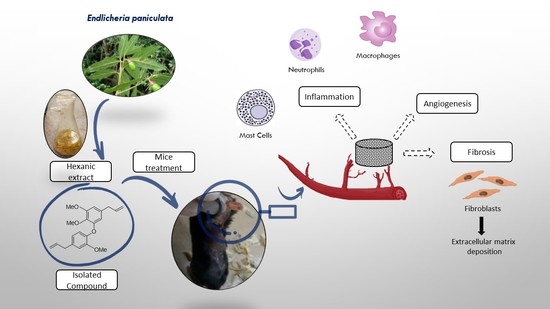
An In Vivo Assessment of the Effect of Hexane Extract from Endlicheria paniculata Branches and Its Main Compound, Methyldehydrodieugenol B, on Murine Sponge-Induced Inflammation
Abstract
1. Introduction
2. Results and Discussion
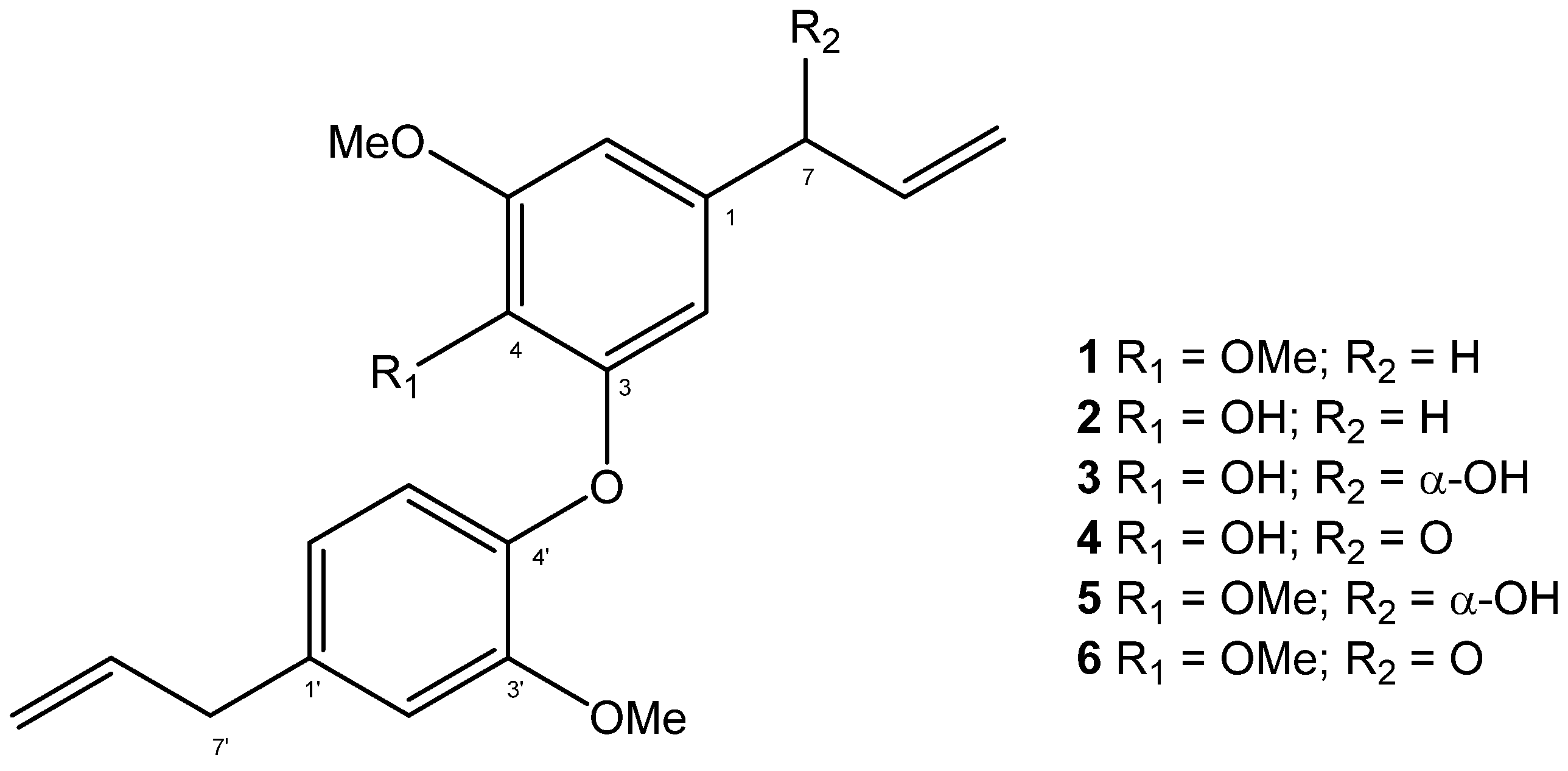
2.1. Chemical Analysis
2.2. In Silico Analysis of the Pharmacokinetic Parameters of Compounds 1–6
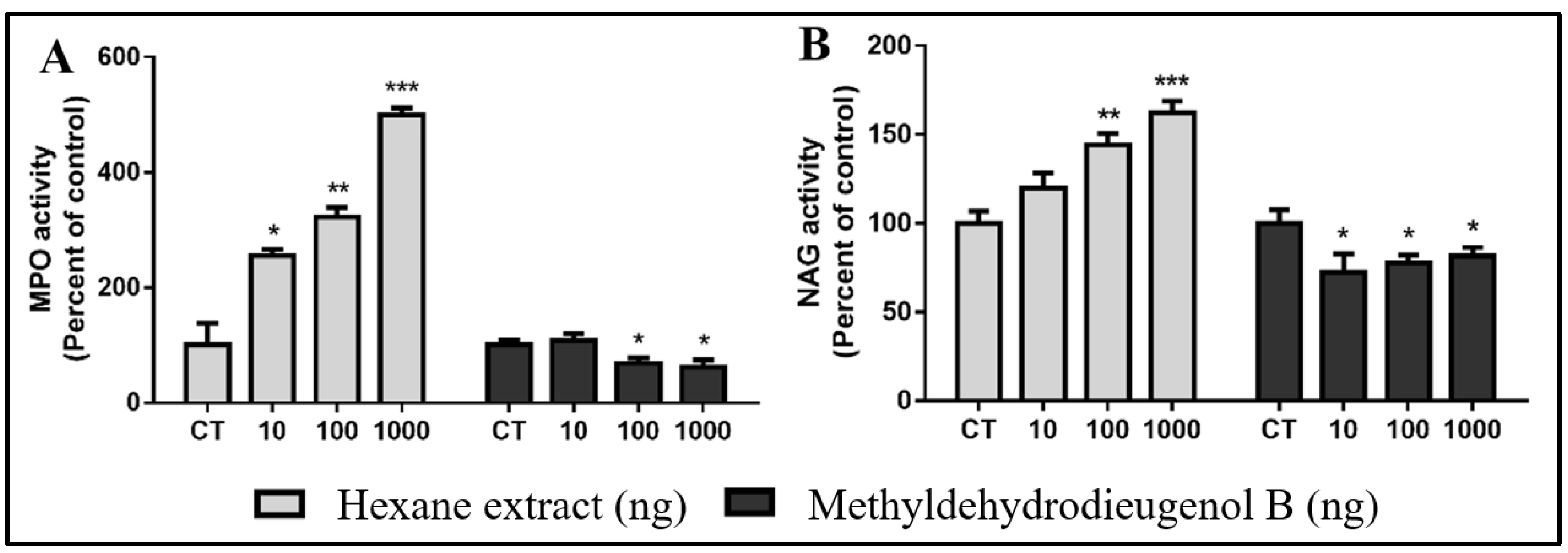
2.3. Evaluation of Inflammatory Response Induced by HEB and Methyldehydrodieugenol B in Sponge Implants
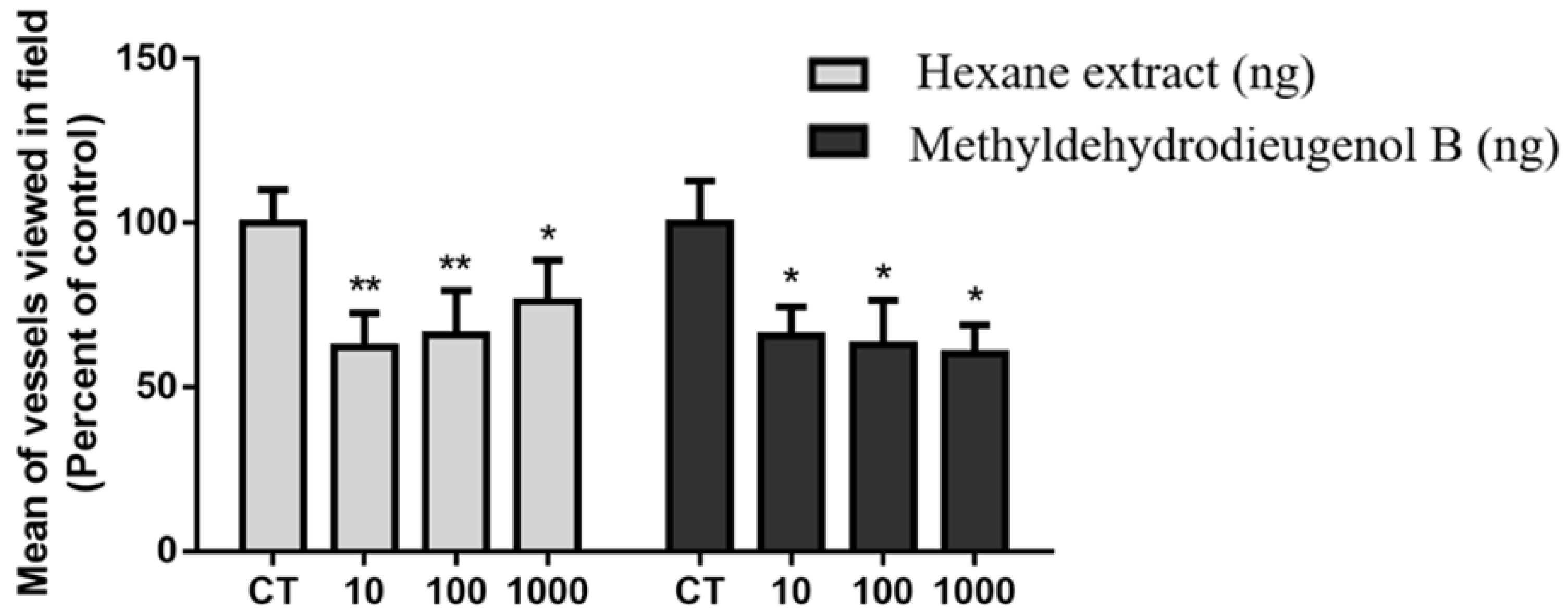
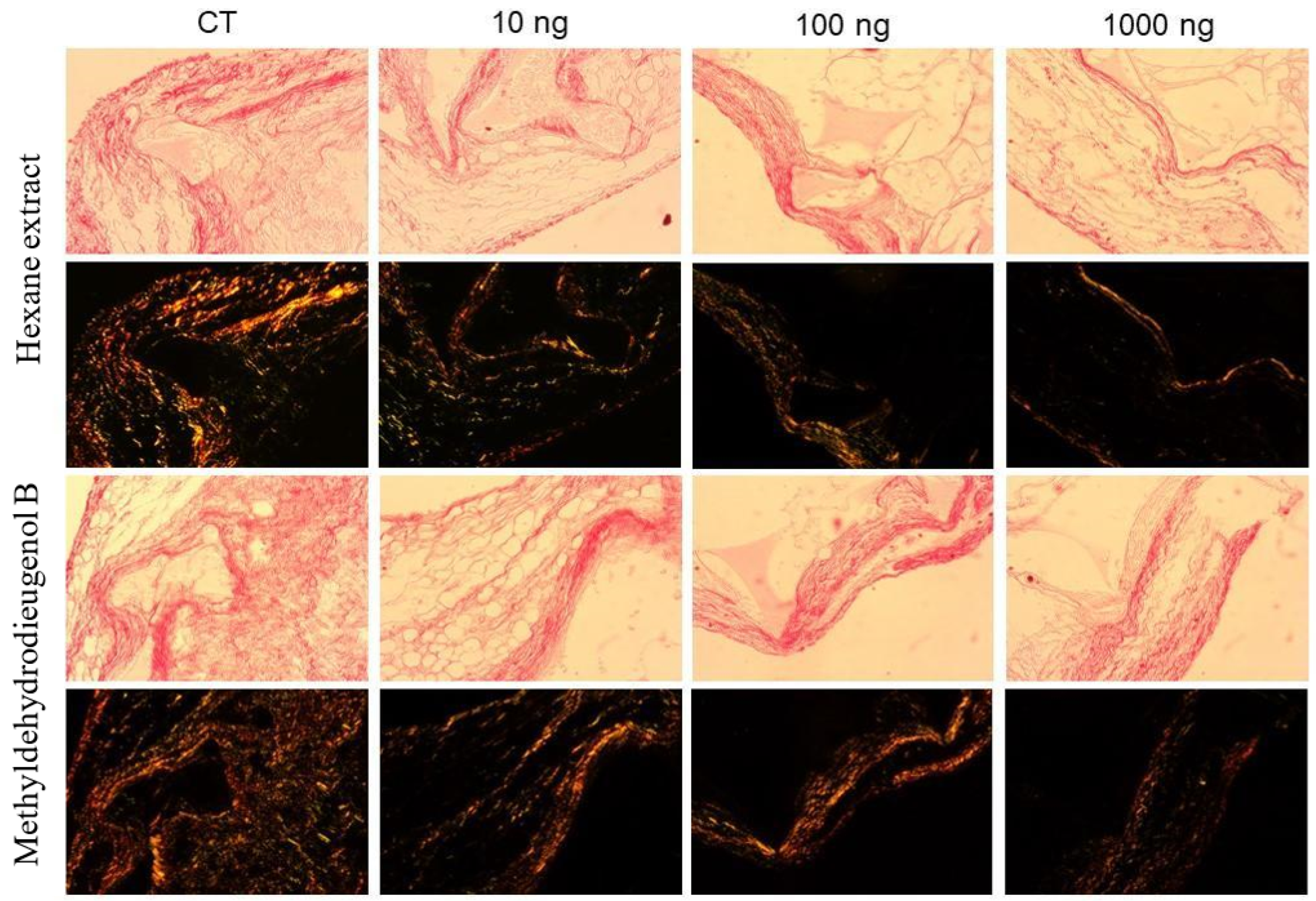
2.4. Antiangiogenic Activity of HEB and Methyldehydrodieugenol B
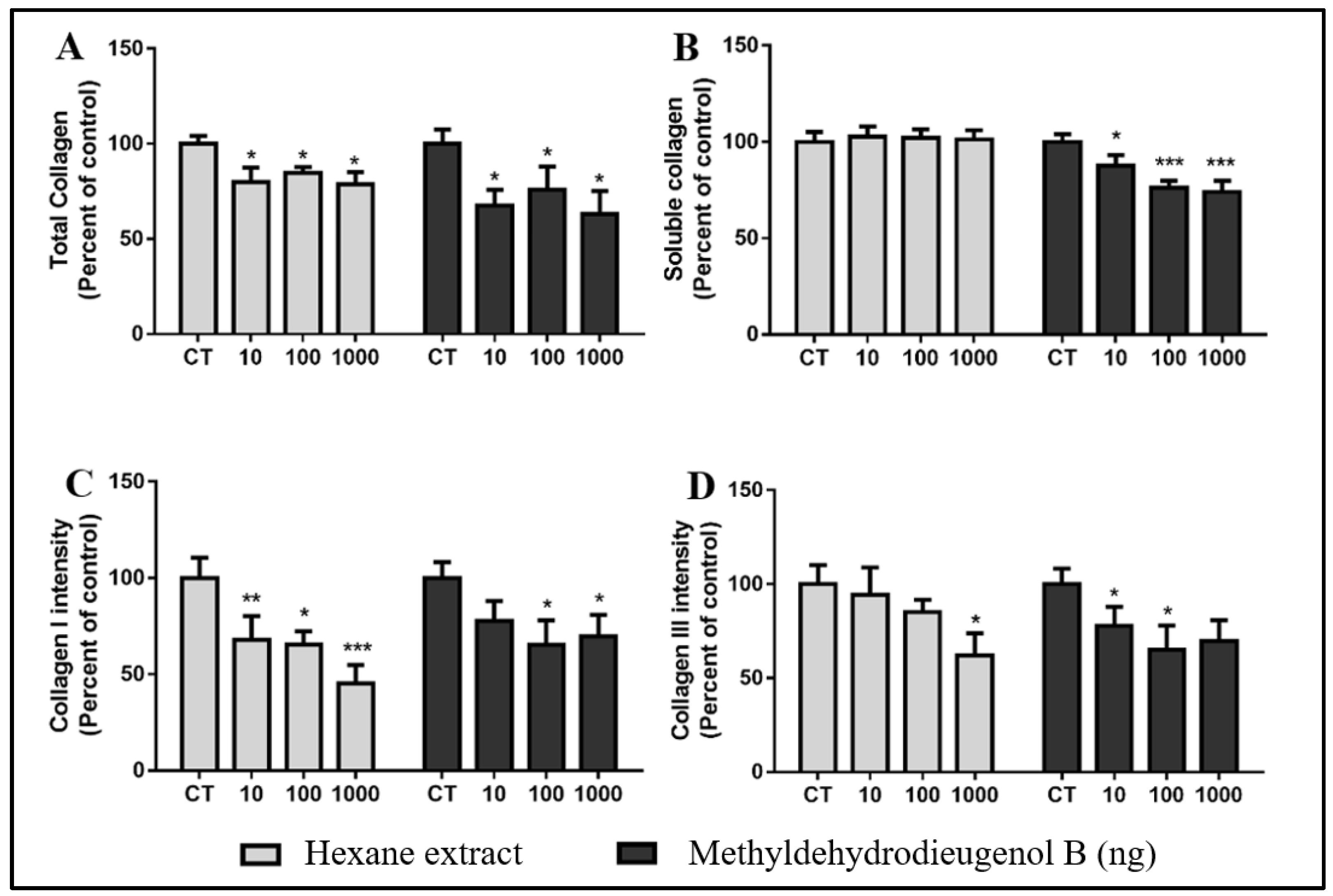
2.5. Anti-Fibrogenic Effects of HEB and Methyldehydrodieugenol B
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction of Plant Material
3.4. Chemical Analysis of Hexane Extract from Branches (HEB) of E. paniculata
3.5. In Silico Analysis of Pharmacokinetic Parameters
3.6. Ethics Statement
3.7. Animals
3.8. Surgical Implantation of the Sponge Discs and Treatment Protocol
3.9. Myeloperoxidase (MPO) and N-acetyl-β-D-glucosaminidase (NAG) Activities
3.10. Histological Analysis and Staining
