Comparison of Salvianolic Acid A Adsorption by Phenylboronic-Acid-Functionalized Montmorillonites with Different Intercalators
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of PMP and PMK
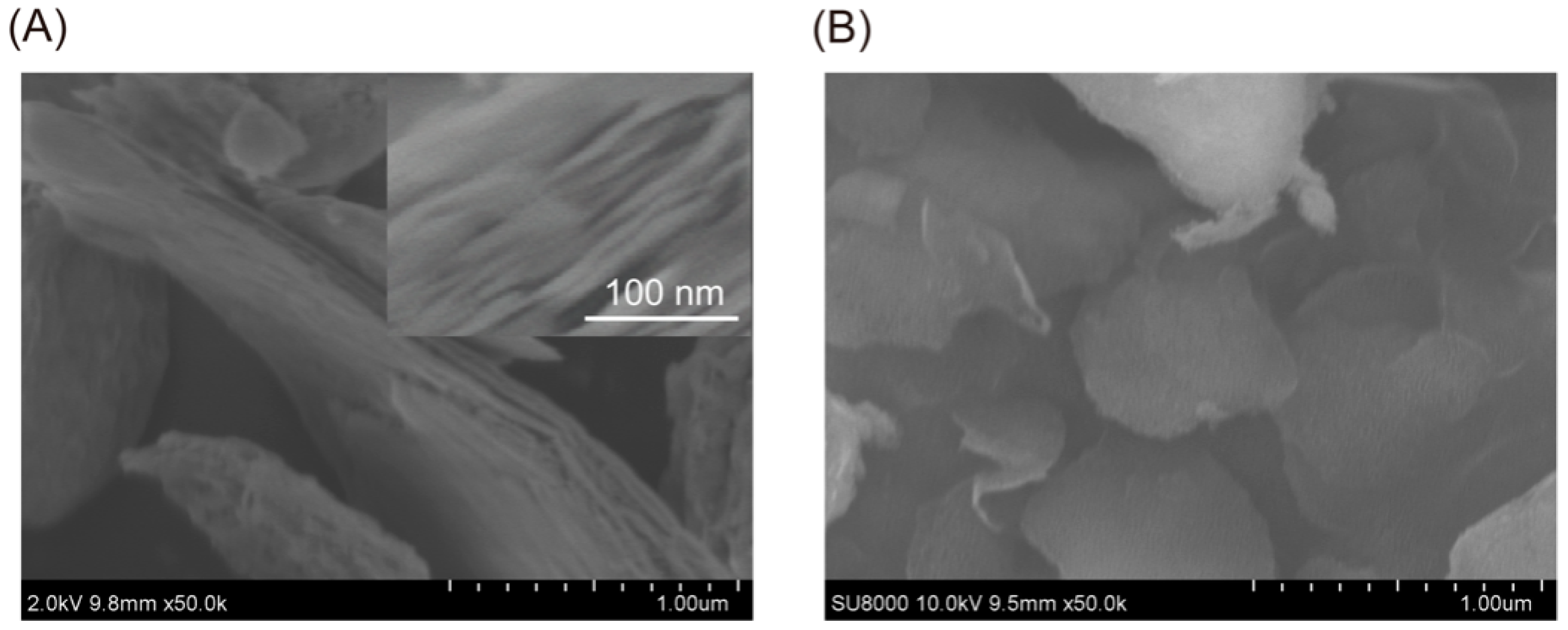
2.1.1. SEM Analysis
2.1.2. XRD Analysis
2.1.3. BET Analysis
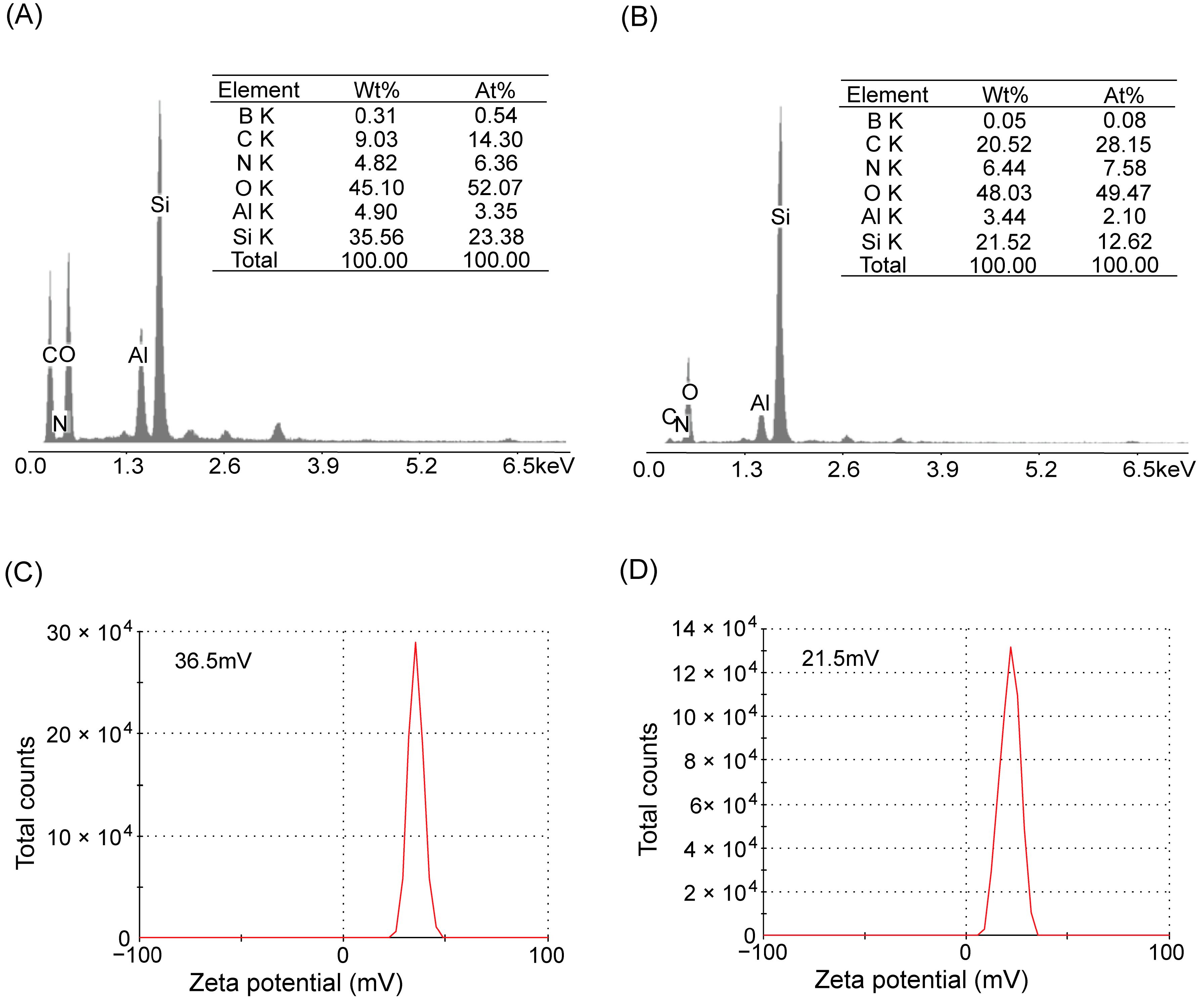
2.1.4. EDX and Zeta Potential Analysis
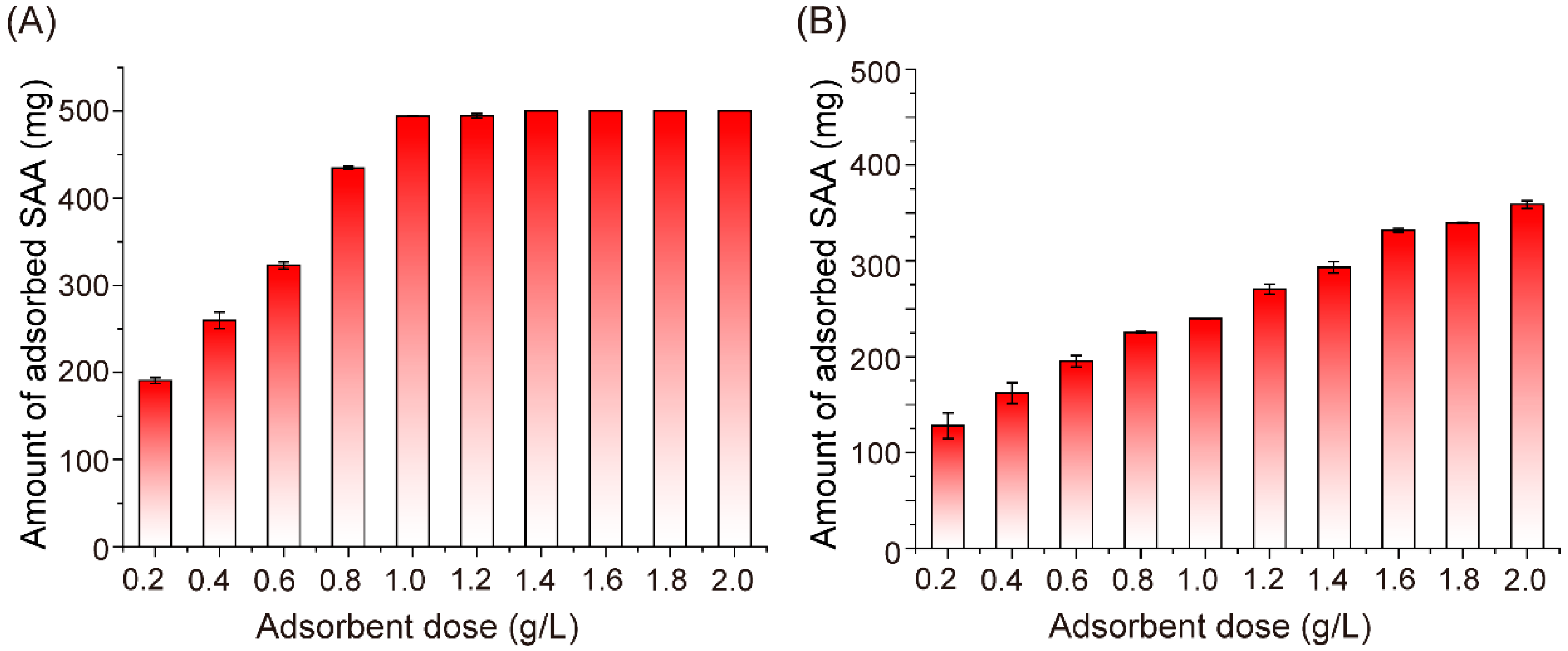
2.2. Effect of Adsorbent Concentration on SAA Adsorption
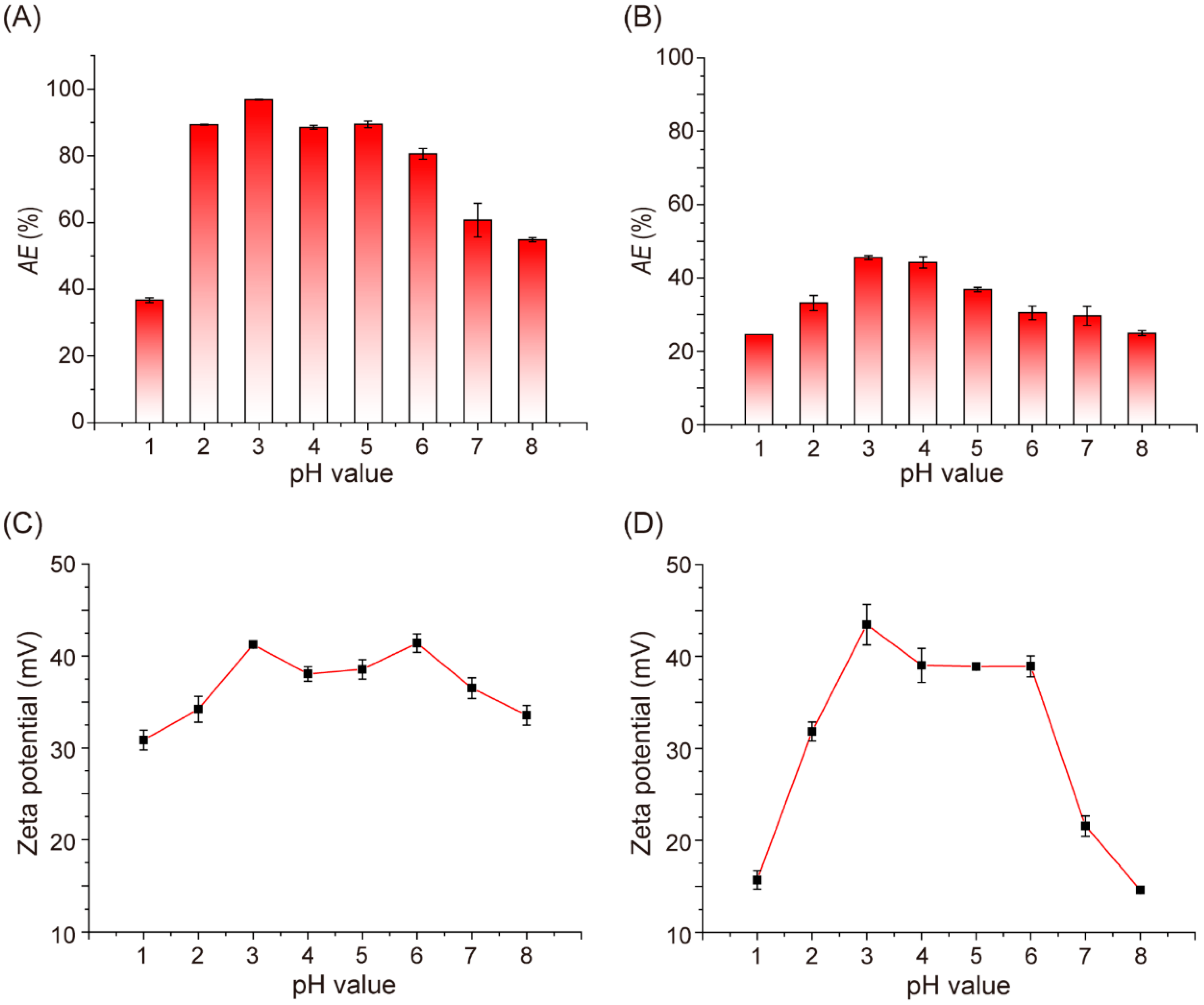
2.3. Effect of pH on SAA Adsorption by PMP and PMK
2.4. Effects of Contact Time and Temperature on SAA Adsorption by PMP and PMK
2.5. Adsorption Isotherms of PMP and PMK
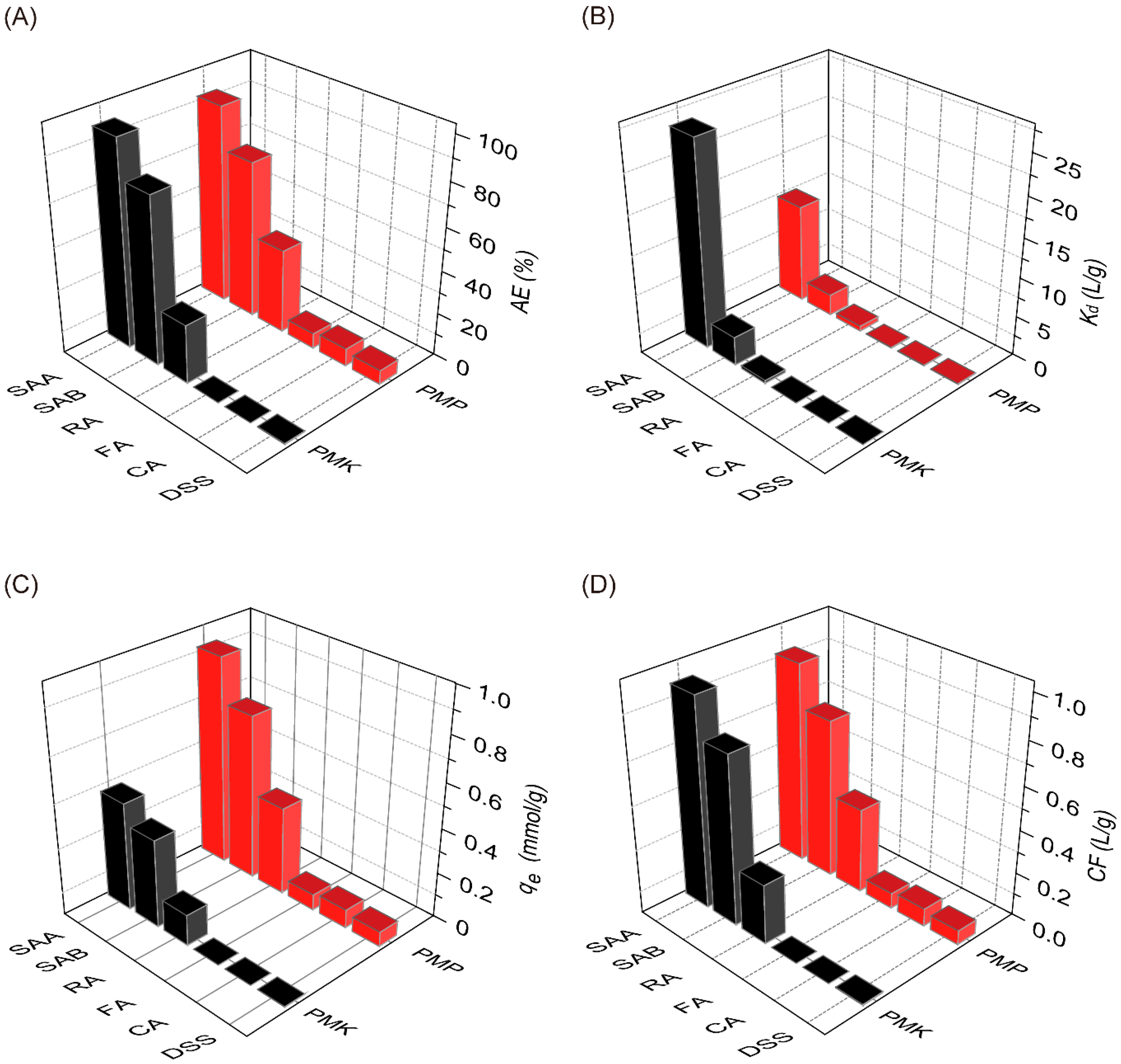
2.6. Selectivity of PMP and PMK
2.7. Desorption Study
3. Materials and Experiments
3.1. Materials
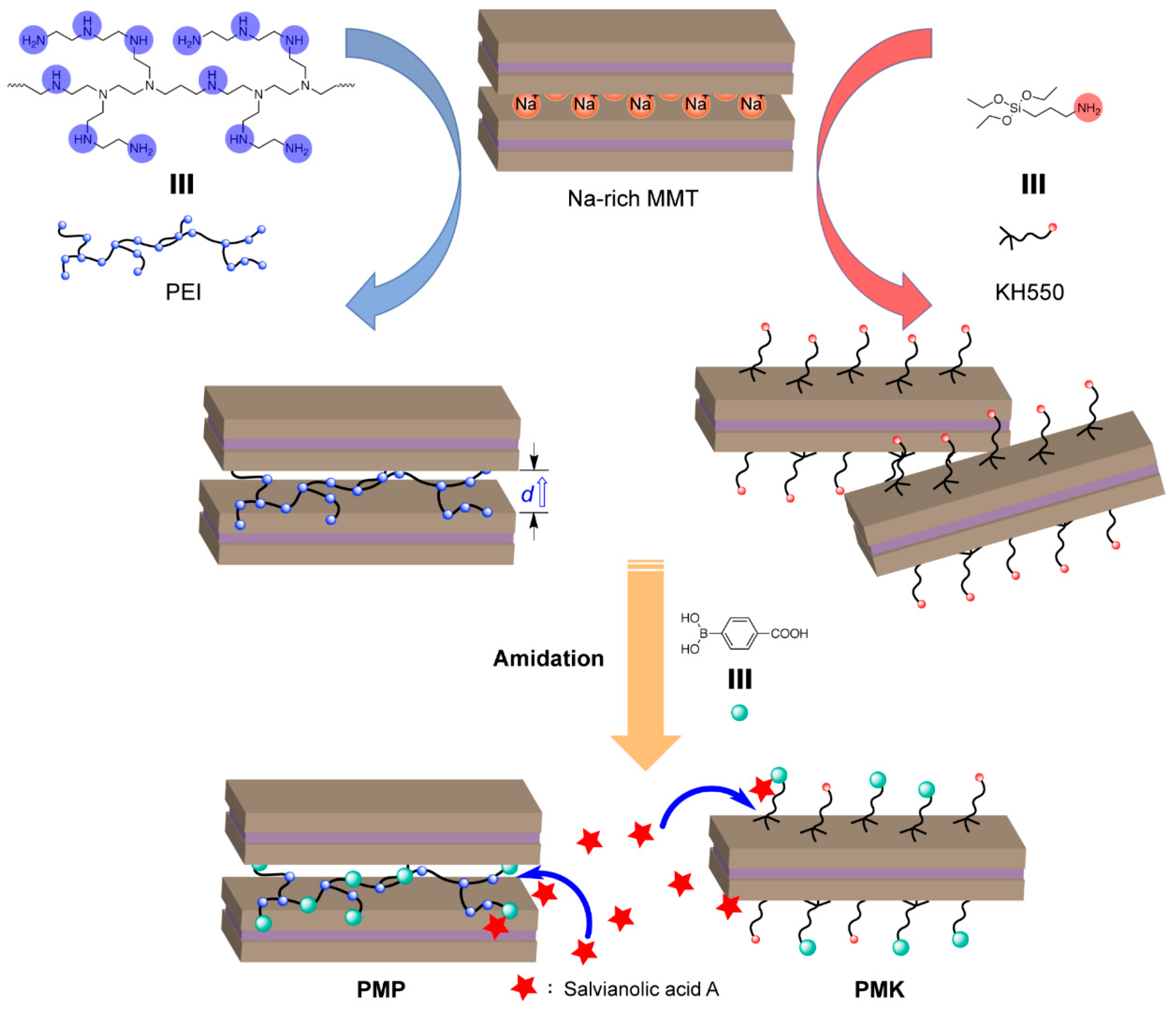
3.2. Preparation of Phenylboronic-Acid-Functionalized Montmorillonites with PEI (PMP)
3.3. Preparation of Phenylboronic-Acid-Functionalized Montmorillonites with KH550 (PMK)
3.4. Adsorption of Salvianolic Acid A by PMP and PMK
3.5. Desorption Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-m.; Xu, S.-w.; Liu, P.-q. Salvia miltiorrhiza burge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Fan, J.-Y.; Horie, Y.; Miura, S.; Cui, D.