A Facile Preparation of Sandwich-Structured Pd/Polypyrrole-Graphene/Pd Catalysts for Formic Acid Electro-Oxidation
Abstract
:1. Introduction
2. Results and Discussion
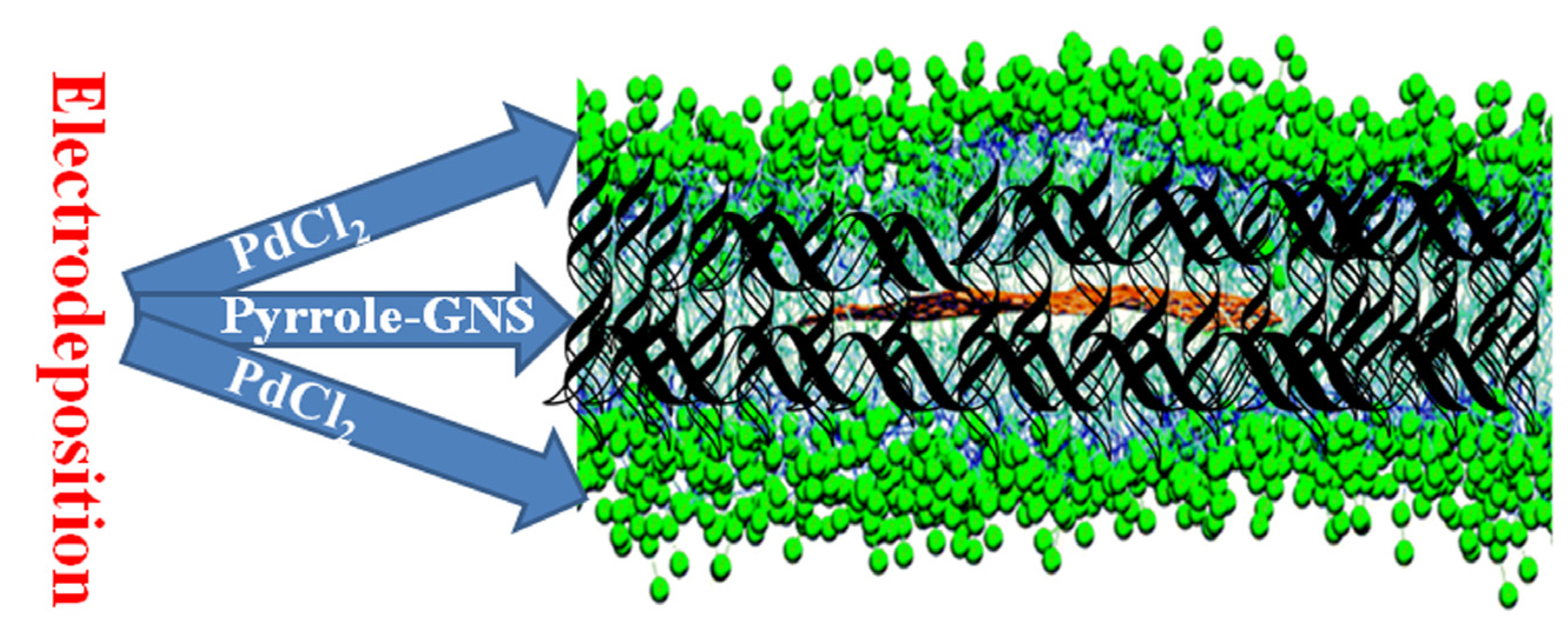
2.1. Morphology and Structure Characterizations
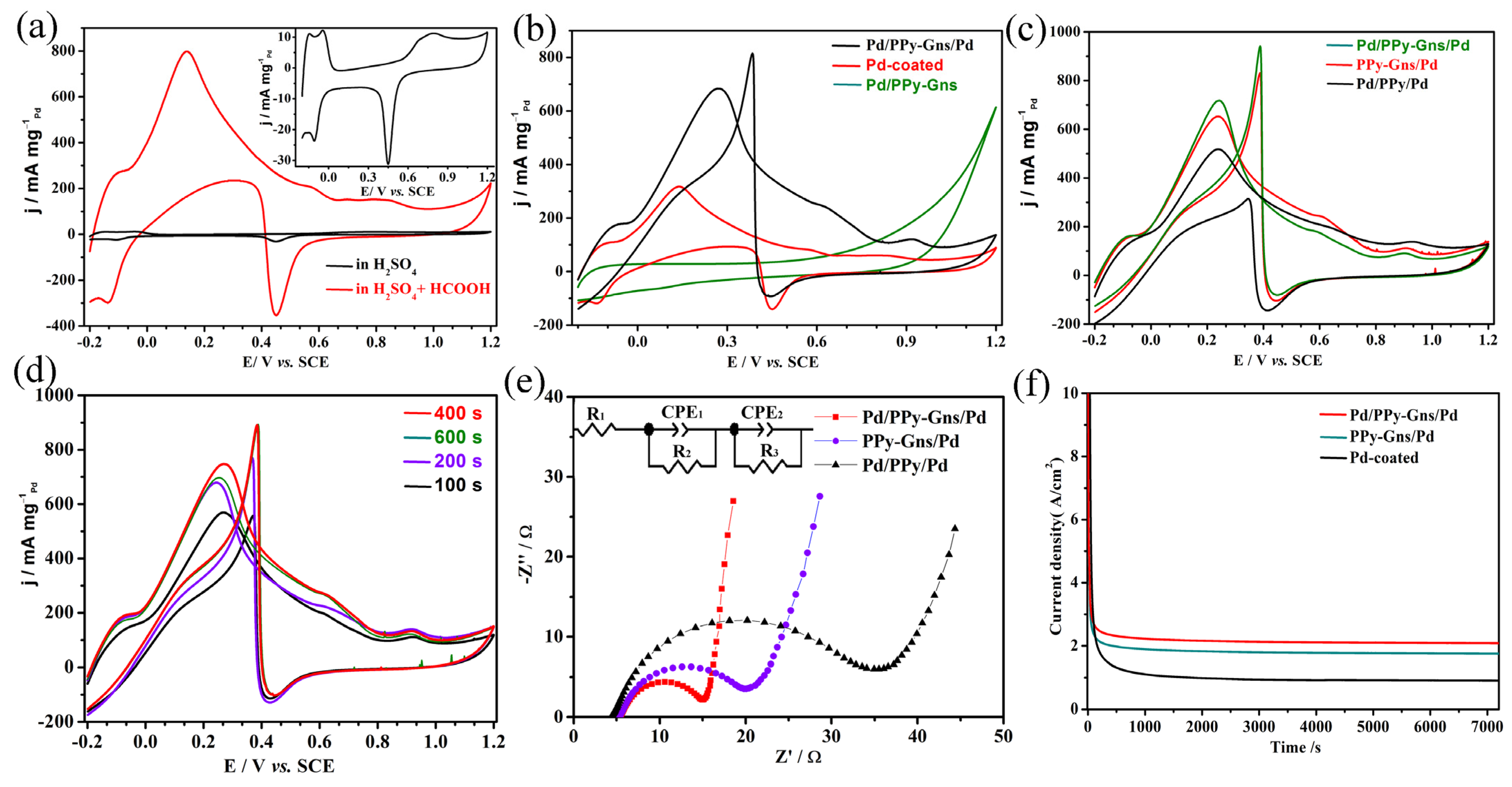
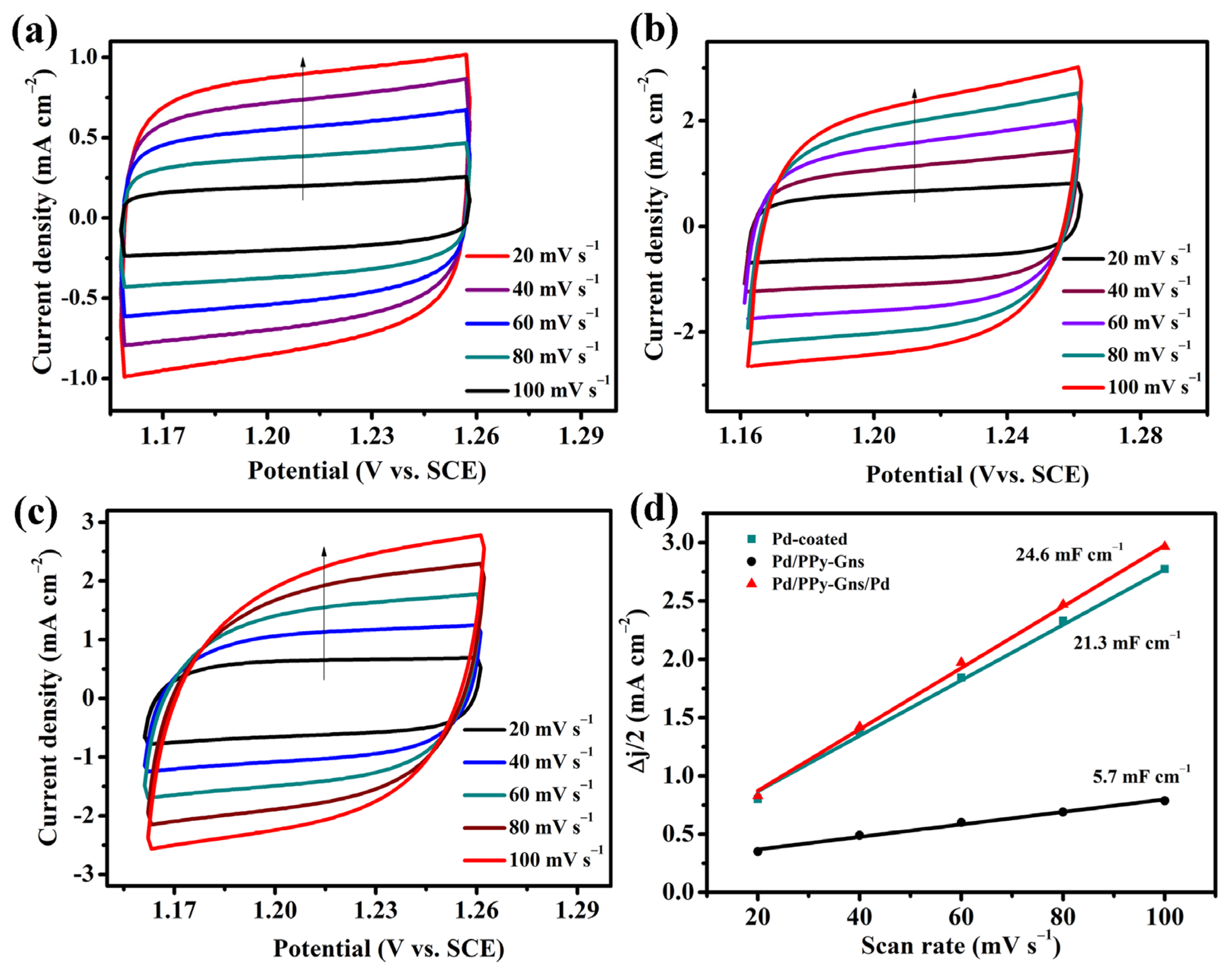
2.2. Electrocatalysis of Pd-Modified Electrode toward Formic Acid Oxidation
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Graphene Oxide and Graphene
3.3. Preparation of Pyrrole-Graphene Mixtures
3.4. Preparation of Modified Electrodes
3.5. Chemoelectrochemical Performance Test of Formic Acid Oxygen
3.6. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Na, Y.; Khadke, P.; Glüsen, A.; Kimiaie, N.; Müller, M.; Krewer, U. A robust methanol concentration sensing technique in direct methanol fuel cells and stacks using cell dynamics. Int. J. Hydrogen Energy 2022, 47, 6237–6246. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Zhu, X.; Zhang, T.; Ye, D.D.; Chen, R.; Liao, Q. Novel superaerophobic anode with fern-shaped Pd nanoarray for high-performance direct formic acid fuel cell. Adv. Funct. Mater. 2022, 32, 2201872. [Google Scholar] [CrossRef]
- Liu, H.; Jia, R.; Qin, C.; Yang, Q.; Tang, Z.; Li, M.; Ma, Z. Anti-CO Poisoning FePtRh nanoflowers with Rh-Rich core and Fe-Rich shell boost methanol oxidation electrocatalysis. Adv. Funct. Mater. 2022, 33, 2210626. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, J.; Xie, H.; Zhang, S.; Zhang, J.; Sun, S.; Luo, P.; Lin, M.; Wang, S.; Pan, Z.; et al. Hydrogen intercalation-induced crystallization of ternary PdNiP alloy nanoparticles for direct formic acid fuel cells. Adv. Energy Mater. 2023, 13, 2203893. [Google Scholar] [CrossRef]