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Straub, R.H.; Schradin, C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public. Health 2016, 2016, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.-E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Souza, R.A.C.; Ferreira, B.A.; Moura, F.B.R.; Costa Silva, T.D.; Cavalcanti, F.; Franca, E.F.; Sousa, R.M.F.; Febronio, J.L.; Lago, J.H.G.; Araújo, F.A.; et al. Dehydrodieugenol B and hexane extract from Endlicheria paniculata regulate inflammation, angiogenesis, and collagen deposition induced by a murine sponge model. Fitoterapia 2020, 147, 104767. [Google Scholar] [CrossRef]
- Domiciano, T.P.; Dalalio, M.M.; Silva, E.L.; Ritter, A.M.; Estevão-Silva, C.F.; Ramos, F.S.; Caparroz-Assef, S.M.; Cuman, R.K.; Bersani-Amado, C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 331–338. [Google Scholar] [CrossRef]
- Filho, A.; Silva, I.S.; Sousa, F.B.M.; de Souza, L.K.M.; Gomes, B.D.S.; Gonçalves, R.L.G.; de Rezende, D.C.; Cunha, F.V.M.; Wong, D.V.T.; Júnior, R.; et al. Inhibition of neutrophil migration and reduction of oxidative stress by ethyl p-coumarate in acute and chronic inflammatory models. Phytomedicine 2019, 57, 9–17. [Google Scholar] [CrossRef]
