Abstract
Four polyoxygenated stigmastanes (1–4) alongside known analogues (7–8) and flavonoids (5–6) were isolated from a dichloromethane/methanol (1:1, v/v) extract of the whole plant of Vernonia kotschyana Sch. Bip. ex Walp. (Asteraceae). Their structures were determined by means of spectroscopic and spectrometric analysis. The relative stereochemistry of the new compounds was established and confirmed via biosynthesis evidence and cyclization of 1 under acidic conditions. A plausible biosynthetic pathway to the new compounds and the chemophenetic significance of the isolated constituents were also discussed. The crude extract, fractions, and compounds (1–3) were assessed for their antibacterial activity against five highly prevalent bacterial strains. The fractions and compounds showed low to moderate activity with minimal inhibitory concentrations (MICs) > 125 µg/mL.
1. Introduction
The genus Vernonia contributes to alleviating malaria and other parasitic illnesses in tropical regions Worldwide [1,2,3]. The species V. amygdalina (locally called “Ndolès” in Cameroon) was the first within the genus to arouse medicinal interests following the uses of plant leaves by wild chimpanzees when experiencing fever [2,4]. Ever since, extensive investigations have reported on the occurrences of sesquiterpenoid lactones and stigmastane-type steroids as chemical markers of the genus [3,4,5,6]. Unlike sesquiterpene lactones, which are distributed throughout the genus, stigmastanes instead occur in few species, including V. guineensis and V. amygdalina [4,5,6,7,8]. Sesquiterpene lactones are able to alkylate cysteine-containing enzymes and proteins, inducing apoptosis in malignant cells [9,10]. Sesquiterpene lactones and related enriched extracts have shown significant potential against various cancer cells [11,12].
The genus Vernonia constitutes a group of 350 species that include Vernonia kotschyana Sch. Bip. Ex Walp, which is the accepted name in the genus of V. adoensis var. kotschyana (Sch.Bip. ex Walp.) G. V. Pope, V. bequaertii De Wild., V. kotschyana Sch. Bip. ex Walp., V. kotschyi Sch. Bip. ex Schweinf., V. leptolepis Bak., and V. woodii O. Hoffm. It is distributed around tropical regions in Africa from Senegal to Cameroon and extending across the continent to Ethiopia [11,12]. It is an herbal remedy used in African folk medicine against digestive insufficiency, colitis, dermatosis, tuberculosis, and headache. The roots of the plant are used in Mali for the treatment of gastritis, stomach ulcer, and wounds. Traditional healers recommend the use of the decoction of dried and powdered roots in the treatment of gastric dysfunction [13]. It was even considered for clinical trials in Mali and has demonstrated efficacy in alleviating gastric ulcers in patients; it therefore has been listed amongst the essential drugs of the country by the Ministry of Health since 2005 [12,14,15]. In the Southwest Region of Cameroon (Lebialem Division), the maceration of leaves in water as well as the decoction of roots of the plant are taken orally for the treatment of gastritis and internal ulcers [16]. A recent study of the total metabolome of V. kotschyana by means of LC-HR-ESI-MS/MS revealed the presence of highly oxygenated stigmastane-type saponins in the roots, including vernocuminosides I-J; vernoamyoside D; vernoniosides D1, D2-D3, and F1-F2; and vernoniamyoside A-D [17] in accordance with previous findings by Sanago et al. 1997 [18], Nergard et al. also revealed the occurrences of pectic arabinogalactan type II in the roots [12,14,15]. Biological studies acknowledge the involvement of the plant saponins and polysaccharides in the immunomodulating inhibition of Helicobacter pylori, cancer cell viability, apoptosis, and ROS production properties of the plant roots [14,15,16,17,19]. Within the frame of the ongoing research project on the search for taxa with antibacterial and antiparasitic activities from Cameroonian rain forests and pharmacopeia [20,21,22], we undertook a phytochemical investigation and antibacterial screening of the whole plant of V. kotschyana. Four hitherto unknown stigmastane-type steroids (1–4) alongside the antibacterial activities of the extract, fractions, and compounds are herein reported.
2. Results and Discussion
The DCM/MeOH (1:1) extract of the whole plant of V. kotschyana was subjected to silica gel flash column chromatography, yielding four main fractions that were further purified via various chromatographic methods to afford eight secondary metabolites, including four new stigmastane-type steroids (1–4) (Figure 1). Their structures were determined by means of extensive spectroscopic and spectrometric analysis.
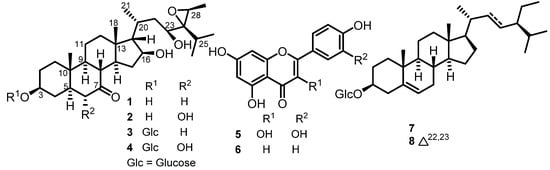
Figure 1.
Structures of isolated compounds 1–8 from V. kotschyana.
