Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis
Abstract
1. Introduction
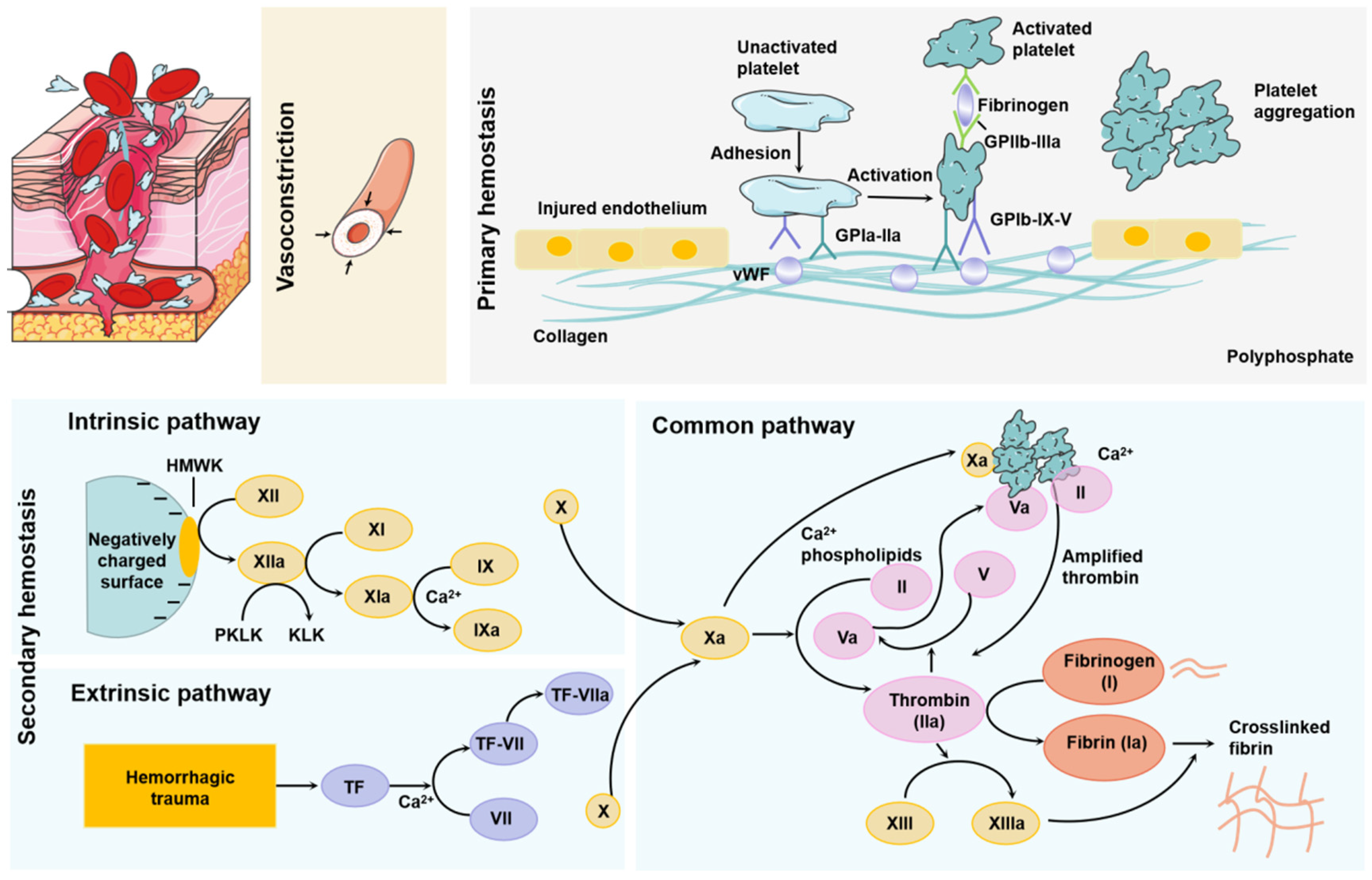
2. Physiological Hemostatic Mechanisms
3. Physical Hemostatic Methods
4. Commonly Used Hemostatic Materials
5. Research and Application of Hemostatic Nanomaterials
5.1. Nanoparticles
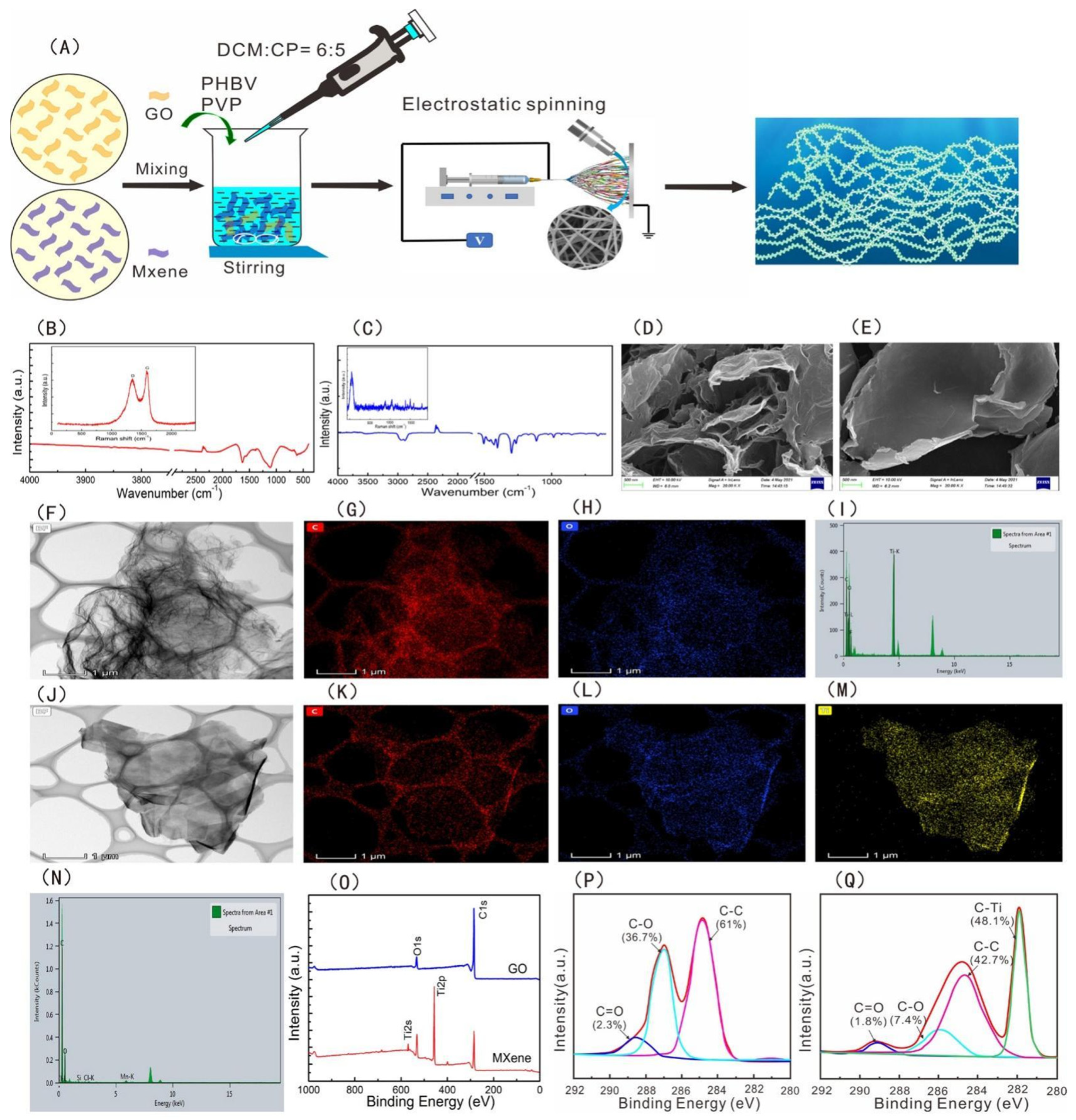
5.2. Nanosheets
5.3. Liposomes
5.4. Nanofibers
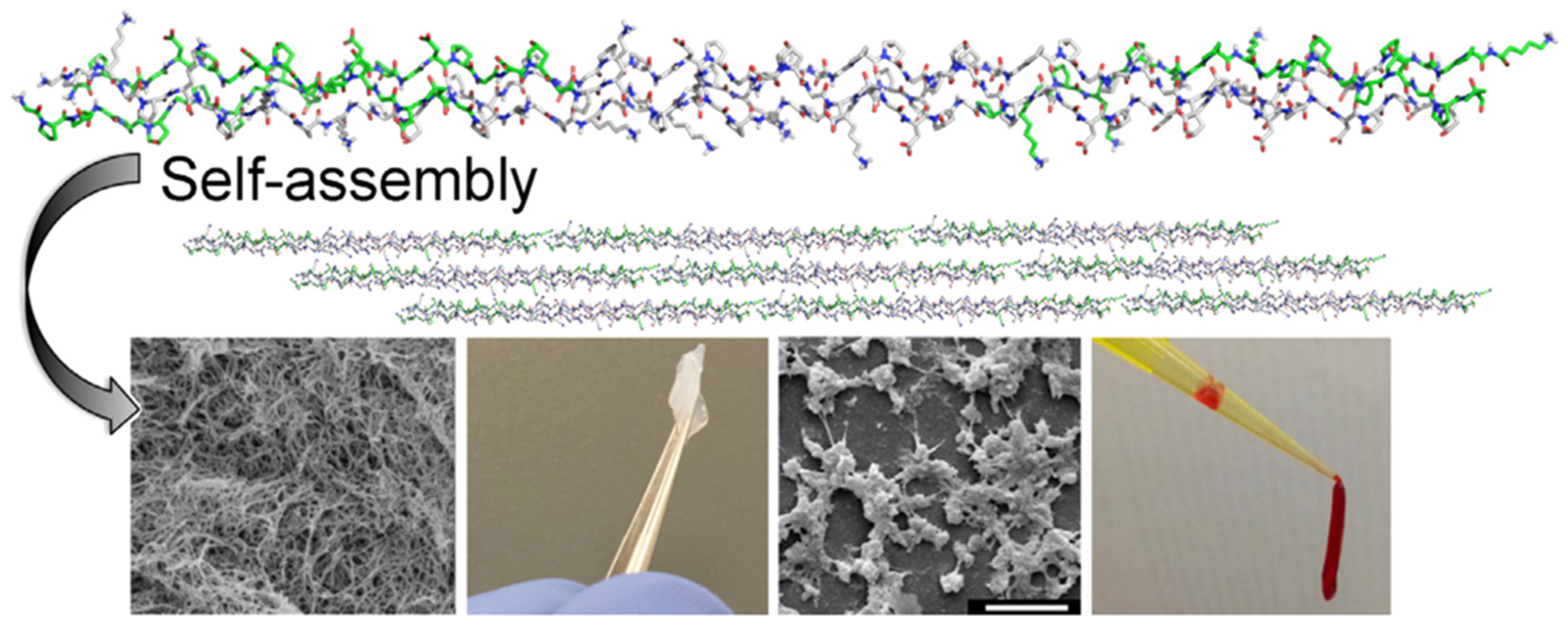
5.5. Self-Assembling Peptides
5.6. Nanocomposite Hydrogel
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma Mon. 2016, 21, 26023. [Google Scholar] [CrossRef]
- Weeks, A. The prevention and treatment of postpartum haemorrhage: What do we know, and where do we go to next? BJOG 2015, 122, 202–210. [Google Scholar] [CrossRef]
- Howe, N.; Cherpelis, B. Obtaining rapid and effective hemostasis: Part I. Update and review of topical hemostatic agents. J. Am. Acad. Dermatol. 2013, 69, 659. [Google Scholar] [CrossRef] [PubMed]
- Gruen, R.L.; Brohi, K.; Schreiber, M.; Balogh, Z.J.; Pitt, V.; Narayan, M.; Maier, R.V. Haemorrhage control in severely injured patients. Lancet 2012, 380, 1099–10108. [Google Scholar] [CrossRef]
- Eissa, R.A.; Saafan, H.A.; Ali, A.E.; Ibrahim, K.M.; Eissa, N.G.; Hamad, M.A.; Pang, C.; Guo, H.; Gao, H.; Elsabahy, M.; et al. Design of nanoconstructs that exhibit enhanced hemostatic efficiency and bioabsorbability. Nanoscale 2022, 14, 10738–10749. [Google Scholar] [CrossRef]
- Wang, X.X.; Liu, Q.; Sui, J.X.; Ramakrishna, S.; Yu, M.; Zhou, Y.; Jiang, X.Y.; Long, Y.Z. Recent Advances in Hemostasis at the Nanoscale. Adv. Healthc. Mater. 2019, 8, 1900823. [Google Scholar] [CrossRef]
- Malik, A.; Rehman, F.U.; Shah, K.U.; Naz, S.S.; Qaisar, S. Hemostatic strategies for uncontrolled bleeding: A comprehensive update. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Binsalah, M.; Devanesan, S.; AlSalhi, M.S.; Nooh, S.; Al-ghamdi, O.; Nooh, N. The effect of ethyl acetate mediated silver nanoparticles from Urtica diocia on hemostasis; in-vitro and in-vivo study. J. Drug Deliv. Sci. Technol. 2022, 76, 103840. [Google Scholar] [CrossRef]
- Simak, J.; De Paoli, S. The effects of nanomaterials on blood coagulation in hemostasis and thrombosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1448. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelium and haemostasis. Hamostaseologie 2015, 35, 11–16. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef]
- Derner, R.; Buckholz, J. Surgical hemostasis by pneumatic ankle tourniquet during 3027 podiatric operations. J. Foot Ankle Surg. 1995, 34, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.Z.; Aijaz, S.; Sheikh, S.; Sattar, S. Randomized trial comparing radial hemostasis techniques; catechol conjugated chitosan pad (InnoSEAL) versus pneumatic compression band. Catheter. Cardiovasc. Interv. 2021, 98, 181–187. [Google Scholar] [CrossRef]
