Poly (Aryl Amino Ketone/Sulfones) with Obvious Electrochromic Effect Prepared by One-Step Low-Cost and Facile Synthesis
Abstract
:1. Introduction
2. Results
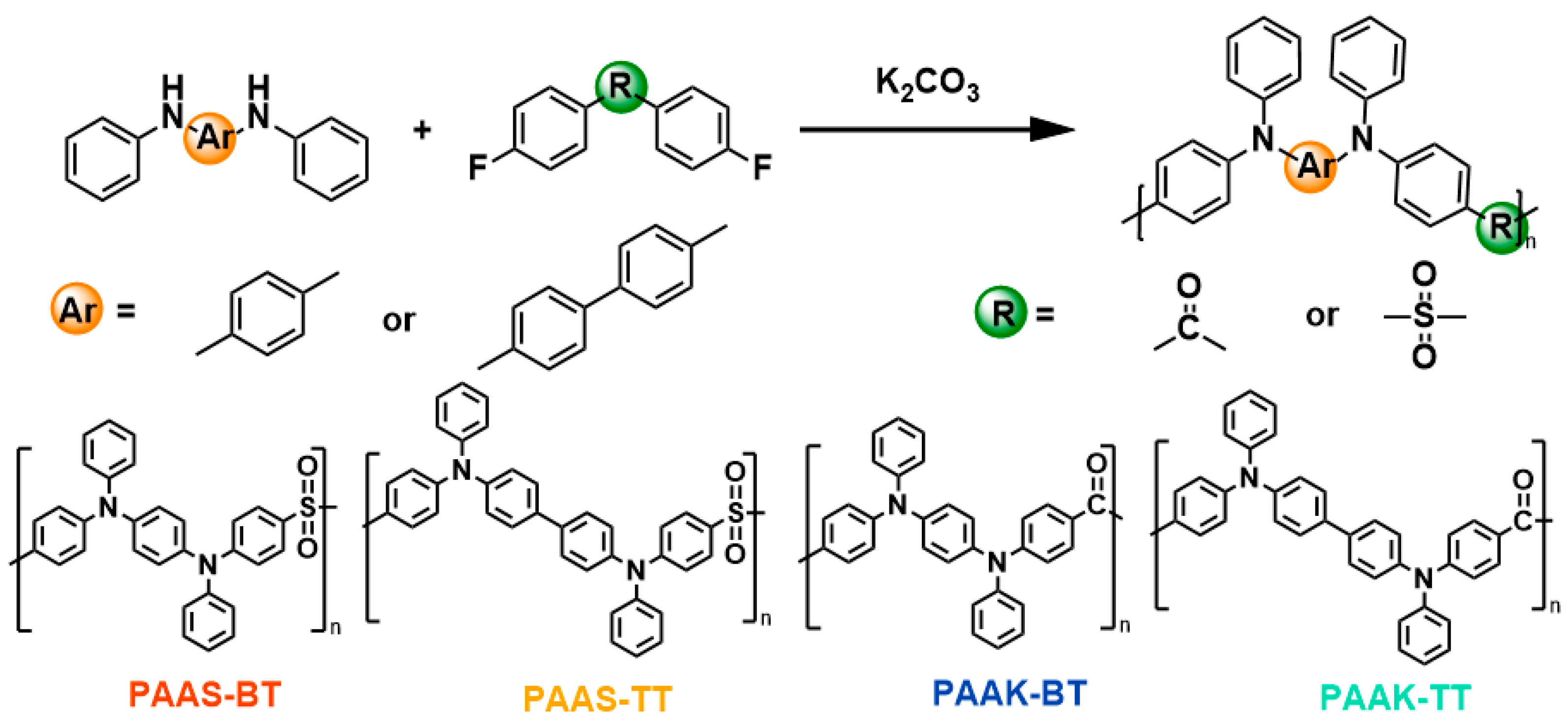
2.1. Design and Synthesis
2.2. Basic Characterization
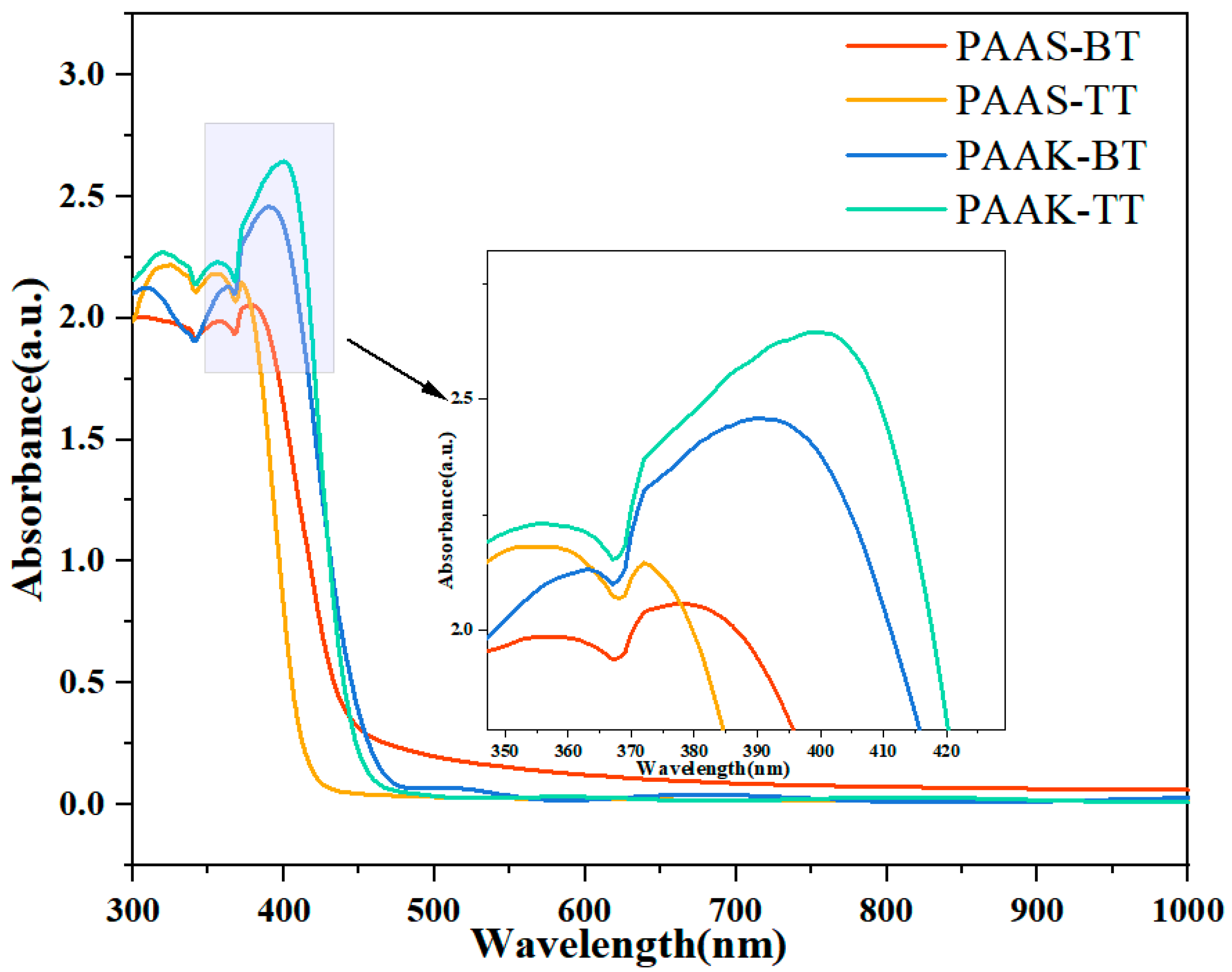
2.3. Optical Properties
2.4. Electrochemical Properties
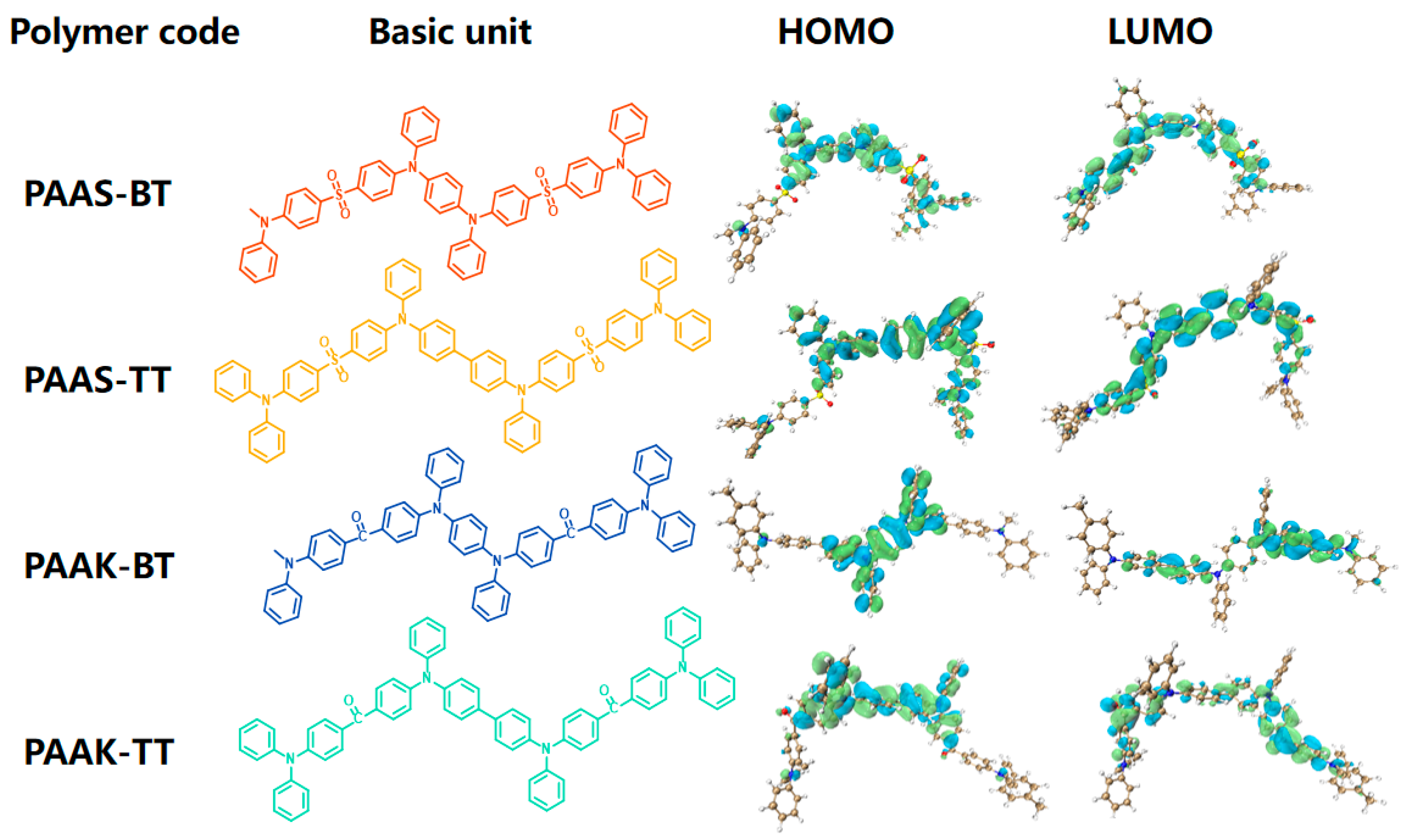
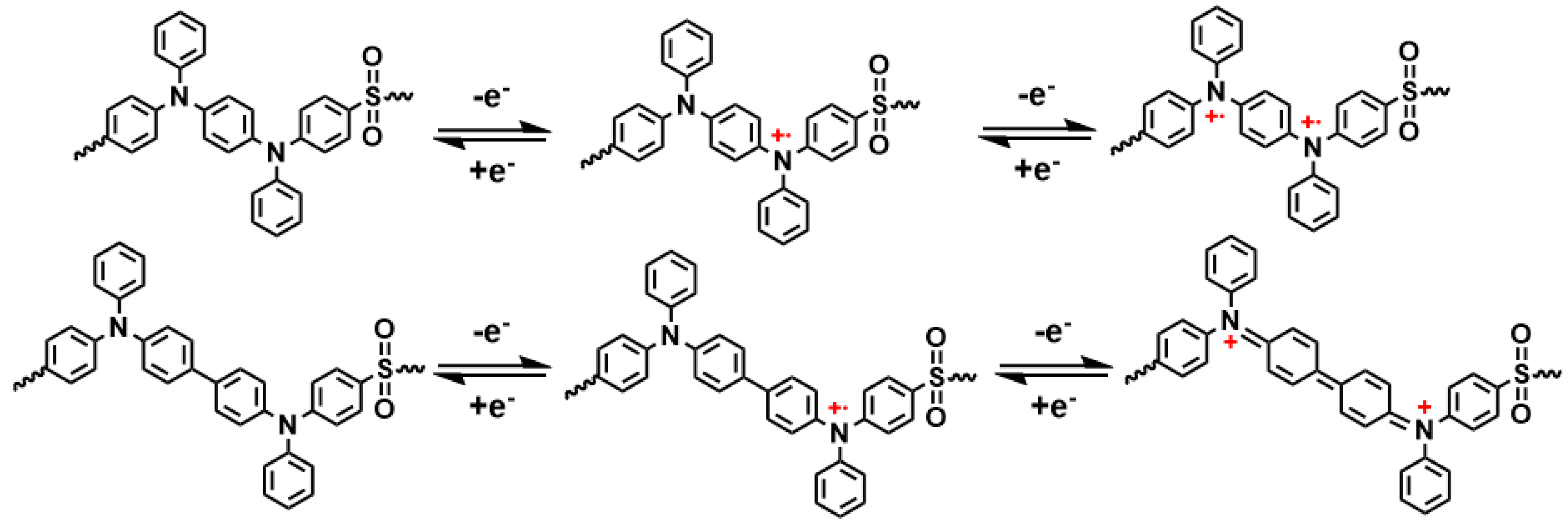
2.5. Theoretical Analysis
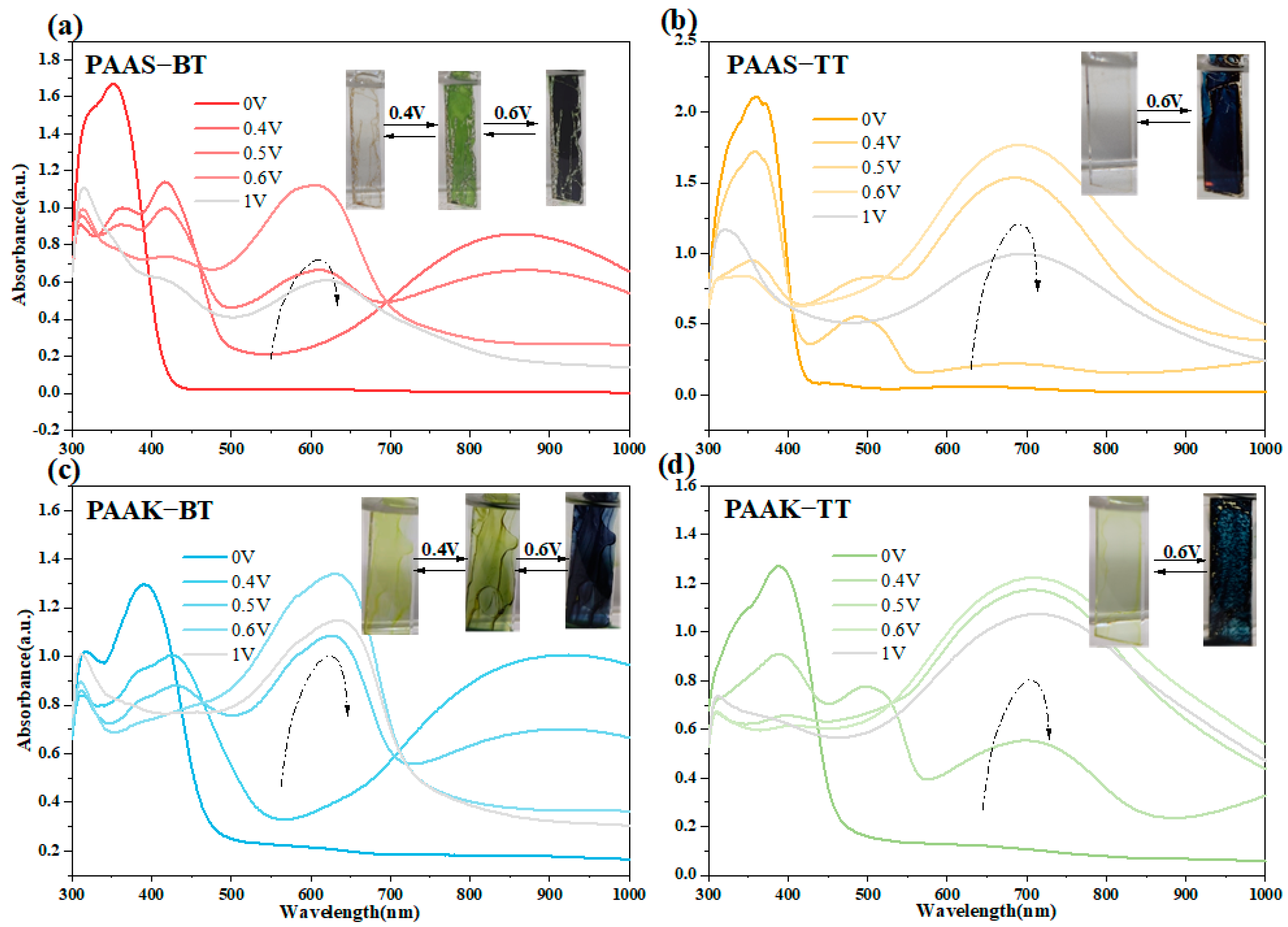
2.6. Spectroelectrochemical Properties
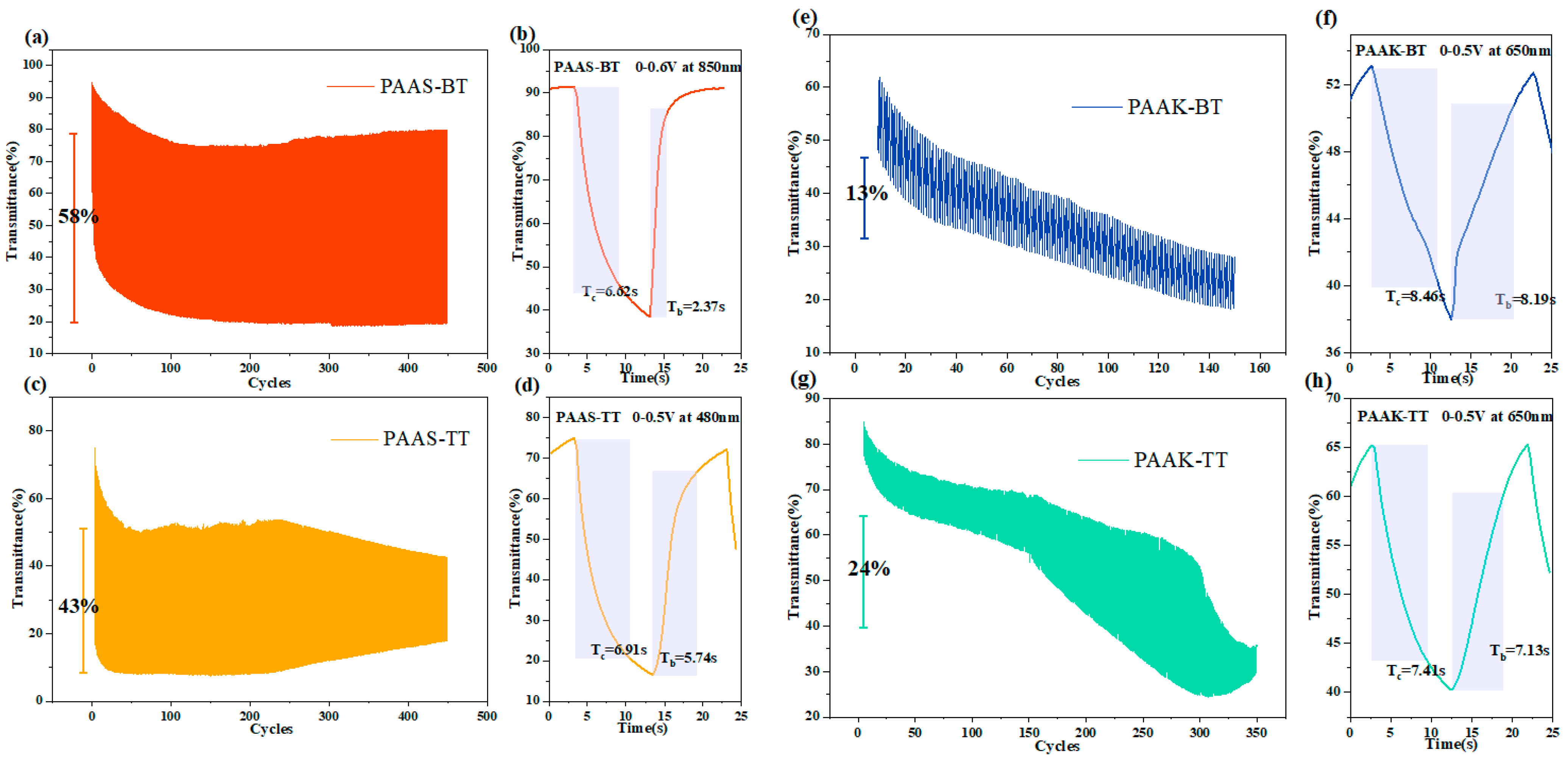
2.7. Electrochromic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Polymer PAAS−BT
3.3. Synthesis of Polymer PAAS−TT
3.4. Synthesis of Polymer PAAK−BT
3.5. Synthesis of Polymer PAAK−TT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lei, P.Y.; Wang, J.H.; Gao, Y.; Hu, C.Y.; Zhang, S.Y.; Tong, X.R.; Wang, Z.P.; Gao, Y.H.; Cai, G.F. An Electrochromic Nickel Phosphate Film for Large-Area Smart Window with Ultra-Large Optical Modulation. Nano-Micro Lett. 2023, 15, 34. [Google Scholar] [CrossRef]