2.1. Structure Elucidation of Compounds
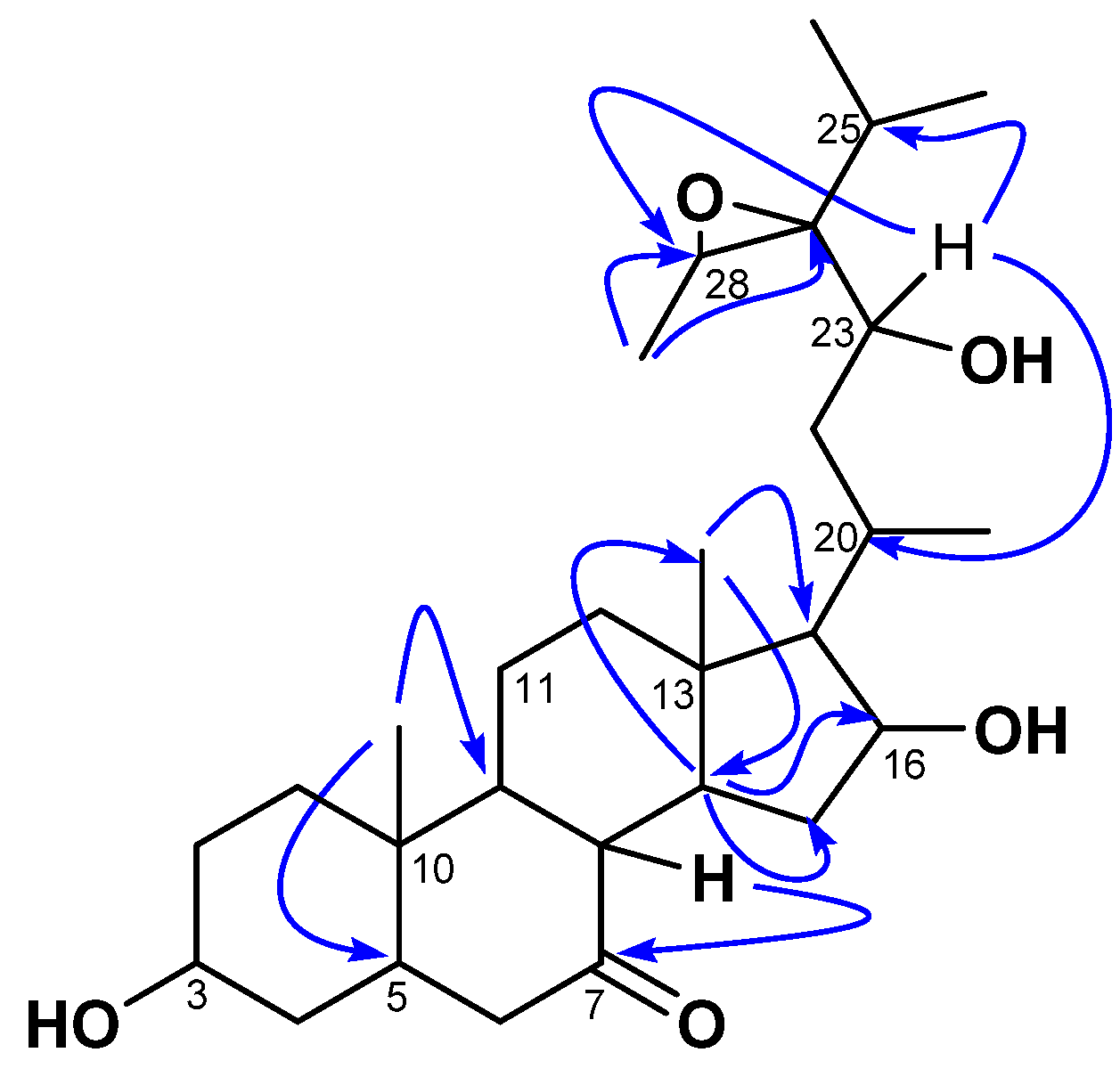
Compound 1 was isolated as a white amorphous powder with an of −23 (c 0.5, MeOH). Its molecular formula (C29H48O5) was determined via HR-ESI-MS, which showed the sodium adduct ion peak [M+Na]+ at m/z 499.3386 (calcd. for C29H48O5Na+, 499.3399). Its IR spectrum showed characteristic absorption bands of hydroxyl (3392 cm−1), carbonyl (1735 cm−1), and aliphatic chains (2940 and 2870 cm−1). The 1H NMR spectrum of 1 was typical of a sterol structure, displaying two angular methyl singlets of stigmastane-type steroids at δH 1.06 (3H, s, H-18) and 1.09 (3H, s, H-19) assigned based on the HMBC interaction from the signal at δH 1.06 to the characteristic carbon C-5 (δC 47.5) of triterpenes [23,24,25]. The stigmastane backbone was confirmed by the 13C NMR spectrum of 1, which revealed the occurrences of 29 carbon atoms (including a ketonic carbonyl group at δC 212.2 (C-7). However, the spectrum showed signals of more than one oxygenated carbon (60–85 ppm) not common in steroids. Moreover, the 13C NMR spectrum also lacked C-C double bond signals to decide on the type of stigmastane skeleton, stigmasterol, or sitosterol; while the 1H NMR spectrum showed more than one signal for methyl doublets. Nevertheless, the HMBC spectrum of 1 evidenced scalar coupling from the methyl H-19 (δH 1.09) to the methine C-9 (δC 55.9), which was part of a spin system formed by H-8/H-9/H-14 as judged by cross-peaks between signals at δH 2.41, 1.08, and 1.47 from the 1H-1H COSY spectrum. Thus, interactions of the latter—mainly from H-9/H-14—helped to locate the carbonyl at C-7. Further interactions from H-14 to C-13 (δC 43.1), C-15 (δC 37.4), and C-16 (δC 72.3) evidenced an oxygenated methine at C-16. The proton H-16 of the latter was part of a spin system along with H-15 (δH 3.26) and H-17 (δH 0.96, m) as judged by correlations between H-15/H-16/H-17 observed in the 1H-1H COSY spectrum. One of the secondary methyls at δH 1.03 and the angular methyl singlet H-18 (δH 1.06) further showed HMBC cross-peaks to C-17 at δH 62.0. The position of the oxygenated methine CH-23 was determined based on 1H-1H COSY correlations between the doublet at δH 1.03 (ultimately assigned to H-21), and the methine H-20 (δH 2.47), then between H-20 and H-17 (δH 0.96) and the methylene H-22 (δH 1.45), and finally between H-22 to H-23 (δH 4.28). The other secondary methyls at δH 1.10 (3H, d, J = 7.0 Hz) and 1.14 (3H, d, J = 7.2 Hz) were part of a spin system with the methine at δH 1.82 (H-25) characteristic of the isopropyl moiety, which is common in stigmastanes [25]. The remaining secondary methyl at δH 14.2 (assigned to H-29) showed HMBC cross-peaks (Figure 2) to two oxygenated methine carbons at δC 69.3 (C-24) and 57.2 (H-28). The relative low resonances of both C-28 and C-24 combined with the molar mass and hydrogen bond deficiency imposed an epoxide between C-24 and C-28 [25,26]. Consequently, the planar structure of 1 was deduced to be 24,28-epoxy-3,6,23-trihydroxystigmastan-7-one.