- Hurcombe, S.D.A.; Radcliffe, R.M.; Cook, V.L.; Divers, T.J. The pathophysiology of uncontrolled hemorrhage in horses. J. Vet. Emerg. Crit. Care 2022, 32, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Ito, K.; Takemura, N.; Oikawa, R.; Koutake, H.; Mihara, F.; Yagi, J.; Nakanishi, M.; Tomio, K.; Oishi, H.; et al. Laparoscopic hemostasis for abdominal brunt massive hemorrhage due to endometriosis. Asian J. Endosc. Surg. 2022, 15, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Shinchi, H.; Maemura, K.; Aikou, T. Ultrasonically activated scalpel is an effective tool for cutting the pancreas in biliary-pancreatic surgery: Experimental and clinical studies. J. Hepatobiliary Pancreat. Surg. 2000, 7, 58–62. [Google Scholar] [CrossRef]
- Cobb, C.M. Lasers in periodontics: A review of the literature. J. Periodontol. 2006, 77, 545–564. [Google Scholar] [CrossRef]
- Reddy, S.; Shenoy, R.; Mandadi, L.R.; Saluja, I.; Thomas, M.S. Effect of blood contamination and various hemostatic procedures on the push-out bond strength of Biodentine when used for furcation perforation repair. J. Conserv. Dent. 2021, 24, 260–264. [Google Scholar]
- Lee, B.R. Laparoscopic total and partial nephrectomy. Int. Braz. J. Urol. 2002, 28, 504–509. [Google Scholar]
- Sah, A.P. Clinical Outcomes and Experience of a Multiyear Consecutive Case Series of Total Knee Arthroplasty Procedures Conducted with a Bipolar Sealer System for Hemostasis. J. Knee Surg. 2022, 35, 1378–1384. [Google Scholar] [CrossRef]
- Hartz, K.M.; Lee, R.Y.; Walsh, L.T.; Dixon, M.E.B.; Moyer, M.T. Endoscopic hemostatic spray for uncontrolled bleeding after complicated endoscopic mucosal resection or endoscopic submucosal dissection: A report of 2 cases. VideoGIE 2021, 6, 481–483. [Google Scholar] [CrossRef]
- Goya, R.; Takemoto, M.; Nyuta, E.; Antoku, Y.; Yamaguchi, A.; Furuta, N.; Eto, A.; Mito, T.; Kurachi, M.; Koga, T.; et al. Efficacy and Safety of Nepcell STM in Achieving Hemostasis After Removal of a 15-Fr Femoral Venous Sheath in Patients Undergoing Cryoballoon Ablation for Atrial Fibrillation. Circ. Rep. 2021, 3, 691–698. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, S.; Zhu, X.; Zhao, Y.; Chen, Y.; Tong, J.; Shi, X.; Du, Y.; Zhong, Z.; Ye, Q. Emerging chitin nanogels/rectorite nanocomposites for safe and effective hemorrhage control. J. Mater. Chem. B 2019, 7, 5096–5103. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Suneetha, M.; Park, G.T.; Babu, A.G.; Han, S.S. Hemostatic, biocompatible, and antibacterial non-animal fungal mushroom-based carboxymethyl chitosan-ZnO nanocomposite for wound-healing applications. Int. J. Biol. Macromol. 2020, 155, 71–80. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Amirthalingam, S.; Mony, U.; Varma, P.K.; Jayakumar, R. Injectable chitosan-nano bioglass composite hemostatic hydrHemostatic, biocompatible, and antibacterial non-animal fungal mushroom-based carboxymethyl chitosan-ZnO nanocomposite for wound-healing applications.ogel for effective bleeding control. Int. J. Biol. Macromol. 