-H.; Ishii, H.; Hibi, T.; Tsuneki, H.; Kimura, I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol. Ther. 2008, 117, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.D.; Liu, N.N.; Zhang, S.; Ma, G.D.; Yang, H.G.; Kong, L.L.; Du, G.H. Salvianolic acid A prevented cerebrovascular endothelial injury caused by acute ischemic stroke through inhibiting the Src signaling pathway. Acta Pharmacol. Sin. 2021, 42, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Zhao, Y.; Yang, X.; Fang, L.; Wang, S.; Shi, L.; Yu, X.; Wang, S.; Yang, H.; et al. Research progress of salvianolic acid A. Zhongguo Zhongyao Zazhi 2011, 36, 2603–2609. [Google Scholar] [PubMed]
- Xu, K.; Liu, H.; Liu, D.; Sheng, C.; Shen, J.; Zhang, W. Synthesis of (+)-salvianolic acid A from sodium Danshensu. Tetrahedron 2018, 74, 5996–6002. [Google Scholar] [CrossRef]
- Villanueva-Bermejo, D.; Zahran, F.; Troconis, D.; Villalva, M.; Reglero, G.; Fornari, T. Selective precipitation of phenolic compounds from Achillea millefolium L. Extracts by supercritical anti-solvent technique. J. Supercrit. Fluids 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Counce, R.M.; Counce, R.W.; Watson, J.S.; Labbe, N.; Tao, J. Recovery of phenolic compounds from switchgrass extract. ACS Sustain. Chem. Eng. 2018, 6, 374–379. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, H.; Wang, J.; Liu, Z.; Bi, J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B 2009, 877, 733–737. [Google Scholar] [CrossRef]