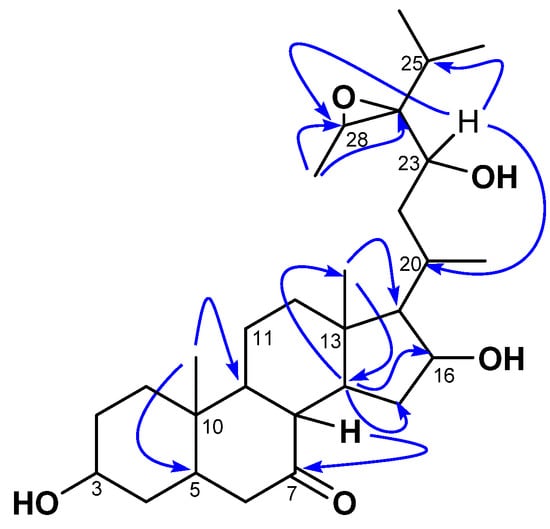
Figure 2.
Key HMBC correlations of kotschyanoside A (1).
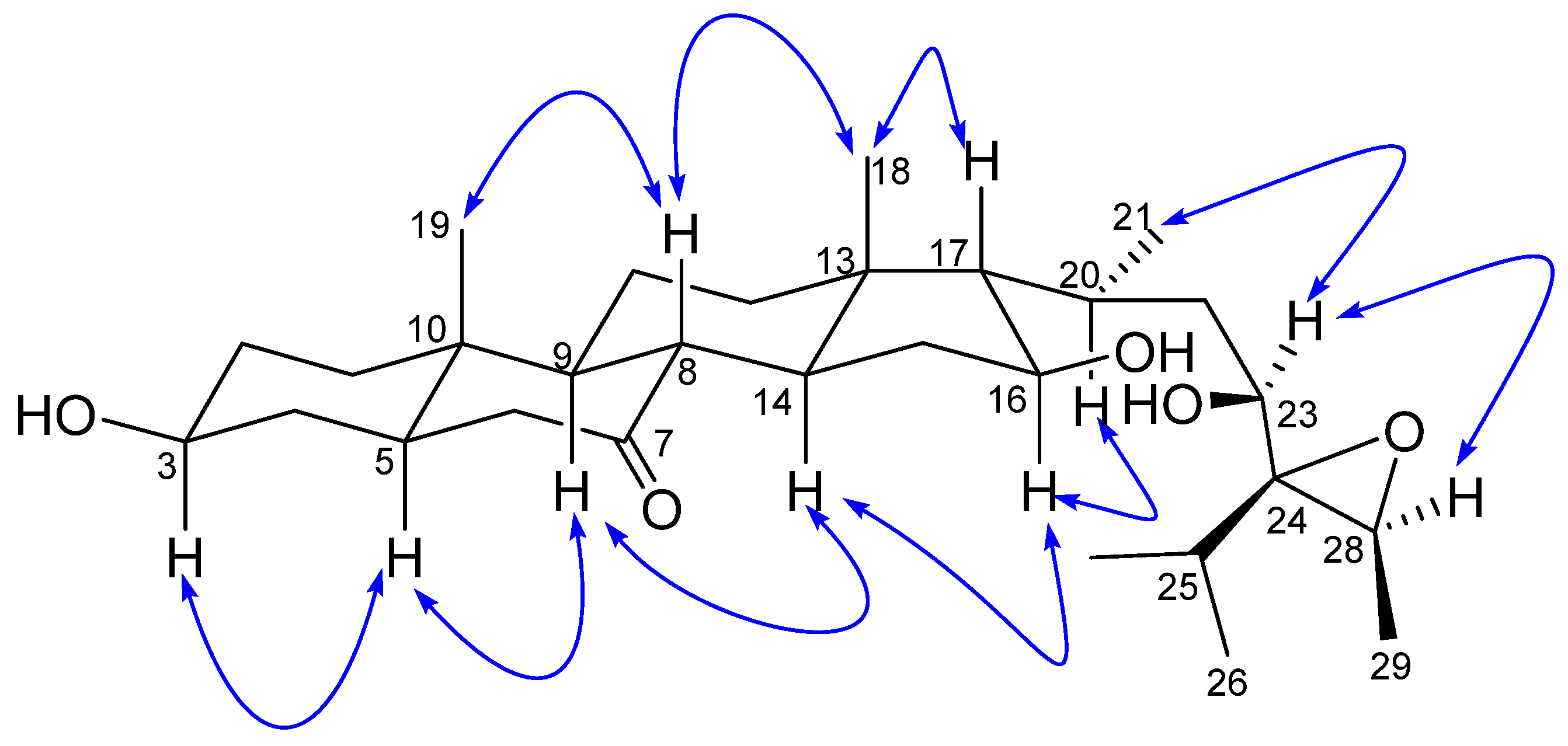
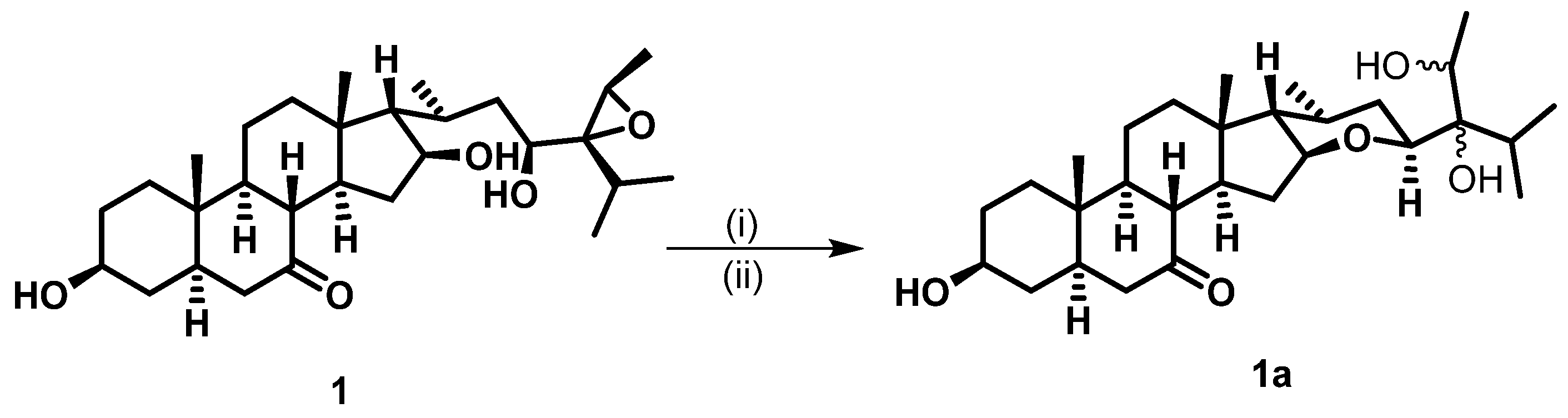
The relative stereochemistry of 1 was established by exploring its ROESY spectrum. Stigmastanes are known to occur in a chair–chair–chair–boat conformer. As a result, the hydroxyl at C-3 was found to be oriented equatorially because of the cross-peak between H-3 and the characteristic H-5 positioned axially. The proton H-17 was suggested to be trans-positioned to H-16 due to the absence of a cross-peak between the two protons in the ROESY spectrum. Further ROESY interactions evidenced correlations between H-21 (biosynthetically α-oriented) and H-23 and H-28 (Figure 3). The isopropyl moiety was β-oriented as in all stigmastanes (Supplementary Materials, Figures S1–S9). The position and stereochemistry of the hydroxyls at C-16 and C-23 were further verified through the cyclization reaction of 1 under an acidic condition (Figure 4). Only pyran or furan are expected when both hydroxyls are in the same side of the plane. Accordingly, the C-16-O-C-23 oxanes (1a) with an opened epoxide at C-24/C-28 was isolated from the reaction mixture. Its NMR data (Figures S11–S16) as well as its mass spectrum (Figure S10) were completely consistent with the expected structure. In fact, its HMBC spectrum showed a strong cross-peak between H-16 (δH 3.95) and C-23 (δC 75.7) establishing the oxane ring moiety. In its 13C NMR spectrum, the characteristic signals at δC 69.3 and 57.2 for the epoxy group disappeared, and the signals for C-24 and C-28 were observed at δC 76.5 and 70.6, respectively. Thus, the structure of compound 1a no longer contained an epoxy group. As a consequence, compound 1 was thus fully characterized as 24,28-epoxy-3,16,23-trihydroxy stigmastan-7-one, for which we proposed the trivial name kotschyanoside A.
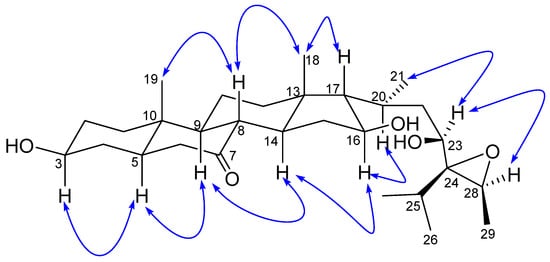
Figure 3.
Key ROESY correlations of kotschyanoside A (1).
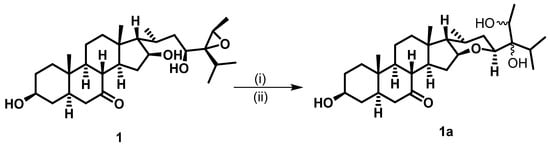
Figure 4.
Cyclization of 1 to 1a under conditions if (i) p-TsOH (50 mg) and (ii) DMF overnight at 90 °C.
Compound 2 was isolated as a white amorphous powder with an of −11.5 (c 0.5, MeOH). Its molecular formula (C29H48O6) was determined via HR-ESI-MS, which showed a sodium adduct ion peak [M+Na]+ at m/z 515.3349 (calcd. for C29H48O6Na+, 515.3343). The molecular mass was 16 Da higher compared to 1, suggesting an additional hydroxyl group present in 2. Its IR spectrum showed absorption bands corresponding