- Howard, E.L.; Osterholm, A.M.; Shen, D.E.; Panchumarti, L.P.; Pinheiro, C.; Reynolds, J.R. Cost-Effective, Flexible, and Colorful Dynamic Displays: Removing Underlying Conducting Layers from Polymer-Based Electrochromic Devices. ACS Appl. Mater. Inter. 2021, 13, 16732–16743. [Google Scholar] [CrossRef]
- Brooke, R.; Freitag, K.; Petsagkourakis, I.; Nilsson, M.; Ersman, P.A. All-Printed Electrochromic Stickers. Macro. Mater. Eng. 2023, 6, 2300044. [Google Scholar] [CrossRef]
- Österholm, A.M.; Shen, D.E.; Gottfried, D.S.; Reynolds, J.R. Full Color Control and High-Resolution Patterning from Inkjet Printable Cyan/Magenta/Yellow Colored-to-Colorless Electrochromic Polymer Inks. Adv. Mater. Technol. 2016, 1, 1600063. [Google Scholar] [CrossRef]
- Lang, A.W.; Osterholm, A.M.; Reynolds, J.R. Paper-Based Electrochromic Devices Enabled by Nanocellulose-Coated Substrates. Adv. Funct. Mater. 2019, 29, 1903487. [Google Scholar] [CrossRef]
- Osterholm, A.M.; Shen, D.E.; Kerszulis, J.A.; Bulloch, R.H.; Kuepfert, M.; Dyer, A.L.; Reynolds, J.R. Four shades of brown: Tuning of electrochromic polymer blends toward high-contrast eyewear. ACS Appl. Mater. Inter. 2015, 7, 1413–1421. [Google Scholar] [CrossRef]
- Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Navigating the Color Palette of Solution-Processable Electrochromic Polymers. Chem. Mater. 2010, 23, 397–415. [Google Scholar] [CrossRef]
- van Mullekom, H.A.M.; Vekemans, J.A.J.M.; Havinga, E.E.; Meijer, E.W. Developments in the chemistry and band gap engineering of donor-acceptor substituted conjugated polymers. Mat. Sci. Eng. 2001, 32, 1–40. [Google Scholar] [CrossRef]
- Shao, Y.J.; Wang, Y.C.; Liou, G.S. An unexpected discovery of a one-pot synthesis for carbazole-based diamine and the electrochromic properties of the derived polymers. Polym. Chem. 2023, 14, 477–485. [Google Scholar] [CrossRef]
- Constantin, C.P.; Damaceanu, M.D. A refreshing perspective on electrochromic materials: Phenoxazine as an opportune moiety towards stable and efficient electrochromic polyimides. Chem. Eng. J. 2023, 465, 142883. [Google Scholar] [CrossRef]
- Jia, X.T.; Chao, D.M.; Berda, E.B.; Wang, S.T.; Yang, R.; Yao, L.; Wang, C. Synthesis and Properties of a Novel Electroactive Poly(aryl ether ketone) Bearing Pendant Aniline Tetramer. Macro. Chem. Phys. 2012, 213, 1475–1481. [Google Scholar] [CrossRef]
- Wang, Q.L.; Li, S.Y.; Zheng, Z.B.; Chen, Z.; Jiang, Z.H. Rapid and effective electrochemical strategy for the synthesis of PAASs/PAAKs electrochromic high-performance polymers. Sol. Energy Mater. Sol. Cells 2023, 254, 112256. [Google Scholar] [CrossRef]
- Xing, Z.; Jia, S.R.; Li, S.Y.; Wang, Q.L.; Zhong, J.D.; Qi, H.Y.; Sun, W.B.; Jiang, Z.H.; Chen, Z. Preparation and characterization of novel high-performance N,N,N′,N′-tetraphenyl-p-phenylenediamine-based poly (ether sulfone)s. Electrochim. Acta 2023, 452, 142316. [Google Scholar] [CrossRef]
- Li, Y.W.; Xue, L.L.; Xia, H.J.; Xu, B.; Wen, S.P.; Tian, W.J. Synthesis and properties of polythiophene derivatives containing triphenylamine moiety and their photovoltaic applications. J Polym. Sci. Pol. Chem. 2008, 46, 3970–3984. [Google Scholar] [CrossRef]
