Efficient and Selective Adsorption of Cationic Dye Malachite Green by Kiwi-Peel-Based Biosorbents
Abstract
1. Introduction
2. Results and Discussion
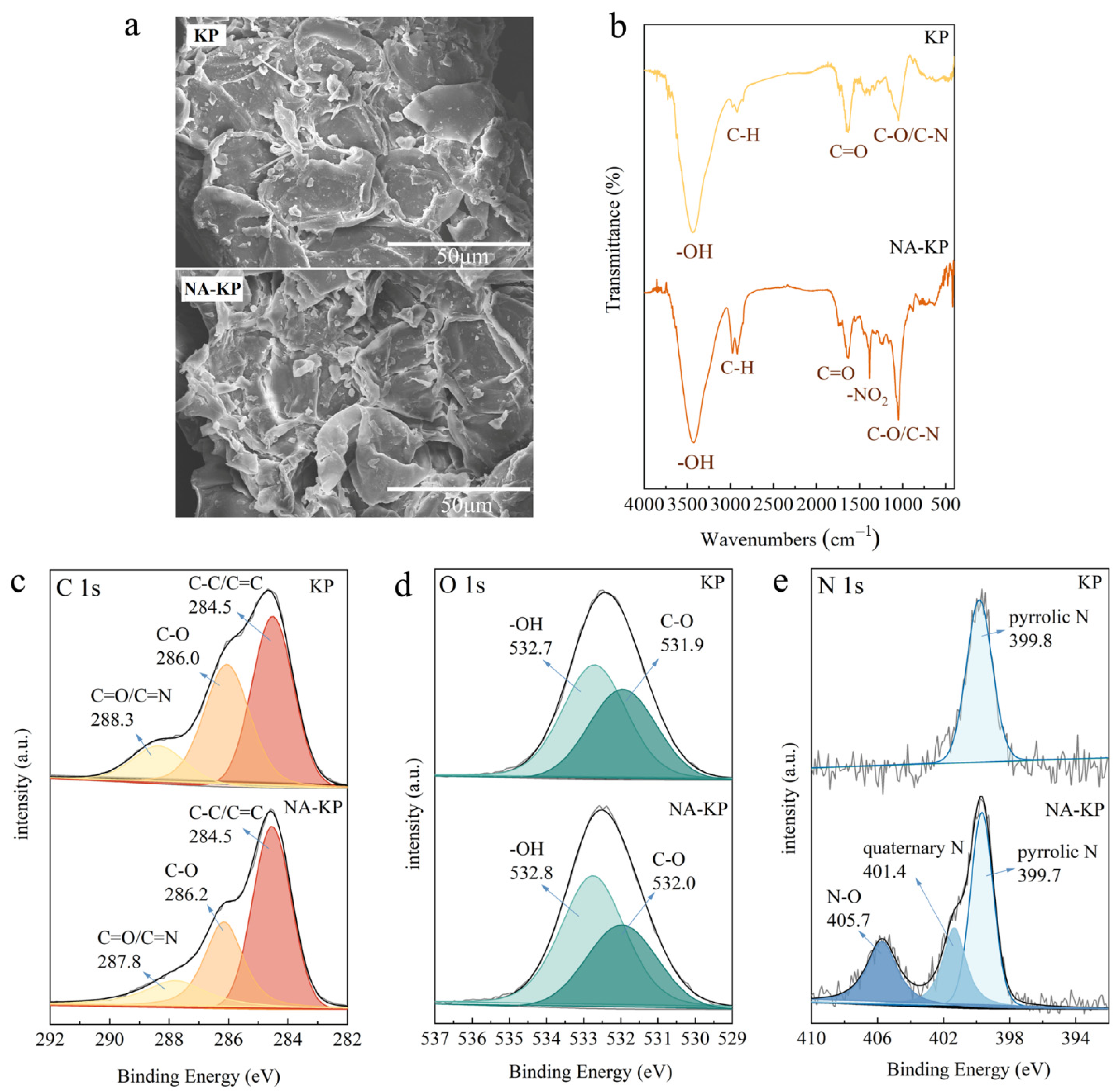
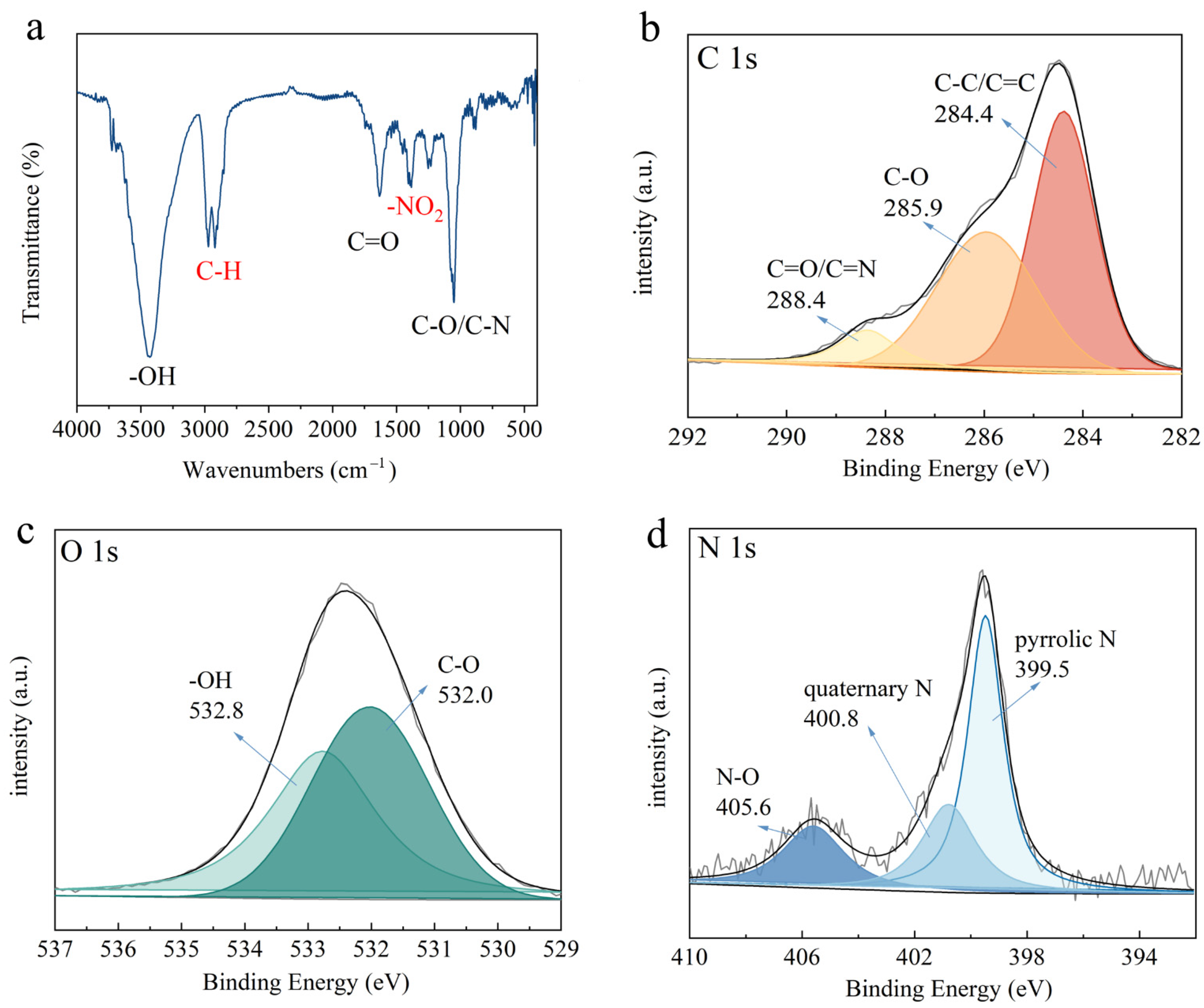
2.1. Characterization of Kiwi-Peel-Based Adsorbents
2.2. Adsorption Kinetics
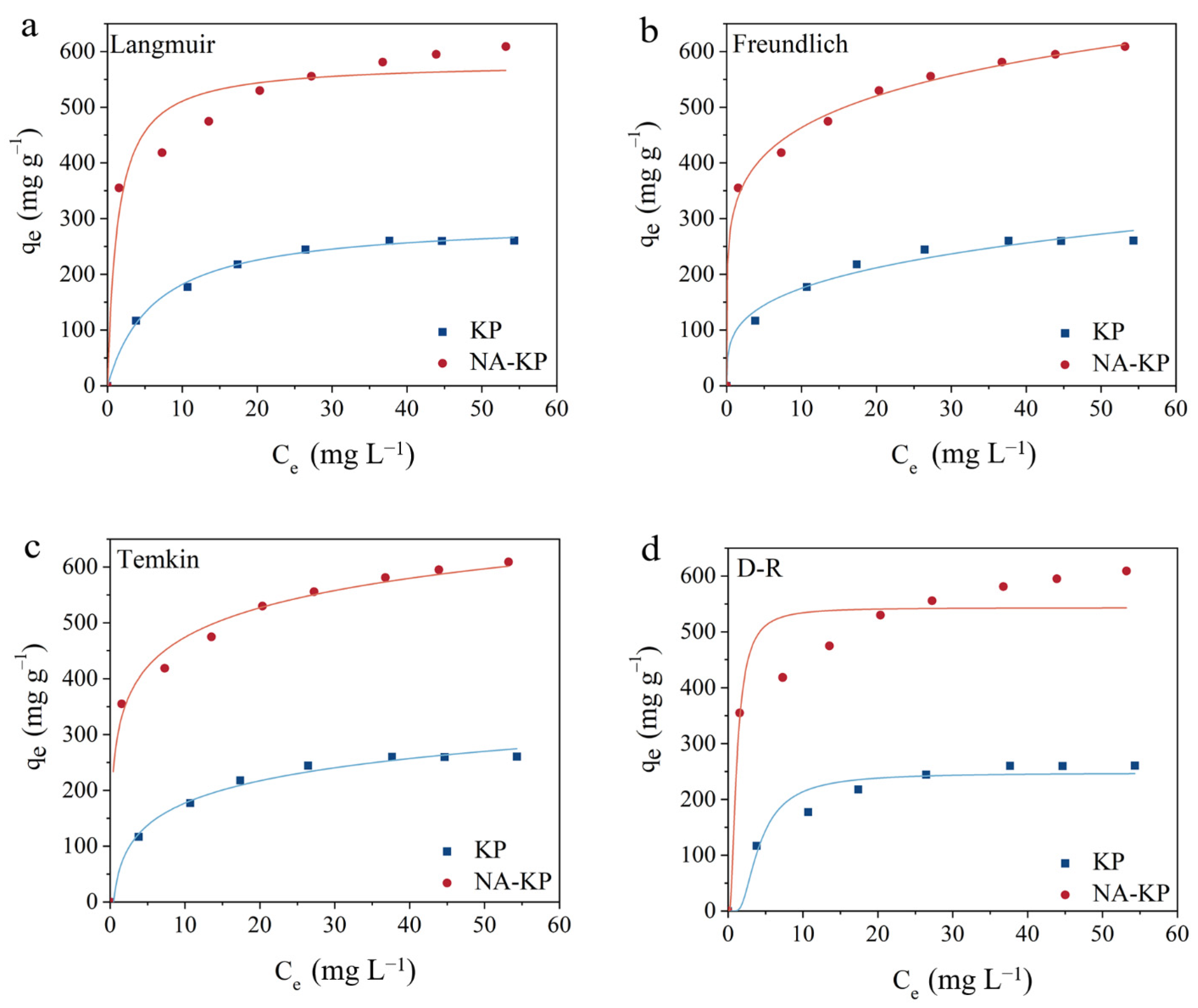
2.3. Adsorption Isotherms
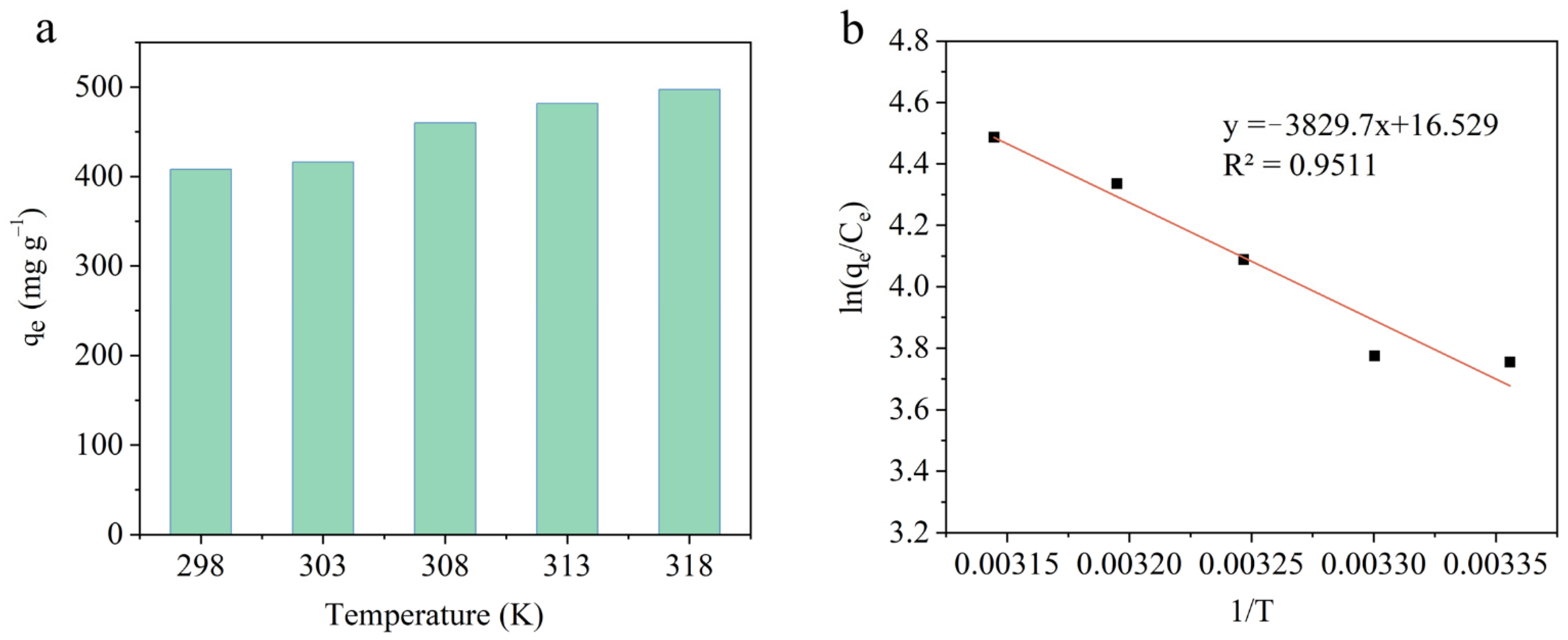
2.4. Adsorption Thermodynamics
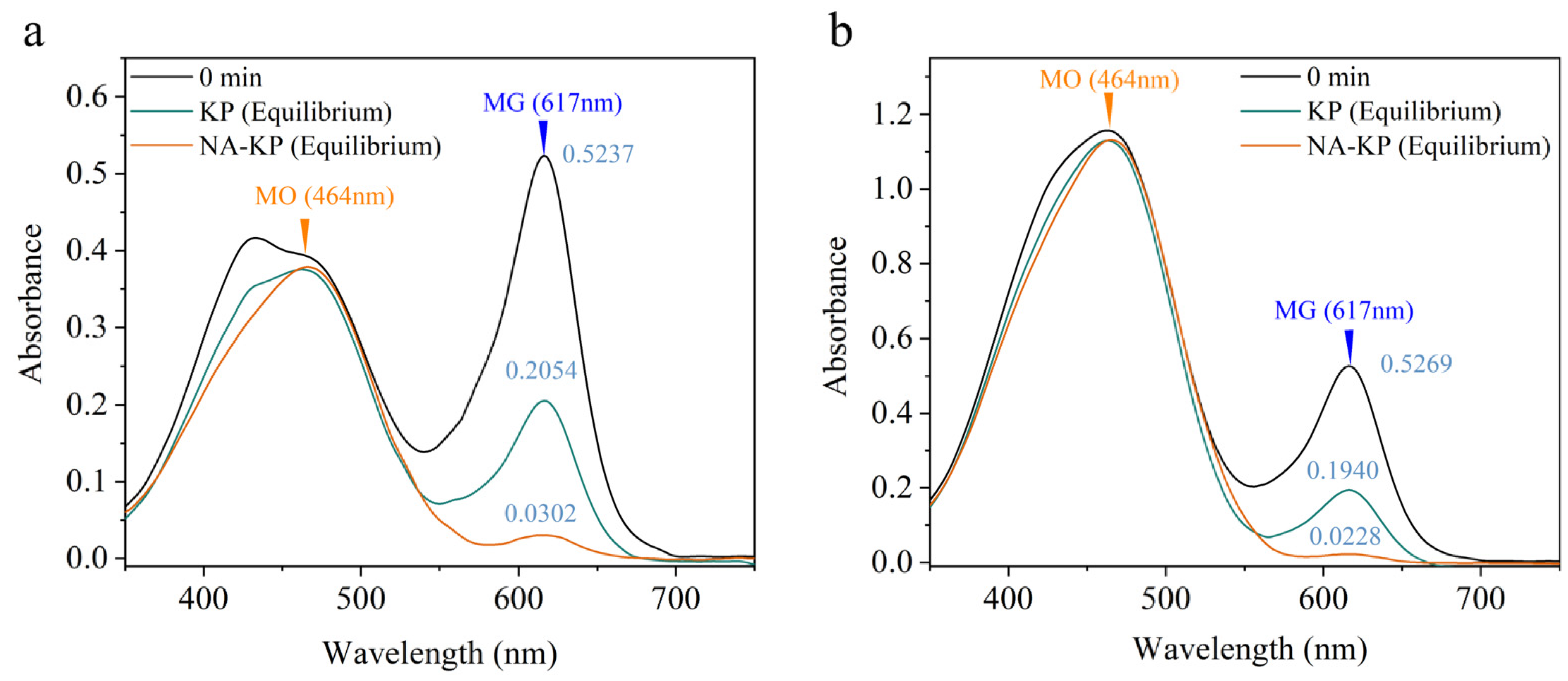
2.5. Selective Adsorption of Cationic Dye by NA-KP
2.6. Adsorption Mechanism of MG by NA-KP
3. Experimental
3.1. Reagents and Materials
3.2. Preparation of Kiwi-Peel-Based Adsorbents
3.3. Characterization
3.4. Batch Adsorption Studies
3.5. Selectivity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, C.; Lai, Z.; Chen, S.; He, D.; Mu, J. Honeycomb-like cork activated carbon with ultra-high adsorption capacity for anionic, cationic and mixed dye: Preparation, performance and mechanism. Bioresour. Technol. 2022, 357, 127363. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.-T.; Jiang, X.-Y.; Yu, J.-G. Efficient and Selective Removal of Organic Cationic Dyes by Peel of Brassica juncea Coss. var. gemmifera Lee et Lin-Based Biochar. Molecules 2023, 28, 3353. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Heo, J.W.; An, L.; Chen, J.; Bae, J.H.; Kim, Y.S. Preparation of amine-functionalized lignins for the selective adsorption of Methylene blue and Congo red. Chemosphere 2022, 295, 133815. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, H.; Han, X.; Han, L.; Xu, Z.; Wang, P. Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules. Molecules 2023, 28, 3010. [Google Scholar] [CrossRef]
- Chen, L.; Mi, B.; He, J.; Li, Y.; Zhou, Z.; Wu, F. Functionalized biochars with highly-efficient malachite green adsorption property produced from banana peels via microwave-assisted pyrolysis. Bioresour. Technol. 2023, 376, 128840. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2021, 281, 119950. [Google Scholar] [CrossRef]