- Yang, S.; Yang, J.; Chung, Y.; Kwon, Y. PdBi bimetallic catalysts including polyvinylpyrrolidone surfactant inducing excellent formic acid oxidation reaction and direct formic acid fuel cell performance. Int. J. Hydrogen Energy 2017, 42, 17211–17220. [Google Scholar] [CrossRef]
- Tohidian, M.; Ghaffarian, S.R. Surface modified multi-walled carbon nanotubes and Nafion nanocomposite membranes for use in fuel cell applications. Polym. Adv. Technol. 2018, 29, 1219–1226. [Google Scholar] [CrossRef]
- Yan, W.; Xiang, Y.; Zhang, J.; Lu, S.; Jiang, S.P. Substantially enhanced power output and durability of direct formic acid fuel cells at elevated temperatures. Adv. Sust. Syst. 2020, 4, 2000065. [Google Scholar] [CrossRef]
- Jałowiecka, M.; Bojarska, Z.; Małolepszy, A.; Makowski, Ł. Mass transport enhancement in direct formic acid fuel cell with a novel channel design. Chem. Eng. J. 2023, 451, 138474. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Yao, M.-S.; Wang, F.; Li, Y.; Collins, S.M.; Chiu, Y.-L.; Du, S. Porous electrodes from self-assembled 3D jointed Pd polyhedra for direct formic acid fuel cells. Chem. Eng. J. 2023, 462, 142244. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Li, H.; Li, Y.; Ye, J.; Cao, Z.; Fang, M.; Kuang, Q.; Zheng, J.; Xie, Z. Stable palladium hydride as a superior anode electrocatalyst for direct formic acid fuel cells. Nano Energy 2018, 44, 127–134. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Dawood, K.M.; El-Deab, M.S.; Al-Andouli, B.E. Efficient direct formic acid fuel cell (DFAFC) anode of nano-sized palladium complex: High durability and activity origin. Appl. Catal. B Environ. 2017, 213, 118–126. [Google Scholar] [CrossRef]
- Singh, H.; Dheeraj, P.B.; Singh, Y.P.; Rathore, G.; Bhardwaj, M. Electrodeposition of porous copper as a substrate for electrocatalytic material. J. Electroanal. Chem. 2017, 785, 1–7. [Google Scholar] [CrossRef]