- Chien, T.Y.; Chen, L.G.; Lee, C.J.; Lee, F.Y.; Wang, C.C. Anti-inflammatory constituents of Zingiber zerumbet. Food Chem. 2008, 110, 584–589. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Grecco, S.S.; Costa-Silva, T.A.; Jerz, G.; de Sousa, F.S.; Londero, V.S.; Galuppo, M.K.; Lima, M.L.; Neves, B.J.; Andrade, C.H.; Tempone, A.G.; et al. Neolignans from leaves of Nectandra leucantha (Lauraceae) display in vitro antitrypanosomal activity via plasma membrane and mitochondrial damages. Chem. Biol. Interact. 2017, 277, 55–61. [Google Scholar] [CrossRef] [PubMed]
- da Costa-Silva, T.A.; Grecco, S.S.; de Sousa, F.S.; Lago, J.H.G.; Martins, E.G.A.; Terrazas, C.A.; Varikuti, S.; Owens, K.L.; Beverley, S.M.; Satoskar, A.R.; et al. Immunomodulatory and antileishmanial activity of phenylpropanoid dimers isolated from Nectandra leucantha. J. Nat. Prod. 2015, 78, 653–657. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, F.S.; Grecco, S.S.; Girola, N.; Azevedo, R.A.; Figueiredo, C.R.; Lago, J.H.G. Neolignans isolated from Nectandra leucantha induce apoptosis in melanoma cells by disturbance in mitochondrial integrity and redox homeostasis. Phytochemistry 2017, 140, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.; Hutt, A.; Kirton, S. In silico prediction of aqueous solubility using simple QSPR models: The importance of phenol and phenol-like moieties. J. Chem. Inform. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast cell biology at molecular level: A comprehensive review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef]
