidant and Anti-HIV Activities of a Brazilian Propolis from Ceará State. Rev. Bras. Farmacogn. 2019, 29, 309–318. [Google Scholar] [CrossRef]
- Ortega, J.T.; Suárez, A.I.; Serrano, M.L.; Baptista, J.; Pujol, F.H.; Rangel, H.R. The Role of the Glycosyl Moiety of Myricetin Derivatives in Anti-HIV-1 Activity in Vitro. AIDS Res. Ther. 2017, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Nakabori, T.; Hikita, H.; Murai, K.; Nozaki, Y.; Kai, Y.; Makino, Y.; Saito, Y.; Tanaka, S.; Wada, H.; Eguchi, H.; et al. Sodium Taurocholate Cotransporting Polypeptide Inhibition Efficiently Blocks Hepatitis B Virus Spread in Mice with a Humanized Liver. Sci. Rep. 2016, 6, 27782. [Google Scholar] [CrossRef]
- Ross, J.A.; Potter, J.D.; Robison, L.L. Infant Leukemia, Topoisomerase II Inhibitors, and the MLL Gene. J. Natl. Cancer Inst. 1994, 86, 1678–1680. [Google Scholar] [CrossRef]
- Ross, J.A.; Potter, J.D.; Reaman, G.H.; Pendergrass, T.W.; Robison, L.L. Maternal Exposure to Potential Inhibitors of DNA Topoisomerase II and Infant Leukemia (United States): Report from the Children’s Cancer Group. Cancer Causes Control 1996, 7, 581–590. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.-Y.; Chen, Y.; Zhang, X.; Wu, Y.; Wang, Z.-X.; Chen, P.-H.; Dai, H.-Q.; Feng, J.; Chatterjee, S.; et al. Quercetin Induces Apoptosis via Downregulation of Vascular Endothelial Growth Factor/Akt Signaling Pathway in Acute Myeloid Leukemia Cells. Front. Pharmacol. 2020, 11, 1972. [Google Scholar] [CrossRef]
- Bonkovsky, H.L. Hepatotoxicity Associated with Supplements Containing Chinese Green Tea (Camellia sinensis). Ann. Intern. Med. 2006, 144, 68–71. [Google Scholar] [CrossRef]
- Kassem, M.E.S.; Ibrahim, L.F.; Hussein, S.R.; El-Sharawy, R.; El-Ansari, M.A.; Hassanane, M.M.; Booles, H.F. Myricitrin and Bioactive Extract of Albizia amara Leaves: DNA Protection and Modulation of Fertility and Antioxidant-Related Genes Expression. Pharm. Biol. 2016, 54, 2404–2409. [Google Scholar] [CrossRef]
- Yan, L.J.; Yang, H.T.; Duan, H.Y.; Wu, J.T.; Qian, P.; Fan, X.W.; Wang, S. Myricitrin Inhibits Vascular Adhesion Molecule Expression in TNF-α-Stimulated Vascular Smooth Muscle Cells. Mol. Med. Rep. 2017, 16, 6354–6359. [Google Scholar] [CrossRef]
- Córdova, M.M.; de Paula Werner, M.F.; da Silva, M.D.; Ruani, A.P.; Pizzolatti, M.G.; Santos, A.R.S. Further Antinociceptive Effects of Myricitrin in Chemical Models of Overt Nociception in Mice. Neurosci. Lett. 2011, 495, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Y.; Ye, X.; Xue, S.; Shi, J.; Pan, J.; Chen, Q. Inhibition Effects and Induction of Apoptosis of Flavonoids on the Prostate Cancer Cell Line PC-3 In Vitro. Food Chem. 2013, 138, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, B.; Wang, L.; Hu, Y.Q.; Lu, W.G.; Yang, L.; Luo, Z.J.; Liu, J. Protective Effects of Myricitrin against Osteoporosis via Reducing Reactive Oxygen Species and Bone-Resorbing Cytokines. Toxicol. Appl. Pharmacol. 