- Nayagam, J.O.P.; Prasanna, K. Utilization of shell-based agricultural waste adsorbents for removing dyes: A review. Chemosphere 2021, 291, 132737. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, W.; Huang, K.; Shao, H.; Qu, C.; Liu, J. One-step synthesis of garlic peel derived biochar by concentrated sulfuric acid: Enhanced adsorption capacities for Enrofloxacin and interfacial interaction mechanisms. Chemosphere 2022, 290, 133263. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2020, 9, 104901. [Google Scholar] [CrossRef]
- Liu, Z.; Khan, T.A.; Islam, M.A.; Tabrez, U. A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon. Bioresour. Technol. 2022, 354, 127168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, L.; Li, W.; Liu, J.; Liu, X.; Huang, K. Insights into enhanced adsorptive removal of Rhodamine B by different chemically modified garlic peels: Comparison, kinetics, isotherms, thermodynamics and mechanism. J. Mol. Liq. 2019, 293, 111516. [Google Scholar] [CrossRef]
- Yaqub, A.; Ajab, H.; Almas, A.; Syed, S.M.; Azam, A.; Khan, M.I.; Awais, M.; Muhammad, I.; Galal, A.M.; Alshahrani, M.Y. Utilization of nano-biosorbents based on pine needles and banana peel for methylene blue removal: Equilibrium, kinetics, thermodynamic study, and application. Biomass Convers. Biorefinery 2022, 12, 1787–1802. [Google Scholar] [CrossRef]
- Stavrinou, A.; Aggelopoulos, C.; Tsakiroglou, C. Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: Adsorption kinetics and equilibrium isotherms as a tool. J. Environ. Chem. Eng. 2018, 6, 6958–6970. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Kim, D.-S. Adsorption of anionic azo dye Congo Red from aqueous solution by Cationic Modified Orange Peel Powder. J. Mol. Liq. 2016, 220, 540–548. [Google Scholar] [CrossRef]