- Han, Y.T.; Xing, Z.; Ma, P.Y.; Li, S.; Wang, C.; Jiang, Z.H.; Chen, Z. Design Rules for Improving the Cycling Stability of High-Performance Donor-Acceptor-Type Electrochromic Polymers. ACS Appl. Mater. Inter. 2020, 12, 7529–7538. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Amb, C.M.; Reynolds, J.R. Spectral Engineering in π-Conjugated Polymers with Intramolecular Donor-Acceptor Interactions. Acc. Chem. Res. 2010, 43, 1396–1407. [Google Scholar] [CrossRef]
- Han, Y.T.; Lin, Y.J.; Sun, D.Y.; Xing, Z.; Jiang, Z.H.; Chen, Z. Poly(aryl amino ketone)-based materials with excellent electrochromic and electrofluorochromic behaviors. Dye. Pigment. 2019, 163, 40–47. [Google Scholar] [CrossRef]
- Quinton, C.; Alain-Rizzo, V.; Dumas-Verdes, C.; Miomandre, F.; Clavier, G.; Audebert, P. Redox- and Protonation-Induced Fluorescence Switch in a New Triphenylamine with Six Stable Active or Non-Active Forms. Chem.-Eur. J. 2015, 21, 2230–2240. [Google Scholar] [CrossRef]
- Wang, H.M.; Hsiao, S.H. Enhancement of Redox Stability and Electrochromic Performance of Aromatic Polyamides by Incorporation of (3,6-Dimethoxycarbazol-9-yl)-triphenylamine Units. J Poly. Sci. Pol. Chem. 2014, 52, 272–286. [Google Scholar] [CrossRef]