2014, 280, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Demigné, C.; Texier, O.; Régérat, F.; Rémésy, C. Bioavailability of Rutin and Quercetin in Rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Jafari, S.M. Biopolymer Nano-Particles and Natural Nano-Carriers for Nano-Encapsulation of Phenolic Compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
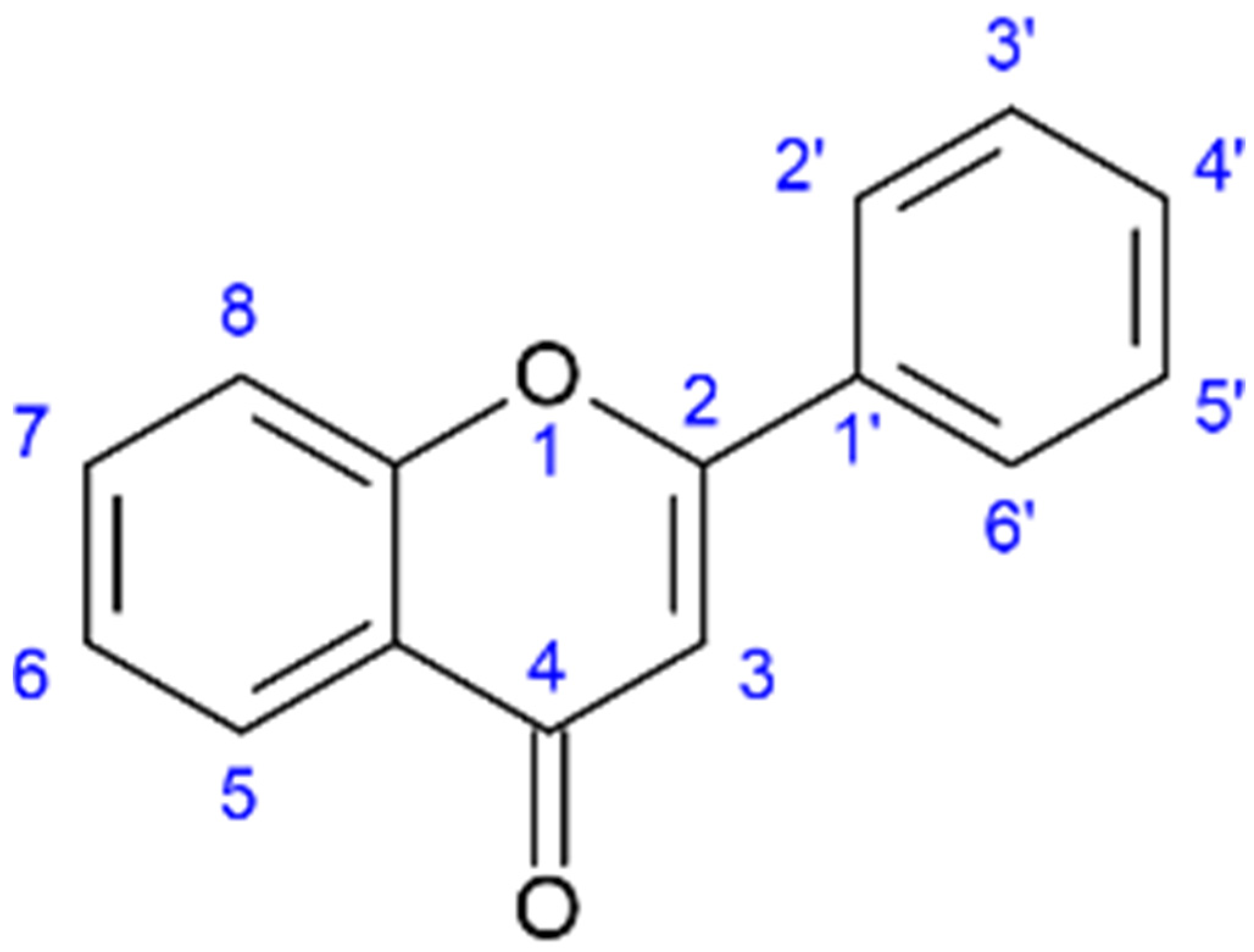


| Name | Synonym | Biomass Origin | Concentration mg/kg DM | Ref. |
|---|---|---|---|---|
| Caffeic acid | 3,4-Dihydroxycinnamic acid | S. europaea L. | 30.4 | [17] |
| S. europaea L. | 28.6 | [46] | ||
| S. fruticosa L. | 31.0 ± 0.8 | [16] | ||
| S. ramosissima J. Woods | 14.4 ± 0.7 | [18] | ||
| Ferulic acid | 3-Methoxy-4-hydroxycinnamic acid | S. europaea L. | 58.2 | [17] |
| S. fruticosa L. | 67.9 ± 1.9 | [16] | ||
| S. ramosissima J. Woods | 28.5 ± 1.4 | [18] | ||
| S. ramosissima J. Woods | 35.7 ± 3.4 | [28] | ||
| Chlorogenic acid | 3-(3,4-Dihydroxycinnamoyl)quinic acid | S. europaea L. | 45.1 | [17] |
| S. europaea L. | 391.7 | [46] | ||
| S. europaea L. | 840.0 | [37] | ||
| S. fruticosa L. | 85.2 ± 1.5 | [16] | ||
| S. ramosissima J. Woods | 16.7 ± 0.8 | [18] | ||
| S. ramosissima J. Woods | 450.8 ± 115.2 | [28] | ||
| Apigenin | 4′,5,7-Trihydroxyflavone | S. fruticosa L. | <QL | [16] |
| S. ramosissima J. Woods | 4.5 ± 0.2 | [18] | ||
| Kaempferol | 3,4′,5,7-Tetrahydroxyflavone | S. fruticosa L. | <QL | [16] |
| S. ramosissima J. Woods | 4.5 ± 0.2 | [18] | ||
| Quercetin | 3,3′,4′,5,7-Pentahydroxyflavone | S. europaea L. | 8.0 | [17] |
| S. europaea L. | 14.8 | [47] | ||
| S. fruticosa L. | 15.6 ± 0.3 | [16] | ||
| S. ramosissima J. Woods | 34.0 ± 1.7 | [18] | ||
| Isorhamnetin | 3,4′,5,7-Tetrahydroxy-3′-methoxyflavone | S. europaea L. | 58.9 | [17] |
| Myricetin | 3,3′,4′,5,5′,7-Hexahydroxyflavone | S. fruticosa L. | 137.0 ± 1.0 | [16] |
| S. ramosissima J. Woods | 465.5 ± 23.3 | [18] | ||
| Isoquercitrin | Quercetin 3-O-glucoside | S. europaea L. | 10.9 | [17] |
| S. fruticosa L. | 531.4 ± 9.4 | [16] | ||
| S. ramosissima J. Woods | 453.7 ± 68.8 | [28] | ||
| Myricitrin | Myricetin 3-O-rhamnoside | S. fruticosa L. | 3314.5 ± 94.8 | [16] |
| Compound | H1N1 Inhibition Target | Inhibition Activity | Ref. |
|---|---|---|---|
| Caffeic acid | A/PR/8/34 (H1N1) infected MDCK | IC50 inhibition: 81.6 ± 8.3 µM | [101] |