- Akkari, I.; Graba, Z.; Bezzi, N.; Merzeg, F.A.; Bait, N.; Ferhati, A.; Kaci, M.M. Biosorption of Basic Red 46 using raw cactus fruit peels: Equilibrium, kinetic and thermodynamic studies. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.-J. Chemically modified sugarcane bagasse-based biocomposites for efficient removal of acid red 1 dye: Kinetics, isotherms, thermodynamics, and desorption studies. Chemosphere 2021, 291, 132796. [Google Scholar] [CrossRef]
- Mohamad Zaidi, N.A.H.; Sallehuddin, F.N.; Lim, L.B.L.; Kooh, M.R.R. Surface modification of Artocarpus odoratissimus leaves using NaOH, SDS and EDTA to enhance adsorption of toxic crystal violet dye. Int. J. Environ. Anal. Chem. 2021, 103, 1836–1854. [Google Scholar] [CrossRef]
- Suhaimi, N.; Kooh, M.R.R.; Lim, C.M.; Chao, C.-T.C.; Chau, Y.-F.C.; Mahadi, A.H.; Chiang, H.-P.; Hassan, N.H.H.; Thotagamuge, R. The Use of Gigantochloa Bamboo-Derived Biochar for the Removal of Methylene Blue from Aqueous Solution. Adsorpt. Sci. Technol. 2022, 2022, 8245797. [Google Scholar] [CrossRef]
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Carbonaceous Adsorbents Derived from Agricultural Sources for the Removal of Pramipexole Pharmaceutical Model Compound from Synthetic Aqueous Solutions. Processes 2021, 9, 253. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, X.; Wang, C.; Sun, H.; Ma, A.; Zhang, G.; Wang, N. Sorption of cadmium onto Mg-Fe Layered Double Hydroxide (LDH)-Kiwi branch biochar. Environ. Pollut. Bioavailab. 2019, 31, 189–197. [Google Scholar] [CrossRef]
- Jiang, L.; Yi, X.; Yang, K.; Li, M.; Rao, H.; Wu, X.; Li, Y.; Gao, C. Comparison of adsorption behavior of Pb(II) by acid–alkali and chitosan modified biochar derived from kiwifruit branch. Hum. Ecol. Risk Assess. Int. J. 2022, 29, 410–426. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K. Polyacrylamide-Grafted Actinidia deliciosa peels powder (PGADP) for the sequestration of crystal violet dye: Isotherms, kinetics and thermodynamic studies. Appl. Water Sci. 2020, 10, 195. [Google Scholar] [CrossRef]