2019, 129, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Mellado, C.; Figueroa, T.; Báez, R.; Castillo, R.; Melendrez, M.; Schulz, B.; Fernández, K. Development of Graphene Oxide Composite Aerogel with Proanthocyanidins with Hemostatic Properties As a Delivery System. ACS Appl. Mater. Interfaces 2018, 10, 7717–7729. [Google Scholar] [CrossRef]
- Kumar, V.A.; Wickremasinghe, N.C.; Shi, S.; Hartgerink, J.D. Nanofibrous Snake Venom Hemostat. ACS Biomater. Sci. Eng. 2015, 1, 1300–1305. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, J.; Zhao, N.; Cai, C.; Qian, Z.; Si, F.; Luo, H.; Guo, J.; Lai, X.; Shao, L.; et al. Superhydrophobic/Superhydrophilic Janus Fabrics Reducing Blood Loss. Adv. Healthc. Mater. 2018, 7, e1701086. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. Commun. 2019, 10, 5562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, J.; Li, M.; Liu, Z.; Wang, X.; Zhang, L.; Wang, Z. Hydrogel-Based Biomaterials Engineered from Natural-Derived Polysaccharides and Proteins for Hemostasis and Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 780187. [Google Scholar] [CrossRef]
- Hangge, P.; Stone, J.; Albadawi, H.; Zhang, Y.S.; Khademhosseini, A.; Oklu, R. Hemostasis and nanotechnology. Cardiovasc. Diagn. Ther. 2017, 7, 267–275. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, H.; Min, N.; Wei, Y.; Li, X.; Li, X. Application and outlook of topical hemostatic materials: A narrative review. Ann. Transl. Med. 2021, 9, 577. [Google Scholar] [CrossRef]
- Thon, J.N.; Italiano, J.E. Platelets: Production, morphology and ultrastructure. Handb. Exp. Pharmacol. 2012, 210, 3–22. [Google Scholar]
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Coupland, L.A.; Crispin, P.J.; Fitzgerald, A.; Nisbet, D.R.; Tsuzuki, T. Non-oxidized cellulose nanofibers as a topical hemostat: In vitro thromboelastometry studies of structure vs function. Carbohydr. Polym. 2021, 265, 118043. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sorrells, M.G.; Lam, W.A.; Neeves, K.B. Physical forces regulating hemostasis and thrombosis: Vessels, cells, and molecules in illustrated review. Res. Pract. Thromb. Haemost. 2021, 5, 12548. [Google Scholar] [CrossRef]
- Fröhlich, E. Action of Nanoparticles on Platelet Activation and Plasmatic Coagulation. Curr. Med. Chem. 2016, 23, 408–430. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Maitz, M.F.; Grasso, S.; Werner, C.; Kanse, S.M. A Positively Charged Surface Triggers Coagulation Activation Through Factor VII Activating Protease (FSAP). ACS Appl. Mater. Interfaces 2017, 9, 40107–40116. [Google Scholar] [CrossRef]