| A/Osaka/2024/09 (H1N1) infected MDCK | IC50 inhibition: 98.8 µM | [101] | |
| A/Osaka/71/11 (H1N1) infected MDCK | IC50 inhibition: 97.1 µM | [101] | |
| Ferulic acid | NA | Docking energy: −7.1 kcal/mol | [84] |
| A/Malaysia/Muar/33/09 NA (H1N1) inhibition (NAI) in vitro assay | IC50 inhibition: 140 µM | [84] | |
| EC50 of A/Malaysia/Muar/33/09 (H1N1) in infected MDCK cells | EC50 inhibition: 1.32 ± 0.08 µM | [84] | |
| Chlorogenic acid | EC50 of A/PR/8/34 (H1N1) in infected MDCK cells | EC50 inhibition: 44.87 µM | [99] |
| NAI by fluorescence-based in vitro assay | IC50 inhibition: 22.13 ± 1.07 µM | [99] | |
| Apigenin | EC50 of A/PR/8/34 (H1N1) in infected MDCK cells | EC50 inhibition: 56.7 ± 11.1 µM | [102] |
| EC50 of A/Toyama/129/11 (H1N1) in infected MDCK cells | EC50 inhibition: 65.9 ± 32.2 µM | [102] | |
| EC50 of A/Toyama/26/11 (H1N1) in infected MDCK cells | EC50 inhibition: 30.0 ± 17.4 µM | [102] | |
| A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 31.6 ± 0.9 µM | [65] | |
| Kaempferol | NA | Docking energy: −6.8 kcal/mol | [103] |
| NAI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [100] | |
| A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 58.6 ± 0.6 µM | [65] | |
| Quercetin | A/PR/8/34 (H1N1) infected MDCK | IC50 inhibition: 274.6 ± 3.3 µM | [101] |
| A/PR/8/34 (H1N1) plaque formation inhibition in vitro assay | IC50 inhibition: 1.40 µM | [104] | |
| NAI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [100] | |
| NA | Docking energy: −6.8 kcal/mol | [103] | |
| A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 58.4 ± 3.8 µM | [65] | |
| Isorhamnetin | HI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [100] |
| NAI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [100] | |
| Myricetin | A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 82.6 ± 8.9 µM | [65] |
| A/PR/8/34 (H1N1) plaque formation inhibition in vitro assay | IC50 inhibition: 0.90 µM | [104] | |
| Isoquercitrin | HI RAW 264.7 cell line in vitro assay | Complete inhibition: 5 µM | [82] |
| NAI by fluorescence-based in vitro assay | IC50 inhibition: 37.1 ± 0.6 µM | [82] | |
| PB2 | Docking energy: −8.0 kcal/mol | [83] | |
| NA | Docking energy: −8.6 kcal/mol | [105] | |
| A/PR/8/34 (H1N1) infected MDCK | IC50 inhibition: 10.6 ± 0.4 µM | [83] | |
| A/WS/33 (H1N1) infected MDCK | IC50 inhibition: 21.4 ± 2.4 µM | [83] | |
| Myricitrin | A/PR/8/34 (H1N1) infected MDCK | 255.6% increase in cell viability at 224.0 µM | [106] |
| Name | HBV/HCV Inhibition Target | Inhibition Activity | Ref. |
|---|---|---|---|
| Caffeic acid | HCV-1 infected Huh7.5.1 cells | IC50 inhibition: 100 ± 20 µM | [132] |
| EHMT2 | Docking energy: −5.9 kcal/mol | [121] | |