- Qian, J.; Kai, G. Application of micro/nanomaterials in adsorption and sensing of active ingredients in traditional Chinese medicine. J. Pharm. Biomed. Anal. 2020, 190, 113548. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, D.-D.; Zhang, J.-W.; Gao, D.; Yang, F.-Q.; Chen, H.; Xia, Z.-N. Amino-terminated supramolecular cucurbit [6] uril pseudorotaxane complexes immobilized on magnetite@silica nanoparticles: A highly efficient sorbent for salvianolic acids. Talanta 2019, 195, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xu, X.; Su, J.; Zeng, W.; Han, B.; Hao, X.; Kai, G. A strategy for effective recovery of salvianolic acid A from Salvia miltiorrhiza (Danshen) through multiple interactions. Compos. Part B 2022, 231, 109563. [Google Scholar] [CrossRef]
- Qian, J.; Zhou, L.; Yang, X.; Hua, D.; Wu, N. Prussian blue analogue functionalized magnetic microgels with ionized chitosan for the cleaning of cesium-contaminated clay. J. Hazard. Mater. 2020, 386, 121965. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liang, G.; Xiao, H.; Qiu, J.; Liu, H.; Ma, S.; Komarneni, S. Immobilization mechanism of As, Mn, Pb and Zn ions in sulfide tailings by the addition of triethylenetetramine-montmorillonite nanocomposite. Chem. Eng. J. 2022, 435, 134817. [Google Scholar] [CrossRef]