to hydroxyl (3392 cm−1), carbonyl (1707 cm−1), and aliphatic chains (2939 and 2871 cm−1). Its NMR data were superimposable on those of compound 1 except for the occurrence of a hydroxylated methine at δH/δC 3.85/74.6 (H-6) in 2 instead of a methylene (δH 2.10/δC 46.8) in 1 (Table 1 and Table 2). The HMBC spectrum of 2 evidenced an important cross-peak from H-6 to a ketone group at δC 211.7. Further interactions from H-6 to C-4 (δC 33.4), C-5 (δC 53.7), and C-10 (δC 36.1) confirmed the position of the new hydroxyl group at C-6. The relative stereochemistry of 2 was also established by means of its ROESY spectrum as identical to that of compound 1. The ROESY cross-peak observed from H-19 (positioned equatorially) to H-6 implied that the hydroxyl at C-6 was positioned axially (Supplementary Materials, Figures S17–S24). Thus, 2 was identified as 24,28-epoxy-3,6,16,23-tetra-hydroxystigmastan-7-one, for which we proposed the trivial name kotschyanoside B.

Table 1.
13C NMR spectroscopic data for compounds 1–4 (δ in ppm).

Table 2.
1H NMR spectroscopic data for compounds 1–4 (δ in ppm).
Compound 3 was obtained as a white amorphous powder with an of −36.8 (c 1.0, MeOH). Its molecular formula (C35H58O10) was determined via HR-ESI-MS, which showed a sodium adduct ion peak [M+Na]+ at m/z 661.3928 (calcd. for C35H58O10Na+, 661.3922). The molecular mass was 162 Da higher compared to 1, suggesting the presence of an additional hexose moiety in 3. Its IR spectrum showed characteristic absorption bands corresponding to hydroxyl (3356 cm−1), carbonyl (1705 cm−1), and aliphatic chains (2935 and 2871 cm−1). Its NMR data were similar to those of 1 except for the presence of a glucosyl group (δC 102.3, 78.1, 77.9, 75.2, 71.0, and 62.8), which accounted for the additional one degree of unsaturation and was identified as β-D-glucopyranoside via comparison of its NMR data with reported values [8,25]. The position of the sugar was defined based on the HMBC interaction from the anomeric proton H-1’ (δH 4.40) to δC 69.3 (C-3), which was further confirmed by the glycosidation shifts in the resonances of C-2, C-3, and C-4 (δC 35.3, 78.6, and 47.0, respectively) as compared to their respective values for compounds 1 and 2 (Table 1). The relative stereochemistry of 3 was consistent with those of 1 and 2 when comparing their ROESY data (Supplementary Materials, Figures S25–S33). Thus, 3 was fully characterized as 24,28-epoxy-3,16,23-trihydroxystigmastan-7-one 3-O-β-D-glucopyranoside, for which we proposed the trivial name kotschyanoside C.