- Jo, A.; Lee, Y.; Lee, C. Electrodeposition of tantalum on carbon black in non-aqueous solution and its electrocatalytic properties. Anal. Chim. Acta 2016, 933, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yuda, A.; Kumar, A. A review of g-C3N4 based catalysts for direct methanol fuel cells. Anal. Chim. Acta 2022, 47, 3371–3395. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Mikolajczuk-Zychora, A.; Mierzwa, B.; Borodzinski, A.; Stobinski, L. A simple method for enhancing the catalytic activity of Pd deposited on carbon nanotubes used in direct formic acid fuel cells. Appl. Surf. Sci. 2019, 476, 806–814. [Google Scholar] [CrossRef]
- Iftikhar, T.; Asif, M.; Aziz, A.; Ashraf, G.; Jun, S.; Li, G.; Liu, H. Topical advances in nanomaterials based electrochemical sensors for resorcinol detection. Trends Environ. Anal. Chem. 2021, 31, e00138. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and promising applications of graphene and porous graphene materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Iftikhar, T.; Xu, Y.; Aziz, A.; Ashraf, G.; Li, G.; Asif, M.; Xiao, F.; Liu, H. Tuning electrocatalytic aptitude by incorporating α-MnO2 Nanorods in Cu-MOF/rGO/CuO hybrids: Electrochemical sensing of resorcinol for practical applications. ACS Appl. Mater. Interfaces 2021, 13, 31462–31473. [Google Scholar] [CrossRef]
- Bao, Y.; Zha, M.; Sun, P.; Hu, G.; Feng, L. PdNi/N-doped graphene aerogel with over wide potential activity for formic acid electrooxidation. J. Energy Chem. 2021, 59, 748–754. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, J.; Yang, C.; Yang, Y.; Zhang, Y.; Yang, F.; Ma, R.; Yang, L.; He, H.; Huang, H. Pd nanocrystals anchored on 3D hybrid architectures constructed from nitrogen-doped graphene and low-defect carbon nanotube as high-performance multifunctional electrocatalysts for formic acid and methanol oxidation. Mater. Today Energy 2020, 16, 100409. [Google Scholar] [CrossRef]