- Barshtein, G.; Arbell, D.; Yedgar, S. Hemodynamic Functionality of Transfused Red Blood Cells in the Microcirculation of Blood Recipients. Front. Physiol. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Kaibara, M. Activation of factor IX by erythrocyte membranes causes intrinsic coagulation. Blood Coagul. Fibrinolysis 2002, 13, 489–496. [Google Scholar] [CrossRef]
- Mehri, R.; Mavriplis, C.; Fenech, M. Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system. PLoS ONE 2018, 13, 0199911. [Google Scholar] [CrossRef]
- Matus, M.F.; Vilos, C.; Cisterna, B.A.; Fuentes, E.; Palomo, I. Nanotechnology and primary hemostasis: Differential effects of nanoparticles on platelet responses. Vascul. Pharmacol. 2018, 101, 1–8. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, J.J.; Bai, Q.; Quan, Y.; Li, Z.; Chen, W.; Gao, Q.; Zhang, Y.; Lu, T. Preparation and hemostatic mechanism of bioactive glass-based membrane-like structure camouflage composite particles. Mater. Des. 2022, 223, 111116. [Google Scholar] [CrossRef]
- Milić, M.; Vuković, B.; Barbir, R.; Pem, B.; Milić, M.; Šerić, V.; Frőhlich, E.; Vinković Vrček, I. Effect of differently coated silver nanoparticles on hemostasis. Platelets 2021, 32, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Potter, T.M.; Rodriguez, J.C.; Neun, B.W.; Ilinskaya, A.N.; Cedrone, E.; Dobrovolskaia, M.A. In Vitro Assessment of Nanoparticle Effects on Blood Coagulation. Methods Mol. Biol. 2018, 1682, 103–124. [Google Scholar]
- Tran, H.D.N.; Moonshi, S.S.; Xu, Z.P.; Ta, H.T. Influence of nanoparticles on the haemostatic balance: Between thrombosis and haemorrhage. Biomater. Sci. 2021, 10, 10–50. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, W.; Xiao, Y.; Du, B.; Zhang, X.; Wen, M.; Lai, C.; Huang, Y.; Sheng, L. Multifunctional, Robust, and Porous PHBV-GO/MXene Composite Membranes with Good Hydrophilicity, Antibacterial Activity, and Platelet Adsorption Performance. Polymers 2021, 13, 3748. [Google Scholar] [CrossRef]
- Shi, S.; Lan, X.; Feng, J.; Ni, Y.; Zhu, M.; Sun, J.; Wang, J. Hydrogel loading 2D montmorillonite exfoliated by anti-inflammatory Lycium barbarum L. polysaccharides for advanced wound dressing. Int. J. Biol. Macromol. 2022, 209, 50–58. [Google Scholar] [CrossRef]
- Nikoobakht, B.; Li, X. Two-dimensional nanomembranes: Can they outperform lower dimensional nanocrystals? ACS Nano 2012, 6, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Modery-Pawlowski, C.L.; Tian, L.L.; Ravikumar, M.; Wong, T.L.; Sen Gupta, A. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials 2013, 34, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Sarkari, M.; Sunderland, R.; St John, A.E.; White, N.J.; Kastrup, C.J. Platelets loaded with liposome-encapsulated thrombin have increased coagulability. J. Thromb. Haemost. 2018, 16, 1226–1235. [Google Scholar] [CrossRef]
- Donovan, A.J.; Kalkowski, J.; Szymusiak, M.; Wang, C.; Smith, S.A.; Klie, R.F.; Morrissey, J.H.; Liu, Y. Artificial Dense Granules: A Procoagulant Liposomal Formulation Modeled after Platelet Polyphosphate Storage Pools. Biomacromolecules 2016, 17, 2572–2581. [Google Scholar] [CrossRef]