Compound 4 was isolated as a white amorphous powder with an of −26.7 (c 1.0, MeOH). Its molecular formula (C35H58O11) was determined via HR-ESI-MS, which showed a sodium adduct ion peak [M+Na]+ at m/z 677.3879 (calcd. for C35H58O11Na+, 677.3871). The molecular mass was 162 Da higher compared to 2, suggesting the presence of an additional hexose moiety in 4. Its IR spectrum showed characteristic absorption bands corresponding to hydroxyl (3354 cm−1), carbonyl (1704 cm−1), and aliphatic chains (2935 and 2871 cm−1). The NMR data of 4 were closely related to those of 3 except for the signal of an additional oxygenated methine at δC/δH 76.5/3.99, which was located at C-6 due to HMBC interactions from the latter to carbons C-8 (48.4), C-5 (δC 55.2), and C-7 (δC 212.3). The relative stereochemistry of 4 was consistent with that of 3 when comparing the ROESY data of both 3 and 4 (Supplementary Materials, Figures S34–S41). Thus, 4 was fully characterized as 24,28-epoxy-3,6,16,23-tetrahydroxystigmastan-7-one 3-O-β-D glucopyranoside, for which we proposed the trivial name kotschyanoside D.
The other isolates were identified via comparison of their spectroscopic data with those reported in the literature as quercetin (5) [27], apigenin (6) [10], and a mixture of β-sitosterol 3-O-β-D-glucopyranoside (7) and stigmasterol 3-O-β-D-glucopyranoside (8) [28].
2.2. Chemophenetic Significance
Overall, eight (1–8) compounds were isolated during the chemical investigations of this species, including new stigmastane-type glycosides with unique oxygenated patterns (1–4), two flavonoids (5–6), and a mixture of two readily available phytosterols (7–8). Sesquiterpenoid and stigmastane-type steroids are the chemophenetic markers of the genus Vernonia [10,29,30]. To the best of our knowledge, this is the third report disclosing the chemical constituents of the studied species. Our investigation allowed us to postulate that sesquiterpenoid lactones were not occurring in V. kotschyana after phytochemical screening tests and LC-MS facilities were used.
The new compounds displayed a new oxygenated pattern in stigmastane-type steroids found in Vernonia. Stigmastanes have been highlighted in some species of the genus, including V. guineesis and V. amygdalina. Mainly, the side chain of Vernonia steroids usually bears a γ-lactone fused to a tetrahydrofuran group resulting from successive oxidation and cyclization processes [4,17]. The new compounds 1–4 could have resulted from the hydrolysis of this well-known moiety in Vernonia species. Moreover, our findings revealed new oxygenated carbons in the steroid skeleton, namely positions C-6 and C-7, indicating that steroids from V. kotschyana originate instead from the stigmasterol backbone. This might represent a new significant chemophenetic marker for plants of the genus Vernonia.
Although flavonoids have been reported in Vernonia species (including V. cinerascens [11] and V. cinarea [31] for compound 5 and V. amygdalina for compound 6 [32]), they are herein reported for the first time from V. kotschyana. This observation provides new insights into the occurrence of flavonoids in the genus Vernonia and the family Asteraceae.
2.3. Biological Results
The antibacterial activities of the crude extract and major fractions (FA–FD) obtained from the main column chromatography, as well as pure compounds obtained in sufficient amounts (1–3), were evaluated against five highly prevalent bacterial strains: Escherichia coli ATCC25322, Staphylococcus aureus ATCC25923, Staphylococcus pneumoniae ATCC461916, Pseudomonas aeruginosa HM801, and the clinical strain Klebsiella pneumonia. The extract and three fractions (FA, FC, and FD) showed weak activity (with MIC > 500 µg/mL), whereas fraction FB was moderately active on S. aureus ATCC25923 (MIC = 250 µg/mL) and P. aeruginosa HM801 (MIC = 250 µg/mL) and moderate on the clinical strain K. pneumonia (MIC = 500 µg/mL). Compounds 1 and 2 displayed moderate activity (125 < MIC < 500 µg/mL) against the tested strains, while compound 3 was totally inactive at the tested concentration.
The biological properties of stigmastane-type steroids and flavonoids are well documented [18], and the presence of both classes of compounds in an extract might strengthen evidence of the medicinal value of V. kotschyana (mainly as potential inhibitors of Helicobacter pylori) for a possible mode of action of the plant in the treatment of gastric ulcer.
3. Experimental
3.1. General Experimental Procedures
Electrospray ionization (ESI) data were obtained on a 1200-series HPLC system or a 1260-series Infinity II HPLC system (Agilent-Technologies, Santa Clara, CA, USA) with a binary pump and integrated diode array detector coupled to an LC/MSD-Trap-XTC-mass spectrometer (Agilent-Technologies) or an LC/MSD Infinity lab LC/MSD (G6125B LC/MSD). High-resolution mass spectra were recorded on a Micromass-Q-ToF-Ultima-3 mass spectrometer (Waters, Milford MA, USA) with a LockSpray interface and a suitable external calibrant using gradients of acetonitrile–water (containing 0.1% formic acid) as the elution system. Infrared (IR) spectra were recorded on an FTIR spectrometer (Bruker Tensor 27) equipped with a diamond ATR unit; the frequency of the absorption is reported in cm−1. NMR data were obtained on a Bruker Avance III 500 HD (1H: 500 MHz, 13C: 125 MHz) or Avance 600 (1H: 600 MHz, 13C: 150 MHz); chemical shifts δ (ppm) were referenced relative to the residual solvent signal and/or tetramethylsilane (TMS).
Compounds were purified via chromatographic methods using silica gel (35–70 μm, Acros Organics, Waltham, MA, USA) and Sephadex LH-20 automated column chromatography on a Büchi Reveleris® X2 with a binary pump and an ELSD detector using flash columns or a Biotage Snap Ultra C18 column with a gradient at various flow rates. Thin-layer chromatography (TLC) was carried out on silica plates (TLC Silica 60 F254 by Merck, Darmstadt, Germany), and spots were detected by spraying with 20% H2SO4 followed by charring at 100 °C.