- Choi, H.G.; Choi, Y.H.; Kim, J.H.; Kim, H.-H.; Kim, S.-H.; Kim, J.A.; Lee, S.M.; Na, M.; Lee, S.H. A new neolignan and lignans from the stems of Lindera obtusiloba Blume and their anti-allergic inflammatory effects. Arch. Pharmacal. Res. 2014, 37, 467–472. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Zhang, J.; Duan, X.; Zhang, Y. Large accumulation of collagen and increased activation of mast cells in hearts of mice with hyperlipidemia. Arq. Bras. Cardiol. 2017, 109, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ito, C.; Masubuchi, S.; Itoigawa, M. Licarin A is a candidate compound for the treatment of immediate hypersensitivity via inhibition of rat mast cell line RBL-2H3 cells. J. Pharm. Pharmacol. 2015, 67, 1723–1732. [Google Scholar] [CrossRef]
- Kang, P.; Kim, K.Y.; Lee, H.S.; Min, S.S.; Seol, G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013, 93, 955–961. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug. Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The role of angiogenesis in cancer treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- de Moura, S.A.L.; Negri, G.; Salatino, A.; Lima, L.D.d.C.; Dourado, L.P.A.; Mendes, J.B.; Andrade, S.P.; Ferreira, M.A.N.D.; Cara, D.C. Aqueous