- Chellapandi, T.; Madhumitha, G.; Roopan, S.M.; Manjupriya, R.; Arunachalapandi, M.; Pouthika, K.; Elamathi, M. Facile synthesis route for visible active g-C3N5/MK30 nanocomposite and its computationally guided photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2023, 307, 122865. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, L.; Zhang, D.; Wang, F.; Sharma, V.K.; Wang, C. Amido-functionalized carboxymethyl chitosan/montmorillonite composite for highly efficient and cost-effective mercury removal from aqueous solution. J. Colloid Interface Sci. 2019, 554, 479–487. [Google Scholar] [CrossRef]
- Su, J.; Qian, J.; Zeng, W.; Wang, Y.; Kai, G. Effective adsorption of salvianolic acids with phenylboronic acid functionalized polyethyleneimine-intercalated montmorillonite. Sep. Purif. Technol. 2023, 311, 123304. [Google Scholar] [CrossRef]
- Egirani, D.E.; Poyi, N.R.; Wessey, N. Synthesis of a copper(II) oxide-montmorillonite composite for lead removal. Int. J. Miner. Metall. Mater. 2019, 26, 803–810. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Tanhaei, B.; Ayati, A.; Ranjbari, S. Substantial improvement in the adsorption behavior of montmorillonite toward tartrazine through hexadecylamine impregnation. Environ. Res. 2022, 204, 111965. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Li, H.; Jin, B.; Li, Z.; Zhang, T.; Zhu, X. Mechanistic insights into ball milling enhanced montmorillonite modification with tetramethylammonium for adsorption of gaseous toluene. Chemosphere 2022, 296, 133962. [Google Scholar] [CrossRef]