| HBV-DNA | IC50 inhibition: 3.9 ± 1.1 µM | [133] | |
| HBsAg in vitro assay in infected HepG2.2.2.15 cells | IC50 inhibition: 12.7 ± 9.9 µM | [133] | |
| HBeAg in vitro assay in infected HepG2.2.2.15 cells | IC50 inhibition: 109.3 ± 56.0 µM | [133] | |
| Ferulic acid | No data | ||
| Chlorogenic acid | EHMT2 | Docking energy: −7.8 kcal/mol | [121] |
| STAT3 | Docking energy: −7.2 kcal/mol | [121] | |
| HBV-DNA | IC50 inhibition: 1.2 ± 0.4 µM | [133] | |
| HBsAg in vitro assay in infected HepG2.2.2.15 cells | IC50 inhibition: 241.5 ± 198.2 µM | [133] | |
| HBeAg in vitro assay in infected HepG2.2.2.15 cells | IC50 inhibition: >1000 µM | [133] | |
| Apigenin | EC50 of HBsAg | EC50 inhibition: 26.3 ± 5.6 µM | [134] |
| EC50 of HBeAg | EC50 inhibition: 47.4 ± 3.3 µM | [134] | |
| Kaempferol | HBV-RT | Docking energy: −9.0 kcal/mol | [66] |
| HBcAg | Docking energy: −9.1 kcal/mol | [66] | |
| Quercetin | HBV-RT | Docking energy: −8.3 kcal/mol | [66] |
| HBcAg | Docking energy: −8.7 kcal/mol | [66] | |
| NTCP | Docking energy: −5.4 kcal/mol | [66] | |
| HBsAg in vitro assay in infected HepG2.2.2.15 cells | 60% inhibition | [66] | |
| HBeAg in vitro assay in infected HepG2.2.2.15 cells | 62% inhibition | [66] | |
| Isorhamnetin | No data | ||
| Myricetin | Infected fulminant hepatitis mice | 66% increased survival rate | [113] |
| Isoquercitrin | No data | ||
| Myricitrin | HBV-RT | Docking energy: −7.7 kcal/mol | [66] |
| HBcAg | Docking energy: −7.1 kcal/mol | [66] | |
| NTCP | Docking energy: −6.7 kcal/mol | [66] | |
| HBsAg in vitro assay in infected HepG2.2.2.15 cells | 44% inhibition | [66] | |
| HBeAg in vitro assay in infected HepG2.2.2.15 cells | 35% inhibition | [66] |
| Name | HIV-1 Inhibition Target | Inhibition Activity | Reference |
|---|---|---|---|
| Caffeic acid | IN assay in infected MT-2 cells | IC50 inhibition: >278 µM | [149] |
| Syncytia formation in Molt-3 cells | 93% inhibtion at 255 µM | [150] | |
| Matrix protein by immunofluorescence analysis | 50% inhibtion at 255 µM | [150] | |
| Capsid protein by immunofluorescence analysis | 57% inhibtion at 255 µM | [150] | |
| RT assay | 32% inhibtion at 255 µM | [150] | |
| Ferulic acid | No inhibitory effect | N/A | [151,152] |
| Chlorogenic acid | IN assay in infected MT-2 cells | IC50 inhibition: >142 µM | [149] |
| RT by colorimetric enzyme immunoassay | IC50 inhibition: 374 µM | [153] | |
| Apigenin | RT by PCR assay | IC50: >37.03 µM | [69] |
| HIV-1 in infected OM-10.1 cells | IC50 inhibition: 12 µM | [148] | |
| Kaempferol | IN integration | IC50 inhibition: 64.7 ± 18.1 µM | [146] |
| IN cleavage | IC50 inhibition: 97.8 ± 9.2 µM | [146] | |
| RT by PCR assay | IC50 inhibition: >34.94 µM | [69] | |