- Wu, J.T.; Fan, Y.Z.; Liou, G.S. Synthesis, characterization and electrochromic properties of novel redox triarylamine-based aromatic polyethers with methoxy protecting groups. Poly. Chem. 2019, 10, 345–350. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.J.; Liou, G.S. Solution-Processable Novel Near-Infrared Electrochromic Aromatic Polyamides Based on Electroactive Tetraphenyl-p-Phenylenediamine Moieties. Chem. Mater. 2009, 21, 4062–4070. [Google Scholar] [CrossRef]
- Halder, S.; Pal, S.; Sivasakthi, P.; Samanta, P.K.; Chakraborty, C. Thiazolothiazole-Containing Conjugated Polymer with Electrochromism and Electrofluorochromism-Based Dual Performance for a Flip-Flop Molecular Logic Gate. Macromolecules 2023, 56, 2319–2327. [Google Scholar] [CrossRef]
- Gao, Z.J.; Kong, L.Q.; Ming, S.L.; Du, H.M.; Zhang, Y.; Zhao, J.S. D-A type ambipolar electrochromic copolymers based on dithienopyrrole, 3,4-propylenedioxythiophene and benzotriazole units with dual fading processes. Eur. Poly. J. 2023, 186, 111866. [Google Scholar] [CrossRef]

| GPC Results a | Thermal Stability (°C) | ||||
|---|---|---|---|---|---|