- Yin, X.; Wang, X.; Wu, H.; Takahashi, H.; Inaba, Y.; Ohnuki, T.; Takeshita, K. Effects of NH4+, K+, Mg2+, and Ca2+ on the Cesium Adsorption/Desorption in Binding Sites of Vermiculitized Biotite. Environ. Sci. Technol. 2017, 51, 13886–13894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, J.; Shi, M.; Xia, M.; Wang, F.; Fu, C. Adsorption of two beta-blocker pollutants on modified montmorillonite with environment-friendly cationic surfactant containing amide group: Batch adsorption experiments and Multiwfn wave function analysis. J. Colloid Interface Sci. 2021, 590, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Baysal, G.; Aydın, H.; Hoşgören, H.; Uzan, S.; Karaer, H. Improvement of Synthesis and Dielectric Properties of Polyurethane/Mt-QASs+ (Novel Synthesis). J. Polym. Environ. 2016, 24, 139–147. [Google Scholar] [CrossRef]
- He, H.P.; Tao, Q.; Zhu, J.X.; Yuan, P.; Shen, W.; Yang, S.Q. Silylation of clay mineral surfaces. Appl. Clay Sci. 2013, 71, 15–20. [Google Scholar] [CrossRef]
- Liu, Z.; He, H. Synthesis and applications of boronate affinity materials: From class selectivity to biomimetic specificity. Acc. Chem. Res. 2017, 50, 2185–2193. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Wang, Y.; Zheng, L.; Jin, Y.; Xiao, H. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022, 298, 120103. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, W.; Ren, Z.; Ning, J.; Xu, Y.-Q.; Wang, Y.; Deng, W.-W. Cost-effective and sensitive indicator-displacement array (IDA) assay for quality monitoring of black tea fermentation. Food Chem. 2023, 403, 134340. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, B.H.; Yang, H.-M.; Seo, B.-K.; Lee, K.-W. Enhanced desorption of cs from clays by a polymeric cation-exchange agent. J. Hazard. Mater. 2017, 327, 127–134. [Google Scholar] [CrossRef]
- Fan, S.; Gao, X.; Zhu, D.; Guo, S.; Li, Z. Enhancement mechanism of the organic nano-montmorillonite and its effect on the properties of wood fiber/HDPE composite. Ind. Crops Prod. 2021, 169, 113634. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetensk. Handl. 1898, 4, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Yang, C.Y.; Chen, C.Y.; Chen, C.Y.; Chen, C.W. The Chemically Crosslinked Metal-Complexed Chitosans for Comparative Adsorptions of Cu(II), Zn(II), Ni(II) and Pb(II) Ions in Aqueous Medium. J. Hazard. Mater. 2009, 163, 1068–1075. [Google Scholar] [CrossRef]
- Chunmei, N.; Wenhui, W.; Zhu, W.; Shumin, L.; Jianquan, W. Adsorption of heavy metal ions from aqueous solution by crosslinked carboxymethyl konjac glucomannan. J. Hazard. Mater. 2007, 141, 209–214. [Google Scholar] [CrossRef]