- Luo, X.; Fu, C.; Guo, Y.; Cai, X.; Luo, X.; Tan, Z.; Xue, R.; Cheng, X.; Shen, S.; Zhang, J. Ultrafine Core@Shell Cu1Au1@Cu1Pd3 nanodots synergized with 3D porous N-doped graphene nanosheets as a high-performance multifunctional electrocatalyst. ACS Nano. 2023, 17, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Z.; Zhong, W.; Yang, W. Hydrothermal direct synthesis of polyaniline, graphene/polyaniline and N-doped graphene/polyaniline hydrogels for high performance flexible supercapacitors. J. Mater. Chem. A 2018, 6, 9245–9256. [Google Scholar] [CrossRef]
- Minisy, I.M.; Gavrilov, N.; Acharya, U.; Moravkova, Z.; Unterweger, C.; Micusik, M.; Filippov, S.K.; Kredatusova, J.; Pasti, I.A.; Breitenbach, S.; et al. Tailoring of carbonized polypyrrole nanotubes core by different polypyrrole shells for oxygen reduction reaction selectivity modification. J. Colloid Interface Sci. 2019, 551, 184–194. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Liu, Y.; Li, T.; Ma, D.; Gao, J.; Yang, J.; Zhou, Y.; Zhang, Y.-F. Microwave assisted polymeric modification of graphite oxide and graphite by poly(allyl diazoacetate-co-acrolein). Mater. Des. 2019, 183, 108116. [Google Scholar] [CrossRef]
- Muzyka, R.; Kwoka, M.; Smędowski, Ł.; Díez, N.; Gryglewicz, G. Oxidation of graphite by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Yang, L.; Lahiri, A.; Krebs, F.; Endres, F. Zinc storage mechanism in polypyrrole electrodeposited froma queous, organic, and ionic liquid electrolytes: An in situ raman spectroelectrochemical study. ACS Appl. Energy Mater. 2022, 5, 3217–3226. [Google Scholar] [CrossRef]