- Arici, T.A. CTAB/H2O2 modified biosorbent for anionic dye from aqueous solutions: Biosorption parameters and mechanism. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.-Y.; Tam, K.C. Selective adsorption and separation of organic dyes using functionalized cellulose nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar] [CrossRef]
- Schio, R.d.R.; Martinello, K.D.B.; Netto, M.S.; Silva, L.F.; Mallmann, E.S.; Dotto, G.L. Adsorption performance of Food Red 17 dye using an eco-friendly material based on Luffa cylindrica and chitosan. J. Mol. Liq. 2021, 349, 118144. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Wang, Y.; Su, W.; Wei, Y.; Wu, W. Adsorption Studies on the Removal of Anionic and Cationic Dyes from Aqueous Solutions Using Discarded Masks and Lignin. Molecules 2023, 28, 3349. [Google Scholar] [CrossRef]
- Min, L.; Zhang, P.; Fan, M.; Xu, X.; Wang, C.; Tang, J.; Sun, H. Efficient degradation of p-nitrophenol by Fe@pomelo peel-derived biochar composites and its mechanism of simultaneous reduction and oxidation process. Chemosphere 2020, 267, 129213. [Google Scholar] [CrossRef]
- Meddour, A.; Azizi, A. Synthesis and spectral investigations of pyridinium picrate. J. Mol. Struct. 2017, 1128, 499–506. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced phosphate removal by zeolite loaded with Mg–Al–La ternary (hydr)oxides from aqueous solutions: Performance and mechanism. Chem. Eng. J. 2018, 357, 33–44. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Qian, T.; Liu, S.; Li, Y.; Hou, Z.; Goodenough, J.B.; Ajayan, P.M.; Yan, C. Oxidizing Vacancies in Nitrogen-Doped Carbon Enhance Air-Cathode Activity. Adv. Mater. 2018, 31, e1803339. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2021, 429, 132166. [Google Scholar] [CrossRef]