- Ide, T.; Hirayama, Y. How boron is adsorbed by D-glucamine: A density functional theory study. Comput. Theor. Chem. 2019, 1150, 85–90. [Google Scholar] [CrossRef]
- Hu, H.; Ren, Z.; Xi, Y.; Fang, L.; Fang, D.; Yang, L.; Shao, P.; Shi, H.; Yu, K.; Luo, X. Insights into the role of cross-linking agents on polymer template effect: A case study of anionic imprinted polymers. Chem. Eng. J. 2021, 420, 129611. [Google Scholar] [CrossRef]
- Haroon, H.; Gardazi, S.M.H.; Butt, T.A.; Pervez, A.; Mahmood, Q.; Bilal, M. Novel lignocellulosic wastes for comparative adsorption of Cr(VI): Equilibrium kinetics and thermodynamic studies. Pol. J. Chem. Technol. 2017, 19, 6–15. [Google Scholar] [CrossRef]
- Zhou, J.; Saeidi, N.; Wick, L.Y.; Kopinke, F.-D.; Georgi, A. Adsorption of polar and ionic organic compounds on activated carbon: Surface chemistry matters. Sci. Total Environ. 2021, 794, 148508. [Google Scholar] [CrossRef]
- Wu, F.; Li, H.; Pan, Y.; Sun, Y.; Pan, J. Bioinspired construction of magnetic nano stirring rods with radially aligned dual mesopores and intrinsic rapid adsorption of palladium. J. Hazard. Mater. 2023, 441, 129917. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M.; Soylak, M. Adsorption of Pb(II) and Cr(III) from aqueous solution on Celtek clay. J. Hazard. Mater. 2007, 144, 41–46. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids Part I Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]