3.2. Plant Material
The whole plant of V. kotschyana was collected in November 2016 at Bamendjing (Mbouda Subdivision), Western Region of Cameroon, and identified by Victor Nana, a retired botanist of the National Herbarium of Cameroon, where a voucher specimen (N° 48782 HNC) was deposited.
3.3. Extraction and Isolation
The whole plant of V. kotschyana (3.7 kg) was macerated in a mixture of CH2Cl2/MeOH (1:1) at room temperature for 48 h, and the extraction process was conducted thrice. The resulting solution was filtered, and the removal of the solvent in vacuo afforded 100.1 g of a semi-solid crude extract. Part of the crude extract (90.5 g) was subjected to silica gel (230–400 mesh) column chromatography using a stepwise gradient of EtOAc in petroleum ether (PE) and MeOH in EtOAc. A total of 95 fractions of 250 mL each were collected and combined based on TLC profiles into 4 main fractions (FA–FD). Fraction FA (2.5 g, PE) was mainly fatty content and was not further investigated. Fractions FB (10 g, PE/EtOAc, 95:5–9:1 v/v) and FC (17.2 g, PE/EtOAc, 9:1–8.5: 1.5 v/v) were combined based on their TLC profiles and labeled FBC (27.2 g). A mass of 27.1 g of this fraction was submitted to open column chromatography over silica gel (70–230 mesh) and eluted with a gradient of acetone in PE to yield compound 1 (1.2 g). The resulting fractions were grouped into three subfractions (FBC1–FBC3) based on their TLC profiles. Subfraction FBC2 (3 g, PE/acetone, 8.5:1.5–7:3 v/v) was further chromatographed over silica gel and eluted with a gradient of acetone in PE to yield compounds 5 (3.0 mg) and 2 (4.8 mg). Fractions FD (55 g, PE/acetone, 95:5–9:1 v/v) was submitted to open column chromatography over silica gel (70–230 mesh) and eluted with a gradient of acetone in PE to yield compound 3 (50.7 mg). The resulting fractions were grouped into four subfractions (FD-S1–FD-S4) based on their TLC profiles. Subfraction FD-S1 (15.6 mg, PE/acetone 3:2–1:1 v/v) was chromatographed over silica gel and eluted with a gradient of acetone in PE to yield compound 6 (2.5 mg). Subfraction FD-S2 (6.3 g, PE/acetone 3:2–1:1 v/v) was chromatographed over silica gel and eluted with a gradient of acetone in PE to obtain the mixture of compounds 7 and 8. Subfractions FD-S3 and FD-S4 (40.2 g, PE/EtOAc 3:2–1:1 v/v) were combined based on their TLC profile and labeled FD-S3, which was purified over a silica gel column and eluted with a gradient of acetone in PE to afford compounds 3 (1.2 g) and 4 (5.3 mg).
3.4. Kotschyanoside A (1)
3.5. Kotschyanoside B (2)
3.6. Kotschyanoside C (3)
3.7. Kotschyanoside D (4)
3.8. Cyclization of Compound 1
A total of 50.0 mg of compound 1 was mixed with p-TsOH (50.0 mg), dissolved in 2 mL of DMF, and stirred for 24 h (overnight) at 90 °C. The solvent was evaporated, and the residual was purified via column chromatography followed by MPLC with a gradient of EtOAc in PE to afford compound 1a (5.6 mg) along with an unresolved complex mixture.
Compound 1a: white amorphous powder 1H NMR (600 MHz, acetone-d6) δH 3.97–3.93 (m, 1H), 3.74 (dd, J = 7.8, 4.8 Hz, 1H), 3.62 (dq, J = 8.4, 6.7 Hz, 1H), 3.43 (d, J = 8.4 Hz, 1H), 2.41 (t, J = 11.3 Hz, 1H), 2.35–2.27 (m, 1H), 1.87 (ddd, J = 10.4, 7.5, 5.1 Hz, 1H), 1.83–1.75 (m, 2H), 1.65 (dddd, J = 28.8, 13.3, 5.6, 3.2 Hz, 3H), 1.46 (dddd, J = 26.2, 12.2, 5.1, 2.6 Hz, 2H), 1.10 (d, J = 6.7 Hz, 3H), 1.01 (s, 3H), 1.00–0.97 (m, 1H), 0.95 (d, J = 6.8 Hz, 3H), 0.93–0.86 (m, 2H), 0.84 (d, J = 6.9 Hz, 3H), 0.77 (d, J = 7.2 Hz, 3H), 0.76 (d, J = 4.7 Hz, 0H), 0.74 (s, 3H). 13C NMR (150 MHz, Acetone-d6) δC 77.1, 76.5, 75.7, 70.6, 69.8, 59.7, 55.7, 55.5, 48.8, 46.6, 45.9, 45.6, 42.1, 38.1, 37.8, 36.1, 36.0, 34.6, 31.8, 31.1, 30.0, 24.3, 22.0, 21.2, 19.9, 18.7, 17.5, 17.2, 14.4, 13.6, 11.1. (+)-ESIMS m/z 499.36 [M+Na]+.
3.9. Bioassay
Five pathogenic bacterial strains were used. The antibacterial assay was performed on five bacteria strains including four standards (Escherichia coli ATCC25322, Staphylococcus aureus ATCC25923, Staphylococcus pneumoniae ATCC461916, and Pseudomonas aeruginosa HM801) and the clinical Klebsiella pneumonia CPC. They were maintained on fresh Mueller Hinton Agar (MHA) for 24 h prior to any antibacterial assay, and the minimal inhibitory concentrations (MICs) of the tested samples were determined as reported previously [33].
4. Conclusions
The chemical investigation of Vernonia kotschyana led to the isolation of four new stigmastane-type glycosides with unique oxygenated patterns (1–4). Compound 1, one of the most active metabolites against the tested microbial strains (125–250 µg/mL), was isolated from the most active fraction, FB (250 μg/mL). These results indicated that V. kotschyana shares similar chemical characteristics with other species of this genus and might provide additional insights into the chemotaxonomic classification of the genus Vernonia. The discovery of more steroids featuring similar chemical diversities as encountered for compounds 1–4 from previously unstudied Vernonia species might provide more information on the suggested occurrence pattern highlighted in the present report. Such cues would trigger the development of strategies to clear up the biosynthesis of steroids as they occur in the Vernonia genus. Ultimately, the recorded bioactivity is an indication to look further toward the validation of health benefits associated with the plant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28135278/s1: NMR, UV, IR, and HR-ESIMS data for compounds 1−4 (Figures S1–S9 and S17–S41); NMR and ESIMS spectra of the derivative 1a (Figures S10–S16); 1D NMR spectra of known compounds 5–8 (Figures S42–S45).