- Nakielski, P.; Pierini, F. Blood interactions with nano- and microfibers: Recent advances, challenges and applications in nano- and microfibrous hemostatic agents. Acta Biomater. 2019, 84, 63–76. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Yu, J.; Ding, B. Nanofibrous hemostatic materials: Structural design, fabrication methods, and hemostatic mechanisms. Acta Biomater. 2022, 154, 49–62. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Chen, Y.; Shen, Y.; Yu, F.; Wang, W.; Yuan, Z.; Morsi, Y.; Wu, J.; Mo, X. Conjugate Electrospun 3D Gelatin Nanofiber Sponge for Rapid Hemostasis. Adv. Healthc. Mater. 2021, 10, 2100918. [Google Scholar] [CrossRef]
- Chen, S.; Carlson, M.A.; Zhang, Y.S.; Hu, Y.; Xie, J. Fabrication of injectable and superelastic nanofiber rectangle matrices (“peanuts”) and their potential applications in hemostasis. Biomaterials 2018, 179, 46–59. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Li, Y.; Qin, Y.; Wen, Y.; Zhang, X. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019, 204, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ye, Z.; Zhu, H.; Zhao, X. Self-Assembling Peptide Nanofibrous Hydrogel on Immediate Hemostasis and Accelerative Osteosis. Biomacromolecules 2015, 16, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.K.; Kassam, H.A.; Lee, R.H.; Bergmeier, W.; Peters, E.B.; Gillis, D.C.; Dandurand, B.R.; Rouan, J.R.; Karver, M.R.; Struble, M.D.; et al. Development of Optimized Tissue-Factor-Targeted Peptide Amphiphile Nanofibers to Slow Noncompressible Torso Hemorrhage. ACS Nano 2020, 14, 6649–6662. [Google Scholar] [CrossRef]
- Morgan, C.E.; Dombrowski, A.W.; Rubert Pérez, C.M.; Bahnson, E.S.; Tsihlis, N.D.; Jiang, W.; Jiang, Q.; Vercammen, J.M.; Prakash, V.S.; Pritts, T.A.; et al. Tissue-Factor Targeted Peptide Amphiphile Nanofibers as an Injectable Therapy to Control Hemorrhage. ACS Nano 2016, 10, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2022, 15, 108. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Mehrali, M.; Thakur, A.; Pennisi, C.P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced Hydrogels for Tissue Engineering: Biomaterials that are Compatible with Load-Bearing and Electroactive Tissues. Adv. Mater. 2017, 29, 1603612. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, S.; Shen, J.; Chen, Y.; Xiao, Y. A Composite Hydrogel Based on Pectin/Cellulose via Chemical Cross-Linking for Hemorrhage. Front. Bioeng. Biotechnol. 2021, 8, 627351. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, Y.; Zhou, Y.; Tan, C.; Liu, M. Gold@Halloysite nanotubes-chitin composite hydrogel with antibacterial and hemostatic activity for wound healing. Bioact. Mater. 2022, 20, 355–367. [Google Scholar] [CrossRef]