| Polymer | Mn(kDa) | Mw(kDa) | Pd | Tg b | Td5 c |
| PAAS−BT | 9.9 | 1.5 | 1.55 | 242 | 446 |
| PAAS−TT | 9.3 | 1.2 | 1.25 | 252 | 443 |
| PAAK−BT | 9.4 | 1.1 | 1.18 | 219 | 530 |
| PAAK−TT | 5.7 | 6.5 | 1.15 | 195 | 526 |
| Experimental | Calculated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oxidation(V) | Energy Level(eV) | Energy Level(eV) | ||||||||
| Polymer | Eonset | E1/2ox,1 | E1/2ox,2 | λonset(nm) | Eg a | HOMO b | LUMO c | HOMO | LUMO | Eg |
| PAAS−BT | 0.45 | 0.55 | 0.82 | 445 | 2.79 | −5.25 | −2.46 | −5.09 | −1.29 | 3.80 |
| PAAS−TT | 0.55 | 0.67 | 413 | 3.00 | −5.35 | −2.35 | −5.08 | −1.32 | 3.76 | |
| PAAK−BT | 0.32 | 0.42 | 0.69 | 452 | 2.74 | −5.12 | −2.38 | −4.91 | −1.53 | 3.38 |
| PAAK−TT | 0.43 | 0.51 | 445 | 2.79 | −5.23 | −2.44 | −4.93 | −1.50 | 3.43 | |
| Polymer | λmax(nm) | Cycles0.8 a | Tc0.9 | Tb0.9 | ∆OD b | Q(mC/cm2) c | CE (cm2/C) d |
|---|---|---|---|---|---|---|---|
| PAAS−BT | 600 | 450 | 6.62 | 2.37 | 0.38 | 2.37 | 160.34 |
| PAAS−TT | 695 | 350 | 6.91 | 5.74 | 0.65 | 4.07 | 159.71 |
| PAAK−BT | 633 | 150 | 8.46 | 8.19 | 0.14 | 6.04 | 23.18 |