extract of brazilian green propolis: Primary components, evaluation of inflammation and wound healing by using subcutaneous implanted sponges. Evid. Based Complement. Alternat. Med. 2011, 2011, 748283. [Google Scholar] [CrossRef]
- Shin, D.S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.O. Effect of sinapic acid against dimethylnitrosamine-induced hepatic fibrosis in rats. Arch. Pharm. Res. 2013, 36, 608–618. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, B.; Cao, B.; Wei, F.; Yu, X.; Li, G.F.; Chen, H.; Wei, L.Q.; Wang, P.L. Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. Int. Immunopharmacol. 2017, 48, 67–75. [Google Scholar] [CrossRef]
- Pulivendala, G.; Bale, S.; Godugu, C. Honokiol: A polyphenol neolignan ameliorates pulmonary fibrosis by inhibiting TGF-β/Smad signaling, matrix proteins and IL-6/CD44/STAT3 axis both in vitro and in vivo. Toxicol. Appl. Pharmacol. 2020, 391, 114913. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis-a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Andrade, S.P.; Fan, T.P.; Lewis, G.P. Quantitative in vivo studies on angiogenesis in a rat sponge model. Br. J. Exp. Pathol. 1987, 68, 755–766. [Google Scholar]
- Cassini-Vieira, P.; Araújo, F.A.; da Costa Dias, F.L.; Russo, R.C.; Andrade, S.P.; Teixeira, M.M.; Barcelos, L.S. iNOS activity modulates inflammation, angiogenesis, and tissue fibrosis in polyether-polyurethane synthetic implants. Mediat. Inflamm. 2015, 2015, 138461. [Google Scholar] [CrossRef]
- Barcelos, L.S.; Talvani, A.; Teixeira, A.S.; Vieira, L.Q.; Cassali, G.D.; Andrade, S.P.; Teixeira, M.M. Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1. J. Leukoc. Biol. 2005, 78, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.A.; Toyama, D.; Henrique-Silva, F.; Araújo, F.D.A. Recombinant sugarcane cystatin CaneCPI-5 down regulates inflammation and promotes angiogenesis and collagen deposition in a mouse subcutaneous sponge model. Int. Immunopharmacol. 2021, 96, 107801. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.A.; Norton Filho, A.F.; Deconte, S.R.; Tomiosso, T.C.; Thevenard, F.; Andrade, S.P.; Lago, J.H.G.; Araújo, F.D.A. Sesquiterpene polygodial from Drimys brasiliensis (Winteraceae) down-regulates implant-induced inflammation and fibrogenesis in mice. J. Nat. Prod. 2020, 83, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
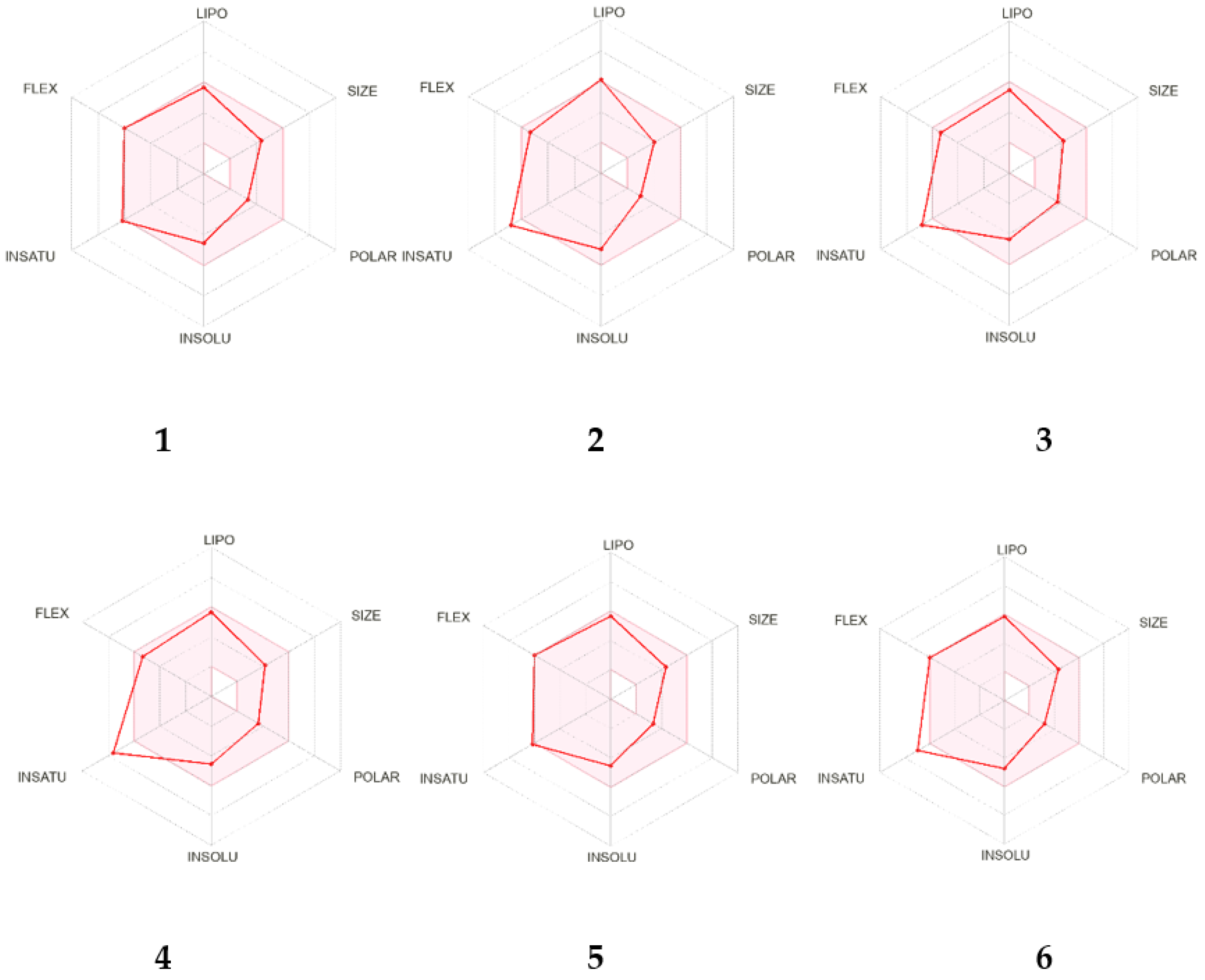


| Parameters | Compounds | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Physicochemical Properties | ||||||
| Num. Heavy atoms | 25 | 24 | 25 | 25 | 26 | 26 |
| Fraction Csp3 | 0.24 | 0.20 | 0.20 | 0.15 | 0.24 | 0.19 |