| PR by fluorescence assay | 62.7% inhibition at 174.7 µM | [154] | |
| Quercetin | RT by ELOSA | IC50 inhibition: 60 µM | [135] |
| PR by oligopeptide cleavage assay | IC50 inhibition: >100 µM | [135] | |
| α-glucosidase inhibition assay | IC50 inhibition: >100 µM | [135] | |
| IN integration | IC50 inhibition: 13.6 ± 3.4 µM | [146] | |
| IN cleavage | IC50 inhibition: 23.6 ± 6.6 µM | [146] | |
| RT by PCR assay | IC50 inhibition: <1.65 µM | [69] | |
| RT by PCR assay | 100% inhibition at 6.62 µM | [69] | |
| RT by ELISA colorimetric enzyme immunoassay | 43.41 ± 4.56% inhibition at 661.7 µM | [155] | |
| Isorhamnetin | HIV-1 replication in H9 lymphocyte cells | EC50 inhibition: 6.6 µM | [154] |
| RT by ELISA colorimetric enzyme immunoassay | 56.99 ± 3.91% inhibition at 632.4 µM | [155] | |
| Myricetin | IN integration | IC50 inhibition: 2.5 ± 1.0 µM | [146] |
| IN cleavage | IC50 inhibition: 7.6 ± 0.6 µM | [146] | |
| RT by PCR assay | IC50 inhibition: <1.57 µM | [69] | |
| RT by PCR assay | 100% inhibition at 6.29 µM | [69] | |
| HIV-1 Blood and lymphocytes infected TZM-bl cells | 87% inhibition at 100 µM | [147] | |
| HIV-1 Blood and lymphocytes infected TZM-bl cells | IC50 inhibition: 20.43 µM | [147] | |
| HIV-1 in infected MT4 cells | EC50 inhibition: 230 µM | [156] | |
| RT assay | IC50 inhibition: 7.6 µM | [156] | |
| RT | Docking energy: −7.0 kcal/mol | [156] | |
| Isoquercitrin | PR by fluorescence assay | 64.4% inhibition at 107.7 µM | [154] |
| Myricitrin | PR by fluorescence assay | 50.4% inhibition at 107.7 µM | [154] |
| HIV-1 in infected MT4 cells | EC50 inhibition: 120 µM | [156] | |
| RT assay | IC50 inhibition: 10.6 µM | [156] | |
| RT | Docking energy: −5.0 kcal/mol | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredsgaard, M.; Kaniki, S.E.K.; Antonopoulou, I.; Chaturvedi, T.; Thomsen, M.H. Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules 2023, 28, 5312. https://doi.org/10.3390/molecules28145312
Fredsgaard M, Kaniki SEK, Antonopoulou I, Chaturvedi T, Thomsen MH. Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules. 2023; 28(14):5312. https://doi.org/10.3390/molecules28145312
Chicago/Turabian StyleFredsgaard, Malthe, Samba Evelyne Kabemba Kaniki, Io Antonopoulou, Tanmay Chaturvedi, and Mette Hedegaard Thomsen. 2023. "Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review" Molecules 28, no. 14: 5312. https://doi.org/10.3390/molecules28145312
APA StyleFredsgaard, M., Kaniki, S. E. K., Antonopoulou, I., Chaturvedi, T., & Thomsen, M. H. (2023). Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules, 28(14), 5312. https://doi.org/10.3390/molecules28145312