Author Contributions
Conceptualization, N.T.W. and G.T.M.B.; Funding acquisition, B.N.L. and N.S.; Investigation, N.T.W., G.T.M.B. and I.M.K.; Project administration, B.N.L. and N.S.; Supervision, S.F.K. and N.S.; Writing—original draft, N.T.W. and I.M.K.; Writing—review and editing, G.T.M.B., J.T. and C.N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Academic Exchange Service (DAAD) through the YaBiNaPA project (No. 57316173) and the Alexander von Humboldt Foundation (3.4-CMR-Hub).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Akinpelu, A.D. Antimicrobial activity of Vernonia amygdalina leaves. Fitoterapia 1999, 70, 432–434. [Google Scholar] [CrossRef]
- Koshimizu, K.; Ohigashi, H.; Huffman, M.A. Use of Vernonia amygdalina by wild chimpanzee: Possible roles of its bitter and related constituents. Physiol. Behav. 1994, 56, 1209–1216. [Google Scholar] [CrossRef]
- Donfack, N.R.A.; Toyang, J.N.; Wabo, K.H.; Tane, P.; Awouafack, D.M.; Kikuchi, H.; Tamokou, J.D.D.; Kuiate, J.R.; Oshima, Y. Stigmastane derivatives from the roots of Vernonia guineensis and their antimicrobial activity. Phytochem. Lett. 2012, 5, 596–599. [Google Scholar] [CrossRef]
- Ohigashi, H.; Jisaka, M.; Takagaki, T.; Nozaki, H.; Tada, T.; Huffman, M.A.; Nishida, T.; Kaji, M.; Koshimizu, K. Bitter principle and a related steroid glucoside from V. amygdalina, a possible medicinal plant for wild chimpanzees. Chem. Biol. Technol. Agric. 1991, 55, 1201–1203. [Google Scholar] [CrossRef]
- Jisaka, M.; Kawanaka, M.; Sugiyama, H.; Takegawa, K.; Huffman, M.A.; Ohigashi, H.; Koshimizu, K. Antischistosomal activities of sesquiterpene lactones and steroid glucosides from Vernonia amygdalina, possibly used by wild chimpanzees against parasite-related diseases. Biosci. Biotechnol. Biochem. 1992, 56, 845–846. [Google Scholar] [CrossRef]
- Jisaka, M.; Ohigashi, H.; Takagaki, T.; Nozaki, H.; Tada, T.; Hirota, M.; Irie, R.; Huffman, M.A.; Nishida, T.; Kaji, M.; et al. Bitter steroid glucosides, vernosides A1, A2, A3 and related B1 from a possible medicinal plant Vernonia amygdalina used by wild chimpanzees. Tetrahedron 1992, 48, 625–632. [Google Scholar] [CrossRef]
- Tchinda, A.T.; Tsopmo, A.; Tane, P.; Ayafor, F.J.; Connolly, J.D.; Sterner, O. Vernoguinosterol and vernoguinoside, trypanocidal stigmastane derivatives from Vernonia guineensis (Asteraceae). Phytochemistry 2002, 59, 371–374. [Google Scholar] [CrossRef]
- Anh, H.L.T.; Vinh, L.B.; Lien, L.T.; Cuong, V.P.; Arai, M.; Ha, P.T.; Lin, N.H.; Dat, H.T.T.; Cuong, V.L.C.; Kim, H.Y. In vitro study on a-amylase and a-glucosidase inhibitory activities of a new stigmastane-type steroid saponin from the leaves of Vernonia amygdalina. Nat. Prod. Res. 2021, 35, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Bitchagno, M.T.G.; Nchiozem-Ngnitedem, V.-A.; Wandji, T.N.; Noulala, T.G.C.; Fobofou, T.A.S.; Lenta, N.B. Plant-Derived Compounds against Microbial Infections and Cancers. In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health; Sharma, K., Mishra, K., Kamal Senapati, K., Danciu, C., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83880-887-7. [Google Scholar]
- Bitchagno, G.T.M.; Koffi, G.J.; Simo, K.I.; Kagho, D.U.K.; Ngouela, A.S.; Lenta, N.B.; Sewald, N. LC-ToF-ESI-MS patterns of hirsutinolide-like sesquiterpenoids present in the Elephantopus mollis Kunth extract and chemophenetic significance of its chemical constituents. Molecules 2021, 26, 4810. [Google Scholar] [CrossRef]
- Toyang, J.N.; Verpoorte, R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef]
- Nergard, C.S.; Matsumoto, T.; Inngjerdingen, M.; Inngjerdingen, K.; Hokputsa, S.; Harding, S.E.; Michaelsen, T.E.; Diallo, D.; Kiyohara, H.; Paulsen, B.S.; et al. Structural and immunological studies of a pectin and a pectic arabinogalactan from Vernonia kotschyana Sch. Bip. ex Walp. (Asteraceae). Carbohydr. Res. 2005, 340, 115–130. [Google Scholar] [CrossRef]
- Diarra, M.L.; Denou, A.; Coulibaly, L.B.; Togola, A.; Sanogo, D.; Sanogo, R.; Traore, M.; Diallo, D.; Noba, K. Caractéristiques botaniques et phytochimiques de Vernonia kotschyana Sch. Bip. ex Walp. mise en culture et utilisée dans le traitement des gastrites et l’ulcère gastroduodénal au Mali. Int. J. Biol. Chem. Sci. 2018, 12, 381–391. [Google Scholar] [CrossRef]
- Nergard, C.S.; Diallo, D.; Michaelsen, T.E.; Malterud, K.E.; Kiyohara, H.; Matsumoto, T.; Yamada, H.; Paulsen, B.S. Isolation, partial characterization and immunomodulating activities of polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp. J. Ethnopharmacol. 2004, 91, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Nergard, C.S.; Kiyohara, H.; Reynolds, J.C.; Thomas-Oates, J.E.; Matsumoto, T.; Yamada, H.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Structure-immunomodulating activity relationships of a pectic arabinogalactan from Vernonia kotschyana Sch Bip. ex Walp. Carbohydr. Res. 2005, 340, 1789–1801. [Google Scholar] [CrossRef]