| Hemostatic Method | Principle | Advantage | Disadvantage |
|---|---|---|---|
| Tourniquet hemostasis [12] | Use a hose to deflate blood vessels to stop bleeding. | Firm and reliable. | Wrapping too much will lead to skin damage, and too long will promote tissue ischemia and necrosis. |
| Pneumatic hemostasis [13] | Filling the tourniquet with gas through a high-efficiency air pump can compress the limbs and block the blood flow. | The operation field of vision is clear to avoid minor structural damage. | It will cause paralysis, shock, pain, skin injury, tissue ischemia, and other adverse reactions. |
| Single ligation hemostasis [14] | Hemostatic forceps were used to stop bleeding. The ligated tissue was completely sheathed by a ligation line and then ligated. | It can accurately stop bleeding by single ligation of blood vessels. | Postoperative bleeding may occur due to inaccurate ligation or falling off, and too tight will cut blood vessels. |
| Suture ligation hemostasis [15] | Penetrating ligature hemostasis. | Prevent the ligature wire from falling off. | The suture takes a long time and consumes a lot of sutures, which may lead to uneven tissue alignment. |
| Electrocoagulation hemostasis [16] | The coagulation current of the probe generates high heat, which promotes the edema of the tissue around the trauma, and the compressed vascular cavity becomes smaller or blocked, forming thrombosis and hemostasis. | Simple, safe, and economical. | Too long will cause the wound to become larger and deeper, resulting in bleeding again. |
| Ultrasonic scalpel hemostasis [17] | Through the ultrasonic system in the handle, the kinetic energy on the cutter rod is amplified to cut the tissue. After the tissue in contact with the cutter head absorbs the ultrasonic energy, the protein hydrogen bond breaks, and then solidifies, denatures, and cuts into one. | The utility model has the advantages of a wide application range, a clear field of vision during operation, fast cutting, and small cutting tissue damage. | Slow operation, high price, and limited cutting range. |
| Laser hemostasis [18] | The use of laser coagulation hemostasis, the use of heat energy to evaporate the water in the cells, promotes the degeneration and contraction of vascular wall collagen, and forms small vascular thrombosis. | It has little damage to surrounding tissues, is effective in hemostasis of capillaries and arterioles, and sterilizes at the same time. | It produces toxic smoke and is easy to adhere to after operation. |
| Microwave knife Hemostasis [19] | The radiation of the microwave knife head generates heat energy, and the tissue absorbing heat energy will solidify and stop bleeding after resection. | The hemostatic effect is obvious; it is not easy to produce a burning taste, the wound heals quickly, and the postoperative bleeding is less. | Only coagulate the blood vessels within 3 mm and temporarily block the blood vessels. |