- Li, Y.; An, Y.; Zhao, R.; Zhong, Y.; Long, S.; Yang, J.; Li, J.; Zheng, H. Synergetic removal of oppositely charged dyes by co-precipitation and amphoteric self-floating capturer: Mechanism investigation by molecular simulation. Chemosphere 2022, 296, 134033. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Kwak, J.-H.; Nzediegwu, C.; Wang, S.; Palansuriya, K.; Kwon, E.E.; Naeth, M.A.; El-Din, M.G.; Ok, Y.S.; Chang, S.X. Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ. Pollut. 2021, 281, 117094. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, M.; Cao, M.; Liu, X.; Yang, H.; Li, Y. A series of novel carbohydrate-based carbon adsorbents were synthesized by self-propagating combustion for tetracycline removal. Bioresour. Technol. 2021, 332, 125059. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Yan, B.; Niu, C. Adsorption of ciprofloxacin from water by pretreated oat hulls: Equilibrium, kinetic, and thermodynamic studies. Ind. Crop. Prod. 2018, 127, 237–250. [Google Scholar] [CrossRef]
- Naboulsi, A.; Kassimi, A.; Yazid, H.; Essebbar, F.-E.; El Himri, M.; El Haddad, M. Adsorption of single and mixed colors by kaolinite clay: Experimental research combined with a theoretical examination using DFT. J. Mol. Struct. 2023, 1276, 134678. [Google Scholar] [CrossRef]
- Isik, B.; Ugraskan, V.; Cakar, F.; Yazici, O. A comparative study on the adsorption of toxic cationic dyes by Judas tree (Cercis siliquastrum) seeds. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Jeong, C. Magnetized chitosan nanocomposite as an effective adsorbent for the removal of methylene blue and malachite green dyes. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Kumari, M.; Chaudhary, G.R.; Chaudhary, S.; Umar, A. Transformation of solid plastic waste to activated carbon fibres for wastewater treatment. Chemosphere 2022, 294, 133692. [Google Scholar] [CrossRef] [PubMed]