- Focho, D.A.; Ndam, W.T.; Fonge, B.A. Medicinal plants of Aguambu—Bamumbu in the Lebialem highlands, SouthWest province of Cameroon. Afr. J. Pharm. Pharmacol. 2008, 3, 1–13. [Google Scholar]
- Vasincua, A.; Luca, V.S.; Charalambous, C.; Neophytou, M.C.; Skalicka-Woźniakg, K.; Mironc, A. LC-HRMS/MS phytochemical profiling of Vernonia kotschyana Sch. Bip. ex Walp.: Potential involvement of highly-oxygenated stigmastane-type saponins in cancer cell viability, apoptosis and intracellular ROS production. S. Afr. J. Bot. 2022, 144, 83–91. [Google Scholar] [CrossRef]
- Sanogo, R.; Germano, M.P.; Tommasi, D.N.; Pizza, C.; Aquino, R. Vernoniosides and an androstane glycoside from Vernonia kotschyana. Phytochemistry 1997, 47, 73–78. [Google Scholar] [CrossRef]
- Inngjerdingen, T.K.; Thöle, C.; Diallo, D.; Paulsen, S.B. Andreas Hensel, A. Inhibition of Helicobacter pylori adhesion to human gastric adenocarcinoma epithelial cells by aqueous extracts and pectic polysaccharides from the roots of Cochlospermum tinctorium A. Rich. and Vernonia kotschyana Sch. Bip. ex Walp. Fitoterapia 2014, 95, 127–132. [Google Scholar] [CrossRef]
- Tameye, N.S.J.; Akak, M.C.; Tabekoueng, B.G.; Mkounga, P.; Bitchagno, M.T.G.; Lenta, N.B.; Sewald, N.; Nkengfack, E.A. Chemical constituents from Diospyros fragrans Gürke (Ebenaceae). Biochem. Syst. Ecol. 2021, 100, 104373. [Google Scholar] [CrossRef]
- Wandji, T.N.; Bitchagno, G.T.M.; Tchamgoue, J.; Stammler, H.-G.; Frese, M.; Lenta, B.N.; Sewald, N.; Kouam, F.S. Furanocoumarins and other constituents from the twigs of Ficus chlamydocarpa Mildbraed & Burret (Moraceae). Phytochem. Lett. 2022, 47, 38–41. [Google Scholar]
- Wouamba, N.S.C.; Happi, M.G.; Lenta, N.B.; Sewald, N.; Kouam, F.S. Vernoguinamide: A new ceramide and other compounds from the root of Vernonia guineensis Benth. and their chemophenetic significance. Biochem. Syst. Ecol. 2020, 88, 103988. [Google Scholar] [CrossRef]
- Ambadiang, M.M.M.; Atontsa, K.C.B.; Tankeo, B.S.; Nayim, P.; Wamba, N.E.B.; Bitchagno, M.T.G.; Mpetga, S.D.J.; Penlap, B.V.; Kuete, V. Bark extract of Cassia sieberiana DC. (Caesalpiniaceae) displayed good antibacterial activity against MDR gram-negative phenotypes in the presence of phenylalanine-arginine β-naphthylamide. BMC Complement. Med. Ther. 2020, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Qi, W.Y.; Hussain, S.H.; Gao, K.; Arfan, M. Highly oxygenated stigmastane-type steroids from the aerial parts of Vernonia anthelmintica Willd. Steroids 2012, 77, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Igile, G.; Oleszek, W.; Jurzysta, M. Vernoniosides D and E, two novel saponins from Vernonia amygdalzna. J. Nat. Prod. 1995, 58, 1438–1443. [Google Scholar] [CrossRef]
- Cioffi, G.; Sanogo, R.; Diallo, D.R.G.; Tommasi, D.N. New Compounds from an extract of Vernonia colorata leaves with anti-inflammatory Activity. J. Nat. Prod. 2004, 67, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wan, C.; Zhou, S. Quercetin—A flavonoid compound from Sarcopyramis bodinieri var delicate with potential apoptotic activity in HepG2 Liver Cancer Cells. Trop. J. Pharm. Res. 2013, 12, 529–533. [Google Scholar] [CrossRef]
- Rai, N.P.; Adhikari, B.B.; Paudel, A.; Masuda, K.; Mckelvey, R.D.; Manandhar, M.D. Phytochemical constituents of the flowers of Sarcococca coriacea of Nepalese origin. J. Nepal Chem. Soc. 2006, 21, 1–7. [Google Scholar] [CrossRef]
- King, B.; Jone, B.S. Chemosystematics of Vernonia series Flexuosae (Vernonieae: Compositae). Bull. Torrey Bot. Club 1982, 109, 279–286. [Google Scholar] [CrossRef]
- Mabry, T.J.; Abdel-Base, Z.; Padolina, G.; Jones, S.B. Systematic implications of flavonoids and sesquiterpene lactones in species of Vernonia. Biochem. Syst. Ecol. 1975, 2, 185–192. [Google Scholar] [CrossRef]
- Youn, J.U.; Miklossy, G.; Chai, X.; Wongwiwatthananukit, S.; Toyama, O.; Thanapat, S.; Turkson, J.; Chang, L.C. Bioactive sesquiterpene lactones and other compounds isolated from Vernonia cinerea. Fitoterapia 2014, 93, 194–200. [Google Scholar] [CrossRef]
- Dagnon, S.; Novkova, Z.; Bojilov, D.; Nedialkov, P.; Kouassi, C.H. Development of surrogate standards approach for the determination of polyphenols in Vernonia amygdalina Del. J. Food Compost. Anal. 2019, 82, 103231. [Google Scholar] [CrossRef]
- Nguengang, R.T.; Tchegnitegni, B.T.; Nono, E.C.N.; Bellier Tabekoueng, G.; Fongang, Y.S.F.; Bankeu, J.J.K.; Chouna, J.R.; Nkenfou, C.N.; Fekam, F.B.; Sewald, N.; et al. Constituents of the Stem Bark of Symphonia globulifera Linn. f. with Antileishmanial and Antibacterial Activities. Molecules 2023, 28, 2473. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).