| Num. Rotatable Bonds | 9 | 8 | 8 | 8 | 9 | 9 |
| Num. H-bonds acceptors | 4 | 4 | 5 | 5 | 5 | 5 |
| Num. H-bonds donors | 0 | 1 | 2 | 1 | 1 | 0 |
| Molar Refractivity | 100.65 | 96.18 | 97.34 | 96.60 | 101.81 | 101.07 |
| TPSA a/Å2 | 36.92 | 47.92 | 68.15 | 64.99 | 57.15 | 53.99 |
| Lipophilicity | ||||||
| log Po/w b | 4.75 | 4.44 | 3.69 | 3.84 | 4.06 | 4.20 |
| Water Solubility | ||||||
| log S | −6.04 | −5.97 | −5.21 | −5.46 | −5.31 | −5.57 |
| Class c | PS | MS | MS | MS | MS | MS |
| Drug-likeness | ||||||
| Lipinski d | Yes | Yes | Yes | Yes | Yes | Yes |
| Ghose e | Yes | Yes | Yes | Yes | Yes | Yes |
| Veber f | Yes | Yes | Yes | Yes | Yes | Yes |
| Egan g | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Medicinal Chemistry | ||||||
| PAINS | 0 alert | 0 alert | 0 alert | 0 alert | 0 alert | 0 alert |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, B.A.; Souza, R.A.C.; de Moura, F.B.R.; Silva, T.d.C.; Adriano, T.d.S.; Franca, E.d.F.; de Sousa, R.M.F.; Araújo, F.d.A.; Lago, J.H.G.; de Oliveira, A. An In Vivo Assessment of the Effect of Hexane Extract from Endlicheria paniculata Branches and Its Main Compound, Methyldehydrodieugenol B, on Murine Sponge-Induced Inflammation. Molecules 2023, 28, 5247. https://doi.org/10.3390/molecules28135247