- Salih, R.; Veličković, Z.; Milošević, M.; Pavlović, V.P.; Cvijetić, I.; Sofrenić, I.V.; Gržetić, J.D.; Marinković, A. Lignin based microspheres for effective dyes removal: Design, synthesis and adsorption mechanism supported with theoretical study. J. Environ. Manag. 2023, 326, 116838. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, E.; Facchi, D.P.; Souza, P.R.; Monteiro, J.P.; Popat, K.C.; Kipper, M.J.; Martins, A.F. A tannin-polymer adsorbent created from the freezing-thawing method for removal of metal-complex acid black 172 and methylene blue from aqueous solutions. J. Mol. Liq. 2022, 351, 118682. [Google Scholar] [CrossRef]
- Usman, M.A.; Khan, A.Y. Selective adsorption of anionic dye from wastewater using polyethyleneimine based macroporous sponge: Batch and continuous studies. J. Hazard. Mater. 2022, 428, 128238. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; Subhan, S.; Muhammad, Y.; Hu, Y.; Huang, M.; Peng, Y.; Zhao, Z.; Zhao, Z. Engineering pH-switchable UiO-66 via in-situ amino acid doping for highly selective adsorption of anionic dyes. Chem. Eng. J. 2020, 395, 124958. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Wu, S.; Jia, G.; Cui, X.; Zhao, P.; Li, Y. Enhanced adsorption of malachite green on hydroxyl functionalized coal: Behaviors and mechanisms. Process. Saf. Environ. Prot. 2022, 163, 48–57. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Wang, Z.; Liu, X. Coadsorption of Cu(II) and tylosin/sulfamethoxazole on biochar stabilized by nano-hydroxyapatite in aqueous environment. Chem. Eng. J. 2020, 381, 122785. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, P.-J. Sono-assisted rapid dye removal by chromium-based metal organic frameworks derived from waste PET bottles: Characterization, kinetics and adsorption isotherms. J. Environ. Chem. Eng. 2021, 9, 106766. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.-H.; Liu, Z.; Liu, J.; Zhu, L.; Liu, X.; Huang, K. Renewable Tb/Eu-Loaded Garlic Peels for Enhanced Adsorption of Enrofloxacin: Kinetics, Isotherms, Thermodynamics, and Mechanism. ACS Sustain. Chem. Eng. 2018, 6, 15264–15272. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Preparation, characterization and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J. Mol. Liq. 2019, 274, 315–327. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Arghavan, S.M.A.; Allahyari, E.; Arghavan, F.S.; Othmani, A.; Nasseh, N. Adsorption of malachite green dye onto almond peel waste: A study focusing on application of the ANN approach for optimization of the effect of environmental parameters. Biomass Convers. Biorefinery. 2022. [Google Scholar] [CrossRef]
- Pathania, D.; Bhat, V.S.; Shivanna, J.M.; Sriram, G.; Kurkuri, M.; Hegde, G. Garlic peel based mesoporous carbon nanospheres for an effective removal of malachite green dye from aqueous solutions: Detailed isotherms and kinetics. Spectrochim. Acta, Part A. 2022, 276, 121197. [Google Scholar] [CrossRef] [PubMed]
- Almufarij, R.S.; Abdulkhair, B.Y.; Salih, M.; Aldosari, H.; Aldayel, N.W. Optimization, Nature, and Mechanism Investigations for the Adsorption of Ciprofloxacin and Malachite Green onto Carbon Nanoparticles Derived from Low-Cost Precursor via a Green Route. Molecules 2022, 27, 4577. [Google Scholar] [CrossRef]
- Hui, T.S.; Zaini, M.A.A. Textile sludge-sawdust chemically produced activated carbon: Equilibrium and dynamics studies of malachite green adsorption. Biomass Convers. Biorefinery 2020, 12, 2847–2859. [Google Scholar] [CrossRef]

| Kinetic Models | Equation | Parameters | The Value of the Parameters | |
|---|---|---|---|---|
| KP | NA-KP | |||
| Pseudo-First-Order | qe,cal (mg g−1) | 218.17 | 332.86 | |
| k1 (min−1) | 0.012 | 0.010 | ||
| R2 | 0.9932 | 0.9943 | ||
| Pseudo-Second-Order | qe,cal (mg g−1) | 254.81 | 401.35 | |
| k2 (g mg−1 min−1) | 5.86 × 10−5 | 2.78 × 10−5 | ||
| h (mg g−1 min−1) | 3.80 | 4.48 | ||
| t1/2 (min) | 66.97 | 89.63 | ||
| R2 | 0.9958 | 0.9927 | ||