| PAAK−TT | 705 | 310 | 7.41 | 7.13 | 0.21 | 6.41 | 32.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Xing, Z.; Wang, Q.; Wang, S.; Chen, Z. Poly (Aryl Amino Ketone/Sulfones) with Obvious Electrochromic Effect Prepared by One-Step Low-Cost and Facile Synthesis. Molecules 2023, 28, 5297. https://doi.org/10.3390/molecules28145297
Jia S, Xing Z, Wang Q, Wang S, Chen Z. Poly (Aryl Amino Ketone/Sulfones) with Obvious Electrochromic Effect Prepared by One-Step Low-Cost and Facile Synthesis. Molecules. 2023; 28(14):5297. https://doi.org/10.3390/molecules28145297
Chicago/Turabian StyleJia, Songrui, Zhen Xing, Qilin Wang, Shiwei Wang, and Zheng Chen. 2023. "Poly (Aryl Amino Ketone/Sulfones) with Obvious Electrochromic Effect Prepared by One-Step Low-Cost and Facile Synthesis" Molecules 28, no. 14: 5297. https://doi.org/10.3390/molecules28145297
APA StyleJia, S., Xing, Z., Wang, Q., Wang, S., & Chen, Z. (2023). Poly (Aryl Amino Ketone/Sulfones) with Obvious Electrochromic Effect Prepared by One-Step Low-Cost and Facile Synthesis. Molecules, 28(14), 5297. https://doi.org/10.3390/molecules28145297