| Samples | BET Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| Na-rich MMT | 201.9 | 7.4 | 0.375 |
| PMP | 55.4 | 11.9 | 0.166 |
| PMK | 69.9 | 8.8 | 0.153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.; Su, J.; Zeng, W.; Wang, Y.; Hu, Y.; Kai, G. Comparison of Salvianolic Acid A Adsorption by Phenylboronic-Acid-Functionalized Montmorillonites with Different Intercalators. Molecules 2023, 28, 5244. https://doi.org/10.3390/molecules28135244
Qian J, Su J, Zeng W, Wang Y, Hu Y, Kai G. Comparison of Salvianolic Acid A Adsorption by Phenylboronic-Acid-Functionalized Montmorillonites with Different Intercalators. Molecules. 2023; 28(13):5244. https://doi.org/10.3390/molecules28135244
Chicago/Turabian StyleQian, Jun, Jiajia Su, Weihuan Zeng, Yue Wang, Yingyuan Hu, and Guoyin Kai. 2023. "Comparison of Salvianolic Acid A Adsorption by Phenylboronic-Acid-Functionalized Montmorillonites with Different Intercalators" Molecules 28, no. 13: 5244. https://doi.org/10.3390/molecules28135244
APA StyleQian, J., Su, J., Zeng, W., Wang, Y., Hu, Y., & Kai, G. (2023). Comparison of Salvianolic Acid A Adsorption by Phenylboronic-Acid-Functionalized Montmorillonites with Different Intercalators. Molecules, 28(13), 5244. https://doi.org/10.3390/molecules28135244