| Elovich | α (mg g−1 min−1) | 9.30 | 9.52 | |
| β (mg g−1) | 0.020 | 0.011 | ||
| R2 | 0.9791 | 0.9822 | ||
| Isotherm Models | Equation | Parameters | The Value of the Parameters | |
|---|---|---|---|---|
| KP | NA-KP | |||
| Langmuir | kL (L mg−1) | 0.16 | 0.73 | |
| qm (mg g−1) | 297.15 | 580.61 | ||
| RL | 0.08–0.39 | 0.02–0.07 | ||
| R2 | 0.9962 | 0.9473 | ||
| Freundlich | kF | 92.84 | 315.62 | |
| 1/n | 0.28 | 0.17 | ||
| R2 | 0.9773 | 0.9960 | ||
| Temkin | kT (L mg−1) | 2.18 | 50.08 | |
| B | 57.56 | 76.25 | ||
| R2 | 0.9904 | 0.9911 | ||
| D–R | qm (mg g−1) | 247.55 | 543.07 | |
| E (kJ mol−1) | 0.44 | 1.29 | ||
| R2 | 0.9464 | 0.9032 | ||
| T (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) |
|---|---|---|---|
| 298 | −9.30 | 31.84 | 0.14 |
| 308 | −9.51 | ||
| 318 | −10.47 | ||
| 328 | −11.28 | ||
| 338 | −11.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, X.; Li, W.; Pei, S.; Ren, Y.; Li, X.; Qu, C.; Wu, C.; Liu, J. Efficient and Selective Adsorption of Cationic Dye Malachite Green by Kiwi-Peel-Based Biosorbents. Molecules 2023, 28, 5310. https://doi.org/10.3390/molecules28145310
Zhao Y, Liu X, Li W, Pei S, Ren Y, Li X, Qu C, Wu C, Liu J. Efficient and Selective Adsorption of Cationic Dye Malachite Green by Kiwi-Peel-Based Biosorbents. Molecules. 2023; 28(14):5310. https://doi.org/10.3390/molecules28145310
Chicago/Turabian StyleZhao, Yanjun, Xintong Liu, Wenhui Li, Suyun Pei, Yifan Ren, Xinyang Li, Chen Qu, Chuandong Wu, and Jiemin Liu. 2023. "Efficient and Selective Adsorption of Cationic Dye Malachite Green by Kiwi-Peel-Based Biosorbents" Molecules 28, no. 14: 5310. https://doi.org/10.3390/molecules28145310
APA StyleZhao, Y., Liu, X., Li, W., Pei, S., Ren, Y., Li, X., Qu, C., Wu, C., & Liu, J. (2023). Efficient and Selective Adsorption of Cationic Dye Malachite Green by Kiwi-Peel-Based Biosorbents. Molecules, 28(14), 5310. https://doi.org/10.3390/molecules28145310