Ferreira BA, Souza RAC, de Moura FBR, Silva TdC, Adriano TdS, Franca EdF, de Sousa RMF, Araújo FdA, Lago JHG, de Oliveira A. An In Vivo Assessment of the Effect of Hexane Extract from Endlicheria paniculata Branches and Its Main Compound, Methyldehydrodieugenol B, on Murine Sponge-Induced Inflammation. Molecules. 2023; 28(13):5247. https://doi.org/10.3390/molecules28135247
Chicago/Turabian StyleFerreira, Bruno Antonio, Rafael Aparecido Carvalho Souza, Francyelle Borges Rosa de Moura, Tiara da Costa Silva, Tais da Silva Adriano, Eduardo de Faria Franca, Raquel Maria Ferreira de Sousa, Fernanda de Assis Araújo, João Henrique Ghilardi Lago, and Alberto de Oliveira. 2023. "An In Vivo Assessment of the Effect of Hexane Extract from Endlicheria paniculata Branches and Its Main Compound, Methyldehydrodieugenol B, on Murine Sponge-Induced Inflammation" Molecules 28, no. 13: 5247. https://doi.org/10.3390/molecules28135247
APA StyleFerreira, B. A., Souza, R. A. C., de Moura, F. B. R., Silva, T. d. C., Adriano, T. d. S., Franca, E. d. F., de Sousa, R. M. F., Araújo, F. d. A., Lago, J. H. G., & de Oliveira, A. (2023). An In Vivo Assessment of the Effect of Hexane Extract from Endlicheria paniculata Branches and Its Main Compound, Methyldehydrodieugenol B, on Murine Sponge-Induced Inflammation. Molecules, 28(13), 5247. https://doi.org/10.3390/molecules28135247