| Radio frequency knife hemostasis [20] | Through RF energy, a small plasma electric field is formed in the electrolyte. After the acceleration energy is sufficient, the energy is transmitted to the tissue to destroy the protein ion bond and coagulate. | The heat effect is small, the damage is small, and the saline drops out at the same time as hemostasis. | It can only solidify blood vessels less than 2 mm. A large amount of normal saline is required in the liquid environment, which makes the operation inconvenient. |
| Argon knife hemostasis [15] | Through the ionized gas conduction of high-frequency current to the tissue, the thermal effect can play a good therapeutic effect. | The gas is stable, the operation does not produce smoke, the fabric damage is small, the continuous solidification is small, and the thermal effect is small. It can form dense eschar, and the hemostatic effect is better. | It can only coagulate blood vessels < 2 mm, which may increase pneumoperitoneum pressure and promote gas embolism and vascular gas embolism. |
| Ablation hemostasis [21] | Using the thermal tissue effect to dehydrate the tissue and further lead to protein degeneration, coagulation, and necrosis, electrocoagulation and electro-resection of the tissue are carried out in surgery, so as to stop bleeding and cutting. | Avoid accidental tissue injury, reduce tissue adhesion, reduce tissue injury and scar, and shorten the postoperative healing time. It is not easy to produce harmful smoke and clear vision during the operation. | The higher the tissue temperature, the deeper the damage and the worse the safety. |
| Type | Hemostatic Materials | Advantage | Disadvantage |
|---|---|---|---|
| Polysaccharide-based Hemostatic Hydrogels [30] | Chitosan | Good biocompatibility and degradability, antibacterial and healing-promoting ability, and excellent hemostatic and adhesive properties. | It can be used in patients with coagulopathy. But hemostasis in the wound of extensive bleeding is not very satisfactory. |
| Hyaluronic Acid | The ability of rapid hemostasis, accelerating wound healing and preventing infection, is similar to that of fibrin glue. | Poor mechanical properties. | |
| Alginate | Can accelerate platelet aggregation to accelerate hemostasis, and has good adhesion. | It is suitable for filling the wound, especially the deep and wide surgical cavity, after endoscopic surgery. | |
| Cellulose | Low cost and excellent mechanical properties. | It is suitable for packaging, application, stuffing, and other operations hemostasis for capillary arterioles and venous bleeding, but it is not suitable for the treatment of peripheral nerve-rich wounds and irregular lacerations. | |