- Thi Hien, H.; Thi Anh Thu, D.; Quang Ngan, P.; Hong Thai, G.; Thanh Trung, D.; Trung, T.; Minh Tan, M.; Truong Giang, H. High NH3 sensing performance of NiO/PPy hybrid nanostructures. Sens. Actuators B Chem. 2021, 340, 129986. [Google Scholar] [CrossRef]
- Bora, C.; Dolui, S.K. Fabrication of polypyrrole/graphene oxide nanocomposites by liquid/liquid interfacial polymerization and evaluation of their optical, electrical and electrochemical properties. Polymer 2012, 53, 923–932. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Y.; Xie, J.; Hu, J.; Wang, K.; Jia, D. Construction of Co2P/CoP@Co@NCNT rich-interface to synergistically promote overall water splitting. Chem. Eng. J. 2022, 430, 132877. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, J.; Hu, J.; Wang, K.; Cao, Y. In Situ Replacement Synthesis of Co@NCNT Encapsulated CoPt3@Co2P heterojunction boosting methanol oxidation and hydrogen evolution. Small 2021, 17, e2104656. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-H.; Zhang, J.; Li, G.; Zhang, J.-M.; Zhang, B.-W.; Jiang, Y.-X.; Sun, S.-G. Tuning atomic Pt site surface on PtAu alloy toward electro-oxidation of formic acid. Mater. Today Energy 2022, 27, 101028. [Google Scholar] [CrossRef]
- Sang, Q.; Yin, S.; Liu, F.; Yin, H.; He, J.; Ding, Y. Highly coordinated Pd overlayers on nanoporous gold for efficient formic acid electro-oxidation. Nano Res. 2021, 14, 3502–3508. [Google Scholar] [CrossRef]
- Jin, L.; Xu, H.; Chen, C.; Shang, H.; Wang, Y.; Wang, C.; Du, Y. Three-dimensional PdCuM (M = Ru, Rh, Ir) trimetallic alloy nanosheets for enhancing methanol oxidation electrocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 42123–42130. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, T.; Aziz, A.; Ashraf, G.; Xu, Y.; Li, G.; Zhang, T.; Asif, M.; Xiao, F.; Liu, H. Engineering MOFs derived metal oxide nanohybrids: Towards electrochemical sensing of catechol in tea samples. Food Chem. 2022, 395, 133642. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Z.; Xie, J.; Hu, J.; Cao, Y.; Hao, A. In situ construction of MnO2-Co3O4 nanosheet heterojunctions on Co@NCNT surfaces for oxygen evolution. Inorg. Chem. 2023, 62, 3532–3540. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Wu, Y.; Zhang, G.; Wu, H.; Lyu, F.; He, J.; Fan, J.; Lu, J.; Li, Y.Y. Identifying Fe as OER active sites and ultralow-cost bifunctional electrocatalysts for overall water splitting. Small 2023, e2301715. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, D.; Feng, X.; Müllen, K. Dispersion of Graphene Sheets in Organic Solvent Supported by Ionic Interactions. Adv. Mater. 2009, 21, 1679–1683. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Qin, W.; Ma, J.; Cao, Y.; Bao, S. A Facile Preparation of Sandwich-Structured Pd/Polypyrrole-Graphene/Pd Catalysts for Formic Acid Electro-Oxidation. Molecules 2023, 28, 5296. https://doi.org/10.3390/molecules28145296
Lu Z, Qin W, Ma J, Cao Y, Bao S. A Facile Preparation of Sandwich-Structured Pd/Polypyrrole-Graphene/Pd Catalysts for Formic Acid Electro-Oxidation. Molecules. 2023; 28(14):5296. https://doi.org/10.3390/molecules28145296
Chicago/Turabian StyleLu, Zhenjiang, Wenjin Qin, Juan Ma, Yali Cao, and Shujuan Bao. 2023. "A Facile Preparation of Sandwich-Structured Pd/Polypyrrole-Graphene/Pd Catalysts for Formic Acid Electro-Oxidation" Molecules 28, no. 14: 5296. https://doi.org/10.3390/molecules28145296
APA StyleLu, Z., Qin, W., Ma, J., Cao, Y., & Bao, S. (2023). A Facile Preparation of Sandwich-Structured Pd/Polypyrrole-Graphene/Pd Catalysts for Formic Acid Electro-Oxidation. Molecules, 28(14), 5296. https://doi.org/10.3390/molecules28145296