| Protein-Based Hemostatic Hydrogels [30] | Gelatin | Effective control of small blood vessel bleeding, absorption by the body within 4–6 weeks, neutral, can be used with a physiological hemostatic agent. | Water swelling may compress nerves; Use around the site of arterial bleeding may cause displacement of sponges; Use in vascular lacunae may cause embolism. |
| Silk | Has unique physical and chemical properties, good mechanical strength, and certain hemostatic abilities. | SF has drawbacks such as brittleness, easy fragmentation, and difficulty in generating a uniform thickness. Further studies are warranted to create a new array of SF-based hemostatic agents. | |
| Elastin | Components of the extracellular matrix in the vasculature, skin, and lung. | It is insoluble and has poor structural stability. | |
| Inorganic hemostatic agent [31] | Zeolites | Contains inert mineral particles. When it is spread on a wound, the inert particles in it absorb water and clot blood factors. | It cannot achieve rapid hemostasis, and is less effective in arterial bleeding and coagulopathic patients. |
| Kaolin | An aluminosilicate clay that activates hemostasis mechanisms. | It cannot achieve rapid hemostasis, and is less effective in arterial bleeding and coagulopathic patients. | |
| Biologically active agents [31] | Fibrin | Fibrin adhesive accelerates the clot tissue in the application area, achieving stable and rapid hemostasis without mixing or other preparation. | The price is high, and there is a risk of disease transmission. |
| Thrombin | Local thrombin activates platelets and constrains blood vessels; simple to use and quick to take effect. | The use of animal thrombin may cause an immune response and increase the likelihood of blood clots forming. | |
| Tranexamic acid | A synthetic lysine analog that acts by blocking the lysine binding site on plasminogen and preventing its activation. | ||
| Recombinant factor VIIa (rFVIIa) | Significantly reduced transfusion volumes. | Resulting in reduced utilization of blood products in patients with coagulation diseases, high cost, potentially harmful effects, and difficult storage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wang, J.; Xu, T.; Yao, H.; Yu, L.; Huang, D. Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis. Molecules 2023, 28, 5264. https://doi.org/10.3390/molecules28135264
Du J, Wang J, Xu T, Yao H, Yu L, Huang D. Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis. Molecules. 2023; 28(13):5264. https://doi.org/10.3390/molecules28135264
Chicago/Turabian StyleDu, Jian, Jingzhong Wang, Tao Xu, Hai Yao, Lili Yu, and Da Huang. 2023. "Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis" Molecules 28, no. 13: 5264. https://doi.org/10.3390/molecules28135264
APA StyleDu, J., Wang, J., Xu, T., Yao, H., Yu, L., & Huang, D. (2023). Hemostasis Strategies and Recent Advances in Nanomaterials for Hemostasis. Molecules, 28(13), 5264. https://doi.org/10.3390/molecules28135264








